Abstract
In Tangier disease, absence of ATP binding cassette transporter A1 (ABCA1) results in reduced plasma HDL and elevated triglyceride (TG) levels. We hypothesized that hepatocyte ABCA1 regulates VLDL TG secretion through nascent HDL production. Silencing of ABCA1 expression in oleate-stimulated rat hepatoma cells resulted in: 1) decreased large nascent HDL (>10 nm diameter) and increased small nascent HDL (<10 nm) formation, 2) increased large buoyant VLDL1 particle secretion, and 3) decreased phosphatidylinositol-3 (PI3) kinase activation. Nascent HDL-containing conditioned medium from rat hepatoma cells or HEK293 cells transfected with ABCA1 was effective in increasing PI3 kinase activation and reducing VLDL TG secretion in ABCA1-silenced hepatoma cells. Addition of isolated large nascent HDL particles to ABCA1-silenced hepatoma cells inhibited VLDL TG secretion to a greater extent than small nascent HDL. Similarly, addition of recombinant HDL, but not human plasma HDL, was effective in attenuating TG secretion and increasing PI3 kinase activation in ABCA1-silenced cells. Collectively, these data suggest that large nascent HDL particles, assembled by hepatic ABCA1, generate a PI3 kinase-mediated autocrine signal that attenuates VLDL maturation and TG secretion. This pathway may explain the elevated plasma TG concentration that occurs in most Tangier subjects and may also account, in part, for the inverse relationship between plasma HDL and TG concentrations in individuals with compromised ABCA1 function.
Keywords: hepatic ATP binding cassette transporter A1, very low density lipoprotein TG secretion, PI3 kinase signaling, ABCA1 silencing, Tangier disease, VLDL assembly
ABCA1 is a membrane protein that transports phospholipid (PL) and free cholesterol (FC) across cell membranes, to combine with lipid-free apolipoprotein (apo)A-I , forming nascent HDL particles (1). Mutations in ABCA1 that inactivate its function cause Tangier disease, which is characterized by a severe HDL deficiency, rapid clearance of apoA-I from plasma, deposition of sterol in macrophage-rich tissues, and premature coronary heart disease (2–5). ABCA1 is expressed in many cells, but hepatocytes are the single most important cell type in determining plasma HDL concentrations, contributing 70–80% of the HDL pool in mice (6, 7). Recently, we have shown that ABCA1 expression is necessary and sufficient for formation of heterogeneous-sized pre-β migrating nascent HDL subspecies (pre-β 1, 2, 3, and 4 HDL), which vary in size from 7.1 to 15.7 nm (8). Although electrophoretic mobility (pre-β vs. α migration) of discoidal nascent HDLs is dependent on cell type and culture conditions, similar distinct-sized subspecies of nascent HDL are formed by most cell types tested, including fibroblasts (9, 10), macrophages, hepatocytes, and ABCA1-expressing human embryonic kidney (HEK)293 cells (11). The importance of nascent HDL size heterogeneity in the physiology and pathophysiology of HDL metabolism is poorly understood.
ABCA1 expression also affects plasma VLDL and LDL concentrations. In addition to the near absence of plasma HDL, many homozygous Tangier disease patients have variable and significantly elevated plasma TG levels as well as reduced LDL concentrations compared with normal controls (2). Similarly, heterozygous Tangier disease patients have decreased HDL cholesterol (HDL-C) and elevated plasma TG levels, but similar plasma LDL concentrations compared with controls (12). Recently, our group documented that targeted deletion of ABCA1 in hepatocytes resulted in many of the phenotypic manifestations of Tangier disease including: 1) severe reduction in plasma HDL (∼20% of normal), 2) reduced (50%) plasma LDL concentrations, 3) increased (2-fold) plasma TG concentrations, and 4) hypercatabolism of apoA-I (6), suggesting that hepatic ABCA1 plays a major role in determining the Tangier disease plasma lipid phenotype, including the higher plasma TG concentrations. It has been suggested that apoC-III and apoA-II enrichment of VLDL results in reduced reactivity with lipoprotein lipase in Tangier subjects (2, 13, 14), leading to increased plasma TG concentrations. Another study has shown that incubation of apoA-I with wild-type, but not ABCA1 knockout, mouse hepatocytes results in decreased secretion of newly synthesized TG, suggesting an inverse relationship between apoA-I-mediated lipid efflux via hepatic ABCA1 and TG secretion (15). However, the underlying mechanism relating ABCA1 deficiency to elevated plasma TG concentrations and hepatic VLDL secretion is poorly understood.
Although liver is a major organ for both HDL and VLDL production, there is no evidence for a direct role of ABCA1 in modulating TG mobilization for nascent VLDL particle assembly. In this work, we hypothesized that loss of ABCA1 function increases VLDL TG secretion indirectly via nascent HDL, the product of ABCA1 activity. Our data suggest that pre-β migrating nascent HDL particles, assembled by ABCA1, generate a phosphatidylinositol-3 (PI3) kinase-mediated signal that attenuates hepatic VLDL maturation and TG secretion.
EXPERIMENTAL PROCEDURES
Cell culture and siRNA transfection
Rat hepatoma McA-RH7777 (McA) cells were cultured in DMEM containing 10% FBS, 2 mM L-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin in 5% CO2 at 37°C. Control and ABCA1 over-expressing HEK293 cells were cultured as described previously (8). Where indicated, 0.8 mM sodium oleate complexed to 0.5% BSA was included in culture medium.
McA cells were seeded at ∼70% confluence and after overnight culture transfected with 25 nM rat ABCA1 ON-TARGET plus SMARTpool small interfering (si)RNA (Dharmacon) or 25 nM negative control siRNA (AllStars Negative, Qiagen) using DharmaFECT1 siRNA transfection reagent according to the manufacturer's protocol. Forty-eight h after siRNA transfection, McA cells were incubated with oleate or lipid-free apoA-I for an additional 12–24 h as described below.
Promoter reporter assay for LXR activity
Two days after transfection with siRNA, McA cells were transiently transfected with 0.2 μg of pCMX-Gal4-LXRαLBD, an expression vector containing liver X receptor (LXR)α ligand binding domain (amino acid 155-447), and 0.2 μg of pMH100 × 4-TK-Luc, a luciferase reporter construct (16, 17), and 1 ng of pRL-CMV, a control vector, using Fugen-6 reagent (Roche Applied Science) according to the manufacturer's protocol. Cells were then incubated in DMEM supplemented with 3% charcoal-stripped serum in the presence and absence of oleate (0.8 mM, Sigma) or T0901317 (5 μM, Sigma) stimulation. After 16 h, the cells were harvested and the luciferase assay was performed using the commercial kits for dual luciferase assay (Promega).
Analysis of radiolabeled TG secretion
Two days after transfection with siRNA, McA cells were incubated with [3H]oleate (10 μCi/ml, Perkin Elmer Life Sciences) ± 0.8 mM oleate (complexed with BSA, fatty acid: BSA molar ratio = 5:1) in radiolabeling medium composed of four parts Met/Cys-deficient DMEM and one part complete DMEM in 5% FBS (18). For the kinetic studies shown in Fig. 1F, siRNA transfected McA cells were pulse-radiolabeled with 1 μCi/ml [14C]glycerol and 5 μCi/ml [3H]acetate (Perkin Elmer Life Sciences) in the same radiolabeling medium. For inhibition of PI3 kinase activity, transfected McA cells were pretreated for 1 h with either 100 nM wortmannin (Calbiochem), 10 μM LY294002 (Calbiochem), or DMSO vehicle (control) before stimulation with [3H]oleate (10 μCi/ml) plus 0.8 mM oleate. For experiments in which nascent HDL formation and VLDL-TG secretion were both monitored (Figs. 4, 5), transfected McA cells were washed with balanced salt solution and the medium was changed to serum-free DMEM containing lipid-free [125I]apoA-I and [3H]oleate (10 μCi/ml) plus 0.8 mM oleate.
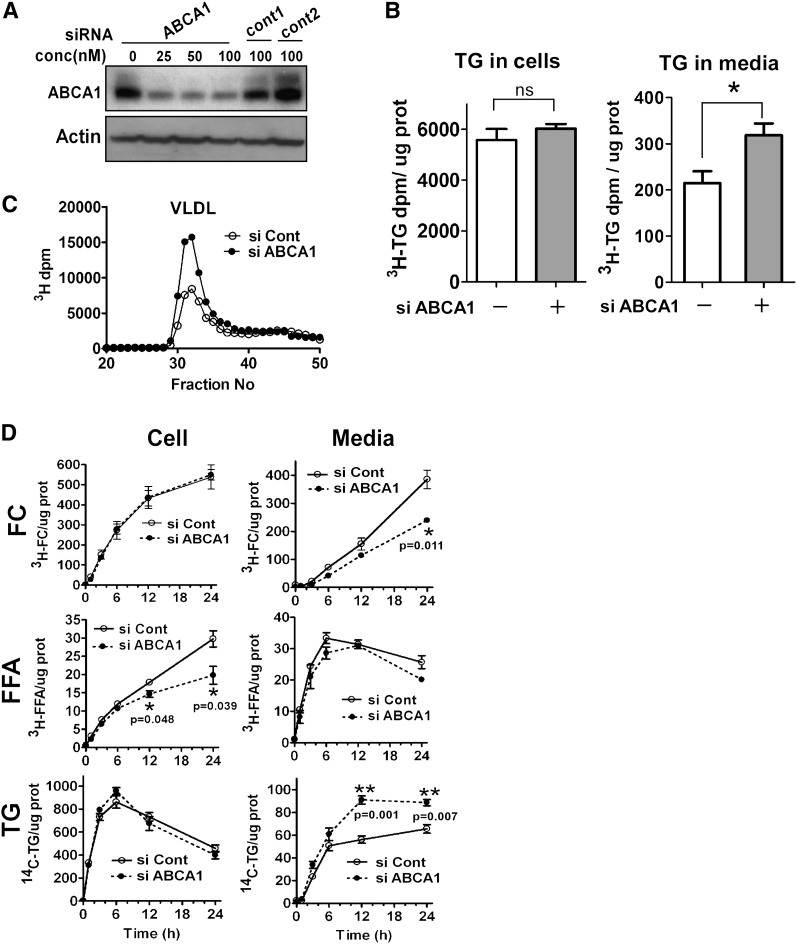
Fig. 1.
