Abstract
The authors examined the association between weight patterns during middle age and incident type 2 diabetes mellitus using a subset (n = 1,476) of the Framingham Heart Study original cohort limited-access data set (1948–2003). Participants diagnosed with diabetes before age 50 years were excluded. A functional principal components analysis of body mass index from age 40 years to age 50 years was used to define weight patterns in terms of overall weight status (normal weight, overweight, or obese), weight change (weight loss, stable weight, or weight gain), and weight cycling. Overall overweight and obesity were associated with higher rates of diabetes (for overall overweight, crude hazard ratio (HR) = 3.2, 95% confidence interval (CI): 2.3, 4.6; for overall obesity, crude HR = 8.8, 95% CI: 6.0, 12.8). Weight cycling was also associated with higher rates of diabetes (crude HR = 1.6, 95% CI: 1.2, 2.1). Neither weight loss nor weight gain was associated with incident diabetes. After adjustment for overall weight status, weight cycling was no longer associated with higher rates of diabetes. This study underscores the importance of obesity in diabetes risk and the importance of preventing the development of overweight and obesity earlier in life.
Keywords: body weight changes, diabetes mellitus, obesity
Overweight and obesity increase risk of type 2 diabetes mellitus in adults (1–4). Weight history provides information about diabetes risk beyond current weight (3–6), and increased duration of obesity is associated with diabetes (5, 6). Weight variability has also been linked to incident diabetes (7) and high fasting glucose levels (8). While the influence of weight variability appears to vary across categories of baseline weight (8), the evidence is mixed as to whether these effects can be explained by attained weight (2, 7). Thus, we quantified the association between weight patterns during middle age and incident diabetes.
MATERIALS AND METHODS
Study population
The institutional review board of Brown University (Providence, Rhode Island) approved this study. We used the limited-access data set of the Framingham Heart Study (FHS) original cohort (https://biolincc.nhlbi.nih.gov/home/). The design of the FHS has been described in detail elsewhere (9). Briefly, in 1948–1952, researchers enrolled 5,209 men and women aged 28–62 years without clinically apparent cardiovascular disease in Framingham, Massachusetts. Biennial study visits included an interview, a clinical examination, and laboratory tests. Of the 5,079 participants who provided informed consent for inclusion in the limited-access data set, we excluded participants who were enrolled in the FHS cohort after the age of 40 years (n = 3,071), those who did not attend FHS examinations after age 50 years (n = 218), and those missing body mass index (BMI; weight (kg)/height (m)2) measurements for more than 4 consecutive years or at 2 or more examinations during this period (n = 213). This resulted in a sample for the characterization of weight patterns of 1,577 adults who were observed from age 40 years to age 50 years.
Characterizing weight patterns
We characterized weight patterns during middle age using principal component scores derived from a functional principal components (PC) analysis of BMI from age 40 years to age 50 years. The limited-access FHS data set included exact BMI data for the majority of participant assessments. For examinations for which exact BMI was not provided, we used a randomly selected weight from the 5-pound range provided to preserve participant confidentiality and used proximally measured height to calculate BMI. We linearly interpolated BMI for intervening ages using the 2 nearest observed BMIs for a window of up to 4 years. For example, for a participant who completed study examinations at ages 39 years and 41 years, we averaged BMIs from those 2 examinations to approximate BMI at age 40 years. We represented each participant's BMI curve as a linear combination of cubic B-spline basis functions (10). Each basis curve is positive and nonzero for only a short time period. Therefore, the basis functions can be thought of as reflecting short periods during middle age, and each participant's BMI trajectory is the weighted sum of all basis functions.
The theory and methodology of functional PC analysis have been described in detail elsewhere (11). This approach applies the traditional PC method to determine the most important ways in which each trajectory varies from the average trajectory. We defined the functional equivalent of a linear combination of the estimated BMI functions and a weighting coefficient function. We chose the weight function to maximize the mean square of the PC scores subject to the continuous analog of the unit sum of squares constraint. For subsequent steps, we chose PC functions using the same procedure but with the additional constraint that each PC function had to be orthogonal to all of the previously chosen PC functions. Thus, the PC functions explain unique modes of variation. Scores for each participant for each PC function reflect the extent to which the participant's trajectory resembles that PC function. We categorized the PC function scores into weight patterns based on interpretability.
