Abstract
Soft drinks and other sweetened beverages may contribute to risk of type 2 diabetes and obesity. However, research has not addressed higher risk and Asian populations. The authors examined the association between soft drinks and juice and the risk of type 2 diabetes among Chinese Singaporeans enrolled in a prospective cohort study of 43,580 participants aged 45–74 years and free of diabetes and other chronic diseases at baseline. The incidence of physician-diagnosed type 2 diabetes was assessed by interview and validated; 2,273 participants developed diabetes during follow-up. After adjustment for potential lifestyle and dietary confounders, participants consuming ≥2 soft drinks per week had a relative risk of type 2 diabetes of 1.42 (95% confidence interval (CI): 1.25, 1.62) compared with those who rarely consumed soft drinks. Similarly, consumption of ≥2 juice beverages per week was associated with an increased risk (relative risk (RR) = 1.29, 95% CI: 1.05, 1.58). The association was modified by 5-year weight gain for ≥2 soft drinks per week among those who gained ≥3 kg (RR = 1.70, 95% CI: 1.34, 2.16) compared with those who gained less weight (RR = 1.20, 95% CI: 1.03, 1.41). Relatively frequent intake of soft drinks and juice is associated with an increased risk for development of type 2 diabetes in Chinese men and women.
Keywords: Asian continental ancestry group; carbonated beverages; diabetes mellitus, type 2
Rates of type 2 diabetes mellitus in Asia are reaching epidemic proportions, with prevalence rates now 3–5 times what they were approximately 30 years ago in Southeast Asia. There is evidence that this increase is occurring rapidly in areas of China and India and in younger age groups (1). In Singapore, the prevalence in Chinese nearly doubled from 1984 (4.7%) to 1998 (8.0%) (2). These increases in Southeast Asia are beyond the increase in rates observed in the United States and other parts of the world (1, 3). Substantive shifts in socioeconomic, demographic, and lifestyle patterns are thought to be responsible (1, 4). This epidemic is predicted to have a large economic burden due to the numerous complications and deaths that would result (5). One well-documented aspect of change that may contribute to diabetes risk in Asia and elsewhere is increased consumption of soft drinks, juice drinks, and other sweetened drinks (6–8). Increased consumption of these beverages in the United States has been linked cross-sectionally and prospectively with obesity in children and adults (6, 9–12), although the evidence is not conclusive (13–15), and has been associated with metabolic disturbances and type 2 diabetes (16–21).
Identifying potential modifiable risk factors in the development of type 2 diabetes is increasingly important because of the growing global burden of the disease. Given the levels of soft drink and other juice drink consumption worldwide (22) and the trends of increased consumption observed in the United States and developing countries in Asia (22–24), dietary interventions or policy changes aimed at reducing levels of consumption could have important public health effects if associations with health outcomes are causal. However, there are few studies on this topic and none that we are aware of outside the United States on high-risk populations.
The Singapore Chinese Health Study is a population-based prospective cohort investigation of over 63,000 Chinese men and women in Singapore. There are no studies we are aware of examining the consumption of soft drinks and juices and the risk of type 2 diabetes among Asians. Therefore, the aim of this paper was to investigate the nature of the association between consumption of soft drinks and juices and the risk of incident type 2 diabetes.
MATERIALS AND METHODS
From April 1993 through December 1998, a total of 63,257 Chinese women and men aged 45–74 years enrolled in the study (25). Study subjects were restricted to the 2 major dialect groups of Chinese in Singapore, that is, the Hokkiens and the Cantonese, who originated from the contiguous provinces of Fujian and Guangdong, respectively, in the southern part of China (26). Participants were residents of government-built housing estates, where 86% of the Singapore population resided during the enrollment period (25). Recruitment occurred by an initial letter informing potential participants of the study and inviting them to participate. Approximately 85% of eligible subjects who were invited responded positively (25). At recruitment, a face-to-face interview was conducted in the subject's home by a trained interviewer using a structured, scanner-readable questionnaire that requested information on demographics, height, weight, use of tobacco, usual physical activity, menstrual and reproductive history (women only), medical history, family history of cancer, and a 165-item food frequency section assessing usual dietary intake during the previous year (25). A follow-up telephone interview took place between 1999 and 2004 for 52,325 cohort members (83% of the recruited cohort), and questions were asked to update tobacco and alcohol use, medical history, and menopausal status of women. The institutional review boards at the National University of Singapore and the University of Minnesota approved this study.
Assessment of soft drink and juice intake and other covariates
A semiquantitative food frequency questionnaire specifically developed for this population assessing 165 commonly consumed food items was administered during the baseline interview. During the interview, the respondent referred to accompanying photographs to select from 8 food frequency categories (ranging from “never or hardly ever” to “two or more times a day”) and 3 portion sizes. The food frequency questionnaire has subsequently been validated against a series of 24-hour dietary recall interviews in a random sample of ≥1,000 participants that occurred on 1 weekday and 1 weekend day approximately 2 months apart (25), as well as selected biomarker studies (27, 28). A range of 0.24–0.79 in correlation coefficients of energy/nutrients was obtained using the 2 methods, and the majority of macronutrients and food groups display correlation coefficients in the high end of this reported range (25).
