Abstract
Both maternal diabetes and obesity have been associated with an increased risk of neural tube defects (NTD), possibly due to a sustained state of hyperglycemia and/or hyperinsulinemia. Data were collected in the Boston University Slone Birth Defects Study (a case-control study) from 1988 to 1998. The authors examined whether high dietary glycemic index (DGI) and high dietary glycemic load (DGL) increased the risk of NTDs in nondiabetic women. Mothers of NTD cases and nonmalformed controls were interviewed in person within 6 months after delivery about diet and other exposures. Odds ratios and 95% confidence intervals were estimated from logistic regression for high DGI (≥60) and high DGL (≥205), with cutpoints determined by cubic spline. Of 698 case mothers, 25% had high DGI and 4% had high DGL. Of 696 control mothers, 15% had high DGI and 2% had high DGL. After adjustment for sociodemographic factors and other dietary factors, the odds ratio for high DGI was 1.5 (95% confidence interval: 1.1, 2.0); for high DGL, it was 1.8 (95% confidence interval: 0.8, 4.0). Diets with proportionally high DGI or DGL may put the developing fetus at risk of an NTD, adding further evidence that hyperglycemia lies within the pathogenic pathway.
Keywords: diet, glycemic index, neural tube defects, pregnancy
Prevalences of obesity and diabetes have been increasing in the United States; in women of all ages, the prevalence of diabetes increased 32% and the prevalence of obesity increased 47% from the early 1990s to the late 1990s (1–3). Among the many pregnancy complications associated with obesity and diabetes is the risk of congenital anomalies, particularly neural tube defects (NTDs) (1, 4–16). How these conditions affect fetal development has not been clearly delineated, but there are some suggestions that hyperglycemia and/or hyperinsulinemia may play a role, since 2 studies have shown that diabetic mothers who have better glycemic control are at lower risk for congenital anomalies (17, 18). Rodent studies have suggested a similar effect, with an increase in malformation rates as levels of glucose increase (19–21). While the increased risk of NTDs associated with obesity appears to be independent of diabetes, a possible mechanism might be hyperglycemia due to insulin resistance in obese women (11).
One approach to studying this potential mechanism in humans is assessing glycemic index, a measure of blood glucose response to carbohydrate ingestion. Carbohydrate-containing foods produce different glucose responses, as reflected by their glycemic index values. Dietary glycemic index (DGI) is a measure of the quality of carbohydrates consumed, and dietary glycemic load (DGL) is a measure of the quantity as well as the quality of carbohydrate consumed; both are markers of aggregate glucose response to dietary intake. To date, the effect of glycemic intake on the risk of NTDs has been considered in only 2 studies (22, 23). In the first study, Shaw et al. (22) found an approximately 2-fold increased risk in women with a high DGI. The DGI effect among obese women was even higher: more than a 4-fold increase in risk of an NTD (22). In the second study, Shaw et al. (23) focused on maternal DGL and found no increased risk. The authors suggested that the lack of an association in the latter study may have been due to the use of a shortened food frequency questionnaire (FFQ), which may not have adequately captured the range of foods contributing to glycemic load.
Using data collected in a large study of risk factors for birth defects, we examined the associations between maternal DGI and DGL and the risk of NTDs in nondiabetic women. In addition, we examined whether the shortened questionnaire sufficiently captured exposure levels necessary to detect an association.
MATERIALS AND METHODS
The Pregnancy Health Interview Study (also called the Slone Birth Defects Study) is an ongoing multicenter case-control surveillance effort designed to evaluate the risks and safety of various antenatal exposures in relation to congenital anomalies. The study methods have been previously described (24, 25). The study was initiated in 1976 as an ongoing surveillance program; cases were ascertained from birth hospitals and tertiary-care hospitals at a number of study sites in the United States and Canada. To identify potential case mothers at the birth hospital, study staff reviewed clinical and surgical logs, reviewed admission and discharge lists, and contacted newborn nurseries and labor and delivery rooms. The mothers’ primary physicians were then asked for permission to contact them. Beginning in 1990, cases from electively terminated pregnancies were included. In 1993, the study investigators began ascertaining infants with no major congenital anomalies to use as controls. The controls came from the same birth hospitals as the cases and were randomly selected from discharge or newborn nursery lists.