siRNA-induced silencing of ABCA1 increases VLDL TG secretion in rat hepatoma cells. A: McA rat hepatoma cells were transfected with either ABCA1 siRNA (0–100 nM) or two control siRNAs for 72 h. Silencing efficiency was assessed by Western blot analysis for ABCA1 expression. All subsequent experiments used 25 nM siRNA to silence ABCA1. B: Control and ABCA1 siRNA transfected McA cells were incubated with radiolabeling medium (10% FBS, 5 μCi/ml 3H-oleate + 0.8 mM oleate) for 24 h. Lipids were extracted from medium and cells, fractionated by TLC, and radiolabeled TG quantified by liquid scintillation spectroscopy. Cellular protein was quantified and radiolabeled TG content was expressed as 3H-TG dpm/μg cell protein (n = 3). C: Conditioned medium (500 µl; pooled, n = 3) from the experiment in B was fractionated by fast protein liquid chromatography and radiolabel in each fraction was determined. VLDL elution position (fractions 30–35) is denoted. D: Control and ABCA1 siRNA transfected McA cells were radiolabeled with [14C]glycerol (1μCi/ml) and [3H]acetate (5μCi/ml) in the presence of 0.8 mM oleate and cell and medium was harvested at 0, 1, 3, 6, 12, and 24 h. Lipids were extracted from cells and medium and separated into individual lipid classes by TLC, followed by radiolabel quantification in individual lipid classes by liquid scintillation spectroscopy. FC, free cholesterol; TG, triglyceride. ABCA1 siRNA, closed circle and dashed line; control siRNA, open circle and solid line. Results are expressed as mean ± SEM of triplicate wells. * (P < 0.05) and ** (P < 0.01) by Student's t-test. ns, not significant.
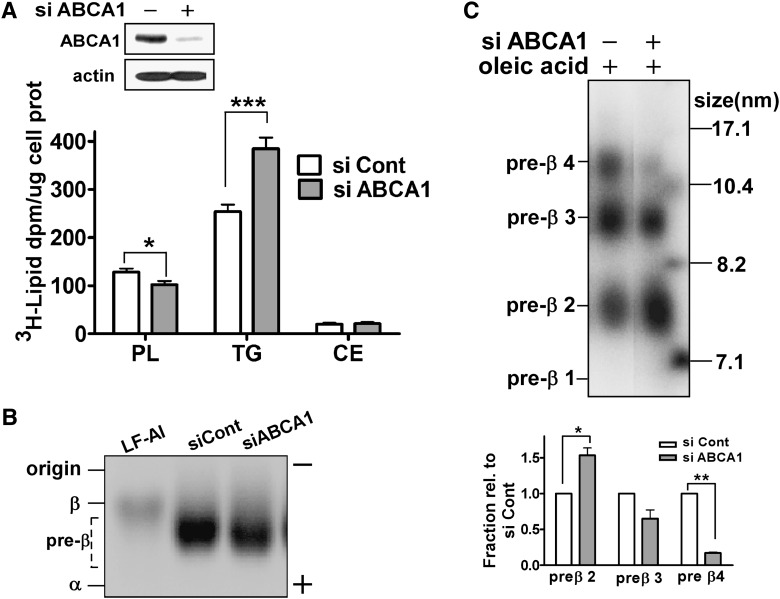
Fig. 4.
Silencing of ABCA1 results in decreased formation of large pre-β HDL concomitant with increased TG secretion. Control and ABCA1 siRNA transfected McA cells were incubated with [3H]oleate (5 µCi/ml) and [125I]apoA-I (10 µg/ml; 105 cpm/µg) in the presence of 0.4 mM oleate in serum-free medium for 24 h. A: ABCA1 expression levels were assessed by Western blot analysis (top). Aliquots of conditioned medium were lipid extracted, lipids (PL, TG, and CE) were separated by TLC, and [3H]oleate incorporated into the lipid fractions was determined (mean ± SEM, n = 4). B: Conditioned media and lipid-free [125I]apoA-I were separated on agarose gels and radioactivity was visualized using a phosphorimager. Human HDL and LDL were used as markers of α and β migration. C: Aliquots (15µl) of conditioned medium were fractionated by NDGGE and nascent HDL particles were visualized using a phosphorimager. Nascent pre-β migrating HDL formation was quantified by phosphorimager analysis and normalized to control siRNA transfected McA cells for each pre-β HDL fraction. Results are expressed as mean ± SEM of triplicate gels. Image is representative data from three independent experiments. * (P < 0.05), ** (P < 0.01), and *** (P < 0.001) by Student's t-test.
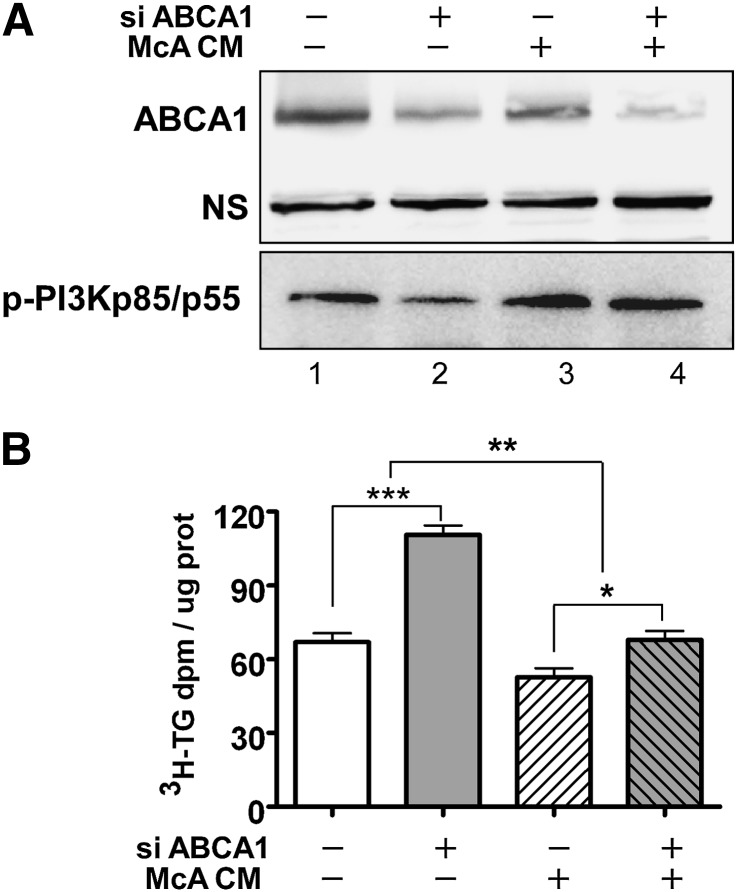
Fig. 5.
Increased TG secretion in ABCA1 silenced McA cells is attenuated by nascent HDL-containing conditioned medium. Conditioned McA medium (+ McA CM) was prepared by incubating 10 μg/ml of apoA-I with McA cells for 24 h; nonconditioned medium (− McA CM) was prepared by incubating the same concentration of apoAI in empty dishes. Conditioned or nonconditioned medium was then transferred to McA cells that had been previously transfected with control or ABCA1 siRNA and cells were then stimulated with 0.4 mM oleate in serum-free radiolabeling medium (containing 5 μCi/ml [3H]oleate) for an additional 12 h. Cells were harvested for Western blot analysis (A) and the medium was harvested to determine the extent of [3H]oleate incorporation into secreted TG (B). A: ABCA1 and p-PI3K p85/p55 expression by Western blot analysis. NS, non-specific band used as load control. B: [3H]TG secretion from control or ABCA1 siRNA-treated McA cells after incubation with conditioned medium from McA cells or nonconditioned medium for 12 h. Results are representative of two separate experiments and are expressed as mean ± SEM of triplicate analyses. * (P < 0.05), ** (P < 0.01), *** (P < 0.001) by Student's t-test.
After the cells were incubated for 12–24 h, lipids were extracted using the Bligh Dyer method (19) and separated by a dual-solvent TLC system. Briefly, the lipid extract was first developed in a polar solvent system (chloroform: methanol: acetic acid: water = 75:45:12:6) until the solvent front had traveled above the origin (∼5 cm), to ensure that any residual unincorporated radiolabel tracer would remain at the origin and not contaminate the phospholipid fraction. Then, the plate was allowed to dry and placed in a neutral solvent system (hexane: ether: acetic acid = 70:30:2). Based on the migration of authentic lipid standards, bands corresponding to PL, FC, FFA, TG, and cholesteryl ester (CE) were cut and [3H] and/or [14C] radioactivity quantified by liquid scintillation spectroscopy. After lipid extraction, cellular protein was assayed by the Lowry method and results were expressed as dpm/μg cell protein.
Western blotting
Immunoblotting of ABCA1 and β-actin were conducted as we previously described using 4–16% gradient SDS-PAGE (6). All the other proteins were separated using 10% SDS-PAGE. After electrophoresis, proteins were transferred to a polyvinylidene difluoride (PVDF) membrane for conventional Western blotting. The polyclonal antibodies targeting p-PI3K p55/p85, p-AKT (protein kinase B) (Ser473), p-AKT (Thr308), p-phosphoinositide-dependent kinase-1 (PDK1) (Ser241), p-extracellular signal-regulated kinase (ERK) (Thr202 /Tyr204), p-cJun NH2-terminal kinase (JNK) (Thr183/Tyr185), t-ERK, and p-mammalian target of rapamycin (mTOR) (Ser2448) were purchased from Cell Signaling Technology. Monoclonal antibody against adipocyte differentiation related protein (ADRP) was purchased from Research Diagnostics, Inc. Goat antibody against bovine large subunit 88 of microsomal triglyceride transfer protein (MTP) (α88-MTP) was a gift from Dr. David Gordon at Bristol-Myers Squibb.
