Abstract
Subjects with increased cholesterol absorption might benefit more from statin therapy combined with a cholesterol absorption inhibitor. We assessed whether baseline cholesterol absorption markers were associated with response to ezetimibe/simvastatin therapy, in terms of LDL-cholesterol (LDL-C) lowering and cholesterol absorption inhibition, in patients with familial hypercholesterolemia (FH). In a posthoc analysis of the two-year ENHANCE trial, we assessed baseline cholesterol-adjusted campesterol (campesterol/TC) and sitosterol/TC ratios in 591 FH patients. Associations with LDL-C changes and changes in cholesterol absorption markers were evaluated by multiple regression analysis. No association was observed between baseline markers of cholesterol absorption and the extent of LDL-C response to ezetimibe/simvastatin therapy (β = 0.020, P = 0.587 for campesterol/TC and β<0.001, P = 0.992 for sitosterol/TC). Ezetimibe/simvastatin treatment reduced campesterol levels by 68% and sitosterol levels by 62%; reductions were most pronounced in subjects with the highest cholesterol absorption markers at baseline, the so-called high absorbers (P < 0.001). Baseline cholesterol absorption status does not determine LDL-C lowering response to ezetimibe/simvastatin therapy in FH, despite more pronounced cholesterol absorption inhibition in high absorbers. Hence, these data do not support the use of baseline absorption markers as a tool to determine optimal cholesterol lowering strategy in FH patients. However, due to the exploratory nature of any posthoc analysis, these results warrant further prospective evaluation in different populations.
Keywords: cholesterol synthesis, statins, familial hypercholesterolemia
Cholesterol homeostasis is regulated by an intricate interaction of cholesterol synthesis, intestinal absorption, biliary removal, and fecal excretion. Plasma levels of the plant sterols campesterol and sitosterol and the cholesterol metabolite cholestanol have been shown to correlate positively with intestinal cholesterol absorption and inversely with endogenous cholesterol synthesis. In contrast, the cholesterol precursor lathosterol has been shown to positively correlate with cholesterol synthesis (1–3). Consequently, these noncholesterol sterols have been used as markers of steady-state cholesterol homeostasis. Based on these markers, a classification of subjects with high and low basal cholesterol absorption or synthesis has been suggested (4, 5). These so-called high and low absorbers and high and low synthesizers have been shown to respond differently to cholesterol lowering treatments in terms of LDL-cholesterol (LDL-C) lowering (6–8) and cardiovascular event rate (9). Hence, it was postulated that high absorbers should not be treated with statins alone but would benefit more from the addition of a cholesterol absorption inhibitor (4).
Differences in basal cholesterol absorption and synthesis, as defined by baseline noncholesterol sterols, have also been suggested to predict LDL-C lowering response in patients with familial hypercholesterolemia (FH) (10–13). Furthermore, a recent study showed a strong negative correlation between the LDL-C response to statins and the response to the subsequent addition of ezetimibe in a population of heterozygous FH patients, indicating that good responders to statins were poor responders to ezetimibe and vice versa (14). The authors hypothesized that this might be explained by variability in basal cholesterol synthesis and absorption. Unfortunately, no markers of basal absorption or synthesis were measured. To address this issue, we performed a posthoc analysis of the ENHANCE trial, in which a population of heterozygous FH patients was treated with either ezetimibe/simvastatin combination therapy or simvastatin alone for a period of 2 years (15). Our primary objective was to evaluate whether FH patients with high baseline absorption markers show stronger LDL-C reductions after ezetimibe/simvastatin therapy compared with patients with low baseline absorption markers. In addition, we investigated whether subjects with high baseline synthesis markers showed more pronounced LDL-C reductions after simvastatin therapy when compared with low synthesizers. Finally, we assessed whether changes in absorption and synthesis markers after treatment with ezetimibe/simvastatin and simvastatin alone differed between patients with different baseline levels of cholesterol absorption and synthesis.
METHODS
Subjects and study design
Data were derived from subjects who participated in the ENHANCE study. Details and outcomes were previously reported (15). In short, in this prospective double-blind, randomized, multicenter 24-month trial, heterozygous FH patients, diagnosed either by genotyping or by WHO diagnostic criteria and aged between 30 and 75 years, were enrolled regardless of prior lipid-lowering therapy. Major exclusion criteria were high-grade stenosis or occlusion of the carotid artery, a history of carotid endarterectomy or carotid stenting, homozygous FH, severe congestive heart failure, cardiac arrhythmia, angina pectoris, or recent cardiovascular events.
After a single-blind 6-week placebo run-in period, a total of 720 subjects with untreated LDL-C levels of 5.43 mmol/l (210 mg/dl) or more were randomized to daily therapy with 80 mg of simvastatin either with placebo or with 10 mg of ezetimibe for a period of two years.
At the end of the placebo run-in period, baseline measurements of lipoproteins and noncholesterol sterols were performed. For this posthoc analysis, we used these baseline data and data obtained after 24 months of therapy of all subjects who completed the trial and who had retrievable noncholesterol sterol levels at baseline and at the end of the study.
Measurement of lipoproteins and noncholesterol sterols
Plasma total cholesterol (TC), HDL-cholesterol, and triglycerides were analyzed using standardized methods at the central laboratory of the trial (PPD Global Central Labs, Highland Heights, Kentucky). LDL-C was calculated using the Friedewald formula. Noncholesterol sterols were quantified by GC-MS.
