Abstract
Our objective was to determine the diagnostic accuracy of a free-breathing diffusion-weighted single-shot echo-planar magnetic resonance imaging (FBDW-SSEPI) technique with parallel imaging and high diffusion factor value (b = 1000 s/mm2) in the detection of primary rectal adenocarcinomas. Thirty-one patients (14M and 17F; mean age 67 years) with histopathologically proven primary rectal adenocarcinomas and 31 patients without rectal malignancies (14M and 17F; mean age 63.6 years) were examined with FBDW-SSEPI (repetition time (TR/echo time (TE) 3900/91 ms, gradient strength 45 mT/m, acquisition time 2 min) at 1.5 T using generalized autocalibrating partially parallel acquisitions (GRAPPA, acceleration factor 2) and a b value of 1000 s/mm2. Apparent diffusion coefficients (ADCs) of rectal adenocarcinomas and normal rectal wall were measured. FBDW-SSEPI images were evaluated for tumour detection by 2 readers. Sensitivity, specificity, accuracy and Youden score for rectal adenocarcinoma detection were calculated with their 95% confidence intervals (CI) for ADC value measurement and visual image analysis. Rectal adenocarcinomas had significantly lower ADCs (mean 1.036 × 10−3 ± 0.107 × 10−3 mm2/s; median 1.015 × 10−3 mm2/s; range (0.827–1.239) × 10−3 mm2/s) compared with the rectal wall of control subjects (mean 1.387 × 10−3 ± 0.106 × 10−3 mm2/s; median 1.385 × 10−3 mm2/s; range (1.176–1.612) × 10−3 mm2/s) (p < 0.0001). Using a threshold value ≤ 1.240 × 10−3 mm2/s, all rectal adenocarcinomas were correctly categorized and 100% sensitivity (31/31; 95% CI 95–100%), 94% specificity (31/33; 95% CI 88–100%), 97% accuracy (60/62; 95% CI 92–100%) and Youden index 0.94 were obtained for the diagnosis of rectal adenocarcinoma. FBDW-SSEPI image analysis allowed depiction of all rectal adenocarcinomas but resulted in 2 false-positive findings, yielding 100% sensitivity (31/31; 95% CI 95–100%), 94% specificity (31/33; 95% CI 88–100%), 97% accuracy (60/62; 95% CI 92–100%) and Youden index 0.94 for the diagnosis of primary rectal adenocarcinoma. We can conclude that FBDW-SSEPI using parallel imaging and high b value may be helpful in the detection of primary rectal adenocarcinomas.
Keywords: Diffusion magnetic resonance imaging, echo-planar imaging, colorectal neoplasms, parallel imaging, diffusion factor, apparent diffusion coefficient, sensitivity and specificity
Introduction
Diffusion-weighted single-shot echo-planar imaging (DW-SSEPI) is used to investigate a variety of intraabdominal and pelvic diseases[1–7]. This technique allows non-invasive characterization of focal lesions depending on their water diffusion properties, independently from T1 and T2 relaxation times and without the need for contrast agent administration[8–10]. When considering colorectal carcinomas, the results of several studies have shown that DW-SSEPI can help to depict and further characterize colorectal abnormalities using either visual evaluation of images or apparent diffusion coefficient (ADC) measurement[2,5,11–14]. To improve the capabilities of DW-SSEPI in that task, several strategies have been developed and many combinations of specific techniques have been evaluated. Among the possible parameters, the selection of a high diffusion factor value (b = 1000 s/mm2) seems to have a marked positive influence on the performances of DW-SSEPI for colorectal tumour detection[2,5,11].
Parallel imaging is a sequence that allows the use of shorter echo time (TE) and may facilitate diffusion-weighted magnetic resonance imaging (MRI) of the abdomen[4,11]. This technique relies on the fact that the signal from a multi-element surface coil contains limited spatial information because of the differing sensitivities of the component coils[15,16]. Parallel imaging reduces the echo-train length in combination with a faster k-space traversal per unit of time[4]. The resulting increased bandwidth per pixel in the phase-encoding direction and the shortened echo-planar imaging train improve image quality, mostly by reducing the background noise and the amount of motion artefacts[4,5,11]. One other advantage of parallel imaging is that it allows the use of multiple acquisition averages while keeping an acquisition time of 3–4 min[12] compared with more than 5 min in the absence of parallel imaging[2]. As a consequence, this technique has been used successfully in conjunction with respiratory-triggered acquisition for the diagnosis of colorectal cancers[12,14].
We recently used a free-breathing (FB) acquisition technique without respiratory triggering with the aim of obtaining an acquisition time of less than 2 min with DW-SSEPI. To our knowledge, the value of FBDW-SSEPI using parallel imaging and a high b value in the detection of rectal adenocarcinomas has not been evaluated so far. Accordingly, the goal of our retrospective study was to determine the diagnostic accuracy of FBDW-SSEPI with parallel imaging and a high diffusion factor value (b = 1000 s/mm2) in the detection of primary adenocarcinoma of the rectum, using endoscopic and surgical findings as standard of reference.
