Abstract
Adducts of benzo[α]pyrene-diolepoxide (BPDE)2 with blood nucleophiles have been used as biomarkers of exposure to polycyclic aromatic hydrocarbons (PAHs). The most popular such assay is a competitive ELISA which employs monoclonal antibody 8E11 to detect benzo[α]pyrene tetrols following hydrolysis of BPDE adducts from lymphocyte DNA or human serum albumin (HSA). Here we use 8E11 as the capture antibody in a sandwich ELISA to detect BPDE-HSA adducts directly in 1 mg samples of HSA or 20 μL of serum/plasma. The assay employs an anti-HSA antibody for detection, which is amplified by an avidin/biotinylated horseradish peroxidase complex. The sandwich ELISA has advantages of specificity and simplicity and is about 10 times more sensitive than the competitive ELISA. To validate the assay, HSA samples were assayed from three populations with known high (coke-oven workers), medium (steel-factory control workers), and low (volunteer subjects) PAH exposures (n = 30). The respective geometric mean levels of BPDE-HSA adducts, i.e., 67.8, 14.7 and 1.93 ng/mg HSA (1,010, 220 and 28.9 fmol BPDE equivalents/mg HSA), were significantly different (p < 0.05). The sandwich ELISA will be useful for screening PAH exposures in large epidemiologic studies and can be extended to other adducts for which capture antibodies are available.
Keywords: biomarker, adduct, polycyclic aromatic hydrocarbons, environmental exposure, human serum albumin
Introductory Statement
Polycyclic aromatic hydrocarbons (PAHs) represent a class of ubiquitous pollutants arising from combustion of hydrocarbon fuels, fires, and cigarette smoke. Several PAHs are carcinogenic in humans and animals, notably benzo[α]pyrene (BaP) [1]. The ultimate carcinogens derived from BaP metabolism are the isomeric benzo[α]pyrene-diol-epoxides (BPDEs) which bind to DNA, proteins, and other macromolecules to form adducts. Adducts of the BPDEs accumulate in blood and can be used as biomarkers of exposure to BaP and other PAHs from the same source [2].
Although DNA adducts of BPDE have been widely investigated in human populations as measures of genetic damage in human cells, adducts of BPDE with human serum albumin (HSA) have also been detected [3]. Because HSA is much more abundant than DNA in blood (30 mg HSA/mL vs. 0.003–0.008 mg DNA/mL) and HSA adducts are not repaired, HSA adducts have inherent advantages over DNA adducts as measures of exposure for epidemiologic studies [4], even though they may not be strictly proportional to genetic damage in target organs. Furthermore, stable HSA adducts have a mean residence time of 28 d in humans, which is sufficiently long to damp the day-to-day variability in exposure often observed in human studies [5]. Yet, despite the relative advantages of BPDE-HSA adducts as biomarkers of exposure to PAHs, these adducts have rarely been used in epidemiologic studies.
Enzyme-linked immunosorbent assays (ELISA) provide an efficient means for screening BPDE-HSA adducts in human studies. One such assay, developed by Santella and coworkers, employs monoclonal antibody 8E11, which was raised against BPDE-I-modified guanosine conjugated with bovine serum albumin but cross reacts with many other large PAHs [3]. Because this assay involves hydrolysis of BPDE adducts to the corresponding BaP tetrols, it can be applied to essentially any hydrolysable BPDE adduct bound to proteins or DNA. As applied to HSA, the assay requires HSA to be isolated from blood, purified, hydrolyzed by treatment with enzymes or acid to generate BaP tetrols, purification of the tetrols, and detection of the tetrols by competitive ELISA. Although each of these steps can result in losses of analyte and can introduce imprecision, this competitive ELISA assay has remained essentially unchanged for 20 years [3; 6; 7]. Early attempts to use the 8E11-based ELISA to measure intact BPDE-HSA adducts, i.e., without hydrolysis to BaP tetrols, resulted in a 5-fold to 20-fold decrease in sensitivity [6; 8].
Given our interest in developing high-throughput methods for screening exposures to PAHs in epidemiologic studies, we sought a simpler and more sensitive ELISA for detecting BPDE-HSA adducts in serum, plasma, or isolated HSA. Towards this end, we developed a sandwich ELISA, which uses 8E11 as the capture antibody and an anti-HSA antibody for detection, and requires only 1 mg of isolated HSA, equivalent to about 20-μL of serum/plasma. Although simpler to use, this sandwich assay is about 10 times more sensitive than the original competitive ELISA. The sandwich ELISA was validated with archived human samples of HSA from workers exposed to PAHs and from control subjects.
Materials and Methods
Chemicals and Reagents
Benzo[α]pyrene-r-7,t-8-dihydrodiol-t-9,10-epoxides (±) [BPDE-I (±), MRI #477] were obtained from Midwest Research Institute, the NCI Chemical Carcinogen Repository (Kansas City, MO). Anhydrous tetrahydrofuran (THF, ≥ 99.9%), carbonate-bicarbonate buffer, Tween 20, human serum albumin (HSA, fraction V, 96–99%) and triethylamine (TEA) were from Sigma-Aldrich (St. Louis, MO). Tris buffered saline (TBS) 10x, Tris base, acetonitrile (Fisher Optima grade, 99.9%), and formic acid (Pierce, 1 mL ampoules, 99+%) were from Fisher Scientific (Pittsburgh, PA). Non-fat dry milk (NFDM) was from Genesee Scientific (San Diego, CA). SuperBlock, ImmunoPure ABC (avidin-biotin complex) staining kits and 1-step ultra tetramethylbenzidine (TMB, the colorimetric substrate for HRP) were from Thermo Scientific (Rockford, IL). Anti-mouse IgG-Fc (rabbit IgG) was from Bethyl Laboratories (Montgomery, TX). Anti-HSA (rabbit IgG, biotin conjugated) and anti-BPDE monoclonal antibody clone 8E11 (mouse IgG) were obtained from Rockland Immunochemicals (Gibertsville, PA) and Trevigen, (Gaithersburg, MD), respectively. All antibodies were polyclonal except 8E11.