Two different questions from the food frequency questionnaire specifically asked study subjects to report the intake frequency of 1) soft drinks such as Coca-Cola (The Coca-Cola Company, Atlanta, Georgia) and 7UP (Dr. Pepper Snapple Group, Plano, Texas), 1 glass, and 2) other fruit and vegetable juices, 1 glass, packet, or hawker portion from 9 predefined categories (never or hardly ever, 1–3 times a month, once a week, 2–3 times a week, 4–6 times a week, once a day, 2–3 times a day, 4–5 times a day, and 6 or more times a day). Hawker centers are ubiquitous in Singapore and other parts of Asia, serve a variety of foods all day long, and resemble fast-food courts in US shopping malls. One glass was assigned a value of 237 mL or approximately 1 cup. However, we note that there is likely heterogeneity in serving size, and our analysis is focused on frequency.
In conjunction with this cohort, the Singapore Food Composition Table was developed, a food-nutrient database that lists the levels of 96 nutritive/nonnutritive components per 100 g of cooked food and beverages in the diet of the Singaporean Chinese. By combining information obtained from the food frequency questionnaire with nutrient values provided in this food-nutrient database, we were able to compute the mean daily intakes of nutrients for each subject (25).
Other known or suspected risk factors for diabetes assessed with the baseline questionnaire included the following: age (years); smoking habits/status (age started/quit, amount, frequency, type); highest educational level reached; body mass index (kg/m2) calculated by using self-reported height and weight; and amount (hours) of moderate (e.g., brisk walking, bicycling on level ground) and strenuous (e.g., jogging, bicycling on hills, tennis) physical activity on a weekly basis. Weight change was calculated by subtracting the baseline weight (kg) from the follow-up weight (kg).
Assessment of diabetes
Self-reported diabetes as diagnosed by a physician was evaluated at baseline, and participants with a history of diagnosed diabetes were excluded from analysis. Diabetes status was assessed again by the following question asked during the follow-up telephone interview: “Have you been told by a doctor that you have diabetes (high blood sugar)?” If yes: “Please also tell me the age at which you were first diagnosed.” Participants were classified as having incident diabetes if they reported developing diabetes anytime between the initial enrollment interview and the follow-up telephone interview that occurred between July 1999 and October 2004. The average follow up time was 5.7 years.
A validation study of the incident diabetes mellitus cases used 2 different methods and is reported in detail in the report by Odegaard et al. (29). First, cases were ascertained through linkage with hospital records in a nationwide, hospital-based discharge summary database, an administrative database in the Singapore Ministry of Health (30). If subjects in the study had been admitted to hospitals for diagnoses carrying diabetes-related International Classification of Diseases, Ninth Revision, codes 250.00–250.92 after recruitment into the cohort, they were considered a valid case. Cases that did not have hospitalization records available with diabetes-related diagnoses were contacted to answer a supplementary questionnaire regarding symptoms, diagnostic tests, and hypoglycemic therapy during a telephone interview. A valid case had the following 3 criteria: 1) confirmed diagnosis later than the baseline interview date, 2) diabetes still present at the time of interview, and 3) use of oral medications or insulin injections to treat diabetes. On the basis of these criteria, we observed a positive predictive value of 99%, as previously described (29).
An alternative approach was used to examine those who did not report being diagnosed with diabetes at the baseline or follow-up interview. As part of an ongoing genome-wide association study of type 2 diabetes in this study population, potential control subjects were randomly selected who answered “no” to the question of diabetes diagnosis at baseline and follow-up and who provided blood samples at their first follow-up interview. Frozen red blood cell samples were shipped to the University of Minnesota on dry ice, where they were analyzed for percentage of hemoglobin A1c (HbA1c) (glycated hemoglobin) in a Clinical Laboratory Improvement Amendments-certified laboratory. HbA1c is measured in ethylenediaminetetraacetic acid-treated whole blood on a Tosoh G7 HPLC Glycohemoglobin Analyzer (Tosoh Medics, Inc., San Francisco, California) using an automated high-performance liquid chromatography method. This method is calibrated utilizing standard values derived by the National Glycohemoglobin Standardization Program. The reference range is 4.3%–6.0% with a laboratory coefficient of variation range of 1.4%–1.9% (31). To date, 2,625 samples have been analyzed, with 148 subjects (5.6% of the sample) having an HbA1c ≥6.5%, meeting the most recent diagnostic guidelines for the presence of diabetes (32). Thus, 94.4% of persons who reported being free of diabetes at baseline and follow-up were below the HbA1c threshold for diabetes, yielding a very high negative predictive value.
Statistical analysis
We excluded from analysis any participants who died before the follow-up interview (n = 7,722); reported baseline diabetes (n = 5,469) or cancer, heart disease, or stroke (n = 5,975); reported implausibly high (>5,000 kcal) or low (<600 kcal) energy intakes; or were lost to follow-up (<0.5%). These exclusions, along with further exclusion of 20 participants whose diabetes status was not clear after the validation effort, left 43,580 participants in the present analyses.
