Abstract
Tumours in the oral cavity and oropharynx differ in presentation and prognosis and the detection of spread of tumour from one subsite to another is essential for the T-staging. This article reviews the anatomy and describes the pattern of spread of different cancers arising in the oral cavity and oropharynx; the imaging findings on computerized tomography and magnetic resonance imaging are also described. Brief mention is made on the role of newer imaging modalities such as [18F]fluorodeoxyglucose-positron emission tomography/computed tomography, perfusion studies and diffusion-weighted magnetic resonance imaging.
Keywords: Anatomy, oral cavity, oropharynx, carcinoma, magnetic resonance imaging, computerized tomography
Introduction
The oral cavity and oropharynx are the uppermost part of the digestive tract and unique in the variety of tissues contained within a small area. Knowledge of anatomy is essential in identifying the site of origin of the tumour, its pattern of spread and behaviour. Carcinoma in this area is treated with surgery, chemotherapy and radiotherapy depending on the T-staging and it is therefore very important to accurately stage the tumour to allow for optimum therapy and to avoid unnecessary morbidity.
Epidemiology
The incidence of cancer of the oral cavity was reported by Parkin et al.[1] as 2.6% of all cancers followed by pharyngeal (oro/hypo) cancers reported with 1.2%. It was more common in men than women; the M/F ratio for mouth cancer was 2.0 and for pharyngeal cancers 4.4. In men, the incidence of oral cavity and pharyngeal cancers is high in western and southern Europe, whereas oral cavity cancers have a higher incidence in south-east Asia, southern Africa and Australia. In women, pharyngeal and oral cavity cancers have a relatively high incidence in south-central Asia and oral cancers have a higher rate in south-east Asia and Australia. These patterns reflect the prevalence of specific risk factors, tobacco/alcohol in south Europe and Africa and chewing of betel nuts in south-central and south-east Asia. In Australia, there is a high rate of lip cancer due to solar radiation. Oral cavity cancer is the sixth leading cause of cancer deaths in the world with cancer of the mouth and pharynx being the most common head and neck tumours in the United States[2].
Anatomy
The oral cavity includes the lips, the hard palate, the upper and lower alveolar ridge, the anterior two-thirds of the tongue, sublingual region, the buccal mucosa, the retromolar trigone and the floor of the mouth (mylohyoid, digastric, geniohyoid muscles)[3]. The retromolar trigone is a small mucosal area on the mandibular ramus behind the posterior molars. This is a junction point between the oral cavity, oropharynx and nasopharynx allowing for complex spread of tumours[4].
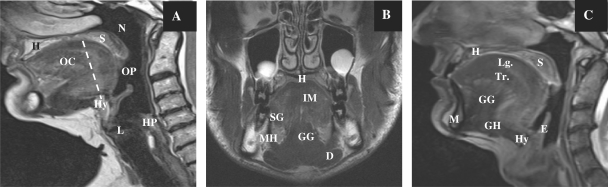
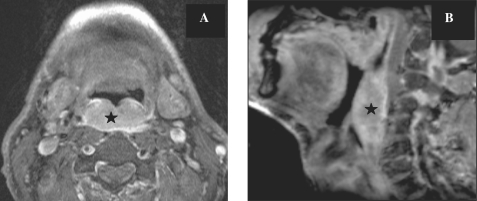
The oral cavity is separated from the oropharynx by an imaginary line drawn across the circumvallate papillae, anterior tonsillar pillars and junction of the hard and soft palate (Fig. 1). The oropharynx contains the posterior third (base) of the tongue, the tonsillar fossa and pillars, the soft palate and the posterior and lateral pharyngeal walls to the level of the hyoid bone inferiorly. The anterior and posterior tonsillar pillars are mucosal folds over the palatoglossus and palatopharyngeal muscles, respectively; the faucial or palatine tonsils are located between the tonsillar pillars bilaterally.
Figure 1.
Sagittal T2-weighted MR image (A) in the median plane showing normal anatomy and the division of the oral cavity and oropharynx. Coronal T2-weighted image (B) and sagittal T2-weighted image (C) showing the various structures in the oral cavity. OC, oral cavity; OP, oropharynx; HP, hypopharynx; L, larynx; N, nasopharynx; H, hard palate; S, soft palate; Hy, hyoid bone; E, epiglottis. Muscles of the tongue: SG, styloglossus muscle; GG, genioglossus muscle; GH, geniohyoideus; IM, intrinsic muscles; Lg, superior longitudinal muscles; Tr, transverse lingual muscles. Muscles of the floor of the mouth: MH, myelohyoid muscle; D, anterior belly of the digastric muscle.
Imaging: when and how
Evaluation of the oral cavity and oropharynx is primarily done by clinical examination and with endoscopy. Diagnosis of cancer is made by biopsy. Cross-sectional imaging is used for staging and allows visualization of the pathology beneath the mucosa, helps to determine the size, thickness and depth of the tumour, detects invasion of neighbouring structures, bone or perineural spread, assesses lymph node metastases, excludes a second tumour and assesses the teeth, and is used for treatment planning and follow-up during and after treatment.