Synthesis of BPDE-HSA Standards
A BPDE stock solution was prepared by dissolving BPDE-I in THF with 5% TEA under nitrogen at a concentration of 3,000 μg/mL. Commercial HSA (1 mg/mL in 0.01 M Tris buffer, pH 7.5) was mixed with BPDE stock in a molar ratio of 1:5 and gently shaken overnight in the dark at room temperature. The reaction mixture was applied to a 30,000 MW Microcon filter (Millipore, MA), washed three times with 400 μL 0.01 M Tris buffer, and diluted to a concentration of 1 mg/mL with 0.01 M tris buffer, pH 7.5. For quantitation purposes, BPDE equivalents were estimated assuming a molecular weight of 66,863 fg BPDE-HSA per fmol, as estimated by mass spectrometry of the BPDE-HSA standard (described below), and one mol BPDE per mol BPDE-HSA.
Mass Spectrometric Characterization of BPDE-HSA Adducts
Samples of commercial HSA before and after reaction with BPDE were characterized with an Agilent 1200 series liquid chromatograph (LC) (Santa Clara, CA) that was connected in-line with an LTQ Orbitrap XL hybrid mass spectrometer (MS) equipped with an Ion Max electrospray ionization source (ESI; Thermo Fisher Scientific, Waltham, MA). The LC employed C8 guard (Poroshell 300SB-C8, 5 μm, 12.5 × 2.1 mm, Agilent) and analytical (75 × 0.5 mm) columns and a 100-μL sample loop. Solvent A was 0.1% formic acid in water and solvent B was 0.1% formic acid in acetonitrile (v/v). Following sample injection, analyte trapping was performed for 5 min with 99.5% A at a flow rate of 90 μL/min. The elution program consisted of a linear gradient from 25% to 95% B over 34 min, isocratic conditions at 95% B for 5 min, a linear gradient to 0.5% B over 1 min, and then isocratic conditions at 0.5% B for 14 min, at a flow rate of 90 μL/min. The column and sample compartments were maintained at 35°C and 10°C, respectively. The MS ESI source parameters were as follows: ion transfer capillary temperature 275°C, normalized sheath gas (nitrogen) flow rate 25%, ESI voltage 2.0 kV, ion transfer capillary voltage 33 V, and tube lens voltage 125 V. Positive ion mass spectra were recorded over the range m/z = 500 to 2,000 using the Orbitrap mass analyzer, in profile format, with full MS automatic gain control target settings of 3×104 and 5×105 charges for the linear ion trap and the Orbitrap, respectively, and an Orbitrap resolution setting of 6×104 (at m/z = 400, FWHM). ESI mass spectra were processed using Xcalibur software (version 4.1, Thermo) and charge state distributions were deconvoluted using ProMass software (version 2.5 SR-1, Novatia, Monmouth Junction, NJ), using the default “large protein” parameters and a background subtraction factor of 1.5.
Human HSA Samples
For validation of the ELISA, 30 archived specimens of HSA from both sexes were obtained from studies of PAH-exposed workers at a steel factory in Northern China [9] and volunteer control subjects from North Carolina, U.S.A. [10]. All blood samples had been obtained with informed consent after approval of protocols by Institutional Review Boards at the institutions where the initial investigations were conducted. The PAH-exposed subjects consisted of 10 top-side coke-oven workers and 10 factory-control workers (both smokers and nonsmokers) from the same steel-making complex in Northern China; these workers had previously been shown to have high and intermediate levels of urinary PAH metabolites, respectively [11]. The volunteer control subjects were nonsmokers. All HSA specimens had previously been isolated from plasma, dialyzed, lyophilized to constant weight, dissolved in distilled water (50 mg/mL) and stored at −80°C prior to analysis.
Standard ELISA Procedures
Unless otherwise specified, standard washing steps were applied throughout the assay, and all reagents, antibodies and HSA samples or standards were loaded at 100 μL/well in 96-well plates (MaxiSorp™, C type, Nunc, NY). Prior to loading with analytes, plates were rinsed and briefly vortexed three times with 200 μL TBS-T (0.05% Tween in TBS) on a micro-plate mixer (Micromix 5, DPC, Flanders, NJ). After loading, plates were vortexed briefly, incubated for 1.5 h (45 min for reduced-volume ELISA) at 37°C and rinsed as described above. Wells were blocked at 37°C with 250 μL/well of either 5% NFDM, Superblock, or 15% NFDM as indicated. A 1-step TMB solution was added and plates were incubated for 45 min before stopping the reaction by addition of 100 μL/well of 2 M sulfuric acid. Colorimetric measurements of TMB were made at 450 nm using a microplate spectrophotometer (ELx800, Bio-Tek, Winooski, VT).