Statistical analyses
Differences in baseline parameters between the treatment groups were compared using an independent Student's t-test. Skewed data were log-transformed prior to testing. Baseline sitosterol, campesterol, and lathosterol levels and changes in these levels can be presented both as cholesterol-adjusted ratios (noncholesterol sterol/TC) and as absolute values. The former is generally performed to eliminate the influence of high lipoprotein concentrations, such as in FH (16). When statin-induced changes in markers of cholesterol absorption and synthesis are concerned, presentation of changes in absolute concentrations has been advocated (17). Therefore, in line with a recent report addressing this issue (18), noncholesterol sterol levels are expressed as follows: high absorbers are defined as subjects with high baseline cholesterol-adjusted campesterol or sitosterol ratios (campesterol/TC and sitosterol/TC, respectively). Similarly, high synthesizers are defined as subjects with high baseline lathosterol/TC ratios. Conversely, when describing the relationship between change in markers of absorption and synthesis on the one hand and change in cholesterol levels on the other, absolute sterol concentrations are preferred, because adjusting for TC levels would mask the outcome variable of interest. Nevertheless, both cholesterol-adjusted and absolute changes in noncholesterol sterols are reported to meet other preferences in the field and to allow for comparisons with previous reports.
We evaluated Pearson's correlations between baseline noncholesterol sterols on the one hand and baseline noncholesterol sterols or LDL-C levels on the other hand. Associations between baseline markers of cholesterol absorption or synthesis and parameters of interest were analyzed by means of multiple regression analysis. Associations with treatment-induced changes in LDL-C and noncholesterol sterols were evaluated for both treatment arms separately. For change in LDL-C, the model was adjusted for baseline LDL-C levels.
The model for studying the association between baseline campesterol or sitosterol levels and their change during treatment was adjusted for baseline lathosterol levels and body mass index (BMI), because these were previously suggested as important determinants (7). Similarly, the association between baseline lathosterol levels and their change during treatment was adjusted for baseline campesterol levels and BMI. Finally, we assessed whether these relationships were merely caused by regression to the mean, as previously described (19). In short, associations between changes in noncholesterol sterols and baseline noncholesterol sterols were evaluated in four models, which included no effect and either or both additive and multiplicative treatment effects. If the model, which included both additive and multiplicative effects fitted better than the additive model only (regression to the mean), changes in noncholesterol sterol levels were considered to be determined by baseline noncholesterol sterol levels instead of being caused by regression to the mean only. Analyses were performed using SPSS 15.0 for Windows software (SPSS Inc., Chicago, IL) and R software (version 2.8.1.0). A P-value <0.05 was considered statistically significant.
RESULTS
As previously reported, in the ENHANCE study, 363 subjects were treated with simvastatin alone, of which 299 completed the trial (15). Three-hundred fifty-seven subjects were treated with ezetimibe/simvastatin therapy, of which 316 completed the trial. In our analyses, we included 289 subjects in the simvastatin group and 302 subjects in the ezetimibe/simvastatin group, also excluding the subjects with missing noncholesterol sterol data.
Baseline characteristics and correlations
Treatment groups did not differ with respect to baseline characteristics, lipoproteins, and cholesterol-adjusted and absolute noncholesterol sterol levels (Table 1).
TABLE 1.
Baseline characteristics, lipids, and noncholesterol sterols
| Simvastatin 80 mg | Ezetimibe/Simvastatin 10/80 mg | P | |
|---|---|---|---|
| Subjects (n) | 289 | 302 | |
| Age (years) | 45.5 ± 9.6 | 46.4 ± 8.9 | 0.271 |
| Male gender (n) | 145 (50.2%) | 161 (53.3%) | 0.583 |
| BMI (kg/m2) | 26.9 ± 4.3 | 27.5 ± 4.7 | 0.108 |
| Total cholesterol (mmol/l) | 10.33 ± 1.86 | 10.35 ± 1.83 | 0.923 |
| LDL-C (mmol/l) | 8.22 ± 1.80 | 8.25 ± 1.75 | 0.805 |
| HDL-cholesterol (mmol/l) | 1.19 ± 0.30 | 1.21 ± 0.29 | 0.494 |
| Triglycerides (mmol/l) | 1.82 [ 0.54 - 6.75] | 1.77 [0.49 – 8.08] | 0.380 |
| Campesterol (mg/dl) | 0.76 ± 0.38 | 0.82 ± 0.46 | 0.118 |
| Sitosterol (mg/dl) | 0.54 ± 0.26 | 0.56 ± 0.27 | 0.376 |
| Lathosterol (mg/dl) | 0.53 ± 0.20 | 0.52 ± 0.21 | 0.392 |
| Campesterol/TC ratio (μg/mg) | 1.90 ± 0.88 | 2.02 ± 1.01 | 0.125 |
| Sitosterol/TC (μg/mg) | 1.36 ± 0.59 | 1.40 ± 0.59 | 0.431 |
| Lathosterol/TC ratio (μg/mg) | 1.35 ± 0.48 | 1.30 ± 0.50 | 0.228 |
Data are presented as means ± SD, median [range], or number (%). Analyses were performed with independent Student's t-test. For gender, Chi2 test was used. As triglyceride data were skewed, data were log-transformed prior to testing; however, untransformed medians and range are presented.
Correlations between baseline markers of cholesterol absorption and synthesis.
Correlations between cholesterol-adjusted markers of absorption and synthesis were statistically significant, although relatively weak (R = −0.17, P < 0.001 for campesterol/TC and lathosterol/TC; R = −0.26, P < 0.001 for sitosterol/TC and lathosterol/TC). Campesterol/TC and sitosterol/TC ratios correlated well with one another (R = 0.85, P < 0.001). Correlations between absolute cholesterol absorption and synthesis markers were not statistically significant (R = −0.003, P = 0.940 for campesterol and lathosterol; R = −0.07, P = 0.071 for sitosterol and lathosterol). Absolute campesterol and sitosterol levels were highly correlated (R = 0.88, P < 0.001).