Computerized tomography (CT) is usually the first imaging modality used to assess and stage tumours of the oral cavity and oropharynx because it is widely available, relatively cheap, quick and easy to perform. Thin section (1 mm) scanning results in good quality coronal and sagittal reconstructions. CT can delineate the size and extent of the primary tumour, and assess bone involvement and metastatic lymph nodes. Disadvantages include radiation exposure, the need to inject iodinated contrast medium, poor soft tissue contrast and artefacts due to dental amalgam.
Magnetic resonance imaging (MRI) is complementary to CT but offers distinct advantages such as better soft tissue contrast, multiplanar capability and reduced artefacts from dental fillings. It is superior in the detection of early bone marrow involvement, pterygopalatine fossa infiltration, prevertebral muscle involvement and perineural tumour spread[5]. MRI entails no radiation and no iodinated contrast medium has to be administered. However, MR contrast medium has to be injected in the evaluation of patients with tumours. Macrocyclic gadolinium chelates at low doses should be administered to avoid the risk of nephrogenic systemic fibrosis in patients with renal insufficiency.
The disadvantages of MRI include the long investigation time with increased risk of motion artefacts and higher cost. Contraindications to MRI include the presence of pacemakers, metal foreign bodies and claustrophobia.
CT and MR imaging appearance
Squamous cell carcinoma
On CT, tumours enhance after administration of contrast material. Moderate enhancement may be difficult to identify particularly when in the anterior part of the floor of the mouth, because of beam hardening artefacts due to a dense mandible or dental amalgam. Large tumours tend to be heterogeneous because of necrosis[6].
On T1-weighted images, the tumour is hypointense or isointense to muscle and on T2-weighted images, it is usually hyperintense. Solid tumours enhance with gadolinium (Fig. 2), whereas necrotic areas within the tumour remain hypointense[2]. The precontrast T1-weighted image is particularly useful in differentiating tumour from surrounding fat, detecting neurovascular bundle encasement and bone marrow involvement[2].
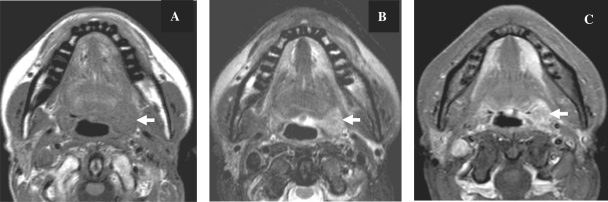
Figure 2.
Axial MR images in a patient with proven cancer of the left tonsil. The tumour (arrow) is well delineated, hypointense on T1-weighted (A), hyperintense on T2-weighted (B) with enhancement after contrast injection (C).
MRI can overestimate the tumour due to haemorrhage and inflammatory changes.
The main drawback of both CT and MR imaging is their lack of specificity; it is not possible to differentiate between tumour and inflammatory changes.
Bone involvement
Cortical bone invasion is depicted as an interruption or an erosion of the peripheral hyperattenuating rim at CT or hypointense rim at MR imaging in all sequences. CT depicts cortical bone invasion best. Subtle cortical erosion is best detected by thin sectional CT with bone algorithm with coronal and sagittal reconstruction. The exact extent of the medullary bone invasion is better defined with MR imaging. Low signal intensity on unenhanced T1-weighted images with high signal on T2-weighted images and enhancement after intravenous (iv) contrast administration may be considered to indicate marrow invasion. These changes are unfortunately non-specific and also seen with peritumoural oedema, inflammation, coexisting periodontal disease, osteomyelitis, radiation fibrosis and osteoradionecrosis[3]. Sensitivity of bone marrow involvement is high at 100%, specificity is however relatively low at about 71–75% with MRI[4]. Invasion of the maxilla is most often seen in retromolar trigone cancers and cancer of the hard palate[3].
Perineural spread
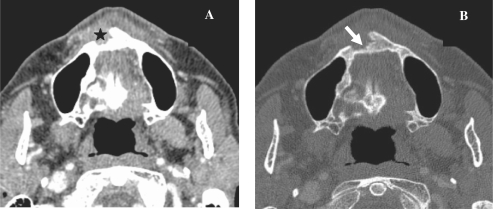
CT can show enlargement of the nerve foramina and replacement of fat in the presence of perineural spread (Fig. 3). MRI can show the enlargement of the nerve and enhancement and replacement of the fat in the pterygopalatine fossa (Fig. 4)[7]. Perineural spread can be antegrade or retrograde and skip lesions may occur. Therefore meticulous evaluation of the skull base is of utmost importance in order to detect perineural spread. However, inflammatory or oedematous enlargement of the nerves can simulate perineural spread so these changes are not specific for perineural spread[8]. Secondary signs of denervation such as atrophy of the muscles innervated by the involved nerve or abnormal enhancement of the denervated muscle can be helpful in making the correct diagnosis.
