Abstract
Following the submission of this article to Cancer Imaging, unfortunately the European manufacturer of ferumoxtran-10 (Guerbet) has withdrawn the product pending further phase III studies. This is secondary to the view of the Committee for Medicinal Products for Human Use that the phase III data did not provide adequate statistical demonstration of the product's efficacy.
Magnetic resonance lymphography holds much promise for the non-invasive evaluation of lymph nodes. The technique utilizes ultrasmall superparamagnetic particles of iron oxide and has been shown to be highly sensitive and specific in the diagnosis of malignant lymph nodes. This article reviews the technique and the performance of magnetic resonance lymphography in studies to date; alternative newer methods of nodal assessment such as fluorodeoxyglucose-positron emission tomography/computed tomography and diffusion-weighted magnetic resonance imaging are also discussed, with emphasis on gynaecological malignancies.
Keywords: Ultrasmall particles of iron oxide, nanoparticles, lymph node metastases, magnetic resonance imaging, diffusion-weighted imaging, PET/CT, cervical cancer, endometrial cancer, vulval cancer, treatment planning
Introduction
Computed tomography (CT) and magnetic resonance imaging (MRI) are the present mainstay of nodal imaging and non-invasive nodal staging but the inherent pitfall of these techniques remains their reliance on nodal size as the main diagnostic criterion. In recent years, magnetic resonance lymphography (MRL) using ultrasmall superparamagnetic particles of iron oxide (USPIO) has emerged as a promising new technique for evaluation of lymph nodes[1]. There is wide-ranging debate on the role of surgical lymphadenectomy in patients with endometrial cancer and thus the need for non-invasive staging becomes ever more important[2,3].
In this article, the importance of accurate nodal evaluation of gynaecological malignancies is highlighted, the technique and interpretation of MRL are reviewed and the potential use of MRL in clinical practice is discussed. Other new techniques such as fluorodeoxyglucose (FDG)-positron emission tomography(PET)/computed tomography and diffusion-weighted imaging (DWI) in nodal assessment are also discussed.
Why is nodal assessment important in cancer management?
Accurate knowledge of the metastatic involvement of regional and distant lymph nodes is crucial in determining the prognostic status[4]. In patients with cervical cancer, survival rates for surgically treated stage IB to IIA disease drop from 85–90% to 50–55% in the presence of lymph node involvement[5]. Similarly, in vulval cancer, the 5-year survival rate is 90% when no nodes are involved, compared with approximately 50% in a patient with positive nodes[6]. Nodal status also has an important impact on planning treatment.
Selecting the primary treatment modality
In cervical cancer, small volume disease confined to the cervix is usually treated surgically. If there is disease beyond the cervix (including cases of suspected nodal involvement), primary chemoradiation is the treatment of choice[7].
Radiotherapy planning
In patients treated with primary chemoradiation, precise mapping of involved nodes can direct the radiotherapy field contour to reduce toxicity to the normal surrounding tissues, such as small bowel and bladder[8].
Operative planning
Knowledge of the sites of lymph node involvement may guide the extent of lymph node dissection, thus reducing the time of surgery, complication rates and associated morbidity. It may also guide lymph node sampling, particularly if nodes are found to lie outside the standard nodal dissection field. Furthermore, if there is no nodal involvement, unnecessary radical surgery can be avoided[9].
Why is there a need for non-invasive nodal assessment?
The gold standard for nodal assessment is surgical lymphadenectomy. However, this results in prolonged surgical time and increases morbidity from associated complications such as lymphoedema. The patient outcomes and surgical complications have been evaluated recently in a large randomized study in the United Kingdom, in which 1408 patients with stage I, surgically treatable endometrial carcinoma were randomized into either having standard surgery (hysterectomy, bilateral salpingo-oophorectomy, peritoneal washings, para-aortic lymph node palpation) or surgery plus lymphadenectomy[2]. The study revealed that there is no significant benefit in overall or recurrence-free survival with pelvic lymphadenectomy. These results corroborated the findings from a similar randomized trial by Benedetti et al.[3] that involved 514 patients with stage I endometrial carcinoma. Surgical lymphadenectomy requires highly specialist surgical input, which may not be available at all centres[2,10]. Thus, a non-invasive method for nodal detection is particularly important for determining prognosis and treatment planning.
The non-invasive assessment of nodes has largely centred on CT and MRI, which provide good anatomical localization but rely on size criteria and node morphology to distinguish metastatic and benign nodes. Morphologic criteria are helpful when present; a large fatty hilum or dense calcification is suggestive of benign disease, whereas central necrosis and irregular contour are more indicative of malignant involvement[11,12]. With respect to size criteria, the cut-off for malignant nodal involvement varies depending on anatomical location. In general, nodes less than 10 mm in short axis diameter are considered benign in pelvic disease[13]. A size ratio may also be used, whereby if the short axis diameter is less than 8 mm, the node is considered benign; if the short axis is greater than 10 mm, it is considered malignant; if the short axis is between 8 and 10 mm, then the ratio of short axis to long axis is measured. If the ratio is greater than 0.8 (in other words a round node), then the node is considered malignant[14].
However, it has been shown that size criteria are unreliable. In cervical cancer, stages IB–IV, 80% of nodal metastases are less than 10 mm in short axis[15]. Similarly, nodal metastases in prostate cancer are mainly found in lymph nodes with short axis less than 8–10 mm, resulting in size and shape criteria producing low sensitivities of 36–40%[16,17].
Thus, CT and MRI are limited in their ability to detect metastatic disease in non-enlarged lymph nodes, and (to some extent) in distinguishing metastatic and benign enlarged lymph nodes. The reported sensitivities of CT and MRI in pelvic malignancies range from 40 to 87% with specificities between 64 and 100%[18,19].
For these reasons, alternative, accurate, reliable non-invasive techniques have been sought to assess nodal status. These newer imaging methods include PET-CT, DWI-MRI and MRL.