The first PC function explained the vast majority of variation in weight trajectories during middle age (95.2%). This PC function reflected differences in overall weight status. Participants with high scores were heavier than average and participants with low scores were lighter than average, regardless of the shape of their trajectory. Guided by standard BMI cutoffs (BMI < 25 for underweight/normal weight, BMI 25–29.9 for overweight, and BMI ≥ 30 for obese (12)), we categorized participants as being, overall, normal weight (PC function 1 score in deciles 1–5), overweight (deciles 6–9), or obese (decile 10). Median mean BMIs from age 40 years to age 50 years for these 3 groups were 22.8 (interquartile range (IQR), 21.5–23.9) for overall normal weight (excluding 20 participants who were consistently underweight), 26.9 (IQR, 25.9–28.3) for overall overweight, and 32.3 (IQR, 31.3–34.2) for overall obesity.
The second PC function reflected weight change. This PC function explained 2.5% of variation in the weight trajectories. Participants with higher scores experienced larger weight gains during middle age, those with median scores experienced slight weight gains, and those with low scores experienced weight losses. We categorized weight change as weight loss (score in quintile 1 on PC function 2), stable weight (quintile 2), and weight gain (quintiles 3–5). Median differences between BMIs at ages 50 and 40 years for these 3 groups were −1.3 (IQR, −2.1 to −0.6) for weight loss, 0.03 (IQR, −0.6 to 0.6) for stable weight, and 1.4 (IQR, 0.6 to 2.4) for weight gain.
The third through eighth PC functions reflected weight cycling at increasing frequency. Each of these PC functions explained 1% or less of the variation in weight trajectories. Participants with both high and low scores for these PC functions exhibit cycling in their BMI curves during middle age but with gains and losses at opposite times. The biennial measurement of weight in the FHS limited our ability to detect weight changes occurring more frequently than every 2 years. However, a participant with a highly variable BMI curve was likely to have that variability captured in the biennial sampling, even if the amplitude or frequency of weight cycles captured differed from those experienced (13). Because of this sampling artifact, we chose to classify cycling at any frequency as cycling. Participants with scores in the highest or lowest 10% for PC functions 3–6 and/or in the highest or lowest 5% for PC function 7 or 8 were considered to have experienced weight cycling during middle age. This reflected cycling of approximately 1 kg/m2 or more.
Ascertainment of diabetes
We considered a nonfasting plasma glucose level of at least 200 mg/dL (11.1 mmol/L) and/or reported treatment with insulin or an oral hypoglycemic agent at a study examination to be indicative of type 2 diabetes. While it is possible that some participants had type 1 diabetes, type 2 diabetes accounts for 90%–95% of diabetes among adults, and diagnosis of type 1 diabetes during middle age is unlikely (14).
Potential confounders
We considered 5 non-time-varying potential confounders: gender, education, family history of diabetes, weight status at age 25 years, and physical activity. At examinations 7 and 8, participants reported their lifetime family history of diabetes. Participants who reported diabetes in a parent, sibling, or both at either examination were considered to have a positive family history of diabetes. To preserve confidentiality, recalled weight at age 25 years was provided in 5-pound (2.3-kg) groupings in the limited-access data set. For the majority of participants, both the minimum and maximum possible BMI calculated from measured height at examination 1 were either in the overweight/obese (BMI ≥ 25) or underweight/normal (BMI < 25) weight range. For the 104 participants whose possible BMI range included 25, we categorized weight status at age 25 years on the basis of a randomly selected weight from the 5-pound range provided. At certain examinations, participants reported the average number of hours per day they spent sleeping, resting, standing, and engaging in increasingly more physical tasks, and the amount of time spent engaging in each activity was weighted by the average metabolic equivalent value (9, p. 159). Physical activity was first assessed when participants were aged 36–48 years (examination 4) and was not assessed again until age 50–62 years (examination 11). When we considered measurements of activity closest to age 50 years, within a 10-year window (ages 45–55 years), 51% of our sample did not have physical activity data available. Therefore, we decided not to include physical activity as a correlate in the main analyses.