Correlations between baseline markers of cholesterol absorption or synthesis and baseline LDL-C levels.
Baseline campesterol/TC and sitosterol/TC ratios were positively correlated with baseline LDL-C, although relationships were weak (R = 0.10, P = 0.014 for campesterol/TC; R = 0.08, P = 0.048 for sitosterol/TC). Conversely, lathosterol/TC ratios showed an inverse relation with baseline LDL-C (R = −0.15, P < 0.001). We also found statistically significant associations between absolute noncholesterol sterols and baseline LDL-C (R = 0.41, P < 0.001 for campesterol; R = 0.44, P < 0.001 for sitosterol and R = 0.30, P < 0.001 for lathosterol, respectively).
Effects of noncholesterol sterols on LDL-C change
Baseline markers of cholesterol absorption and synthesis do not predict LDL-C change after ezetimibe/simvastatin or simvastatin monotherapy.
We found no association between baseline campesterol/TC or sitosterol/TC ratios and LDL-C change after both ezetimibe/simvastatin therapy (P = 0.587 and P = 0.992, respectively) and simvastatin monotherapy (P = 0.287 and P = 0.871, respectively). Similarly, there was no significant association between baseline lathosterol/TC ratios and LDL-C change after both ezetimibe/simvastatin therapy (P = 0.154) and simvastatin monotherapy (P = 0.927). This also applied when absolute noncholesterol levels were used (Table 2).
TABLE 2.
Associations between noncholesterol sterols and LDL-C change
| Simvastatin 80 mg (N = 289) |
Ezetimibe/Simvastatin 10/80 mg (N = 302) |
|||
|---|---|---|---|---|
| Baseline Noncholesterol Sterols | β | P | β | P |
| Campesterol (mg/dl) | −0.072 | 0.166 | 0.009 | 0.825 |
| Sitosterol (mg/dl) | −0.020 | 0.705 | −0.010 | 0.806 |
| Lathosterol (mg/dl) | −0.001 | 0.981 | 0.057 | 0.148 |
| Campesterol/TC (μg/mg) | −0.051 | 0.287 | 0.020 | 0.587 |
| Sitosterol/TC (μg/mg) | 0.008 | 0.871 | <0.001 | 0.992 |
| Lathosterol/TC (μg/mg) | 0.005 | 0.927 | 0.053 | 0.154 |
| Change in Sterols | Simvastatin 80 mg (N = 289) | Ezetimibe/Simvastatin 10/80 mg (N = 302) | ||
| Δ Campesterol 2y (mg/dl) | 0.235 | <0.001 | 0.081 | 0.047 |
| Δ Sitosterol 2y (mg/dl) | 0.206 | <0.001 | 0.125 | 0.003 |
| Δ Lathosterol 2y (mg/dl) | 0.232 | <0.001 | 0.157 | <0.001 |
| Δ Campesterol/TC 2y (μg/ml) | −0.180 | <0.001 | −0.013 | 0.736 |
| Δ Sitosterol/TC 2y (μg/ml) | −0.193 | <0.001 | −0.021 | 0.570 |
| Δ Lathosterol/TC 2y (μg/ml) | 0.135 | 0.001 | 0.083 | 0.026 |
Data were analyzed in a multiple regression model with LDL-C change from baseline as dependent variable and the noncholesterol sterol and baseline LDL-C as independent variables. In addition, associations between change in absolute noncholesterol sterol levels and LDL-C change were analyzed. In this model, LDL change from baseline served as dependent variable and change in the noncholesterol sterol and baseline LDL-C as independent variables. β represents the standardized β coefficient.
In contrast, changes in absolute campesterol, sitosterol, and lathosterol levels were significantly associated with LDL-C change in both treatment groups (Table 2). Changes in campesterol/TC and sitosterol/TC ratios were associated with LDL-C change in the ezetimibe/simvastatin-treated group only, whereas changes in lathosterol/TC ratios were significantly associated with LDL-C change in both treatment groups (Table 2).
Addition of ezetimibe to simvastatin results in incremental LDL-C reductions irrespective of baseline cholesterol absorption or synthesis.
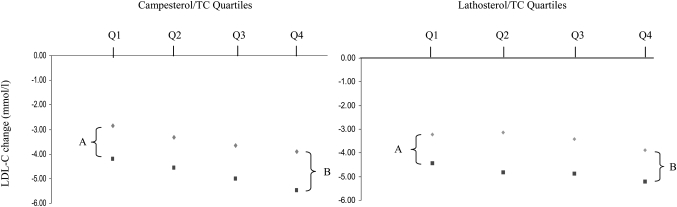
To evaluate whether addition of ezetimibe to simvastatin results in significantly stronger LDL-C reductions in high absorbers as compared with low absorbers, subjects were stratified into quartiles according to baseline campesterol/TC ratios (supplementary Table I). Subsequently, we compared differences in mean LDL-C change between the two treatments within the highest and lowest campesterol/TC quartiles. No significant differences were found; in the highest quartile (Q4), the difference in mean LDL-C change between subjects treated with ezetimibe/simvastatin and those treated with simvastatin alone was 1.49 ± 0.27 mmol/l, whereas this difference between the treatment groups was 1.33 ± 0.17 mmol/l in the lowest quartile (Q1) (P = 0.928, Fig. 1). This also applied when sitosterol/TC quartiles were used as a marker of baseline cholesterol absorption (data not shown). Similarly, no differences were found between the two treatments for high versus low synthesizers (supplementary Table II), as the differences in mean LDL-C change between the two treatments within the lowest and highest lathosterol/TC quartiles were not significant (1.31 ± 0.26 mmol/l in Q4 vs. 1.20 ± 0.20 mmol/l in Q1, P = 0.741, Fig. 1). Similar results were obtained when baseline absolute noncholesterol levels were used (data not shown).
