The acute phase response (APR) is induced by infection, inflammation, trauma, and malignancy. The APR is characterized by alterations in hepatic protein synthesis leading to increases in specific plasma proteins, positive acute phase proteins, and decreases in other plasma proteins, negative acute phase proteins (1). Many disorders that induce the APR are associated with an increased risk of atherosclerosis (for example, rheumatoid arthritis, systemic lupus erythematosus, psoriasis, chronic dental infections, HIV infections). Moreover, it is now well recognized that common metabolic disorders including obesity, Type 2 diabetes, metabolic syndrome, and atherosclerosis induce a low-grade APR with elevations in positive acute phase proteins such as C-reactive protein and serum amyloid A (2). In addition to changes in hepatic protein synthesis and serum protein levels, the APR also induces marked changes in lipid metabolism; for example, plasma triglyceride levels increase and HDL levels decrease (for detailed review, see reference 3).
It is likely that there are multiple mechanisms by which the APR leads to an increased risk of atherosclerosis, but it is increasingly clear that alterations in HDL structure and function may play a significant role. During the APR, plasma HDL cholesterol and apolipoprotein (Apo) AI levels are reduced in humans (3). In addition, there are both increases and decreases in the content of various proteins associated with HDL (Table 1) (3). For example, paraoxonase 1 levels decrease, which would decrease the ability of HDL to prevent the oxidation of LDL (4, 5). Conversely, platelet-activating factor acetyl hydrolase increases, which would lead to the increased production of pro-atherogenic lipids (6, 7). The lipid composition of HDL is also altered during the APR with a decrease in cholesterol esters and phospholipids and an increase in triglycerides, unesterified cholesterol, ceramides, and glucosylceramides (3). Finally, many of the transport proteins and enzymes that play important roles in HDL metabolism are altered during the APR (LCAT, cholesteryl ester transfer protein, and hepatic lipase decrease, while secretory phospholipase A2 (sPLA2) and endothelial cell lipase increase) (3). Given these extensive alterations in the structure of HDL, one would anticipate that the function of HDL would be altered during the APR and that these functional changes could contribute to the increased risk of atherosclerosis that occurs with the APR.
TABLE 1.
Changes in HDL protein composition during the acute phase response
| Increased | Decreased |
|---|---|
| Serum Amyloid A (SAA) | Apo A-I |
| Apo J (clusterin) | Apo A-II |
| Secretory phospholipase A2 (SPLA2) | Apo C I, II, and III |
| Apo E | Apo M |
| Ceruloplasmin | LCAT |
| Apo A-VI and V | CETP |
| PAF-acetylhydrolase | Paraoxonase 1 (PON 1) |
| PLTP (human) | PLTP (rat) |
| Lipopolysaccharide binding protein (LBP) | Transferrin |
| Bactericidal/permeability increasing protein (BPI) | Hepatic lipase |
Although HDL has many putative functions, the ability of HDL to inhibit the oxidation of LDL and to facilitate reverse cholesterol transport are thought to be the major mechanisms accounting for the anti-atherogenic properties of HDL. Studies have shown that HDL obtained from individuals undergoing an APR is not as effective as HDL obtained from normal individuals in protecting LDL from oxidation (for review, see reference 8). Additionally, several in vitro studies have demonstrated that acute phase HDL obtained from humans and animal models does not facilitate the efflux of cholesterol from macrophages as effectively as normal HDL (9–12). In fact, studies have shown that acute phase HDL actually delivered cholesterol to macrophages (10).
Two recent publications (one published in this issue of the Journal of Lipid Research) have now clearly demonstrated a decrease in reverse cholesterol transport in vivo in animals administered LPS, a TLR4 ligand and a model of gram negative infections, which induces the APR (11, 13). In both of these studies, reverse cholesterol transport was measured by loading macrophages in vitro with radioactive cholesterol and then injecting these cholesterol loaded macrophages into intact mice. The appearance of radioactive cholesterol in the plasma, liver, and stool is then quantitated over time to determine the rate of reverse cholesterol transport. In both studies, the authors observed that LPS treatment results in a marked decrease in total reverse cholesterol transport (i.e., radioactive cholesterol and bile acids in the collected feces). Additionally, more modest decreases in labeled cholesterol in the plasma were also observed in the LPS treated animals, suggesting that the efflux of cholesterol from macrophages to lipoprotein acceptors, the initial step in reverse cholesterol transport, was also adversely affected by LPS treatment.