Materials and methods
Study group
Written informed consent was prospectively obtained from all patients, who agreed to have their personal medical and imaging data used for research purpose. All procedures were performed in our department which is located in a tertiary care and teaching hospital, in accordance with institutional review board guidelines. Because of the retrospective nature of this study, which included cases collected in a routine clinical setting under an already approved protocol, no specific final approval by our institutional review board was needed.
From January 2007 to March 2009, our MR imaging database was retrospectively queried to identify all cases of patients referred for MR evaluation of rectal adenocarcinoma. January 2007 was selected because FBDW-SSEPI MRI using the parallel imaging technique and high b value has been systematically used as part of our rectal neoplasm MR protocol since that date. A total of 42 consecutive patients were identified. Of these, 11 patients were excluded because of insufficient confirmation of the nature of the rectal lesions or because MR and surgical resection of the rectal tumour were performed more than 1 month apart. Thus, the final cohort comprised 31 patients. There were 14 men and 17 women with a mean age of 67 years (range 43–84 years; median 69 years). The standard of reference for determining the actual nature of the rectal lesions being examined was established by a radiologist who served as a study coordinator who had access to the patient's records, including the pathology reports and the entire imaging history. The study coordinator was not involved in the image analysis process. All malignant rectal neoplasms had histopathological confirmation after surgical removal and were primary adenocarcinomas. Of these, 17 were moderately differentiated, 12 were well differentiated and 2 were poorly differentiated adenocarcinomas. The rectal adenocarcinomas were located at the lower third (n = 14), middle third (n = 9), upper third (n = 5) of the rectum or at the rectosigmoid junction (n = 3). The values for the diameter of the rectal adenocarcinomas as defined by the largest diameter measured during histopathological examination ranged from 10 to 62 mm (mean 28.2 mm; median 27 mm). The rectal adenocarcinomas were classified as T1 (n = 4), T2 (n = 8), T3 (n = 15) and T4 (n = 4). A control group was made, which consisted of 31 patients who had MR imaging of the pelvis during the same period with the same protocol. Control patients were selected by the study coordinator. There were 14 men and 17 women, with a mean age of 63.6 years (range 30–81 years; median 65 years). They had MR imaging of the pelvis for evaluation of a pelvic mass (n = 11), suspected peritoneal carcinomatosis (n = 10), suspected anoperineal fistula tract (n = 7) and suspected recurrence of a rectal cancer at the anatomotic site (n = 3). All control patients were definitely excluded to have rectal carcinoma by optical colonoscopy (n = 17), optical rectoscopy (n = 8) or by follow-up evaluation, including clinical examination and pelvic MR imaging (n = 6).
MR imaging
All MR examinations were performed with the same protocol. MR imaging was performed with a 1.5-T clinical MR unit (Magnetom Avanto MRB15 version, Siemens Healthcare, Erlangen, Germany) with 18 receiver channels, using one anterior torso phased-array coil with 6 channels and 2 posterior spine clusters with 3 channels each, with the patient in a supine position. The gradient strength of the magnet was 45 mT/m with a maximal gradient slope of 200 T/m per s. A high-resolution FB T2-weighted turbo spin-echo (TSE) sequence with respiratory triggering using prospective acquisition correction (PACE) and 3D volumetric interpolated breath-hold (VIBE) gradient-echo before and after intravenous administration of a gadolinium chelate sequence were obtained for all patients.
FBDW-SSEPI was performed with a fat-suppressed single-shot spin-echo echo-planar diffusion-weighted technique in the axial plane with 3 gradient factors (b values 0, 500 and 1000 s/mm2) within the same acquisition. The diffusion gradients were applied in 3 orthogonal directions along the 3 main axes of the magnet bore (i.e. frequency, phase and slice select directions). The single-shot echo-planar imaging (EPI) readout was preceded by a diffusion-sensitizing block consisting of two 180° radiofrequency pulses. Parallel imaging with generalized autocalibrating partially parallel acquisition (GRAPPA) was used with an acceleration factor (or reduction factor) of 2 and 16 calibration lines. Consequently, the parallel imaging data sets consisted of 136 phase encodes compared with 256 in a conventional acquisition. By comparison with the acquisition time needed for conventional scans, this resulted in an acquisition time reduction of approximately 44% for the parallel scans[17]. Fat suppression was obtained with a frequency-selective fat saturation to reduce chemical shift artefacts. The other parameters were as follows: repetition time (TR)/echo time (TE) 3900 ms/91 ms; echo spacing 0.83 ms; matrix size 182 × 192; 6/8 partial Fourier acquisition; section thickness 5 mm; intersection gap 1 mm; voxel size 2.1 × 2.0 × 5.0 mm; field of view 340–400 mm; number of signal averages 4; no respiratory triggering; EPI factor 182; receiver bandwidth 1.302 Hz/pixel; 25 axial sections acquired; acquisition time 120 s. Partially parallel imaging datasets were reconstructed using a GRAPPA-based algorithm. No specific bowel preparation was used before MR examination and no antispasmodic agents were given to the patients.
