Abstract
Organochlorines are environmentally persistent contaminants that readily cross the placenta, posing a potential risk to the developing fetus. Evidence for neurodevelopmental effects at low levels of these compounds is growing, though few studies have focused on behavioral outcomes. The authors investigated the association between prenatal polychlorinated biphenyl (PCB) and p,p′-dichlorodiphenyl dichloroethylene (p,p′-DDE) levels and behaviors associated with attention deficit hyperactivity disorder (ADHD), measured with the Conners’ Rating Scale for Teachers (CRS-T), in a cohort of 607 children aged 7–11 years (median age, 8.2 years) born in 1993–1998 to mothers residing near a PCB-contaminated harbor in New Bedford, Massachusetts. The median umbilical cord serum level of the sum of 4 prevalent PCB congeners (118, 138, 153, and 180) was 0.19 ng/g serum (range, 0.01–4.41 ng/g serum). The authors found higher risk for ADHD-like behaviors assessed with the CRS-T at higher levels of PCBs and p,p′-DDE. For example, the authors found higher risk of atypical behavior on the Conners’ ADHD Index for the highest quartile of the sum of 4 PCB congeners versus the lowest quartile (risk ratio = 1.76, 95% confidence interval: 1.06, 2.92) and a similar relation for p,p′-DDE. These results support an association between low-level prenatal organochlorine exposure and ADHD-like behaviors in childhood.
Keywords: attention deficit disorder with hyperactivity, dichlorodiphenyl dichloroethylene, maternal exposure, polychlorinated biphenyls
Attention deficit hyperactivity disorder (ADHD) is the most common childhood neurobehavioral disorder, affecting 5%–10% of children worldwide (1, 2). Symptoms often persist into adolescence and adulthood and are associated with substantial social and medical costs (1, 2). The etiology of ADHD is poorly understood, and identifying modifiable risk factors, including environmental neurotoxicants, is of public health importance.
Organochlorines, including polychlorinated biphenyls (PCBs) and p,p′-dichlorodiphenyl dichloroethylene (p,p′-DDE), have been shown to cross the placenta and have been associated with neurobehavioral effects in children (3). In the literature, investigators have reported associations between PCB levels and measures of attention and impulsivity among infants, school-age children, and adults (4–11). To our knowledge, associations between p,p′-DDE exposure and behaviors associated with ADHD have not been previously reported.
Behavioral rating scales are used to clinically diagnose ADHD in conjunction with other measures, including physical and neurologic examination and family and school assessments (12). Rating scales use a standardized behavioral assessment form; when administered in a population-based sample, they may be less affected by access to health care or other sources of diagnostic bias.
The purpose of the current study was to prospectively investigate the relation between prenatal exposure to PCBs and p,p′-DDE and behaviors associated with ADHD, assessed using the Conners’ Rating Scale for Teachers (CRS-T), among children born to mothers residing near a PCB-contaminated harbor.
MATERIALS AND METHODS
Study population
Participants in this longitudinal cohort study were children born between 1993 and 1998 to English- or Portuguese-speaking mothers aged 18 years or older who resided near a PCB-contaminated harbor in New Bedford, Massachusetts (13). The parent cohort included 788 mother-infant pairs recruited from a local hospital with approximately 2,000 births per year; approximately 10% of mothers were available for recruitment during times when a study examiner was on-site and met study eligibility criteria. Mother-infant pairs were eligible to participate if the mother was aged 18 years or older, had lived in one of 4 towns (New Bedford, Acushnet, Fairhaven, or Dartmouth) adjacent to the contaminated New Bedford Harbor for at least the duration of her pregnancy, and spoke English or Portuguese. Infants too ill to undergo neonatal examination or born by cesarean section were excluded from the study. Multiple births were also excluded from the current analysis. Follow-up neurodevelopmental assessments were performed at school age (approximately 8 years) in 607 children from the 788 mother-infant pairs in the birth cohort (77%); 590 of these children had a CRS-T evaluation, and 573 also had data on umbilical cord serum organochlorine levels.
Laboratory measurements of exposure
Umbilical cord blood samples for organochlorine analyses were collected at the infant's birth; the serum fraction was removed after centrifugation and stored at −20°C. All sample analyses were performed at the Harvard School of Public Health Organic Chemistry Laboratory (Boston, Massachusetts). Laboratory personnel were blinded to infant outcomes. Umbilical cord serum samples were analyzed for 51 individual PCB congeners and p,p′-DDE. Laboratory analytic methods and quality control procedures are described elsewhere (13). Briefly, liquid-liquid extraction was used, and the extracts were analyzed by gas chromatography with electron capture detection using an internal standard. Primary and confirmatory capillary columns were used, and where results differed, the lower value was reported.
PCB concentrations were calculated as individual congeners, after the amount of analyte in the procedural blank was subtracted, and as the sum of all congeners assayed (ΣPCB) in units of ng/g serum. Lipid content could not be determined for study subjects because of insufficient sample volume (1.5–4 mL). Therefore, lipid content was measured in 12 randomly selected cord blood samples from discarded anonymous samples collected at the study recruitment site; values were reproducible (1.7 g/L; standard deviation, 0.3) and were consistent with the lipid content in cord blood reported elsewhere (1.8 g/L; standard deviation, 0.07) (14).
The method detection limits for individual PCBs ranged from 0.002 ng/g serum to 0.04 ng/g serum, with most limits being less than 0.01 ng/g; the method detection limit for p,p′-DDE in serum was 0.07 ng/g (13). We used quantifiable values below the detection limit to optimize statistical power and avoid biased exposure estimates associated with censoring at the method detection limit (15). Reproducibility of serum analyses was good; over the 5 years of analysis, the ΣPCB within-batch coefficient of variation was 3% and the between-batch coefficient of variation was 20%, with similar performance for p,p′-DDE.
Outcome assessment
The CRS-T (16) is a 59-item questionnaire used to evaluate problem behaviors. Four of the 13 CRS-T subscales are considered measures of behaviors associated with ADHD: 1) Conners’ ADHD Index, 2) Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) Inattentive, 3) DSM-IV Hyperactive-Impulsive, and 4) DSM-IV Total (both subtypes combined). CRS-T scores are standardized to age- and sex-adjusted T scores with a mean of 50 and a standard deviation of 10; a higher score indicates more adverse behavior.