Although these two papers clearly demonstrate that LPS treatment reduces reverse cholesterol transport, it would be very important to determine if other stimulants that induce the APR also reduce reverse cholesterol transport. Even more important would be to determine if inducing a mild APR, similar to what is observed in the large number of patients with obesity, diabetes, atherosclerosis, and other disease states, also leads to defects in reverse cholesterol transport. It is worth noting that McGillicuddy et al. (11) observed that a relatively low dose of LPS (0.3 mg/kg), which did not increase serum cytokine levels, decreased reverse cholesterol transport in mice. Additionally, McGillicuddy et al. (11) reported that HDL isolated from humans treated with low doses of LPS, that induced flu-like symptoms with elevations of serum cytokines, did not facilitate cholesterol efflux from macrophages. These observations suggest that alterations in reverse cholesterol transport occur even during a mild APR.
Reverse cholesterol transport is a complex process with multiple steps. The paper by Annema et al. (13) examined several proteins that increase during the APR to determine whether increasing their levels in plasma affected reverse cholesterol transport. They provide new data showing that increasing serum amyloid A and myeloperoxidase to levels similar to those observed during the APR, decrease reverse cholesterol transport. In contrast, increasing sPLA2 did not inhibit reverse cholesterol transport, but did decrease the levels of labeled cholesterol in the plasma, which suggests that the increase in sPLA2 may alter the structure or levels of HDL and thereby affect the efflux of cholesterol from macrophages. It should be noted that the changes in serum amyloid A, myeloperoxidase, and sPLA2 can quantitatively account for only a modest portion of the inhibitory effects on reverse cholesterol transport observed with LPS treatment, indicating that LPS induces other changes in lipid metabolism that are also playing key roles in this process.
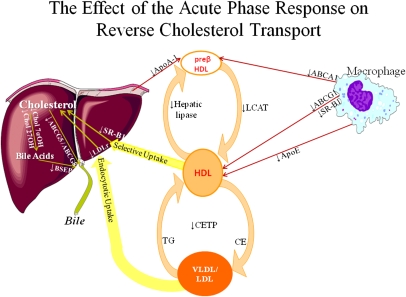
In addition to reductions in HDL and Apo AI levels and alterations in HDL composition and structure, our laboratory and others have demonstrated that LPS and other stimuli that induce an APR affect many of the key sites required for reverse cholesterol transport (Fig. 1). First, LPS decreases the expression of ABCA1, ABCG1, and scavenger receptor class B type 1, transporters involved in the efflux of cholesterol from macrophages to HDL (11, 14–16). Additionally, Apo E expression in macrophages is also decreased and could further contribute to the decrease in cholesterol efflux (17, 18). Second, LPS decreases plasma LCAT activity, which could lead to a decrease in the esterification of cholesterol in HDL particles, thereby reducing the maturation of HDL and the ability of HDL to accept additional cholesterol from macrophages (19, 20). Third, although not relevant for reverse cholesterol transport in mice, studies have shown that LPS decreases cholesteryl ester transfer protein, which would inhibit the transfer of cholesterol from HDL to VLDL and LDL, an important pathway of reverse cholesterol transport in humans (21, 22). Fourth, LPS treatment reduces scavenger receptor class B type 1 expression in the liver, which could decrease the hepatic uptake of cholesterol esters from HDL (23). Fifth, LPS treatment decreases cholesterol 7α hydroxylase and cholesterol 27 hydroxylase, the key enzymes which catalyze the conversion of cholesterol to bile acids, a major pathway of cholesterol excretion (24–26). Finally, LPS treatment decreases the expression of the transporters that secrete cholesterol (ABCG5 and ABCG8) and bile acids (ABCB11/BSEP) into the bile (11, 16, 27, 28). It is likely that the combined effect of these changes in lipid metabolism, and others that have not yet been identified, together contribute to the reduction in reverse cholesterol transport that occurs during the APR. It is therefore not surprising that the studies of Annema et al. did not find a single dominant factor that accounted for the LPS induced decrease in reverse cholesterol transport. Rather, it is likely that the decrease in reverse cholesterol transport that occurs during the APR is multifactorial.
Fig. 1.
The effect of the acute phase response on reverse cholesterol transport.