Sandwich ELISA Design
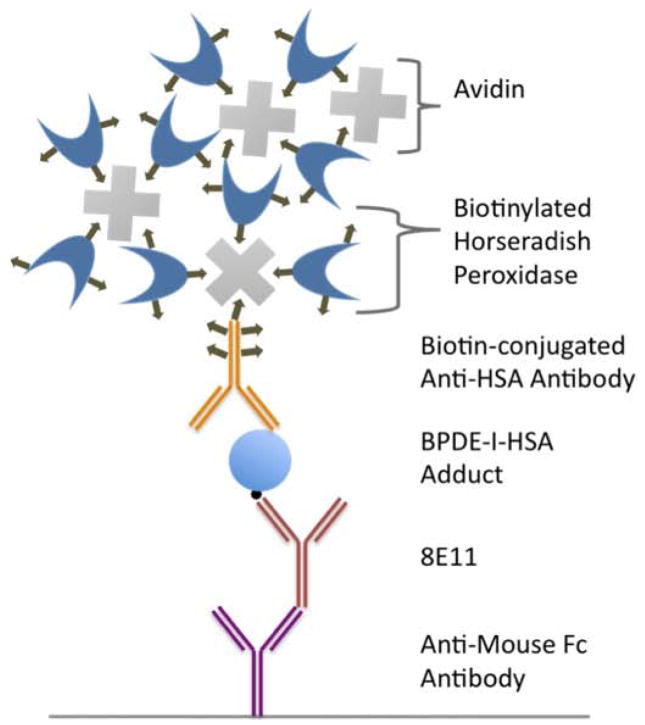
The sandwich ELISA design is illustrated in Figure 1. Anti-mouse IgG-Fc (rabbit IgG) at 5 μg/mL in 0.1 M carbonate-bicarbonate buffer was coated into the 96-well plate at 4°C overnight. After blocking with SuperBlock, monoclonal antibody 8E11 at 0.5 μg/mL was added and the plate was incubated. After loading standards of BPDE-HSA adducts into the wells and incubating, biotin-conjugated anti-HSA (rabbit IgG) at 1 μg/mL in blocking buffer was loaded and the plate was incubated again. ABC reagent, prepared in TBS-T, was added to the wells and the plate was incubated for 30 min at room temperature and then rinsed 5 times.
Figure 1.
Illustration of the sandwich ELISA design. The design incorporates an anti-mouse-IgG-Fc to increase the effective concentration of 8E11. Signals are amplified with an avidin-biotin HRP complex.
Reduced-Volume ELISA
To reduce background signals from unadducted HSA, the sandwich ELISA was modified slightly by reducing the volume of reagents to 20 μL/well. After coating the 96-well plate (50 μL/well) with anti-mouse IgG-Fc (rabbit IgG) diluted to 1 μg/mL with 0.1 M carbonate-bicarbonate buffer, the plate was incubated at 4°C overnight. The plate was blocked with 15% NFDM dissolved in TBS-T and monoclonal antibody 8E11 at 3 μg/mL in blocking buffer was added. After incubating the plate, the BPDE-HSA-adduct sample or unmodified-HSA sample was loaded in a 20-μL volume and the plate was incubated for 1.5 h. The assay then proceeded as described above except that biotin conjugated antibody was prepared with SuperBlock, and 8.5 M acetic acid with 0.5 M sulfuric acid (10 μL) was used to stop the final reaction.
This reduced-volume ELISA was used to measure BPDE-HSA adducts in samples of HSA from PAH-exposed workers and volunteer control subjects (in duplicate) after blinding of the analyst as to exposure status and randomization of samples. Wells containing sample HSA without monoclonal antibody 8E11 were used as individual controls for all specimens of HSA.
Statistical Analyses
Dose-response curves were fitted by the variable slope sigmoid function, , where y is the absorbance, min is the minimum response plateau, max is the maximum response plateau, logIC50 is the log (base 10) of IC50, Hillslope is the slope of the curve, and x is the log (base 10) concentration of BPDE-HSA (Qtiplot; ProIndep Serv, Romania). Descriptive statistics and pairwise t-tests with Bonferroni correction [by one way analysis of variance (ANOVA)] were performed with STATA 10 (StataCorp, TX), using log-transformed (base e) data to satisfy normality assumptions. Normality and homogeneity of variance were confirmed by Shapiro-Wilks and Bartlett’s tests, also using STATA 10. Values below the detection limit were imputed a value of half the detection limit for statistical analyses.
Limits of detection and quantitation were defined, respectively, as the mean of 12 blank values obtained with BPDE-HSA standards and spiked samples of either 1 mg control HSA or 20 μL control serum, plus either 3 times or 10 times the respective standard deviation [12]. The following detection and quantitation limits for the reduced-volume ELISA were estimated: standards - 0.124 and 0.619 ng BPDE-HSA (1.8 and 9.26 fmol BPDE equivalents), 1 mg HSA - 0.704 and 2.31 ng BPDE-HSA (10.5 and 34.5 fmol BPDE equivalents), and 20 μL serum - 0.637 and 2.54 ng BPDE-HSA (9.52 and 38.0 fmol BPDE equivalents). To estimate the precision of the assay, duplicate reference standards at 5 ng and 80 ng BPDE-HSA/well (75 and 1200 fmol BPDE equivalents/well) were prepared in each plate on 5 different days. The coefficients of variation (CV) estimated from one-way ANOVA of the 5 data pairs (in natural scale) were as follows: intra-assay CV – 8.71% (80 ng/well) and 32.2% (5 ng/well) and inter-assay CV – 10.7% (80 ng/well) and 15.0% (5 ng/well).