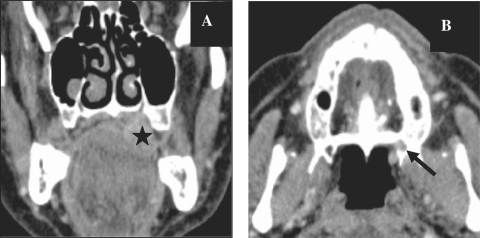
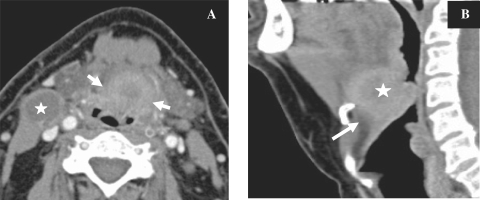
Figure 3.
CT images, coronal reconstruction (A) and axial images (B) showing a histologically proven case of adenoid cystic carcinoma of the hard palate (star) with extension along the greater palatine nerve and replacement of fat (arrow) in the greater palatine foramen. Compare the normal foramen on the right side.
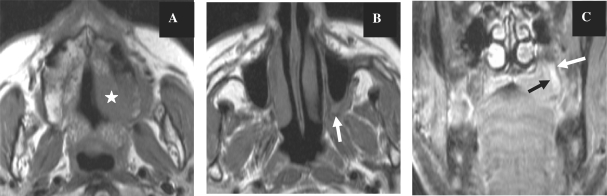
Figure 4.
Axial T1-weighted MR images before contrast injection (A,B) showing the hypointense tumour of the hard palate on the left (star) with loss of normal fat signal in the pterygopalatine fossa on the left (arrow) in a patient with proven lymphoma and perineural extension of the tumour. The coronal image after gadolinium injection with fat suppression (C) shows enhancement of the greater palatine nerve on the left (arrows).
Lymph node staging
The status of the lymph nodes is the most significant predictor of outcome in patients who have squamous cell carcinoma (SCC) of the oral cavity[2]. The oral tongue and floor of the mouth are rich in lymphatics and tumours in these areas have a higher risk of nodal metastases compared with the hard palate and alveolar ridge. Level I–III nodes are at highest risk from oral cancers. In previously untreated neck cancers, metastases in nodes at level IV and V are rare in the absence of lymph node involvement at levels I–III.
Most metastatic nodes are abnormally enlarged. For level II nodes, the size criterion used is more than 1.5 cm transversal short axis; for all other nodes, more than 1 cm is pathological. Normal-sized nodes can have focal metastases or necrosis that is more easily seen on CT than on MRI[2]. Necrosis should be differentiated from the fat hilus of lymph nodes by measuring the Hounsfield units and looking at the shape of the lymph node. Other features include heterogeneous enhancement and stranding or involvement of the adjacent soft tissue if extracapsular nodal spread is present. This is more common in larger nodes. The current staging system for the neck does not take into account the presence of extracapsular spread but is based on the site, number and laterality of the lymph nodes relative to the primary tumour[2].
N staging of lymph nodes
Nx regional lymph nodes cannot be assessed
N0 no nodes
N1 ipsilateral lymph node 3 cm or less in greatest dimension
N2a ipsilateral lymph node between 3 and 6 cm
N2b multiple ipsilateral lymph nodes less than 6 cm
N2c bilateral or contralateral lymph nodes less than 6 cm
N3 lymph nodes larger than 6cm
Cancers of the oral cavity
SCC accounts for 90–95% of tumours. Other tumours include minor salivary gland tumours, lymphomas and rare tumours such as melanomas, liposarcomas, rhabdomyosarcomas[2,6]. SCC of the oral cavity tends to spread locally with invasion of surrounding structures; the risk and pattern of spread varies with the anatomic location of the tumour[6]. Adenoid cystic carcinoma is well known for its propensity to perineural spread but this is not specific and is also observed in squamous cell cancer which is more common[8].
T-staging of oral cavity cancer (TNM 2009)
T-staging of the tumour depends on size criteria and extension of the tumour in neighbouring structures. Tx indicates that the primary tumour cannot be assessed, T0 no evidence of tumour, T1 tumours are less than 2 cm in the greatest dimension, T2 between 2 and 4 cm, T3 more than 4 cm. T4a tumours show invasion of the cortical bone, deep or extrinsic muscles of the tongue, maxillary sinus and skin of the face. T4b tumours show infiltration of the masticator space, pterygoid plates, skull base or encasement of the internal carotid artery.
Lips
SCC of the lip is the most common malignant neoplasm of the oral cavity with an incidence of 40%. Cancer of the lip usually arises from the vermillion border and can spread to involve adjacent skin or underlying muscles such as the orbicularis oris muscle. Early lesions are easier to detect clinically. Imaging plays a role with more advanced lesions with indeterminate margins. The role of imaging is to determine the full extent of the lesion including possible perineural spread, lymph node metastases and the integrity of the adjacent bone. If there is invasion of bone, the carcinoma is upstaged to a T4[4].
On CT and MRI the primary tumour may appear as a mass with or without areas of ulceration. Subtle bone erosion usually occurs along the buccal surface of the mandibular or maxillary alveolar ridge and is best detected with CT. Large tumours may extend directly into the mandible or involve the mental nerve without cortical bone destruction[3].