Person-years for each participant were calculated from the year of recruitment to the year of reported type 2 diabetes diagnosis or the year of follow-up telephone interview for those who did not report diabetes diagnoses. Relative risks per category of soft drink and juice consumption were estimated by Cox proportional hazards regression models with simultaneous adjustment for demographic, lifestyle, and dietary variables. All regression analyses were conducted by using SAS, version 9.1, statistical software (PROC TPHREG; SAS Institute, Inc., Cary, North Carolina). There was no evidence that proportional hazards assumptions were violated as indicated by the lack of significant interaction between the predictors and a function of survival time in the model. Soft drink and juice categories were based on intakes that allowed for logical cutpoints and provided sufficient participants and cases per category and are as follows: never or hardly ever (0 servings), monthly (1–3 servings a month), 1 time a week, and 2 or more times a week. We combined all participants reporting 2–3 servings a week and above because of a lack of statistical power in the above levels. However, the associations that we observed at 2–3 servings a week in the population for both soft drink and juice consumption persisted regardless of how consumption was categorized and did not materially differ in magnitude from the combination of 2–3 servings a week category with the collective upper categories. The top categories of soft drink and juice are defined by their median. Tests for trend were performed by assigning the median value of soft drink or juice consumption to the respective categories and entering this as a continuous variable into the models.
Four main models were constructed including risk factors known to be associated with type 2 diabetes, with the final 2 models including body mass index (kg/m2), total energy intake, and weight gain, which may be on the causal pathway between the beverage intakes and type 2 diabetes risk. Model 1 included baseline age (<50, 50–54, 55–59, 60–64, ≥65 years), year of interview (1993–1995 and 1996–1998), dialect (Hokkiens vs. Cantonese), and sex. Model 2 included the variables in model 1 plus education (none, primary, secondary or more); smoking (no, former, current); alcohol intake (no, monthly, weekly, daily); moderate activity (0, 0.5–3 hours/week, ≥4 hours/week) and strenuous activity (0, 0.5–2 hours/week, >2 hours/week); total dairy intake as quintiles (g/day); fiber intake (g/day); saturated fat (g/day); and coffee (nondaily, once per day, 2–3 times/day, ≥4 times/day), plus adjustment for the soft drink or juice variable that was not the main exposure of interest. Model 3 included those variables in model 2, plus baseline body mass index (kg/m2 as the original body mass index and its quadratic term (body mass index2)) and total energy intake, as these may represent mediators rather than confounders. Similarly, model 4 included the variables in model 3 plus weight gain (kg) continuously.
We also calculated the mean weight change per level of beverage consumption between the baseline period and follow-up. The mean weight change was calculated as the difference in kilograms between baseline weight and the reported weight during the follow-up interview. General linear modeling was used (PROC GLM; SAS Institute, Inc.) for these weight-gain analyses with the same demographic and lifestyle covariate adjustments as described above, plus adjustment for time between baseline and follow-up interview, and for total intakes (g/day) of fruits, vegetables, dairy products, meat, candy, and desserts.
We hypothesized that there might be a biologically plausible interaction between soft drink intake and weight gain, with accelerated diabetes risk among those high in soft drink consumption and relatively high in body weight gain over time, so we tested for an interaction between soft drink intake and weight gain as a continuous variable. For presentation of the analysis, the soft drink categories were collapsed into <2 drinks/week (referent) and ≥2 drinks/week, and weight gain was transformed from a continuous variable into a dichotomous variable of the top quarter (n = 11,922; 596 cases) of weight gain in participants (≥3 kg over follow-up) and all other participants (<3 kg over follow-up).
RESULTS
Of 43,580 men and women with a mean age of 54.8 (standard deviation, 7.5) years and a mean follow-up of 5.7 years, 2,273 developed type 2 diabetes (approximately 5.2%). The characteristics of the study population according to consumption of soft drinks and juice are presented in Table 1. Participants with a higher intake of soft drinks were younger and more likely to be male, with higher body mass indexes and lower levels of physical activity, higher levels of smoking and alcohol consumption, higher total energy intake, and lower dietary fiber intake. Participants with higher levels of juice intake were younger and more likely to be male, with higher levels of physical activity and education, higher levels of smoking and alcohol consumption, and higher total energy and fiber intake.
Table 1.