Results and Discussion
Characterization of the BPDE-HSA Standard
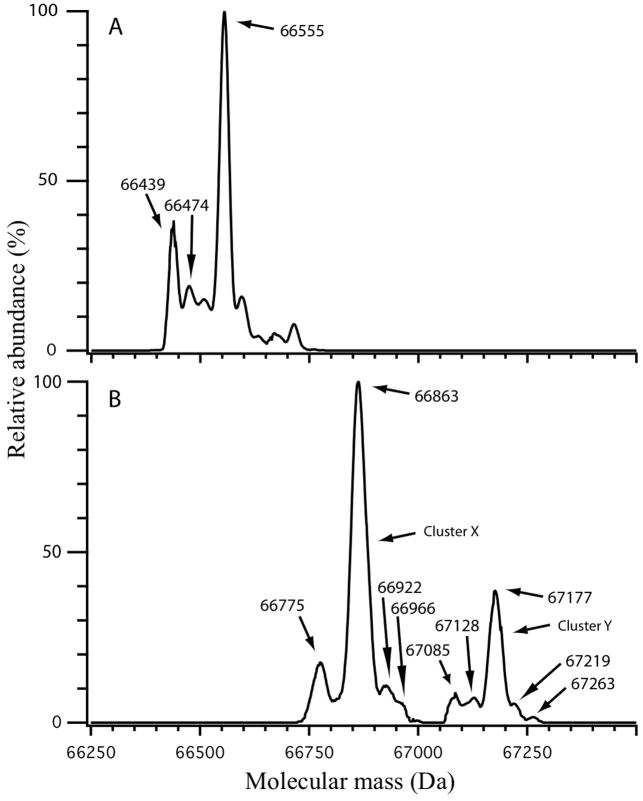
We quantified the modification level of the BPDE-HSA standard by ESI MS of the intact proteins, as illustrated in Figure 2. There was little overlap between deconvoluted mass spectra of commercial HSA (Figure 2A) and the BPDE-HSA standard obtained by reaction of commercial HSA with BPDE-I (Figure 2B), suggesting that the standard contained BPDE-HSA adducts in high yield. The most prominent mass in the spectrum of commercial HSA (Figure 2A) was 66,555 Da, representing the cysteinylated adduct at HSA-Cys34, whereas mercaptalbumin was represented by the smaller peak at 66,439 Da [13]. In contrast, the mass spectrum of the BPDE-HSA standard was dominated by peaks at 66,863 Da and 67,177 Da, which are labeled in Figure 2B as cluster X and cluster Y, respectively. The mass differences between clusters X and Y and the prominent peak in commercial HSA were 308 Da (66,863 Da minus 66,555 Da) and 622 Da (67,177 Da minus control HSA at 66,555 Da), respectively. Because the theoretical mass change for addition of one mol of BPDE to one mol of HSA is 302.3 Da, we conclude that cluster X most likely represents one BPDE adduct per HSA molecule and cluster Y most likely represents two BPDE adducts per HSA molecule. Based on the relative abundances of the corresponding ions in the raw spectra (not shown), roughly 80% of the BPDE-HSA standard contained a single BPDE modification and 20% contained two BPDE modifications. Thus, the average level of BPDE modification was about 1.2 mol of BPDE/mol HSA. Since stable covalent BPDE binding has been observed at His146 of HSA and relatively unstable ester adducts have been observed at Asp187 and Glu188 of HSA [14; 15], it is reasonable to expect that more than one BPDE molecule could be bound to a single HSA molecule. Also, since repeated analysis of the BPDE-HSA standard showed that adducts were stable under prolonged periods of laboratory storage (results not shown), it appears that most of the adducts in our standard represent the stable modification at His146 of HSA.
Figure 2.
Deconvoluted mass spectra of commercial HSA (A) and the BPDE-HSA standard (B). Masses in (A) at 66,439 Da and 66,555 Da are mercaptalbumin and HSA which has been cysteinylated at HSA-Cys34. The mass difference between cluster X in (B) at 66,683 Da and cysteinylated HSA in (A) is 308 Da and between cluster Y in (B) at 67,177 Da and cysteinylated HSA is 622 Da. Since the theoretical mass change after addition of one mol of BPDE to one mol of HSA is 302.3 Da, clusters X and Y probably represent cysteinylated HSA which has been modified with one and two BPDE molecules, respectively.
Response of the Sandwich ELISA
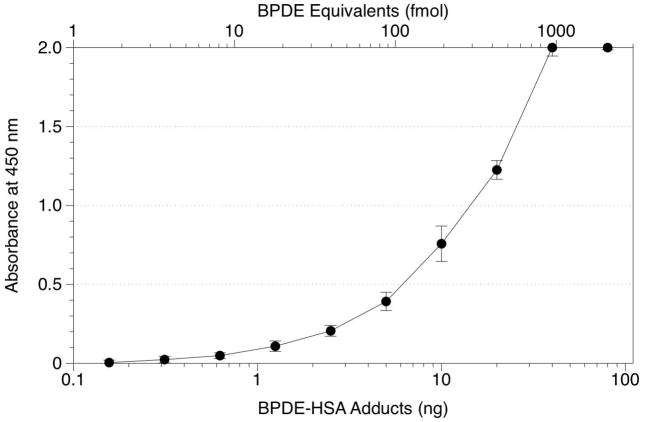
The sandwich ELISA (Figure 1) incorporated several elements to enhance sensitivity of the original competitive ELISA format. These included coating the wells with a tether antibody, anti-mouse IgG-Fc (rabbit IgG), and substituting an ABC-horseradish peroxidase (HRP) detection system for the alkaline phosphatase (AP)-p-nitrophenylphosphate system. By coating the wells with a tether antibody, we sought to minimize denaturation and loss of specificity [16] and also to free the 8E11 capture antibody from any constraints that might be imposed by its direct attachment to the wells. Introduction of the ABC-HRP detection system to replace AP-p-nitrophenylphosphate should theoretically amplify the detection of BPDE-HSA adducts by a factor of 4 because one avidin molecule is capable of binding 4 biotin molecules. The response curve is shown in Figure 3. With 10 ng BPDE-HSA/well (150 fmol BPDE equivalents/well), the sandwich ELISA produced an absorbance of 0.76 O.D. units and the detection limit for standard solutions was estimated to be 0.1 ng BPDE-HSA/well (1.5 fmol BPDE-HSA equivalents/well). Attempts to increase sensitivity by substituting a fluorescent AP substrate (AttoPhos®, Promega, Madison, WI) [17] for p-nitrophenylphosphate were unsuccessful (data not shown).