The present study was restricted to data collected from 1988 through 1998, when dietary intake was assessed by means of a 99-item Willett FFQ. It included subjects recruited from the greater metropolitan areas of Boston, Massachusetts, Philadelphia, Pennsylvania, and Toronto, Ontario, Canada. Cases of NTD (livebirths and stillbirths) included cases of spina bifida, anencephaly, rachischisis, and encephalocele. Though there is some debate as to whether encephaloceles should be classified as NTDs from an epidemiologic perspective, they were included in this analysis because they often are more similar to spina bifida and anencephaly than they are different (26). Conjoined twins and infants with amniotic bands, body wall defects, cloacal exstrophy, bladder exstrophy, chromosomal anomalies, or a known syndrome were excluded. Cases whose diagnosis could not be confirmed through medical record review were also excluded. Of those mothers who were eligible and were asked to participate, mothers of 80% of cases and 68% of controls agreed to be interviewed.
Interviews were conducted in person by trained nurses within 6 months of delivery. The interview consisted of questions on medication and vitamin use, illnesses during pregnancy, reproductive history, demographic information, and diet. Dietary intake was measured with the Willett FFQ, which has been validated for use in epidemiologic studies (27). Because neural tube closure occurs in the first 6 weeks of gestation, before many women recognize that they are pregnant, investigators focused the dietary questions on the 6-month period before pregnancy in an effort to capture usual dietary patterns in this very early period of gestation. Subjects who left more than 3 FFQ questions blank were considered to have not completed the FFQ and were excluded from the analyses. In addition, women who reported an extreme caloric intake (<500 kcal/day or >3,800 kcal/day) or reported consuming an implausible amount of food on a daily basis were excluded.
Glycemic index values for individual foods were obtained from the Harvard University Food Composition Database, which is derived from the US Department of Agriculture database (28). Carbohydrate-containing foods differ in their effects on blood glucose levels, as reflected by their glycemic index values. For example, the glycemic index of white rice is higher than that of brown rice. To calculate a subject's DGL, we multiplied each food's glycemic index by the number of daily servings and by carbohydrate content and then summed the data across all foods consumed. To calculate a subject's DGI, we divided DGL by the total amount of carbohydrate consumed. The quality of carbohydrate intake is reflected in DGI, whereas both quality and quantity are reflected in DGL (29, 30). For example, diets with high intakes of cola have a high DGI but a low DGL, because sugared sodas have a large effect on blood sugar but contain relatively little carbohydrate. In models that examined DGL, we controlled for the effect of dietary fiber in order to take into account the type of carbohydrate consumed. All dietary variables, including DGI, DGL, fiber, and folic acid, were adjusted for energy intake using the residual method (31, 32).
Sociodemographic and behavioral factors were taken into consideration, including: race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other), education (less than high school, high school, more than high school), maternal age (<20, 20–29, 30–39, or 40–49 years), prepregnancy weight (<50, 50–59, 60–69, 70–79, or ≥80 kg), use of a vitamin containing folic acid (92% multivitamin and 8% single component) during the month after the last menstrual period (yes/no), dietary folate (μg; continuous variable), dietary fiber (g; continuous variable), income in 2005 US dollars (<$15,000, $15,000–$29,999, $30,000–$44,999, or ≥$45,000), and study center (Boston, Philadelphia, or Toronto). We compared case and control mothers for DGI and DGL with a cubic-spline logistic model that adjusted for sociodemographic and behavioral factors to identify cutoff values within the distribution of DGI and DGL for dichotomization. In subsequent case-control comparisons, we utilized these dichotomizations of maternal DGI and DGL intakes to estimate odds ratios using unconditional logistic regression. Crude and adjusted odds ratios were calculated for all NTDs combined, as well as for individual NTD subgroups (anencephaly/rachischisis, spina bifida, and encephalocele). NTDs were classified as isolated or associated, the latter based on concomitant major malformations that are not part of the NTD sequence (e.g., clubfoot). Analyses were also stratified according to maternal weight. A cutpoint of 80 kg was used to identify the heaviest women, since previous research had identified this weight as the cutoff at which the risk of NTDs increases (10). Beginning in 1993, data on height were collected, and this allowed for stratification according to body mass index (weight (kg)/height (m)2). We hypothesized that DGI and DGL would be most strongly associated with an increased risk in heavy women, assuming they would be more insulin-resistant.
To compare the long 99-item Willett FFQ to the shortened 58-item Willett FFQ used in the other NTD study (23), we simulated the shortened questionnaire by removing those items that were only included in the long FFQ and recalculated DGI and DGL. Examples of items that were removed from the long FFQ are: pastries, doughnuts, jam, oatmeal, bagels, muffins, pancakes, popcorn, raisins, prunes, and sherbet. Because previous studies of DGI and DGL in relation to NTD risk examined quartiles of intake, we created quartiles for both the long FFQ and the simulated shortened FFQ based on the control distribution for DGI and DGL, which allowed comparisons of the ranges covered by each quartile with our spline-determined cutoff values and the ranges covered in the 2 previous studies (22, 23). All analysis were performed using SAS 9.1 software (33).