Baseline Characteristics According to Frequency of Soft Drink and Juice Consumption in the Singapore Chinese Health Study, 1993–2004a
| Characteristic | Frequency of Soft Drink Consumption |
Ptrend | |||
| Almost Never (n = 32,060) | 1–3/Month (n = 4,514) | 1/Week (n = 2,389) | 2–≥3/Weekb (n = 4,617) | ||
| No. of cases | 1,615 | 247 | 111 | 300 | |
| No. of person-years | 185,645 | 25,285 | 13,104 | 25,140 | |
| Soft drinks/week | 0 | 0.5 | 1.0 | 5.2 | <0.0001 |
| Age, years | 55.9 | 54.1 | 53.0 | 52.7 | <0.0001 |
| Sex, female (%) | 59.0 | 59.0 | 54.0 | 44.1 | <0.0001 |
| Body mass index, kg/m2 | 22.9 | 23.1 | 23.2 | 23.3 | <0.0001 |
| Moderate activity, minutes/week | 54.0 | 46.0 | 42.0 | 40.0 | <0.0001 |
| Education, % secondary | 31.9 | 36.0 | 43.9 | 40.4 | <0.0001 |
| Alcohol, drinks/week | 0.9 | 0.8 | 1.0 | 1.4 | <0.0001 |
| Smoking, ever (%) | 26.6 | 24.3 | 26.1 | 34.0 | <0.0001 |
| Energy intake, kcal/day | 1,525 | 1,585 | 1,700 | 1,901 | <0.0001 |
| Saturated fat, % kcal | 8.8 | 9.2 | 9.5 | 9.4 | <0.0001 |
| Carbohydrate, % kcal | 59.2 | 58.5 | 58.0 | 58.8 | <0.0001 |
| Dietary fiber, g/1,000 kcal | 8.4 | 8.1 | 8.0 | 7.5 | <0.0001 |
| Frequency of Juice Consumption |
|||||
| Almost Never (n = 35,719) | 1–3/Month (n = 4,399) | 1/Week (n = 1,791) | 2–≥3/Week (n = 1,671) | ||
| No. of cases | 1,871 | 223 | 80 | 99 | |
| No. of person-years | 205,272 | 24,603 | 10,030 | 9,269 | |
| Juice drinks/week | 0 | 0.5 | 1.0 | 3.8 | |
| Age, years | 55.8 | 52.7 | 52.3 | 52.9 | <0.0001 |
| Sex, female (%) | 59.0 | 52.0 | 50.4 | 46.2 | <0.0001 |
| Body mass index, kg/m2 | 23.0 | 23.2 | 23.1 | 23.1 | 0.01 |
| Moderate activity, minutes/week | 50.0 | 52.0 | 55.0 | 65.0 | <0.0001 |
| Education, % secondary | 30.6 | 44.4 | 52.3 | 53.1 | <0.0001 |
| Alcohol, drinks/week | 0.9 | 1.0 | 1.3 | 1.6 | <0.0001 |
| Smoking, ever (%) | 26.7 | 28.7 | 27.6 | 31.0 | 0.003 |
| Energy intake, kcal/day | 1,540 | 1,661 | 1,856 | 1,929 | <0.0001 |
| Saturated fat, % kcal | 8.8 | 9.3 | 9.5 | 9.3 | <0.0001 |
| Carbohydrate, % kcal | 59.3 | 58.3 | 56.9 | 57.7 | <0.0001 |
| Dietary fiber, g/1,000 kcal | 8.1 | 8.3 | 8.8 | 9.3 | <0.0001 |
Data are means unless noted as percentages (%).
The highest category of consumption (2–≥3/week) is defined by the median value.
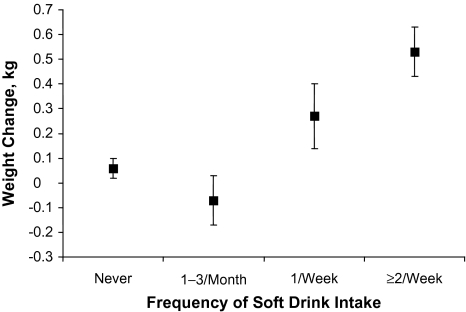
The overall mean weight change for the cohort was 0.10 (standard error, 0.03) kg. Participants in the highest category of soft drink consumption had a subtle but significant increase in weight (0.53 kg) compared with those who did not consume soft drinks or reported only monthly consumption (P < 0.001) (Figure 1). There was no association between intake of juice drinks and change in mean weight between baseline and follow-up.
Figure 1.
Mean weight change by soft drink intake category in the Singapore Chinese Health Study, 1993–2004. Results were adjusted for age, sex, dialect, year of interview, person-years, education, smoking, alcohol, body mass index, and total intakes (g/day) of fruits, vegetables, dairy products, meat, candy, and desserts, as well as dietary fiber, saturated fat, juice, and coffee. Bars represent the standard error of the estimated mean weight change between the baseline and follow-up interviews. The mean follow-up time was 5.7 years.
The relative risks for incident type 2 diabetes mellitus by soft drink and juice intake are given in Table 2. In all models, increasing consumption of soft drinks and juice is associated with an increased risk of incident type 2 diabetes mellitus. We observed a 42% increased risk in participants reporting ≥2 soft drinks per week (relative risk (RR) = 1.42, 95% confidence interval (CI): 1.25, 1.62) compared with those consuming no soft drinks after adjustment for demographic, lifestyle, and dietary factors. This association was slightly attenuated in model 3 upon adjustment for body mass index (kg/m2) and total energy intake (RR = 1.34, 95% CI: 1.17, 1.52) but was not further attenuated upon adjustment for weight change. Similarly, we observed a 29% increased risk in participants reporting ≥2 juice drinks per week (RR = 1.29, 95% CI: 1.05, 1.58; Ptrend = 0.03) compared with those consuming no juice drinks after adjustment for demographic, lifestyle, and dietary factors. Further adjustment for body mass index (kg/m2) and energy intake did not materially change this observation (RR = 1.24, 95% CI: 1.01, 1.53). Hypothesized tests for interaction between beverage intakes and body mass index, sex, and age, as well as stratification efforts, provided no evidence of any effect modification. An analysis excluding diabetes cases with less than 2 years of follow-up time did not materially alter the results.