FDG-PET/CT and DWI-MRI: performance in nodal assessment of gynaecological malignancies
FDG-PET/CT
In the late 1990s, PET with [18F]FDG emerged as a novel imaging technique that allowed detection of tumours based on metabolic activity. Its inherent pitfall was its lack of anatomic detail. This was overcome by integrating PET with CT. FDG-PET/CT is now a routine imaging technique for a variety of tumours including lymphoma and lung cancer. There have been several studies evaluating its potential role in nodal assessment of gynaecological malignancies. Early reports were encouraging. Reinhardt et al.[20] performed a study comparing the diagnostic accuracy of MRI with FDG-PET in the detection of metastatic lymph nodes in 35 patients with stage IB or II cervical cancer, prior to radical hysterectomy and lymphadenectomy. On a patient basis, they obtained sensitivities of 0.91 on FDG-PET and 0.73 on MRI and specificities of 1.0 on FDG-PET and 0.83 on MRI. On a patient basis, the positive predictive values (PPV) of PET and MRI were not statistically different (1.0 versus 0.67) However, when the number of metastatic nodal sites were compared, there was a significant improvement in PPV using PET (0.90 on PET versus 0.64 on MRI, p < 0.05). In a more recent study, 60 patients with early stage cervical cancer (stage IA2–IIA) who were deemed not to have nodal metastases on MRI underwent preoperative FDG-PET[21]. Of 10 histologically confirmed nodal metastases, only one was detected on FDG-PET. The metastatic focus within the node measured 5×6 mm. The nodes that proved to be false-negatives contained metastatic deposits up to 6×7 mm in diameter. In contrast, Sironi et al.[22] found that for overall patient-based assessment, FDG-PET/CT had a sensitivity of 73%, specificity of 97% and PPV of 92% for detection of malignant nodes in early stage cervical carcinoma. This group also found that the threshold size for detection of metastatic nodes on FDG-PET/CT was 5 mm; below this size, FDG-PET/CT became unreliable. A meta-analysis of publications evaluating the use of FDG-PET in early cervical cancer showed a pooled sensitivity and specificity of 79% and 99% compared with 72% and 96% for MRI[23]. Conflicting results from studies performed in early stage cervical carcinoma imply that FDG-PET/CT does not have a clear role in these cases, most likely due to the presence of metastases in small nodes in this subset of patients.
In advanced stage cervical cancer (higher than stage IB), FDG-PET/CT has been found to have a more valuable role. These patients are more likely to have para-aortic lymph node involvement and several authors have reported improved sensitivities in the detection of para-aortic nodal metastases using FDG-PET compared with CT alone[24,25].
There have been a small number of studies investigating the use of FDG-PET/CT in endometrial cancer. Kitajima et al.[26] performed FDG-PET/CT in 40 patients with stage I endometrial cancer, all of whom subsequently underwent pelvic lymphadenectomy with or without para-aortic lymhadenectomy. They found that overall, PET/CT was only moderately sensitive (53%) but very specific (99.6%). The sensitivity for detection of nodes less than 4 mm was 16.7% and this improves to 93.3% in nodes larger than 10 mm. The same group have also evaluated the accuracy of PET/CT using contrast-enhanced CT with similar results[27].
DWI-MRI
Diffusion-weighted MRI is reliant on differences in molecular water mobility in extracellular spaces and provides information about the cellularity of tissue. Studies that have assessed the use of DWI-MRI for determination of nodal status have yielded mixed results. Nakai et al.[28] did not observe a significant difference between benign and malignant nodes at 1.5 T. However, they did note that DWI-MRI can aid in the detection of lymph nodes. This is in contrast to Lin et al.[29] who observed a significant difference in the apparent diffusion coefficient values at 3 T. Thoeny et al.[30] have evaluated the use of MRL and DW-MRI in patients with bladder and prostate cancer using a combined method. The advantage of this technique appears to be easier identification of lymph nodes which can then be characterized on the MRL images. This work is described in more detail later in the section on the future developments of MRL.
MRL technique and interpretation
MRL uses a lymph node specific contrast agent, ferumoxtran-10, that is comprised of ultrasmall superparamagnetic particles of USPIO. These 30–50 nm nanoparticles, coated with a dextran to prolong circulation time, are administered intravenously where they extravasate into the interstitial space and are transported to lymph nodes via the lymphatic system. Approximately 24–36 h post contrast administration, macrophages in normal lymph nodes internalize the USPIO particles, resulting in a drop in signal intensity on T2*W sequences due to a susceptibility effect of the iron oxide. Metastatic nodes with tumour cells infiltrating and replacing macrophages do not produce this drop in signal[1,31].
Although MRL scanning protocols vary between institutions, patients in our institution attend the MRI department twice. The initial MRI incorporates standard high-resolution sequences for staging the primary tumour. The standard staging sequences are then supplemented with additional axial and axial oblique sequences, using the iron-sensitive T2*W sequence for the nodal staging, and iron-insensitive T1W images to detect any fatty nodal hila. The field of view for the nodal staging sequences corresponds to lymph node drainage of the tumour. For endometrial, cervical and upper vaginal cancers, axial images along the line of the iliac vessels are performed along with oblique planes parallel to the psoas muscles. In lower vaginal and vulval carcinoma, the field of view is adjusted to include the inguinal regions and includes a coronal T2*W image to help with surgical correlation of the nodes. Following intravenous infusion of the USPIO contrast medium, the patient re-attends for a second scan in 24–36 h, when the nodal imaging sequences are repeated identically to allow direct comparison between the pre- and post-contrast images[1].
There are variations in the uptake patterns for benign and malignant nodes (Tables 1 and 2)[32]. Generally, benign nodes show homogenous or mildly heterogeneous signal loss post contrast (Fig. 1). Malignant nodes show no signal loss, which is either diffuse or focal (Figs. 2 and 3)[33–35].
Table 1.
