Abstract
Glucosylceramide synthase (GCS or GlcT-1), converting ceramide to glucosylceramide, is a key enzyme for the synthesis of glycosphingolipids. Due to its diverse roles in physiology and diseases, GCS may be a disease marker and drug target. Current assays for enzymes including GCS are based on reactions conducted in a test tube using enzyme preparations. Measurement of enzyme activity in laboratory-made conditions cannot directly evaluate the role of GCS in cells. Here, we introduce a new approach to determine GCS cellular activity using fluorescent NBD C6-ceramide in vivo. Cellular GCS transfers UDP-glucose to NBD C6-ceramide and produces NBD C6-glucosylceramide. C6-glucosylceramide is then separated from C6-ceramide by thin-layer chromatography and both are then quantitated by spectrophotometer. This cell-based method is able to quantitate glucosylceramide in pmol range, produced by approximately 50,000 cells or 1.0 mg tissue. This method has been used successfully to evaluate the degrees of GCS enzyme in cells and in tumors subjected to gene manipulation and chemical inhibition. These data indicate that this cell-based fluorescent method is direct, reproducible, and simple for assessing ceramide glycosylation. It is applicable to validate GCS activity in drug-resistant cancers and in other disorders.
Keywords: ceramide, enzyme, glucosylceramide, cells, tumor, thin-layer chromatograph
Glucosylceramide synthase (GCS) (UDP-glucose ceramide glucosyltransferase, UGCG or GlcT-1) is a key enzyme for the synthesis of glycosphingolipids (GSLs) (1–3). GCS (EC2.41.80) transfers glucose residues of UDP-glucose to ceramide (Cer) and produces glucosylceramide (GlcCer) (1). Cer glycosylation by GCS is the first and limited step for GSL synthesis and GlcCer serves as a core structure for more than 300 glycolipids in vertebrates (1, 4, 5). GSLs are highly located in GSL-enriched microdomains (GEMs) and rafts of membrane; these play crucial roles in the properties and biological functions of cells (6, 7). In addition to their roles as building blocks of membranes, GSLs modulate signal transduction and cell adhesion; GSLs are involved in cell proliferation, differentiation, immunoresponse, and oncogenic transformation (8–11). Inhibition of GCS, decreasing GlcCer accumulation, is a treatment option for Gaucher's disease (12). Cer, as a lipid second messenger, mediates many cell-stress responses in embryo development and in tumorigenesis (3, 13–15). Cer-induced apoptosis is correlated to the efficacy of anticancer regimens such as anthracyclines, taxanes, Vinca alkaloid, tumor necrosis factor-α and irradiation (16–18). Recent studies indicate that an enhanced expression of GCS is a cause of cancer drug resistance (19–21) and diabetic insulin resistance (22, 23). Inhibition of Cer glycosylation on GCS is a therapeutic approach to improve treatments for cancer (16, 20) and type 2 diabetes (23).
GCS measurement is essential for evaluating the role of Cer glycosylation in cell functions and for monitoring therapeutic efficiency. After Basu et al.'s work (1), several additional methods have been reported (24–27). Employing UDP-[3H]glucose to track glucose transferring has significantly increased the sensitivity of enzyme measurement and characterized the role of GCS in cancer drug resistance (25, 28, 29). Employing 6-N-[7-nitrobenz-2-oxa-1,3-diazol-4-yl] aminocaproyl-sphingosine (NBD C6-Cer) as cell permeable acceptor for UDP-glucose, TLC (26, 30) or HPLC (27) can measure NBD C6-Cer and N-hexanoyl-NBD-GlcCer (NBD C6-GlcCer) simultaneously. The HPLC method based on NBD C6-Cer has been shown to be a highly sensitive, rapid, and reproducible assay for GCS (27). However, current enzyme assays, including these for GCS, conduct enzymatic reactions in test tubes using classical in vitro conditions and enzyme extracts. Thus, the activity measured in vitro may partially represent GCS cellular activities and lead to an incomplete understanding of its roles in cell functions. In the present study, we report a new TLC-based method that conducts the GCS enzymatic reaction with NBD C6-Cer intracellularly.
EXPERIMENTAL PROCEDURES
Materials
NBD C6-Cer and NBD C6-Cer complexed to BSA (Cer:BSA, 1:4) were purchased from Invitrogen (Carlsbad, CA). NBD C6-GlcCer, NBD C6-GalCer (N-hexanoyl-NBD-galactosylceramide), and D-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol HCl (D-threo-PDMP) were purchased from Matreya (Pleasant Gap, PA). [3H]UDP-glucose (40 Ci/mmol) was purchased from American Radiolabeled Chemicals (St. Louis, MO). Anti-human GCS rabbit serum (GCS 6.2) was kindly provided by Dr. D. L. Marks and Dr. R. E. Pagano (Mayo Clinic and Foundation, Rochester, MN). Other chemicals except indicated were from Sigma-Aldrich (St. Louis. MO) and Fisher Scientific (Pittsburgh, PA).
Cell culture
Drug-resistant NCI/ADR-RES human ovary cancer cells (formerly designated MCF-7/AdrR) (31) were kindly provided by Dr. Kenneth Cowan (University of Nebraska Medical Center Eppley Cancer Center, Omaha, NE) and Dr. Merrill Goldsmith (National Cancer Institute, Bethesda, MD). Doxorubicin-selected SW620Ad (32) human colon cancer cells were kindly provided by Drs. Susan Bates and Antonio Fojo (National Cancer Institute). NCI/ADR-RES/GCS and NCI/ADR-RES/asGCS cells were established by introduction of human GCS gene or its antisense sequence into parental NCI/ADR-RES cells, respectively (19). Cells were maintained in RPMI-1640 medium (Invitrogen) with supplement of 10% FBS, 100 units/ml penicillin, 100 µg/ml streptomycin, and 584 mg/liter L-glutamine. Geneticin (G-418, 400 µg/ml) was added to above culture medium for NCI/ADR-RES/GCS and NCI/ADR-RES/asGCS cells. Cells were cultured in an incubator humidified with 95% air and 5% CO2 at 37°C.
Western blot
Western blot was carried out as described previously (19). Briefly, cells (2 × 106 cells/100 mm dish) were grown in 10% culture medium for 48 h and harvested using NP40 cell lysis buffer (Biosource, Camarillo, CA). Equal amount of proteins (50 µg/lane) were resolved using 4–20% gradient SDS-PAGE (Invitrogen). The transferred blots were blocked with 5% fat-free milk PBS and immunoblotted with anti-GCS serum at 1:500 dilution, at 4°C for overnight. The antigen-antibody in blots was detected by using secondary antibody-conjugated HRP and enzyme-linked chemiluminescence plus substrate (GE Healthcare). The amount of protein was measured using the bicinchoninic acid protein assay (Pierce, Rockford, IL) with BSA as a standard.