Figure 3.
Standard curves obtained from the sandwich ELISA using 1 mg HSA per well spiked with increasing amounts of the BPDE-HSA standard. Error bars represent standard deviations of duplicate samples.
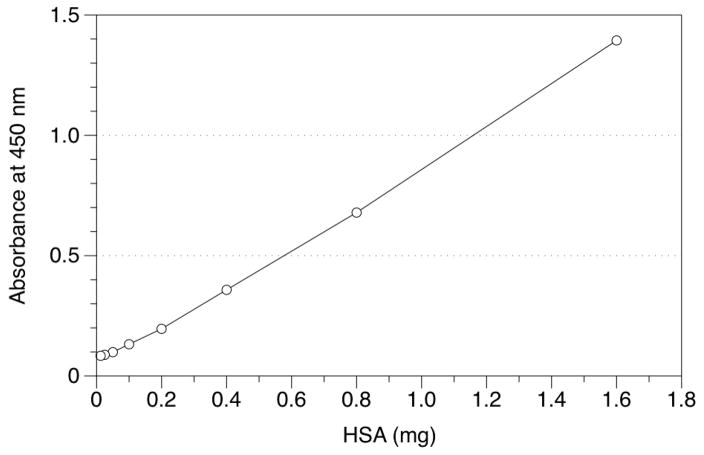
Reducing Background Effects of HSA
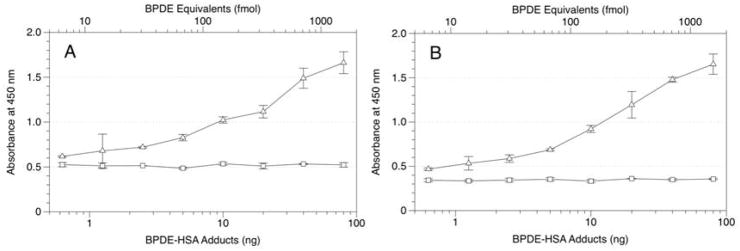
Because adduct levels in human HSA are expected to be about one molecule of BPDE-HSA per 106 HSA molecules, unadducted HSA can produce substantial background signals due to non-specific binding of HSA to the antibodies and internal surfaces of the wells. To illustrate this effect, Figure 4 shows the increase in background signals with increasing quantities of control HSA added to each well. We sought to reduce this background signal by reducing the total reagent volume from 100 μL to 20 μL, thereby reducing the effective surface area of each well, while also increasing the concentration of NFDM from 5% to 15% in the blocking buffer (to increase competition of NFDM with HSA for nonspecific binding). Figure 5 shows response curves for this modified protocol when applied to 1 mg samples of HSA spiked with BPDE-HSA (Figure 5A) or 20 μL of human serum spiked with BPDE-HSA (Figure 5B). (Note that 20 μL of human serum contains about 0.84 mg of HSA). In both figures, signals from control samples of 1 mg HSA or 20 μL serum (without addition of 8E11) did not increase with increasing amounts of BPDE-HSA adducts. Also, a stable background of about 0.5 O.D. units was attained after only 45 min of incubation, which was sufficient for the assay to achieve full sensitivity. These results indicate that modifications to the sandwich ELISA effectively controlled the background signal and increased the signal-to-noise ratio in the presence of high concentrations of unadducted HSA. The detection limit after the modification is about 0.7 ng BPDE-HSA/mg HSA (10 fmol BPDE equivalents/mg HSA). This can be compared to an estimated detection limit of roughly 10–20 ng BPDE-HSA/mg HSA (150–300 fmol BPDE equivalents/mg HSA) for the conventional competitive ELISA [7; 18]. (Because the competitive ELISA is based upon measurement of BaP tetrols that are extracted with variable efficiency after being liberated from adducts, actual detection limits are difficult to estimate for this assay).
Figure 4.
Relative background signals from non-specific binding of HSA in the sandwich ELISA. Reactions were stopped at 8 min (rather than 45 min) to avoid signal saturation.
Figure 5.
Response curves obtained from the low-volume ELISA design using either 1 mg HSA per well (A) or 20 μL of human serum per well (B) spiked with increasing amounts of the BPDE-HSA standard: △ - BPDE-HSA adducts; □ - controls (without 8E11). HSA solutions were prepared at 50 mg/mL; both HSA and serum contained 15% NFDM and 0.1% Tween 20. Error bars represent standard deviations of duplicates.