Although a prolonged decrease in reverse cholesterol transport likely has adverse effects leading to an increased risk of atherosclerosis, one can speculate that the short term effects of decreasing reverse cholesterol transport during the APR may be beneficial. During infections and trauma it may be beneficial to increase the quantity of cholesterol in macrophages and other cells that are required to fight off infections or heal tissue injuries (3). Thus, the short-term changes in HDL function that occur during the APR may have evolved as a beneficial response, but if prolonged, may lead to harmful consequences.
Footnotes
See referenced article, J. Lipid Res. 2010, 51: 743–754.
REFERENCES
- 1.Gabay C., Kushner I.1999. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 340: 448–454 [DOI] [PubMed] [Google Scholar]
- 2.Hotamisligil G. S.2006. Inflammation and metabolic disorders. Nature. 444: 860–867 [DOI] [PubMed] [Google Scholar]
- 3.Khovidhunkit W., Kim M. S., Memon R. A., Shigenaga J. K., Moser A. H., Feingold K. R., Grunfeld C. 2004. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J. Lipid Res. 45: 1169–1196 [DOI] [PubMed] [Google Scholar]
- 4.Feingold K. R., Memon R. A., Moser A. H., Grunfeld C. 1998. Paraoxonase activity in the serum and hepatic mRNA levels decrease during the acute phase response. Atherosclerosis. 139: 307–315 [DOI] [PubMed] [Google Scholar]
- 5.Van Lenten B. J., Hama S. Y., de Beer F. C., Stafforini D. M., McIntyre T. M., Prescott S. M., La Du B. N., Fogelman A. M., Navab M. 1995. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J. Clin. Invest. 96: 2758–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao Y., Stafforini D. M., Zimmerman G. A., McIntyre T. M., Prescott S. M. 1998. Expression of plasma platelet-activating factor acetylhydrolase is transcriptionally regulated by mediators of inflammation. J. Biol. Chem. 273: 4012–4020 [DOI] [PubMed] [Google Scholar]
- 7.Memon R. A., Fuller J., Moser A. H., Feingold K. R., Grunfeld C. 1999. In vivo regulation of plasma platelet-activating factor acetylhydrolase during the acute phase response. Am. J. Physiol. 277: R94–R103 [DOI] [PubMed] [Google Scholar]
- 8.Navab M., Anantharamaiah G. M., Reddy S. T., Van Lenten B. J., Fogelman A. M. 2009. HDL as a biomarker, potential therapeutic target, and therapy. Diabetes. 58: 2711–2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banka C. L., Yuan T., de Beer M. C., Kindy M., Curtiss L. K., de Beer F. C. 1995. Serum amyloid A (SAA): influence on HDL-mediated cellular cholesterol efflux. J. Lipid Res. 36: 1058–1065 [PubMed] [Google Scholar]
- 10.Khovidhunkit W., Shigenaga J. K., Moser A. H., Feingold K. R., Grunfeld C. 2001. Cholesterol efflux by acute-phase high density lipoprotein: role of lecithin: cholesterol acyltransferase. J. Lipid Res. 42: 967–975 [PubMed] [Google Scholar]
- 11.McGillicuddy F. C., de la Llera Moya M., Hinkle C. C., Joshi M. R., Chiquoine E. H., Billheimer J. T., Rothblat G. H., Reilly M. P. 2009. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 119: 1135–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pussinen P. J., Jauhiainen M., Vilkuna-Rautiainen T., Sundvall J., Vesanen M., Mattila K., Palosuo T., Alfthan G., Asikainen S. 2004. Periodontitis decreases the antiatherogenic potency of high density lipoprotein. J. Lipid Res. 45: 139–147 [DOI] [PubMed] [Google Scholar]
- 13.Annema W., Nijstad N., Tolle M., de Boer J. F., Buijs R. V., Heeringa P., van der Giet M., Tietge U. J. 2010. Myeloperoxidase and serum amyloid A contribute to impaired in vivo reverse cholesterol transport during the acute phase response, but not group IIA secretory phospholipase A2. J. Lipid Res. 51: 743–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baranova I., Vishnyakova T., Bocharov A., Chen Z., Remaley A. T., Stonik J., Eggerman T. L., Patterson A. P. 2002. Lipopolysaccharide down regulates both scavenger receptor B1 and ATP binding cassette transporter A1 in RAW cells. Infect. Immun. 70: 2995–3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buechler C., Ritter M., Quoc C. D., Agildere A., Schmitz G. 1999. Lipopolysaccharide inhibits the expression of the scavenger receptor Cla-1 in human monocytes and macrophages. Biochem. Biophys. Res. Commun. 262: 251–254 [DOI] [PubMed] [Google Scholar]
