Abstract
The specifics of nascent HDL remodeling within the plasma compartment remain poorly understood. We developed an in vitro assay to monitor the lipid transfer between model nascent HDL (LpA-I) and plasma lipoproteins. Incubation of α-125I-LpA-I with plasma resulted in association of LpA-I with existing plasma HDL, whereas incubation with TD plasma or LDL resulted in conversion of α-125I-LpA-I to preβ-HDL. To further investigate the dynamics of lipid transfer, nascent LpA-I were labeled with cell-derived [3 H]cholesterol (UC) or [3H]phosphatidylcholine (PC) and incubated with plasma at 37°C. The majority of UC and PC were rapidly transferred to apolipoprotein B (apoB). Subsequently, UC was redistributed to HDL for esterification before being returned to apoB. The presence of a phospholipid transfer protein (PLTP) stimulator or purified PLTP promoted PC transfer to apoB. Conversely, PC transfer was abolished in plasma from PLTP−/− mice. Injection of 125I-LpA-I into rabbits resulted in a rapid size redistribution of 125I-LpA-I. The majority of [3H]UC from labeled r(HDL) was esterified in vivo within HDL, whereas a minority was found in LDL. These data suggest that apoB plays a major role in nascent HDL remodeling by accepting their lipids and donating UC to the LCAT reaction. The finding that nascent particles were depleted of their lipids and remodeled in the presence of plasma lipoproteins raises questions about their stability and subsequent interaction with LCAT.
Keywords: nascent apoA-I-containing particle, remodeling of high density lipoprotein, origin of high density lipoprotein, ATP binding cassette transporter A1, lecithin:cholesterol acyltransferase, phospholipid transfer protein
The process of lipidation of apolipoprotein A-I (apoA-I) by the ABCA1 transporter is functionally important in the biogenesis of HDL and in regulating lipid transport and metabolism, which, in turn, is critical for normal human physiology. This process is believed to be one of the major mechanisms by which HDL may protect against atherosclerotic cardiovascular disease (1, 2). As a result, the molecular mechanisms underlying the origin of plasma HDL have been a subject of intense study.
Although it is well established that the liver and intestine are major sources of newly secreted HDL (3, 4), there is little information on the metabolism of nascent HDL, their interaction with resident plasma lipoproteins, and their effect on lipid transport. Although discoidal nascent HDLs are believed to be critical intermediates between lipid-poor apoA-I and mature spherical HDL, the accurate detection and analysis of these nascent particles has proven difficult because they are rapidly remodeled by plasma factors and are subsequently found at relatively low concentrations in the plasma of most species.
Generally it is thought that upon entering the plasma, nascent HDL acquire phospholipids (PLs) and unesterified cholesterol (UCs) and associate with LCAT and other plasma factors, including phospholipid transfer protein (PLTP) and cholesteryl ester transfer protein (CETP), for the completion of the maturation cycle. The pioneering biophysical and biochemical studies by Forte and colleagues (5–7), have shown that nascent HDLs are defined by their ability to be transformed into mature plasma HDL by the action of LCAT. Indeed, LCAT alone appears sufficient to introduce heterogeneity into the size distribution of HDL particles. Newly secreted HDL generated by hepatocyte HepG2 are remarkably similar to HDL isolated from patients with familial LCAT deficiency (5). Addition of LCAT alone to these particles produces a plasma-like subclass distribution.
Previous studies from our laboratory and others (8–11) have documented that incubation of lipid-free apoA-I with different cell types expressing ABCA1 leads to the formation of heterogeneous nascent apoA-I-containing particles in the HDL size range. The availability of these models of nascent HDL has revived the interest in elucidating the pathways governing the formation, maturation, and remodeling of nascent HDL. In a recent detailed study, Mulya et al. (12) used a similar model of nascent HDL and documented that initial interaction of apoA-I with ABCA1 impacts the in vivo metabolic fate of these particles.
It remains unclear whether apolipoprotein exchange and/or lipid transfer between nascent HDL and other plasma lipoproteins affects the structural characteristics of the nascent particles and their subsequent remodeling within the plasma compartment. Studies by von Eckardstein and Assman and colleagues (13, 14) have shown that cell-derived UC cycles between HDL subfractions and LDL for its effective esterification in plasma. This is in accordance with earlier studies by Francone, Gurakar, and Fielding (15) demonstrating that preβ-HDL acts as an initial acceptor of cellular cholesterol and shuttles it into a series of larger preβ particles and ultimately to α-migrating particles that contain LCAT for esterification. However, the interrelationship between the observed cholesterol cycles and nascent HDL remodeling has not been examined.
In this report, we performed more detailed studies to better define the nature and the specifics of the lipid transfer activity between model nascent HDL and plasma lipoproteins. Furthermore, we examined whether the process of lipid transfer impacts the remodeling of these nascent particles within the plasma compartment.
MATERIALS AND METHODS
Samples
Blood samples were obtained from normolipidemic male subjects with apoE3/3 phenotype after an overnight fast. Blood was drawn from the antecubital vein into tubes containing EDTA (final concentration: 1.5 mg/ml). Collection tubes were immediately placed on ice before centrifugation (3,000 rpm, 15 min, 4°C). Plasma from Tangier disease (TD) subjects were provided by Dr. Gerd Assmann, Institute of Clinical Chemistry, Munster, Germany. Plasma from subjects with CETP deficiency (Int 14A homozygote) with apoE3/3 phenotype were provided by Dr. Hitoshi Chiba from Hokkaido University School of Medicine, Sapporo, Japan. Plasma samples from PLTP-deficient mice were obtained from the Animal Care Center, National Institute for Health and Welfare, Helsinki, Finland (Dr. Christian Ehnholm). Clinical characteristics of human subjects are shown in Table 1.
Human plasma apoA-I
Purified plasma apoA-I (Biodesign) was resolubilized in 4 M guanidine-HCl and dialyzed extensively against PBS buffer. Freshly resolubilized apoA-I was iodinated with 125iodine by IODO-GEN® (Pierce) to a specific activity of 800–1500 cpm/ng apoA-I and used within 48 h.
Preparation of radiolabeled nascent LpA-I
Nascent apoA-I-containing (LpA-I) particles were prepared as previously described (11). Briefly, human fibroblasts were stimulated with 22OH/9CRA and incubated with 10 µg/ml 125I-apoA-I for 24 h at 37°C. Alternatively, fibroblasts were labeled with [3H] cholesterol (5 µCi/ml) or [3H] choline (10 µCi/ml) for 24 h, stimulated with 22OH/9CRA, and incubated with 10 µg/ml apoA-I for 24 h at 37°C. Media containing either 125I-LpA-I, [3H] UC-labeled LpA-I, or [3H] phospholipid (PL)-labeled LpA-I were recovered and concentrated with a size exclusion centrifugal filter (spiral ultrafiltration cartridge, MWCO 50,000; Amicon). Concentrated medium was washed three times with PBS containing protease inhibitors (Roche Diagnostics). LpA-I particles were further dialyzed (MWCO 50,000) to remove any remaining lipid-free apoA-I. The integrity of isolated LpA-I particles was verified by two-dimensional polyacrylamide nondenaturing gradient gel electrophoresis (2D-PAGGE) and used within 48 h.
Preparation of reconstituted HDL particles
Complexes comprising apoA-I, POPC, and cholesterol at a 1:100:5 molar ratio were prepared using the sodium cholate dialysis method as described by Jonas, Steinmetz, and Churgay (16). [3H] UC was added to a specific activity of ∼2 × 104 cpm/μg apoA-I. Reconstituted HDL [r(HDL)] particles were concentrated by ultrafiltration (spiral ultrafiltration cartridge, MWCO 50,000) and dialyzed against PBS (MWCO 50,000) to discard any lipid-free apoA-I or proteolytic peptides. ApoA-I lipid complex formation was verified by 2D-PAGGE. Particles were used within 48 h.