Immunoprecipitation of apoB
For metabolic radiolabeling of apoB, siRNA transfected McA cells were preincubated in Met/Cys-deficient medium for 30 min. The medium was changed to labeling medium containing 50 μCi/ml of [35S]Met/Cys (Perkin Elmer Life Sciences) in the presence of 0.8 mM oleate for 4 h. ApoB in total cell extracts and medium was immunoprecipitated with 5 μl of goat anti-human apoB antibody (Academy Bio-Medical Co.) in immunoprecipitation buffer [1% Triton X-100, 0.5% NP 40, 150 mM NaCl, 1 mM EDTA, protease and phosphatase inhibitors (Sigma) in 10 mM Tris buffer (pH 7.4)]. The samples were then incubated with rotation at 4°C for 18 h, after which 20 μl of protein G-Sepharose beads (Amersham 50:50 slurry) were added and the incubation was continued for an additional 2 h at 4°C. Beads were collected by centrifugation at 10,000 rpm for 20 s and washed three times with immunoprecipitation buffer. Proteins were eluted from the beads by heating (70°C for 15 min) in 1× SDS-PAGE sample buffer with DTT and subjected to 4–8% gradient SDS-PAGE. The dried gel was visualized using a phosphorimager.
Density gradient ultracentrifugation of ApoB-containing lipoproteins
Transfected McA cells (3 × 106 cells in 10 cm plate) were radiolabeled with [35S]Met/Cys for 4 h. ApoB-containing lipoproteins in the conditioned medium were separated by cumulative rate flotation ultracentrifugation as described by Wang et al. (20). Briefly, 4 ml of the sample, adjusted to d = 1.10 g/ml with solid KBr, was overlaid with 3 ml of d = 1.065 g/ml NaBr, 3 ml of d = 1.02 g/ml NaBr, and 3 ml of d = 1.006 g/ml NaCl in a Beckman SW40 centrifuge tube. After centrifugation at 40,000 rpm for 148 min at 20°C, VLDL1 (Sf > 100) was collected as the top 1 ml of the gradient. Following subsequent centrifugation at 37,000 rpm for 18 h at 15°C, VLDL2 (Sf 20–100) and the other lipoproteins were collected from the top into 12 one-ml fractions. ApoB in individual fractions or pooled fractions was immunoprecipitated, resolved by SDS-PAGE, and visualized using a phosphorimager.
Lipoprotein size analysis
To determine the size of VLDL particles secreted from McA cells, 5 ml of conditioned medium from 3 × 106 cells incubated for 24 h were concentrated to 0.5 ml using an Amicon Ultra-10 concentrator. The concentrated VLDL fraction was overlayered with saline and spun at 100,000 g for 4 h to float the VLDL, which was then collected by tube-slicing. The volume was adjusted to 0.5 ml and the VLDL analyzed using a Zetasizer nano S® dynamic light scattering instrument (Malvern). Particle sizes are reported as median peak diameter using volume analysis.
Nascent HDL formation
Lipid-free apoA-I was prepared and radiolabeled as previously described (8) and verified to be authentic human apoA-I by mass spectrometry (21) and SDS-PAGE analysis. ApoA-I preparations contained <1 molecule of PL per molecule of apoA-I as assessed by phosphorus analysis (22). siRNA-transfected McA cells or ABCA1-expressing HEK were incubated with 10 μg/ml of lipid-free [125I]apoA-I (105 cpm/μg) in serum-free medium for 24 h. Immediately before incubation, [125I]apoA-I was heated to 60°C for 30 min and then cooled to room temperature to standardize the conformational state of apoA-I (8, 23). The size distribution of nascent HDL particles in conditioned medium was determined by 4–30% nondenaturing gradient gel (NDGGE) at 10°C for 1,400 V/h. After electrophoresis, gels were imaged using a phosphorimager to visualize the nascent HDL. To determine the electrophoretic mobility of McA cell-generated nascent HDL particles, 15 μl of medium was analyzed using a Paragon lipoprotein agarose gel electrophoresis system (Beckman), according to the manufacturer's instructions. The remainder of the conditioned medium was fractionated by size-exclusion chromatography using three Superdex 200 HR fast protein liquid chromatography (FPLC) columns in series as described previously (8).
rHDL preparation and human HDL isolation
Recombinant (r)HDLs were prepared from lipid free apoA-I and egg yolk PC using a cholate dialysis procedure as described previously (24). To obtain different sized rHDL particles, initial apoA-I to PC molar ratios of 1:30 for small rHDL (< 9.6 nm, rHDLsm) and 1:160 for large rHDL (>9.6 nm, rHDLlg) were used. After extensive dialysis to remove cholate, electrophoretic mobility and rHDL particle size were determined using agarose gel electrophoresis and NDGGE (4–30%), respectively (8). rHDLs were used for experiments without further purification. To isolate human HDL (hHDL), human plasma lipoproteins were isolated by ultracentrifugation at d<1.25g/ml and lipoproteins were fractionated by FPLC using a Superose 6 column. The peak three fractions of the HDL elution region were pooled and used for experiments.
Statistical analyses
Results are presented as mean ± SEM. Data were statistically analyzed using Student's t-test or one way ANOVA with Tukey's multiple comparison test to isolate individual differences. Analyses were performed using GraphPad® Prism software.
RESULTS
Silencing of ABCA1 in McA rat hepatoma cells increases VLDL TG secretion
McA cells were chosen for these studies because they robustly secrete VLDL upon incubation with oleic acid (20, 25). In addition, McA cells incubated with apoA-I assemble nascent HDL particles that are similar in size to the discrete-sized populations of nascent HDL assembled by HEK293 cells expressing ABCA1 (11).
To reduce ABCA1 expression in McA cells, we used siRNA. A dose-response study demonstrated that ABCA1 expression was reduced by ∼70% after transfection with 25–100 nM ABCA1 siRNA compared with two different control siRNAs (Fig. 1A). For all subsequent studies, we used 25 nM siRNA. To determine the effect of ABCA1 silencing on secretion of newly synthesized TG, [3H]oleate was added to control or ABCA1-silenced McA cells in the presence of 0.8 mM oleate to stimulate VLDL secretion. In agreement with a previous study (26), <5% of newly synthesized TG was secreted into the medium, whereas the remainder was cell associated (Fig. 1B). Compared with control, silencing of ABCA1 was associated with a ∼2-fold increase in TG secretion (Fig. 1B). However, the amount of radiolabel incorporated into cellular TG was similar between control and ABCA1 siRNA transfected cells. The TG increase observed in whole medium upon silencing of ABCA1 was confined to the VLDL peak as shown by FPLC analysis (Fig. 1C).
To further investigate the kinetics of lipid synthesis and secretion with ABCA1 silencing, McA cells were pulse radiolabeled with [3H]acetic acid and [14C]glycerol in the presence of oleate (0.8 mM) and the incorporation of radiolabel into newly synthesized lipid in the cells and secretion into medium was followed for 24 h (Fig. 1D). Silencing of ABCA1 was associated with decreased [3H]FC in the medium, consistent with reduced ABCA1-dependent cholesterol efflux. Accumulation of [3H]FFA was lower in ABCA1-silenced McA cells compared with control cells starting at the 12 h time point. Interestingly, the decrease in cellular FFA occurred over a similar time course as an increased secretion of [14C]TG into medium, suggesting that incorporation of newly synthesized FFA into a secretory pool of TG may be augmented in ABCA1-silenced cells. Radiolabeled CE and PL accumulation in cells and medium was similar for control and ABCA1 silenced cells (data not shown). Collectively, these data suggest that reduced expression of ABCA1 selectively increases hepatocyte TG secretion.
Silencing of ABCA1 in McA rat hepatoma cells increases VLDL size
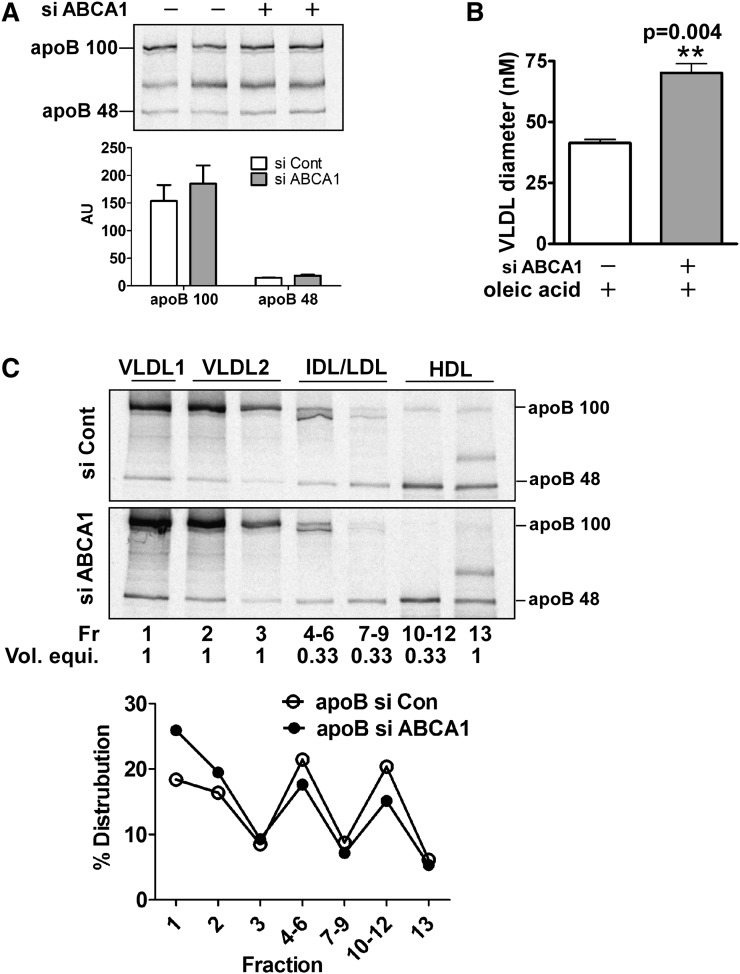
To explore the effect of ABCA1 silencing on apoB secretion, control and ABCA1-silenced McA cells were metabolically radiolabeled with [35S]Met/Cys for 4 h in the presence of oleic acid. Newly synthesized apoB48 and apoB100 in the medium was modestly increased by ABCA1 silencing (∼10–15%) compared with control siRNA (Fig. 2A). Because the ∼2-fold increase in VLDL TG secretion (Fig. 1B–D) was associated with only a marginal increase of apoB secretion, we postulated that ABCA1 silencing caused the secretion of larger VLDL particles rather than an increased number of VLDL particles. To test this, VLDL particle size was measured by dynamic laser light scattering. VLDL in medium from cells treated with ABCA1 siRNA averaged 62.7 ± 4.6 nm in diameter, a 1.6-fold increase relative to VLDL isolated from the medium of control siRNA transfected cells (38.7 ± 0.5 nm) (Fig. 2B). To further support these results, cells were radiolabeled with [35S]Met/Cys and conditioned medium was fractionated using KBr/NaBr step density gradient ultracentrifugation into VLDL1, VLDL2, IDL, LDL, and HDL. Relative to control cells, ABCA1 silencing resulted in an increased distribution of apoB100 and apoB48 in VLDL1 (Sf 100–400) versus VLDL2 (Sf 20–100) (Fig. 2C). As anticipated from results of previous studies (27), some apoB48 is distributed in the HDL fraction of oleate-stimulated McA cells due to poor lipidation of apoB48 particles. Together, these data demonstrate that silencing of ABCA1 in McA cells results in the secretion of larger VLDL particles, suggesting that the second step of VLDL assembly is augmented with ABCA1 deficiency.