RESULTS
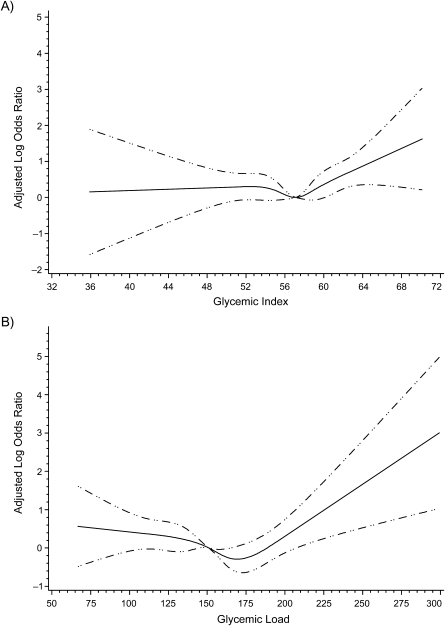
After exclusion of 53 mothers who reported either diabetes or gestational diabetes and 30 mothers who reported an extreme caloric intake or consuming more than 20 different items daily, there were 698 cases and 696 controls. Compared with controls, case mothers were more likely to be young, less educated, and obese, to have lower incomes, and to have not taken a multivitamin containing folic acid (Table 1). Figure 1 shows cubic splines with the adjusted odds ratio plotted for each level of DGI or DGL. We used an odds ratio of 1.5 (0.405 on the log scale) to determine the cutoff value for dichotomization. Therefore, high DGI was defined as ≥60 and high DGL as ≥205.
Table 1.
Characteristics of Women Who Had an Infant With a Neural Tube Defect and Women Whose Infants Had No Major Malformations, United States and Canada, 1988–1998
| Controls |
Cases |
Total | |||
| No. | % | No. | % | ||
| Race/ethnicity | |||||
| White | 635 | 91.2 | 619 | 88.7 | 1,254 |
| Black | 34 | 4.9 | 37 | 5.3 | 71 |
| Other | 13 | 1.9 | 17 | 2.4 | 30 |
| Hispanic | 14 | 2.0 | 23 | 3.3 | 37 |
| Missing data | 0 | 0.0 | 2 | 0.3 | 2 |
| Age, years | |||||
| <20 | 19 | 2.7 | 46 | 6.6 | 65 |
| 20–29 | 304 | 43.7 | 370 | 53.0 | 674 |
| 30–39 | 361 | 51.9 | 266 | 38.1 | 627 |
| 40–49 | 12 | 1.7 | 16 | 2.3 | 28 |
| Education | |||||
| Less than high school | 33 | 4.7 | 100 | 14.3 | 133 |
| High school graduation | 142 | 20.4 | 233 | 33.4 | 375 |
| More than high school | 521 | 74.9 | 365 | 52.3 | 886 |
| Weight, kg | |||||
| <50 | 79 | 11.4 | 81 | 11.6 | 160 |
| 50–59 | 239 | 34.3 | 243 | 34.8 | 482 |
| 60–69 | 196 | 28.2 | 157 | 22.5 | 353 |
| 70–79 | 109 | 15.7 | 111 | 15.9 | 220 |
| ≥80 | 73 | 10.5 | 106 | 15.2 | 179 |
| Folic acid supplementation | |||||
| None | 481 | 69.1 | 571 | 81.8 | 1,052 |
| Anya | 215 | 30.9 | 127 | 18.2 | 342 |
| Income (2005 US dollars) | |||||
| Missing data | 24 | 3.4 | 23 | 3.3 | 47 |
| <15,000 | 37 | 5.3 | 60 | 8.6 | 97 |
| 15,000–29,999 | 47 | 6.8 | 122 | 17.5 | 169 |
| 30,000–44,999 | 75 | 10.8 | 50 | 7.2 | 125 |
| ≥45,000 | 513 | 73.7 | 443 | 63.5 | 956 |
| Study center | |||||
| Boston, Massachusetts | 229 | 32.9 | 294 | 42.1 | 523 |
| Philadelphia, Pennsylvania | 159 | 22.8 | 189 | 27.1 | 348 |
| Toronto, Ontario, Canada | 308 | 44.3 | 215 | 30.8 | 523 |
From use of multivitamins or single vitamins during the month after the last menstrual period.
Figure 1.
Adjusted cubic splines for A) glycemic index and B) glycemic load, United States and Canada, 1988–1998. The splines were adjusted for maternal race/ethnicity, education, age, folic acid supplementation, dietary folic acid, income, study center, and (in glycemic load models only) fiber. Dashed lines, 95% confidence interval.