Image analysis
For this retrospective case–control study, FBDW-SSEPI images and trace images obtained with the 3 different b values were reviewed by 2 abdominal radiologists with 18 and 16 years of experience in interpreting MR images of the abdomen and pelvis. To avoid review bias, they were both blinded to the results of biological tests, clinical and histopathological data and the results of other imaging techniques. Agreement was reached by consensus reading. For the retrospective analysis MR images were reviewed using a PACS workstation (Directview, 10.1 sp1 version, Carestream Health Inc, Rochester, NY) with no visible information relative to the patients.
ADC measurement
Trace images were obtained for each b value and a pixel-based ADC map was created from all diffusion weightings and directions with a commercially available workstation (MMWP with the Syngo Software, Siemens Healthcare). The ADC values were calculated using a mono-exponential fitting algorithm, with integrated software by linear regression analysis of the function S = S0exp(−b × ADC), where S is the signal intensity of the rectal area being evaluated after application of the diffusion gradient and S0 is the signal intensity on the diffusion-weighted image acquired at b = 0 s/mm2[10]. All b values (i.e., 0, 500 and 1000 s/mm2) were used for ADC calculation by integrated software. Mean ADC values were measured after drawing free-hand a region of interest (ROI) that included, when present, the entire tumour using the FBDW-SSEPI axial image obtained with b = 0 s/mm2. ROIs were placed at a level of slice on which the rectal tumours had their largest axial dimensions. ROI size varied according to rectal tumour axial dimensions. ADCs were measured 3 times and the 3 measurements were averaged for each patient. To ensure that the same areas were measured, regions of interest were copied and pasted from DW images to ADC maps. In the absence of visible rectal tumour, ROIs were randomly drawn to encompass normal-appearing portions of the rectal wall and to record their ADCs.
Tumour detection
FBDW-SSEPI images were reviewed in consensus using a black and white reversed contrast. Adenocarcinoma of the rectum was considered to exist (positive finding) when the presence of a well-defined area with dark signal on inverted grey-scale images obtained at b = 1000 s/mm2, which was sharply demarcated from surrounding tissues, was agreed by the 2 reviewers. Adenocarcinoma of the rectum was considered absent (negative finding) when such a dark signal area was not visible.
Statistical analysis
The distributions of ADCs were displayed in box plots. A non-parametric Mann–Whitney test was used to search for differences in ADCs between the 2 independent groups of patients. A P value of less than 0.05 was considered to indicate statistical significance. Optimal ADC threshold values for the diagnosis of rectal adenocarcinoma were determined using binary logistic regression and receiver operating characteristic curve analyses. Sensitivities, specificities, accuracies and Youden indexes for the diagnosis of rectal adenocarcinoma were subsequently calculated with their corresponding 95% confidence intervals (CIs) for 2 ADC threshold values depending on the expected effect. The capabilities of FBDW-SSEPI images for the diagnosis of rectal adenocarcinoma were evaluated in terms of sensitivity, specificity, accuracy and Youden index with their corresponding 95% CIs. The sensitivity for rectal adenocarcinoma detection was defined as the true-positive rate, that is, the number of patients with rectal adenocarcinoma who were correctly identified as having the disease at FBDW-SSEPI divided by the number of patients with rectal adenocarcinoma who were actually present in our study population. Specificity was calculated on a per patient basis. Accuracy was defined as the number of cases of rectal adenocarcinoma that were correctly diagnosed in our study population. The Youden index was defined as sensitivity plus specificity minus 1 and represented the proportion of patients correctly classified at FBDW-SSEPI. Statistical analysis was performed with commercially available software (SPSS 10.0 for Windows; SPSS, Chicago, IL, USA).