Table 2.
Relative Risks of Incident Type 2 Diabetes Mellitus According to Soft Drink and Juice Consumption in the Singapore Chinese Health Study, 1993–2004
| Characteristic | Frequency of Consumption |
Ptrend | |||||||
| Almost Never |
1–3/Month |
1/Week |
2–≥3/Weeka |
||||||
| Relative Risk | 95% Confidence Interval | Relative Risk | 95% Confidence Interval | Relative Risk | 95% Confidence Interval | Relative Risk | 95% Confidence Interval | ||
| Soft drink consumption | |||||||||
| No. of cases | 1,615 | 247 | 111 | 300 | |||||
| No. of person-yearsb | 185,645 | 25,285 | 13,104 | 25,140 | |||||
| Model 1c | 1.0 | Referent | 1.14 | 0.99, 1.30 | 1.03 | 0.85, 1.25 | 1.46 | 1.29, 1.66 | <0.0001 |
| Model 2d | 1.0 | Referent | 1.14 | 0.99, 1.30 | 1.02 | 0.84, 1.23 | 1.42 | 1.25, 1.62 | <0.0001 |
| Model 3e | 1.0 | Referent | 1.11 | 0.97, 1.26 | 0.98 | 0.81, 1.29 | 1.34 | 1.17, 1.52 | <0.0001 |
| Juice consumption | |||||||||
| No. of cases | 1,871 | 223 | 80 | 99 | |||||
| No. of person-yearsb | 205,272 | 24,603 | 10,030 | 9,269 | |||||
| Model 1 | 1.0 | Referent | 1.04 | 0.90, 1.19 | 0.95 | 0.76, 1.18 | 1.23 | 1.01, 1.51 | 0.08 |
| Model 2 | 1.0 | Referent | 1.04 | 0.90, 1.19 | 0.98 | 0.78, 1.22 | 1.29 | 1.05, 1.58 | 0.03 |
| Model 3 | 1.0 | Referent | 1.00 | 0.87, 1.16 | 0.94 | 0.75, 1.18 | 1.24 | 1.01, 1.53 | 0.09 |
| Model 4f | 1.0 | Referent | 1.00 | 0.87, 1.16 | 0.94 | 0.75, 1.18 | 1.24 | 1.01, 1.53 | 0.09 |
The highest category of consumption (2–≥3/week) is defined by the median value.
Person-year follow-up time.
Model 1: adjusted for age, sex, dialect, and year of interview.
Model 2: model 1 + educational level, smoking status, alcohol use, physical activity, saturated fat intake, dietary fiber intake, dairy intake, juice or soft drink intake depending on model, and coffee consumption.
Model 3: model 2 + body mass index (kg/m2) and energy intake (kcal/day).
Model 4: model 3 + weight gain (kg) continuously.
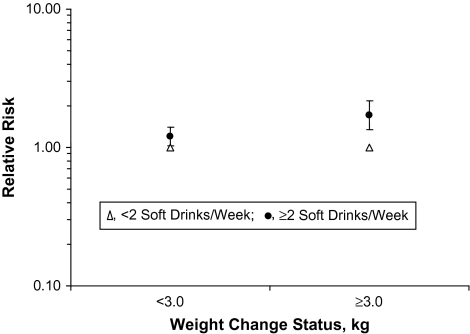
A test for interaction between soft drink intake and weight gain over time was statistically significant (P = 0.007). The relative risks of diabetes by soft drink intake stratified by weight gain category are presented in Figure 2 and include the same variables from model 3. Incidence rates per 10,000 person-years of follow-up time were 84 for those who gained <3 kg and reported <2 soft drinks per week, 110 for <3 kg and ≥2 soft drinks per week, 101 for gaining ≥3 kg and <2 soft drinks per week, and 148 for those who gained ≥3 kg and ≥2 soft drinks per week. Diabetes risk appears more pronounced among those who reported gaining at least a moderate amount of weight over time (≥3 kg) and also were in the highest soft drink consumption group. In this group, we observed a relative risk increase of 70% (RR = 1.70, 95% CI: 1.34, 2.16) compared with those with lower soft drink intakes after adjustment for demographic and lifestyle characteristics plus body mass index and energy intake. Those who gained less weight but were in the highest soft drink consumption category had a smaller increase in risk (RR = 1.20, 95% CI: 1.03, 1.40). A test for interaction between juice consumption and weight gain was not significant (P = 0.51).
Figure 2.
Relative risks of type 2 diabetes by soft drink intake stratified by follow-up time weight change status in the Singapore Chinese Health Study, 1993–2004. Results were adjusted for demographic, lifestyle, and dietary characteristics plus body mass index and energy intake. The mean follow-up time was 5.7 years. A 3.0-kg or greater weight gain represents the top quarter of weight gain in the population and corresponds to 11,922 participants and 596 diabetes cases.