Isolation and radiolabeling of preβ1-LpA-I particles generated by HepG2
LpA-I particles generated by HepG2 were prepared as previously described (11). HepG2 grown in 100 mm diameter dishes were incubated with 10 µg/ml 125I-apoA-I for 24 h. Media containing 125I-LpA-I were recovered, concentrated, and dialyzed as described above. To isolate preβ1-LpA-I particles, 125I-LpA-I samples were further dialyzed against a solution containing 150 mM NaCl, 10 mM Tris-HCl, and 0.01% EDTA at pH 8.0. After dialysis, the density of the solution was adjusted to 1.21 g/ml using solid KBr. The 125I-LpA-I preparation was then transferred to an ultracentrifuge tube (Beckman) and overlaid with an equal volume of KBr solution of the same density. The sample was subjected to ultracentrifugation at 48,000 rpm for 21 h in a 50.2 Ti rotor (Beckman) at 4°C. The bottom 1.5 ml fraction containing 125I-preβ1-LpA-I particles was collected by tube slicing and was dialyzed extensively against 0.01 M NH4HCO3. Alternatively, HepG2 were labeled with 15 µCi/ml of [3H]cholesterol for 24 h and then incubated with 10 µg/ml of apoA-I for 24 h. [3H]UC-labeled preβ1-LpA-I particles were isolated by ultracentrifugation as described above. The integrity of isolated LpA-I particles was verified by analysis with 2D-PAGGE and used within 48 h.
In vitro incubation
125I-LpA-I, lipid-free 125I-apoA-I, [3H]UC-labeled LpA-I, or [3H]PL-labeled LpA-I were incubated with plasma (1 µg LpA-I: 10 µg of plasma apoA-I) at 37°C for the indicated time in the presence or absence of either 2 mM LCAT inhibitor (DTNB), 10 mM PLTP stimulator [4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (AEBSF)], or 5 µl/ml CETP-antibody (TP2). Inhibition of LCAT and CETP was verified by standard methodology (17, 18). Addition of AEBSF stimulated PLTP activity 3-fold as measured by PLTP activity assay (19). In parallel experiments, isolated LDL or HDL3 (100 µg protein) was incubated with 10 µg 125I-LpA-I for 3 h at 37°C. After incubation, lipid-free apoA-I was removed by ultrafiltration (spiral ultrafiltration cartridge, MWCO 50,000).
In vitro lipid transfer assay
Cell-derived [3H]UC-labeled LpA-I or [3H]PL-labeled LpA-I were incubated with plasma (1 µg LpA-I:10 µg plasma apoA-I) at 37°C or 4°C for the indicated times in the absence or presence of either DTNB, AEBSF, or purified PLTP. After incubation, plasma apoB was precipitated with an equal volume of 13% polyethylene glycol (PEG) 6000, and the lipids from the supernatants (HDL) and precipitates (apoB) were extracted. [3H]UC, [3H]cholesteryl ester (CE), [3H]phosphatidylcholine (PC), and [3H] sphingomyelin (SM) were separated by TLC and were assayed for radioactivity.
In vivo turnover study
In vivo studies were performed on female New Zealand White rabbits (3.5–5 kg). Rabbits were chosen as an animal model because of higher levels of LDL-C as compared with mice and the presence of the plasma remodeling factors CETP, LCAT, and PLTP. Rabbits were injected with 1.5–5 × 108cpm 125I-apoA-I, 125I-LpA-I, or [3H]UC-r(HDL) in the central artery of the left ear. Blood samples were drawn from the marginal vein of the right ear 10 min, 15 min, 30 min, 1 h, 3 h, 4 h, 6 h, and 8 h postinjection, as indicated. Samples were immediately placed on ice and centrifuged at 3,000 rpm for 15 min at 4°C.
Lipid and lipoprotein assays
Cholesterol and triglyceride concentrations were determined enzymatically on an autoanalyzer (Cobas Mira, Roche Molecular Biochemicals). HDL-cholesterol concentration was determined by measuring cholesterol in the supernatant after precipitation of apoB-containing lipoproteins with heparin-manganese from the d > 1.006 g/ml fraction prepared by ultracentrifugation. Plasma apoA-I, apoE, and apoB concentrations were determined by nephelometry (Behring Nephelometer 100 Analyzer) or by ELISA. ApoA-I concentration in nascent LpA-I was determined by ELISA. Phospholipid concentrations in nascent LpA-I were determined by ESI-MS as we have previously described (20). 2D-PAGGE and non-denaturing (ND)-PAGGE were performed as described previously (18). Human apoB- and HDL-associated UC and total cholesterol mass were measured according to the manufacturer's protocol (Wako). Rabbit plasma lipoproteins were separated by HPLC on a Superose-6HR column, and cholesterol content was determined enzymatically (Infinity™ kit; Thermo Electron). LCAT activity was assayed using standard methodology (17). CETP and PLTP activities were determined as described previously (18, 19). Human plasma PLTP was purified as described previously (21).
Statistical analysis
Statistical analyses were performed with SigmaPlot statistical software (Jandel Corporation). Data were expressed as mean ± SD. Student's t-test was used for comparisons between groups.
RESULTS
Structural characteristics and composition of model nascent LpA-I
Nascent LpA-I were generated by incubation of lipid-free apoA-I with 22OH/9CRA stimulated human fibroblasts as described in Materials and Methods. As shown in Fig. 1A, four species of nascent LpA-I particles were routinely observed. These nascent particles exhibited α-electrophoretic mobility with diameters of 7.5–18 nm. These nascent LpA-I subfractions were designated α1-LpA-I, α2-LpA-I, α3-LpA-I, and α4-LpA-I as indicated (Fig. 1A) (8, 11). The largest proportion of nascent LpA-I was associated with α2-LpA-I, which had an average particle size of 14 nm. Mass analysis of LpA-I species generated by human fibroblasts showed an UC to PL molar ratio of 1:7. Additionally, the percentage of phospholipid subclass composition of LpA-I was as follows: PC, 51 ± 1%; SM, 16 ± 1%; PE, 15 ± 0.6%; LPC, 4.4 ± 1.3%; and PI, 14 ± 0.2%, as we have reported previously (8). The α-electrophoretic mobility of LpA-I could be attributable to the higher content of PI (8, 9). Chemical cross-linking of the apoA-I molecules in LpA-I revealed one, two, three, or four molecules of apoA-I per particle, as we have reported previously (10, 22).
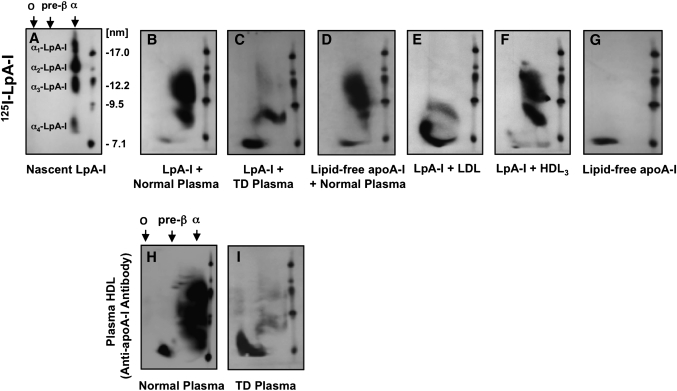
Fig. 1.