- 16.Khovidhunkit W., Moser A. H., Shigenaga J. K., Grunfeld C., Feingold K. R. 2003. Endotoxin down-regulates ABCG5 and ABCG8 in mouse liver and ABCA1 and ABCG1 in J774 murine macrophages: differential role of LXR. J. Lipid Res. 44: 1728–1736 [DOI] [PubMed] [Google Scholar]
- 17.Gafencu A. V., Robciuc M. R., Fuior E., Zannis V. I., Kardassis D., Simionescu M. 2007. Inflammatory signaling pathways regulating ApoE gene expression in macrophages. J. Biol. Chem. 282: 21776–21785 [DOI] [PubMed] [Google Scholar]
- 18.Werb Z., Chin J. R. 1983. Endotoxin suppresses expression of apoprotein E by mouse macrophages in vivo and in culture. A biochemical and genetic study. J. Biol. Chem. 258: 10642–10648 [PubMed] [Google Scholar]
- 19.Auerbach B. J., Parks J. S. 1989. Lipoprotein abnormalities associated with lipopolysaccharide-induced lecithin: cholesterol acyltransferase and lipase deficiency. J. Biol. Chem. 264: 10264–10270 [PubMed] [Google Scholar]
- 20.Ly H., Francone O. L., Fielding C. J., Shigenaga J. K., Moser A. H., Grunfeld C., Feingold K. R. 1995. Endotoxin and TNF lead to reduced plasma LCAT activity and decreased hepatic LCAT mRNA levels in Syrian hamsters. J. Lipid Res. 36: 1254–1263 [PubMed] [Google Scholar]
- 21.Hardardottir I., Moser A. H., Fuller J., Fielding C., Feingold K., Grunfeld C. 1996. Endotoxin and cytokines decrease serum levels and extra hepatic protein and mRNA levels of cholesteryl ester transfer protein in syrian hamsters. J. Clin. Invest. 97: 2585–2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masucci-Magoulas L., Moulin P., Jiang X. C., Richardson H., Walsh A., Breslow J. L., Tall A. 1995. Decreased cholesteryl ester transfer protein (CETP) mRNA and protein and increased high density lipoprotein following lipopolysaccharide administration in human CETP transgenic mice. J. Clin. Invest. 95: 1587–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khovidhunkit W., Moser A. H., Shigenaga J. K., Grunfeld C., Feingold K. R. 2001. Regulation of scavenger receptor class B type I in hamster liver and Hep3B cells by endotoxin and cytokines. J. Lipid Res. 42: 1636–1644 [PubMed] [Google Scholar]
- 24.Dikopoulos N., Weidenbach H., Adler G., Schmid R. M. 2003. Lipopolysaccharide represses cholesterol 7-alpha hydroxylase and induces binding activity to the bile acid response element II. Eur. J. Clin. Invest. 33: 58–64 [DOI] [PubMed] [Google Scholar]
- 25.Feingold K. R., Spady D. K., Pollock A. S., Moser A. H., Grunfeld C. 1996. Endotoxin, TNF, and IL-1 decrease cholesterol 7 alpha-hydroxylase mRNA levels and activity. J. Lipid Res. 37: 223–228 [PubMed] [Google Scholar]
- 26.Memon R. A., Moser A. H., Shigenaga J. K., Grunfeld C., Feingold K. R. 2001. In vivo and in vitro regulation of sterol 27-hydroxylase in the liver during the acute phase response. Potential role of hepatocyte nuclear factor-1. J. Biol. Chem. 276: 30118–30126 [DOI] [PubMed] [Google Scholar]
- 27.Hartmann G., Cheung A. K., Piquette-Miller M. 2002. Inflammatory cytokines, but not bile acids, regulate expression of murine hepatic anion transporters in endotoxemia. J. Pharmacol. Exp. Ther. 303: 273–281 [DOI] [PubMed] [Google Scholar]
- 28.Vos T. A., Hooiveld G. J., Koning H., Childs S., Meijer D. K., Moshage H., Jansen P. L., Muller M. 1998. Up-regulation of the multidrug resistance genes, Mrp1 and Mdr1b, and down-regulation of the organic anion transporter, Mrp2, and the bile salt transporter, Spgp, in endotoxemic rat liver. Hepatology. 28: 1637–1644 [DOI] [PubMed] [Google Scholar]