DISCUSSION
In this large prospective cohort of Chinese men and women in Singapore, we observed a positive association between soft drink and juice consumption and increased risk of type 2 diabetes. We also observed subtle but significant overall weight gain associated with soft drink consumption. Furthermore, the association of soft drink consumption and diabetes risk appeared to vary by level of weight gain, with a stronger association among those in the high soft drink category who also had moderate to high (≥3 kg) weight gains.
Our results are consistent with those from the few prospective studies on this topic (16, 17, 20, 21), while one study found no association (33). They are also in agreement with those from cross-sectional studies (16, 19). To our knowledge, there are no trials examining the effects of sugar-sweetened beverage intake on risk of diabetes. Studies examining how soft drink and juice type drinks contribute to or cause obesity are growing in number (6, 10, 17, 34), although the precise mechanisms for any effects on energy balance are a matter of continued investigation (13–15).
Although obesity is the strongest modifiable risk factor for type 2 diabetes, the associations we observed were not explained by adjustment for total energy intake, body mass index, and weight change in the main analysis without stratification. These findings are in contrast with those from another study on this topic that suggested that the association between soft drinks and type 2 diabetes, but not juice drinks and type 2 diabetes, was modified by body mass index (21). Our stratified results suggest that higher soft drink consumption is associated with increased risk of diabetes independent of body mass index or level of weight gain, and that the combination of higher weight gain and higher soft drink intake appears to be at least additive for increasing diabetes risk. Therefore, our findings may support other mechanisms in addition to energy balance.
Ingestion of soft drinks and juices tends to cause rapid increase in blood sugar and insulin relative to many other beverages and foods (35). When portion sizes and frequency of intake are taken into account, the chronic impact of postprandial glycemia and insulinemia due to frequent ingestion of these high-sugar beverages could have deleterious effects on the pancreatic beta cells and therefore raise the risk of type 2 diabetes in susceptible individuals. Indeed, a diet of high glycemic index foods and beverages has been shown to be a risk factor for type 2 diabetes in some cohort studies (36–38), but not in others (39). Related research suggests that this type of diet may be more pernicious for diabetes risk when combined with overweight or obese statuses (40, 41). Others have hypothesized that this possible effect, if causal, may be exacerbated in susceptible populations (42).
Additionally, our finding that juice consumption is associated with increased diabetes risk has been examined with mixed findings (17, 20, 21, 33). Fruit punch, but not fruit juice, was associated with an increased risk of diabetes in the Nurses’ Health Study II (17), while sweetened juice was associated with an increased risk in a Finnish population (20), and there was no association between juice and diabetes risk in another US cohort (33). The Black Women's Health Study also found a positive association, which, similar to our study, persisted after adjusting for weight gain and body mass index (21). It is possible that juice, especially those highly processed with added sugar, may have a similar consequential metabolic action as soft drinks in type 2 diabetes pathophysiology. Essentially, when components of fruit, such as naturally occurring soluble fiber, vitamins, minerals, and phytochemicals, along with other known and unknown components of whole fruit, are removed or diminished in processing and sugar/sweeteners are concentrated, the juice product will be nutritionally poor and energy-dense relative to the original whole fruit. However, we interpret our juice findings cautiously and note that the actual juice consumed and its nutritional composition are variable and not directly measured in our study.
To our knowledge, this is the first large prospective study addressing the topic of soft drink and juice consumption and incident type 2 diabetes in an Asian population. The combination of a generally sedentary lifestyle and dietary pattern that has increasingly more Western influences with high susceptibility to diabetes in this population suggests that these results may be of particular relevance to dietary modification and prevention approaches in Singapore, as well as similar populations. Other strengths of this study include the high participant response rate and the facts that less than 0.5% of participants eligible for analysis were lost to follow-up, the data were obtained through a detailed face-to-face interview including a food frequency questionnaire specific to this population, and the diabetes case status was validated with a high positive and negative predictive value. The advanced and high level of medical care for all residents of Singapore likely contributes to the high validity of diabetes self-report in this study.
Limitations include potential misclassification of the exposures due to self-report; this would likely bias the results toward the null assuming it is nondifferential in nature. Residual confounding as an explanation also needs to be considered, specifically with lack of data on family history of diabetes as well as with self-reported dietary, body mass index (baseline and follow-up), and physical activity variables. Furthermore, interpretation of weight change and the influence of adjustment for body mass index and weight change in the diabetes risk models should be cautious because of the use of 2 self-reports in this cohort. Finally, these results may only apply to physician-diagnosed diabetes. Even with high levels of validity, there is potential for numerous undiagnosed cases of type 2 diabetes due to the nature of the disease. If increased soft drink or juice consumption led to increased symptomatic diabetes and physician diagnosis, the associations could be overestimated. Conversely, if the beverage habits were not related to diagnosis of type 2 diabetes, the estimates could be underestimated.