We also evaluated 3 time-varying potential confounders. We used the most recent assessment to categorize these variables at every year of age. Current smokers included persons who reported having smoked cigarettes, pipes, and/or cigars since the previous FHS examination. We categorized participants as never smokers, current smokers, former smokers who had quit smoking within the past 4 years, and former smokers who had quit smoking more than 4 years previously. Alcohol data were provided in the FHS limited-access data set as ounces per week or month or as numbers of various types of alcoholic drinks consumed per week. We converted these data into average grams of ethanol consumed per day and categorized daily alcohol consumption as none, moderate (≤15 g/day for women and ≤30 g/day for men), or heavier (>15 g/day for women and >30 g/day for men). We classified women as never or ever users of any type of hormones.
Statistical analyses
Of the 1,577 participants included in the characterization of weight patterns, we excluded those who were consistently underweight (n = 20), those with diabetes diagnosed before age 50 years (n = 23), and those with missing data on covariates (n = 58). This resulted in a sample of 1,476 adults for analysis of the association between weight patterns and incident diabetes. Because the first PC function represented the most important mode of variation and explained the vast majority of variation in weight trajectories during middle age, we describe the characteristics of the sample in relation to overall weight status (Table 1).
Table 1.
Characteristicsa of Participants at Age 50 Years in Relation to Overall Weight Status During Middle Age in a Subset (n = 1,476) of the Framingham Heart Study Original Cohort Limited-Access Data Set, 1948–2003
| Normal Weight (n = 722) | Overweight (n = 601) | Obese (n = 153) | |
| Female gender | 69.1 | 39.3 | 47.7 |
| Education | |||
| Less than high school | 25.5 | 33.1 | 33.3 |
| Completion of high school | 39.8 | 37.9 | 47.1 |
| More than high school | 34.8 | 29.0 | 19.6 |
| Overweight or obese at age 25 years | 5.5 | 30.3 | 69.9 |
| Family history of diabetes | 17.7 | 20.1 | 20.3 |
| Smoking statusb | |||
| Never smoker | 24.7 | 26.1 | 34.6 |
| Current smoker | 60.1 | 55.9 | 51.0 |
| Former smoker who quit ≤4 years previously | 6.1 | 6.8 | 2.0 |
| Former smoker who quit >4 years previously | 9.1 | 11.2 | 12.4 |
| Daily alcohol consumptionb | |||
| None | 18.1 | 18.3 | 30.7 |
| Moderate | 54.3 | 57.2 | 52.9 |
| Heavier | 27.6 | 24.5 | 16.3 |
| Ever use of hormones (women)b | 23.3 | 17.8 | 19.2 |
| Physical activityc | 33.7 (5.4) | 34.5 (6.3) | 35.0 (7.5) |
| Missing data on physical activity | 53.5 | 47.9 | 54.9 |
| Weight changes during middle age | |||
| Weight loss | 14.5 | 25.1 | 19.6 |
| Stable weight | 20.6 | 19.1 | 18.3 |
| Weight gain | 64.8 | 55.7 | 62.1 |
| Weight cycling during middle age | 43.2 | 58.7 | 78.4 |
All data are percentages unless otherwise specified.
Based on the most recent assessment before or at age 50 years.
Mean value (standard deviation) for participants with available data on activity level near age 50 years, based on the assessment closest to age 50 years within the age range 45–55 years.
We calculated the number of events and person-years of follow-up from age 50 years to the first examination with an indication of diabetes or the last examination attended. Kaplan-Meier estimators (15) provided graphical comparisons of incident diabetes across weight patterns. We estimated incidence rate ratios for diabetes using Cox proportional hazards models (15). We estimated the crude association between each weight pattern and diabetes (Table 2, models 1–3), the associations adjusted for the other weight patterns (model 4), and finally the association between the weight patterns and incident diabetes adjusted for confounding (model 5). We controlled for confounding by means of multivariable adjustment. If the inclusion of a potential confounder changed an estimated hazard ratio by approximately 10%, the covariate that produced the greatest change in the hazard ratio was retained in the model. Model-building continued until the estimated hazard ratios were not modified by inclusion of additional confounders. We evaluated the characteristics listed in Table 1 for confounding (except for physical activity); confounders included in the adjusted model are given in a footnote to Table 2. Regression analyses were conducted in SAS (version 9.1.3; SAS Institute, Inc., Cary, North Carolina). The functional PC analysis was conducted in R (R Project for Statistical Computing, Vienna, Austria (www.r-project.org)), and useful functional data analysis packages are available for download (ftp://ego.psych.mcgill.ca/pub/ramsay/FDAfuns/R/fda.zip).