Floor of the mouth
Most SCCs originate within 2 cm of the anterior midline floor of the mouth[6]. They spread beneath the mucosa into the sublingual gland and can obstruct the Wharton duct resulting in submandibular gland sialadenitis. This is often the first clinical manifestation and therefore careful examination of the floor of the mouth is mandatory in cases of an enlarged submandibular gland (Fig. 5). Invasion of the mylohyoid muscle signifies submandibular spread. Superiorly and posteriorly the tumours tend to involve the ventral surface of the tongue, the adjacent lingual neurovascular bundle and the base of the tongue. Anteriorly and laterally, the neoplasm may advance into the adjacent gingival mucosa and then destroy the lingual cortex and involve the marrow of the mandible[3].
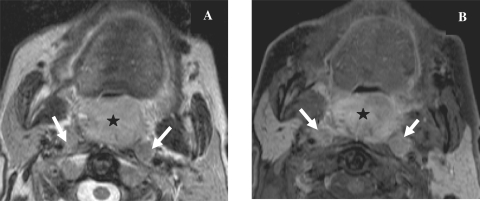
Figure 5.
Axial T2-weighted MR image of a patient with histologically proven squamous cell cancer of the floor of the mouth showing the tumour with involvement of the mandible and extension into the adjacent soft tissues anteriorly (star). Note obstruction with dilatation of the Wharton duct on both sides (arrows).
The less common tumours arising from the lateral floor of the mouth show similar dissemination patterns. Advanced lesions may extend beyond the oral cavity and infiltrate the contiguous spaces of the suprahyoid neck particularly the parapharyngeal space and the masticator space. The medial pterygoid muscle inserts into the inner cortex of the angle of the mandible, and can be invaded by tumours of the lateral floor of the mouth. The prevalence of lymph node metastases is between 30% and 70%[3]. Lymph nodes that should be assessed include level I to III nodes[4].
Retromolar trigone
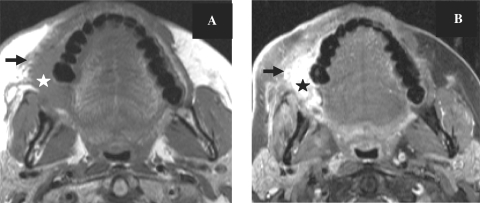
Retromolar trigone tumours (Fig. 6) account for 7% of tumours affecting the oral cavity. Cancers originating in the retromolar trigone can spread to any of the adjacent structures, including the tonsils, the base of the tongue, along the pterygomandibular raphe superiorly to the temporalis muscle, medial to the pterygomandibular space where the lingual and inferior alveolar nerves run, inferiorly into the floor of the mouth or anteriorly from the medial pterygoid plate into the pterygopalatine fossa[7].
Figure 6.
Axial T1-weighted MR images before (A) and after contrast administration with fat saturation (B) in a patient with proven cancer of the retromolar trigone on the right (star). Note infiltration of the buccinator muscle and extension along the alveolar ridge (arrow).
Oral tongue
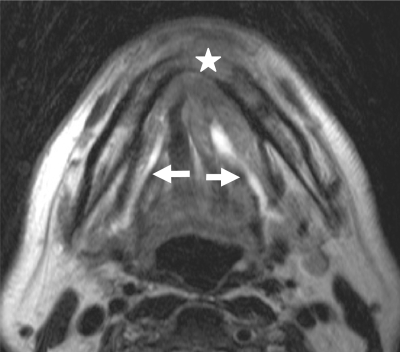
The tongue is composed of intrinsic and extrinsic muscle fibres and a midline fibrous septum extending from the hyoglossus membrane and the midportion of the hyoid bone. The main bulk of the tongue is made up of the four interdigitating intrinsic muscles: the superior and inferior longitudinal, transverse and oblique muscles. The extrinsic muscles originate from bony attachments external to the tongue and include the genioglossus, styloglossus, hyoglossus and palatoglossus muscles. These interdigitate with the intrinsic muscles (Fig. 1).
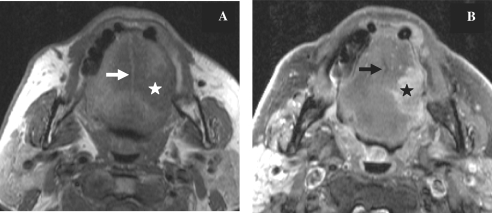
Nearly all SCCs of the oral tongue occur on the lateral and undersurface of the tongue. Most of the lateral border lesions occur on the middle and posterior thirds of the oral tongue[6]. SCCs tend to remain in the tongue until they are quite large. The prognosis is generally better than that of carcinoma of the tongue base. Large tumours tend to grow into the glossotonsillar sulcus, base of the tongue, tonsillar fossa and floor of the mouth. In addition to routine axial images, coronal MR images may provide additional information about the exact extent of tongue neoplasms and their relationship to the neurovascular bundle. Infiltrating lesions extend medially in the tongue itself. They first invade the muscles located laterally (the hyoglossus, styloglossus and palatoglossus muscles). Then they reach successively the lingual pedicle, the genioglossus muscle, the lingual septum and the contralateral half of the tongue. Tumour extension across the midline with involvement of the contralateral lingual artery or nerve makes surgical resection for cure difficult because of the morbidity associated with total glossectomy (Fig. 7). Tumours may also extend in the anteroposterior direction, from the tip to the base of the tongue.