In conclusion, we observed a significant increased risk of type 2 diabetes in Chinese men and women of Singapore with higher relative consumption of soft drinks and juices. The association was stronger in those who gained weight but persisted regardless of weight change status or body mass index. We also observed modest but significant weight gain among those consuming higher levels of soft drinks. Further analyses and clinical investigations are needed to understand which mechanisms may be involved with respect to the impact of soft drinks and juices on diabetes risk. Nonetheless, public health and practitioner efforts to reduce the consumption of nutritionally poor soft drinks and certain juices, especially with increased marketing and consumption patterns across the globe, may help to prevent type 2 diabetes.
Acknowledgments
Author affiliations: University of Minnesota, Minneapolis, Minnesota (Andrew O. Odegaard, Kazuko Arakawa, Mimi C. Yu, Mark A. Pereira); and National University of Singapore, Singapore, Singapore (Woon-Puay Koh).
Funding was from the National Institutes of Health (NCI R01 CA55069, R35 CA53890, R01 CA80205, and R01 DK080720).
The authors thank Siew-Hong Low of the National University of Singapore for supervising the fieldwork of the Singapore Chinese Health Study and Kazuko Arakawa and Renwei Wang for the development and maintenance of the cohort study database.
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- HbA1c
hemoglobin A1c
- RR
relative risk
References
- 1.Yoon KH, Lee JH, Kim JW, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368(9548):1681–1688. doi: 10.1016/S0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- 2.Lee WR. The changing demography of diabetes mellitus in Singapore. Diabetes Res Clin Pract. 2000;50(suppl 2):S35–S39. doi: 10.1016/s0168-8227(00)00184-4. [DOI] [PubMed] [Google Scholar]
- 3.Gregg EW, Cadwell BL, Cheng YJ, et al. Trends in the prevalence and ratio of diagnosed to undiagnosed diabetes according to obesity levels in the U.S. Diabetes Care. 2004;27(12):2806–2812. doi: 10.2337/diacare.27.12.2806. [DOI] [PubMed] [Google Scholar]
- 4.Cheung BM, Thomas GN. The metabolic syndrome and vascular disease in Asia. Cardiovasc Hematol Disord Drug Targets. 2007;7(2):79–85. doi: 10.2174/187152907780830914. [DOI] [PubMed] [Google Scholar]
- 5.Annual Data Report; Atlas of Chronic Kidney Disease & End-Stage Renal Disease in the United States. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health; 2007. [Google Scholar]
- 6.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79(4):537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 7.Ismail AI, Tanzer JM, Dingle JL. Current trends of sugar consumption in developing societies. Community Dent Oral Epidemiol. 1997;25(6):438–443. doi: 10.1111/j.1600-0528.1997.tb01735.x. [DOI] [PubMed] [Google Scholar]
- 8.Basu M. Diabetes, obesity and soft drinks. Natl Med J India. 2007;20(2):102–103. [PubMed] [Google Scholar]
- 9.Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet. 2001;357(9255):505–508. doi: 10.1016/S0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- 10.Berkey CS, Rockett HR, Field AE, et al. Sugar-added beverages and adolescent weight change. Obes Res. 2004;12(5):778–788. doi: 10.1038/oby.2004.94. [DOI] [PubMed] [Google Scholar]
- 11.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84(2):274–288. doi: 10.1093/ajcn/84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross LS, Li L, Ford ES, et al. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: an ecologic assessment. Am J Clin Nutr. 2004;79(5):774–779. doi: 10.1093/ajcn/79.5.774. [DOI] [PubMed] [Google Scholar]
- 13.Pereira MA. The possible role of sugar-sweetened beverages in obesity etiology: a review of the evidence. Int J Obes (Lond) 2006;30(suppl 3):S28–S36. [Google Scholar]
- 14.Drewnowski A, Bellisle F. Liquid calories, sugar, and body weight. Am J Clin Nutr. 2007;85(3):651–661. doi: 10.1093/ajcn/85.3.651. [DOI] [PubMed] [Google Scholar]
- 15.Bachman CM, Baranowski T, Nicklas TA. Is there an association between sweetened beverages and adiposity? Nutr Rev. 2006;64(4):153–174. doi: 10.1111/j.1753-4887.2006.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 16.Dhingra R, Sullivan L, Jacques PF, et al. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation. 2007;116(5):480–488. doi: 10.1161/CIRCULATIONAHA.107.689935. [DOI] [PubMed] [Google Scholar]
- 17.Schulze MB, Manson JE, Ludwig DS, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292(8):927–934. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 18.Schulze MB, Hoffmann K, Manson JE, et al. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr. 2005;82(3):675–684. doi: 10.1093/ajcn.82.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida M, McKeown NM, Rogers G, et al. Surrogate markers of insulin resistance are associated with consumption of sugar-sweetened drinks and fruit juice in middle and older-aged adults. J Nutr. 2007;137(9):2121–2127. doi: 10.1093/jn/137.9.2121. [DOI] [PubMed] [Google Scholar]