Various described patterns of USPIO uptake and their interpretation in benign nodes (reprinted with permission © RSNA. Narayanan P, Iyngkaran T, Sohaib SA, Reznek RH, Rockall AG. Pearls and pitfalls of MR lymphography in gynecologic malignancy. Radiographics 2009; 29: 1057–69)
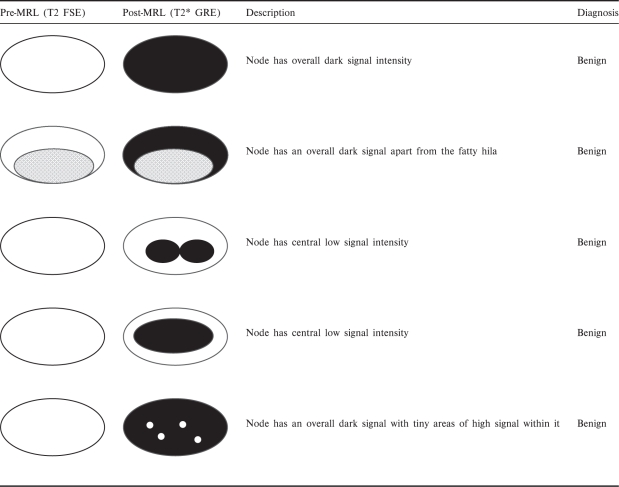 |
Table 2.
Various described patterns of USPIO uptake and their interpretation in metastatic nodes (reprinted with permission © RSNA. Narayanan P, Iyngkaran T, Sohaib SA, Reznek RH, Rockall AG. Pearls and pitfalls of MR lymphography in gynecologic malignancy. Radiographics 2009; 29: 1057–69)
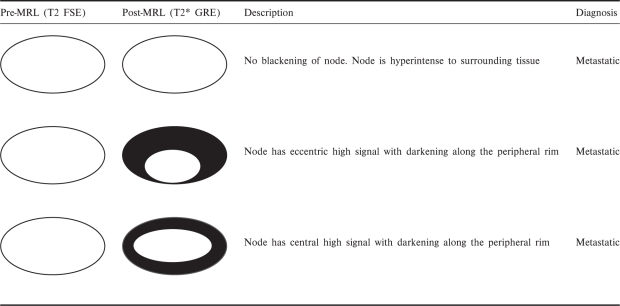 |
Figure 1.
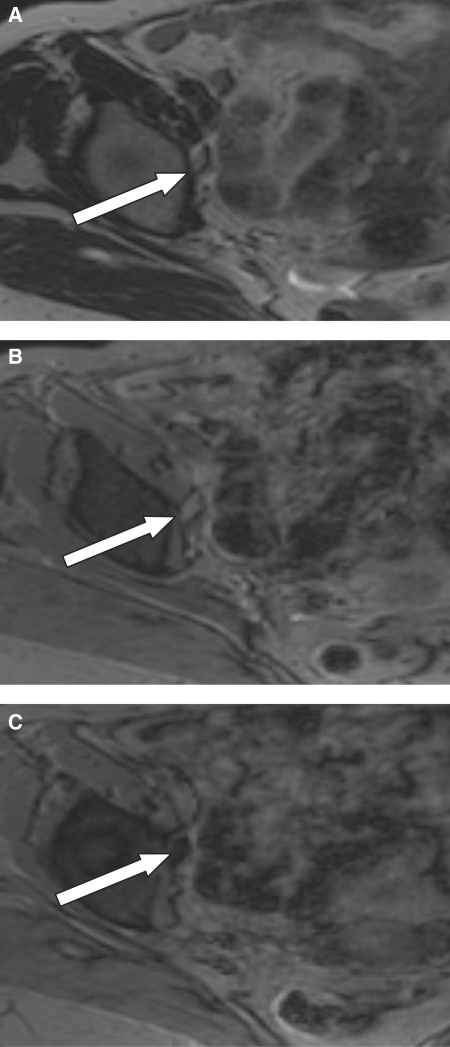
An 86-year-old patient with vulval carcinoma. A small right obturator lymph node is demonstrated on (a) axial T2W and (b) axial pre-USPIO T2*W images (white arrows). (c) Axial post-contrast T2*W image demonstrating that the node returns a homogeneous low signal intensity following the administration of USPIO. The appearances are consistent with a benign node.
Figure 2.
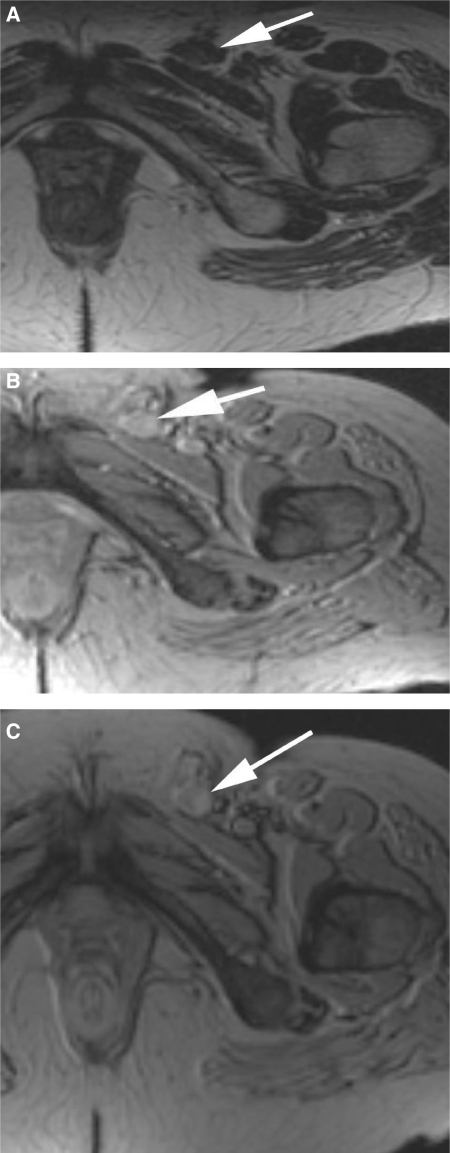
A 61-year-old patient with vulval carcinoma. (a) Axial T2W image shows a left inguinal lymph node that measured just over 1 cm in short axis (white arrow). (b) The node on the precontrast T2*W study. (c) Following USPIO administration, the axial T2*W image shows there is no uptake of contrast agent resulting in a uniformly bright node. The appearances are consistent with a malignant node which was histologically proven.