Fig. 2.
Silencing of ABCA1 promotes secretion of larger VLDL (i.e., VLDL1) with minimal increase in total apoB secretion. Control and ABCA1 siRNA-transfected McA cells were metabolically radiolabeled with [35S]Met (50μCi/ml) for 4 h in the presence of 0.8 mM oleate. A: Conditioned medium was immunoprecipitated with antibody to apoB. The immunoprecipitated proteins were separated by 4–8% SDS-PAGE and visualized with a phosphorimager. Relative intensities (mean ± range) of apoB100 and apoB48 were quantified and are shown under the image. AU, arbitrary units. B: VLDL from conditioned medium was floated by ultracentrifugation at d = 1.006g/ml and particle size was measured by dynamic laser light scatter. Results are expressed as mean ± SEM of triplicate analyses. ** (P < 0.01). C: [35S]Met radiolabeled conditioned medium was subjected to density gradient ultracentifugation to fractionate the indicated lipoprotein species. ApoB was immunoprecipitated from one ml of individual or pooled fractions, the immunoprecipitated proteins were separated by SDS-PAGE and radiolabel in protein bands was visualized using a phosphorimager. Because fractions 4–6, 7–9, and 10–12 were pooled (i.e., 3 ml total), the volume equivalent for each lane on the gel is indicated below the fraction number. Positions of lipoprotein classes in the gradient are denoted at the top of the gel and migration positions of apoB100 and apoB48 are shown on the right side of the gel image. VLDL1 = Sf 100–400, VLDL2 = Sf 20–100. Images were quantified using a phosphorimager and relative intensities of apoB (B100 + B48) in individual or pooled fractions are presented as a percentage distribution in the gradient. Intensities for the pooled fractions were multiplied by three to correct for the difference in total volume between individual (1 ml) and pooled fractions (3 ml).
Silencing of ABCA1 in McA rat hepatoma cells does not lead to increased LXR activation
We originally hypothesized that a deficiency of ABCA1 will reduce ABCA1-dependent FC efflux, which in turn may cause cellular accumulation of oxysterols that are potential endogenous ligands for LXRs. LXRs are cholesterol-sensing nuclear receptors that stimulate lipogenic target gene expression upon ligand binding, resulting in increased FFA synthesis, elevation of plasma TG, and liver steatosis (28, 29). To test whether LXR ligation is increased when ABCA1 is silenced, a LXR ligand-binding domain reporter construct was transfected into control and ABCA1-silenced cells. Contrary to our expected outcome, endogenous ligation of LXRα was unaffected by ABCA1 silencing (supplementary Fig. I). As a positive control, the synthetic LXR agonist T0901317 was used to stimulate LXR ligation. As expected, T0901317 treatment of cells stimulated LXR binding to the reporter construct for control and ABCA1 silenced cells. Surprisingly, LXR binding was less in ABCA1-silenced versus control cells. Based on these data, we ruled out the possibility that elevated TG secretion in ABCA1 silenced cells is driven by oxysterol-mediated LXR activation.
Elevated TG secretion in ABCA1-silenced McA rat hepatoma cells is dependent on PI3 kinase inhibition
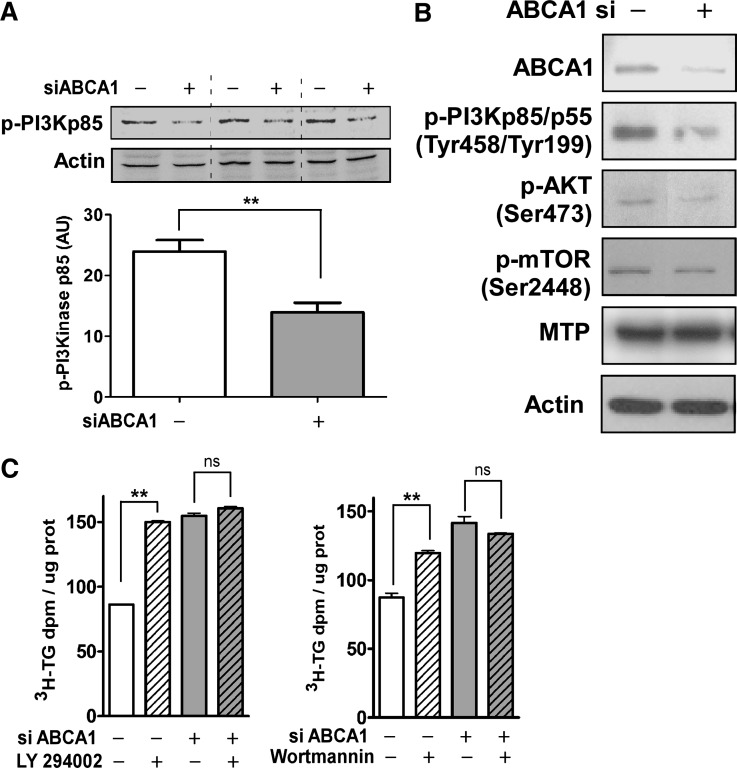
Increased VLDL secretion can be achieved through inhibition of PI3 kinase activation (30, 31). Hence, we determined whether PI3 kinase activation was involved in the increased TG secretion from ABCA1-silenced oleate-stimulated McA cells. PI3 kinase p85/55 phosphorylation was attenuated by ∼72% in oleate-stimulated ABCA1 silenced cells compared with control siRNA treated cells (Fig. 3A). Despite the considerable decrease in PI3 kinase activation in ABCA1-silenced McA cells, the decrease in phosphorylation of downstream target proteins of PI3 kinase, such as PKB/Akt and mTOR, was less robust. The expression of MTP, an essential protein for lipid transfer to VLDL (20), remained unchanged (Fig. 3B).
Fig. 3.
ABCA1 silencing is associated with decreased activation of PI3 kinase and increased TG secretion. Control and ABCA1 siRNA transfected McA cells were incubated with 0.8 mM oleate for 24 h. A: PI3 kinase activation was determined by Western blot analysis of p-PI3 kinase p85/p55. Data represent mean ± SEM for three samples obtained in two separate experiments. B: Cells were harvested and Western blotted for ABCA1, p-PI3 kinase p85/p55, p-AKT, p-mTOR, MTP, and β-actin (loading control) as indicated. C: Control or ABCA1 siRNA transfected McA cells were incubated with vehicle (DMSO), LY294002 (10 μM), or wortmannin (100 nM) as indicated for 1 h before addition of radiolabeling medium ([3H]oleate (5 μCi/ml) + 0.8 mM oleate). After additional 12 h incubation, lipids were extracted from medium and radiolabel incorporation into TG was quantified by TLC. Data (mean ± SEM, n = 3–4) were normalized to cellular protein content. ** (P < 0.01) by Student's t-test; ns, not significant.
To determine the extent to which decreased PI3 kinase activation is linked to the increase in TG secretion in ABCA1-silenced McA cells, we used two pharmacological inhibitors of PI3 kinase activity [Ly 294002 (Ly) and wortmannin]. In the absence of Ly, ABCA1 silencing resulted in a 2-fold increase in TG secretion compared with control, as expected (Fig. 3C, left panel). In the presence of Ly, TG secretion was unchanged for cells treated with ABCA1 siRNA; however, control siRNA-treated cells responded to Ly treatment with an increase in TG secretion that was similar in magnitude to that of cells treated with ABCA1 siRNA (Fig. 3C, left panel). Similar results were observed in wortmannin-treated control and ABCA1-silenced cells (Fig. 3C, right panel). These data suggest that silencing of ABCA1 attenuates PI3 kinase signaling to a similar degree to that observed with chemical inhibition, resulting in greater VLDL-TG secretion.
Elevated TG secretion in ABCA1-silenced McA rat hepatoma cells is independent of MEK/ERK and mTOR signaling
Several studies have reported that control of VLDL secretion can be achieved through mTOR (32, 33) and MEK/ERK signaling (34). We investigated whether MEK/ERK phosphorylation was mediating the increase in VLDL-TG secretion when ABCA1 was silenced. Even though ERK was decreased with oleate stimulation compared with nonstimulated cells, there was only a small decrease in ERK activation in ABCA1-silenced McA cells compared with control cells (supplementary Fig. IIA). In addition, inhibition of ERK phosphorylation with a specific MEK/ERK inhibitor (U0126) had minimal impact on VLDL-TG secretion relative to the striking increase in VLDL TG secretion with ABCA1 silencing (supplementary Fig. IIB). Phosphorylation of mTOR appeared reduced in ABCA1-silenced cells compared with control cells (supplementary Fig. IIA). Treatment of cells with rapamycin to inhibit mTOR resulted in decreased cellular TG and increased TG secretion but failed to abolish the increase in TG secretion caused by ABCA1 silencing (supplementary Fig. IIC). Taken together, these results suggest that increased TG secretion in ABCA1-silenced McA cells is dependent on inhibition of PI3 kinase and minimally affected by MEK/ERK or mTOR pathways.
Pre-β-HDLs generate a PI3 kinase-mediated autocrine signal resulting in reduced VLDL TG secretion in McA rat hepatoma cells
Previously, we demonstrated that incubation of apoA-I with a nonhepatic HEK293 cell line expressing ABCA1 is necessary and sufficient for generation of multiple nascent HDL particles that vary in size, lipid, and apoA-I content (8). A similar size range of nascent HDL particles was also observed when apoA-I was incubated with McA cells (11). We hypothesized that silencing of ABCA1 in McA cells results in reduced nascent HDL formation that normally attenuates VLDL TG secretion via a PI3 kinase signaling pathway, resulting in increased VLDL TG secretion.