Several subjects had multiple CRS-T assessments because: 1) teachers from 2 different grades completed assessments—the assessment from the teacher at the higher grade was included; 2) a primary teacher and a secondary teacher (i.e., special education teacher) completed assessments—the assessment from the primary teacher was included; or 3) multiple primary teachers in the same grade completed assessments—the mean score across primary teachers was calculated.
Statistical analysis
The 4 behavioral outcomes were investigated in relation to the following 3 congener groups: 1) the sum of all 51 PCB congeners measured (ΣPCB51); 2) the sum of 4 prevalent PCB congeners: 118, 138, 153, and 180 (ΣPCB4); and 3) the computed toxic equivalent for the sum of the 5 dioxin-like mono-ortho PCB congeners measured: 105, 118, 156, 167, and 189, computed on a lipid basis and weighted with toxic equivalency factors (17). We included the toxic equivalent group to investigate a potentially distinct biologic mechanism for the effect of dioxin-like congeners on neurodevelopment. We also investigated associations with p,p′-DDE.
Associations between organochlorines and CRS-T measures were estimated using multivariable regression analysis. Continuous CRS-T T scores were log-transformed to better satisfy model assumptions (i.e., homoscedasticity) and were modeled using multivariable linear regression. To facilitate interpretation on the original scale, we computed a change in CRS-T T score as the difference in the predicted outcome (on the original scale) at the 95th percentile of exposure as compared with the fifth percentile, at the median or reference level of the covariates. In addition, to identify those children with more extreme behavioral patterns suggestive of possible ADHD diagnosis, we dichotomized outcomes at the 86th percentile (T score ≥ 61), which identifies children with mildly to markedly atypical scores indicative of a possible or significant behavioral problem (16). We used a log risk model to estimate the associations between the biomarker levels and the risk of an elevated CRS-T score. Exposure-response relations were investigated with nonparametric models (i.e., penalized splines) and by categorizing exposure into quartiles.
Data on covariates came from maternal and pediatric medical records (including lead screening) and study questionnaires administered 2 weeks after birth and at the school-age follow-up. Data on lead levels were abstracted from pediatric medical records—lead screening is mandatory in Massachusetts among children aged 3 years or younger under the Lead Poisoning Prevention and Control Act (18). The school-age examination also included assessment of maternal intelligence and depression using the Kaufman Brief Intelligence Test and the Beck Depression Inventory, respectively, and assessment of family and home characteristics using the Home Observation for Measurement of the Environment (HOME) score (19). Potential confounders considered were characteristics of the mother (age at child's birth, prenatal smoking and alcohol consumption, prenatal diet (local and overall fish consumption), illicit drug use in the year prior to the child's birth, breastfeeding, parity, and, when the child was of school age, education, marital status, intelligence quotient, and depression) and the child (age at examination, sex, race, ADHD medication use, and peak and mean 12- to 36-month blood lead levels). Household income and quality of the home environment (HOME score) when the child was of school age were also considered. Inclusion of covariates in multivariable models was based on a priori considerations of covariate associations with exposure and outcome, model fit (statistically significant partial f test at α < 0.10), and whether covariate inclusion meaningfully affected the organochlorine exposure effect estimate.
To check the sensitivity of our estimates to the influence of parent-reported ADHD medication use, we repeated the analyses in the subset of children who were not reported to have a history of ADHD medication use. We also conducted a sensitivity analysis to assess the influence of missing covariate data on organochlorine effect estimates by imputing covariate data (PROC MIANALYZE, Unix SAS, version 9.1.3; SAS Institute Inc., Cary, North Carolina).
The study protocol was reviewed and approved by the human subjects committees of the Harvard School of Public Health and Brigham and Women's Hospital (Boston, Massachusetts) and of Southcoast Hospitals Group (New Bedford, Massachusetts). Written informed consent was obtained from all participating families before study evaluation.
RESULTS
Table 1 shows summary statistics for umbilical cord serum PCB and p,p′-DDE levels in the 573 children with both exposure and outcome data. Levels for the sum of 4 prevalent PCB congeners (ΣPCB4: 118, 138, 153, and 180) comprised almost half of the sum of all measured congeners (ΣPCB51). The 3 PCB measures were also highly correlated with each other, with Spearman correlation coefficients ranging between 0.87 and 0.92. Table 1 also shows correlations between the 4 CRS-T outcome measures. Although the Conners’ ADHD Index and the DSM-IV Total index have only 3 items in common, children's scores on these indices were highly correlated (Spearman correlation coefficient = 0.96).
Table 1.
Umbilical Cord Serum Organochlorine Levels and Scores on 4 Subscales of the Conners’ Rating Scale for Teachers Among 8-Year-Old Children Born in New Bedford, Massachusetts, With Data on Both Exposure and Outcome Measures (n = 573), 1993–1998
| Mean (Standard Deviation) | Median | Range | Spearman Correlation Coefficient |
||||
| ΣPCB51a | ΣPCB4b | Toxic Equivalentc | p,p′-DDE | ||||
| ΣPCB51, ng/g serum | 0.57 (0.92) | 0.40 | 0.07–18.14 | 1 | 0.92 | 0.87 | 0.59 |
| ΣPCB4, ng/g serum | 0.26 (0.31) | 0.19 | 0.01–4.41 | 1 | 0.90 | 0.64 | |
| Toxic equivalent, pg/g lipid | 7.20 (11.73) | 4.45 | 0.00–151.49 | 1 | 0.57 | ||
| p,p′-DDE, ng/g serum | 0.50 (1.04) | 0.31 | 0.00–14.93 | 1 | |||
| Conners’ ADHD | DSM-IV Inattentive | DSM-IV Hyperactive | DSM-IV Total | ||||
| Conners’ ADHD Index | 53.19 (11.07) | 50 | 41–90 | 1 | 0.89 | 0.82 | 0.96 |
| DSM-IV Inattentive | 53.51 (10.74) | 50 | 40–90 | 1 | 0.63 | 0.94 | |
| DSM-IV Hyperactive-Impulsive | 51.74 (10.14) | 47 | 42–90 | 1 | 0.83 | ||
| DSM-IV Total | 53.04 (10.16) | 50 | 41–90 | 1 |
Abbreviations: ADHD, attention deficit hyperactivity disorder; p,p′-DDE, p,p′-dichlorodiphenyl dichloroethylene; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; PCBs, polychlorinated biphenyls.
Sum of all measured PCB congeners.
Sum of PCB congeners 118, 138, 153, and 180.
Toxic equivalency factor-weighted sum of mono-ortho PCB congeners 105, 118, 156, 167, and 189, measured in pg/g lipid.