Effect of plasma lipoproteins on the particle size distribution of model nascent LpA-I. Nascent 125I-LpA-I (A) or lipid-free 125I-apoA-I (G) (10 µg) was incubated with 100 µl of normal plasma (B, D) or TD plasma (C) for 3 h at 37°C. Isolated LDL (E) or HDL3 (F) (100 µg protein) was incubated with 10 µg of 125I-LpA-I for 3 h at 37°C. After incubation, lipid-free 125I-apoA-I was removed by ultrafiltration (MWCO 50,000) as described in Materials and Methods. Samples were separated by 2D-PAGGE, and 125I-apoA-I was detected by autoradiography. Nascent 125I-LpA-I (A) or lipid-free 125I-apoA-I (G) incubated in PBS for 3 h at 37°C are shown as controls. Normal plasma (H) or TD plasma (I) (50 µl) were separated by 2D-PAGGE, and apoA-I was detected by anti-apoA-I antibody. Molecular size markers are shown.
We believe that the present model of nascent HDL may be useful for the study of apolipoprotein exchange and/or lipid transfer as it recapitulates to an extent in vivo nascent HDL while permitting manipulation of protein and lipid components. Previously, we used these model nascent particles to examine their cholesterol esterification kinetics (11), and more recently, similar particles were used to investigate their in vivo metabolic fate in human apoA-I transgenic mice (12).
In vitro remodeling of model nascent LpA-I: particle size distribution analysis
To determine whether the particle size distribution of model nascent LpA-I was affected by the presence of plasma lipoproteins, 125I-LpA-I were incubated with normolipidemic plasma (1 µg LpA-I:10 µg plasma apoA-I) at 37°C for 3 h, based on the assumption that the in vivo nascent HDL pool represented ∼10% of total plasma apoA-I mass (23). As shown in Fig. 1, when incubated with normolipidemic plasma (Table 1), α-125I-LpA-I shifted from diameters of 7.5–18 nm (Fig. 1A), to rapidly associate with existing plasma HDL-sized lipoproteins (Fig. 1B). This is consistent with the size distribution of plasma apoA-I-containing particles detected with an anti-apoA-I antibody (Fig. 1H). In contrast, in the absence of plasma HDL, such as the case of TD (Table 1, Fig. 1I), LpA-I were converted to both smaller α-HDL and preβ-HDL particles (Fig. 1C). Similarly, in the presence of isolated LDL, the majority of LpA-I were converted to preβ-HDL (Fig. 1E), whereas upon incubation with HDL3, LpA-I were converted to particles corresponding in size to HDL3 (Fig. 1F). Interestingly, both nascent 125I-LpA-I and lipid-free 125I-apoA-I exhibited similar patterns of association with normolipidemic plasma (Fig. 1B, D, respectively). We did not observe any significant change in the size distribution of LpA-I incubated alone for 3 h at 37°C compared with LpA-I kept at 4°C (data not shown).
TABLE 1.
Levels of cholesterol, triglycerides, and apolipoproteins in normolipidemic, TD, and CETP-deficient subjects
| Subjects | Cholesterol | Triglycerides | HDL-Cholesterol | ApoB | ApoA-I | ApoE |
|---|---|---|---|---|---|---|
| mmol/L | mmol/L | mmol/L | mg/dl | mg/dl | mg/dl | |
| Controls (n = 6) | 4.45 ± 0.83 | 1.23 ± 0.42 | 1.41 ± 0.42 | 88 ± 14 | 147 ± 13 | 3.6 ± 0.4 |
| TD | ||||||
| TD1 | 3.50 | 2.10 | 0.09 | 87 | 3 | 2.4 |
| TD2 | 3.20 | 1.67 | 0.10 | 79 | 5 | 2.7 |
| TD3 | 3.67 | 1.50 | 0.13 | 86 | 3 | 2.2 |
| CETP deficiency | ||||||
| CETPD1 | 5.33 | 0.72 | 3.26 | 70 | 182 | 8.7 |
| CETPD2 | 5.07 | 3.69 | 3.00 | 77 | 193 | 6.1 |
CETPD, CETP deficiency.
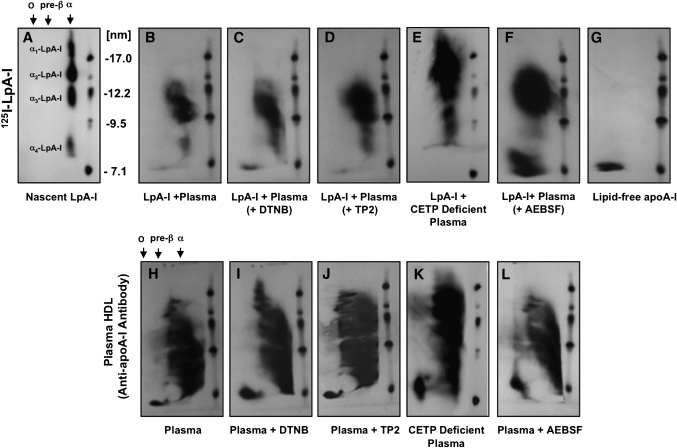
Next, we examined whether inhibition and/or stimulation of the plasma factors LCAT, CETP, and PLTP could affect the observed changes of α-125I-LpA-I size distribution following incubation with plasma. Incubations were carried out as described in Fig. 1, in the presence of a LCAT inhibitor (DTNB), a CETP inhibitor (TP2) anti-CETP antibody, or a PLTP stimulator (AEBSF). As shown in Fig. 2, incubations in the presence of DTNB or TP2 (Fig. 2C, D) did not affect the particle size distribution of 125I-LpA-I associated with plasma HDL (Fig. 2B). This is consistent with the result showing that nascent 125I-LpA-I have similar association patterns with normal and CETP-deficient plasma (Fig. 2B, E, respectively). CETP-deficient plasma was characterized by larger HDL particles as detected by apoA-I antibody (Fig. 2K). In contrast, incubation of 125I-LpA-I with a normolipidemic plasma in the presence of a PLTP stimulator (AEBSF) resulted in the transformation of a significant proportion of the 125I-LpA-I previously associated with α-HDL-sized particles to preβ-HDL migrating species (Fig. 2F). This was consistent with the apparent increase of plasma preβ-HDL levels following AEBSF treatment as detected by apoA-I antibody (Fig. 2L) compared with untreated plasma (Fig. 2H). The presence of DTNB and TP2 resulted in the inhibition of LCAT and CETP by 95 ± 3% and 68 ± 5%, respectively, whereas AEBSF increased PLTP activity by 300 ± 23%.
Fig. 2.
Effect of inhibition of LCAT and CETP or stimulation of PLTP on the particle size distribution of model nascent LpA-I. Nascent 125I-LpA-I (10 μg) (A) were incubated with 100 μl normal plasma alone for 3 h at 37°C (B) or in the presence of 2 mM LCAT inhibitor (DTNB) (C), 5 μl/ml of anti-CETP antibody (TP2) (D), 100 μl of plasma from CETP-deficient subject (E) or 10 mM PLTP stimulator (AEBSF) (F). Lipid-free apoA-I was removed from samples as described in Fig. 1. Samples were separated by 2D-PAGGE, and 125I-apoA-I was detected by autoradiography. Lipid-free 125I-apoA-I (G) is shown as a control. Additionally, 50 ml aliquots of samples were separated by 2D-PAGGE, and apoA-I was detected by anti-apoA-I antibody (H–L). Molecular size markers are shown.