Table 2.
Relation of Incident Type 2 Diabetes to Weight Patterns During Middle Age in a Subset (n = 1,476) of the Framingham Heart Study Original Cohort Limited-Access Data Set, 1948–2003
| No. of Participants | No. of Cases | Person-Years of Follow-up | Model 1: Overall Weight Status |
Model 2: Weight Changes |
Model 3: Weight Cycling |
Model 4: All Weight Patterns |
Model 5: All Weight Patterns, Adjusted for Confoundinga |
||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | ||||
| Overall weight status | |||||||||||||
| Normal weight | 722 | 46 | 18,454 | 1 | Referent | 1 | Referent | 1 | Referent | ||||
| Overweight | 601 | 108 | 13,793 | 3.2 | 2.3, 4.6 | 3.2 | 2.3, 4.6 | 2.9 | 2.0, 4.1 | ||||
| Obese | 153 | 63 | 3,112 | 8.8 | 6.0, 12.8 | 8.5 | 5.7, 12.6 | 7.7 | 4.9, 12.1 | ||||
| Weight changes during middle age | |||||||||||||
| Weight loss | 286 | 41 | 6,151 | 1.2 | 0.8, 1.9 | 1.0 | 0.6, 1.5 | 1.1 | 0.7, 1.8 | ||||
| Stable weight | 292 | 38 | 6,753 | 1 | Referent | 1 | Referent | 1 | Referent | ||||
| Weight gain | 898 | 138 | 22,454 | 1.1 | 0.7, 1.5 | 1.1 | 0.8, 1.5 | 1.2 | 0.8, 1.7 | ||||
| Weight cycling during middle age | |||||||||||||
| No cycling | 691 | 80 | 17,116 | 1 | Referent | 1 | Referent | 1 | Referent | ||||
| Cycling | 785 | 137 | 18,243 | 1.6 | 1.2, 2.1 | 1.1 | 0.8, 1.5 | 1.1 | 0.8, 1.5 | ||||
Abbreviations: CI, confidence interval; HR, hazard ratio.
Adjusted for weight status at age 25 years (normal weight/underweight, overweight/obese), gender, ever use of hormones (women; updated every 2 years), alcohol consumption (none, moderate, or heavier; updated every 2 years), smoking (never, current, quit up to 4 years previously, or quit more than 4 years previously; updated every 2 years), and education (less than high school, completion of high school, more than high school).
RESULTS
Characteristics of the sample at age 50 years in relation to overall weight status during middle age are shown in Table 1. The gender distribution varied across overall weight status: Persons with overall normal weight trajectories were more likely to be female. Overall weight status was inversely associated with education. Persons who were overweight or obese during middle age were more likely to have been overweight or obese at age 25 years. Participants with an overall obese weight pattern were more likely to abstain from alcohol use and smoking. The proportion of participants with a family history of diabetes was slightly higher for participants who were overweight or obese during middle age. Weight cycling was more common among heavier persons.