Baseline characteristics of the study participants and their unadjusted associations with the 4 CRS-T measures (dichotomized at the 86th percentile) are displayed in Table 2. Higher risk of ADHD-like behaviors was found among school-aged children with mothers who were younger (<20 years) at the child's birth, less educated (less than a high school diploma), and unmarried and who smoked during pregnancy, used illicit drugs in the year before birth, had a lower intelligence quotient, and had more depression symptoms. Children were also at higher risk if they were examined at an older age, came from a lower-income household, had never been breastfed or were breastfed for less than 1 month, were nonwhite, and had no siblings living in the house. A higher HOME score was associated with lower risk of ADHD-like behaviors.
Table 2.
Baseline Characteristics of Mothers and School-Age (8 Years) Children Born in New Bedford, Massachusetts, and Associations With Children's Scores on 4 Subscales of the Conners’ Rating Scale for Teachers (n = 590), 1993–1998a
| No. of Subjects | % | Mean (Standard Deviation) | Conners’ ADHD Index |
DSM-IV Inattentive |
DSM-IV Hyperactive-Impulsive |
DSM-IV Combined |
|||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | ||||
| Maternal characteristics | |||||||||||
| Age at child's birth, years | |||||||||||
| <20 | 79 | 13.4 | 2.0 | 1.3, 3.1 | 1.8 | 1.3, 2.5 | 2.0 | 1.3, 3.0 | 2.0 | 1.4, 2.9 | |
| 20–29 | 307 | 52.0 | 1 | 1 | 1 | 1 | |||||
| 30–34 | 129 | 21.9 | 0.6 | 0.4, 1.0 | 0.7 | 0.5, 1.1 | 0.5 | 0.3, 1.0 | 0.5 | 0.3, 0.8 | |
| ≥35 | 75 | 12.7 | 0.9 | 0.5, 1.6 | 0.9 | 0.6, 1.5 | 0.8 | 0.4, 1.6 | 1.0 | 0.6, 1.6 | |
| Education at child's school age (6 values missing) | |||||||||||
| Less than 12th grade | 64 | 11.0 | 1.8 | 1.2, 2.6 | 1.4 | 0.9, 2.1 | 1.4 | 0.8, 2.4 | 1.5 | 0.9, 2.3 | |
| High school graduation | 187 | 32.0 | 1 | 1 | 1 | 1 | |||||
| Some college | 333 | 57.0 | 0.8 | 0.6, 1.1 | 0.9 | 0.6, 1.2 | 0.8 | 0.5, 1.2 | 0.7 | 0.5, 1.1 | |
| Annual household income at child's school age (8 values missing) | |||||||||||
| <$20,000 | 121 | 20.8 | 2.3 | 1.6, 3.3 | 2.0 | 1.4, 2.9 | 2.3 | 1.4, 3.6 | 2.3 | 1.5, 3.4 | |
| $20,000–$39,999 | 159 | 27.3 | 2.0 | 1.4, 2.9 | 2.0 | 1.4, 2.8 | 2.0 | 1.3, 3.1 | 2.2 | 1.5, 3.3 | |
| ≥$40,000 | 302 | 51.9 | 1 | 1 | 1 | 1 | |||||
| Marital status at child's school age | |||||||||||
| Married | 345 | 58.5 | 1 | 1 | 1 | 1 | |||||
| Never married/separated/divorced | 245 | 41.5 | 2.0 | 1.5, 2.7 | 1.9 | 1.4, 2.5 | 1.9 | 1.3, 2.8 | 2.1 | 1.5, 2.9 | |
| Smoking during pregnancy (42 values missing) | |||||||||||
| Yes | 164 | 29.9 | 2.2 | 1.6, 3.0 | 2.2 | 1.6, 3.0 | 2.1 | 1.4, 3.1 | 2.5 | 1.8, 3.5 | |
| No | 384 | 70.1 | 1 | 1 | 1 | 1 | |||||
| Alcohol consumption during pregnancy, servings/month (85 values missing) | |||||||||||
| <1 | 453 | 89.7 | 1 | 1 | 1 | 1 | |||||
| 1–2 | 12 | 2.4 | 0.7 | 0.2, 2.7 | 1.0 | 0.4, 2.8 | 1.0 | 0.3, 3.5 | 1.2 | 0.4, 3.2 | |
| >2 | 40 | 7.9 | 0.8 | 0.4, 1.6 | 1.0 | 0.6, 1.8 | 0.7 | 0.3, 1.7 | 0.8 | 0.4, 1.7 | |
| Use of illicit drugs during year prior to birth (87 values missing) | |||||||||||
| Yes | 73 | 14.5 | 1.7 | 1.2, 2.5 | 1.9 | 1.3, 2.6 | 2.0 | 1.3, 3.0 | 2.1 | 1.4, 3.0 | |
| No | 430 | 85.5 | 1 | 1 | 1 | 1 | |||||
| Maternal intelligence quotientb (3 values missing) | 97.2 (11.2) | 0.98 | 0.97, 1.00 | 0.98 | 0.97, 0.99 | 0.99 | 0.97, 1.00 | 0.99 | 0.97, 0.99 | ||
| Maternal depressionb (2 values missing) | 8.4 (8.8) | 1.02 | 1.00, 1.03 | 1.02 | 1.01, 1.04 | 1.02 | 1.00, 1.03 | 1.02 | 1.01, 1.04 | ||
| HOME score (11 values missing) | 45.6 (5.4) | 0.93 | 0.91, 0.96 | 0.93 | 0.91, 0.96 | 0.94 | 0.91, 0.97 | 0.93 | 0.91, 0.95 | ||
| Child's characteristics | |||||||||||
| Gestational age, weeks (2 values missing) | 39.7 (1.3) | 1.1 | 0.9, 1.2 | 1.2 | 1.0, 1.3 | 1.0 | 0.8, 1.1 | 1.1 | 0.9, 1.2 | ||
| Age at examination, years | 8.4 (0.7) | 1.3 | 1.1, 1.6 | 1.1 | 0.9, 1.3 | 1.3 | 1.0, 1.6 | 1.3 | 1.1, 1.6 | ||
| Sex | |||||||||||
| Male | 302 | 51.2 | 1.2 | 0.9, 1.7 | 1.1 | 0.8, 1.5 | 1.0 | 0.8, 1.6 | 1.0 | 0.8, 1.4 | |
| Female | 288 | 48.8 | 1 | 1 | 1 | 1 | |||||
| Race/ethnicity (11 values missing) | |||||||||||
| White | 398 | 68.7 | 1 | 1 | 1 | 1 | |||||
| Black | 37 | 6.4 | 1.1 | 0.6, 2.0 | 1.0 | 0.5, 1.8 | 1.0 | 0.4, 2.4 | 1.2 | 0.6, 2.3 | |
| Hispanic | 57 | 9.8 | 1.5 | 0.9, 2.3 | 1.4 | 0.9, 2.2 | 1.4 | 0.8, 2.6 | 1.8 | 1.1, 2.7 | |
| Cape Verdean | 63 | 10.9 | 1.1 | 0.7, 1.8 | 0.9 | 0.5, 1.5 | 1.5 | 0.9, 2.7 | 1.2 | 0.7, 2.0 | |
| Other | 24 | 4.2 | 1.8 | 1.1, 3.2 | 1.7 | 1.0, 2.9 | 1.9 | 0.9, 3.9 | 1.9 | 1.0, 3.4 | |