Figure 3.
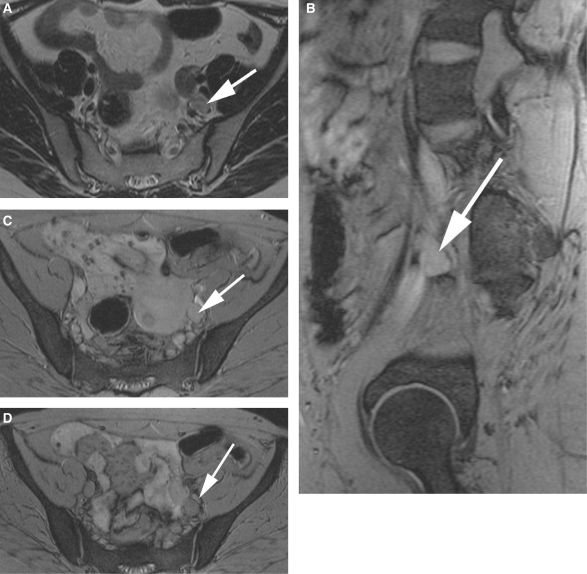
A 55-year-old patient with endometrial carcinoma. A left interiliac node is seen on the axial T2W image (a; white arrow). On the pre-USPIO T2*W sequences, the node is identifiable (white arrows) on both the sagittal (b) and axial images (c). Following contrast administration, there is no evidence of contrast uptake within this node (d; white arrow) in keeping with a malignant node.
Performance of MRL
This non-invasive imaging modality can be performed in the outpatient setting, making it easily accessible. Various studies have shown that it has a high sensitivity and specificity for detection of metastatic lymph nodes. A meta-analysis by Will et al.[36] in 2006 showed an overall sensitivity of 88% and a specificity of 96% for all tumour types; MRL was shown to have a higher diagnostic accuracy compared with standard MRI. Table 3 provides an overview of studies that have investigated the performance of MRL in pelvic malignancies[30,37–41].
Table 3.
Studies on the performance of MRL in pelvic malignancies
| Primary malignancy | Study type/number of patients | Performance of MRL | Reference |
|---|---|---|---|
| Genitourinary | Prospective/30 | Sensitivity 100%; specificity 80% | Bellin et al.[37], 1998 |
| Prostate | Prospective comparison with contrast-enhanced CT+standard MRI/80 | Sensitivity improved from 35.4% to 90.5% (node by node basis) and 100% (patient by patient basis); specificity improved from 90.4% to 97.8% | Harisinghani et al.[38], 2003 |
| Gynaecological | Prospective/9 | Sensitivity 33%, specificity 99% (node by node basis); sensitivity 25%, specificity 80% (patient by patient basis) | Keller et al.[39], 2004 |
| Gynaecological | Prospective comparison with standard MRI/44 | Sensitivity improved from 29% to 82–93% (node by node basis) and from 27% to 91–100% (patient by patient basis); specificity >95% maintained | Rockall et al.[40], 2005 |
| Prostate | Prospective comparison with contrast-enhanced CT/375 | Sensitivity improved from 34% to 82%; specificity changed from 97% to 93% (patient by patient basis) | Heesakkers et al.[41], 2008 |
| Bladder and prostate | Prospective/21 | Sensitivity 80%, specificity 73% (patient by patient basis) | Thoeny et al.[30], 2009 |
The low sensitivities of MRL seen in the early study by Keller et al.[39] may have been due to low patient numbers and total nodal metastases. Low sensitivities using size criteria in the study by Rockall et al.[40] probably reflects the early disease stage of many of the patients, with few nodes measuring above the threshold for size criteria.
The slightly worse performance of MRL in the study by Thoeny et al.[30] compared with the work of Harisinghani et al.[38] may be explained by differences in observer experience in MRL; in addition, the former study recruited fewer patients with advanced disease, which may influence the diagnostic performance.
Disadvantages and pitfalls of MRL
One of the drawbacks of MRL is the need for two attendances to the MRI department, particularly during the learning phase, thus potentially increasing cost. Furthermore, interpretation of images is time-consuming as node by node comparison has to be made between pre- and post-contrast imaging. Some of these problems may be overcome in the future by improvements in training and the use of DWI-MRI alongside the MRL.
Mild to moderate side effects, mainly headaches, flushing and back pain have been reported, but these are of short duration. Other reported adverse effects include urticaria, muscle cramps, diarrhoea and nausea. Vasovagal reactions and more severe rashes are very infrequent[30,40,42].
Diagnostic pitfalls of MRL have been described and may result in errors in interpretation[32]. These pitfalls are most often due to difficulty in characterization of nodes that have intermediate levels of uptake of the contrast rather than complete uptake (i.e. a black node) or non-uptake (i.e. a white node). Partial uptake patterns require the reader to make a judgment on whether the node is benign or malignant and this can result in diagnostic errors. It may be possible to overcome some of these errors by training and experience.
Pitfalls may result in false-positive and -negative results[1,41,43].
False-positive readings that may be overcome with training include the misinterpretation of a normal fatty hilum of a node (Fig. 4), or confusing a bright blood vessel for a lymph node. More challenging to overcome is the problem of misinterpretation of areas of hyperplasia or granulomatous fibrosis within a node which may result in reduced USPIO uptake and thus be considered malignant due to lack of signal loss on T2*W imaging[37,40,43].
Figure 4.