Results
ADC values
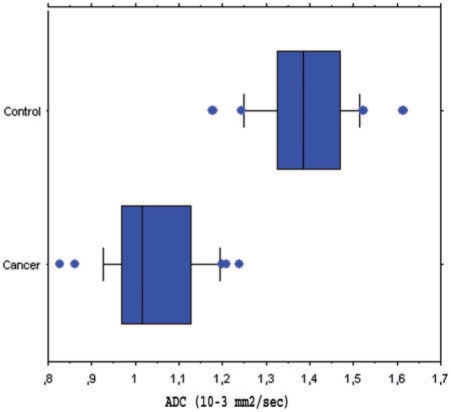
Adenocarcinomas of the rectum had significantly lower ADCs (mean 1.036 × 10−3 ± 0.107 × 10−3 mm2/s; median 1.015 × 10−3 mm2/s; range (0.827–1.239)× 10−3 mm2/s) by comparison with the rectal wall of control subjects (mean 1.387 × 10−3 ± 0.106 × 10−3 mm2/s; median 1.385 × 10−3 mm2/s; range (1.176–1.612) × 10−3 mm2/s) (P < 0.0001). Although the ADC values for the 2 groups of patients differed significantly, some degrees of overlap were found (Fig. 1). Using a threshold value ≤ 1.240 × 10−3 mm2/s, 100% (31/31) of rectal adenocarcinomas were correctly categorized, whereas 2 control patients were false-positive for the diagnosis of malignant tumour. These 2 patients were found to have normal rectum at optical colonoscopy. Using this threshold value that maximized sensitivity and accuracy, ADC measurement had 100% sensitivity (31/31; 95% CI 95–100%), 94% specificity (31/33; 95% CI 88–100%), 97% accuracy (60/62; 95% CI 92–100%) and Youden index 0.94 for the diagnosis of rectal adenocarcinoma. Using a threshold value ≤ 1.176 × 10−3 mm2/s that maximized specificity, ADC measurement had 100% specificity (31/31; 95% CI 99–100%), 84% sensitivity (26/31; 95% CI 75–93%), 92% accuracy (57/62; 95% CI 85–99%) and a Youden index 0.84.
Figure 1.
Box plots of ADC values, which differed significantly between rectal adenocarcinomas (cancer) and normal rectal wall (control). Boxes stretch across the interquartile range (IR), i.e. from lower quartile (Q1) to upper quartile (Q2); whiskers show smallest data point that is greater than (Q1−1.5 × IR) and largest data point that is smaller than (Q2 + 1.5 × IR). The vertical line through each box represents the median value. Blue dots indicate outliers. ADC values of rectal adenocarcinomas overlapped with those of normal rectal wall.
The numbers of rectal adenocarcinomas in each differentiation subtype category were too small to perform valid statistical analysis to determine whether ADCs differed significantly between differentiation subtypes.
Tumour detection
Of the 31 rectal adenocarcinomas, all (31/31, 100%) were considered present by the 2 readers during visual analysis of FBDW-SSEPI images (Fig. 2). In the control group, the 2 readers agreed upon the absence of rectal adenocarcinoma in 29 patients (Fig. 3), whereas 2 patients were erroneously considered as having rectal tumours. In these 2 false-positive cases, the middle third of the rectum showed well-defined, hemi-circumferential areas with dark signal on black and white reversed contrast images. One false-positive finding was found in a 49-year-old woman who was also false-positive for the presence of rectal cancer using ADC calculation because of an ADC value of 1.176 × 10−3 mm2/s. This patient who had MR imaging of the pelvis because of perineal pain with perianal inflammation and suspected Crohn disease, had a normal optical colonoscopy and was definitely excluded from having Crohn disease or rectal tumour (Fig. 4). The other false-positive finding was found in a 46-year-old man who had MR imaging of the pelvis because of suspected peritoneal carcinomatosis. This patient, with an ADC value of 1.341 × 10−3 mm2/s, was definitely excluded from having colorectal cancer or any other colorectal disease after a normal optical colonoscopy. FBDW-SSEPI image analysis yielded 100% sensitivity (31/31; 95% CI 95–100%), 94% specificity (31/33; 95% CI 88–100%), 97% accuracy (60/62; 95% CI 92–100%) and Youden index 0.94 for the diagnosis of rectal adenocarcinoma.
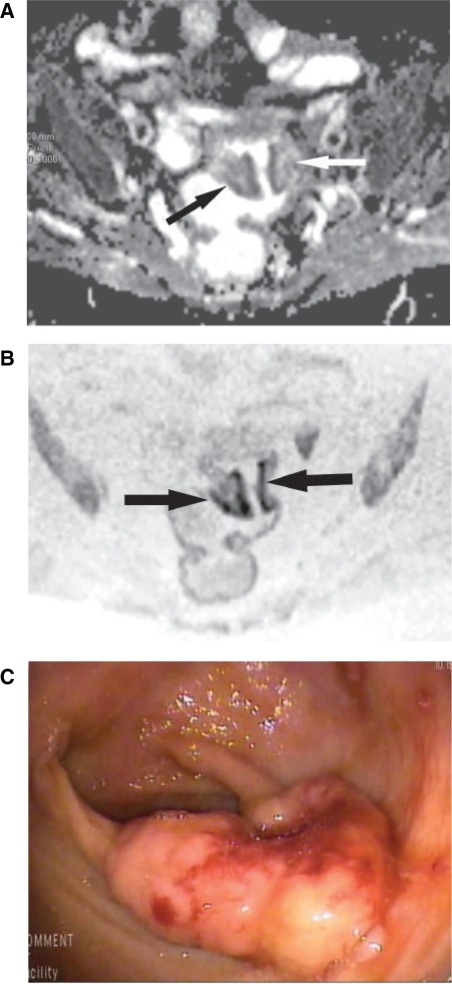
Figure 2.