| Duration of breastfeeding, months (3 values missing) | |||||||||||
| Never breastfed or <1 | 377 | 64.2 | 1.8 | 1.2, 2.7 | 1.8 | 1.2, 2.7 | 1.6 | 1.0, 2.7 | 1.9 | 1.2, 3.0 | |
| 1–3 | 62 | 10.6 | 1.0 | 0.5, 2.0 | 0.9 | 0.5, 1.9 | 0.8 | 0.3, 2.0 | 1.1 | 0.5, 2.4 | |
| ≥4 | 148 | 25.2 | 1 | 1 | 1 | 1 | |||||
| Type of school | |||||||||||
| Public | 535 | 90.7 | 1 | 1 | 1 | 1 | |||||
| Private or parochial | 55 | 9.3 | 1.4 | 0.3, 1.2 | 1.3 | 0.5, 1.5 | 1.4 | 0.4, 1.6 | 1.4 | 0.4, 1.4 | |
| No. of siblings living in house | |||||||||||
| 0 | 86 | 14.6 | 1 | 1 | 1 | 1 | |||||
| 1 | 277 | 47.0 | 0.6 | 0.4, 0.9 | 0.7 | 0.5, 1.0 | 0.5 | 0.3, 0.8 | 1.0 | 0.4, 0.9 | |
| ≥2 | 227 | 38.5 | 0.6 | 0.4, 0.9 | 0.8 | 0.6, 1.2 | 0.5 | 0.3, 0.8 | 1.0 | 0.5, 1.0 | |
Abbreviations: ADHD, attention deficit hyperactivity disorder; CI, confidence interval; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; HOME, Home Observation for Measurement of the Environment; RR, risk ratio.
Scores were dichotomized at the 86th percentile. A higher score indicates more severe behavioral problems.
Maternal intelligence quotient was measured with the Kaufman Brief Intelligence Test, and maternal depression was measured with the Beck Depression Inventory.
Splines relating continuous exposures to log-transformed outcomes showed approximately linear associations. Therefore, we report results from linear regression models of the association between organochlorines (modeled as a continuous variable) and log-transformed CRS-T scores in Table 3. There was very little difference between the unadjusted slopes for all subjects with exposure and outcome data and the unadjusted slopes in the subset of subjects with nonmissing covariate data. In the covariate-adjusted model, there was a consistent positive association between organochlorines and increased ADHD-like behaviors. Multicontaminant models (including p,p′-DDE in PCB models and PCB in p,p′-DDE models) produced a slight attenuation of, though not a meaningful change in, the effect estimates (data not shown). In addition, lead did not confound the associations between organochlorines and ADHD-like behaviors.
Table 3.
Associations Between Umbilical Cord Serum Organochlorine Levels and Log-Transformed Scores on 4 Subscales of the Conners’ Rating Scale for Teachers (n = 573) Among Children Born in New Bedford, Massachusetts, 1993–1998
| No. of Subjects | Sum of 51 PCBs (0.40 ng/g, 0.14–1.30)b |
Sum of 4 PCBs (0.19 ng/g, 0.05–0.68) |
Toxic Equivalenta (0.004 ng/g, 0.001–0.019) |
p,p′-DDE (0.31 ng/g, 0.11–1.32) |
|||||
| β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | ||
| Conners’ ADHD Index | |||||||||
| Unadjusted | 573 | 0.02 | 0.00, 0.03 | 0.01 | −0.04, 0.06 | 0.95 | −0.39, 2.29 | 0.00 | −0.01, 0.02 |
| Unadjusted 2c | 487 | 0.02 | 0.00, 0.03 | 0.02 | −0.03, 0.08 | 1.18 | −0.19, 2.54 | 0.00 | −0.01, 0.02 |
| Adjustedd | 487 | 0.03 | 0.01, 0.04 | 0.08 | 0.03, 0.13 | 2.38 | 1.05, 3.71 | 0.01 | 0.00, 0.03 |
| Change in scoree | 487 | 1.54 | 2.44 | 2.07 | 0.73 | ||||
| DSM-IV Inattentive | |||||||||
| Unadjusted | 573 | 0.01 | −0.01, 0.02 | −0.01 | −0.06, 0.04 | 0.37 | −0.95, 1.70 | 0.00 | −0.02, 0.01 |
| Unadjusted 2 | 521 | 0.01 | −0.01, 0.02 | 0.00 | −0.05, 0.05 | 0.53 | −0.79, 1.86 | 0.00 | −0.01, 0.02 |
| Adjusted | 521 | 0.01 | 0.00, 0.03 | 0.04 | −0.01, 0.09 | 1.17 | −0.11, 2.45 | 0.01 | −0.01, 0.02 |
| Change in score | 521 | 0.75 | 1.14 | 1.01 | 0.41 | ||||
| DSM-IV Hyperactive-Impulsive | |||||||||
| Unadjusted | 573 | 0.02 | 0.00, 0.03 | 0.03 | −0.02, 0.08 | 1.45 | 0.23, 2.67 | 0.01 | 0.00, 0.02 |
| Unadjusted 2 | 521 | 0.02 | 0.00, 0.03 | 0.04 | −0.01, 0.08 | 1.57 | 0.34, 2.79 | 0.01 | −0.01, 0.02 |
| Adjusted | 521 | 0.03 | 0.01, 0.04 | 0.08 | 0.03, 0.12 | 2.46 | 1.26, 3.66 | 0.02 | 0.00, 0.03 |
| Change in score | 521 | 1.49 | 2.37 | 2.08 | 0.91 | ||||
| DSM-IV Total | |||||||||
| Unadjusted | 573 | 0.01 | 0.00, 0.03 | 0.02 | −0.03, 0.06 | 1.08 | −0.17, 2.33 | 0.01 | −0.01, 0.02 |
| Unadjusted 2 | 521 | 0.01 | 0.00, 0.03 | 0.02 | −0.02, 0.07 | 1.21 | −0.04, 2.45 | 0.01 | −0.01, 0.02 |
| Adjusted | 521 | 0.02 | 0.01, 0.04 | 0.06 | 0.01, 0.11 | 1.98 | 0.79, 3.17 | 0.01 | 0.00, 0.03 |
| Change in score | 521 | 1.21 | 1.89 | 1.70 | 0.85 | ||||
Abbreviations: ADHD, attention deficit hyperactivity disorder; CI, confidence interval; p,p′-DDE, p,p′-dichlorodiphenyl dichloroethylene; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; HOME, Home Observation for Measurement of the Environment; PCB(s), polychlorinated biphenyl(s).