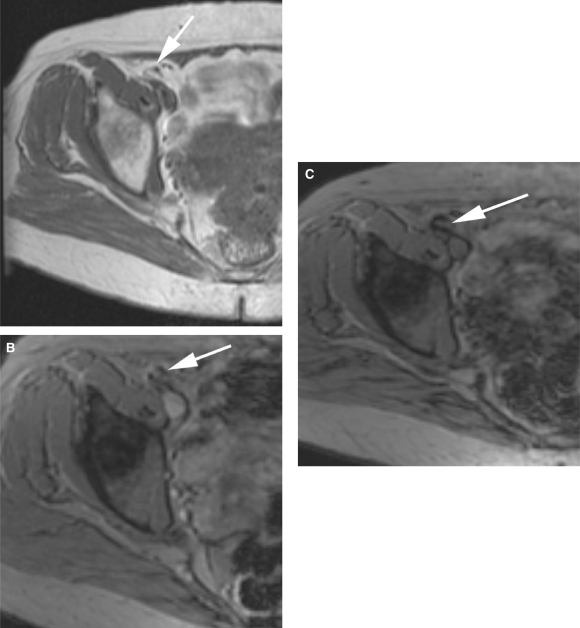
A pre-USPIO T1W image shows a right external iliac node with a fatty hilum (a; white arrow). (b) The node on a pre-USPIO T2*W image (white arrow). On a post-contrast image, the node is seen to have a focal area of high signal due to the fatty hilum (c; white arrow) and demonstrates a potential pitfall in the interpretation of MRL.
False-negative results in gynaecological malignancies have been shown to be caused by failure in identifying a malignant node in the parametrium[40]. Other reasons for false-negatives include the presence of micrometastases which are too small for the spatial resolution of MRI[38,39], and the blooming/susceptibility artefact, which produces an apparent enlargement of nodes on post-contrast T2*W imaging, potentially obscuring small metastatic foci.
Finally, if the post-contrast scan is performed before 24 h, there may be insufficient uptake of nanoparticles by normal macrophages, which may also lead to interpretation errors.
Future developments in MRL
Use of DWI
Thoeny et al.[30] recently addressed some of the issues associated with interpretation errors by studying whether the addition of DWI improved the diagnostic accuracy of MRL and reduced interpretation time. It has been shown that lymph nodes return a high signal intensity on DWI-MRI, and some publications have reported that malignant lymph nodes show restricted diffusion[20,22]. In Thoeny’s study, 20 patients had conventional MRI sequences at 3 T with DWI before and after USPIO administration, followed by extended pelvic lymphadenectomy at the time of their primary surgery. Image interpretation was then performed by a classic method by 2 experienced MR readers commencing with the pre- and post-USPIO sequences, followed by the combined USPIO-DWI sequences. This step-wise reading method was compared with a new reading method, by 3 independent readers, who analysed the USPIO-DWI images first for non-continuous hyperintense structures, then using the information from this to guide their MRL analysis.
The study reported that the addition of DWI significantly reduced the time of analysis from an average of 80 min in the classic method to 13 min in the new method, whilst the diagnostic accuracy remained comparable at 90%. Furthermore, there was significant inter-observer agreement between the readers in the new method, suggesting that interpretation using this technique is reproducible[30].
Although this novel technique appears promising and would substantially reduce reporting times, the results need to be substantiated by further larger studies. The effect of increased scanning time and cost-effectiveness also need to be considered.
Use of higher field strengths
A further development in the improvement of the diagnostic accuracy of MRL could be brought about by using higher magnetic field strengths. Heesakkers et al.[44] enrolled 48 patients with prostate cancer into a study that compared image quality between 1.5 T and 3.0 T ferumoxtran-10 enhanced MR imaging. They found that the 3.0 T images showed significantly improved muscle-fat contrast, vessel-fat contrast, lymph node border delineation and total image quality, thus suggesting that this may allow more accurate detection of small positive lymph nodes[44].
Use in delineating the primary tumour
There has been interest in the role of MRL in delineating the primary tumour. In a study by Laghi et al.[45] looking at patients with uterine malignancies, a significant reduction in myometrial and stromal signal intensity with MRL allowed better tumour definition. This may subsequently allow for more accurate T staging of the primary tumour. Although these findings are exciting and may have potential for tumour staging, larger studies will need to be performed in the future to substantiate the conclusions.
The future use of MRL in clinical practice
MRL has the potential to play an invaluable role in the management of gynaecological malignancies.
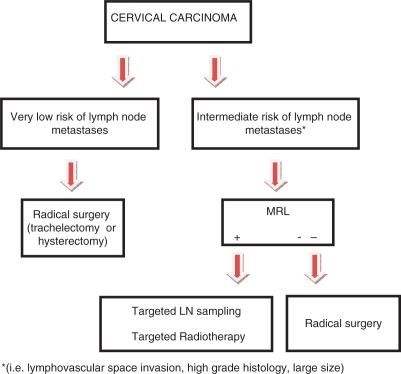
Cervical Cancer (Fig. 5)
Figure 5.
This flow diagram illustrates the potential place of MRL in the treatment planning of patients with cervical cancer.
In patients with early stage cervical cancer, including those who wish to undergo fertility-conserving surgery (trachelectomy), radical surgery should be undertaken only if there is organ-confined disease with no evidence of nodal metastases[46]. In some cases, when the tumour size is very small and the histology is low grade with no lymphovascular space invasion, the clinical team may wish to proceed with radical surgery without MRL, as the risk of nodal metastases is very low. However, if there is a clinical risk of nodal metastatic disease, then MRL may be used to rule out or identify nodal metastases. If MRL is negative, then radical surgery could be undertaken. If nodal metastasis is suspected on MRL, then targeted nodal sampling could be used to confirm this. Furthermore, MRL could detect nodes lying outside of the standard field of dissection, thus guiding lymph node dissection and sampling. Alternatively, the patient could be offered targeted nodal radiotherapy as part of a primary chemoradiotherapy regime. Thus, MRL could play an important role by potentially limiting the extent of lymph node dissection in patients with early stage cervical cancer and more accurately stratifying patients who are suitable for radical surgery or trachelectomy.
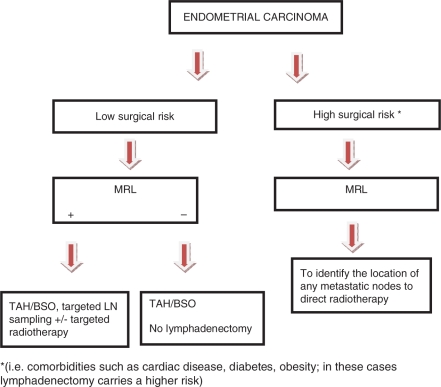
Endometrial cancer (Fig. 6)
Figure 6.