Fig. 1.
Change in LDL-C by campesterol/TC and lathosterol/TC quartiles. Differences in mean LDL-C change after simvastatin (diamond) and ezetimibe/simvastatin (square) therapy within the lowest (A) and highest (B) campesterol/TC and lathosterol/TC quartiles. A is not significantly different from B, as analyzed by an independent sample t-test (P = 0.928 for campesterol/TC and P = 0.741 for lathosterol/TC).
Effects of baseline noncholesterols on markers of cholesterol absorption and synthesis
Overall change in markers of cholesterol absorption and synthesis after ezetimibe/simvastatin and simvastatin monotherapy.
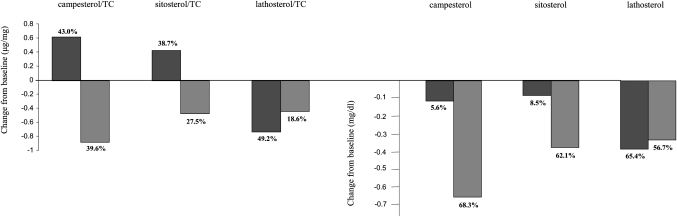
Campesterol/TC ratios changed by −0.88 ± 0.82 μg/mg (−39.6% ± 21.1%) in the ezetimibe/simvastatin group compared with 0.62 ± 0.69 μg/mg (43.0% ± 56.2%) in the simvastatin group (P < 0.001); sitosterol/TC ratios changed by −0.42 ± 0.41 μg/mg (−27.5% ± 25.0%) in the combination group, compared with 0.43 ± 0.53 μg/mg (38.7% ± 52.1%) in the simvastatin group (P < 0.001). Lathosterol/TC ratios changed by −0.37 ± 0.56 μg/mg (−18.6% ± 90.8%) in the ezetimibe/simvastatin group compared with −0.73 ± 0.52 μg/mg (−49.2% ± 67.8%) in the simvastatin group (P < 0.001).
Absolute campesterol levels changed by −0.58 ± 0.41 mg/dl (−68.3% ± 13.3%) in the ezetimibe/simvastatin group versus −0.11 ± 0.25 mg/dl (−5.6% ± 42.1%) in the simvastatin group (P < 0.001); sitosterol levels changed by −0.36 ± 0.21 mg/dl (−62.1% ± 14.3%) in the ezetimibe/simvastatin group versus −0.08 ± 0.17 mg/dl (−8.5% ± 38.9%) in the simvastatin group (P < 0.001). Absolute lathosterol levels changed by −0.32 ± 0.21 mg/dl (−56.7% ± 48.7%) in the ezetimibe/simvastatin group versus −0.37 ± 0.21 mg/dl (−65.4% ± 46.8%) in the simvastatin group (P = 0.03). Data are presented graphically in Fig. 2.
Fig. 2.
Change in cholesterol-adjusted and absolute noncholesterol sterols after 2 years of treatment with simvastatin (black) and ezetimibe/simvastatin (gray).
Baseline lathosterol, but not sitosterol or campesterol, levels predict reduction in cholesterol synthesis after simvastatin and ezetimibe/simvastatin therapy.
We investigated whether the observed reductions in cholesterol synthesis, as reflected by lathosterol/TC ratios, were most pronounced in subjects with the highest baseline lathosterol/TC levels, the so-called high synthesizers. This was indeed the case (P < 0.001 for both treatments, Table 3). This also applied to reductions in absolute lathosterol levels (P < 0.001 for both treatments, Table 3). These significant relationships were not attributable to regression to the mean only, but indeed showed a relationship with baseline levels, as determined by comparison of the above-mentioned models proposed by Chen et al. (14).
TABLE 3.
Associations between baseline noncholesterol sterols and change in noncholesterol sterols
| Change in Noncholesterol Sterols | Simvastatin 80 mg (N = 289) |
Ezetimibe/Simvastatin 10/80 mg (N = 302) |
||
|---|---|---|---|---|
| β | P | β | P | |
| Δ Lathosterol/TC, μg/mga | ||||
| Lathosterol/TC, μg/mg | −0.752 | <0.001 | −0.623 | <0.001 |
| Campesterol/TC, μg/mg | −0.043 | 0.316 | −0.049 | 0.313 |
| Sitosterol/TC, μg/mg | −0.051 | 0.247 | −0.048 | 0.336 |
| Δ Lathosterol, mg/dlb | ||||
| Lathosterol, mg/dl | −0.853 | <0.001 | −0.832 | <0.001 |
| Campesterol, mg/dl | −0.035 | 0.310 | −0.027 | 0.435 |
| Sitosterol, mg/dl | −0.025 | 0.470 | −0.025 | 0.475 |
| Δ Campesterol/TC, μg/mgc | ||||
| Campesterol/TC, μg/mg | −0.210 | 0.001 | −0.898 | <0.001 |
| Sitosterol/TC, μg/mg | −0.189 | 0.003 | −0.695 | <0.001 |
| Lathosterol/TC, μg/mg | −0.122 | 0.050 | −0.043 | 0.175 |
| Δ Campesterol, mg/dld | ||||
| Campesterol, mg/dl | −0.676 | <0.001 | −0.973 | <0.001 |
| Sitosterol, mg/dl | −0.567 | <0.001 | −0.821 | <0.001 |
| Lathosterol, mg/dl | −0.116 | 0.015 | −0.005 | 0.754 |
| Δ Sitosterol/TC, μg/mge | ||||
| Sitosterol/TC, μg/mg | −0.164 | 0.009 | −0.719 | <0.001 |
| Campesterol/TC, μg/mg | −0.117 | 0.057 | −0.632 | <0.001 |
| Lathosterol/TC, μg/mg | −0.122 | 0.053 | −0.052 | 0.286 |
| Δ Sitosterol, mg/dl f | ||||
| Sitosterol, mg/dl | −0.589 | <0.001 | −0.940 | <0.001 |
| Campesterol, mg/dl | −0.543 | <0.001 | −0.829 | <0.001 |
| Lathosterol, mg/dl | −0.118 | 0.025 | −0.013 | 0.570 |
Data were analyzed by multiple regression. β represents the standardized β coefficient.