Toxic equivalency factor-weighted sum of mono-ortho PCB congeners 105, 118, 156, 167, and 189, measured in ng/g lipid.
Median serum (or lipid) concentration, 5th–95th percentile range.
Unadjusted estimates in the subset of subjects with nonmissing covariate data.
Conners’ ADHD Index was adjusted for child's age and sex and for maternal age, marital status, smoking during pregnancy, alcohol consumption during pregnancy, local fish consumption during pregnancy, and illicit drug use. All DSM-IV outcomes were adjusted for child's age and sex and for maternal age, marital status, smoking during pregnancy, and HOME score.
Adjusted change in Conners' Rating Scale for Teachers T score from the 5th percentile of exposure to the 95th percentile, computed on the natural (non-log-transformed) scale at the reference level for categorical covariates and the median value for continuous covariates.
Change in score, computed as the difference in CRS-T score associated with an increase from the fifth percentile of exposure to the 95th percentile, also increased with organochlorine exposure (Table 3), indicating more adverse behavior with higher exposure. For example, an increase in the sum of 4 PCBs (ΣPCB4) from the fifth percentile of exposure to the 95th (0.6 ng/g serum) resulted in an estimated 2.4-point increase in the Conners’ ADHD Index score. Change in score associated with ΣPCB4 was lower for the DSM-IV Inattentive index (1.1-point increase) than for the Hyperactive/Impulsive index (2.4-point increase) and the DSM-IV Total index (1.9-point increase). The other PCB categories (ΣPCB51, toxic equivalent) and p,p′-DDE were associated with slightly smaller effect estimates (Table 3).
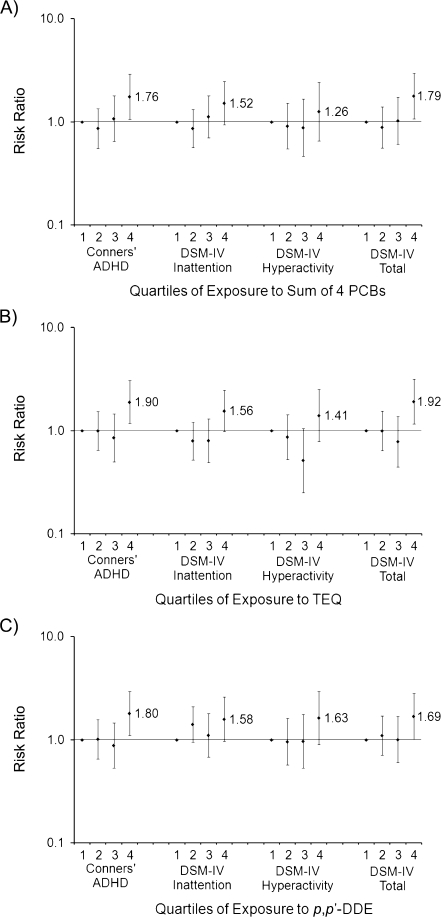
Figure 1 shows risk ratios and 95% confidence intervals for ADHD-like behavior dichotomized at the 86th percentile (mildly to markedly atypical scores) across quartiles of exposure to the sum of 4 PCBs, dioxin-like PCBs (toxic equivalent), and p,p′-DDE. Across the 3 exposures, risk of ADHD-like behaviors increased by 26%–92% for the highest quartiles of exposure versus the lowest quartiles. Risk associated with PCB and p,p′-DDE exposure was highest for outcomes representing behavior related to the combined measures—Conners' ADHD Index and DSM-IV Total (69%–92%). While the P value for the trend across exposure quartiles was significant for the majority of the plots, this increase did not appear monotonic across quartiles; an apparent threshold of effect was observed at the highest exposure quartile (e.g., ΣPCB4 ranging from 0.30 ng/g serum to 4.4 ng/g serum).
Figure 1.
Adjusted risk ratios for behaviors associated with attention deficit hyperactivity disorder (ADHD) among school-aged children (n = 573) born in New Bedford, Massachusetts, in 1993–1998, according to umbilical cord serum levels of A) the sum of 4 polychlorinated biphenyl (PCB) congeners (118, 138, 153, 180), B) the sum of mono-ortho toxic equivalency factor-weighted PCB congeners (TEQ), and C) p,p′-dichlorodiphenyl dichloroethylene (p,p′-DDE). Behaviors associated with ADHD were assessed using 4 subscales of the Conners’ Rating Scale for Teachers: Conners’ ADHD Index, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) Inattention, DSM-IV Impulsive-Hyperactive, and DSM-IV Total. Values were dichotomized at the 86th percentile. Results for Conners’ ADHD Index were adjusted for child's age and sex and for maternal age, marital status, smoking during pregnancy, alcohol consumption during pregnancy, local fish consumption during pregnancy, and illicit drug use. All DSM-IV outcomes were adjusted for child's age and sex and for maternal age, marital status, smoking during pregnancy, and Home Observation for Measurement of the Environment score. A statistically significant P value for trend (P < 0.05) was detected for A) the sum of 4 PCBs and all outcomes, except for DSM-IV Hyperactivity; B) TEQ and all outcomes; and C) p,p′-DDE and Conners’ ADHD Index and DSM-IV Total. Bars, 95% confidence interval.
Forty-five children were reported by their parents to have a history of ADHD medication use. Sensitivity analysis excluding ADHD medication users and imputing missing covariate data did not change the estimates of effect for organochlorines and CRS-T.