This flow diagram illustrates the potential place of MRL in the treatment planning of endometrial cancer.
As routine surgical lymphadenectomy has recently been shown to be of limited value in improving outcomes in patients with endometrial carcinoma[2,15], MRL could have a significant role in identifying metastatic nodes in both high- and low-risk surgical patients. This would improve prognostication in addition to allowing improved planning of radiotherapy treatment fields. Where surgical lymph node dissection is to be undertaken, MRL may direct the surgeon to any suspicious nodes.
Vulval cancer
Bilateral inguinal lymph node dissection is part of the standard management in patients with vulval carcinoma. However, it has been shown that between 75 and 90% of patients with early stage disease do not have lymph node involvement and undergo unnecessary surgery[9]. MRL could potentially limit or prevent such radical lymphadenectomy in this group of patients, although there has been limited research into the use of MRL in vulval cancer.
Conclusion
It is well established that a more precise, non-invasive method of nodal imaging in gynaecologic cancer is required. MRL, using USPIO, is one such technique that has been shown in a small number of studies to be highly sensitive and specific for nodal evaluation.
Standardised and formal training in image acquisition and image interpretation are vital to optimize the diagnostic capabilities of this imaging technique, and indeed, technical developments continue to be made. PET/CT and MRL are being jointly evaluated as tools for detection of nodal metastases in patients with gynaecological malignancy in two studies: the American College of Radiology Imaging Network (ACRIN) (cervix cancer), and a study supported by Cancer Research UK, in patients with cervix or endometrial cancer. Although there does appear to be a role for this technique in the near future, licensing is still awaited.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Narayanan P, Iyngkaran T, Sohaib SA, Reznek RH, Rockall AG. Magnetic resonance lymphography: a novel technique for lymph node assessment in gynecologic malignancies. Cancer Biomark. 2009;5:81–8. doi: 10.3233/CBM-2009-0543. [DOI] [PubMed] [Google Scholar]
- 2.Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373:125–36. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benedetti PP, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–16. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 4.Kosary CL. FIGO stage, histology, histologic grade, age and race as prognostic factors in determining survival for cancers of the female gynecological system: an analysis of 1973–87 SEER cases of cancers of the endometrium, cervix, ovary, vulva, and vagina. Semin Surg Oncol. 1994;10:31–46. doi: 10.1002/ssu.2980100107. doi:10.1002/ssu.2980100107. PMid:8115784. [DOI] [PubMed] [Google Scholar]
- 5.Kaur H, Silverman PM, Iyer RB, Verschraegen CF, Eifel PJ, Charnsangavej C. Diagnosis, staging, and surveillance of cervical carcinoma. AJR Am J Roentgenol. 2003;180:1621–31. doi: 10.2214/ajr.180.6.1801621. [DOI] [PubMed] [Google Scholar]
- 6.Ghurani GB, Penalver MA. An update on vulvar cancer. Am J Obstet Gynecol. 2001;185:294–9. doi: 10.1067/mob.2001.117401. doi:10.1067/mob.2001.117401. PMid:11518882. [DOI] [PubMed] [Google Scholar]
- 7.Hoskin PJ, Symonds P. Uterine malignancies. Clin Oncol. 2008;20:387. doi: 10.1016/j.clon.2008.05.001. doi:10.1016/j.clon.2008.05.001. PMid:18555672. [DOI] [PubMed] [Google Scholar]
- 8.Taylor A, Rockall AG, Reznek RH, Powell ME. Mapping pelvic lymph nodes: guidelines for delineation in intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:1604–12. doi: 10.1016/j.ijrobp.2005.05.062. doi:10.1016/j.ijrobp.2005.05.062. PMid:16198509. [DOI] [PubMed] [Google Scholar]
- 9.Cavanagh D, Hoffman MS. Controversies in the management of vulvar carcinoma. Br J Obstet Gynaecol. 1996;103:293–300. doi: 10.1111/j.1471-0528.1996.tb09731.x. [DOI] [PubMed] [Google Scholar]
- 10.Lagasse LD, Creasman WT, Shingleton HM, Ford JH, Blessing JA. Results and complications of operative staging in cervical cancer: experience of the Gynecologic Oncology Group. Gynecol Oncol. 1980;9:90–8. doi: 10.1016/0090-8258(80)90013-x. doi:10.1016/0090-8258(80)90013-X. PMid: . [DOI] [PubMed] [Google Scholar]
- 11.Yang WT, Lam WW, Yu MY, Cheung TH, Metreweli C. Comparison of dynamic helical CT and dynamic MR imaging in the evaluation of pelvic lymph nodes in cervical carcinoma. AJR Am J Roentgenol. 2000;175:759–66. doi: 10.2214/ajr.175.3.1750759. [DOI] [PubMed] [Google Scholar]
- 12.Brown G, Richards CJ, Bourne MW, et al. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology. 2003;227:371–7. doi: 10.1148/radiol.2272011747. doi:10.1148/radiol.2272011747. PMid:12732695. [DOI] [PubMed] [Google Scholar]
- 13.Vinnicombe SJ, Norman AR, Nicolson V, Husband JE. Normal pelvic lymph nodes: evaluation with CT after bipedal lymphangiography. Radiology. 1995;194:349–55. doi: 10.1148/radiology.194.2.7824709. [DOI] [PubMed] [Google Scholar]