Dynamics of transfer and esterification of nascent LpA-I cholesterol content
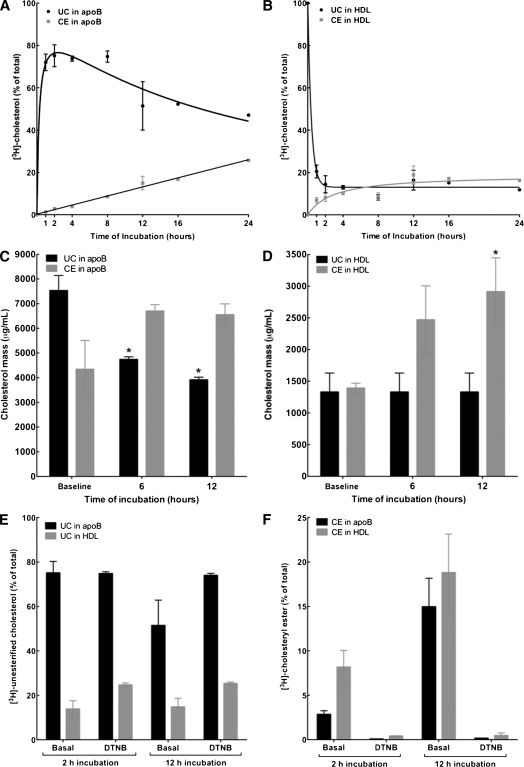
Based on the observation that nascent LpA-I were remodeled to smaller particles, including preβ-HDL, upon incubation with TD plasma or isolated LDL fraction (Fig. 1), the question was raised whether the change in the size distribution of LpA-I was accompanied by cholesterol transfer between nascent particles and plasma lipoproteins. To respond to this, we developed an in vitro assay to monitor the dynamics of cholesterol transfer between nascent LpA-I and plasma lipoproteins, as well as its esterification. Fibroblasts were labeled with [3H]UC and incubated with apoA-I to produce nascent LpA-I, as described in Materials and Methods. Nascent LpA-I were incubated with normolipidemic plasma (1 µg LpA-I:10 µg plasma apoA-I) at 37°C for various time periods as described above. Subsequently, plasma apoB was precipitated with PEG 6000 and the lipids from the supernatants (HDL) and precipitates (apoB) were extracted. [3H]UC and [3H]CE were separated by TLC and assayed for radioactivity. To verify that under the present lipid transfer assay nascent LpA-I did not artifactually associate with plasma apoB, the assay was carried out using 125I-LpA-I. No significant 125I-LpA-I was found associated with apoB over a 24 h incubation period (see supplementary Fig. I). Conversely, a time course analysis of cholesterol transfer showed that after a 1 h incubation, the majority (75%) of [3H]UC from LpA-I was transferred to plasma apoB, which decreased progressively after an 8 h incubation period (Fig. 3A). This was consistent with a proportional loss of [3H]UC content from LpA-I at the 1 h time period (Fig. 3B). At the same time, a significant proportion of [3H]UC was esterified by LCAT within the HDL fraction (Fig. 3B) and was subsequently transferred to apoB by CETP in a time-dependent manner (Fig. 3A). To examine whether the exchange of [3H]UC between nascent LpA-I, apoB, and HDL reflected the movement of actual UC and CE mass between plasma lipoproteins, the change in UC and CE mass in the apoB and HDL fractions was monitored over time. As shown in Fig. 3C, UC mass in plasma apoB was significantly decreased after 6 and 12 h incubation at 37°C, whereas the mass of CE was significantly increased after 6 and 12 h as compared with baseline. At the same time, there was a 2-fold increase in the mass of CE in HDL after 6 and 12 h incubation compared with baseline (Fig. 3D). Incubation at 37°C in the presence of DTNB did not inhibit the transfer of [3H]UC content from LpA-I to apoB (Fig. 3E), but as expected, did suppress esterification of cholesterol (Fig. 3F).
Fig. 3.
Dynamics of transfer, redistribution, and esterification of cell-derived cholesterol content of nascent LpA-I. Nascent LpA-I were labeled with cell-derived [3H]cholesterol and incubated with a normolipidemic plasma for various time periods at 37°C (1 µg LpA-I:10 µg plasma apoA-I). After incubation, apoB-containing particles were precipitated by PEG 6000, and the fractions of HDL and apoB-containing particles were dialyzed. After lipid extraction, [3H]UC and [3H]CE from apoB (A) and HDL (B) fractions were separated by TLC and assayed for radioactivity. Plotted values indicate percentage of total [3H]cholesterol associated with each fraction as UC or CE (mean ± SD of triplicate measures). At time 0 h, 100% of [3H]cholesterol was found as UC in the HDL fraction as [3H]UC-labeled LpA-I. C and D: [3H]UC-labeled LpA-I was incubated with plasma as described above for 6 and 12 h at 37°C. After incubation, apoB was precipitated by PEG, both apoB and HDL fractions were dialyzed, and UC and CE mass in apoB and HDL fractions was determined. Plotted values indicate micrograms of CE or UC per milliliter of plasma (mean ± SD of triplicate measures), *P < 0.05 versus baseline values. E and F: [3H]UC-labeled LpA-I was incubated with plasma as described above for 2 and 12 h at 37°C in the presence or absence of 2 mM LCAT inhibitor (DTNB). After incubation, apoB was precipitated by PEG. After lipid extraction, [3H]UC and [3H]CE were separated by TLC and assayed for radioactivity. Plotted values are mean ± SD of triplicate measures.
Additionally, we observed that preβ1-LpA-I generated by incubation of lipid-free 125I-apoA-I with HepG2 were similarly transformed to larger particles by associating with existing plasma HDL (see supplementary Fig. IIA). Again, this conversion seemed to be independent of LCAT because the presence of DTNB did not prevent remodeling. Consistent with the fibroblast LpA-I model, cell-derived [3H]UC from labeled preβ1-LpA-I were transferred to apoB-containing lipoproteins and subsequently esterified within plasma HDL (see supplementary Fig. IIB, C).
Although the lipid exchange properties of apoB within the plasma environment have not yet been defined, we obtained evidence that both isolated LDL and small unilamellar vesicles (100 nm) present at an equal phospholipid concentration are efficient acceptors of [3H]UC content of LpA-I, as assessed by FPLC separation (data not shown). Furthermore, the transfer of [3H]UC-LpA-I to isolated LDL occurred in the absence of mature HDL. This is consistent with the finding that the transfer of UC content from LpA-I to plasma apoB was preserved even in the absence of mature HDL, such as the case with TD subjects (see supplementary Fig. III). More thorough investigations are required to determine the structural characteristics of apoB responsible for the initial lipid exchange process.
Remodeling of model nascent LpA-I particles by PLTP
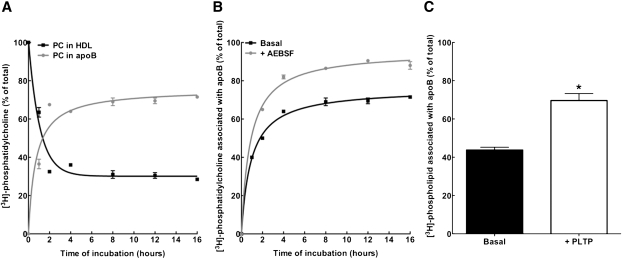
We obtained evidence that incubation of 125I-LpA-I with plasma in the presence of a PLTP stimulator (AEBSF) resulted in the conversion of a significant proportion of 125I-LpA-I associated with α-HDL to preβ-HDL migrating species (Fig. 2F). To determine whether the change in LpA-I particle size distribution was accompanied by phospholipid depletion of these particles, we investigated the dynamics of phospholipid transfer between nascent LpA-I and plasma lipoproteins. Nascent LpA-I were labeled with cell-derived [3H]phospholipids as described in Materials and Methods. Radiolabeled LpA-I were incubated with normolipidemic plasma (1 µg LpA-I:10 µg plasma apoA-I) at 37°C for various time periods. Plasma apoB was precipitated as described above, and [3H]PC and [3H]SM were separated by TLC and assayed for radioactivity. As shown in Fig. 4A, >40% of [3H]PC content of LpA-I was transferred to plasma apoB within a 1 h incubation period, reaching saturation at 4 h with a maximum of 65% [3H]PC transfer to apoB. At the same time, <15% of [3H]SM from LpA-I was transferred to apoB over a 16 h incubation period (data not shown). To determine whether the PC depletion of LpA-I was mediated by PLTP activity, incubations were carried out in the presence of a PLTP stimulator (AEBSF) or purified human plasma PLTP. As shown in Fig. 4B and C, both AEBSF and purified PLTP significantly increased the transfer of [3H]PC to apoB. Conversely, the transfer of [3H]PC from nascent LpA-I to isolated LDL was drastically reduced in the presence of plasma from mice lacking PLTP (Fig. 5A). Consistently, incubation of 125I-LpA-I with plasma from wild-type, but not PLTP knockout, mice in the presence of AEBSF resulted in the conversion of a significant proportion of 125I-LpA-I associated with α-HDL-sized particles to preβ-HDL (Fig. 5B).