Figure 7.
Axial T1-weighted MR images before (A) and after contrast injection with fat saturation (B) in a patient with cancer of the tongue on the left side (star). The tumour does not cross the midline which is represented by the fatty signal intensity of the lingual septum (arrow).
Because of the anatomic disposition of the genioglossus muscle, it constitutes a natural route for advanced lesions to extend towards the anterior floor of the mouth. Middle-third lesions invade the lateral floor of the mouth and mandible. Lesions of the posterior third grow into the floor of the mouth, the glossotonsillar sulcus, the oropharyngeal tonsil and underlying deep spaces. Lesions may extend superiorly to the soft palate via the palatoglossus muscle and from the soft palate to the nasopharynx via the veli palatini muscles. Detection of posterior extension into the base of the tongue is important since it can change treatment. The oral tongue is mobile and therefore evaluation is easier in the sagittal and coronal planes than on axial images.
T-staging and tumour thickness is important for the correct choice of therapy. Tumour thickness or depth of tumour can predict the presence of cervical lymph node metastases[9–11]. Cancer of the tongue is known to have a high incidence of nodal metastases even in the early T1 and T2 stages. The pathophysiology of nodal metastases is not known but could be related to the abundant submucosal lymphatic vessels. The incidence of nodal metastases increases rapidly when the muscles of the tongue are infiltrated and postulated explanations include the more invasive behaviour of deeply infiltrating cancer or the contraction of the tongue muscles which could promote lymphatic spread[9].
Tumour thickness is also reported to influence the local recurrence rate. The most important risk factor for local recurrence is the histological resection margin, the deep margin is frequently the site of inadequate resection because it is more difficult to assess accurately with palpation. Therefore, the deeper the infiltration, the higher the chances of an inadequate resection margin[9].
Incidence of subclinical nodal metastases with tumour thickness of 3 mm has been reported to be 8% with no local recurrence. For tumour thickness between 3 and 9 mm, the incidence of subclinical nodal metastases reported was 44% with 7% local recurrence. Tumour thickness more than 9 mm was associated with subclinical nodal metastases in 53% and local recurrence in 24%[11].
Hard palate
Here it is important to assess the degree of bone invasion and to exclude perineural spread for these tumours. Adenoid cystic cancers in this area have a propensity for perineural spread along the greater and lesser palatine nerves into the pterygopalatine fossa (Fig. 3). Perineural spread can also occur with lymphoma (Fig. 4). From the pterygopalatine fossa, tumour can travel along the maxillary nerve into the foramen rotundum or anteriorly into the infraorbital foramen or along the vidian nerve in the vidian canal.
Although SCC of the hard palate is often confined to its site of origin at the time of diagnosis, advanced tumours may invade the maxilla, nasal cavity, buccal mucosa, tongue or retromolar trigone. Therefore, coronal sections are extremely useful for evaluating these tumours. Lymphatic spread occurs along the facial and retropharyngeal lymph nodes and along the upper jugular chain.
Buccal region including gingival and alveolar ridges
Although less than 10% of oral cavity tumours arise in this region, proximity of these tumours to bony cortical margins is worrying due to potential bony invasion and perineural spread (Fig. 8). Buccal carcinomas usually arise along the lateral margins and common routes of spread include lateral submucosal extension along the buccinator muscle towards the ptyeromandibular raphe and subsequent bony involvement. SCC of the buccal mucosa tends to invade the masticator space, the buccinator muscle with subsequent involvement of the skin, the anterior tonsillar pillars and the soft palate. Infiltration of the medial pterygoid muscle causes trismus. Pathways of lymphatic spread are variable and include the submandibular, facial, intraparotid and preauricular nodes. SCC of the gingiva frequently occurs in the molar and premolar regions along the gingival margin of a tooth and bone destruction is a frequent finding. Nearly 50% of patients have level II lymph node involvement at presentation.
Figure 8.
Axial CT images in soft tissue (A) and bone window (B) showing the histologically confirmed alveolar SCC (star) and the bony erosion (arrow).
Cancer of the oropharynx
Oropharyngeal SCCs are usually poorly differentiated and locally advanced at the time of clinical presentation. The overall incidence of cervical lymph node metastases is 50–70%[3]. Submucosal tumour extension within the oropharynx is common. Extension across the midline usually precludes surgical cure in tongue base tumours. The commonest sites of cancers in the oropharynx are the tonsils and the tongue base[7]. Tonsils are a prime site for lymphoma.