To test this hypothesis, we used experimental conditions that allowed us to simultaneously analyze VLDL TG secretion and nascent HDL formation using [3H]oleate (with 0.4 mM oleate) and [125I]apoA-I, respectively, in serum-free medium. Under these conditions, we observed a 2-fold increase in radiolabeled TG secretion and a significant decrease in PL secretion (Fig. 4A). The decrease in PL secretion from ABCA1-silenced cells may be due to reduced ABCA1-dependent PL efflux that is only apparent in serum-free medium. Silencing of ABCA1 did not affect electrophoretic mobility of nascent HDL, which was in the pre-β position (Fig. 4B). Separation of conditioned medium by NDGGE demonstrated silencing of ABCA1 caused a preferential loss of larger nascent HDLs (>10 nm, pre-β3 and pre-β4) and an accumulation of pre-β2 nascent HDL (Fig. 4C). These results demonstrate an association between decreased formation of large nascent HDLs and increased TG secretion in ABCA1-silenced cells.
To determine whether the attenuation of PI3 kinase activation and the resulting increase in TG secretion in ABCA1-silenced cells could be reversed by exogenously supplied nascent HDL particles, conditioned medium containing nascent HDL or nonconditioned medium containing only lipid-free apoA-I were separately prepared using cultures of McA cells or empty dishes, respectively. Lipid-free apoA-I (non-conditioned medium) did not alter PI3 kinase signaling or TG secretion patterns in cells treated with control siRNA (Fig. 5A, lanes 1 and 3; 5B, bars 1 and 3). However, nascent HDL-containing conditioned medium increased PI3 kinase phosphorylation (Fig. 5A, lane 4) and decreased TG secretion (Fig. 5B, bar 4) to levels observed in control siRNA-treated cells. To rule out the possibility that decreased radiolabeled TG in McA cell-conditioned medium was due to lipolysis, lipase activity in medium was measured using [3H]triolein emulsion particles as substrate (35). The absence of lipase activity above background levels in McA cell-conditioned medium (data not shown) suggested that the decrease in radiolabeled TG in the medium of ABCA1-silenced cells was due to secretion and not lipolysis. Collectively, these data suggest the existence of a novel signaling pathway in which nascent HDLs generated by ABCA1 attenuate VLDL TG secretion.
Larger nascent HDLs selectively decrease VLDL TG secretion
Next, we addressed two fundamental questions regarding the ability of nascent HDLs to attenuate VLDL TG secretion: 1) is this a unique property of nascent HDLs of hepatic origin, and 2) are different-sized nascent HDLs equally effective in reducing TG secretion?
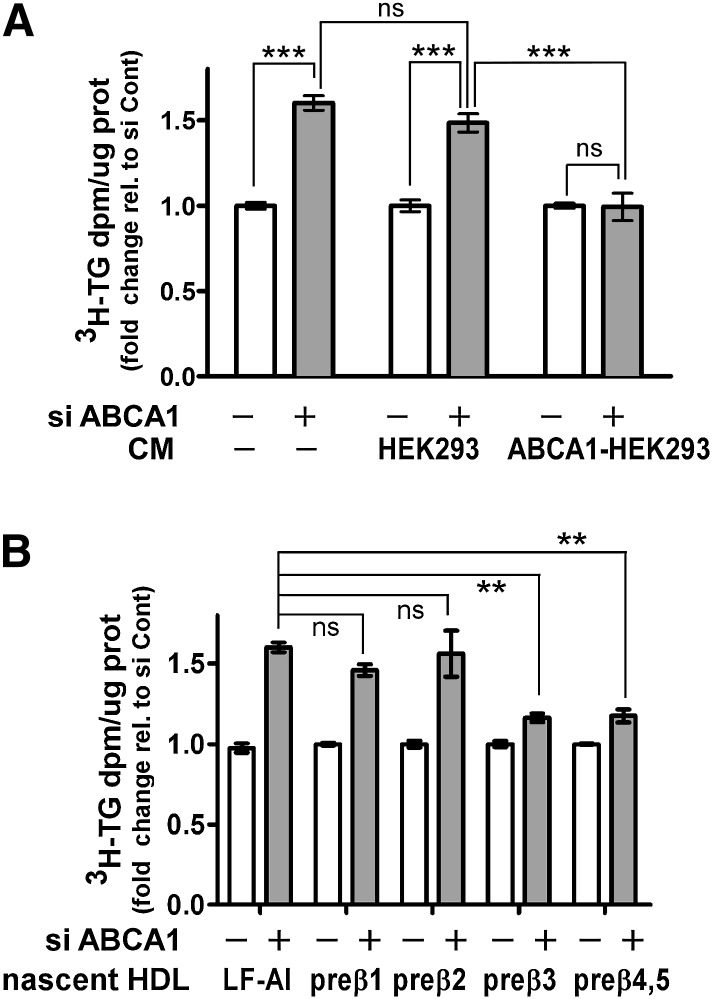
To answer the first question, conditioned medium of nonhepatic origin was prepared from ABCA1-expressing or control HEK293 cells, followed by addition of conditioned medium to oleate-stimulated control and ABCA1-silenced McA cells. In the absence of conditioned medium, ABCA1-silenced cells demonstrated increased TG secretion, as anticipated (Fig. 6A, first two bars). Similar results were obtained when conditioned medium from control HEK293 (i.e., not expressing ABCA1) cells was added to ABCA1-silenced McA cells (Fig. 6A, bars 3–4). However, when conditioned medium from ABCA1-expressing HEK293 cells was added to ABCA1-silenced McA cells, TG secretion was reduced to the levels observed in control siRNA-treated cells (Fig. 6A, bars 5–6). Hence, the ability to reduce TG secretion was not a unique property of nascent HDL of hepatic origin. In addition, this experiment excludes the possibility that the results obtained in Fig. 5B were due to an unidentified secretory factor(s) from McA cells that was responsible for lowering TG secretion.
Fig. 6.
Large nascent HDLs attenuate the increased TG secretion induced by ABCA1 silencing. A: Conditioned medium was prepared by incubating 10 μg apoA-I /ml with control and ABCA1-expressing HEK 293 cells for 24 h. Nonconditioned medium (−CM) was prepared by incubating 10 µg/ml of apoA-I with empty dishes for 24 h. The conditioned or nonconditioned medium along with [3H]oleate + 0.4 mM oleate was then added to McA cells that had been previously transfected with control or ABCA1 siRNA (25 nM for 48 h). After additional 12 h incubation, [3H]TG secretion into the medium was quantified as described in Fig. 1 legend. B: Conditioned medium from ABCA1-expressing HEK293 cells was prepared as described in A and fractionated by high resolution FPLC into individual subfractions of nascent HDL as described previously (8). McA cells that had been previously transfected with control or ABCA1 siRNA (25 nM for 48 h) were incubated with individual nascent HDL subfractions (2 µg protein/ml) in the presence of [3H]oleate + 0.8 mM oleate for an additional 12 h and the amount of newly synthesized TG secreted into the medium was quantified. Results are normalized to control siRNA transfected cells and are expressed as mean ± SEM of triplicate analyses. ** (P < 0.01), *** (P < 0.001); ns, not significant, by one-way ANOVA (Tukey's multiple comparison test).
To address whether all nascent HDL subfractions are equally effective in reducing TG secretion in ABCA1-silenced McA cells, we fractionated conditioned medium from ABCA1-HEK293 cells by FPLC and isolated and concentrated individual nascent HDL species (pre-β1–5). Individual (pre-β1, 2, 3) or pooled (pre-β4 and 5) nascent HDL were incubated with oleate-stimulated control and ABCA1-silenced McA cells. The larger (>10 nm; pre-β 3, 4, and 5) nascent HDL significantly attenuated TG secretion, whereas the smaller pre-β1 and 2 particles had no significant impact on TG secretion (Fig. 6B).
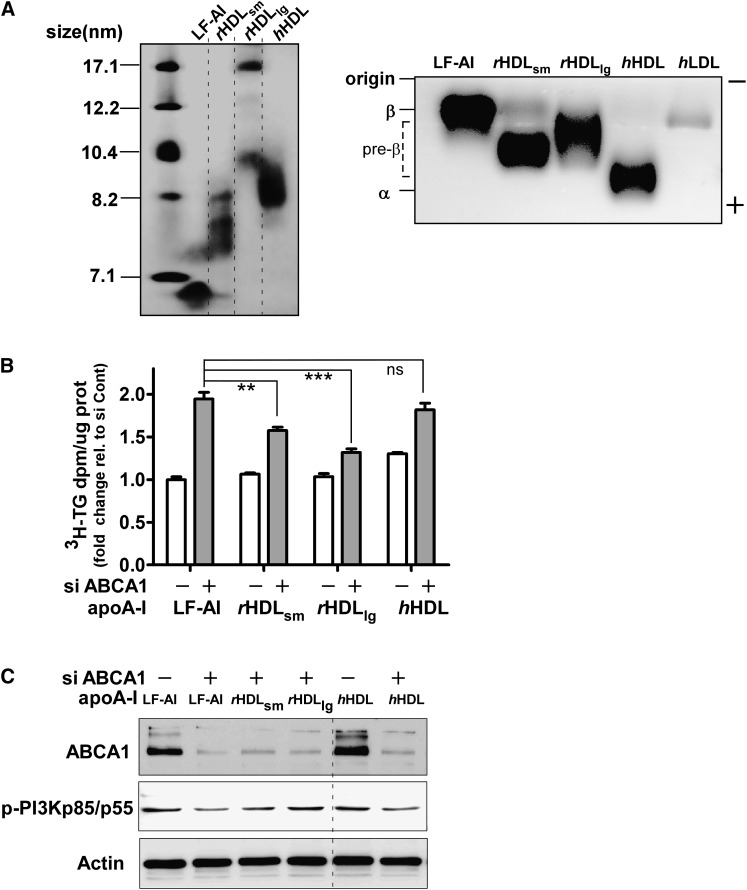
Finally, to determine whether cell-free assembly of model nascent HDL particles (i.e., rHDL) would also attenuate TG secretion in ABCA1-silenced McA cells, we incubated small rHDL (rHDLsm <10 nm) and large rHDL (rHDLlg >10 nm) as well as human plasma HDL with control and ABCA1-silenced McA cells and measured TG secretion and PI3 kinase activation. rHDLsm were heterogeous in size, ranging between 7.1 and ∼8.2 nm, and rHDLlg were composed of two major particles, 17 and 10 nm (Fig. 7A, left panel). As anticipated, both rHDLs migrated in the pre-β range on agarose gels, whereas hHDL migrated in the α position (Fig. 7A, right panel). Addition of rHDL particles, but not hHDL, resulted in a significant reduction in TG secretion relative to lipid-free apoA-I in ABCA1-silenced McA cells, with a greater reduction in TG secretion observed with rHDLlg compared with rHDLsm (Fig. 7B). Furthermore, reduced PI3 kinase activation in ABCA1-silenced McA was restored with rHDL incubation, but not with lipid-free apoA-I or hHDL (Fig. 7C). Based on these combined results, we propose that large nascent HDLs assembled by ABCA1 generate an autocrine signal that contributes to diminished TG secretion.