An 80-year-old woman with adenocarcinoma of the upper third of the rectum, at the rectosigmoid junction. (A) Axial ADC map obtained with b = 1000 s/mm2 shows tumour (arrows). ADC is 1.195 × 10−3 mm2/s. (B) Axial FBDW-SSEPI image obtained with b = 1000 s/mm2 and displayed using black and white reversed contrast shows dark well-defined areas (arrows) that were correctly classified as rectal cancer by the 2 reviewers (true-positive case). (C) Optical colonoscopic view confirms the rectal tumour that was classified as a T2 well-differentiated adenocarcinoma at histopathologic analysis after surgical resection.
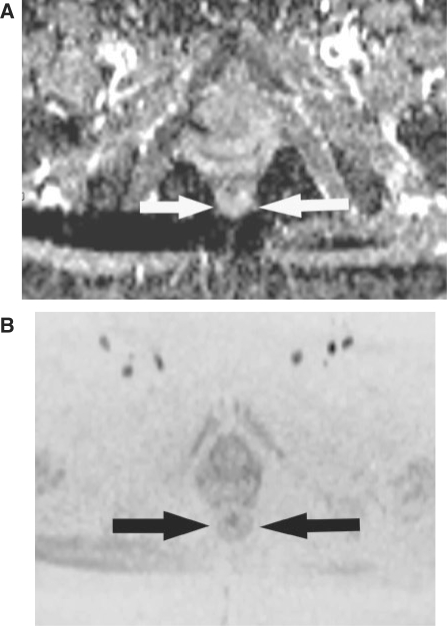
Figure 3.
A 56-year-old woman without rectal adenocarcinoma who had pelvic MR imaging for the evaluation of uterine fibroids (control subject). (A) Axial ADC map obtained with b = 1000 s/mm2 shows normal rectal wall (arrows). ADC is 1.364 × 10−3 mm2/s. (B) An axial FBDW-SSEPI image obtained with b = 1000 s/mm2 and displayed using black and white reversed contrast; no dark areas are visible within the rectal wall (arrows). This case was correctly classified as normal by the 2 reviewers (true-negative case).
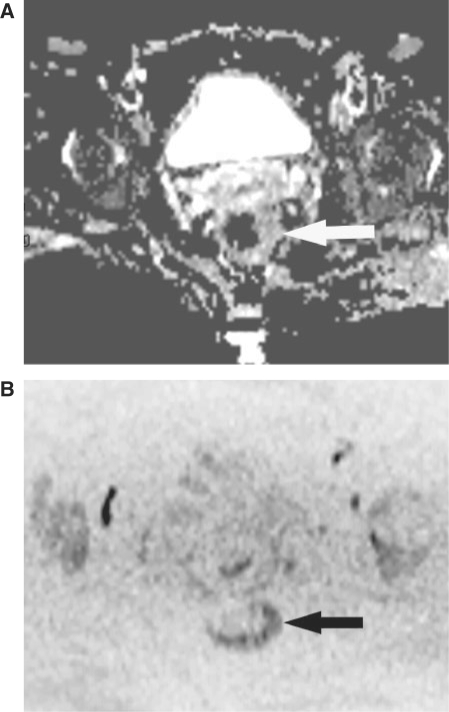
Figure 4.
A 49-year-old woman without rectal adenocarcinoma who had pelvic MR imaging for pelvic pain and suspected Crohn disease (control subject). (A) Axial ADC map obtained with b = 1000 s/mm2 shows asymmetric thickening of the rectal wall (arrow). ADC is 1.176 × 10−3 mm2/s. (B) Axial FBDW-SSEPI image obtained with b = 1000 s/mm2 and black and white reversed contrast shows a dark well-defined area at the left lateral aspect of the rectal wall (arrows) which was erroneously classified as rectal cancer by the 2 reviewers (false-positive case). Optical colonoscopy excluded Crohn disease and rectal cancer.