- 14.Jager GJ, Barentsz JO, Oosterhof GO, Witjes JA, Ruijs SJ. Pelvic adenopathy in prostatic and urinary bladder carcinoma: MR imaging with a three-dimensional TI-weighted magnetization-prepared-rapid gradient-echo sequence. AJR Am J Roentgenol. 1996;167:1503–7. doi: 10.2214/ajr.167.6.8956585. [DOI] [PubMed] [Google Scholar]
- 15.Benedetti-Panici P, Maneschi F, Scambia G, et al. Lymphatic spread of cervical cancer: an anatomical and pathological study based on 225 radical hysterectomies with systematic pelvic and aortic lymphadenectomy. Gynecol Oncol. 1996;62:19–24. doi: 10.1006/gyno.1996.0184. doi:10.1006/gyno.1996.0184. PMid:8690286. [DOI] [PubMed] [Google Scholar]
- 16.Heesakkers RA, Jager GJ, Hovels AM, et al. Prostate cancer: detection of lymph node metastases outside the routine surgical area with ferumoxtran-10-enhanced MR imaging. Radiology. 2009;251:408–14. doi: 10.1148/radiol.2512071018. doi:10.1148/radiol.2512071018. PMid:19401573. [DOI] [PubMed] [Google Scholar]
- 17.Hovels AM, Heesakkers RA, Adang EM, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008;63:387–95. doi: 10.1016/j.crad.2007.05.022. doi:10.1016/j.crad.2007.05.022. PMid:18325358. [DOI] [PubMed] [Google Scholar]
- 18.Koh DM, Hughes M, Husband JE. Cross-sectional imaging of nodal metastases in the abdomen and pelvis. Abdom Imaging. 2006;31:632–43. doi: 10.1007/s00261-006-9022-2. doi:10.1007/s00261-006-9022-2. PMid:16897278. [DOI] [PubMed] [Google Scholar]
- 19.Williams AD, Cousins C, Soutter WP, et al. Detection of pelvic lymph node metastases in gynecologic malignancy: a comparison of CT, MR imaging, and positron emission tomography. AJR Am J Roentgenol. 2001;177:343–8. doi: 10.2214/ajr.177.2.1770343. [DOI] [PubMed] [Google Scholar]
- 20.Reinhardt MJ, Ehritt-Braun C, Vogelgesang D, et al. Metastatic lymph nodes in patients with cervical cancer: detection with MR imaging and FDG PET. Radiology. 2001;218:776–82. doi: 10.1148/radiology.218.3.r01mr19776. [DOI] [PubMed] [Google Scholar]
- 21.Chou HH, Chang TC, Yen TC, et al. Low value of [18F]-fluoro-2-deoxy-D-glucose positron emission tomography in primary staging of early-stage cervical cancer before radical hysterectomy. J Clin Oncol. 2006;24:123–8. doi: 10.1200/JCO.2005.03.5964. doi:10.1200/JCO.2005.03.5964. PMid:16382121. [DOI] [PubMed] [Google Scholar]
- 22.Sironi S, Buda A, Picchio M, et al. Lymph node metastasis in patients with clinical early-stage cervical cancer: detection with integrated FDG PET/CT. Radiology. 2006;238:272–9. doi: 10.1148/radiol.2381041799. doi:10.1148/radiol.2381041799. PMid:16304090. [DOI] [PubMed] [Google Scholar]
- 23.Havrilesky LJ, Kulasingam SL, Matchar DB, Myers ER. FDG-PET for management of cervical and ovarian cancer. Gynecol Oncol. 2005;97:183–91. doi: 10.1016/j.ygyno.2004.12.007. doi:10.1016/j.ygyno.2004.12.007. PMid:15790456. [DOI] [PubMed] [Google Scholar]
- 24.Sugawara Y, Eisbruch A, Kosuda S, Recker BE, Kison PV, Wahl RL. Evaluation of FDG PET in patients with cervical cancer. J Nucl Med. 1999;40:1125–31. [PubMed] [Google Scholar]
- 25.Loft A, Berthelsen AK, Roed H, et al. The diagnostic value of PET/CT scanning in patients with cervical cancer: a prospective study. Gynecol Oncol. 2007;106:29–34. doi: 10.1016/j.ygyno.2007.03.027. doi:10.1016/j.ygyno.2007.03.027. PMid:17482666. [DOI] [PubMed] [Google Scholar]
- 26.Kitajima K, Murakami K, Yamasaki E, et al. Accuracy of 18F-FDG PET/CT in detecting pelvic and paraaortic lymph node metastasis in patients with endometrial cancer. AJR Am J Roentgenol. 2008;190:1652–8. doi: 10.2214/AJR.07.3372. doi:10.2214/AJR.07.3372. PMid:18492920. [DOI] [PubMed] [Google Scholar]
- 27.Kitajima K, Murakami K, Yamasaki E, Kaji Y, Sugimura K. Accuracy of integrated FDG-PET/contrast-enhanced CT in detecting pelvic and paraaortic lymph node metastasis in patients with uterine cancer. Eur Radiol. 2009;19:1529–36. doi: 10.1007/s00330-008-1271-8. doi:10.1007/s00330-008-1271-8. PMid:19184037. [DOI] [PubMed] [Google Scholar]
- 28.Nakai G, Matsuki M, Inada Y, et al. Detection and evaluation of pelvic lymph nodes in patients with gynecologic malignancies using body diffusion-weighted magnetic resonance imaging. J Comput Assist Tomogr. 2008;32:764–8. doi: 10.1097/RCT.0b013e318153fd43. doi:10.1097/RCT.0b013e318153fd43. PMid:18830107. [DOI] [PubMed] [Google Scholar]
- 29.Lin G, Ho KC, Wang JJ, et al. Detection of lymph node metastasis in cervical and uterine cancers by diffusion-weighted magnetic resonance imaging at 3T. J Magn Reson Imaging. 2008;28:128–35. doi: 10.1002/jmri.21412. doi:10.1002/jmri.21412. PMid:18581404. [DOI] [PubMed] [Google Scholar]
- 30.Thoeny HC, Triantafyllou M, Birkhaeuser FD, et al. Combined ultrasmall superparamagnetic particles of iron oxide-enhanced and diffusion-weighted magnetic resonance imaging reliably detect pelvic lymph node metastases in normal-sized nodes of bladder and prostate cancer patients. Eur Urol. 2009;55:761–9. doi: 10.1016/j.eururo.2008.12.034. doi:10.1016/j.eururo.2008.12.034. PMid:19144456. [DOI] [PubMed] [Google Scholar]