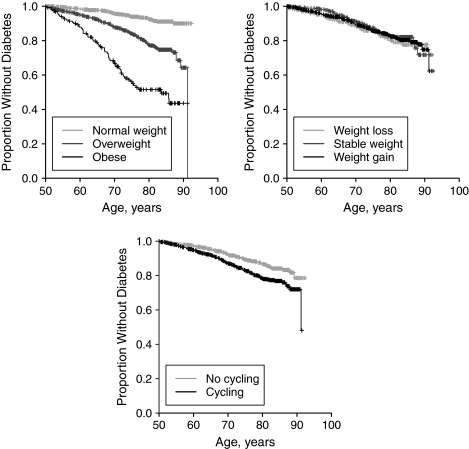
Overall, 217 cases of type 2 diabetes were diagnosed during 35,359 person-years of follow-up. Participants with diabetes were diagnosed at a median age of 67.8 years (IQR, 59.8–74.4), while those without diabetes were followed to a median age of 77.9 years (IQR, 67.6–83.7). Overall weight status and weight cycling were both crudely associated with incident diabetes (Table 2, models 1 and 3; Figure 1). Neither weight loss nor weight gain during middle age was associated with incident diabetes (Table 2, model 2). After adjustment for weight change, weight cycling, and confounders, adults who were overweight during middle age had 2.9 times the rate of diabetes incidence (95% confidence interval (CI): 2.0, 4.1) as those who were normal weight during middle age, and adults who were obese had 7.7 times the rate of diabetes (95% CI: 4.9, 12.1 (Table 2, model 5; Figure 1)). Weight cycling was not associated with incident diabetes when overall weight status was included in the model (Table 2, models 4 and 5).
Figure 1.
Relation of incident type 2 diabetes mellitus to overall weight status (top left panel), weight change (top right panel), and weight cycling (bottom panel) during middle age in a subset (n = 1,476) of the Framingham Heart Study original cohort limited-access data set, 1948–2003. For definitions of categories, see text.
DISCUSSION
In this study, overall weight status during middle age was strongly associated with the development of type 2 diabetes. Our findings add to the body of evidence linking overweight and obesity in adulthood to incident diabetes (1–4). However, while many previous studies used only a few weight assessments (2–6), we showed an effect of weight trajectory over a period of 11 years on subsequent diabetes using functional methods to characterize weight histories. Functional PC analysis captured variation in participants’ entire weight histories throughout the period under study, thus allowing us to characterize weight as a time-varying and cumulative exposure. This is a strength of our study in light of evidence that the duration of overweight or obesity increases risk of diabetes (5, 6).
Weight cycling was crudely associated with increased rates of diabetes but not after adjustment for overall weight status. Our results confirm those of Field et al.’s analysis (2) of Nurses’ Health Study II. Field et al. found that while weight cycling was associated with incident diabetes at the crude level, women who experienced weight cycling had a higher mean BMI, and weight cycling had no effect on diabetes after adjustment for BMI (2). Taken together, these findings suggest that weight status plays a larger role in diabetes risk than do changes in weight.
There is strong clinical trial evidence that modest weight loss can delay or prevent the development of diabetes among overweight and obese adults (16), and observational research has found lower rates of diabetes among persons with intentional weight loss but not among persons with unintentional weight loss (17). A limitation of the current study was the lack of information about intentionality of weight changes, which could explain why we did not find a protective effect of weight loss during middle age on incident diabetes. Additionally, weight losses that are not maintained do not lead to reduced risk of diabetes (18). In another study, Weyer et al. (19) found that although fasting glucose and 2-hour glucose values improved with weight loss, values returned to pre-weight-loss levels with weight regain.
We additionally explored the impact of weight status proximal to diabetes diagnosis by considering participants’ weight status after age 50 years, updated at biennial study examinations (underweight/normal weight (BMI < 25), overweight (BMI 25–29.9), or obese (BMI ≥ 30) (12)). The estimated hazard ratios for weight change and weight cycling were unchanged from the multivariate-adjusted model (Table 2, model 5) when current weight status was included in the model, but the estimated hazard ratios for overall overweight and overall obesity were somewhat attenuated (for overall overweight, hazard ratio = 2.6, 95% CI: 1.8, 3.8; for overall obesity, hazard ratio = 6.9, 95% CI: 4.2, 11.3), indicating that weight status during middle age may act at least partially through weight status later in life. These results confirm previous research on the relevance of recent weight to diabetes incidence (1) and strengthen our findings regarding the impact of weight status during middle age on subsequent diabetes.