Corrected for baseline lathosterol/TC and BMI.
Corrected for baseline lathosterol and BMI.
Corrected for baseline campesterol/TC, lathosterol/TC, and BMI.
Corrected for baseline campesterol, lathosterol, and BMI.
Corrected for baseline sitosterol/TC, lathosterol/TC, and BMI.
Corrected for baseline sitosterol, lathosterol, and BMI.
Reductions in lathosterol/TC levels after both treatments did not differ between high and low absorbers, because baseline campesterol/TC levels were not significantly associated with change in lathosterol/TC levels in either of the treatment arms (Table 3). This also applied to changes in absolute lathosterol levels.
Baseline campesterol and sitosterol levels predict both simvastatin-induced increases and ezetimibe/simvastatin-induced decreases in markers of cholesterol absorption.
We tested the hypothesis whether the observed decreases in cholesterol absorption, as reflected by campesterol/TC and sitosterol/TC ratios, after ezetimibe/simvastatin therapy were more pronounced in high absorbers as compared with low absorbers. Indeed, reductions in campesterol/TC and sitosterol/TC ratios were the strongest in subjects with high baseline campesterol/TC and sitosterol/TC levels in the ezetimibe/simvastatin group (P < 0.001 for both, Table 3).
We also assessed whether the simvastatin-induced increases in campesterol/TC and sitosterol/TC ratios differed between high and low cholesterol absorbers. We found that low absorbers show more pronounced increases in campesterol/TC and sitosterol/TC ratios after simvastatin therapy compared with high absorbers (P < 0.001, Table 3). These relationships were also significant when absolute campesterol and sitosterol levels were used (Table 3) and were not merely caused by regression to the mean (data not shown).
DISCUSSION
This posthoc analysis demonstrates that in subjects with FH, baseline noncholesterol sterols as indicators of basal cholesterol absorption and synthesis do not predict the LDL-C lowering response to treatment with ezetimibe/simvastatin or simvastatin alone. Furthermore, so-called high and low absorbers benefit equally from the addition of ezetimibe to simvastatin in terms of LDL-C lowering, despite stronger cholesterol absorption inhibition in high absorbers.
Previous reports suggest that high synthesizers show more pronounced cholesterol synthesis inhibition and subsequent LDL-C reductions after statin therapy (6), whereas high absorbers show more pronounced LDL-C reductions after cholesterol absorption inhibiting strategies (6, 7, 20). These effects have also been described in FH, although mostly in small-scale studies (11–13, 21).
Some of the above-mentioned studies showed that baseline noncholesterol sterols were more strongly correlated with changes in synthesis and absorption markers, whereas respective differences in serum cholesterol levels were markedly less (6, 7, 22). Nevertheless, based on these reports, it has been suggested that in subjects with high baseline absorption markers, statin treatment needs to be combined with cholesterol absorption inhibition to achieve effective serum cholesterol lowering (4, 6) and that quantification of baseline noncholesterol sterols might be used as a clinical tool to customize cholesterol-lowering therapy in the individual hypercholesterolemic patient (5).
Our data do not support this suggestion, because high synthesizers indeed experienced stronger cholesterol synthesis inhibition after simvastatin therapy compared with low synthesizers, but this did not result in more pronounced LDL-C lowering. Similarly, high absorbers showed more pronounced cholesterol absorption inhibition in the ezetimibe/simvastatin group compared with low absorbers, again without experiencing stronger LDL-C reductions. This discrepancy might be attributed to the fact that in previous studies, LDL-C responses were mostly not corrected for baseline LDL-C concentrations, an important predictor of LDL-C reduction during lipid-lowering therapy (23, 24). In our study, baseline absolute noncholesterol sterol levels were also strongly associated with LDL-C reductions in both treatment groups (P < 0.01 for each of the noncholesterol sterols; data not shown). Nevertheless, these associations were abolished once corrected for baseline LDL-C levels (Table 2). Our finding is in line with that of other studies in which the LDL-C responses after treatment with statins (18) and intake of plant sterols (25) were also adjusted for baseline LDL-C levels. This highlights the importance of baseline LDL-C as a determinant for LDL-C lowering response, rather than baseline markers of cholesterol metabolism, even more so, because these were weakly correlated at baseline in our study.