Immunohistochemistry
Cells (25,000 cells/chamber) were grown in 4-chamber slide with 10% FBS culture medium for 48 h. After methanol fixation, slides were blocked and then incubated with anti-GCS serum (1:100) in block solution (Vector Laboratories, Burlingame, CA), overnight at 4°C. GCS-antibody on cells was recognized by Alexa Fluor®488 goat anti-rabbit IgG (Invitrogen). Cell nuclei were counterstained with DAPI (4′, 6 diamidino-2-phenylindole) in mounting solution (Vector Laboratories). The slides were observed using Nikon TE-2000 phase contrast microscope, and the images were captured by a Retiga 2300TM monochrome digital camera using IPLabTM image analysis program (Scanalytics Inc., Rockville, MD).
Cellular Cer glycosylation assay
The assays were performed as described previously with modification (21, 26, 30). Briefly, cells (5 × 105 cells/well) were grown 24 h in 6-well plates with 10% FBS RPMI-1640 medium. To inhibit GCS enzyme, NCI/ADR-RES cells were exposed to D-threo-PDMP (2.5 and 10 µM) in 5% FBS RPMI-1640 medium for 4 h at 37°C. Cells were switched to 500 µl of 1% BSA RPMI-1640 medium containing 500 µM NBD C6-Cer complexed to BSA (equal to 100 µM NBD C6-Cer), unless otherwise indicated. After 2 h incubation at 37°C, cells were then rinsed twice with ice-cold PBS (pH 7.4), scraped with 1.3 ml of ice-cold acidic methanol (acetic acid: methanol, 1:50, v/v) and transferred to glass vials. Lipids were extracted by the addition of chloroform (1.3 ml) and water (1.3 ml). The organic lower-phase collected was evaporated to dry with nitrogen gas, and resuspended in 100 µl of chloroform/methanol (1:1, v/v). Equal fluorescent units (4,000 FU in 10 µl per lane) of samples were applied to partisil HPTLC plates with fluorescent indicator (Cat. no 4802-400/4806-410, Whatman, Florham Park, NJ). The samples were resolved using chloroform/methanol/3.5 N ammonium hydroxide (85:15:1, v/v/v) and visualized with an AlphaImager HP imaging system (Alpha Innotech, San Leandro, CA). Fluorescent chromatograms were captured by the AlphaImager, and were inverted using the Adobe Photoshop CS2 program. TLC areas, aligned with bands of lipid standards on the fluorescent chromatograms, were scraped into 96-well plates with 200 µl of methanol. The fluorescence was measured using Synergy HT multi-detection microplate reader (BioTek, Winnooski, VT) at λexcitation 466 and λemission 539 nm. The amounts of GlcCer and Cer were quantitated with each standard curve. Constants for linear correlation, cellular accumulation, and enzyme Km were calculated using Prism V4 program (GraphPad software, San Diego, CA).
To distinguish GalCer and GlcCer, the borate-impregnated plates were prepared as described previously (33, 34). After spraying 1% aqueous sodium tetraborate, the HPTLC plates were activated at 120°C for 30 min. GSLs on the borate-impregnated plates were resolved with the solvent system of chloroform/methanol/water (100:30:4, v/v/v). Fluorescent GalCer, GlcCer, and Cer were identified and measured as described above. Anisaldehyde was used for chemical detection of lipids on the borate-impregnated plates (33).
Tumor-bearing mice and MBO-asGCS treatments
All animal experiments were approved by the Animal Care and Use Committee of the University of Louisiana at Monroe and were in accordance with the National Institutes of Health guidelines. Athymic nude mice of Foxn1nu/Foxn1+ (female, 4–5 weeks) were purchased from Harlan (Indianapolis, IN). Cell suspensions of NCI/ADR-RES, NCI/ADR-RES and SW620Ad (three to five passages, 1 × 106 cells in 20 µl of medium per mouse) were implanted subcutaneously in the left flank of mice, respectively. The treatments started once tumor reached approximately 0.4 cm in the randomized groups of NCI/ADR-RES and SW620Ad (6 mice/group). MBO-asGCS (mixed backbone oligonucleotide against human GCS) (35, 36) was administered intratumorally, at the dose of 1 mg/kg, every three days, five times. A GCS inhibitor D-threo-PDMP dissolved in medium was administered intratumorally when tumors reached ∼0.8 cm in mice inoculated with NCI/ADR-RES cells. The control groups were administered medium only.
To analyze Cer glycosylation close to in vivo condition, tumor cell suspensions were prepared immediately after resection (<10 min) as described previously with modification (37, 38). Tumor tissues (∼50 mg) were pieced (< 1 mm) in 100 µl of RPMI-1640 medium containing collagenase IV (500 units/ml). Glycosylation was carried out in 200 µl of RPMI-1640 medium containing NBD C6-Cer and collagenase IV (250 units/ml) at 37°C with shaking (20 rpm) for two h. Final concentration of NBD C6-Cer in reactions was 2 mM NBD C6-Cer complexed to BSA (equal to 400 µM of NBD C6-Cer), unless otherwise indicated. Cellular accumulation of NBD sphingolipids was observed on smear slides during incubation. Lipids extracted were resuspended in 100 µl of chloroform/methanol (1:1, v/v), and equal amounts lipids (4,000 FU in 10 µl per lane) were subjected to TLC separation and fluorescent measurement.
GCS radioenzymatic assay
GCS radioenzymatic assay was performed as previously described (29). Cells were homogenized by sonication in lysis buffer (50 mM Tris-HCl, pH 7.4, 1.0 µg/ml leupeptin, 10 µg/ml aprotinin, 25 µM phenylmethylsulfonyl fluoride. Microsomes were isolated by centrifugation (129,000 g, 60 min). The enzyme assay, containing 50 µg microsomal proteins in 0.2 ml, was performed in a shaking water bath at 37°C for 60 min. The reaction contained a liposomal substrate composed of C6-Cer (1.0 mM), phosphatidylcholine (3.6 mM), and brain sulfatides (0.9 mM). Other reaction components included sodium phosphate buffer (0.1 M) pH 7.8, EDTA (2.0 mM), MgCl2 (10 mM), dithiothreitol (1.0 mM), β-NAD (2.0 mM), and [3H]UDP-glucose (0.5 mM). The radiolabeled and unlabeled UDP-glucose were diluted to achieve the desired radio-specific activity (4,700 dpm/nmol) in assays. To terminate the reactions, 0.5 ml isopropanol and 0.4 ml Na2SO4 were added into tubes on ice. After addition of 3 ml t-butyl methyl ether and centrifugation, 0.5 ml of the upper phase that contained GlcCer was mixed with 4.5 ml EcoLume for analysis of radioactivity by liquid scintillation spectroscopy.