Measuring BPDE-HSA Adducts in Human Samples
To validate the sandwich assay, archived HSA samples from subjects exposed to high, medium, and low (control) levels of PAHs were randomized and assayed with the analyst blinded as to exposure status. Descriptive statistics are shown in Table 1 for the assays of PAH-exposed and control subjects. Adducts were detected in all 20 of the PAH-exposed workers (high-exposure and medium-exposure groups) and in 7 of 9 of the volunteer control subjects. Adduct levels spanned a 200-fold range across specimens of HSA from workers with high and medium exposure to PAHs (1.4–257 ng BPDE-HSA/mg HSA equivalent to 21–3,850 fmol BPDE equivalents/mg). Pairwise t-tests detected significant differences across the three groups (p < 0.05 for all pairwise tests). These results demonstrate that the optimized sandwich ELISA can be used as a screening tool to quantify PAH exposures in PAH-exposed and control populations.
Table 1.
Concentrations of BPDE-HSA adducts in groups of PAH-exposed and unexposed persons from the current study.
| ng BPDE-HSA/mg HSA (fmol BPDE equivalents/mg HSA) |
|||||
|---|---|---|---|---|---|
| Exposure Group | PAH Exposure | n | Geometric Mean | Geometric SD | Range |
| Coke-oven workers | High | 10 | 67.8* (1,010) | 1.99 | 22.4–257 (336–3,800) |
| Steel-factory control workers | Medium | 10 | 14.7* (220) | 2.63 | 1.4–53.5 (21.0–801) |
| Unexposed controlsa | Low | 10 | 1.93* (28.9) | 2.68 | 0.5–15.3 (7.5–229) |
p-value <0.05 versus both other groups. Comparisons were made by ANOVA of logged data using pairwise t-tests with Bonferroni correction.
Adducts not detected (< 1 ng/mg HSA) in two control subjects.
It is informative to compare levels of BPDE-HSA adducts from our investigation to those reported in previous studies which used the competitive ELISA to measure BPDE-HSA adducts in PAH-exposed workers and controls. We found four such studies, namely, Kure et al. [19], who reported adduct levels in coke-oven workers and rural controls, Lee et al. [3], who reported adduct levels in foundry workers, roofers and controls, and Sherson et al. [20] and Omland [21], who reported adduct levels in foundry workers and controls. Results from these studies and the current investigation are summarized in Table 2. For comparison, geometric mean (GM) or median adduct levels are shown as fmol BPDE equivalents/mg HSA. For the Kure et al. and Lee et al. studies, GM concentrations were estimated from the reported mean and SD values according to the relationship, GM = (mean)2/sqrt(mean2+SD2). Absolute adduct concentrations varied widely across the published studies, even in nonsmoking control subjects, where levels ranged from 28.9 fmol BPDE equivalents/mg HSA in the current study to 3,280 fmol BPDE equivalents/mg HSA in the Lee et al. study. To reduce effects of inter-laboratory differences, Table 2 also shows fold ratios of BPDE-HSA levels measured in each group of exposed workers compared to those measured concurrently in control subjects. Using the sandwich assay, we found fold ratios of 35 for the coke-oven workers and 7.6 for the steel-factory control workers compared to controls. While these fold ratios are considerably larger than those from the other studies, which used the competitive ELISA (fold-ratio range: 0.83–5.6), the results should be regarded as preliminary given the small numbers of subjects involved and difficulties in choosing control subjects for PAHs, which are ubiquitous contaminants of air, water, food, and tobacco smoke. Nonetheless, the combination of lower control levels of BPDE-HSA adducts and higher fold ratios observed with the sandwich ELISA suggests that this assay will be useful for characterizing PAH exposures in epidemiologic investigations.
Table 2.
Equivalent concentrations of BPDE-HSA adduct from the current study and from previous studies of PAH-exposed-exposed workers and control subjects which employed the competitive ELISA format.
| Study | ELISA | Exposure Group | Location | No. Subj. | BPDE Equivalents (fmol/mg HSA) | Fold Ratio (Exposed: Controls) |
|---|---|---|---|---|---|---|
| Current | Sandwich | Coke-oven workers (smoking & nonsmoking) | China | 10 | 1010 (GM) | 35 |
| Sandwich | Steel-factory control workers (smoking & nonsmoking) | China | 10 | 220 (GM) | 7.6 | |
| Sandwich | Unexposed controls (nonsmoking) | U.S.A. | 10 | 28.9 (GM) | ||
| Kure et al. (19) | Competitive | Coke-oven workers (smoking & nonsmoking) | Norway | 38 | 3920 (GM) | 1.5 |
| Competitive | Controls (smoking & nonsmoking) | Norway | 45 | 2620 (GM) | ||
| Lee et al. (3) | Competitive | Foundry workers (nonsmoking) | Finland | 13 | 5020 (GM) | 1.4 |
| Competitive | Controls (nonsmoking) | Finland | 10 | 3600 (GM) | ||
| Competitive | Roofers (nonsmoking) | U.S.A. | 12 | 4020 (GM) | 1.5 | |
| Competitive | Controls (nonsmoking) | U.S.A. | 12 | 3280 (GM) | ||
| Omland et al. (21) | Competitive | Foundry workers (nonsmoking) | Denmark | 25 | 580 (median) | 0.83 |
| Competitive | Controls (nonsmoking) | Denmark | 26 | 700 (median) | ||
| Sherson et al. (20) | Competitive | Foundry workers (nonsmoking) | Denmark | 19 | 200 (median) | 5.6 |
| Competitive | Controls (nonsmoking) | Denmark | 19 | 35.9 (median) | ||
Legend: GM, geometric mean.