Of note, baseline noncholesterol sterol levels in our study were within the same range as previously described in FH (10, 26, 27). Equal to these reports, absolute levels were higher than in non-FH populations, likely due to markedly higher lipoprotein concentrations, whereas cholesterol-adjusted levels were similar to those in the normal population (28). Furthermore, we also analyzed cholestanol to define high and low absorbers, as this cholesterol metabolite has also been used as a marker of cholesterol absorption in several reports to which we refer throughout this manuscript. This yielded the same results as the plant sterols, campesterol and sitosterol (data not shown), which rules out the possibility that divergent results were attributable to differences in quantification and types of noncholesterol sterols or to the population of FH in our study.
Although we did not find an association between baseline noncholesterol sterol levels and LDL-C change, we did observe significant associations between change in noncholesterol sterols and LDL-C change (Table 2). These findings confirm very recent results on the effects of rosuvastatin and atorvastatin on markers of cholesterol synthesis and absorption (18). In that posthoc analysis, baseline noncholesterol sterols did not correlate with the cholesterol lowering response to either of the statins, whereas alterations in absolute noncholesterol sterol levels did. These analyses were correctly adjusted for baseline cholesterol levels and are in line with our findings. In addition, the two statins were most effective in subjects with the greatest reductions in cholesterol synthesis markers and no increase in cholesterol absorption markers during statin therapy. Subjects in which the converse was true were the poorest responders. Hence, the authors concluded that statin therapy was most effective in subjects who are unable to upregulate cholesterol absorption during statin therapy. Although we can confirm these observations in our study (data not shown), the observed increases in plant sterol levels might be merely due to diminished biliary sterol secretion during statin therapy, resulting in a smaller intestinal sterol pool instead of increased cholesterol absorption, as previously suggested (17). Although statin-induced upregulation of cholesterol absorption has been extensively described, based on statin-induced increases in plant sterol levels (6, 16, 4), this is less well established by studies in which cholesterol absorption was directly measured. In fact, some of these studies actually indicate that statins do not increase cholesterol absorption at all (29, 30). Thus, statin-induced changes in plant sterol levels might not be a reflection of changes in cholesterol absorption but merely of one of the mechanisms by which statins achieve cholesterol lowering. Therefore, it is not surprising that these changes are associated with LDL-C change. Altogether, whether changes in plant sterols are valid markers of cholesterol absorption during statin therapy remains to be established. In any case, we and others (18) show that baseline markers of cholesterol absorption and synthesis do not predict LDL-C lowering during statin therapy. The present study demonstrates that this also applies to ezetimibe combined with simvastatin.
The inhibitory effect of ezetimibe on intestinal sterol absorption has been well established (31). However, data were not known for ezetimibe combined with statins until a very recent multiple crossover study in 39 mildly hypercholesterolemic men (30). This study showed that 10/20 mg ezetimibe/simvastatin reduced fractional cholesterol absorption by approximately 60%. This reduction was not statistically different from the 65% reduction achieved by 10 mg ezetimibe monotherapy. Unfortunately, noncholesterol sterol levels were not reported in that study. In fact, the effects of ezetimibe/simvastatin on changes of markers of cholesterol absorption and synthesis have also been poorly studied. Our study is the first to evaluate these effects over a longer term. The observed changes are consistent with recent results in patients with primary hypercholesterolemia treated with ezetimibe/simvastatin for 12 weeks (32). This implies that short-term changes in noncholesterol sterol levels are likely to be sustained in the long term, similarly in FH patients as in patients with primary hypercholesterolemia.
Associations between noncholesterol sterol levels and carotid intima media thickness (cIMT) as a marker of atherosclerosis are unknown, except for a very recent report in 600 individuals without cardiovascular disease (33). Because cIMT was a primary endpoint in the original ENHANCE study, it would seem tempting to evaluate posthoc associations of noncholesterol sterol levels with cIMT. However, cIMT values might have been influenced by long-term pretreatment with statins in the ENHANCE study population (15), which makes the interpretation of such associations even more difficult, next to the general drawbacks of posthoc testing. Therefore, these analyses have been left out of consideration in the present study.
Finally, several aspects of this study merit caution. First of all, exploration of the effects of ezetimibe/simvastatin and simvastatin alone on noncholesterol sterol levels, or the association of the latter with LDL-C changes, was not a prespecified objective of the original ENHANCE study. Because posthoc analyses are not corrected for multiple comparisons, they may have yielded statistically significant results merely by chance. Furthermore, because the ENHANCE study lacked an ezetimibe monotherapy arm, we were not able to evaluate the individual ensuing responses to the addition of ezetimibe to ongoing statin treatment. In addition, plasma noncholesterol sterols are indirect markers of cholesterol metabolism. Hence, the treatment-induced changes in markers of cholesterol synthesis and absorption should be confirmed with direct measurements. Moreover, we were not able to correct our analyses for any possible differences in dietary plant sterol intake between the treatment groups, because dietary data were lacking. Finally, our study population consisted of FH patients. Although sterol metabolism is similar to normal subjects and plasma noncholesterol sterols have been used as surrogate markers of cholesterol metabolism also in FH (26, 34), the LDL-C lowering response in FH may not solely depend on inhibition of cholesterol synthesis (11). In this respect, we cannot exclude that statin-related findings in the FH population might not be directly applicable to the general population.
In summary, our data imply that baseline noncholesterol sterol levels do not predict LDL-C lowering response to ezetimibe/simvastatin or simvastatin monotherapy in FH. Hence, we do not support quantification of baseline noncholesterol sterols as a tool in clinical practice to customize cholesterol lowering strategy in these patients. However, results from any posthoc analysis are by nature exploratory and the results in these FH patients do not necessarily apply to other populations. Therefore, our findings warrant further testing in prospective, randomized controlled trials in different populations.