HPLC/MS of GlcCer measurement
Endogenous GlcCer species of cells were measured using normal phase HPLC coupled to atmospheric pressure chemical ionization-mass spectrometry (LC/MS) by the Lipidomics Core Facility at the Medical University of South Carolina (Charleston, SC) (15, 39). Cells of NCI/ADR-RES, NCI/ADR-RES/GCS, and NCI/ADR-RES/asGCS (5 × 106 cells/100-mm dish) were cultured in 10% FBS RPMI-1640 medium for 48 h and then collected for LC/MS assay. GlcCer and Cer were normalized with proteins.
RESULTS
Quantitation of Cer glycosylation in cells
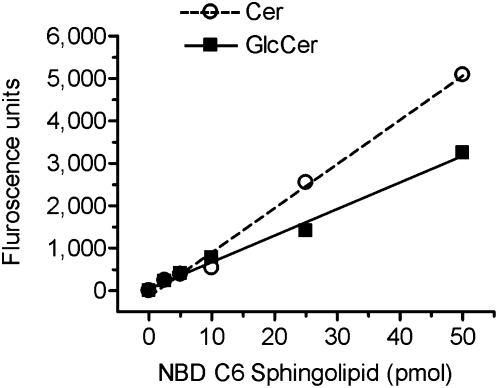
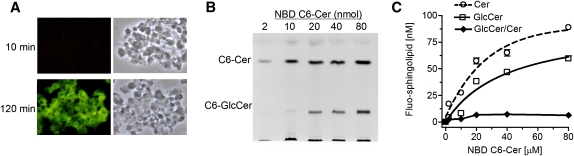
In this study, commercially available fluorescent substrate NBD C6-Cer was used as an acceptor for glucose conversion catalyzed by GCS. NBD C6-Cer complexed to BSA is water-soluble and has high cell permeability. To quantitate GlcCer and Cer generated during cellular glycosylation, correlations of fluorescent intensities to the amounts of NBD C6-GlcCer and NBD C6-Cer were examined using TLC separation and spectrophotometer. A mixture of NBD C6-GlcCer and NBD C6-Cer (mol/mol) was resolved on HPTLC plates in the mobile phase (chloroform/methanol/3.5 N ammonium hydroxide; 85:15:1, v/v/v). NBD C6-GlcCer and NBD C6-Cer were identified on fluorescence chromatograms and quantitatively determined by using fluorescence spectrophotometer. As shown in Fig. 1, fluorescent intensities of GlcCer and Cer detected are linearly correlated to the pmols of NBD C6-GlcCer and NBD C6-Cer spotted, respectively. The correlation coefficients (r2 of linear regression) for GlcCer and Cer are 0.989 and 0.991. These data indicate that TLC-spectrophotometry is a reliable measurement for NBD GlcCer and NBD Cer, at the levels of 2.5 to 50 pmols range.
Fig. 1.
Quantitation of NBD C6-Cer and C6-GlcCer after TLC separation. Increasing amounts of mixture of NBD C6-Cer and NBD C6-GlcCer (1:1, mol/mol) were resolved by TLC. The identified C6-Cer and C6-GlcCer were quantitated by fluorescent spectrophotometer. Each data point represents mean ± SD of three independent experiments. The correlation coefficient for Cer (dash line) is 0.991 ± 0.002, and for GlcCer (solid line) is 0.989 ± 0.002.
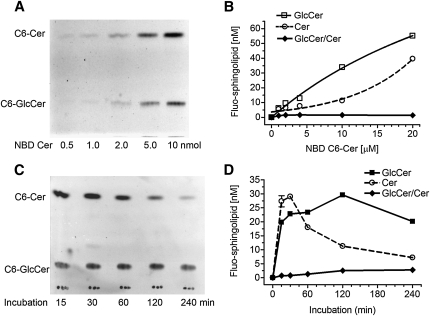
Quantitation of Cer glycosylation was verified using human NCI/ADR-RES cell line that has a high level of GCS (19, 28). We found that culture cells in 1% BSA RPMI-1640 medium containing NBD C6-Cer was optimal. Cellular accumulation of NBD C6-Cer in this condition was much higher than that in cells cultured with 5% FBS medium or in cells incubated with 1% BSA RPMI-1640 medium after trypsinization. We examined whether cellular GlcCer and Cer are correlated to the amounts of NBD C6-Cer added in the medium. As shown in Fig. 2A, the intensities of cellular Cer and GlcCer generated on fluorescent chromatograms were enhanced with the increasing concentrations of NBD C6-Cer in reactions. Measurements revealed a good correlation of cellular GlcCer or Cer to NBD Cer added in reactions (Fig. 2B). The correlation coefficient for cellular Cer is 0.97 (r2 of sigmoidal dose-response with variable slope), and its cellular accumulation rate is 5.10%. The correlation coefficient for cellular GlcCer is 0.99 and the Km value for human GCS is 38.77 µM. The GlcCer/Cer ratios were constant, approximately 1.5 during these reactions (10 to 100 µM of NBD C6-Cer). This indicates this method is capable of quantitative determination of cellular Cer and GlcCer, in 5 to 60 pmol ranges.
Fig. 2.
Determination of Cer glycosylation in cells. NCI/ADR-RES cells were grown in 10% FBS medium for 24 h and switched to 1% BSA RPMI-1640 medium containing NBD C6-Cer for enzymatic reaction. A: Fluorescent chromatogram and (B) spectrophotometry measurements of GlcCer after glycosylation with increasing concentrations of NBD C6-Cer for 2 h. The correlation coefficient for cellular C6-Cer (dashed line) to NBD C6-Cer in medium is 0.97, and for cellular C6-GlcCer (solid line) to NBD C6-Cer in medium is 0.99. C: Fluorescent chromatogram and (D) spectrophotometry measurement of time-dependent Cer glycosylation. NCI/ADR-RES cells were incubated with NBD C6-Cer (100 µM) in 500 µl medium for indicated periods. Results represent the mean ± SD of three independent enzyme reactions in triplicate.