High exposure to DGI and DGL (Table 2) was more prevalent in cases than in controls. A doubling in risk of NTDs was found for women with high DGI compared with lower glycemic intakes. After adjustment for maternal race/ethnicity, education, maternal age, multivitamin usage, dietary folic acid, income, and study center, the odds ratio was lower, but a 50% increase in risk remained (odds ratio = 1.5, 95% confidence interval (CI): 1.1, 2.0). Women with high DGL were found to have an adjusted 80% elevated risk of NTDs (odds ratio = 1.8, 95% CI: 0.8, 4.0), but the 95% confidence interval included 1.0—possibly because of the smaller numbers in the high-DGL group. No single confounder accounted for the difference seen between the crude and adjusted estimates. When analyses were restricted to the 573 isolated NTDs, the results did not change materially (data not shown).
Table 2.
Odds Ratios for Risk of Neural Tube Defects According to Maternal Glycemic Index and Glycemic Load, United States and Canada, 1988–1998
| Controls |
Cases |
OR | 95% CI | Adjusted ORa | 95% CI | |||
| No. | % | No. | % | |||||
| All neural tube defects | ||||||||
| Glycemic index | ||||||||
| Low (<60) | 594 | 85.3 | 522 | 74.8 | Reference | Reference | ||
| High (≥60) | 102 | 14.7 | 176 | 25.2 | 2.0 | 1.5, 2.6 | 1.5 | 1.1, 2.0 |
| Glycemic load | ||||||||
| Low (<205) | 683 | 98.1 | 668 | 95.7 | Reference | Reference | ||
| High (≥205) | 13 | 1.9 | 30 | 4.3 | 2.4 | 1.2, 4.6 | 1.8 | 0.8, 4.0 |
| Maternal weight ≥80 kg | ||||||||
| Glycemic index | ||||||||
| Low (<60) | 61 | 83.6 | 72 | 67.9 | Reference | Reference | ||
| High (≥60) | 12 | 16.4 | 34 | 32.1 | 2.4 | 1.1, 5.0 | 1.6 | 0.6, 3.9 |
| Glycemic load | ||||||||
| Low (<205) | 67 | 91.8 | 96 | 90.6 | Reference | Reference | ||
| High (≥205) | 6 | 8.2 | 10 | 9.4 | 1.2 | 0.4, 3.4 | 0.6 | 0.2, 2.3 |
| Maternal weight <80 kg | ||||||||
| Glycemic index | ||||||||
| Low (<60) | 533 | 85.6 | 450 | 76.0 | Reference | Reference | ||
| High (≥60) | 90 | 14.4 | 142 | 24.0 | 1.9 | 1.4, 2.5 | 1.5 | 1.1, 2.1 |
| Glycemic load | ||||||||
| Low (<205) | 616 | 98.9 | 572 | 96.6 | Reference | Reference | ||
| High (≥205) | 7 | 1.1 | 20 | 3.4 | 3.5 | 1.3, 9.1 | 2.6 | 1.0, 7.1 |
| Maternal BMIb ≥30c | ||||||||
| Glycemic index | ||||||||
| Low (<60) | 53 | 82.8 | 23 | 63.9 | Reference | Reference | ||
| High (≥60) | 11 | 17.2 | 13 | 36.1 | 2.7 | 1.1, 7.0 | 2.0 | 0.6, 7.3 |
| Glycemic load | ||||||||
| Low (<205) | 59 | 92.2 | 32 | 88.9 | Reference | Reference | ||
| High (≥205) | 5 | 7.8 | 4 | 11.1 | 1.5 | 0.4, 5.9 | 0.9 | 0.2, 4.7 |
| Maternal BMI <30c | ||||||||
| Glycemic index | ||||||||
| Low (<60) | 540 | 85.6 | 138 | 74.6 | Reference | Reference | ||
| High (≥60) | 91 | 14.4 | 47 | 25.4 | 2.0 | 1.4, 3.0 | 1.7 | 1.1, 2.7 |
| Glycemic load | ||||||||
| Low (<205) | 623 | 98.7 | 177 | 95.7 | Reference | Reference | ||
| High (≥205) | 8 | 1.3 | 8 | 4.3 | 3.8 | 1.4, 10.5 | 3.3 | 1.0, 10.6 |
Abbreviations: BMI, body mass index; CI, confidence interval; OR, odds ratio.
Adjusted for maternal race/ethnicity, education, age, folic acid supplementation, dietary folic acid, income, study center, and (in glycemic load models only) fiber.
Weight (kg)/height (m)2.
BMI data were available only from 1993 onwards.