- 20.Montonen J, Järvinen R, Knekt P, et al. Consumption of sweetened beverages and intakes of fructose and glucose predict type 2 diabetes occurrence. J Nutr. 2007;137(6):1447–1454. doi: 10.1093/jn/137.6.1447. [DOI] [PubMed] [Google Scholar]
- 21.Palmer JR, Boggs DA, Krishnan S, et al. Sugar-sweetened beverages and incidence of type 2 diabetes mellitus in African American women. Arch Intern Med. 2008;168(14):1487–1492. doi: 10.1001/archinte.168.14.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zenith International's Report on Global Soft Drinks. Bath, United Kingdom: Zenith International, Ltd; 2008. [Google Scholar]
- 23.Agri-Food—Past, Present & Future Report: Singapore. Ottawa, Canada: Canadian Department of Agriculture; 2007. [Google Scholar]
- 24.Kiang MYP. Trends in Food Consumption. Singapore, Singapore: Department of Statistics, Singapore Government; 1998. [Google Scholar]
- 25.Hankin JH, Stram DO, Arakawa K, et al. Singapore Chinese Health Study: development, validation, and calibration of the quantitative food frequency questionnaire. Nutr Cancer. 2001;39(2):187–195. doi: 10.1207/S15327914nc392_5. [DOI] [PubMed] [Google Scholar]
- 26.Koh WP, Yuan JM, Sun CL, et al. Middle-aged and older Chinese men and women in Singapore who smoke have less healthy diets and lifestyles than nonsmokers. J Nutr. 2005;135(10):2473–2477. doi: 10.1093/jn/135.10.2473. [DOI] [PubMed] [Google Scholar]
- 27.Seow A, Shi CY, Chung FL, et al. Urinary total isothiocyanate (ITC) in a population-based sample of middle-aged and older Chinese in Singapore: relationship with dietary total ITC and glutathione S-transferase M1/T1/P1 genotypes. Cancer Epidemiol Biomarkers Prev. 1998;7(9):775–781. [PubMed] [Google Scholar]
- 28.Seow A, Shi CY, Franke AA, et al. Isoflavonoid levels in spot urine are associated with frequency of dietary soy intake in a population-based sample of middle-aged and older Chinese in Singapore. Cancer Epidemiol Biomarkers Prev. 1998;7(2):135–140. [PubMed] [Google Scholar]
- 29.Odegaard AO, Pereira MA, Koh WP, et al. Coffee, tea, and incident type 2 diabetes: the Singapore Chinese Health Study. Am J Clin Nutr. 2008;88(4):979–985. doi: 10.1093/ajcn/88.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heng DM, Lee J, Chew SK, et al. Incidence of ischaemic heart disease and stroke in Chinese, Malays and Indians in Singapore: Singapore Cardiovascular Cohort Study. Ann Acad Med Singapore. 2000;29(2):231–236. [PubMed] [Google Scholar]
- 31.Steffes MW, Cleary P, Goldstein D, et al. Hemoglobin A1c measurements over nearly two decades: sustaining comparable values throughout the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications Study. Clin Chem. 2005;51(4):753–758. doi: 10.1373/clinchem.2004.042143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paynter NP, Yeh HC, Voutilainen S, et al. Coffee and sweetened beverage consumption and the risk of type 2 diabetes mellitus: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2006;164(11):1075–1084. doi: 10.1093/aje/kwj323. [DOI] [PubMed] [Google Scholar]
- 34.DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes Relat Metab Disord. 2000;24(6):794–800. doi: 10.1038/sj.ijo.0801229. [DOI] [PubMed] [Google Scholar]
- 35.Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care. 2008;31(12):2281–2283. doi: 10.2337/dc08-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schulze MB, Liu S, Rimm EB, et al. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am J Clin Nutr. 2004;80(2):348–356. doi: 10.1093/ajcn/80.2.348. [DOI] [PubMed] [Google Scholar]
- 37.Salmerón J, Manson JE, Stampfer MJ, et al. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA. 1997;277(6):472–477. doi: 10.1001/jama.1997.03540300040031. [DOI] [PubMed] [Google Scholar]
- 38.Salmerón J, Ascherio A, Rimm EB, et al. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care. 1997;20(4):545–550. doi: 10.2337/diacare.20.4.545. [DOI] [PubMed] [Google Scholar]
- 39.Meyer KA, Kushi LH, Jacobs DR, Jr, et al. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr. 2000;71(4):921–930. doi: 10.1093/ajcn/71.4.921. [DOI] [PubMed] [Google Scholar]
- 40.Hodge AM, English DR, O'Dea K, et al. Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care. 2004;27(11):2701–2706. doi: 10.2337/diacare.27.11.2701. [DOI] [PubMed] [Google Scholar]
- 41.Villegas R, Liu S, Gao YT, et al. Prospective study of dietary carbohydrates, glycemic index, glycemic load, and incidence of type 2 diabetes mellitus in middle-aged Chinese women. Arch Intern Med. 2007;167(21):2310–2316. doi: 10.1001/archinte.167.21.2310. [DOI] [PubMed] [Google Scholar]
- 42.Willett W, Manson J, Liu S. Glycemic index, glycemic load, and risk of type 2 diabetes. Am J Clin Nutr. 2002;76(suppl 1):274S–280S. doi: 10.1093/ajcn/76/1.274S. [DOI] [PubMed] [Google Scholar]