Different from test tube assay in vitro, NBD C6-Cer, a substrate for GCS in cells, is not under saturation once glycosylation starts. Available cellular C6-Cer is undergoing dynamic changes, briefly depending on cell uptake and metabolism during incubation. We measured cellular GlcCer after increasing periods of incubation, to find out the optimal conditions for ceramide glycosylation. As shown in Fig. 2C and D, Cer uptake increased during the first 30 min and then decreased, whereas GlcCer production constantly increased during the first 120 min of incubation. After two h, Cer accumulation was only 43% of maximum (12.5 vs. 28.9 pmol); however, cellular GlcCer generated reached a plateau, ∼30 pmol in cells. The GC/Cer ratios were 1.4 to 2.4 from one to two h of incubations. Therefore, we optimized Cer glycosylation using a 2 h incubation of cells with NBD C6-Cer in 1% BSA RPMI-1640 medium. GlcCer/Cer ratio that consistently represents GlcCer generated in the presence of cellular Cer has been used to evaluate GCS activity.
Validation of Cer glycosylation in cells that express different levels of GCS
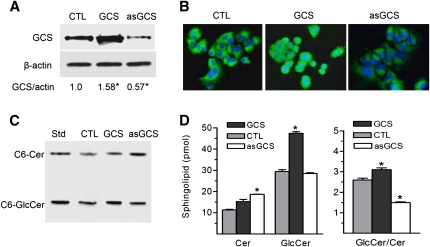
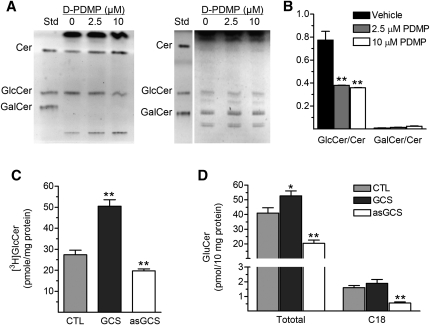
In addition to NBD C6-Cer, available amounts of UDP-glucose and other factors in cells also can affect GlcCer production. To examine whether these interfere with enzyme measurement, we assessed Cer glycosylation in cells expressing GCS in different degrees. NCI/ADR-RES/GCS cells were transfected with human GCS gene and NCI/ADR-RES/asGCS cells were transfected with antisense GCS sequence, respectively (19). As detected by Western blot and immunostaining (Fig. 3A, B), GCS protein was reduced to 57% in NCI/ADR-RES/asGCS and increased to 158% in NCI/ADR-RES/GCS cells, as compared with parental NCI/ADR-RES cells. Using the assay established, we found that C6-Cer level was significantly increased in NCI/ADR/RES/asGCS cells (19 vs. 11 pmol/106 cells, P < 0.05) and C6-GlcCer level was increased in NCI/ADR/RES/GCS cells (47 vs. 29 pmol/106 cells, P < 0.05), as compared with the parental cells (Fig. 3C, D). Consistence with GCS protein levels, GlcCer/Cer ratio was increased to 124% (3.1 vs. 2.5, P < 0.05) in NCI/ADR-RES/GCS and decreased to 60% (1.5 vs. 2.5, P < 0.05) in NCI/ADR-RES asGCS cells, respectively, as compared with NCI/ADR-RES cells. In addition to GlcCer, Cer is possibly converted to GalCer by galactosylceramide synthase (UDP-galactose:ceramide galactosyltransferase, CGT) (40, 41). We tested whether NBD C6-GalCer generated in cells interfere GlcCer measurement. In fluorescent chromatogram of borate-impregnated HPTLC plates, GalCer intensities generated in NCI/ADR-RES cells were close to the background (Fig. 4A, left panel). D-threo-PDMP, a specific inhibitor of GCS enzyme (42, 43), significantly decreased NBD C6-GlcCer (Fig. 4A, left panel) and endogenous GlcCer (Fig. 4A, right panel) ∼2-fold (Fig. 4B), captured by fluorescent and chemical detections. However, cells produced only 1 to 6% of NBD GalCer, as compared with the intensities of NBD GlcCer (Fig. 4B) in corresponding conditions.
Fig. 3.
Cer glycosylation in cell lines that express different degrees of GCS. Human GCS gene and its antisense sequence were introduced into NCI/ADR-RES cells (CTL, control) and established NCI/ADR-RES (GCS) and NCI/ADR-RES (asGCS) cell lines. A: Western blot. The detergent-soluble proteins (50 µg protein/lane) were resolved using 4–20% gradient SDS-PAGE. The transferred membranes were immunoblotted with GCS antiserum (1:1000) and detected by ECL. β-actin was used as endpoint control; GCS protein levels were represented by ratios of GCS/actin densities. * P > 0.01 compared with NCI/ADR-RES cells (CTL). B: Immunostaining. GCS protein was recognized by anti-GCS serum and Alexa 488 conjugated second antibody (green). DAPI nuclear-counter staining (blue) was used as endpoint control. C: Fluorescent chromatogram and (D) measurement of Cer glycosylation in GCS transfectants. Cells were grown overnight in 10% FBS medium then incubated with NBD C6-Cer (100 µM) in 1% BSA RPMI-1640 medium at 37°C for 2 h. Fluorescent C6-Cer and C6-GlcCer were identified by commercial standard (Std). * P < 0.001 compared with NCI/ADR-RES parental cells (CTL). Results are the mean ± SD of three independent experiments.
Fig. 4.
Comparison of Cer glycosylation in cells. A: GalCer generated in cellular glycosylation of NBD C6-Cer. After 4 h D-threo-PDMP treatments (D-PDMP, 2.5, 10 µM), NCI/ADR-RES cells were incubated with NBD C6-Cer (100 µM, 2 h). Sphingolipids were resolved on borate-impregnated HPTLC plates in solvent system of chloroform/methanol/water (100:30:4, v/v/v) and visualized under UV exposure (left) or with anisaldehyde (right). Std, standards of NBD C6-sphingolipids. B: Ratio of GlcCer/Cer versus GalCer/Cer in cells treated with D-threo-PDMP. The intensities of Cer, GlcCer, and GalCer were measured by using fluorescent spectrophotometer after TLC separation as described above. ** P < 0.001 as compared with vehicle treatment. C: GCS radioenzymatic assay. Proteins for enzyme reactions were extracted from NCI/ADR-RES (CTL, control), NCI/ADR-RES/GCS (GCS), and NCI/ADR/RES/asGCS (asGCS) cells. ** P < 0.01 compared with CTL). D: Endogenous total GlcCers and C18-GlcCer analyzed using LC/MS. Lipids were extracted from NCI/ADR-RES (CTL, control), NCI/ADR-RES/GCS (GCS), and NCI/ADR-RES/asGCS (asGCS) cells. * P < 0.01 compared with CTL). * P < 0.05, ** P < 0.01 compared with NCI/ADR-RES cells (CTL). Results are the mean ± SD of three independent experiments.