When the data were stratified by type of NTD, a similar pattern of risks was observed for each type, but these findings were unstable because of smaller numbers. For DGI, the odds ratios for spina bifida, anencephaly, and encephalocele were 1.5 (95% CI: 1.1, 2.2), 1.3 (95% CI: 0.8, 2.2), and 1.9 (95% CI: 1.0, 3.8), respectively. For DGL, the odds ratios for spina bifida and encephalocele were 2.1 (95% CI: 0.9, 5.0) and 4.3 (95% CI: 1.2, 15.5), respectively (there were only 2 exposed anencephaly cases). Because nonmalformed controls were not ascertained from 1988 to 1992, subanalyses were performed on the 221 cases and 696 controls from the later years. Adjusted results were similar, with odds ratios of 1.8 (95% CI: 1.2, 2.7) for DGI and 2.4 (95% CI: 1.0, 6.1) for DGL.
After restricting the analysis to the heaviest women (≥80 kg), we found that odds ratios were not higher than those observed for all cases, but numbers were small and confidence intervals were wide. Likewise, in the subset of 221 case women and 695 control women who had height information available, odds ratios for women with a body mass index greater than or equal to 30 were not further elevated for DGL. The odds ratio for DGI was slightly higher among women with the highest body mass indexes, but the 95% confidence interval did not exclude effects among leaner women.
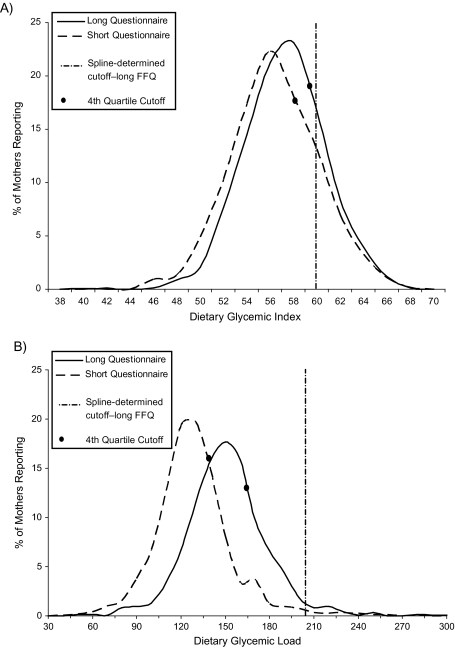
Figure 2 compares results obtained using the long Willett FFQ with those obtained from the simulated shortened FFQ. For DGI, the median and cutoff values for the fourth quartile did not change considerably. The median value for DGL, on the other hand, changed appreciably (long FFQ: 149.9; short FFQ: 127.1), with an approximate 40-point shift to the left when the simulated shortened questionnaire was used. Thus, the cutoff value for the fourth quartile of DGL was considerably higher (165.6) for the long FFQ than for the simulated shortened FFQ (138.8); both were well below the spline-determined cutoff of 205.
Figure 2.
Comparison of results obtained by means of a long food frequency questionnaire (FFQ) as compared with a simulated shortened FFQ for A) glycemic index and B) glycemic load, United States and Canada, 1988–1998.
DISCUSSION
Women with the highest intakes of DGI and DGL were at least 1.5 times as likely to have offspring affected with an NTD, but part of these associations could be attributed to several different socioeconomic and behavioral factors. After these confounders were controlled, modest associations remained, though only the risk estimated for high DGI had a lower bound that excluded 1.0. No subtypes of NTDs appeared to be affected differentially, but odds ratios were unstable because of small numbers.
As we noted above, calculations for DGL take into account the amount of carbohydrate and the glycemic index for each food and therefore can be considered a measure of both the quantity and quality of dietary carbohydrates (34). DGI, on the other hand, measures only the quality of carbohydrate consumed (34). We had hypothesized that a high-glycemic diet in early pregnancy would produce a hyperglycemic and/or hyperinsulinemic state that would then be passed on to the developing fetus and could result in an NTD. Fetal responses to hyperglycemia have not been studied in humans, but animal studies have suggested that hyperglycemia can induce nutritional deficiencies, yolk sac vascular impairment, production of oxygen free radicals, and embryonic depletion of inositol, which have all been linked to an increased risk of NTDs (19, 35–37). Our findings for both poor quality and high quantity of carbohydrate intake (high DGL) support our hypothesis, but the prevalence of such carbohydrate intakes was less than 5%, resulting in unstable odds ratio estimates that include the possibility of no effect.