Fig. 4.
Effect of PLTP on phospholipid transfer between nascent LpA-I and plasma apoB-containing lipoproteins. A: Nascent LpA-I was labeled with cell-derived [3H]phospholipid and incubated with normolipidemic plasma for various time periods at 37°C (1 µg LpA-I:10 µg plasma apoA-I). After incubation, plasma apoB-containing particles were precipitated by PEG, and both HDL and apoB-containing fractions were dialyzed. After lipid extraction, [3H]PC from apoB and HDL fractions was separated by TLC and assayed for radioactivity. Before incubation, 100% of [3H]PC was found in the HDL fraction as [3H]PL-labeled LpA-I. B: [3H]PL-labeled LpA-I was incubated with plasma as described in A in the absence or presence of 10 mM PLTP stimulator (AEBSF). After incubation, the content of [3H]PC in apoB was determined by TLC separation. C: [3H]PL-labeled LpA-I was incubated with plasma as described in A for 2 h at 37°C in the absence or presence of 150 μl purified human plasma PLTP. After incubation, the content of [3H]PC in apoB was determined by TLC separation. Plotted values are mean ± SD of triplicate measures.
Fig. 5.
Defective transfer of nascent LpA-I PC content and impaired conversion to preβ1-LpA-I in the presence of plasma from mice lacking PLTP. A: Nascent LpA-I (15 µg apoA-I) labeled with cell-derived [3H]PL was incubated with 100 µg of human LDL in the presence of plasma (100 µL) from normal or PLTP-deficient mice for 6 h at 37°C in the presence or absence of 10 mM PLTP stimulator (AEBSF). After incubation, apoB was precipitated with PEG 6000. After lipid extraction, [3H]PC associated with apoB was separated by TLC and assayed for radioactivity. Plotted values are mean ± SD of triplicate measures. P < 0.001. B: Nascent 125I-LpA-I (10 µg apoA-I) was incubated with plasma (100 µL) from normal or PLTP-deficient mice in the presence of 10 mM AEBSF for 6 h at 37°C. After incubation, lipid-free apoA-I was removed by ultrafiltration (MWCO 50,000). Samples were separated by 2D-PAGGE, and 125I-apoA-I was detected directly by autoradiography. Nascent 125I-LpA-I incubated in PBS for 6 h at 37°C is shown as control. Molecular size markers are shown.
In vivo remodeling of model nascent LpA-I and reconstituted HDL in rabbits
In an attempt to determine the extent to which the changes of LpA-I particle size distribution could occur in vivo, nascent 125I-LpA-I or lipid-free 125I-apoA-I were injected into rabbits, and the changes in the particle size distribution were assessed by ND-PAGGE. This study was not designed to examine in detail the in vivo metabolic fate of nascent LpA-I, but rather, to provide evidence to support the in vitro data presented above.
Nascent 125I-LpA-I and lipid-free 125I-apoA-I were prepared as described in Materials and Methods, and rabbits were injected with either lipid-free 125I-apoA-I (250 µg) or 125I-LpA-I (250 µg), which represents ∼0.20% of the total plasma apoA-I mass in rabbits (24). Serum was drawn from injected rabbits at the indicated times, and the particle size was assessed by ND-PAGGE. As shown in Fig. 6A, at the earliest time point examined (10 min), the larger nascent 125I-LpA-I with particle diameter of 11–18 nm associated rapidly with larger and medium-sized rabbit HDL. Remodeling of particles occurred as a function of time whereby more apoA-I was shifted from 14 to 10 nm particles with increased incubation. This is consistent with the size distribution of rabbit apoA-I-containing particles detected with an anti-apoA-I antibody (Fig. 6C). At the same time, smaller nascent 125I-LpA-I with particle diameter <9 nm were converted to larger particles during the first hour after injection. Interestingly, lipid-free 125I-apoA-I was rapidly incorporated into existing rabbit HDL-sized particles, and this distribution was conserved over the 6 h time course of the study (Fig. 6B).
Fig. 6.
Particle size distribution of model nascent 125I-LpA-I after intravenous injection into rabbits. A: Serum samples were harvested at the indicated time points after injection of 125I-LpA-I and 35–50 μl aliquots were separated by 5–35% ND-PAGGE. Size distribution of 125I-LpA-I was detected directly by autoradiography. 125I-LpA-I before injection is shown. B: Lipid-free 125I-apoA-I was injected into rabbits and detected as described for 125I-LpA-I. Lipid-free 125I-apoA-I before injection is shown. C: Serum (50 μl) from rabbits obtained before injection was separated by 5–35% ND-PAGGE, and apoA-I-containing particles were detected by anti-rabbit apoA-I antibody.
In vivo transfer and esterification of r(HDL) cholesterol content
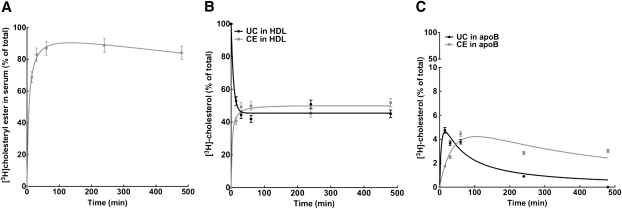
To further examine whether the transfer of unesterified cholesterol content from nascent HDL particles to apoB-containing lipoproteins could occur in vivo, we carried out experiments in rabbits, which have greater apoB-containing lipoprotein levels compared with mice. In this experiment, we used [3H]UC radiolabeled r(HDL) because of the difficulty to obtain sufficient [3H]UC labeling of LpA-I.
Rabbits were injected with 8 mg of [3H]UC-r(HDL), which represents ∼6% of the total plasma apoA-I mass in rabbits (24). 2D-PAGGE analysis showed that r(HDL) exhibited preβ electrophoretic mobility with a mean particle diameter of 12.5 nm. Serum was drawn at the indicated times and subjected to size-exclusion chromatography. As shown in Fig. 7A, at the earliest time point examined (15 min), the majority of [3H]cholesterol was found distributed within the HDL-sized fraction, with a significant portion found in the LDL fraction. Importantly, the noninjected [3H]UC-r(HDL) was distributed exclusively in the HDL size range. The distribution of [3H]cholesterol (as measured by cpm) among lipoproteins was virtually identical to the distribution of lipoprotein cholesterol mass in the noninjected rabbits, present mostly in the HDL fraction (Fig. 7B). [3H]cholesterol in both LDL and HDL fractions decreased progressively over the 8 h time course of study (Fig. 6A). Additionally, analysis of [3H]UC and [3H]CE in whole plasma showed that, at the earliest time point examined (15 min), >70% of cholesterol was esterified (Fig. 8A). As expected based on lipoprotein abundance, the majority of [3H]CE was found associated with the HDL fraction (Fig. 8B). At the earliest time point (15 min), a minor proportion of [3H]UC was found associated with LDL, which decreased over 8 h as LDL became enriched in [3H]CE in a time-dependent fashion (Fig. 8C).
Fig. 7.
Size-exclusion chromatography of rabbit serum after injection of [3H]UC-labeled reconstituted HDL. A: Serum samples were harvested at the indicated time points after injection of [3H]UC-labeled r(HDL) as described in Materials and Methods. Serum samples (250 μl) were applied to a Superose 6 column, and the amount of [3H]cholesterol in each fraction was determined. The elution profile of [3H]UC-labeled r(HDL) before injection is shown. B: Serum (250 μl) obtained from a rabbit before injection was applied to a Superose 6 column, and the total lipoprotein cholesterol content was determined. The elution position of VLDL, LDL, and HDL are shown.