- 31.Barentsz JO, Futterer JJ, Takahashi S. Use of ultrasmall superparamagnetic iron oxide in lymph node MR imaging in prostate cancer patients. Eur J Radiol. 2007;63:369–72. doi: 10.1016/j.ejrad.2007.06.025. doi:10.1016/j.ejrad.2007.06.025. PMid:17689215. [DOI] [PubMed] [Google Scholar]
- 32.Narayanan P, Iyngkaran T, Sohaib SA, Reznek RH, Rockall AG. Pearls and pitfalls of MR lymphography in gynecologic malignancy. Radiographics. 2009;29:1057–69. doi: 10.1148/rg.294085231. doi:10.1148/rg.294085231. PMid:19605656. [DOI] [PubMed] [Google Scholar]
- 33.Harisinghani MG, Saksena MA, Hahn PF, et al. Ferumoxtran-10-enhanced MR lymphangiography: does contrast-enhanced imaging alone suffice for accurate lymph node characterization? AJR Am J Roentgenol. 2006;186:144–8. doi: 10.2214/AJR.04.1287. doi:10.2214/AJR.04.1287. PMid:16357394. [DOI] [PubMed] [Google Scholar]
- 34.Saksena MA, Saokar A, Harisinghani MG. Lymphotropic nanoparticle enhanced MR imaging (LNMRI) technique for lymph node imaging. Eur J Radiol. 2006;58:367–74. doi: 10.1016/j.ejrad.2005.12.041. doi:10.1016/j.ejrad.2005.12.041. PMid:16472955. [DOI] [PubMed] [Google Scholar]
- 35.Koh DM, Brown G, Temple L, et al. Rectal cancer: mesorectal lymph nodes at MR imaging with USPIO versus histopathologic findings–initial observations. Radiology. 2004;231:91–9. doi: 10.1148/radiol.2311030142. doi:10.1148/radiol.2311030142. PMid:14976266. [DOI] [PubMed] [Google Scholar]
- 36.Will O, Purkayastha S, Chan C, et al. Diagnostic precision of nanoparticle-enhanced MRI for lymph-node metastases: a meta-analysis. Lancet Oncol. 2006;7:52–60. doi: 10.1016/S1470-2045(05)70537-4. doi:10.1016/S1470-2045(05)70537-4. PMid: [DOI] [PubMed] [Google Scholar]
- 37.Bellin MF, Roy C, Kinkel K, et al. Lymph node metastases: safety and effectiveness of MR imaging with ultrasmall superparamagnetic iron oxide particles–initial clinical experience. Radiology. 1998;207:799–808. doi: 10.1148/radiology.207.3.9609907. [DOI] [PubMed] [Google Scholar]
- 38.Harisinghani MG, Barentsz J, Hahn PF, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003;348:2491–9. doi: 10.1056/NEJMoa022749. doi:10.1056/NEJMoa022749. PMid:12815134. [DOI] [PubMed] [Google Scholar]
- 39.Keller TM, Michel SC, Frohlich J, Fink D, Caduff R, Marincek B, et al. USPIO-enhanced MRI for preoperative staging of gynecological pelvic tumors: preliminary results. Eur Radiol. 2004;14:937–44. doi: 10.1007/s00330-004-2258-8. doi:10.1007/s00330-004-2258-8. PMid:14991323. [DOI] [PubMed] [Google Scholar]
- 40.Rockall AG, Sohaib SA, Harisinghani MG, et al. Diagnostic performance of nanoparticle-enhanced magnetic resonance imaging in the diagnosis of lymph node metastases in patients with endometrial and cervical cancer. J Clin Oncol. 2005;23:2813–21. doi: 10.1200/JCO.2005.07.166. doi:10.1200/JCO.2005.07.166. PMid:15837995. [DOI] [PubMed] [Google Scholar]
- 41.Heesakkers RA, Hovels AM, Jager GJ, et al. MRI with a lymph-node-specific contrast agent as an alternative to CT scan and lymph-node dissection in patients with prostate cancer: a prospective multicohort study. Lancet Oncol. 2008;9:850–6. doi: 10.1016/S1470-2045(08)70203-1. doi:10.1016/S1470-2045(08)70203-1. PMid: [DOI] [PubMed] [Google Scholar]
- 42.Anzai Y, Piccoli CW, Outwater EK, et al. Evaluation of neck and body metastases to nodes with ferumoxtran 10-enhanced MR imaging: phase III safety and efficacy study. Radiology. 2003;228:777–88. doi: 10.1148/radiol.2283020872. doi:10.1148/radiol.2283020872. PMid:12954896. [DOI] [PubMed] [Google Scholar]
- 43.Saksena M, Harisinghani M, Hahn P, et al. Comparison of lymphotropic nanoparticle-enhanced MRI sequences in patients with various primary cancers. AJR Am J Roentgenol. 2006;187:W582–8. doi: 10.2214/AJR.05.0873. doi:10.2214/AJR.05.0873. PMid:17114509. [DOI] [PubMed] [Google Scholar]
- 44.Heesakkers RA, Futterer JJ, Hovels AM, et al. Prostate cancer evaluated with ferumoxtran-10-enhanced T2*-weighted MR Imaging at 1.5 and 3.0 T: early experience. Radiology. 2006;239:481–7. doi: 10.1148/radiol.2392050411. doi:10.1148/radiol.2392050411. PMid:16641354. [DOI] [PubMed] [Google Scholar]
- 45.Laghi A, Paolantonio P, Panebianco V, et al. Decrease of signal intensity of myometrium and cervical stroma after ultrasmall superparamagnetic iron oxide (USPIO) particles administration: an MR finding with potential benefits in T staging of uterine neoplasms. Invest Radiol. 2004;39:666–70. doi: 10.1097/00004424-200411000-00004. doi:10.1097/00004424-200411000-00004. PMid:15486527. [DOI] [PubMed] [Google Scholar]
- 46.Shepherd JH, Milliken DA. Conservative surgery for carcinoma of the cervix. Clin Oncol (R Coll Radiol) 2008;20:395–400. doi: 10.1016/j.clon.2008.05.002. [DOI] [PubMed] [Google Scholar]