Fig. 7.
Large rHDL, but not hHDL, attenuate TG secretion in ABCA1-silenced McA cells. A: Small (rHDLsm) and large (rHDLlg) recombinant HDL and human HDL were characterized for size by NDGGE (left) and electrophoretic mobility by agarose gel electrophoresis (right). Proteins were visualized by Coomassie Brilliant Blue G-250 staining. B: Control or ABCA1 siRNA transfected (25 nM for 48 h) McA cells were incubated with 10µg protein/ml of either lipid-free apoA-I (LF-AI), rHDL, or hHDL in the presence of [3H]oleate + 0.8 mM oleate for 12 h and the amount of newly synthesized TG secreted into the medium was quantified. C: Cellular protein from the experiment in B was harvested and ABCA1 and p-PI3K p85/p55 expression was examined by Western blot analysis. Western blot data for control siRNA rHDL incubations were similar to that for the lipid-free apoA-I incubation and are not shown. ** (P < 0.01), *** (P < 0.001); ns, not significant, by one-way ANOVA (Tukey's multiple comparison test).
DISCUSSION
The goal of this study was to determine the role of hepatic ABCA1 expression in the regulation of VLDL TG secretion. Although we now understand how absence of ABCA1 function leads to low plasma HDL concentrations in Tangier subjects, a molecular explanation for the increased plasma TG concentrations in these patients is not clear. One suggestion is that apoC-III and apoA-II enrichment of VLDL inhibits intravascular lipolysis of VLDL, resulting in increased plasma TG concentrations (14). However, this hypothesis has not been rigorously tested. An alternative unexplored hypothesis is that hepatic overproduction of VLDL leads to elevated plasma TG concentrations. Individuals with type 2 diabetes have a plasma lipid phenotype similar to that of Tangier disease heterozygotes, including increased plasma TG, decreased plasma HDL, and a predominance of smaller HDL particles in plasma (12, 13, 36). Type 2 diabetics also have overproduction of larger TG- enriched VLDL1 particles with little change in smaller VLDL2 particles (37). In addition, cells from Tangier subjects have a defect in Golgi-to-plasma-membrane vesicular trafficking that potentially could affect VLDL secretion (38). These observations led us to hypothesize that the increase in plasma TG in the absence of ABCA1 may result from hepatic overproduction of VLDL. This hypothesis was particularly attractive because hepatocytes are a quantitatively important cell type in both VLDL and HDL production. In this study, we demonstrate that silencing of ABCA1 in McA cells results in decreased formation of large nascent HDL particles with concomitant increase in smaller nascent HDLs, increased secretion of large VLDL1 particles, and attenuated PI3 kinase activation. Furthermore, addition of large nascent pre-β-migrating HDL particles to ABCA1-silenced cells results in decreased TG secretion and increased PI3 kinase activation, suggesting a novel pathway in which hepatic ABCA1 regulates VLDL TG secretion through PI3 kinase-dependent HDL signaling.
Assembly of apoB with lipid to form VLDL particles in the liver is a complex process composed of at least two distinct steps. The first step involves cotranslational apoB lipidation by MTP forming a small dense primordial apoB-containing preVLDL in the endoplasmic reticulum (20, 39). This preVLDL fuses with lipid droplets in the endoplasmic reticulum or Golgi compartments (i.e., second step), maturing into a VLDL-sized particle referred to as VLDL2 (Sf 20–100) (40). Available evidence suggests that MTP is also required for generation of the second step lipid droplets (20, 41–43). If sufficient TG is available in the secretory compartment, second step assembly can result in larger TG-enriched VLDL1 particles (Sf 100–400) that is one of the hallmarks of type 2 diabetes (44). The second step of VLDL assembly is inhibited by PI3 kinase activation, such that inhibitors of PI3 kinase (i.e., wortmannin and Ly 294002) enhance second step assembly and secretion of larger TG-enriched VLDL1 particles (30, 31). Our results suggest that silencing of ABCA1 in McA cells selectively augments second step VLDL assembly based on the following observations: 1) secretion of larger TG- enriched VLDL1 particles with a minimal increase in VLDL particle number (Fig. 2), 2) decreased PI3 kinase phosphorylation (Fig. 3A), and 3) insensitivity of TG secretion to PI3 kinase inhibitors in ABCA1 silenced cells (Fig. 3C).
Insulin diminishes VLDL secretion through several signaling pathways, including MEK/ERK (30, 45) and PI3 kinase pathways (30, 31, 46, 47). Insulin stimulation appears to specifically inhibit the second step of VLDL assembly such that insulin resistance results in increased hepatic production of VLDL1 (37). Most of insulin's inhibitory effect on VLDL secretion appears to be at the level of MTP expression (48, 49). Recently, Kamagate et al. (50) reported that MTP is a target of the transcription factor forkhead box O1 (FoxO1) and that signaling through IRS-1/PI3K/Akt results in phosphorylation of FoxO, which induces translocation of FoxO1 from the nucleus to the cytosol and results in decreased transcriptional activation of the MTP gene. In addition, insulin-mediated MEK/ERK activation causes inhibition of MTP activity, limiting TG availability and apoB stability in HepG2 cells (51, 52). However, these pathways do not explain the increased TG secretion with silencing of ABCA1 since MTP expression was similar (Fig. 3) and MEK/ERK activation (supplementary Fig. II) was minimally affected in ABCA1-silenced McA cells versus control siRNA-treated cells.
mTOR is a serine/threonine kinase that regulates multiple cellular functions and acts as a sensor of cellular nutrient and energy levels (53). Rapamycin is an inhibitor of mTOR and is used clinically as an immunosuppressive drug to reduce the risk of kidney transplant rejection. A significant side effect of rapamycin treatment is hyperlipidemia that involves hepatic overproduction of TG (32, 33). Rapamycin treatment of McA cells did not affect the increased TG secretion observed in ABCA1 versus control siRNA-treated cells (supplemental Fig. IIC), suggesting that the mTOR pathway was not responsible for the increased TG secretion in ABCA1-silenced cells.
Another possible explanation for increased TG secretion in ABCA1-silenced McA cells may relate to an increase in an intracellular pool of cholesterol that stimulates VLDL secretion. In support of this concept, Khan et al. (54) reported that inhibition of cholesterol biosynthesis in rats given statin treatment resulted in a decrease in intracellular cholesterol and a significant decrease in VLDL TG secretion. In addition, Sahoo et al. (15) showed that apoA-I-dependent ABCA1 cholesterol efflux was associated with decreased TG secretion in primary mouse and rat hepatocytes and McA cells. Taken together, these studies suggest a regulatory pool of cholesterol may be involved in controlling VLDL TG secretion. Our results do not support an increase in cellular FC or CE content in ABCA1-silenced cells, although appearance of FC in the medium was decreased (Fig. 1D). However, this does exclude the possibility that a small regulatory pool of cholesterol might not be detected by our experimental procedures. For instance, a small increase in cellular FC in ABCA1-silenced cells may result in an increase in one or more oxysterols that could activate LXR and increase TG synthesis (28, 55). However, we did not observe an increase in cellular TG synthesis (Fig. 1B) and could find no evidence for increased LXR activation in ABCA1-silenced cells (supplementary Fig. I), suggesting that LXR activation does not explain our experimental results.
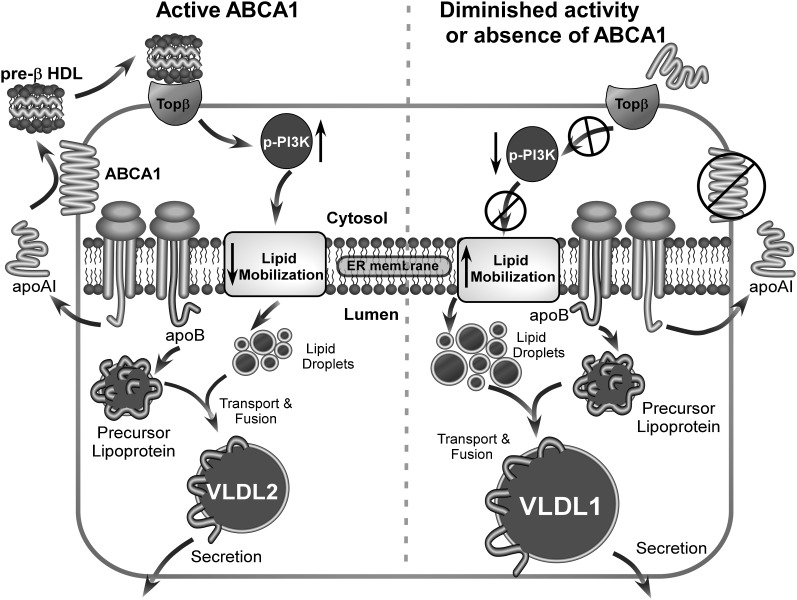
Because ABCA1 has no known role in TG transport, we hypothesized that silencing of ABCA1 reduced nascent HDL production, which in turn resulted in increased TG secretion. In support of our hypothesis, addition of nascent HDL particles or rHDLs to ABCA1-silenced McA cells reduced TG secretion (Figs. 5–7). Furthermore, reduction in TG secretion occurs with the addition of nascent HDL particles generated by hepatic as well as nonhepatic cell lines and larger nascent HDL particles are more potent in reducing TG secretion compared with smaller ones (Fig. 6, 7). This trend was also observed for rHDL particles (Fig. 7B). There is a well-accepted inverse relationship between plasma HDL and TG concentrations in the general population. This relationship is also observed in heterozygous Tangier disease subjects (12). The extent to which low HDL concentrations in individuals with ABCA1 polymorphisms is linked to elevated plasma TG concentrations in these same subjects is unknown. However, our data suggest that there may be a direct link as summarized in Fig. 8. Heterozygous Tangier disease subjects have fewer large HDL particles in plasma. Our results suggest that this may be due to decreased hepatic ABCA1 function, resulting in decreased assembly of large nascent HDL particles (Fig. 4C). We hypothesize that these particles normally transmit signals through a PI3 kinase-mediated pathway to decrease VLDL TG secretion (Fig. 8, left). We speculate that a putative receptor for pre-β HDL particles (i.e., Topβ, target of pre-β HDL) transmits the signal to activate PI3 kinase and reduces lipid mobilization for VLDL secretion, and that larger pre-β migrating HDL particles preferentially interact with the putative Topβ. In situations of diminished ABCA1 function, large nascent HDL particles are reduced in concentration (Fig. 4C), resulting in diminished PI3 kinase activation (Fig. 5A, 7C), increased lipid mobilization, and increased VLDL TG secretion (Fig. 8, right).