Fig. 8.
Distribution and esterification of [3H]cholesterol-labeled reconstituted HDL after intravenous injection into rabbits. A: Serum samples were harvested at the indicated time points after injection of [3H]UC-labeled r(HDL) as described in Materials and Methods. After lipid extraction, [3H]UC and [3H]CE were separated by TLC and assayed for radioactivity. The percentage of total serum content of [3H]CE at the indicated times after injection was determined. Plotted values are mean ± SD of triplicate measures. Serum samples (250 μl) were applied to a Superose 6 column, and the amount of [3H]UC and [3H]CE in both the HDL (B) and LDL (C) peaks was determined by TLC. Before injection, 100% of [3H]cholesterol was found in the HDL fraction as [3H]UC-r(HDL).
DISCUSSION
It is generally thought that after entering plasma, nascent HDL acquire unesterified cholesterol and associate with LCAT, leading to their conversion to mature HDL. However, the fate, route, and physiological relevance of this proposed pathway remains enigmatic. In this report, we present evidence that in vitro incubation of model nascent LpA-I with human plasma changed the LpA-I size distribution by promoting rapid association of LpA-I with existing plasma HDL. However, in the absence of HDL (TD plasma) or in the presence of isolated LDL, the majority of nascent LpA-I were remodeled to smaller α-migrating particles and preβ-HDL species (Fig. 1). This is consistent with previous findings by Jonas, Bottum, and Kezdy (25), where r(HDL) underwent major structural rearrangements upon addition of LDL. It is possible that the interaction of nascent LpA-I with plasma lipoproteins may lead to “shedding” of apoA-I from the nascent particles as they are progressively depleted of lipid to yield lipid-poor apoA-I species, including preβ1-LpA-I and lipid-free apoA-I. Subsequently, these delipidated “remnant” particles associate rapidly with the resident plasma HDL pool. This concept is supported by the in vitro lipid transfer assay showing that the majority of radiolabeled UC content of nascent LpA-I was rapidly transferred to plasma apoB-containing lipoproteins and subsequently redistributed to HDL for its esterification by LCAT before being transferred back as CE to apoB by CETP (Fig. 3). At the same time, the PC content of nascent LpA-I was also transferred to plasma apoB-containing lipoproteins (Fig. 4). Importantly, the finding that the exchange of [3H]UC between nascent LpA-I, apoB, and HDL reflected the movement of actual UC and CE mass between plasma lipoproteins (Fig. 3C, D) supports the concept that UC is transferred from nascent LpA-I to plasma apoB-containing lipoproteins independent of its equilibration with unlabeled UC.
Although the lipid transfer properties of these model nascent particles within the plasma environment have not yet been defined, we have conducted the lipid transfer assays under conditions approaching those in vivo. This is based on the assumption that the nascent HDL pool represents the daily production of apoA-I in normolipidemic subjects (10 mg/kg/day), which approximates 10% of total plasma apoA-I mass (23). Our finding that most of the nascent LpA-I [3H]UC content is transferred to apoB within 1 h of incubation (Fig. 3) is in agreement with a previous study showing that the majority of cell-derived [3H]UC is transferred to LDL independently of its diffusional equilibration with unlabeled UC (13). In normal plasma, 75% of UC is present in LDL. Although the interrelationship between the transfer of [3H]UC and [3H]PC contents from LpA-I to plasma apoB is unknown, we observed that [3H]UC transfer to apoB was preserved at 4°C, whereas [3H]PC transfer was substantially decreased (data not shown). This is consistent with the finding that PLTP promotes the transfer of [3H]PC contents from LpA-I to plasma apoB and mediates the conversion of nascent LpA-I to preβ-HDL (Figs. 2, 4, and 5). Our results are in accordance with earlier studies by Jonas, Rye, and colleagues (26, 27), showing that supplementation with PLTP accelerates the transfer of phospholipids from r(HDL) to LDL to generate small discoidal particles. Furthermore, the transfer of cholesterol and phospholipid between r(HDL) and isolated LDL has been found to be determined by the properties and concentrations of both the donor and acceptor particles. A similar study by Meng, Sparks, and Marcel (28) shows that the rates of cholesterol transfer from r(HDL) to LDL are higher than in the opposite direction, in particular, for the smaller r(HDL) (7.8 nm). This suggests that the lipid transfer process cannot be explained by a passive aqueous diffusion model, as proposed by Nichols and Pagano (29). It is likely that the structure and composition of nascent LpA-I, as well as the interaction with plasma factors, including PLTP, have a significant effect on lipid transfer to other plasma lipoproteins. This is in accordance with previous studies documenting that phospholipids and PLTP play a key role in HDL remodeling and preβ-HDL formation (30–33).
Defining the molecular nature of nascent HDL remodeling in vivo is key for understanding how HDL originate. We obtained evidence that injection of nascent LpA-I into rabbits resulted in a rapid change in the LpA-I size distribution following interactions with preexisting rabbit HDL (Fig. 6). This is in agreement with a recent detailed study by Mulya et al. (12) that showed that a similar model of nascent LpA-I was rapidly remodeled in human apoA-I transgenic mice. Whereas larger nascent LpA-I particles were remodeled to smaller particles, smaller LpA-I particles were enlarged. In turn, particle enlargement resulted in increased liver and decreased kidney catabolism. Despite the lower apoB levels in rabbits compared with humans, which represents a limitation to this study of lipid transfer between nascent LpA-I and apoB, we demonstrated that the [3H]UC content of injected r(HDL) was rapidly redistributed to both rabbit HDL- and LDL-sized particles (Fig. 7). Furthermore, we observed that [3H]UC associated with HDL was rapidly esterified and a minor proportion of CE was transferred to LDL (Fig. 8). Future studies are thus required in monkeys and humans, which are characterized by higher LDL levels, to better define the lipid transfer process between nascent HDL and apoB.
Earlier studies by Francone, Gurakar, and Fielding (15) have documented that a minor subspecies of human HDL that migrates with preβ mobility on agarose gel can remove free cholesterol from cultured fibroblasts at a faster rate than α-migrating HDL, which comprises the bulk of plasma HDL. Furthermore, it was documented that preβ-HDL particles were present in the peripheral lymph of dogs (34) and the interstitial space (35), suggesting a key role for these particles in the initial removal of cholesterol. Our findings suggest that preβ1-LpA-I generated by HepG2 behave similarly as nascent α-LpA-I particles in donating their UC content to plasma apoB and associating with existing plasma HDL independently of LCAT activity (see supplementary Fig. II). It is possible, however, that other plasma factors could be involved in the conversion of preβ1-LpA-I to α-migrating particles. This is supported by our finding that the conversion of nascent LpA-I to larger α-migrating HDL was found to be impaired in TD plasma (Fig. 1C). Indeed, an earlier study by Huang et al. (36) documented that normal plasma contains a factor that converts preβ1-LpA-I to α-LpA-I but that this factor is absent in TD plasma.
Although the structural features of nascent LpA-I required to form mature HDL are as yet unknown, this study raises important questions regarding the stability of nascent particles in the plasma environment: a) how do the nascent particles interact with LCAT if they are depleted of their cholesterol and phospholipid content in the presence of other lipoproteins; b) do the newly formed HDL exist only transiently in the plasma compartment while both their apoA-I and lipid components are incorporated into the resident plasma HDL pool; and c) is the structural integrity of nascent particles preserved during their association with the resident plasma HDL pool? The fact that both the UC and PC contents of LpA-I were transferred to apoB-containing lipoproteins and subsequently distributed to HDL suggests that LDL plays a central role in nascent HDL remodeling. This is in accordance with earlier studies documenting that apoB acquires large amounts of UC upon entering the plasma compartment, UC likely derived from cell membranes (37, 38).