To further validate cell-based ceramide glycosylation, we used other methods to measure GCS activities in these cells. Using [3H]UDP-glucose and prepared GCS protein, we found that GCS enzyme activity was increased to 184% (50.5 vs. 27.4 pmol/h/mg protein, P < 0.001) in NCI/ADR-RES/GCS, and decreased to 79% (19.7 vs. 27.4 pmol/h/mg protein, P < 0.01) in NCI/ADR-RES/asGCS cells, respectively, as compared with NCI/ADR-RES (Fig. 4C). LC/MS analysis showed that all species of GlcCer and C18-GlcCer were decreased to 49% (20.4 vs. 41 pmol/10 mg protein) and 34% (5.5 vs. 16) in NCI/ADR-RES/asGCS cells; GlcCers and C18-GlcCer were increased to 126% (52.8 vs. 41) and 118% (18.9 vs. 16) in NCI/ADR-RES/GCS cells, respectively, compared with NCI/ADR-RES cells (Fig. 4D). The GlcCer/Cer ratios measured by cell-based TLC are similar to the results of other assays and more consistent with GCS protein levels in these cells. These data indicate that other factors have fewer effects on glycosylation of NBD C6-Cer in cells.
Determination of Cer glycosylation in tissues
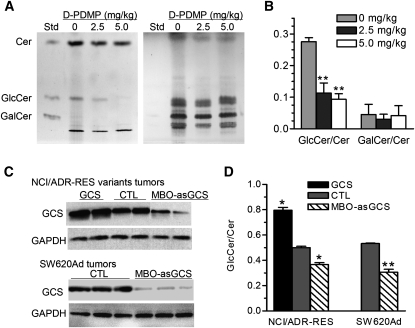
After examination of Cer glycosylation in varied conditions, we found that incubation of NBD C6-Cer with freshly prepared cell suspension is a reliable approach to assess GCS enzyme in tissues. Suspending tissues has been frequently used to isolate stem cells from different tissues (37, 38). However, whether tissue suspension is applicable for enzyme assay has been barely tested. We pieced tumors immediately after resection and carried out glycosylation of NBD C6-Cer in these cell suspensions. It was found that NBD sphingolipid was accumulated in cells after 2 h incubation (Fig. 5A). Suspended tumor cells took up NBD C6-Cer and produced NBD C6-GlcCer (Fig. 5B). Quantitative determination of these showed cellular Cer or GlcCer had a good correlation to NBD C6-Cer concentrations added in the reactions (Fig. 5C). A correlation coefficient for cellular ceramide is 0.97 (r2 of sigmoidal dose-response with variable slope), and the accumulation rate of Cer is 1.4% under those conditions. The coefficient for produced GlcCer is 0.95 and the GCS Km in tumor of NCI/ADR-RES is 40.55 µM, which is similar to that measured in cells (38.77 µM, Fig. 2B). This method was able to detect GlcCer generated in 1 mg of tumor tissues (not shown). In order to verify the influence of GalCer on GlcCer assessment, we tested NBD C6-GalCer generation in tumors treated with D-threo-PDMP. We found that the intensities of C6-GalCer generated in tumor suspensions are close to the background (Fig. 6A). D-threo- PDMP decreased GlcCer generation and reduced the GlcCer/Cer ratios to 27% (0.11 vs. 0.27 P < 0.001, as compared with vehicle (Fig. 6B), but this did not significantly affect C6-GalCer (Fig. 6A, B). These data indicate tissue suspension prepared in this study is useful for GCS assessment.
Fig. 5.
Quantitation of Cer glycosylation in tumors. A: Sphingolipid accumulation in tumor cells. NCI/ADR-RES tumors suspended were incubated with NBD C6-Cer (400 µM) and observed under microscope in fluorescent (left panel, green) and visible light (right panel). B: Chromatogram of Cer glycosylation in tumors suspended. The suspensions of NCI/ADR-RES tumors (200 µl in 0.3 mg/µl) were incubated with increasing concentrations of NBD C6-Cer for 120 min. C: Quantitation of Cer glycosylation in tumors suspended. Intensities of GlcCer and Cer described above were measured using spectrophotometer. Each data point represents mean ± SD of three independent enzyme reactions in triplicate. The correlation coefficient for the cellular C6-Cer (open circle) to NBD C6-Cer in medium is 0.96 and for cellular C6-GlcCer (open square) is 0.95.
Fig. 6.
GCS activity in tumors. A: GlcCer and GalCer generated in glycosylation of NBD C6-Cer. After D-threo-PDMP administration (intratumoral injection two times per day), NCI/ADR-RES tumors suspended were incubated with NBD C6-Cer (400 µM in 200 µl) for 2 h. Sphingolipids were subjected to borate-impregnated TLC separation and visualized under UV exposure (left) or with anisaldehyde (right). Std, standards of NBD C6-sphingolipids. B: Ratio of GlcCer/Cer versus GalCer/Cer after D-threo PDMP treatments and glycosylation described in A. C: GCS protein levels in tumors. Detergent-soluble proteins (50 µg protein/lane) of tumors were subjected to Western blot analysis. GAPDH was used as endpoint control. D: GCS activities in tumor tissues. Cer glycosylation was performed by incubating cell suspension with NBD C6-Cer (400 µM in 200 µl) at 37°C for 2 h. Suspensions were prepared from tumors (50 mg) of NCI/ADR-RES/GCS (GCS), NCI/ADR-RES (CTL, control), NCI/ADR-RES treated with MBO-asGCS (MBO-asGCS), SW620Ad (CTL), and SW620Ad treated with MBO-asGCS (MBO-asGCS) (3–5 mice per each group), respectively. Results are the mean ± SD of three independent experiments in triplicate. * P < 0.001 compared with NCI/ADR-RES or SW620Ad treated with medium (CTL), respectively.
Furthermore, we examined ceramide glycosylation in tumors with GCS gene manipulation. GCS protein was overexpressed in NCI/ADR-RES/GCS tumors, as compared with NCI/ADR-RES (saline treatment, Fig. 6C, top panel). MBO-asGCS treatment suppressed GCS gene expression and significantly decreased its protein levels in NCI/ADR-RES and SW620Ad tumor xenografts (Fig. 6C). Assessment of NBD C6-Cer glycosylation in tumor suspensions found that the GlcCer/Cer ratios were increased to 140% (0.79 vs. 0.5, P < 0.001 as compared with control) in NCI/ADR-RES/GCS tumors, and were decreased to 73% (0.367 vs. 0.50, P < 0.001) in NCI/ADR-RES tumors treated with MBO-asGCS (Fig. 6D). In SW620Ad tumors, the GlcCer/Cer ratio was decreased to 56% (0.30 vs. 0.53, P < 0.001) after MBO-asGCS treatment (Fig. 6D). These data indicate that the GlcCer/Cer ratios in these tumors are consistent with the GCS protein levels, representing GCS activities in vivo.