Fig. 8.
Summary diagram of experimental results. In the presence of active ABCA1 (left), lipid free apoA-I is secreted by hepatocytes and lipidated by hepatocyte ABCA1, forming nascent HDL particles. Large nascent HDLs (>10 nm diameter) bind to a putative receptor (Topβ, target of pre-β), which in turn stimulates PI3 kinase activation and results in reduced lipid mobilization and secretion of normal-sized VLDL particles (i.e., VLDL2). In contrast, in the absence of hepatic ABCA1 or diminished ABCA1 activity (right), newly secreted lipid-free apoA-I fails to form large nascent HDL particles. The absence of large nascent HDL particle formation leads to diminished signaling through Topβ, resulting in reduced PI3 kinase activation, increased lipid mobilization, and increased secretion of larger TG-enriched VLDL particles (VLDL1).
Another explanation for the inverse relationship between plasma HDL and TG in humans and animal species that express active cholesteryl ester transfer protein relates to intravascular metabolism. Cholesteryl ester transfer protein can exchange TG in VLDL for CE in HDL, followed by lipolysis of the TG in HDL by hepatic lipase (56, 57). This results in smaller HDL particles and the generation of pre-β HDL. Thus, when TG concentrations and/or cholesteryl ester transfer protein activity are elevated, HDL concentrations are reduced.
In summary, our data suggest a novel pathway in which hepatic ABCA1 regulates VLDL TG secretion through PI3 kinase-dependent nascent HDL signaling. This pathway may contribute to the inverse association between reduced plasma HDL and increased plasma TG concentrations in individuals with compromised ABCA1 function due to genetic mutations in ABCA1 or other metabolic conditions that compromise nascent HDL assembly by hepatocytes.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Karen Klein for editing the manuscript.
Footnotes
Abbreviations:
- ADRP
- adipocyte differentiation related protein
- apo
- apolipoprotein
- CE
- cholesteryl ester
- ERK
- extracellular signal-regulated kinase
- FC
- free cholesterol
- FPLC
- fast protein liquid chromatography
- hHDL
- human HDL
- HEK
- human embryonic kidney
- HDL-C
- HDL cholesterol
- LXR
- liver X receptor
- McA
- McArdle RH7777
- mTOR
- mammalian target of rapamycin
- MTP
- microsomal triglyceride transfer protein
- NDGGE
- non-denaturating gradient gel electrophoresis
- PI3
- phosphatidylinositide-3
- PL
- phospholipid
- rHDL
- recombinant HDL
- siRNA
- small interfering RNA
- TG
- triglyceride
This work was supported by the National Institutes of Health grants HL 49373, HL 54176, and AT 27820 (JSP) and AHA 0825445E (postdoctoral fellowship to S. Chung). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two figures.
REFERENCES
- 1.Broccardo C., Luciani M., Chimini G. 1999. The ABCA subclass of mammalian transporters. Biochim. Biophys. Acta. 1461: 395–404. [DOI] [PubMed] [Google Scholar]
- 2.Assman G., von Eckardstein A., Brewer H. B., Jr 2001. Familial Analphalipoproteinemia: Tangier Disease. The Metabolic and Molecular Bases of Inherited Disease. Scriver C. R., Beaudet A. L., Sly W. S., Valle D., Childs B., Kinzler K. W., Volkman B. F., McGraw-Hill, New York: 2937–2960. [Google Scholar]
- 3.Brooks-Wilson A., Marcil M., Clee S.M., Zhang L.H., Roomp K., van Dam M., Yu C., Brewer J. A., Collins H. O., Molhuizen O., et al. 1999. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat. Genet. 22: 336–345. [DOI] [PubMed] [Google Scholar]
- 4.Rust S., Rosier M., Funke H., Real J., Amoura Z., Piette J. C., Deleuze J. F., Brewer H. B., Duverger N., Denefle P., et al. 1999. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat. Genet. 22: 352–355. [DOI] [PubMed] [Google Scholar]
- 5.Bodzioch M., Orso E., Klucken J., Langmann T., Bottcher A., Diederich W., Drobnik W., Barlage S., Buchler C., Porsch-Ozcurumez M., et al. 1999. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat. Genet. 22: 347–351. [DOI] [PubMed] [Google Scholar]
- 6.Timmins J. M., Lee J. Y., Boudyguina E., Kluckman K. D., Brunham L. R., Mulya A., Gebre A. K., Coutinho J. M., Colvin P. L., Smith T. L., et al. 2005. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J. Clin. Invest. 115: 1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunham L. R., Kruit J. K., Iqbal J., Fievet C., Timmins J. M., Pape T. D., Coburn B. A., Bissada N., Staels B., Groen A. K., et al. 2006. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J. Clin. Invest. 116: 1052–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mulya A., Lee J. Y., Gebre A. K., Thomas M. J., Colvin P. L., Parks J. S. 2007. Minimal lipidation of pre-beta HDL by ABCA1 results in reduced ability to interact with ABCA1. Arterioscler. Thromb. Vasc. Biol. 27: 1828–1836. [DOI] [PubMed] [Google Scholar]
- 9.Hassan H. H., Denis M., Lee D. Y., Iatan I., Nyholt D., Ruel I., Krimbou L., Genest J. 2007. Identification of an ABCA1-dependent phospholipid-rich plasma membrane apolipoprotein A-I binding site for nascent HDL formation: implications for current models of HDL biogenesis. J. Lipid Res. 48: 2428–2442. [DOI] [PubMed] [Google Scholar]
- 10.Hassan H. H., Bailey D., Lee D. Y., Iatan I., Hafiane A., Ruel I., Krimbou L., Genest J. 2008. Quantitative analysis of ABCA1-dependent compartmentalization and trafficking of apolipoprotein A-I: implications for determining cellular kinetics of nascent high density lipoprotein biogenesis. J. Biol. Chem. 283: 11164–11175. [DOI] [PubMed] [Google Scholar]
- 11.Mulya A., Lee J.Y., Gebre A.K., Boudyguina E.Y., Chung S.K., Smith T.L., Colvin P.L., Jiang X.c., Parks J.S. 2008. Initial interaction of ApoA-I with ATP binding cassette transporter A1 (ABCA1) impacts in vivo metabolic fate of nascent HDL. J. Lipid Res. 49: 2390–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clee S.M., Kastelein J. J., van Dam M., Marcil M., Roomp K., Zwarts K. Y., Collins J. A., Roelants R., Tamasawa N., Stulc T., et al. 2000. Age and residual cholesterol efflux affect HDL cholesterol levels and coronary artery disease in ABCA1 heterozygotes. J. Clin. Invest. 106: 1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asztalos B. F., Brousseau M. E., McNamara J. R., Horvath K. V., Roheim P. S., Schaefer E. J. 2001. Subpopulations of high density lipoproteins in homozygous and heterozygous Tangier disease. Atherosclerosis. 156: 217–225. [DOI] [PubMed] [Google Scholar]
- 14.Alaupovic P., Knight-Gibson C., Wang C. S., Downs D., Koren E., Brewer H. B., Jr., Gregg R. E. 1991. Isolation and characterization of an apoA-II-containing lipoprotein (LP-A-II:B complex) from plasma very low density lipoproteins of patients with Tangier disease and type V hyperlipoproteinemia. J. Lipid Res. 32: 9–19. [PubMed] [Google Scholar]
- 15.Sahoo D., Trischuk T. C., Chan T., Drover V. A., Ho S., Chimini G., Agellon L. B., Agnihotri R., Francis G. A., Lehner R. 2004. ABCA1-dependent lipid efflux to apolipoprotein A-I mediates HDL particle formation and decreases VLDL secretion from murine hepatocytes. J. Lipid Res. 45: 1122–1131. [DOI] [PubMed] [Google Scholar]
- 16.Cheng J. B., Motola D. L., Mangelsdorf D. J., Russell D. W. 2003. De-orphanization of cytochrome P450 2R1: a microsomal vitamin D 25-hydroxilase. J. Biol. Chem. 278: 38084–38093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willy P. J., Umesono K., Ong E. S., Evans R. M., Heyman R. A., Mangelsdorf D. J. 1995. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 9: 1033–1045. [DOI] [PubMed] [Google Scholar]
- 18.Shelness G. S., Hou L., Ledford A. S., Parks J. S., Weinberg R. B. 2003. Identification of the lipoprotein initiating domain of apolipoprotein B. J. Biol. Chem. 278: 44702–44707. [DOI] [PubMed] [Google Scholar]
- 19.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y., Tran K., Yao Z. 1999. The activity of microsomal triglyceride transfer protein is essential for accumulation of triglyceride within microsomes in McA-RH7777 cells. A unified model for the assembly of very low density lipoproteins. J. Biol. Chem. 274: 27793–27800. [DOI] [PubMed] [Google Scholar]
- 21.Owen J. S., Bharadwaj M. S., Thomas M. J., Bhat S., Samuel M. P., Sorci-Thomas M. G. 2007. Ratio determination of plasma wild-type and L159R apoA-I using mass spectrometry: tools for studying apoA-IFin. J. Lipid Res. 48: 226–234. [DOI] [PubMed] [Google Scholar]
- 22.Lee J. Y., Lanningham-Foster L., Boudyguina E. Y., Smith T. L., Young E. R., Colvin P. L., Thomas M. J., Parks J. S. 2004. Prebeta high density lipoprotein has two metabolic fates in human apolipoprotein A-I transgenic mice. J. Lipid Res. 45: 716–728. [DOI] [PubMed] [Google Scholar]