DISCUSSION
In this cohort of children born to mothers residing adjacent to a PCB-contaminated harbor, prenatal umbilical cord blood PCB levels were low relative to other population-based PCB studies (13, 20). Despite low exposure levels, we detected moderate associations between PCB and p,p′-DDE levels and ADHD-like behaviors in this population.
Previous studies of PCBs and child behavior have largely focused on psychometric test batteries for which associations with clinical ADHD have not been established. Neuropsychological test batteries were not used in this analysis and may measure something different than behaviors observed by a child's teacher; however, our findings are broadly consistent with PCB-associated increases in inattention and impulsive responding observed with psychometric testing. For example, associations between prenatal PCB exposure and increased reaction time were found in the Faroe Islands (4) (in the presence of high mercury exposure) using the Neuropsychological Evaluation System Continuous Performance Test at age 7 years and in Dutch children (11) using the Simple Reaction Time Test at age 9 years, suggesting a possible impact on attention. Studies of Michigan children born to consumers of PCB-contaminated fish found no association between prenatal PCB exposures and sustained attention (defined as the ability to maintain focus and alertness over time), measured with a continuous performance test at ages 4 and 11 years (6, 21), but did find an association with focused attention (defined as the maintenance of attention in the presence of distractions), using a digit cancellation task (6) and the freedom-from-distractibility scale of the Wechsler Intelligence Scale for Children at age 11 years (5). Investigators from the Oswego Newborn and Infant Development Project reported associations between prenatal PCBs and errors of commission (false-positive responses) on the Continuous Performance Test at 4.5, 8, and 9.5 years of age, suggesting a potential impairment of response inhibition (9, 10). This was supported by observed associations of PCBs with poorer performance on the Differential Reinforcement of Low Rates of Response test (in the same cohort at age 9.5 years), which measures the ability to withhold a rewarded response for a specific time delay, another test of response inhibition (22).
In addition, we recently reported associations between prenatal PCB and p,p′-DDE exposures and poor attention in early infancy in the New Bedford cohort using items selected a priori from the Neonatal Behavioral Assessment Scale, including alertness, quality of alert responsiveness, and cost of attention (8).
In the only study of the association between PCBs and clinically diagnosed ADHD, Lee et al. (23) reported null findings. However, that study, which used data from the National Health and Nutrition Examination Survey, had a number of limitations (24): Firstly, PCB exposure assessment was confined to only 1 PCB congener (PCB 126) which is coplanar, present at extremely low levels (parts per trillion) in population samples, and not representative of the likely toxic mechanism of the more prevalent non-dioxin-like and mono-ortho PCBs found in most population-based samples. For PCBs, it has been postulated that the non-dioxin-like congeners may be the ones that are more deleterious to neurocognitive functions (25, 26). Secondly, the design was cross-sectional; therefore, Lee et al. were unable to evaluate the effect of prenatal organochlorine exposure, which may be the most critical exposure period. Thirdly, ADHD diagnosis was based on parental reports, the validity of which is unclear.
In animal studies, investigators have reported associations between PCBs and ADHD-like behaviors in rodents and nonhuman primates (27–29), and laboratory studies show that PCBs can disrupt dopamine levels and dopaminergic function in the brain (30, 31); dopamine system dysfunction has also been linked with ADHD (32). Organochlorines, including PCBs, have also been shown to reduce circulating levels of thyroid hormones in animals (33) and humans (34, 35). Associations between these hormone disruptions and ADHD-like behaviors have been demonstrated (36–38), though not consistently (39, 40).
This analysis focused on ADHD-associated behaviors assessed with a teacher's behavioral rating scale, rather than clinically diagnosed ADHD. Although behavioral checklists like the CRS-T may not be associated with clinical ADHD on an individual basis, they are used in conjunction with other guidelines for clinically diagnosing this disorder and characterizing the functional consequences of its symptoms (inattention, hyperactivity, impulsivity). The CRS-T assesses ADHD-like behaviors on a continuous scale—a strength of this study, since ADHD may be best viewed on a behavioral continuum rather than as a categorical disorder (41). Assessing continuously distributed outcomes rather than clinical diagnoses, in addition to improving power, also prevents classification to a diagnosis with a potentially arbitrary cutoff or subjective guidelines that may change over time (42). In addition, deficits on neurobehavioral examinations may indicate early stages of a disorder or more mild cases of this disorder, which a clinical diagnosis would tend to miss (42).
Though the observed associations between organochlorines and continuous CRS-T outcomes were modest (e.g., a 2.4-point increase in the Conners’ ADHD Index for a 0.6-ng/g increase in the sum of 4 PCBs (Table 3)), a shift in group mean is less important for its average change in the population and more important for the change represented at the tails of the distribution, where clinical disease is detected (42). Thus, even a small association between prenatal organochlorines and a health indicator such as increased ADHD-associated behavior in childhood can signal substantial changes in the prevalence of clinically evident ADHD in the population. In fact, with dichotomized CRS-T measures, children in the upper 75% of exposure (ΣPCB4 ranging from 0.30 ng/g to 4.4 ng/g) had a substantially increased risk (risk ratio = 1.8, 95% confidence interval: 1.1, 2.9) of possible or significant behavioral problems on the Conners’ ADHD Index (Figure 1).
Covariate data were missing for a notable proportion of study subjects (10%–15% of those with exposure and outcome data). While this probably reduced the precision of our estimates, we ruled out selection bias by showing that the association between PCBs and ADHD-like behaviors was not meaningfully different for the subsets with and without complete covariate data (Table 3). In addition, imputing covariate data did not change our findings (data not shown).
Notably, in our population higher age was associated with higher risk of ADHD-like behaviors. High-risk children may have been more difficult to schedule or follow up, which could explain why they were examined at older ages.
We found evidence for negative confounding by covariates in the associations between organochlorines and ADHD-like behaviors, as shown by stronger adjusted exposure-outcome estimates than unadjusted estimates (Table 3). Residual confounding due to incomplete adjustment for negative confounders would potentially lead to underestimation of the true exposure-outcome association.
In summary, associations between prenatal exposure to organochlorines, including PCBs and p,p′-DDE, and ADHD-like behaviors were found using a teacher-administered rating scale. Given the burden these behaviors place on the medical and educational system and on the family, identification of modifiable risk factors is a public health priority. Future work in the New Bedford cohort will be directed toward finding consistency between these results and those obtained using other neurobehavioral tests.