We also hypothesized that nondiabetic obese women would be most likely to have some degree of insulin resistance and would therefore pass even higher levels of glucose on to the fetus, putting them at greater risk for NTDs. Our data did not support this hypothesis. Rather, we observed stronger effects of DGL in the nonobese women. One possible explanation for these findings is that glycemic intake would be overridden in obese women because their NTD risk is already elevated, perhaps similarly to diabetic women. However, 1 study has shown that the effect of high DGI was confined to obese women. Shaw et al. (22) found an odds ratio of 1.9 (95% CI: 1.3, 2.7) for women in the highest quartile of DGI exposure, and this risk increased to 4.1 (95% CI: 1.0, 15.7) when the analysis was restricted to women with a body mass index greater than 29. In a more recent study (23), the same investigators found no relation between DGL and increased NTD risk. However, our comparison of DGI and DGL measurements from the long and simulated shortened FFQs revealed a possible explanation for the discrepancy in the 2 Shaw et al. studies (22, 23).
The shortened questionnaire appeared to be limited in capturing high levels of exposure. This was especially true for DGL, where the simulated shortened FFQ produced a 40-point shift to the left. When using the spline, we identified a cutoff value of 205 for the highest intake group, whereas cutoffs based on quartiles would have been 165.6 for the long questionnaire and 138.8 for the simulated shortened questionnaire in the controls. Thus, the use of quartiles would further limit the ability to identify persons at highest risk. In the Shaw et al. study in which the shortened FFQ was used (23), the cutoff value for the highest quartile was 151.43 for DGL. In contrast, the cubic spline from our data revealed no increased risk of NTDs at a DGL of 151. In addition, had quartiles been created using our data, a value of 151.43 would have fallen into our second quartile, indicating that persons in the highest quartile of the Shaw et al. study were not necessarily those at highest risk (23).
The strengths of this study are its large size and the efforts made to collect accurate exposure histories by conducting in-person interviews relatively soon after delivery. FFQs are the standard method of determining dietary intakes in retrospective epidemiologic studies. We used the validated long version of the Willett FFQ, which appears to better capture carbohydrate intakes than the shortened version. While our analysis focused on DGI and DGL, we cannot rule out the possibility that other nutrients that correlate with DGI or DGL may be responsible for the associations we observed. Reporting accuracy is always a concern in case-control studies, but the relatively short interval between delivery and interview probably reduced it. During the time frame of the study (1988–1998), there was little knowledge about carbohydrate intake as a risk factor for birth defects, suggesting that reporting accuracy would not have differed for cases and controls. Another possible limitation is that obese women tend to underreport their dietary intake (38). Since case mothers were more likely to be obese, the observed odds ratios may have been biased downward. Such underreporting might also explain the lack of stronger associations among the heaviest women. In addition, the lack of height information for 1988–1993 prevented us from calculating body mass index for the entire sample, which would have been a better tool for assessing obesity. The recall interval for cases involving pregnancy termination was shorter than that for other study subjects, raising the possibility of differential misclassification of exposure between cases and controls. However, when we restricted comparisons to liveborn subjects, findings did not change appreciably.
Diets with proportionally high amounts of carbohydrates may put the developing fetus at risk of an NTD. Our findings add to those of a previous study in humans and several experimental animal studies in providing evidence that hyperglycemia lies within the pathogenic pathway of NTDs.
Acknowledgments
Author affiliations: Slone Epidemiology Center, Boston University, Boston, Massachusetts (Mahsa M. Yazdy, Allen A. Mitchell, Martha M. Werler); and Program on Genomics and Nutrition, Department of Epidemiology, School of Public Health, University of California, Los Angeles, Los Angeles, California (Simin Liu).
This work was supported by the National Institute of Child Health and Human Development (grant HD047652).
The authors thank Dawn Jacobs, Fiona Rice, Rita Krolak, Kathleen Sheehan, Claire Coughlin, Moira Quinn, Nancy Rodriguez, Carolina Tejedor Meyers, and Nastia Dynkin for their assistance in data collection and computer programming.
This article received the Student Prize Paper Award and was presented at the 22nd annual meeting of the Society for Pediatric and Perinatal Epidemiologic Research, Anaheim, California, June 23, 2009.