T-staging of oropharyngeal cancer (TNM 2009)
T-staging of oropharyngeal cancer depends on tumour size and local infiltration. Tx when the primary tumour cannot be assessed, T1 tumours are less than 2 cm in the greatest dimension, T2 between 2 and 4 cm, T3 more than 4 cm. T4a tumours show invasion of the larynx, deep/extrinsic muscles of the tongue, medial pterygoid muscle, hard palate and mandible. T4b tumours show invasion of the lateral pterygoid muscles, pterygoid plates, lateral nasopharynx, skull base or encasement of the internal carotid artery.
The lymph nodes in the upper jugular and spinal accesssory chain and the retropharyngeal nodes are most commonly involved by oropharyngeal cancers. Oropharyngeal metastases most commonly are to level I–IV with level II being the most common site. Nodal metastases to both sides of the neck are common because of rich lymphatics.
Tonsils
Tumours arising from the tonsillar pillars and the tonsils are grouped together. SCC of the tonsils is the most common tumour in this region followed by lymphoma (Fig. 2). Nodal drainage of tonsils is primarily unilateral to levels II and III. Tonsillar carcinoma can present as unilateral metastatic lymph nodes[2]. It is prone to spread posterolateraly to the lateral pharyngeal wall, parapharyngeal space and pterygoid muscles, inferiorly to the glossotonsillar sulcus, base of the tongue, floor of the mouth and superiorly to the soft palate and nasopharynx.
Base of the tongue
The base of the tongue is the posterior third of the tongue and it extends inferiorly to end at the level of the vallecula and houses the lingual tonsil. Tumours here are often occult and asymptomatic, the lesions are often large by the time they cause symptoms such as dysphagia or otalgia. Some patients present with lymph node metastases without signs of a primary tumour. Invasion can be deep or the lesions can be exophytic and protrude into the airway. Small lesions are difficult to detect due to the surrounding lymphoid tissue which also enhances[2].
SCC in this location is an aggressive, deeply infiltrative tumour with a 75% incidence of lymph node metastases at presentation. It may extend laterally to involve the mandible and medial pterygoid muscles, superiorly to involve the tonsillar fossa and soft palate, anteriorly to involve the mobile tongue and floor of the mouth and inferiorly to involve the vallecula, preepiglottic space, and epiglottis or portions of the hypopharynx. Neoplasms and normal lymphoid tissue at the base of the tongue can be confused at both CT and MRI because of their T2 signal characteristics and enhancement patterns, and deep endoscopic biopsies are the only way to differentiate between the two. In addition to routine axial images, sagittal and coronal MR images may provide an excellent appreciation of the volume of the tumour in the tongue base[3]. Cancer of the base of the tongue can invade the preepiglottic fat space and this may require a supraglottic laryngectomy if not a total laryngectomy (Fig. 9). Since the combined resection of the base of the tongue and larynx diminishes the quality of life with compromised swallowing and speaking, the correct diagnosis is very important[3]. The preepiglottic fat is best assessed with sagittal T1-weighted scans and axial T1-weighted scans in which soft tissue signal intensity within the fat suggests cancerous involvement. Occasionally, adjacent inflammation or peritumoural oedema or partial volume effects might simulate preepiglottic fat invasion[7].
Figure 9.
Axial CT image (A) in a patient with proven SCC of the base of the tongue showing the tumour (arrows) with central necrosis and a necrotic lymph node on the right side (star). The tumour extends into the valleculae on both sides and displaces the epiglottis posteriorly. Sagittal reconstruction (B) showing the tumour (star) with infiltration of the preepiglottic space (arrow).
Lymph nodes commonly involved are level II to IV nodes. Involvement can be bilateral due to rich lymphatics and can be seen in up to 30% of patients[2].
Posterior pharyngeal wall
Carcinomas of the posterior oropharyngeal wall have the worst prognosis of all squamous cell oral cavity and oropharyngeal carcinomas[3]. These tumours spread both in a caudal direction into the hypopharynx and a cranial direction into the nasopharynx. They commonly present as a flat, thick tumour spreading at the mucosal surface usually involving both the oropharynx and hypopharynx and are readily diagnosed on endoscopy. On axial CT or MR images, they appear as thickening of the posterior wall of the pharynx (Fig. 10). They commonly spread submucosally, invading the retropharyngeal fat. The prevertebral fascia acts as a barrier to tumour spread and invasion of the prevertebral muscles is uncommon at initial presentation. Invasion of the prevertebral muscles, the longus colli and longus capiti muscles precludes surgical resection. This can, however, be difficult to assess since abnormal signal intensity, attenuation values and enhancement of the muscles which could indicate involvement can be misleading, and the surgical evaluation at the time of panendoscopy or open exploration remains the gold standard[7]. Demonstration of a retropharyngeal fat plane has a high negative predictive value for tumour involvement[5].
Figure 10.
Axial (A) and sagittal (B) T1-weighted MR images after contrast injection with fat saturation showing the enhancing tumour of the posterior pharangeal wall presenting as a submucosal mass crossing the midline.
Soft palate
Soft palate tumours present as soft tissue swelling and can be difficult to assess radiologically unless they are large (Fig. 11). They can spread laterally, anteriorly or superiorly. Minor salivary gland tumours are often associated with perineural spread and may extend along the lesser palatine nerve to involve the pterygopalatine fossa or along the trigeminal nerve branches to the cavernous sinus and Meckel cave. Neural involvement may be discontinuous[5].