Acknowledgments
Author affiliations: Department of Environmental Health, Harvard School of Public Health, Boston, Massachusetts (Sharon K. Sagiv, David C. Bellinger, Larisa M. Altshul, Susan A. Korrick); Channing Laboratory, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts (Sharon K. Sagiv, Susan A. Korrick); Department of Biostatistics and Computational Biology, School of Medicine and Dentistry, University of Rochester, Rochester, New York (Sally W. Thurston); Children's Hospital, Harvard Medical School, Boston, Massachusetts (David C. Bellinger); and Department of Environmental and Occupational Health, Rollins School of Public Health, Emory University, Atlanta, Georgia (Paige E. Tolbert).
This work was supported by the National Institutes of Health (grants P42 ES05947, R01 ES014864, and T32 MH073122 to S. S.).
The authors thank Cristina Kehoe and Wendy Atkinson for data collection; Diane Sredl, Changzhong Chen, Catrina Crociani, and Elizabeth Wood for database management and programming; and Debbie Raposo and the labor and delivery staff at St. Luke's Hospital for support with data collection.
Conflict of interest: none declared.
Glossary
Abbreviations
- ADHD
attention deficit hyperactivity disorder
- CRS-T
Conners’ Rating Scale for Teachers
- p,p′-DDE
p,p′-dichlorodiphenyl dichloroethylene
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition
- HOME
Home Observation for Measurement of the Environment
- PCB(s)
polychlorinated biphenyl(s)
References
- 1.Faraone SV, Sergeant J, Gillberg C, et al. The worldwide prevalence of ADHD: is it an American condition? World Psychiatry. 2003;2(2):104–113. [PMC free article] [PubMed] [Google Scholar]
- 2.Pelham WE, Foster EM, Robb JA. The economic impact of attention-deficit/hyperactivity disorder in children and adolescents. J Pediatr Psychol. 2007;32(6):711–727. doi: 10.1093/jpepsy/jsm022. [DOI] [PubMed] [Google Scholar]
- 3.Schantz SL, Widholm JJ, Rice DC. Effects of PCB exposure on neuropsychological function in children. Environ Health Perspect. 2003;111(3):357–376. doi: 10.1289/ehp.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grandjean P, Weihe P, Burse VW, et al. Neurobehavioral deficits associated with PCB in 7-year-old children prenatally exposed to seafood neurotoxicants. Neurotoxicol Teratol. 2001;23(4):305–317. doi: 10.1016/s0892-0362(01)00155-6. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson JL, Jacobson SW. Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N Engl J Med. 1996;335(11):783–789. doi: 10.1056/NEJM199609123351104. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson JL, Jacobson SW. Prenatal exposure to polychlorinated biphenyls and attention at school age. J Pediatr. 2003;143(6):780–788. doi: 10.1067/S0022-3476(03)00577-8. [DOI] [PubMed] [Google Scholar]
- 7.Peper M, Klett M, Morgenstern R. Neuropsychological effects of chronic low-dose exposure to polychlorinated biphenyls (PCBs): a cross-sectional study [electronic article] Environ Health. 2005;4(1):22. doi: 10.1186/1476-069X-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sagiv SK, Nugent JK, Brazelton TB, et al. Prenatal organochlorine exposure and measures of behavior in infancy using the Neonatal Behavioral Assessment Scale (NBAS) Environ Health Perspect. 2008;116(5):666–673. doi: 10.1289/ehp.10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart P, Fitzgerald S, Reihman J, et al. Prenatal PCB exposure, the corpus callosum, and response inhibition. Environ Health Perspect. 2003;111(13):1670–1677. doi: 10.1289/ehp.6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart P, Reihman J, Gump B, et al. Response inhibition at 8 and 9 1/2 years of age in children prenatally exposed to PCBs. Neurotoxicol Teratol. 2005;27(6):771–780. doi: 10.1016/j.ntt.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Vreugdenhil HJ, Mulder PG, Emmen HH, et al. Effects of perinatal exposure to PCBs on neuropsychological functions in the Rotterdam cohort at 9 years of age. Neuropsychology. 2004;18(1):185–193. doi: 10.1037/0894-4105.18.1.185. [DOI] [PubMed] [Google Scholar]
- 12.American Academy of Pediatrics. Clinical practice guideline: diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder. Pediatrics. 2000;105(5):1158–1170. doi: 10.1542/peds.105.5.1158. [DOI] [PubMed] [Google Scholar]
- 13.Korrick SA, Altshul LM, Tolbert PE, et al. Measurement of PCBs, DDE, and hexachlorobenzene in cord blood from infants born in towns adjacent to a PCB-contaminated waste site. J Expo Anal Environ Epidemiol. 2000;10(6):743–754. doi: 10.1038/sj.jea.7500120. [DOI] [PubMed] [Google Scholar]
- 14.Denkins YM, Woods J, Whitty JE, et al. Effects of gestational alcohol exposure on the fatty acid composition of umbilical cord serum in humans. Am J Clin Nutr. 2000;71(1 suppl):300S–306S. doi: 10.1093/ajcn/71.1.300s. [DOI] [PubMed] [Google Scholar]
- 15.Kim R, Aro A, Rotnitzky A, et al. K x-ray fluorescence measurements of bone lead concentration: the analysis of low-level data. Phys Med Biol. 1995;40(9):1475–1485. doi: 10.1088/0031-9155/40/9/007. [DOI] [PubMed] [Google Scholar]
- 16.Conners CK. Conners’ Rating Scales-Revised Technical Manual. North Tonawanda, NY: Multi-Health Systems; 1997. [Google Scholar]
- 17.Van den Berg M, Birnbaum L, Bosveld AT, et al. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect. 1998;106(12):775–792. doi: 10.1289/ehp.98106775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bureau of Environmental Health, Massachusetts Department of Public Health. Childhood Blood Lead Levels. Boston, MA: Bureau of Environmental Health, Massachusetts Department of Public Health; 2009. ( http://matracking.ehs.state.ma.us/Health_Data/Childhood_Blood_Lead_Levels.html). (Accessed November 3, 2009) [Google Scholar]
- 19.Caldwell B, Bradley R. Home Observation for Measurement of the Environment—Revised Edition. Little Rock, AR: University of Arkansas; 1984. [Google Scholar]