Supplementary Material
Footnotes
Abbreviations:
- BMI
- body mass index
- cIMT
- carotid intima media thickness
- FH
- familial hypercholesterolemia
- LDL-C
- LDL-cholesterol
- TC
- total cholesterol
Non-cholesterol sterol measurements were performed and funded by Schering-Plough Research Institute.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two tables.
REFERENCES
- 1.Kempen H. J., Glatz J. F., Gevers Leuven, H. A. van der Voort, and M. B. Katan J. A. 1988. Serum lathosterol concentration is an indicator of whole-body cholesterol synthesis in humans. J. Lipid Res. 29: 1149–1155 [PubMed] [Google Scholar]
- 2.Miettinen T. A., Tilvis R. S., Kesaniemi Y. A. 1990. Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am. J. Epidemiol. 131: 20–31 [DOI] [PubMed] [Google Scholar]
- 3.Tilvis R. S., Miettinen T. A. 1986. Serum plant sterols and their relation to cholesterol absorption. Am. J. Clin. Nutr. 43: 92–97 [DOI] [PubMed] [Google Scholar]
- 4.Miettinen T. A., Gylling H. 2005. Effect of statins on noncholesterol sterol levels: implications for use of plant stanols and sterols. Am. J. Cardiol. 96(1A): 40D–46D [DOI] [PubMed] [Google Scholar]
- 5.Hoenig M. R., Rolfe B. E., Campbell J. H. 2006. Cholestanol: a serum marker to guide LDL cholesterol-lowering therapy. Atherosclerosis. 184: 247–254 [DOI] [PubMed] [Google Scholar]
- 6.Miettinen T. A., Strandberg T. E., Gylling H. 2000. Noncholesterol sterols and cholesterol lowering by long-term simvastatin treatment in coronary patients: relation to basal serum cholestanol. Arterioscler. Thromb. Vasc. Biol. 20: 1340–1346 [DOI] [PubMed] [Google Scholar]
- 7.Gylling H., Miettinen T. A. 2002. Baseline intestinal absorption and synthesis of cholesterol regulate its response to hypolipidaemic treatments in coronary patients. Atherosclerosis. 160: 477–481 [DOI] [PubMed] [Google Scholar]
- 8.Thompson G. R., O'Neill F., Seed M. 2002. Why some patients respond poorly to statins and how this might be remedied. Eur. Heart J. 23: 200–206 [DOI] [PubMed] [Google Scholar]
- 9.Miettinen T. A., Gylling H., Strandberg T., Sarna S. 1998. Baseline serum cholestanol as predictor of recurrent coronary events in subgroup of Scandinavian simvastatin survival study. Finnish 4S Investigators. BMJ. 316: 1127–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vuorio A. F., Gylling H., Turtola H., Kontula K., Ketonen P., Miettinen T. A. 2000. Stanol ester margarine alone and with simvastatin lowers serum cholesterol in families with familial hypercholesterolemia caused by the FH-North Karelia mutation. Arterioscler. Thromb. Vasc. Biol. 20: 500–506 [DOI] [PubMed] [Google Scholar]
- 11.Naoumova R. P., Marais A. D., Mountney J., Firth J. C., Rendell N. B., Taylor G. W., Thompson G. R. 1996. Plasma mevalonic acid, an index of cholesterol synthesis in vivo, and responsiveness to HMG-CoA reductase inhibitors in familial hypercholesterolaemia. Atherosclerosis. 119: 203–213 [DOI] [PubMed] [Google Scholar]
- 12.O'Neill F. H., Patel D. D., Knight B. L., Neuwirth C. K., Bourbon M., Soutar A. K., Taylor G. W., Thompson G. R., Naoumova R. P. 2001. Determinants of variable response to statin treatment in patients with refractory familial hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 21: 832–837 [DOI] [PubMed] [Google Scholar]
- 13.Fuentes F., Lopez-Miranda J., Garcia A., Perez-Martinez P., Moreno J., Cofan M., Caballero J., Paniagua J. A., Ros E., Perez-Jimenez F. 2008. Basal plasma concentrations of plant sterols can predict LDL-C response to sitosterol in patients with familial hypercholesterolemia. Eur. J. Clin. Nutr. 62: 495–501 [DOI] [PubMed] [Google Scholar]
- 14.Pisciotta L., Fasano T., Bellocchio A., Bocchi L., Sallo R., Fresa R., Colangeli I., Cantafora A., Calandra S., Bertolini S. 2007. Effect of ezetimibe coadministered with statins in genotype-confirmed heterozygous FH patients. Atherosclerosis. 194: e116–e122 [DOI] [PubMed] [Google Scholar]
- 15.Kastelein J. J., Akdim F., Stroes E. S., Zwinderman A. H., Bots M. L., Stalenhoef A. F., Visseren F. L., Sijbrands E. J., Trip M. D., Stein E. A., et al. 2008. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N. Engl. J. Med. 358: 1431–1443 [DOI] [PubMed] [Google Scholar]
- 16.Miettinen T. A., Gylling H. 2003. Synthesis and absorption markers of cholesterol in serum and lipoproteins during a large dose of statin treatment. Eur. J. Clin. Invest. 33: 976–982 [DOI] [PubMed] [Google Scholar]
- 17.Chan Y. M., Varady K. A., Lin Y., Trautwein E., Mensink R. P., Plat J., Jones P. J. 2006. Plasma concentrations of plant sterols: physiology and relationship with coronary heart disease. Nutr. Rev. 64: 385–402 [DOI] [PubMed] [Google Scholar]