It is well documented that LCAT plays a pivotal role in the reverse cholesterol transport process by maintaining a cholesterol concentration gradient between cell membranes and the plasma compartment (39). The transfer of UC content from nascent particles to apoB, which is cleared via the LDL receptor, may represent an LCAT-independent reverse cholesterol transport process. This is supported by the observation that the in vitro transfer of UC content from nascent LpA-I to apoB was preserved in the presence of LCAT inhibitor (DTNB) (Fig. 3C). This is in agreement with clinical studies showing that LDL of patients with LCAT deficiency possess higher amounts of UC, indicating that the in vivo transfer of UC to LDL occurs in the absence of LCAT (14).
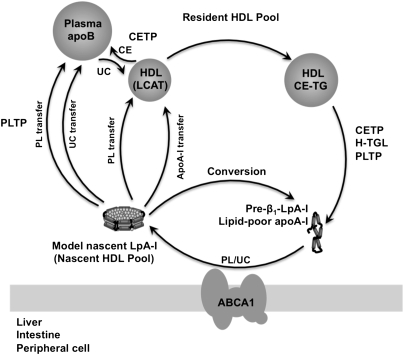
This study has provided a biochemical basis of the nascent HDL remodeling pathway that involves plasma apoB-containing lipoproteins and PLTP. As illustrated in Fig. 9, the UC content of the model nascent HDL pool was transferred to plasma apoB, which was then redistributed to HDL for esterification by LCAT. Subsequently, CEs were transferred back to apoB by CETP. At the same time, PLTP mediated the depletion of the PC content of nascent HDL, leading to the incorporation of lipid-poor apoA-I into the plasma resident HDL pool or conversion to preβ1-LpA-I. Continuous remodeling of the HDL resident pool by CETP, H-TGL, and PLTP contributes to the generation of preβ1-LpA-I or lipid-free apoA-I. Importantly, our results raise questions regarding the stability and structural integrity of the nascent particles within the plasma environment, the significance of which deserves further investigation.
Fig. 9.
A proposed model for nascent HDL remodeling within the plasma compartment. Nascent HDL particles derived from direct secretion by the liver/intestine or generated by the interaction of lipid-poor apoA-I with peripheral cells through the ABCA1 transporter transfer their lipid to both apoB-containing particles and the plasma resident HDL pool. The UC content of the nascent HDL pool is transferred to apoB-containing particles, which then redistributes to HDL for its effective esterification by LCAT before being transferred back to plasma apoB by CETP. Although the mechanisms underlying this process are presently unknown, it is possible that nascent HDL remodeling may lead to “shedding” of apoA-I from nascent lipoprotein particles as they are progressively depleted of phospholipids by PLTP to yield lipid-poor apoA-I. In turn, lipid-poor apoA-I associates rapidly with the resident HDL pool or converts to preβ1-LpA-I. Continuous remodeling of the resident HDL pool by CETP, H-TGL, and PLTP contributes to the generation of preβ1-LpA-I. FC, free cholesterol; TG, triglycerides; H-TGL, hepatic lipase.
Supplementary Material
Acknowledgments
The authors thank Dr. Gerd Assmann, Dr. Hitoshi Chiba, and Dr. Yves L. Marcel for kindly providing plasma from Tangier disease subjects, plasma from CETP deficiency subjects, and anti-CETP antibody (TP2).
Footnotes
Abbreviations:
- 2D-PAGGE
- two-dimensional polyacrylamide nondenaturing gradient gel electrophoresis
- AEBSF
- 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride
- apo
- apolipoprotein
- CE
- cholesteryl ester
- CETP
- cholesteryl ester transfer protein
- LpA-I
- nascent apoA-I-containing particle
- ND
- non-denaturing
- PC
- phosphatidylcholine
- PEG
- polyethylene glycol
- PL
- phospholipid
- PLTP
- phospholipid transfer protein
- r(HDL)
- reconstituted apoA-I-containing proteoliposome
- TD
- Tangier disease
- UC
- unesterified cholesterol
This work was supported by Grant MOP 15042 from the Canadian Institutes of Health Research and the Heart and Stroke Foundation of Canada. J.G. holds the McGill University-Novartis Chair in Cardiology.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures.
REFERENCES
- 1.Brewer H. B., Santamarina-Fojo S. 2003. New insights into the role of the adenosine triphosphate-binding cassette transporters in high-density lipoprotein metabolism and reverse cholesterol transport. Am. J. Cardiol. 91: 3E–11E [DOI] [PubMed] [Google Scholar]
- 2.Tall A. R.1993. Plasma cholesteryl ester transfer protein. J. Lipid Res. 34: 1255–1274 [PubMed] [Google Scholar]
- 3.Brunham L. R., Kruit J. K., Iqbal J., Fievet C., Timmins J. M., Page T. D., Coburn B. A., Bissada N., Staels B., Groen A. K., et al. 2006. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J. Clin. Invest. 116: 1052–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Timmins J. M., Lee J. Y., Boudyguina E., Kluckman K. D., Brunham L. R., Mulya A., Gebre A. K., Cutinho J. M., Colvin P. L., Smith T. L., et al. 2005. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J. Clin. Invest. 115: 1333–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCall M. R., Nichols A. V., Blanche P. J., Shore V. G., Forte T. M. 1989. Lecithin:cholesterol acyltransferase-induce transformation of HepG2 lipoproteins. J. Lipid Res. 30: 1579–1589 [PubMed] [Google Scholar]
- 6.Nichols A. V., Blanche P. J., Gong E. L., Shore V. G., Forte T. M. 1985. Molecular pathways in the transformation of model discoidal lipoprotein complexes induced by lecithin:cholesterol acyltransferase. Biochim. Biophys. Acta. 834: 285–300 [DOI] [PubMed] [Google Scholar]
- 7.Thrift R. N., Forte T. M., Cahoon B. E., Shore V. G. 1986. Characterization of lipoproteins produced by the human liver cell line, HepG2, under defined conditions. J. Lipid Res. 27: 236–250 [PubMed] [Google Scholar]
- 8.Denis M., Haidar B., Marcil M., Bouvier M., Krimbou L., Genest J. 2004. Molecular and cellular physiology of apolipoprotein A-I lipidation by the ATP-binding cassette transporter A1 (ABCA1). J. Biol. Chem. 279: 7384–7394 [DOI] [PubMed] [Google Scholar]
- 9.Duong P. T., Collins H. L., Nickel M., Lund-Katz S., Rothblat G. H., Phillips M. C. 2006. Characterization of nascent HDL particles and microparticles formed by ABCA1-mediated efflux of cellular lipids to apoA-i. J. Lipid Res. 47: 832–843 [DOI] [PubMed] [Google Scholar]
- 10.Hassan H. H., Bailey D., Lee D. Y., Iatan I., Hafiane A., Ruel I., Krimbou L., Genest J. 2008. Quantitative analysis of ABCA1-dependent compartmentalization and trafficking of apolipoprotein A-I: implications for determining cellular kinetics of nascent high density lipoprotein biogenesis. J. Biol. Chem. 283: 11164–11175 [DOI] [PubMed] [Google Scholar]
- 11.Krimbou L., Hajj Hassan H., Blain S., Rashid S., Denis M., Marcil M., Genest J. 2005. Biogenesis and speciation of nascent apoA-I-containing particles in various cell lines. J. Lipid Res. 46: 1668–1677 [DOI] [PubMed] [Google Scholar]