DISCUSSION
In the present study, we developed a simple and reliable method to directly quantitate ceramide glycosylation catalyzed by GCS in cells and in tissues. It provides a new approach to assess enzyme activity such as GCS in vivo and to evaluate its actual roles in cell functions. Assessing activity of enzyme in cells is essential to verify the effects of enzyme on physiology and diseases; however, it is more difficult than in laboratory-made conditions. Due to the dynamic changes in uptake, substrate with high cellular permeability is a primary determinant for GCS enzymatic assay in vivo. NBD C6-Cer has been used to assay GCS activity in vitro (26, 27, 44, 45) and track Cer translocation in cells (30, 46). In the present study, NBD C6-Cer was efficiently taken up in different human cancer cell lines and in tumor suspensions. NBD C6-Cer accumulated in cells and tumor suspension at the rates of 5% (100 µM in reaction) and 1.4% (400 µM in reaction), respectively. Cellular NBD C6-Cer is linearly correlated to these added in the reactions (r2 = 0.99). More importantly, it sequentially provided adequate amounts of substrates for Cer glycosylation by GCS in these enzymatic reactions (Figs. 2, 5). The levels of GlcCer produced were linearly correlated to NBD C6-Cer concentrations added in reactions of cells (r2 = 0.99, Fig. 2) and tumor suspension (r2 = 0.95, Fig. 5). These results point out that NBD C6-Cer is a suitable substrate for Cer glycosylation in vivo and assessment of NBD C6-Cer glycosylation is an applicable approach to measure GCS activity intracellularly.
In cells, Cer can be converted to GlcCer and GalCer in glycosylation, to sphingosine with ceramidases, and to sphingomyelin with sphingomyelin synthase. Among these, the conversion of NBD C6-Cer and UDP-galactose to NBD C6-GalCer by CGT shares the same cellular processes, including uptake and transporting to endoplasmic reticulum, as the conversion of Cer and UDP-glucose to GlcCer. NBD C6-GalCer has a similar property as NBD C6-GlcCer, and can possibly interfere with the measurement of GC on TLC-spectrophotometry. C6-Cer has been used as substrate for CGT assay (40, 41). Interestingly, we found only a few amounts of GalCer were generated either in cells or in tumors incubated with NBD C6-Cer, even when GCS was inhibited (Figs. 4A, B, 6A, B). GlcCer was the dominant product in all glycosylations of NBD C6-Cer conducted either in cells or in tissue suspensions. These suggest that glycosylation of NBD C6-Cer in the condition tested herein is reliable and reproducible to assess GCS activity in vivo. Caution is needed when applying this method to assess GCS in cells and tissues that have high levels of CGT, such as glioma (47), brain, and kidneys.
Previous reports suggest that several other factors including endogenous UDP-glucose, ceramide subcellular localization, and coenzymes may modulate Cer glycosylation (1). Cell glycosylation of Cer is more sophisticated and GlcCer production is the sum of series of cell biological and biochemical processes. Many factors in these processes may limit the direct-indication of NBD C6-GlcCer for GCS activity. This study clearly indicates that NBD C6-GlcCer generated substantially represents GCS enzyme activity, at least in tested cells and tissues. We found that the Km value of human GCS (38.8 µM) in NCI/ADR-RES cells is higher than the rat GCS Km (14.3 µM) using NBD C6-Cer in test-tube assay (45). In tumors generated from NCI-ADR-RE cells, the GCS Km is 40.6 µM, very close to that measured in the cells. Using this new method, we successfully detected the GCS activities in cells and tumors that express GCS protein in different degrees (Figs. 3, 6) or in which GCS has been inhibited by D-threo-PDMP (Figs. 4A, B, 6A, B). Compared with radioenzymatic assay or endogenous GlcCer assessment, the GlcCer/Cer ratios (GCS activity) using this method are more close to the GCS protein levels in cells (Figs. 3, 4C, D). We also found that the GlcCer/Cer ratios tested with tissue suspensions are highly corresponded to the GCS protein levels in tumor tissues (Fig. 6). This study indicates that applying NBD C6-Cer as a substrate for TLC-based assay can quantitatively determine GCS activity in as little as 5 × 104 cells and 1 mg tumor tissue.
We used Cer glycosylation by GCS as a sample, exploring the possibility to assess enzyme in vivo directly. This study demonstrated that it is feasible to use suitable substrate with a system to evaluate enzyme in cells and tissues. For GCS assay, introducing radioactive labeled ceramide as substrate and HPLC separation into the condition used in this study may significantly increase the sensitivity of Cer glycosylation in vivo. The cell-based fluorescent TLC developed in the present study is a simple, reproducible, and direct means for GCS analysis. In addition to cell study, this method may be applicable to evaluating GCS roles in tissues, predicting drug resistance for cancer, and insulin resistance for type 2 diabetes.
Acknowledgments
The authors thank C. Senkal and Dr. B. Ogretmen (Biochemistry & Molecular Biology, Medical University of South Carolina) for the HPLC/MS analyses of GlcCer and Cer, and Drs. D. L. Markes and R. E. Pagano (Mayo Clinic and Foundation) for anti-GCS antibody.