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- DGI
dietary glycemic index
- DGL
dietary glycemic load
- FFQ
food frequency questionnaire
- NTD
neural tube defect
References
- 1.Feig DS, Palda VA. Type 2 diabetes in pregnancy: a growing concern. Lancet. 2002;359(9318):1690–1692. doi: 10.1016/S0140-6736(02)08599-9. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Ford ES, Bowman BA, et al. Diabetes trends in the U.S.: 1990–1998. Diabetes Care. 2000;23(9):1278–1283. doi: 10.2337/diacare.23.9.1278. [DOI] [PubMed] [Google Scholar]
- 3.Mokdad AH, Serdula MK, Dietz WH, et al. The spread of the obesity epidemic in the United States, 1991–1998. JAMA. 1999;282(16):1519–1522. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- 4.Källén K. Maternal smoking, body mass index, and neural tube defects. Am J Epidemiol. 1998;147(12):1103–1111. doi: 10.1093/oxfordjournals.aje.a009408. [DOI] [PubMed] [Google Scholar]
- 5.Shaw GM, Velie EM, Schaffer D. Risk of neural tube defect-affected pregnancies among obese women. JAMA. 1996;275(14):1093–1096. doi: 10.1001/jama.1996.03530380035028. [DOI] [PubMed] [Google Scholar]
- 6.Waller DK, Mills JL, Simpson JL, et al. Are obese women at higher risk for producing malformed offspring? Am J Obstet Gynecol. 1994;170(2):541–548. doi: 10.1016/s0002-9378(94)70224-1. [DOI] [PubMed] [Google Scholar]
- 7.Waller DK, Shaw GM, Rasmussen SA, et al. Prepregnancy obesity as a risk factor for structural birth defects. Arch Pediatr Adolesc Med. 2007;161(8):745–750. doi: 10.1001/archpedi.161.8.745. [DOI] [PubMed] [Google Scholar]
- 8.Watkins ML, Edmonds L, McClearn A, et al. The surveillance of birth defects: the usefulness of the revised US standard birth certificate. Am J Public Health. 1996;86(5):731–734. doi: 10.2105/ajph.86.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watkins ML, Rasmussen SA, Honein MA, et al. Maternal obesity and risk for birth defects. Pediatrics. 2003;111(5):1152–1158. [PubMed] [Google Scholar]
- 10.Werler MM, Louik C, Shapiro S, et al. Prepregnant weight in relation to risk of neural tube defects. JAMA. 1996;275(14):1089–1092. doi: 10.1001/jama.1996.03530380031027. [DOI] [PubMed] [Google Scholar]
- 11.Hendricks KA, Nuno OM, Suarez L, et al. Effects of hyperinsulinemia and obesity on risk of neural tube defects among Mexican Americans. Epidemiology. 2001;12(6):630–635. doi: 10.1097/00001648-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Becerra JE, Khoury MJ, Cordero JF, et al. Diabetes mellitus during pregnancy and the risks for specific birth defects: a population-based case-control study. Pediatrics. 1990;85(1):1–9. [PubMed] [Google Scholar]
- 13.Janssen PA, Rothman I, Schwartz SM. Congenital malformations in newborns of women with established and gestational diabetes in Washington state, 1984–91. Paediatr Perinat Epidemiol. 1996;10(1):52–63. doi: 10.1111/j.1365-3016.1996.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 14.Moore LL, Singer MR, Bradlee ML, et al. A prospective study of the risk of congenital defects associated with maternal obesity and diabetes mellitus. Epidemiology. 2000;11(6):689–694. doi: 10.1097/00001648-200011000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Ramos-Arroyo MA, Rodriguez-Pinilla E, Cordero JF. Maternal diabetes: the risk for specific birth defects. Eur J Epidemiol. 1992;8(4):503–508. doi: 10.1007/BF00146367. [DOI] [PubMed] [Google Scholar]
- 16.Galtier-Dereure F, Boegner C, Bringer J. Obesity and pregnancy: complications and cost. Am J Clin Nutr. 2000;71(5 suppl):1242S–1248S. doi: 10.1093/ajcn/71.5.1242s. [DOI] [PubMed] [Google Scholar]
- 17.Fuhrmann K, Reiher H, Semmler K, et al. Prevention of congenital malformations in infants of insulin-dependent diabetic mothers. Diabetes Care. 1983;6(3):219–223. doi: 10.2337/diacare.6.3.219. [DOI] [PubMed] [Google Scholar]
- 18.Miller E, Hare JW, Cloherty JP, et al. Elevated maternal hemoglobin A1c in early pregnancy and major congenital anomalies in infants of diabetic mothers. N Engl J Med. 1981;304(22):1331–1334. doi: 10.1056/NEJM198105283042204. [DOI] [PubMed] [Google Scholar]