Discussion
The main objective of our retrospective study was to determine the diagnostic accuracy of FBDW-SSEPI using parallel imaging and a high b value in the detection of primary adenocarcinoma of the rectum, which to our knowledge has not yet been investigated. Our results show that this MR imaging technique is a highly accurate tool for that task. We found 100% sensitivity and 97% accuracy either with ADC measurement or visual evaluation of FBDW-SSEPI images, while keeping an acceptable examination time of 2 min. Our results are consistent with those of other studies using different MR techniques. In the study by Nasu et al.[11] involving 40 cases of rectal cancers, all were detected with DW-SSEPI using a breath-hold acquisition in association with sensitivity-encoding (SENSE) parallel imaging and a b value of 1000 s/mm2. In the study by Ono et al.[14] involving 27 cases of colorectal cancers, the 10 cases of rectal adenocarcinomas were all detected with DW-SSEPI using a respiratory-triggered acquisition with SENSE parallel imaging and a b value of 1000 s/mm2, resulting in 100% sensitivity for rectal cancer detection. Ichikawa et al.[2] who had 3 false-negative cases in a series of 33 colorectal cancers including 14 rectal cancers, reported a slightly lower sensitivity of 91% (30 tumours detected out of 33) for the diagnosis of colorectal cancer with DW-SSEPI. The results of Ichikawa et al.[2] were obtained in the absence of parallel imaging with an FB technique and a b value of 1000 s/mm2.
Despite highly statistically significant differences in mean ADC values between rectal adenocarcinomas and normal rectal wall, we found some degrees of overlap between the 2 groups. However, using a threshold value of less than 1.240 × 10−3 mm2/s that maximized accuracy and sensitivity, we found only 2 cases of false-positive finding. This result compares favourably with the 7 false-positive cases found by Hosonuma et al.[12], yielding 65% specificity in their study using parallel imaging (GRAPPA), respiratory-triggered acquisition and a b value of 800 s/mm2. Although direct comparison between results obtained in 2 different studies may be difficult because patients' demographics may vary, this may suggest a trend of superiority of FBDW-SSEPI over the technique used by Hosonuma et al.
The concept behind DW-SSEPI has been extensively described by Le Bihan et al.[8] and Turner et al.[10]. It has been demonstrated that the ADC value is a quantitative evaluation of intravoxel incoherent motion, which corresponds to a combination of diffusion and perfusion effects[8,10,18]. When the b value increases, the perfusion effect decreases accordingly and, for high b values, the ADC corresponds predominantly to the diffusion effect (i.e. the local environment of the water molecules responsible for MR images)[8,10]. It is currently admitted that lower ADC values of rectal adenocarcinomas relate to their high cellularity, whereas higher ACD values of normal rectal wall may relate to a relatively unrestricted motion of water molecules within a more prominent fluid content in large extracellular spaces[10].
In our study, all patients were investigated by means of parallel imaging technique with GRAPPA, which is an autocalibrating technique because the coil calibration necessary for the parallel imaging reconstruction is built into the accelerated scan. As a consequence, the reconstruction is not affected by patient motion between scans or by gradient nonlinearity in the high-resolution scan. By comparison with other parallel imaging techniques, GRAPPA has a number of advantages for our specific application. Griswold et al.[17] showed that parallel imaging techniques that belong to GRAPPA group are more robust than SENSE-like parallel imaging techniques. In addition, the quality of image reconstruction obtained by the former group of parallel imaging techniques is not as sensitive to errors in estimation of coil sensitivities as the latter. When the signal is received by an array of coils, scanning can be accelerated by the acquisition of fewer phase-encoding data points, and the missing data can be synthesized after acquisition using spatial-encoding information of all the coil elements, which is the basic concept of parallel imaging. As a limitation, however, a decreased signal-to-noise ratio is obtained[15]. The use of FB acquisition that relies on multiple acquisition averages is of value as it helps to increase the signal-to-noise ratio[19]. Another advantage of parallel imaging is that it reduces scanning time not by changing either the k-space bandwidth or the trajectory but by increasing the sampling interval along the phase-encoding axis. However, this advantage does not apply to EPI imaging. Conversely, when using EPI, parallel imaging allows the acquisition of a higher volume, which results in an increased number of slices for the same TR value.
The assessment of T stage and circumferential resection margin in patients with rectal adenocarcinoma relies primarily on MR imaging[20]. However, it was not our goal to investigate the value of FBDW-SSEPI in that task. Such evaluation requires high-definition images that allow visualization of the mesorectal fascia and perirectal fat. Because FBDW-SSEPI results in low contrast images, this technique cannot replace conventional MR sequences that provide more anatomical information. However, the added value of FBDW-SSEPI when used to implement conventional MR sequences is unknown and should warrant further investigations. In this regard, the major limitation of MR imaging in the evaluation of rectal carcinoma is the difficulty in differentiating stage T2 and stage T3 tumours. In addition, overstaging is a frequent cause of misdiagnosis[20]. The main reason for these 2 difficulties is the presence of desmoplastic tumour reaction, which may mimic actual tumour spread[20]. Fusion technique, which combines diffusion-weighted images and T2-weighted fast spin-echo images should thus be tested as a problem-solving technique to differentiate between non-malignant spiculations (i.e. those due to nontumorous desmoplastic reaction) and actual malignant spiculations (i.e. those that contain malignant tumour cells).