Figure 11.
Axial T2-weighted MR image (A) and axial T1-weighted image (B) after contrast injection with fat suppression showing the large soft palate tumour (star) with enlargement of the retropharyngeal lymph nodes on both sides (arrows) in a patient with cancer of the uvula.
Bone erosion is an unusual finding and is present only in advanced soft palate carcinoma invading the hard palate. Extensive invasion of the parapharyngeal and masticator spaces may be associated with carotid artery encasement or extension superiorly along the fascial and muscle planes into the skull base. If the tumour encompasses more than 270 degrees of the circumference of the carotid artery on axial images it becomes unlikely that it can be removed without resecting the vessel. Lymph node metastases occur primarily in the upper jugular or retropharyngeal nodes. It is present in 60% of tumours at presentation[2].
Disease specific follow-up
The current goal of treatment is to achieve locoregional control with chemotherapy, radiotherapy and surgery to enable organ preservation with maintenance of function[5]. Cancers of the oral cavity and oropharynx can recur locally, present with recurrent lymph node metastases or less commonly distant metastases. Follow-up imaging should be performed at regular intervals for the first 2 years. It is advisable to wait 3 months after completion of therapy before performing morphological imaging studies.
Radiological features indicating recurrence include a mass-like lesion with or without enhancement, abnormality along the margins of previous resection or reconstruction, bone invasion and perineural spread. Clinical and radiological assessment is difficult due to treatment changes such as oedema, fibrosis and distortion of anatomy due to surgery. Newer imaging techniques have already shown promising results in the detection of tumour recurrence where conventional imaging failed. These techniques are described briefly in the next section.
Newer imaging modalities
[18F]Fluorodeoxyglucose-positron emission tomography/CT
[18F]Fluorodeoxyglucose (FDG)-positron emission tomography (PET)/CT is a well-established tool for the assessment of both lymph node involvement and recurrences. It has been found superior to conventional imaging workup in the evaluation of patients with head and neck malignancies. It can be used in pretreatment staging, treatment monitoring and evaluation of the treatment response. Current clinical practice, however, does not favour the use of PET in staging of all newly diagnosed SCCs, it can, however, detect metastases and other primary tumours more than 1 cm in size[12]. Inflammation cannot be differentiated from tumour and specificity can further be reduced due to physiological uptake in lymphoid tissue, nasal mucosa, salivary glands and muscle activity.
Diffusion-weighted MR imaging
This relies on the relative movement of water in different biological tissues. In normal tissues or tissues with vasogenic oedema, there is no limitation of water diffusion. In tissue with cytotoxic oedema or hypercellular areas, there is diffusion restriction which can be assessed both qualitatively and quantitatively. While conventional MR imaging is usually adequate for demonstrating the primary tumour before treatment, diffusion-weighted imaging can aid in the post-treatment evaluation since post-treatment changes can be difficult to delineate from small foci of tumour recurrence as they can both enhance similarly when using conventional MR or CT. Post-treatment tissue is oedematous and relatively hypocellular and thus does not demonstrate restricted diffusion, whereas tumour recurrence contains areas of increased cellularity and should demonstrate restriction of diffusion[12–14]. The reported sensitivity, specificity and accuracy for detecting malignancy were 94.6%, 95.9% and 95.5%, respectively[13].
Perfusion imaging
This can be performed with CT or MRI and evaluates dynamic microscopic blood flow changes through a region of interest. Changes in signal intensity (MRI) or attenuation (CT) are measured during a dynamic contrast infusion[12]. Oropharyngeal tumour tissues reportedly demonstrate increased blood volume and blood flow with decreased times in comparison with normal tissues[15]. Blood flow and blood volume within recurrent tumour tissue are also reported to be elevated in comparison with therapy-altered tissue with corresponding decreases in transit time[16].
Conclusion
Treatment of patients with cancer of the oral cavity and oropharynx is a challenge for the head and neck surgeon and oncologist. Therefore, accurate diagnosis and staging of these cancers are prerequisites for choice of optimal treatment. CT and MRI are excellent imaging techniques for tumour detection and for providing information on the exact extension.