Specificity of ELISA for BPDE-HSA Adducts
Although 8E11 is a monoclonal antibody raised against BPDE-modified antigens, it is known to cross-react with a range of PAHs and PAH adducts, particularly those with 4 or more rings [3]. While such cross reactivity is not necessarily a disadvantage, in the sense that exposures to PAHs rather than to BPDE are generally of interest, it follows that levels of BPDE-HSA adducts per se can be overestimated by any 8E11-based ELISA. However, the extent of overestimation should be greater for the competitive ELISA than for the sandwich ELISA because the sandwich design is specific for HSA adducts and cannot detect free PAH tetrols, which can arise from both BPDE-HSA adducts and other sources. Some support for the increased specificity of the sandwich design is given in Table 2 where the level of BPDE equivalents measured in control subjects with the sandwich ELISA (GM = 28.9 fmol BPDE equivalents/mg HSA) was roughly a hundred-fold less than the median value for controls in the 4 studies which used the competitive ELISA (2,620 fmol BPDE equivalents/mg HSA).
The specificity of the sandwich ELISA also encourages use of human serum or plasma rather than purified HSA for applications in epidemiologic studies. While direct assays of serum or plasma simplify sample processing, they also open the possibility that binding between 8E11 and PAH adducts may be reduced by matrix effects. Although we saw no signs of such effects in preliminary experiments (Figure 5), more extensive testing of serum and plasma versus purified HSA is warranted in the various populations and specimens of interest.
Extending the Sandwich ELISA to Other HSA Adducts
Competitive ELISAs have been used to quantify a number of protein adducts associated with different chemical exposures, including formaldehyde [22], aflatoxin [23], and other mycotoxins [24]. It is reasonable to expect that our sandwich ELISA design could easily be extended to other HSA adducts by using existing antibodies to capture adducts of interest. Also, a sandwich ELISA that incorporates an adduct-specific capture antibody can be applied to advanced platforms for automation and simultaneous measurement of multiple adducts. For example, a lab-on-a-chip microfluidic device with automated loading and mixing of reagents would vastly increase the throughput of ELISA assays for HSA adducts. Furthermore, the low-volume requirement of the sandwich ELISA (20 μL of serum/plasma) would minimize the use of valuable archived specimens.
Footnotes
Financial support for this work was provided by grant U54ES016115 from the U.S. National Institute for Environmental Health Sciences (NIEHS) through the trans-NIH Genes, Environment and Health Initiative. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institute of Environmental Health Sciences or the National Institutes of Health.
ABC, avidin-biotin complex; ANOVA, analysis of variance; AP, alkaline phosphatase; BaP, benzo[α]pyrene; BPDE, benzo[α]pyrene-diol-epoxide (±); BPDE-I, benzo[α]pyrene-r-7,t-8-dihydrodiol-t-9,10-epoxide (±);BPDE-HSA, HSA adduct of BPDE; CV, coefficient of variation; ELISA, enzyme-linked immunosorbent assay; ESI, electrospray ionization; GM, geometric mean; HRP, horseradish peroxidase; HSA, human serum albumin; LC, liquid chromatograph; MS, mass spectrometer; NFDM, nonfat dry milk; NSB, non-specific binding; PAHs, polycyclic aromatic hydrocarbons; TBS, tris buffered saline; TEA, triethylamine; THF, tetrahydrofuran; TMB, tetramethylbenzidine.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Division of Toxicology and Environmental Medicine. Toxicological Profile for Polycyclic Aromatic Hydrocarbons (PAHs) Agency for Toxic Substances and Disease Registry; Atlanta: 1995. [PubMed] [Google Scholar]
- 2.Tang D, Phillips DH, Stampfer M, Mooney LA, Hsu Y, Cho S, Tsai WY, Ma J, Cole KJ, She MN, Perera FP. Association between carcinogen-DNA adducts in white blood cells and lung cancer risk in the physicians health study. Cancer Research. 2001;61:6708–6712. [PubMed] [Google Scholar]
- 3.Lee BM, Yin BY, Herbert R, Hemminki K, Perera FP, Santella RM. Immunologic measurement of polycyclic aromatic hydrocarbon-albumin adducts in foundry workers and roofers. Scand J Work Environ Health. 1991;17:190–4. doi: 10.5271/sjweh.1711. [DOI] [PubMed] [Google Scholar]
- 4.Tornqvist M, Fred C, Haglund J, Helleberg H, Paulsson B, Rydberg P. Protein adducts: quantitative and qualitative aspects of their formation, analysis and applications. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences. 2002;778:279–308. doi: 10.1016/s1570-0232(02)00172-1. [DOI] [PubMed] [Google Scholar]
- 5.Lin YS, Kupper LL, Rappaport SM. Air samples versus biomarkers for epidemiology. Occup Environ Med. 2005;62:750–760. doi: 10.1136/oem.2004.013102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee BM, Santella RM. Quantitation of protein adducts as a marker of genotoxic exposure: immunologic detection of benzo[a]pyrene--globin adducts in mice. Carcinogenesis. 1988;9:1773–7. doi: 10.1093/carcin/9.10.1773. [DOI] [PubMed] [Google Scholar]