The major limitation of high b value DW-SSEPI images is their low resolution that makes localization of rectal lesions difficult. As pointed out by Nishie et al.[13], this specific weakness of DW-SSEPI is due to the fact that most pelvic organs show little signal with this MR technique. However, lesion localization was not the end point of our study, which was focused on lesion detection. Similarly, the use of FB acquisition for SSEPI has not gained wide acceptance among the radiologic community. In this regard, breath-hold and respiratory-triggered acquisitions are more frequently used[11,12]. The main advocated reason is that misregistration between the different acquisitions obtained at the various b values may result in erroneous ADC values. However, to the best of our knowledge, there is no study in the literature that can substantiate this hypothesis for pelvic imaging. In addition, we found ADC values that were within the range of previously reported values obtained with breath-hold and respiratory-triggered acquisitions[11,12] and that correlated with the status of the patients (i.e. cancer vs normal). This suggests that if any misregistration exists, it has little effect on the calculated ADC values for the diagnosis of primary rectal adenocarcinomas.
Several limitations may be raised with respect to our study. The first one relates to the fact that we did not investigate to what extent anistropy was present on FBDW-SSEPI. However, this issue has been already addressed by Nasu et al.[11] who did not find any difference between the images obtained with different directions for motion probing gradients, supporting the fact that rectal tumours diffuse isotropically. A second limitation is that we did not include patients with inflammatory rectal diseases or with benign or dysplastic polyps. It may be possible that distinction between such diseases and rectal tumours may be difficult and that more pronounced degrees of overlap may exist in terms of ADC values. A third limitation is that we did not compare FBDW-SSEPI images with more conventional MR sequences, so that the actual role of this technique and its added value in rectal tumour detection is not known. However, this latter concern has been addressed by Rao et al.[5] who found improved performances for rectal cancer detection with a combination of DW-SSEPI and T2-weighted fast spin-echo MR images compared with T2-weighted fast spin-echo MR images alone. A fourth limitation is that we did not correlate ADC values with the degree of histological differentiation. Insufficient numbers of rectal adenocarcinomas in each differentiation subtype category were present to perform valid statistical analysis to determine whether ADCs differed significantly between differentiation subtypes. Therefore, further studies are needed to determine to what extent FBDW-SSEPI can help predict the degree of differentiation of primary rectal adenocarcinomas. The fifth limitation relates to the fact that the ADC values and threshold values we found in our study may be specific to our MR technique. We agree that it may reasonably be argued that our results may apply to our FBDW-SSEPI technique with high b value only because calculated ADC values depend on the b values selected.
In conclusion, the results of our study show that FBDW-SSEPI using parallel imaging and high b value may be helpful in the detection of primary adenocarcinomas of the rectum. A large-scale clinical study including several pathologic conditions of the rectum is warranted to determine the potential value and limitations of FBDW-SSEPI imaging in the detection and further characterization of rectal adenocarcinoma.
References
- 1.Ichikawa T, Haradome H, Hachiya J, Nitatori T, Araki T. Diffusion-weighted MR imaging with single-shot echo-planar imaging in the upper abdomen: preliminary clinical experience in 61 patients. Abdom Imaging. 1999;24:456–61. doi: 10.1007/s002619900539. doi:10.1007/s002619900539. PMid:10475927. [DOI] [PubMed] [Google Scholar]
- 2.Ichikawa T, Ertuk SM, Motosugi U, et al. High-b-value diffusion-weighted MRI in colorectal cancer. AJR Am J Roentgenol. 2006;187:181–4. doi: 10.2214/AJR.05.1005. doi:10.2214/AJR.05.1005. PMid:16794174. [DOI] [PubMed] [Google Scholar]
- 3.Ichikawa T, Haradome H, Hachiya J, Nitatori T, Araki T. Diffusion-weighted MR imaging with a single-shot echoplanar sequence: detection and characterization of focal hepatic lesions. AJR Am J Roentgenol. 1998;170:397–402. doi: 10.2214/ajr.170.2.9456953. [DOI] [PubMed] [Google Scholar]