- 23.Tall A. R., Shipley G. G., Small D. M. 1976. Conformational and thermodynamic properties of apo A-1 of human plasma high density lipoproteins. J. Biol. Chem. 251: 3749–3755. [PubMed] [Google Scholar]
- 24.Parks J. S., Gebre A. K., Furbee J. W. 1999. Lecithin-cholesterol acyltransferase. Assay of cholesterol esterification and phospholipase A2 activities. Methods Mol. Biol. 109: 123–131. [DOI] [PubMed] [Google Scholar]
- 25.Boren J., Rustaeus S., Olofsson S. O. 1994. Studies on the assembly of apolipoprotein B-100- and B-48-containing very low density lipoproteins in McA-RH7777 cells. J. Biol. Chem. 269: 25879–25888. [PubMed] [Google Scholar]
- 26.Parks J. S., Johnson F. L., Wilson M. D., Rudel L. L. 1990. Effect of fish oil diet on hepatic lipid metabolism in nonhuman primates: lowering of secretion of hepatic triglyceride but not apoB. J. Lipid Res. 31: 455–466. [PubMed] [Google Scholar]
- 27.Xiao Q., Elovson J., Schumaker V. N. 2000. Rat McA-RH7777 cells efficiently assemble rat apolipoprotein B-48 or larger fragments into VLDL but not human apolipoprotein B of any size. J. Lipid Res. 41: 116–125. [PubMed] [Google Scholar]
- 28.Zelcer N., Tontonoz P. 2006. Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Invest. 116: 607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grefhorst A., Elzinga B. M., Voshol P. J., Plosch T., Kok T., Bloks V. W., van der Sluijs F. H., Havekes L. M., Romijn J. A., Verkade H. J., et al. 2002. Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. J. Biol. Chem. 277: 34182–34190. [DOI] [PubMed] [Google Scholar]
- 30.Brown A. M., Gibbons G. F. 2001. Insulin inhibits the maturation phase of VLDL assembly via a phosphoinositide 3-kinase-mediated event. Arterioscler. Thromb. Vasc. Biol. 21: 1656–1661. [DOI] [PubMed] [Google Scholar]
- 31.Sparks J. D., Phung T. L., Bolognino M., Sparks C. E. 1996. Insulin-mediated inhibition of apolipoprotein B secretion requires an intracellular trafficking event and phosphatidylinositol 3-kinase activation: studies with brefeldin A and wortmannin in primary cultures of rat hepatocytes. Biochem. J. 313: 567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aggarwal D., Fernandez M. L., Soliman G. A. 2006. Rapamycin, an mTOR inhibitor, disrupts triglyceride metabolism in guinea pigs. Metabolism. 55: 794–802. [DOI] [PubMed] [Google Scholar]
- 33.Morrisett J.D., bdel-Fattah G., Kahan B.D. 2003. Sirolimus changes lipid concentrations and lipoprotein metabolism in kidney transplant recipients. Transplant. Proc. 35: 143S–150S. [DOI] [PubMed] [Google Scholar]
- 34.Tsai J., Qiu W., Kohen-Avramoglu R., Adeli K. 2007. MEK-ERK inhibition corrects the defect in VLDL assembly in HepG2 cells: potential role of ERK in VLDL-ApoB100 particle assembly. Arterioscler. Thromb. Vasc. Biol. 27: 211–218. [DOI] [PubMed] [Google Scholar]
- 35.Wilcox R. W., Thuren T., Sisson P., Kucera G. L., Waite M. 1991. Hydrolysis of neutral lipid substrates by rat hepatic lipase. Lipids. 26: 283–288. [DOI] [PubMed] [Google Scholar]
- 36.Ginsberg H. N. 2000. Insulin resistance and cardiovascular disease. J. Clin. Invest. 106: 453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adiels M., Boren J., Caslake M. J., Stewart P., Soro A., Westerbacka J., Wennberg B., Olofsson S. O., Packard C., Taskinen M. R. 2005. Overproduction of VLDL1 driven by hyperglycemia is a dominant feature of diabetic dyslipidemia. Arterioscler. Thromb. Vasc. Biol. 25: 1697–1703. [DOI] [PubMed] [Google Scholar]
- 38.Orso E., Broccardo C., Kaminski W. E., Bottcher A., Liebisch G., Drobnik W., Gotz A., Chambenoit O., Diederich W., Langmann T., et al. 2000. Transport of lipids from golgi to plasma membrane is defective in tangier disease patients and Abc1-deficient mice. Nat. Genet. 24: 192–196. [DOI] [PubMed] [Google Scholar]
- 39.Shelness G. S., Ledford A. S. 2005. Evolution and mechanism of apolipoprotein B-containing lipoprotein assembly. Curr. Opin. Lipidol. 16: 325–332. [DOI] [PubMed] [Google Scholar]
- 40.Gusarova V., Seo J., Sullivan M. L., Watkins S. C., Brodsky J. L., Fisher E. A. 2007. Golgi-associated maturation of very low density lipoproteins involves conformational changes in apolipoprotein B, but is not dependent on apolipoprotein E. J. Biol. Chem. 282: 19453–19462. [DOI] [PubMed] [Google Scholar]
- 41.Kulinski A., Rustaeus S., Vance J. E. 2002. Microsomal triacylglycerol transfer protein is required for lumenal accretion of triacylglycerol not associated with ApoB, as well as for ApoB lipidation. J. Biol. Chem. 277: 31516–31525. [DOI] [PubMed] [Google Scholar]
- 42.Pan M., Liang Js J. S., Fisher E. A., Ginsberg H. N. 2002. The late addition of core lipids to nascent apolipoprotein B100, resulting in the assembly and secretion of triglyceride-rich lipoproteins, is independent of both microsomal triglyceride transfer protein activity and new triglyceride synthesis. J. Biol. Chem. 277: 4413–4421. [DOI] [PubMed] [Google Scholar]
- 43.Stillemark P., Boren J., Andersson M., Larsson T., Rustaeus S., Karlsson K. A., Olofsson S. O. 2000. The assembly and secretion of apolipoprotein B-48-containing very low density lipoproteins in McA-RH7777 cells. J. Biol. Chem. 275: 10506–10513. [DOI] [PubMed] [Google Scholar]
- 44.Adiels M., Olofsson S. O., Taskinen M. R., Boren J. 2006. Diabetic dyslipidaemia. Curr. Opin. Lipidol. 17: 238–246. [DOI] [PubMed] [Google Scholar]
- 45.Sparks C. E., Sparks J. D., Bolognino M., Salhanick A., Strumph P. S., Amatruda J. M. 1986. Insulin effects on apolipoprotein B lipoprotein synthesis and secretion by primary cultures of rat hepatocytes. Metabolism. 35: 1128–1136. [DOI] [PubMed] [Google Scholar]
- 46.Chirieac D. V., Davidson N. O., Sparks C. E., Sparks J. D. 2006. PI3-kinase activity modulates apo B available for hepatic VLDL production in apobec-1−/− mice. Am. J. Physiol. Gastrointest. Liver Physiol. 291: G382–G388. [DOI] [PubMed] [Google Scholar]
- 47.Phung T. L., Roncone A., Jensen K. L., Sparks C. E., Sparks J. D. 1997. Phosphoinositide 3-kinase activity is necessary for insulin-dependent inhibition of apolipoprotein B secretion by rat hepatocytes and localizes to the endoplasmic reticulum. J. Biol. Chem. 272: 30693–30702. [DOI] [PubMed] [Google Scholar]
- 48.Au C. S., Wagner A., Chong T., Qiu W., Sparks J. D., Adeli K. 2004. Insulin regulates hepatic apolipoprotein B production independent of the mass or activity of Akt1/PKBalpha. Metabolism. 53: 228–235. [DOI] [PubMed] [Google Scholar]
- 49.Lin M. C. M., Gordon D., Wetterau J. R. 1995. Microsomal triglyceride transfer protein (MTP) regulation in HepG2 cells: insulin negatively regulates MTP gene expression. J. Lipid Res. 36: 1073–1081. [PubMed] [Google Scholar]
- 50.Kamagate A., Qu S., Perdomo G., Su D., Kim D. H., Slusher S., Meseck M., Dong H. H. 2008. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J. Clin. Invest. 118: 2347–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allister E. M., Borradaile N. M., Edwards J. Y., Huff M. W. 2005. Inhibition of microsomal triglyceride transfer protein expression and apolipoprotein B100 secretion by the citrus flavonoid naringenin and by insulin involves activation of the mitogen-activated protein kinase pathway in hepatocytes. Diabetes. 54: 1676–1683. [DOI] [PubMed] [Google Scholar]
- 52.Au W. S., Kung H. F., Lin M. C. 2003. Regulation of microsomal triglyceride transfer protein gene by insulin in HepG2 cells: roles of MAPKerk and MAPKp38. Diabetes. 52: 1073–1080. [DOI] [PubMed] [Google Scholar]
- 53.Hay N., Sonenberg N. 2004. Upstream and downstream of mTOR. Genes Dev. 18: 1926–1945. [DOI] [PubMed] [Google Scholar]
- 54.Khan B. V., Fungwe T. V., Wilcox H. G., Heimberg M. 1990. Cholesterol is required for the secretion of the very-low-density lipoprotein: in vivo studies. Biochim. Biophys. Acta. 1044: 297–304. [DOI] [PubMed] [Google Scholar]
- 55.Schultz J. R., Tu H., Luk A., Repa J. J., Medina J. C., Li L., Schwendner S., Wang S., Thoolen M., Mangelsdorf D. J., et al. 2000. Role of LXRs in control of lipogenesis. Genes Dev. 14: 2831–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barter P. J., Brewer H. B., Jr, Chapman M. J., Hennekens C. H., Rader D. J., Tall A. R. 2003. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 23: 160–167. [DOI] [PubMed] [Google Scholar]
- 57.Oliveira H. C., Ma L., Milne R., Marcovina S. M., Inazu A., Mabuchi H., Tall A. R. 1997. Cholesteryl ester transfer protein activity enhances plasma cholesteryl ester formation. Studies in CETP transgenic mice and human genetic CETP deficiency. Arterioscler. Thromb. Vasc. Biol. 17: 1045–1052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.