Footnotes
Abbreviations:
- Cer
- ceramide
- D-threo-PDMP
- D-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol HCl
- GCS
- glucosylceramide synthase
- GEM
- GSL-enriched microdomains
- GSL
- glycosphingolipid
- GlcCer
- glucosylceramide
- MBO-asGCS
- mixed backbone oligonucleotide against human GCS
- NBD C6-Cer
- 6-N-[7-nitrobenz-2-oxa-1,3-diazol-4-yl] aminocaproyl-sphingosine
- NBD C6-GlcCer
- N-hexanoyl-NBD-GlcCer
This work was supported by Public Health Service/National Institutes of Health grant P20 RR16456 from the National Center for Research Resources (Y.Y.L, S.M.J), and Department of Defense Breast Cancer Research Program DAMD17-01-1-0536 (Y.Y.L.). This work was partially supported by United States Public Health Service/National Institute of General Medical Sciences grant GM77391 (M.C.C). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
REFERENCES
- 1.Basu S., Kaufman B., Roseman S. 1968. Enzymatic synthesis of ceramide-glucose and ceramide-lactose by glycosyltransferases from embryonic chicken brain. J. Biol. Chem. 243: 5802–5804 [PubMed] [Google Scholar]
- 2.Basu S., Kaufman B., Roseman S. 1973. Enzymatic synthesis of glucocerebroside by a glucosyltransferase from embryonic chicken brain. J. Biol. Chem. 248: 1388–1394 [PubMed] [Google Scholar]
- 3.Yamashita T., Wada R., Sasaki T., Deng C., Bierfreund U., Sandhoff K., Proia R. L. 1999. A vital role for glycosphingolipid synthesis during development and differentiation. Proc. Natl. Acad. Sci. USA. 96: 9142–9147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basu S., Basu M., Dastgheib S., Hawes J. W. 1999. Biosynthesis and regulation of glycosphingolipids. Comprehensive Natural Products Chemistry. Pinto B. M., editor Pergamon, New York: 107–128 [Google Scholar]
- 5.Yu R. K., Nakatani Y., Yanagisawa M. 2009. The role of glycosphingolipid metabolism in the developing brain. J. Lipid Res. 50(Suppl): S440–S445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fishman P. H., Brady R. O. 1976. Biosynthesis and function of gangliosides. Science. 194: 906–915 [DOI] [PubMed] [Google Scholar]
- 7.Yoshizaki F., Nakayama H., Iwahara C., Takamori K., Ogawa H., Iwabuchi K. 2008. Role of glycosphingolipid-enriched microdomains in innate immunity: microdomain-dependent phagocytic cell functions. Biochim. Biophys. Acta. 1780: 383–392 [DOI] [PubMed] [Google Scholar]
- 8.Hakomori S.1981. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu. Rev. Biochem. 50: 733–764 [DOI] [PubMed] [Google Scholar]
- 9.Basu S., Das K., Basu M. 2000. Glycosyltransferases in glycosphingolipid biosynthesis. Oligosaccharides in Chemistry and Biology-A Comprehensive Handbook. Ernst P. S. B., Hart G., Wiley, Verlag GmbH, Weinheim, Germany: 329–347 [Google Scholar]
- 10.Kasahara K., Sanai Y. 2000. Functional roles of glycosphingolipids in signal transduction via lipid rafts. Glycoconj. J. 17: 153–162 [DOI] [PubMed] [Google Scholar]
- 11.Hakomori Si S. I.2002. Inaugural article: the glycosynapse. Proc. Natl. Acad. Sci. USA. 99: 225–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McEachern K. A., Fung J., Komarnitsky S., Siegel C. S., Chuang W. L., Hutto E., Shayman J. A., Grabowski G. A., Aerts J. M., Cheng S. H., et al. 2007. A specific and potent inhibitor of glucosylceramide synthase for substrate inhibition therapy of Gaucher disease. Mol. Genet. Metab. 91: 259–267 [DOI] [PubMed] [Google Scholar]
- 13.Bose R., Verheij M., Haimovitz-Friedman A., Scotto K., Fuks Z., Kolesnick R. 1995. Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell. 82: 405–414 [DOI] [PubMed] [Google Scholar]
- 14.Hannun Y. A.1996. Functions of ceramide in coordinating cellular responses to stress. Science. 274: 1855–1859 [DOI] [PubMed] [Google Scholar]
- 15.Koybasi S., Senkal C. E., Sundararaj K., Spassieva S., Bielawski J., Osta W., Day T. A., Jiang J. C., Jazwinski S. M., Hannun Y. A., et al. 2004. Defects in cell growth regulation by C18:0-ceramide and longevity assurance gene 1 in human head and neck squamous cell carcinomas. J. Biol. Chem. 279: 44311–44319 [DOI] [PubMed] [Google Scholar]
- 16.Senchenkov A., Litvak D. A., Cabot M. C. 2001. Targeting ceramide metabolism–a strategy for overcoming drug resistance. J. Natl. Cancer Inst. 93: 347–357 [DOI] [PubMed] [Google Scholar]
- 17.Ogretmen B., Hannun Y. A. 2001. Updates on functions of ceramide in chemotherapy-induced cell death and in multidrug resistance. Drug Resist. Updat. 4: 368–377 [DOI] [PubMed] [Google Scholar]
- 18.Kolesnick R., Altieri D., Fuks Z. 2007. A CERTain role for ceramide in taxane-induced cell death. Cancer Cell. 11: 473–475 [DOI] [PubMed] [Google Scholar]
- 19.Liu Y. Y., Han T. Y., Giuliano A. E., Cabot M. C. 2001. Ceramide glycosylation potentiates cellular multidrug resistance. FASEB J. 15: 719–730 [DOI] [PubMed] [Google Scholar]
- 20.Reynolds C. P., Maurer B. J., Kolesnick R. N. 2004. Ceramide synthesis and metabolism as a target for cancer therapy. Cancer Lett. 206: 169–180 [DOI] [PubMed] [Google Scholar]
- 21.Liu Y. Y., Yu J. Y., Yin D., Patwardhan G. A., Gupta V., Hirabayashi Y., Holleran W. M., Giuliano A. E., Jazwinski S. M., Gouaze-Andersson V., et al. 2008. A role for ceramide in driving cancer cell resistance to doxorubicin. FASEB J. 22: 2541–2551 [DOI] [PubMed] [Google Scholar]
- 22.Zador I. Z., Deshmukh G. D., Kunkel R., Johnson K., Radin N. S., Shayman J. A. 1993. A role for glycosphingolipid accumulation in the renal hypertrophy of streptozotocin-induced diabetes mellitus. J. Clin. Invest. 91: 797–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao H., Przybylska M., Wu I. H., Zhang J., Siegel C., Komarnitsky S., Yew N. S., Cheng S. H. 2007. Inhibiting glycosphingolipid synthesis improves glycemic control and insulin sensitivity in animal models of type 2 diabetes. Diabetes. 56: 1210–1218 [DOI] [PubMed] [Google Scholar]
- 24.Basu M., De T., Das K. K., Kyle J. W., Chon H. C., Schaeper R. J., Basu S. 1987. Glycosyltransferases involved in glycolipid biosynthesis. Methods in Enzymology. Ginsburg V., editor Academic Press, New York: 575–607 [DOI] [PubMed] [Google Scholar]