Height and weight were measured biannually according to standardized protocols, thus avoiding some of the known biases associated with self-reported weight (20). Although BMI does not differentiate between lean mass and fat mass, BMI is highly correlated with waist circumference and is similarly related to total and abdominal fat (21). The biennial measurement of weight in the FHS limited our ability to detect weight changes occurring more frequently than every 2 years. However, a participant with a highly variable BMI curve was likely to have that variability captured in the biennial sampling, even if the amplitude or frequency of weight cycles captured differed from those experienced (13). Because of this sampling artifact, we chose to classify cycling at any frequency as cycling. Field et al. (2) defined severe and mild cycling in terms of larger amounts of weight than we did in the current study. We conducted a sensitivity analysis by redefining weight cycling to reflect weight cycles of approximately 2 kg/m2 or more. While the crude estimate of association was modestly stronger, this association was no longer significant after adjustment for the other weight patterns and confounders (data not shown).
We considered a nonfasting plasma glucose level of at least 200 mg/dL (11.1 mmol/L) and/or treatment with insulin or an oral hypoglycemic agent at a study examination to be indicative of type 2 diabetes. Type 2 diabetes accounts for 90%–95% of diabetes in adults, and diagnosis of type 1 diabetes during middle age is unlikely (14). Fasting glucose measurements were not available, and our definition of diabetes may have been less sensitive than the criteria recommended by the American Diabetes Association (14) and may have been more likely to identify more severe cases of diabetes. While the reliance on casual glucose measurement is a known limitation of the FHS original cohort, similar definitions have been used in previous investigations with this cohort (1, 22, 23). Because the onset of diabetes may occur 4–7 years before clinical diagnosis (24), use of prospectively measured plasma glucose levels reduces the potential for an extended latency period due to clinical surveillance. Diabetes status was updated at study examinations, and there may have been misclassification of diagnosis date. We conducted a sensitivity analysis in which the date of diagnosis was assumed to be 1 year before the first examination at which diabetes was detected; results were essentially identical to these (data not shown), indicating that misclassification of diagnosis date is unlikely to explain our findings.
There is potential for residual confounding owing to measurement error and unmeasured variables. While smoking, alcohol consumption, and hormone use were assessed regularly, we could not update these variables between examinations, which could potentially have resulted in misclassification at the time of diagnosis. To assess the potential impact of error in recalled weight, we also classified weight status at age 25 years after adjusting BMI for the average number of pounds recalled for groups defined by gender and weight status at the time of recall (20). We found that adjusted models using this variable resulted in very similar estimated hazard ratios for diabetes in relation to the weight patterns (data not shown). Information about current or previous pregnancies in the FHS limited-access data set is limited, yet history of gestational diabetes is a risk factor for type 2 diabetes (25). Gestational diabetes has been linked to a history of weight cycling, though not after adjustment for weight changes during adulthood (26). While our inability to control for confounding by these variables may have inflated our estimated association between weight patterns in middle age and diabetes, our estimated associations between overall weight status and incident diabetes are of similar magnitude to previous research (7). In this sample, among persons with available information on physical activity, activity level was not associated with diabetes (data not shown). Given the limited potential of physical activity to confound the results in this study, the infrequency of measurement, and the high proportion of participants missing data on physical activity, we decided not to include physical activity in our main analyses. Because the sample was fairly lean (average BMIs at ages 40 and 50 years were 25.2 and 25.9, respectively), we were unable to differentiate between levels of obesity. The relation between weight and diabetes may differ among racial/ethnic groups (27), and analyses of more diverse cohorts are needed.
In this study, we found that overweight and obesity over a 10-year period during middle age were associated with increased rates of diabetes. Although modest weight loss can delay or prevent the development of diabetes among overweight and obese adults (16), many people are unable to maintain weight losses (28). While our results suggest that weight regain and subsequent weight loss attempts may not increase diabetes risk, weight cycling has been associated with increased cardiovascular and all-cause mortality (29–31). Given the strong link between obesity and diabetes (2–4), primary prevention of overweight and obesity earlier in life remains a priority.