- 12.Mulya A., Lee J. Y., Gebre A. K., Boudyguina E., Chung S. K., Smith T. L., Colvin P. L., Jiang X. C., Parks J. S. 2008. Initial interaction of apoA-I with ABCA1 impacts in vivo metabolic fate of nascent HDL. J. Lipid Res. 49: 2390–2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Y., vonEckardstein A., Assman G. 1993. Cell-derived unesterified cholesterol cycles between different HDLs and LDL for its effective esterification in plasma. Arterioscler. Thromb. 13: 445–458 [DOI] [PubMed] [Google Scholar]
- 14.von Eckardstein A., Huang Y., Wu S., Funke H., Noseda G., Assman G. 1995. Reverse cholesterol transport in plasma of patients with different forms of familial HDL deficiency. Arterioscler. Thromb. Vasc. Biol. 15: 691–703 [DOI] [PubMed] [Google Scholar]
- 15.Francone O. L., Gurakar A., Fielding C. 1989. Distribution and functions of lecithin:cholesterol acyltransferase and cholesteryl ester transfer protein in plasma lipoproteins. Evidence for a functional unit containing these activities together with apolipoproteins A-I and D that catalyzes the esterification and transfer of cell-derived cholesterol. J. Biol. Chem. 264: 7066–7072 [PubMed] [Google Scholar]
- 16.Jonas A., Steinmetz A., Churgay L. 1993. The number of amphipathic alpha-helical segments of apoliproteins A-I, E, and A-IV determines the size and functional properties of their reconstituted lipoprotein particles. J. Biol. Chem. 268: 1596–1602 [PubMed] [Google Scholar]
- 17.Chisholm J. W., Gebre A. K., Parks J. S. 1999. Characterization of C-terminal histidine-tagged human recombinant lecithin: cholesterol acyltransferase. J. Lipid Res. 40: 1512–1519 [PubMed] [Google Scholar]
- 18.Krimbou L., Tremblay M., Jacques H., Davignon J., Cohn J. S. 1998. In vitro factors affecting the concentration of gamma LpE in human plasma. J. Lipid Res. 39: 861–872 [PubMed] [Google Scholar]
- 19.Jauhiainen M., Ehnholm C. 2005. Determination of human plasma phospholipid transfer protein mass and activity. Methods. 36: 97–101 [DOI] [PubMed] [Google Scholar]
- 20.Lee C. Y., Lesimple A., Larsen A., Mamer O., Genest J. 2005. ESI-MS quantitation of increased sphingomyelin in Niemann-Pick disease type B HDL. J. Lipid Res. 46: 1213–1228 [DOI] [PubMed] [Google Scholar]
- 21.Jauhiainen M., Huuskonen J., Baumann M., Metso J., Oka T., Egashira T., Hattori H., M. Olkkonene V., Ehnholm C. 1999. Phospholipid transfer protein (PLTP) causes proteolytic cleavage of apolipoprotein A-I. J. Lipid Res. 40: 654–664 [PubMed] [Google Scholar]
- 22.Krimbou L., Marcil M., Genest J. 2006. New insights into the biogenesis of human high-density lipoproteins. Curr. Opin. Lipidol. 17: 258–267 [DOI] [PubMed] [Google Scholar]
- 23.Batal R., Tremblay M., Krimbou L., Mamer O., Davignon J., Genest J., Cohn J. S. 1998. Familial HDL deficiency characterized by hypercatabolism of mature apoA-I but not proapoA-I. Arterioscler. Thromb. Vasc. Biol. 18: 655–664 [DOI] [PubMed] [Google Scholar]
- 24.Kee P., Rye K. A., Taylor J. L., Barrett P. H., Barter P. J. 2002. Metabolism of apoA-I as lipid-free protein or as component of discoidal and spherical reconstituted HDLs: studies in wild-type and hepatic lipase transgenic rabbits. Arterioscler. Thromb. Vasc. Biol. 22: 1912–1917 [DOI] [PubMed] [Google Scholar]
- 25.Jonas A., Bottum K., Kezdy K. 1991. Transformations of reconstituted high-density lipoprotein subclasses as a function of temperature or LDL concentration. Biochim. Biophys. Acta. 1085: 71–76 [DOI] [PubMed] [Google Scholar]
- 26.Bottum K., Jonas A. 1995. Cholesterol transfer from low density lipoproteins to reconstituted high density lipoproteins is determined by the properties and concentrations of both particles. Biochemistry. 34: 7264–7270 [DOI] [PubMed] [Google Scholar]
- 27.Jonas A., Kezdy K., Williams M., Rye K. 1988. Lipid transfers between reconstituted high density lipoprotein complexes and low density lipoproteins: effects of plasma protein factors. J. Lipid Res. 29: 1349–1357 [PubMed] [Google Scholar]
- 28.Meng Q-H., Sparks D. L., Marcel Y. L. 1995. Effect of LpA-I composition and structure on cholesterol transfer between lipoproteins. J. Biol. Chem. 270: 4280–4287 [DOI] [PubMed] [Google Scholar]
- 29.Nichols J. W., Pagano R. E. 1982. Use of resonance energy transfer to study the kinetics of amphiphile transfer between vesicles. Biochemistry. 21: 1720–1726 [DOI] [PubMed] [Google Scholar]
- 30.Jauhiainen M., Metso J., Pahlman R., Blomqvist S., vanTol A., Ehnholm C. 1993. Human plasma phospholipid transfer protein causes high density lipoprotein conversion. J. Biol. Chem. 268: 4032–4036 [PubMed] [Google Scholar]
- 31.Lie J., deCrom R., Jauhiainen M., vanGent T., vanHaperen R., Scheek L., Jansen H., Ehnholm C., vanTol A. 2001. Evaluation of phospholipid transfer protein and cholesteryl ester transfer protein as contributors to the generation of pre beta-high-density lipoproteins. Biochem. J. 360: 379–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Settasatian N., Duong M., Curtiss L. K., Ehnholm C., Jauhiainen M., Huuskonen J., Rye K. A. 2001. The mechanism of the remodeling of high density lipoproteins by phospholipid transfer protein. J. Biol. Chem. 276: 26898–26905 [DOI] [PubMed] [Google Scholar]
- 33.von Eckardstein A., Jauhiainen M., Huang Y., Metso J., Langer C., Pussinen P., Wu S., Ehnholm C., Assman G. 1996. Phospholipid transfer protein mediated conversion of high density lipoproteins generates pre beta 1-HDL. Biochim. Biophys. Acta. 1301: 255–262 [DOI] [PubMed] [Google Scholar]
- 34.Lefevre M., Sloop C. H., Roheim P. S. 1988. Characterization of dog prenodal peripheral lymph lipoproteins. Evidence for the peripheral formation of lipoprotein-unassociated apoA-I with slow pre-beta electrophoretic mobility. J. Lipid Res. 29: 1139–1148 [PubMed] [Google Scholar]
- 35.Barter P. J., Rye K. A. 1996. Molecular mechanisms of reverse cholesterol transport. Curr. Opin. Lipidol. 7: 82–87 [DOI] [PubMed] [Google Scholar]
- 36.Huang Y., vonEckardstein A., Wu S., Langer C., Assmann G. 1995. Generation of pre-beta 1-HDL and conversion into alpha-HDL. Evidence for disturbed HDL conversion in Tangier disease. Arterioscler. Thromb. Vasc. Biol. 15: 1746–1754 [DOI] [PubMed] [Google Scholar]
- 37.Faergeman O., Havel R. J. 1975. Metabolism of cholesteryl esters of rat very low density lipoproteins. J. Clin. Invest. 55: 1210–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamilton R. L., Moorehouse A., Havel R. J. 1991. Isolation and properties of nascent lipoproteins from highly purified rat hepatocytic Golgi fractions. J. Lipid Res. 32: 529–543 [PubMed] [Google Scholar]
- 39.Tall A. R.1990. Plasma high density lipoproteins. Metabolism and relationship to atherogenesis. J. Clin. Invest. 86: 379–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.