- 7.Wu HC, Wang Q, Wang LW, Yang HI, Ahsan H, Tsai WY, Wang LY, Chen SY, Chen CJ, Santella RM. Polycyclic aromatic hydrocarbon- and aflatoxin-albumin adducts, hepatitis B virus infection and hepatocellular carcinoma in Taiwan. Cancer Letters. 2007;252:104–14. doi: 10.1016/j.canlet.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wild CP, Jiang YZ, Sabbioni G, Chapot B, Montesano R. Evaluation of methods for quantitation of aflatoxin-albumin adducts and their application to human exposure assessment. Cancer Res. 1990;50:245–51. [PubMed] [Google Scholar]
- 9.Waidyanatha S, Zheng Y, Rappaport SM. Determination of polycyclic aromatic hydrocarbons in urine of coke oven workers by headspace solid phase microextraction and gas chromatography-mass spectrometry. Chemico-Biological Interactions. 2003;145:165–74. doi: 10.1016/s0009-2797(02)00255-7. [DOI] [PubMed] [Google Scholar]
- 10.Lin YS, McKelvey W, Waidyanatha S, Rappaport SM. Variability of albumin adducts of 1,4-benzoquinone, a toxic metabolite of benzene, in human volunteers. Biomarkers. 2006;11:14–27. doi: 10.1080/13547500500382975. [DOI] [PubMed] [Google Scholar]
- 11.Serdar B, Waidyanatha S, Zheng Y, Rappaport SM. Simultaneous determination of urinary 1- and 2-naphthols, 3- and 9-phenanthrols, and 1-pyrenol in coke oven workers. Biomarkers. 2003;8:93–109. doi: 10.1080/1354750021000046570. [DOI] [PubMed] [Google Scholar]
- 12.Steiner JM, Williams DA, Moeller EM, Melgarejo T. Development and validation of an enzyme-linked immunosorbent assay for feline trypsin-like immunoreactivity. American Journal of Veterinary Research. 2000;61:620–3. doi: 10.2460/ajvr.2000.61.620. [DOI] [PubMed] [Google Scholar]
- 13.Beck JL, Ambahera S, Yong SR, Sheil MM, de Jersey J, Ralph SF. Direct observation of covalent adducts with Cys34 of human serum albumin using mass spectrometry. Anal Biochem. 2004;325:326–336. doi: 10.1016/j.ab.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 14.Day BW, Skipper PL, Zaia J, Singh K, Tannenbaum SR. Enantiospecificity of covalent adduct formation by benzo[a]pyrene anti-diol epoxide with human serum albumin. Chemical Research in Toxicology. 1994;7:829–35. doi: 10.1021/tx00042a017. [DOI] [PubMed] [Google Scholar]
- 15.Ozbal CC, Skipper PL, Yu MC, London SJ, Dasari RR, Tannenbaum SR. Quantification of (7S,8R)-dihydroxy-(9R,10S)-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene adducts in human serum albumin by laser-induced fluorescence: implications for the in vivo metabolism of benzo[a]pyrene. Cancer Epidemiology, Biomarkers and Prevention. 2000;9:733–739. [PubMed] [Google Scholar]
- 16.Butler JE. Solid supports in enzyme-linked immunosorbent assay and other solid-phase immunoassays. Methods. 2000;22:4–23. doi: 10.1006/meth.2000.1031. [DOI] [PubMed] [Google Scholar]
- 17.Mumford JL, Williams K, Wilcosky TC, Everson RB, Young TL, Santella RM. A sensitive color ELISA for detecting polycyclic aromatic hydrocarbon-DNA adducts in human tissues. Mutat Res. 1996;359:171–7. doi: 10.1016/s0165-1161(96)90264-2. [DOI] [PubMed] [Google Scholar]
- 18.Santella RM, Perera FP, Young TL, Zhang YJ, Chiamprasert S, Tang D, Wang LW, Beachman A, Lin JH, DeLeo VA. Polycyclic aromatic hydrocarbon-DNA and protein adducts in coal tar treated patients and controls and their relationship to glutathione S-transferase genotype. Mutation Research. 1995;334:117–124. doi: 10.1016/0165-1161(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 19.Kure EH, Andreassen A, Ovrebo S, Grzybowska E, Fiala Z, Strozyk M, Chorazy M, Haugen A. Benzo(a)pyrene-albumin adducts in humans exposed to polycyclic aromatic hydrocarbons in an industrial area of Poland. Occupational and Environmental Medicine. 1997;54:662–6. doi: 10.1136/oem.54.9.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherson D, Sabro P, Sigsgaard T, Johansen F, Autrup H. Biological monitoring of foundry workers exposed to polycyclic aromatic hydrocarbons. Br J Ind Med. 1990;47:448–53. doi: 10.1136/oem.47.7.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omland O, Sherson D, Hansen AM, Sigsgaard T, Autrup H, Overgaard E. Exposure of iron foundry workers to polycyclic aromatic hydrocarbons: benzo(a)pyrene-albumin adducts and 1-hydroxypyrene as biomarkers for exposure. Occupational and Environmental Medicine. 1994;51:513–8. doi: 10.1136/oem.51.8.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Wang J, Konig R, Ansari GA, Khan MF. Formaldehyde-protein conjugate-specific antibodies in rats exposed to formaldehyde. J Toxicol Environ Health A. 2007;70:1071–5. doi: 10.1080/15287390601172155. [DOI] [PubMed] [Google Scholar]
- 23.Wu HC, Wang Q, Yang HI, Ahsan H, Tsai WY, Wang LY, Chen SY, Chen CJ, Santella RM. Aflatoxin B1 exposure, hepatitis B virus infection, and hepatocellular carcinoma in Taiwan. Cancer Epidemiology, Biomarkers and Prevention. 2009;18:846–53. doi: 10.1158/1055-9965.EPI-08-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hooper DG, Bolton VE, Guilford FT, Straus DC. Mycotoxin detection in human samples from patients exposed to environmental molds. Int J Mol Sci. 2009;10:1465–75. doi: 10.3390/ijms10041465. [DOI] [PMC free article] [PubMed] [Google Scholar]