- 4.Taouli B, Martin AJ, Qayyum A, et al. Parallel imaging and diffusion tensor imaging for diffusion-weighted MRI of the liver: preliminary experience in healthy volunteers. AJR Am J Roentgenol. 2004;183:677–80. doi: 10.2214/ajr.183.3.1830677. [DOI] [PubMed] [Google Scholar]
- 5.Rao SX, Zeng MS, Chen CZ, et al. The value of diffusion-weighted imaging in combination with T2-weighted imaging for rectal cancer detection. Eur J Radiol. 2008;65:299–303. doi: 10.1016/j.ejrad.2007.04.001. doi:10.1016/j.ejrad.2007.04.001. PMid:17498902. [DOI] [PubMed] [Google Scholar]
- 6.Bruegel M, Holzapfel K, Gaa J, et al. Characterization of focal liver lesions by ADC measurements using a respiratory triggered diffusion-weighted single-shot echo-planar MR imaging technique. Eur Radiol. 2008;18:477–85. doi: 10.1007/s00330-007-0785-9. doi:10.1007/s00330-007-0785-9. PMid:17960390. [DOI] [PubMed] [Google Scholar]
- 7.Yamada I, Aung W, Himeno Y, Nakagawa T, Shibuya H. Diffusion coefficients in abdominal organs and hepatic lesions: evaluation with intravoxel incoherent motion echo-planar MR imaging. Radiology. 1999;210:617–23. doi: 10.1148/radiology.210.3.r99fe17617. [DOI] [PubMed] [Google Scholar]
- 8.Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168:497–505. doi: 10.1148/radiology.168.2.3393671. [DOI] [PubMed] [Google Scholar]
- 9.Thoeny HC, de Keyzer F. Extracranial applications of diffusion-weighted magnetic resonance imaging. Eur Radiol. 2007;17:407–14. doi: 10.1007/s00330-006-0547-0. [DOI] [PubMed] [Google Scholar]
- 10.Turner R, Le Bihan D, Maier J, Vavrek R, Hedges LK, Pekar J. Echo-planar imaging of intravoxel incoherent motion. Radiology. 1990;177:407–14. doi: 10.1148/radiology.177.2.2217777. [DOI] [PubMed] [Google Scholar]
- 11.Nasu K, Kuroki Y, Kuroki S, Murakami K, Nawano S, Moriyama N. Diffusion-weighted single shot echo planar imaging of colorectal cancer using a sensitivity-encoding technique. Jpn J Clin Oncol. 2004;34:620–6. doi: 10.1093/jjco/hyh108. doi:10.1093/jjco/hyh108. PMid:15591461. [DOI] [PubMed] [Google Scholar]
- 12.Hosonuma T, Tozaki M, Ichiba N, et al. Clinical usefulness of diffusion-weighted imaging using low and high b-values to detect rectal cancers. Magn Reson Med Sci. 2006;5:173–7. doi: 10.2463/mrms.5.173. doi:10.2463/mrms.5.173. PMid:17332707. [DOI] [PubMed] [Google Scholar]
- 13.Nishie A, Stolpen AH, Obuchi M, Kuehn DM, Dagit A, Andresen K. Evaluation of locally recurrent pelvic malignancy: performance of T2- and diffusion-weighted MRI with image fusion. J Magn Reson Imaging. 2008;28:705–13. doi: 10.1002/jmri.21486. doi:10.1002/jmri.21486. PMid:18777555. [DOI] [PubMed] [Google Scholar]
- 14.Ono K, Ochiai R, Yoshida T, et al. Comparison of diffusion-weighted MRI and 2-[fluorine-18]-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) for detecting primary colorectal cancer and regional lymph node metastases. J Magn Reson Imaging. 2009;29:336–40. doi: 10.1002/jmri.21638. doi:10.1002/jmri.21638. PMid:19161185. [DOI] [PubMed] [Google Scholar]
- 15.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42:952–62. doi:10.1002/(SICI)1522-2594(199911)42:5<952::AID-MRM16>3.3.CO;2-J. [PubMed] [Google Scholar]
- 16.Bammer R, Keeling SL, Augustin M, et al. Improved diffusion-weighted single-shot echo-planar imaging (EPI) in stroke using sensitivity encoding (SENSE) Magn Reson Med. 2001;46:548–54. doi: 10.1002/mrm.1226. doi:10.1002/mrm.1226. PMid:11550248. [DOI] [PubMed] [Google Scholar]
- 17.Griswold MA, Jakob PM, Heidemann RM, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002;47:1202–10. doi: 10.1002/mrm.10171. doi:10.1002/mrm.10171. PMid:12111967. [DOI] [PubMed] [Google Scholar]
- 18.Huwart L, Michoux N, Van Beers BE. Magnetic resonance imaging of angiogenesis in tumors. J Radiol. 2007;88:331–8. doi: 10.1016/s0221-0363(07)89829-5. doi:10.1016/S0221-0363(07)89829-5. [DOI] [PubMed] [Google Scholar]
- 19.Jones DK, Basser PJ. “Squashing peanuts and smashing pumpkins”: how noise distorts diffusion-weighted MR data. Magn Reson Med. 2004;52:979–93. doi: 10.1002/mrm.20283. doi:10.1002/mrm.20283. PMid:15508154. [DOI] [PubMed] [Google Scholar]
- 20.Vliegen RFA, Beets GL, von Meyenfeldt MF, et al. Rectal cancer: MR imaging in local staging – is gadolinium-based contrast material helpful? Radiology. 2005;234:179–88. doi: 10.1148/radiol.2341031403. doi:10.1148/radiol.2341031403. PMid:15550372. [DOI] [PubMed] [Google Scholar]