- 18.van Himbergen T. M., Matthan N. R., Resteghini N. A., Otokozawa S., Ai M., Stein E. A., Jones P. H., Schaefer E. J. 2009. Comparison of the effects of maximal dose atorvastatin and rosuvastatin therapy on cholesterol synthesis and absorption markers. J. Lipid Res. 50: 730–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen S., Cox C., Cui L. 1998. A more flexible regression-to-the-mean model with possible stratification. Biometrics. 54: 939–947 [PubMed] [Google Scholar]
- 20.Mussner M. J., Parhofer K. G., von Bergmann K., Schwandt P., Broedl U., Otto C. 2002. Effects of phytosterol ester-enriched margarine on plasma lipoproteins in mild to moderate hypercholesterolemia are related to basal cholesterol and fat intake. Metabolism. 51: 189–194 [DOI] [PubMed] [Google Scholar]
- 21.Ketomaki A., Gylling H., Miettinen T. A. 2005. Non-cholesterol sterols in serum, lipoproteins, and red cells in statin-treated FH subjects off and on plant stanol and sterol ester spreads. Clin. Chim. Acta. 353: 75–86 [DOI] [PubMed] [Google Scholar]
- 22.Miettinen T. A., Gylling H. 2002. Ineffective decrease of serum cholesterol by simvastatin in a subgroup of hypercholesterolemic coronary patients. Atherosclerosis. 164: 147–152 [DOI] [PubMed] [Google Scholar]
- 23.Lahoz C., Pena R., Mostaza J. M., Laguna F., Garcia-Iglesias M. F., Taboada M., Pinto X. 2005. Baseline levels of low-density lipoprotein cholesterol and lipoprotein (a) and the AvaII polymorphism of the low-density lipoprotein receptor gene influence the response of low-density lipoprotein cholesterol to pravastatin treatment. Metabolism. 54: 741–747 [DOI] [PubMed] [Google Scholar]
- 24.Leitersdorf E., Eisenberg S., Eliav O., Friedlander Y., Berkman N., Dann E. J., Landsberger D., Sehayek E., Meiner V., Wurm M. 1993. Genetic determinants of responsiveness to the HMG-CoA reductase inhibitor fluvastatin in patients with molecularly defined heterozygous familial hypercholesterolemia. Circulation. 87(4, Suppl) III35–III44 [PubMed] [Google Scholar]
- 25.Houweling A. H., Vanstone C. A., Trautwein E. A., Duchateau G. S., Jones P. J. 2009. Baseline plasma plant sterol concentrations do not predict changes in serum lipids, C-reactive protein (CRP) and plasma plant sterols following intake of a plant sterol-enriched food. Eur. J. Clin. Nutr. 63: 543–551 [DOI] [PubMed] [Google Scholar]
- 26.Gylling H., Kuusi T., Vanhanen H., Miettinen T. A. 1989. Apolipoprotein E phenotype and cholesterol metabolism in familial hypercholesterolemia. Atherosclerosis. 80: 27–32 [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Otin A. L., Cofan M., Junyent M., Recalde D., Cenarro A., Pocovi M., Ros E., Civeira F. 2007. Increased intestinal cholesterol absorption in autosomal dominant hypercholesterolemia and no mutations in the low-density lipoprotein receptor or apolipoprotein B genes. J. Clin. Endocrinol. Metab. 92: 3667–3673 [DOI] [PubMed] [Google Scholar]
- 28.Pinedo S., Vissers M. N., von Bergmann K., Elharchaoui K., Lutjohann D., Luben R., Wareham N. J., Kastelein J. J., Khaw K. T., Boekholdt S. M. 2007. Plasma levels of plant sterols and the risk of coronary artery disease: the prospective EPIC-Norfolk Population Study. J. Lipid Res. 48: 139–144 [DOI] [PubMed] [Google Scholar]
- 29.Smith J. L., Roach P. D., Wittenberg L. N., Riottot M., Pillay S. P., Nestel P. J., Nathanson L. K. 2000. Effects of simvastatin on hepatic cholesterol metabolism, bile lithogenicity and bile acid hydrophobicity in patients with gallstones. J. Gastroenterol. Hepatol. 15: 871–879 [DOI] [PubMed] [Google Scholar]
- 30.Sudhop T., Reber M., Tribble D., Sapre A., Taggart W., Gibbons P., Musliner T., von Bergmann K., Lutjohann D. Changes in cholesterol absorption and cholesterol synthesis caused by ezetimibe and/or simvastatin in men. J. Lipid Res. Epub ahead of print. April 20, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sudhop T., Lutjohann D., Kodal A., Igel M., Tribble D. L., Shah S., Perevozskaya, and K. von Bergmann I. 2002. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 106: 1943–1948 [DOI] [PubMed] [Google Scholar]
- 32.Assmann G., Kannenberg F., Ramey D. R., Musliner T. A., Gutkin S. W., Veltri E. P. 2008. Effects of ezetimibe, simvastatin, atorvastatin, and ezetimibe-statin therapies on non-cholesterol sterols in patients with primary hypercholesterolemia. Curr. Med. Res. Opin. 24: 249–259 [DOI] [PubMed] [Google Scholar]
- 33.Weingärtner O., Lütjohann D., Rogacev K., Blömer L., Grenner Y., Girndt M., Böhm M., Fliser D., Laufs U., Heine G. H. 2008. Endogenous cholesterol synthesis is associated with increased carotid intima-media-thickness. Circulation Suppl. 118: S_407 [Google Scholar]
- 34.Gylling H., Miettinen T. A. 1989. Absorption and metabolism of cholesterol in familial hypercholesterolaemia. Clin. Sci. (Lond.). 76: 297–301 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.