- 19.Reece EA, Homko CJ, Wu YK. Multifactorial basis of the syndrome of diabetic embryopathy. Teratology. 1996;54(4):171–182. doi: 10.1002/(SICI)1096-9926(199610)54:4<171::AID-TERA1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Reece EA, Homko CJ, Wu YK, et al. The role of free radicals and membrane lipids in diabetes-induced congenital malformations. J Soc Gynecol Investig. 1998;5(4):178–187. doi: 10.1016/s1071-5576(98)00008-2. [DOI] [PubMed] [Google Scholar]
- 21.Sadler TW. Effects of maternal diabetes on early embryogenesis: I. The teratogenic potential of diabetic serum. Teratology. 1980;21(3):339–347. doi: 10.1002/tera.1420210310. [DOI] [PubMed] [Google Scholar]
- 22.Shaw GM, Quach T, Nelson V, et al. Neural tube defects associated with maternal periconceptional dietary intake of simple sugars and glycemic index. Am J Clin Nutr. 2003;78(5):972–978. doi: 10.1093/ajcn/78.5.972. [DOI] [PubMed] [Google Scholar]
- 23.Shaw GM, Carmichael SL, Laurent C, et al. Periconceptional glycaemic load and intake of sugars and their association with neural tube defects in offspring. Paediatr Perinat Epidemiol. 2008;22(6):514–519. doi: 10.1111/j.1365-3016.2008.00964.x. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell AA, Rosenberg L, Shapiro S, et al. Birth defects related to bendectin use in pregnancy. I. Oral clefts and cardiac defects. JAMA. 1981;245(22):2311–2314. [PubMed] [Google Scholar]
- 25.Werler MM, Hayes C, Louik C, et al. Multivitamin supplementation and risk of birth defects. Am J Epidemiol. 1999;150(7):675–682. doi: 10.1093/oxfordjournals.aje.a010070. [DOI] [PubMed] [Google Scholar]
- 26.Rowland CA, Correa A, Cragan JD, et al. Are encephaloceles neural tube defects? Pediatrics. 2006;118(3):916–923. doi: 10.1542/peds.2005-1739. [DOI] [PubMed] [Google Scholar]
- 27.Brown JE, Buzzard IM, Jacobs DR, Jr, et al. A food frequency questionnaire can detect pregnancy-related changes in diet. J Am Diet Assoc. 1996;96(3):262–266. doi: 10.1016/S0002-8223(96)00078-8. [DOI] [PubMed] [Google Scholar]
- 28.Composition of Foods: Raw, Processed, Prepared, 1963–1992. (USDA Handbook no. 8) Washington, DC: US GPO; 1993. US Department of Agriculture. [Google Scholar]
- 29.Neuhouser ML, Tinker LF, Thomson C, et al. Development of a glycemic index database for food frequency questionnaires used in epidemiologic studies. J Nutr. 2006;136(6):1604–1609. doi: 10.1093/jn/136.6.1604. [DOI] [PubMed] [Google Scholar]
- 30.Willett W, Manson J, Liu S. Glycemic index, glycemic load, and risk of type 2 diabetes. Am J Clin Nutr. 2002;76(1):274S–280S. doi: 10.1093/ajcn/76/1.274S. [DOI] [PubMed] [Google Scholar]
- 31.Willett W. Nutritional Epidemiology. 2nd ed. New York, NY: Oxford University Press; 1998. [Google Scholar]
- 32.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124(1):17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 33.SAS Institute Inc. SAS, Version 9.1.3. Cary, NC: SAS Institute Inc; 2000. [Google Scholar]
- 34.Liu S, Manson JE, Stampfer MJ, et al. Dietary glycemic load assessed by food-frequency questionnaire in relation to plasma high-density-lipoprotein cholesterol and fasting plasma triacylglycerols in postmenopausal women. Am J Clin Nutr. 2001;73(3):560–566. doi: 10.1093/ajcn/73.3.560. [DOI] [PubMed] [Google Scholar]
- 35.Wentzel P, Wentzel CR, Gäreskog MB, et al. Induction of embryonic dysmorphogenesis by high glucose concentration, disturbed inositol metabolism, and inhibited protein kinase C activity. Teratology. 2001;63(5):193–201. doi: 10.1002/tera.1034. [DOI] [PubMed] [Google Scholar]
- 36.Beemster P, Groenen P, Steegers-Theunissen R. Involvement of inositol in reproduction. Nutr Rev. 2002;60(3):80–87. doi: 10.1301/00296640260042748. [DOI] [PubMed] [Google Scholar]
- 37.Greene ND, Copp AJ. Inositol prevents folate-resistant neural tube defects in the mouse. Nat Med. 1997;3(1):60–66. doi: 10.1038/nm0197-60. [DOI] [PubMed] [Google Scholar]
- 38.Braam LA, Ocké MC, Bueno-de-Mesquita HB, et al. Determinants of obesity-related underreporting of energy intake. Am J Epidemiol. 1998;147(11):1081–1086. doi: 10.1093/oxfordjournals.aje.a009402. [DOI] [PubMed] [Google Scholar]