The evaluation of the neck after treatment, however, poses a special problem using CT and MRI. In this particular setting newer modalities such as PET-CT, diffusion-weighted MRI and perfusion imaging either with CT or MR are promising tools for detection of residual tumour or recurrence. However, larger scale studies are required to confirm these initial promising results.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer. 1999;80:827–41. doi: 10.1002/(sici)1097-0215(19990315)80:6<827::aid-ijc6>3.0.co;2-p. doi:10.1002/(SICI)1097-0215(19990315)80:6<827::AID-IJC6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 2.Stambuk HE, Karimi S, Lee N, Patel SG. Oral cavity and oropharynx tumors. Radiol Clin N Am. 2007;45:1–20. doi: 10.1016/j.rcl.2006.10.010. doi:10.1016/j.rcl.2006.10.010. PMid:17157621. [DOI] [PubMed] [Google Scholar]
- 3.Becker M. Oral cavity, oropharynx and hypopharynx. Semin Roentgenol. 2000;1:21–30. doi: 10.1016/s0037-198x(00)80029-2. [DOI] [PubMed] [Google Scholar]
- 4.Kirsch C. Oral cavity cancer. Top Magn Reson Imaging. 2007;18:269–80. doi: 10.1097/RMR.0b013e3181572caa. doi:10.1097/RMR.0b013e3181572caa. PMid:17893592. [DOI] [PubMed] [Google Scholar]
- 5.Zima AJ, Wesolowski JR, Ibrahim M, Lassig AAD, Lassig J, Mukerji SK. Magnetic resonance imaging of oropharyngeal cancer. Top Magn Reson Imaging. 2007;18:237–42. doi: 10.1097/RMR.0b013e318157112a. doi:10.1097/RMR.0b013e318157112a. PMid:17893589. [DOI] [PubMed] [Google Scholar]
- 6.Sigal R, Zagdanski A-M, Schwaab G, et al. CT and MR imaging of squamous cell carcinoma of the tongue and floor of the mouth. Radiographics. 1996;16:787–810. doi: 10.1148/radiographics.16.4.8835972. [DOI] [PubMed] [Google Scholar]
- 7.Yousem DM, Chalian AA. Oral cavity and pharynx. Radiol Clin N Am. 1998;36:967–81. doi: 10.1016/s0033-8389(05)70071-3. doi:10.1016/S0033-8389(05)70071-3. [DOI] [PubMed] [Google Scholar]
- 8.Sigal R, Monnet O, de Baere T, et al. Adenoid cystic carcinoma of the head and neck: evaluation with MR imaging and clinical-pathologic correlation in 27 patients. Radiology. 1992;184:95–101. doi: 10.1148/radiology.184.1.1319079. [DOI] [PubMed] [Google Scholar]
- 9.Yuen APW, Lam KY, Wei WI, et al. A comparison of the prognostic significance of tumor diameter, length, width, thickness, area, volume, and clinicopathological features of oral tongue carcinoma. Am J Surg. 2000;180:139–43. doi: 10.1016/s0002-9610(00)00433-5. doi:10.1016/S0002-9610(00)00433-5. [DOI] [PubMed] [Google Scholar]
- 10.Fukano H, Matsuura H, Hasegawa Y, Nakamura S. Depth of invasion as a predictive factor for cervical lymph node metastasis. Head Neck. 1997;19:205–10. doi: 10.1002/(sici)1097-0347(199705)19:3<205::aid-hed7>3.0.co;2-6. doi:10.1002/(SICI)1097-0347(199705)19:3<205::AID-HED7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Yuen APW, Lam KY, Lam LK, et al. Prognostic factors of clinically stage I and II oral tongue carcinoma – a comparative study of stage, thickness, shape, growth pattern, invasive front malignancy, grading, Martinez-Gimeno score, and pathologic features. Head Neck. 2002;24:513–20. doi: 10.1002/hed.10094. doi:10.1002/hed.10094. [DOI] [PubMed] [Google Scholar]
- 12.Shah GV, Wesolowski JR, Ansari SA, Mukherji SK. New directions in head and neck imaging. J Surg Onk. 2008;97:644–8. doi: 10.1002/jso.21022. doi:10.1002/jso.21022. PMid:18493943. [DOI] [PubMed] [Google Scholar]
- 13.Vandecaveye V, Keyzer FD, Nuyts S, et al. Detection of head and neck squamous cell carcinoma with diffusion weighted MRI after (chemo)radiotherapy: correlation between radiologic and histopathologic findings. Int J Radiat Oncol Biol Phys. 2007;67:960–71. doi: 10.1016/j.ijrobp.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 14.Razek AAKA, Kandeel AY, Soliman N, et al. Role of diffusion-weighted echo-planar MR imaging in differentiation of residual or recurrent head and neck tumors and posttreatment changes. Am J Neuroradiol. 2007;28:1146–52. doi: 10.3174/ajnr.A0491. doi:10.3174/ajnr.A0491. PMid:17569975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gandhi D, Hoeffner EG, Carlos RC, et al. Computed tomography perfusion of squamous cell carcinoma of the upper aerodigestive tract: initial results. J Comput Assist Tomogr. 2003;27:687–93. doi: 10.1097/00004728-200309000-00005. doi:10.1097/00004728-200309000-00005. PMid:14501359. [DOI] [PubMed] [Google Scholar]
- 16.Bisdas S, Baghi M, Smolarz A, et al. Quantitative measurements of perfusion and permeability of oropharyngeal and oral cavity cancer, recurrent disease and associated lymph nodes using first pass contrast-enhanced computer tomography studies. Invest Radiol. 2007;42:172–9. doi: 10.1097/01.rli.0000252496.74242.0b. doi:10.1097/01.rli.0000252496.74242.0b. PMid:17287647. [DOI] [PubMed] [Google Scholar]