Acknowledgments
Author affiliations: Department of Community Health, Warren Alpert Medical School, Brown University, Providence, Rhode Island (Molly E. Waring, Charles B. Eaton, Thomas M. Lasater, Kate L. Lapane); Center for Primary Care and Prevention, Memorial Hospital of Rhode Island, Pawtucket, Rhode Island (Molly E. Waring, Charles B. Eaton); Department of Quantitative Health Sciences, University of Massachusetts Medical School, Worcester, Massachusetts (Molly E. Waring); Department of Family Medicine, Warren Alpert Medical School, Brown University, Providence, Rhode Island (Charles B. Eaton); Institute for Community Health Promotion, Brown University, Providence, Rhode Island (Thomas M. Lasater); and Department of Epidemiology and Community Health, Medical College of Virginia, Virginia Commonwealth University, Richmond, Virginia (Kate L. Lapane).
The Framingham Heart Study (FHS) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the FHS investigators. The current study was unfunded.
This research was carried out using a limited-access data set obtained from the NHLBI. The authors acknowledge the enormous contributions of the FHS staff in creating and maintaining this data set.
This paper does not necessarily reflect the opinions or views of the FHS staff or the NHLBI.
Conflict of interest: none declared.
Glossary
Abbreviations
- BMI
body mass index
- CI
confidence interval
- FHS
Framingham Heart Study
- IQR
interquartile range
- PC
principal components
References
- 1.Wilson PW, D'Agostino RB, Sullivan L, et al. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162(16):1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 2.Field AE, Manson JE, Laird N, et al. Weight cycling and the risk of developing type 2 diabetes among adult women in the United States. Obes Res. 2004;12(2):267–274. doi: 10.1038/oby.2004.34. [DOI] [PubMed] [Google Scholar]
- 3.Schienkiewitz A, Schulze MB, Hoffmann K, et al. Body mass index history and risk of type 2 diabetes: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr. 2006;84(2):427–433. doi: 10.1093/ajcn/84.1.427. [DOI] [PubMed] [Google Scholar]
- 4.Colditz GA, Willett WC, Rotnitzky A, et al. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122(7):481–486. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 5.Wannamethee SG, Shaper AG. Weight change and duration of overweight and obesity in the incidence of type 2 diabetes. Diabetes Care. 1999;22(8):1266–1272. doi: 10.2337/diacare.22.8.1266. [DOI] [PubMed] [Google Scholar]
- 6.Carlsson S, Persson P, Alvarsson M, et al. Weight history, glucose intolerance, and insulin levels in middle-aged Swedish men. Am J Epidemiol. 1998;148(6):539–545. doi: 10.1093/oxfordjournals.aje.a009679. [DOI] [PubMed] [Google Scholar]
- 7.Brancati FL, Wang N, Mead LA, et al. Body weight patterns from 20 to 49 years of age and subsequent risk for diabetes mellitus: the Johns Hopkins Precursors Study. Arch Intern Med. 1999;159(9):957–963. doi: 10.1001/archinte.159.9.957. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H, Tamakoshi K, Yatsuya H, et al. Long-term body weight fluctuation is associated with metabolic syndrome independent of current body mass index among Japanese men. Circ J. 2005;69(1):13–18. doi: 10.1253/circj.69.13. [DOI] [PubMed] [Google Scholar]
- 9.Dawber TR. The Framingham Study: The Epidemiology of Atherosclerotic Disease. Cambridge, MA: Harvard University Press; 1980. [Google Scholar]
- 10.Ramsay JO, Silverman BW. From functional data to smooth functions. In: Ramsay JO, Silverman BW, editors. Functional Data Analysis. 2nd ed. New York, NY: Springer Science+Business Media, LLC; 2006. pp. 37–58. [Google Scholar]
- 11.Ramsay JO, Silverman BW. Principal components analysis for functional data. In: Ramsay JO, Silverman BW, editors. Functional Data Analysis. 2nd ed. New York, NY: Springer Science+Business Media, LLC; 2006. pp. 147–172. [Google Scholar]