- 20.Longnecker MP, Wolff MS, Gladen BC, et al. Comparison of polychlorinated biphenyl levels across studies of human neurodevelopment. Environ Health Perspect. 2003;111(1):65–70. doi: 10.1289/ehp.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobson JL, Jacobson SW, Padgett RJ, et al. Effects of prenatal PCB exposure on cognitive procession efficiency and sustained attention. Dev Psychol. 1992;28(2):297–306. [Google Scholar]
- 22.Stewart PW, Sargent DM, Reihman J, et al. Response inhibition during Differential Reinforcement of Low Rates (DRL) schedules may be sensitive to low-level polychlorinated biphenyl, methylmercury, and lead exposure in children. Environ Health Perspect. 2006;114(12):1923–1929. doi: 10.1289/ehp.9216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee DH, Jacobs DR, Porta M. Association of serum concentrations of persistent organic pollutants with the prevalence of learning disability and attention deficit disorder. J Epidemiol Community Health. 2007;61(7):591–596. doi: 10.1136/jech.2006.054700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korrick SA, Bellinger DC. Invited commentary: persistent organic pollutants and childhood learning and behavioural disorders. J Epidemiol Community Health. 2007;61(7):564–565. doi: 10.1136/jech.2006.058073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer LJ, Seegal RF, Ganey PE, et al. Symposium overview: toxicity of non-coplanar PCBs. Toxicol Sci. 1998;41(1):49–61. doi: 10.1006/toxs.1997.2386. [DOI] [PubMed] [Google Scholar]
- 26.Schantz SL, Seo BW, Moshtaghian J, et al. Effects of gestational and lactational exposure to TCDD or coplanar PCBs on spatial learning. Neurotoxicol Teratol. 1996;18(3):305–313. doi: 10.1016/s0892-0362(96)90033-1. [DOI] [PubMed] [Google Scholar]
- 27.Berger DF, Lombardo JP, Jeffers PM, et al. Hyperactivity and impulsiveness in rats fed diets supplemented with either Aroclor 1248 or PCB-contaminated St. Lawrence river fish. Behav Brain Res. 2001;126(1-2):1–11. doi: 10.1016/s0166-4328(01)00244-3. [DOI] [PubMed] [Google Scholar]
- 28.Holene E, Nafstad I, Skaare JU, et al. Behavioural hyperactivity in rats following postnatal exposure to sub-toxic doses of polychlorinated biphenyl congeners 153 and 126. Behav Brain Res. 1998;94(1):213–224. doi: 10.1016/s0166-4328(97)00181-2. [DOI] [PubMed] [Google Scholar]
- 29.Rice DC. Parallels between attention deficit hyperactivity disorder and behavioral deficits produced by neurotoxic exposure in monkeys. Environ Health Perspect. 2000;108(suppl 3):405–408. doi: 10.1289/ehp.00108s3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seegal RF, Brosch KO, Okoniewski RJ. Effects of in utero and lactational exposure of the laboratory rat to 2,4,2′,4′- and 3,4,3′,4′-tetrachlorobiphenyl on dopamine function. Toxicol Appl Pharmacol. 1997;146(1):95–103. doi: 10.1006/taap.1997.8226. [DOI] [PubMed] [Google Scholar]
- 31.Seegal RF, Okoniewski RJ, Brosch KO, et al. Polychlorinated biphenyls alter extraneuronal but not tissue dopamine concentrations in adult rat striatum: an in vivo microdialysis study. Environ Health Perspect. 2002;110(11):1113–1117. doi: 10.1289/ehp.021101113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faraone SV, Biederman J. Neurobiology of attention-deficit hyperactivity disorder. Biol Psychiatry. 1998;44(10):951–958. doi: 10.1016/s0006-3223(98)00240-6. [DOI] [PubMed] [Google Scholar]
- 33.Sinjari T, Darnerud PO. Hydroxylated polychlorinated biphenyls: placental transfer and effects on thyroxine in the foetal mouse. Xenobiotica. 1998;28(1):21–30. doi: 10.1080/004982598239722. [DOI] [PubMed] [Google Scholar]
- 34.Koopman-Esseboom C, Morse DC, Weisglas-Kuperus N, et al. Effects of dioxins and polychlorinated biphenyls on thyroid hormone status of pregnant women and their infants. Pediatr Res. 1994;36(4):468–473. doi: 10.1203/00006450-199410000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Takser L, Mergler D, Baldwin M, et al. Thyroid hormones in pregnancy in relation to environmental exposure to organochlorine compounds and mercury. Environ Health Perspect. 2005;113(8):1039–1045. doi: 10.1289/ehp.7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alvarez-Pedrerol M, Ribas-Fitó N, Torrent M, et al. TSH concentration within the normal range is associated with cognitive function and ADHD symptoms in healthy preschoolers. Clin Endocrinol (Oxf) 2007;66(6):890–898. doi: 10.1111/j.1365-2265.2007.02871.x. [DOI] [PubMed] [Google Scholar]
- 37.Hauser P, Zametkin AJ, Martinez P, et al. Attention deficit-hyperactivity disorder in people with generalized resistance to thyroid hormone. N Engl J Med. 1993;328(14):997–1001. doi: 10.1056/NEJM199304083281403. [DOI] [PubMed] [Google Scholar]
- 38.Matochik JA, Zametkin AJ, Cohen RM, et al. Abnormalities in sustained attention and anterior cingulate metabolism in subjects with resistance to thyroid hormone. Brain Res. 1996;723(1-2):23–28. doi: 10.1016/0006-8993(96)00177-1. [DOI] [PubMed] [Google Scholar]
- 39.Stein MA, Weiss RE. Thyroid function tests and neurocognitive functioning in children referred for attention deficit/hyperactivity disorder. Psychoneuroendocrinology. 2003;28(3):304–316. doi: 10.1016/s0306-4530(02)00024-0. [DOI] [PubMed] [Google Scholar]
- 40.Toren P, Karasik A, Eldar S, et al. Thyroid function in attention deficit and hyperactivity disorder. J Psychiatr Res. 1997;31(3):359–363. doi: 10.1016/s0022-3956(96)00061-1. [DOI] [PubMed] [Google Scholar]
- 41.Levy F, Hay DA, McStephen M, et al. Attention-deficit hyperactivity disorder: a category or a continuum? Genetic analysis of a large-scale twin study. J Am Acad Child Adolesc Psychiatry. 1997;36(6):737–744. doi: 10.1097/00004583-199706000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Bellinger DC. What is an adverse effect? A possible resolution of clinical and epidemiological perspectives on neurobehavioral toxicity. Environ Res. 2004;95(3):394–405. doi: 10.1016/j.envres.2003.07.013. [DOI] [PubMed] [Google Scholar]