- 25.Shukla G. S., Radin N. S. 1990. Glucosyceramide synthase of mouse kidney: further characterization with an improved assay method. Arch. Biochem. Biophys. 283: 372–378 [DOI] [PubMed] [Google Scholar]
- 26.Ichikawa S., Sakiyama H., Suzuki G., Hidari K. I., Hirabayashi Y. 1996. Expression cloning of a cDNA for human ceramide glucosyltransferase that catalyzes the first glycosylation step of glycosphingolipid synthesis. Proc. Natl. Acad. Sci. USA. 93: 4638–4643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashi Y., Horibata Y., Sakaguchi K., Okino N., Ito M. 2005. A sensitive and reproducible assay to measure the activity of glucosylceramide synthase and lactosylceramide synthase using HPLC and fluorescent substrates. Anal. Biochem. 345: 181–186 [DOI] [PubMed] [Google Scholar]
- 28.Lavie Y., Cao H., Volner A., Lucci A., Han T. Y., Geffen V., Giuliano A. E., Cabot M. C. 1997. Agents that reverse multidrug resistance, tamoxifen, verapamil, and cyclosporin A, block glycosphingolipid metabolism by inhibiting ceramide glycosylation in human cancer cells. J. Biol. Chem. 272: 1682–1687 [DOI] [PubMed] [Google Scholar]
- 29.Liu Y. Y., Han T. Y., Giuliano A. E., Cabot M. C. 1999. Expression of glucosylceramide synthase, converting ceramide to glucosylceramide, confers adriamycin resistance in human breast cancer cells. J. Biol. Chem. 274: 1140–1146 [DOI] [PubMed] [Google Scholar]
- 30.Bourteele S., Hausser A., Doppler H., Horn-Muller J., Ropke C., Schwarzmann G., Pfizenmaier K., Muller G. 1998. Tumor necrosis factor induces ceramide oscillations and negatively controls sphingolipid synthases by caspases in apoptotic Kym-1 cells. J. Biol. Chem. 273: 31245–31251 [DOI] [PubMed] [Google Scholar]
- 31.Fairchild C. R., Ivy S. P., Kao-Shan C. S., Whang-Peng J., Rosen N., Israel M. A., Melera P. W., Cowan K. H., Goldsmith M. E. 1987. Isolation of amplified and overexpressed DNA sequences from adriamycin-resistant human breast cancer cells. Cancer Res. 47: 5141–5148 [PubMed] [Google Scholar]
- 32.Lai G. M., Chen Y. N., Mickley L. A., Fojo A. T., Bates S. E. 1991. P-glycoprotein expression and schedule dependence of adriamycin cytotoxicity in human colon carcinoma cell lines. Int. J. Cancer. 49: 696–703 [DOI] [PubMed] [Google Scholar]
- 33.Backhed F., Alsen B., Roche N., Angstrom J., von Euler A., Breimer M. E., Westerlund-Wikstrom B., Teneberg S., Richter-Dahlfors A. 2002. Identification of target tissue glycosphingolipid receptors for uropathogenic, F1C-fimbriated Escherichia coli and its role in mucosal inflammation. J. Biol. Chem. 277: 18198–18205 [DOI] [PubMed] [Google Scholar]
- 34.Breimer M. E., Hansson G. C., Karlsson K. A., Leffler H. 1983. The preparative separation of sialic acid-containing lipids from sulphate group-containing glycolipids from small intestine of different animals. Analysis by thin-layer chromatography and detection of novel species. J. Biochem. 93: 1473–1485 [DOI] [PubMed] [Google Scholar]
- 35.Liu Y. Y., Han T. Y., Yu J. Y., Bitterman A., Le A., Giuliano A. E., Cabot M. C. 2004. Oligonucleotides blocking glucosylceramide synthase expression selectively reverse drug resistance in cancer cells. J. Lipid Res. 45: 933–940 [DOI] [PubMed] [Google Scholar]
- 36.Patwardhan G. A., Zhang Q. J., Yin D., Gupta V., Bao J., Senkal C. E., Ogretmen B., Cabot M. C., Shah G. V., Sylvester P. W., et al. 2009. A new mixed-backbone oligonucleotide against glucosylceramide synthase sensitizes multidrug-resistant tumors to apoptosis. PLoS One. 4: e6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Hajj M., Wicha M. S., Benito-Hernandez A., Morrison S. J., Clarke M. F. 2003. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA. 100: 3983–3988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho R. W., Wang X., Diehn M., Shedden K., Chen G. Y., Sherlock G., Gurney A., Lewicki J., Clarke M. F. 2008. Isolation and molecular characterization of cancer stem cells in MMTV-Wnt-1 murine breast tumors. Stem Cells. 26: 364–371 [DOI] [PubMed] [Google Scholar]
- 39.Kraveka J. M., Li L., Bielawski J., Obeid L. M., Ogretmen B. 2003. Involvement of endogenous ceramide in the inhibition of telomerase activity and induction of morphologic differentiation in response to all-trans-retinoic acid in human neuroblastoma cells. Arch. Biochem. Biophys. 419: 110–119 [DOI] [PubMed] [Google Scholar]
- 40.Kapitonov D., Yu R. K. 1997. Cloning, characterization, and expression of human ceramide galactosyltransferase cDNA. Biochem. Biophys. Res. Commun. 232: 449–453 [DOI] [PubMed] [Google Scholar]
- 41.Basu S., Schultz A. M., Basu M., Roseman S. 1971. Enzymatic synthesis of galactocerebroside by a galactosyltransferase from embryonic chicken brain. J. Biol. Chem. 246: 4272–4279 [PubMed] [Google Scholar]
- 42.Inokuchi J., Radin N. S. 1987. Preparation of the active isomer of 1-phenyl-2-decanoylamino-3-morpholino-1-propanol, inhibitor of murine glucocerebroside synthetase. J. Lipid Res. 28: 565–571 [PubMed] [Google Scholar]
- 43.Boyle P. J., Ma R., Tuteja N., Banerjee S., Basu S. 2006. Apoptosis of human breast carcinoma cells in the presence of cis-platin and L-/D-PPMP: IV. Modulation of replication complexes and glycolipid: glycosyltransferases. Glycoconj. J. 23: 175–187 [DOI] [PubMed] [Google Scholar]
- 44.Lipsky N. G., Pagano R. E. 1983. Sphingolipid metabolism in cultured fibroblasts: microscopic and biochemical studies employing a fluorescent ceramide analogue. Proc. Natl. Acad. Sci. USA. 80: 2608–2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crespo P. M., Silvestre D. C., Gil G. A., Maccioni H. J., Daniotti J. L., Caputto B. L. 2008. c-Fos activates glucosylceramide synthase and glycolipid synthesis in PC12 cells. J. Biol. Chem. 283: 31163–31171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lipsky N. G., Pagano R. E. 1985. A vital stain for the Golgi apparatus. Science. 228: 745–747 [DOI] [PubMed] [Google Scholar]
- 47.Popko B., Pearl D. K., Walker D. M., Comas T. C., Baerwald K. D., Burger P. C., Scheithauer B. W., Yates A. J. 2002. Molecular markers that identify human astrocytomas and oligodendrogliomas. J. Neuropathol. Exp. Neurol. 61: 329–338 [DOI] [PubMed] [Google Scholar]