- 12.National Institutes of Health. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. (Report no. 6, suppl 2) Baton Rouge, LA: North American Association for the Study of Obesity; 1998. [PubMed] [Google Scholar]
- 13.Ebert DS, Musgrave FK, Peachey D, et al., editors. Texturing and Modeling: A Procedural Approach. 3rd ed. San Francisco, CA: Morgan Kaufmann Publishers; 2003. pp. 52–53. [Google Scholar]
- 14.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27(suppl 1):S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 15.Armitage P, Berry G, Matthews JNS. Survival analysis. In: Armitage P, Berry G, Matthews JNS, editors. Statistical Methods in Medical Research. 4th ed. Cornwall, United Kingdom: Blackwell Publishing Company Ltd; 2002. pp. 568–590. [Google Scholar]
- 16.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Will JC, Williamson DF, Ford ES, et al. Intentional weight loss and 13-year diabetes incidence in overweight adults. Am J Public Health. 2002;92(8):1245–1248. doi: 10.2105/ajph.92.8.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore LL, Visioni AJ, Wilson PW, et al. Can sustained weight loss in overweight individuals reduce the risk of diabetes mellitus? Epidemiology. 2000;11(3):269–273. doi: 10.1097/00001648-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Weyer C, Hanson K, Bogardus C, et al. Long-term changes in insulin action and insulin secretion associated with gain, loss, regain and maintenance of body weight. Diabetologia. 2000;43(1):36–46. doi: 10.1007/s001250050005. [DOI] [PubMed] [Google Scholar]
- 20.Perry GS, Byers TE, Mokdad AH, et al. The validity of self-reports of past body weights by U.S. adults. Epidemiology. 1995;6(1):61–66. doi: 10.1097/00001648-199501000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Janssen I, Heymsfield SB, Allison DB, et al. Body mass index and waist circumference independently contribute to the prediction of nonabdominal, abdominal subcutaneous, and visceral fat. Am J Clin Nutr. 2002;75(4):683–688. doi: 10.1093/ajcn/75.4.683. [DOI] [PubMed] [Google Scholar]
- 22.Fox CS, Coady S, Sorlie PD, et al. Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation. 2007;115(12):1544–1550. doi: 10.1161/CIRCULATIONAHA.106.658948. [DOI] [PubMed] [Google Scholar]
- 23.Fox CS, Coady S, Sorlie PD, et al. Trends in cardiovascular complications of diabetes. JAMA. 2004;292(20):2495–2499. doi: 10.1001/jama.292.20.2495. [DOI] [PubMed] [Google Scholar]
- 24.Harris MI, Klein R, Welborn TA, et al. Onset of NIDDM occurs at least 4–7 yr before clinical diagnosis. Diabetes Care. 1992;15(7):815–819. doi: 10.2337/diacare.15.7.815. [DOI] [PubMed] [Google Scholar]
- 25.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25(10):1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 26.Rudra CB, Sorensen TK, Leisenring WM, et al. Weight characteristics and height in relation to risk of gestational diabetes mellitus. Am J Epidemiol. 2007;165(3):302–308. doi: 10.1093/aje/kwk007. [DOI] [PubMed] [Google Scholar]
- 27.Resnick HE, Valsania P, Halter JB, et al. Differential effects of BMI on diabetes risk among black and white Americans. Diabetes Care. 1998;21(11):1828–1835. doi: 10.2337/diacare.21.11.1828. [DOI] [PubMed] [Google Scholar]
- 28.Anderson JW, Konz EC, Frederich RC, et al. Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr. 2001;74(5):579–584. doi: 10.1093/ajcn/74.5.579. [DOI] [PubMed] [Google Scholar]
- 29.Diaz VA, Mainous AG, III, Everett CJ. The association between weight fluctuation and mortality: results from a population-based cohort study. J Community Health. 2005;30(3):153–165. doi: 10.1007/s10900-004-1955-1. [DOI] [PubMed] [Google Scholar]
- 30.Rzehak P, Meisinger C, Woelke G, et al. Weight change, weight cycling and mortality in the ERFORT Male Cohort Study. Eur J Epidemiol. 2007;22(10):665–673. doi: 10.1007/s10654-007-9167-5. [DOI] [PubMed] [Google Scholar]
- 31.Wannamethee SG, Shaper AG, Walker M. Weight change, weight fluctuation, and mortality. Arch Intern Med. 2002;162(22):2575–2580. doi: 10.1001/archinte.162.22.2575. [DOI] [PubMed] [Google Scholar]