Abstract
Handedness is the clearest example of behavioral lateralization in humans. It is not known whether the obvious asymmetry manifested by hand preference is associated with similar asymmetry in brain activation during movement. We examined the functional activation in cortical motor areas during movement of the dominant and nondominant hand in groups of right-handed and left-handed subjects and found that use of the dominant hand was associated with a greater volume of activation in the contralateral motor cortex. Furthermore, there was a separate relation between the degree of handedness and the extent of functional lateralization in the motor cortex. The patterns of functional activation associated with the direction and degree of handedness suggest that these aspects are independent and are coded separately in the brain.
Nine of every 10 humans prefer to use the right hand; this proportion has been consistent across cultures and over time (1, 2). Hand preference is the most striking example of behavioral asymmetry or lateralization in humans; in fact, this lateralization defines handedness. Studies on handedness have concentrated on its relation to language (3, 4) and on its environmental and genetic determinants (5). However, it is not known whether the behavioral lateralization manifested in handedness is associated with an asymmetry in the activation of motor areas of the brain during movement. There are examples in humans and in other species whereby superior behavioral performance is associated with an increase in the volume of neural tissue devoted to that behavior. These include, in the human, the volume of motor and somatosensory cortex related to the hand compared with the foot or trunk‖ (6) and, in rodents, the volume of cortex devoted to the vibrissae (8). Studies using quantitative anatomical methods to examine changes in the volume of the contralateral human motor cortex related to use of the dominant hand reached inconsistent conclusions (9, 10). Only one previous functional imaging study has addressed explicitly the issue of handedness (11); however, in that study, all comparisons were within hemisphere, and asymmetric activation associated with the use of a particular hand was not examined for methodological reasons. Preliminary results using magnetoencephalography suggest that there may be an increase in the size of the hand area related to use of the dominant hand (12). In the current study, we used functional MRI in right-handed (RH) and left-handed (LH) subjects to test the hypothesis that use of the dominant hand, compared with the nondominant hand, is associated with a greater volume of functional activation in contralateral cortical motor areas.
METHODS
Behavioral Task.
Thirteen subjects [seven RH (three male, four female) and six LH (four male, two female), aged 19–34 years (mean 25.5 years)] took part in the study. There were two behavioral tasks. At the beginning of each task, the second through fifth fingers of the subject’s prone semi-flexed hand rested on four low force push buttons mounted on a small pad. The visual instruction was through the use of four annuli arranged horizontally, one corresponding to each finger, displayed on a computer screen and visible through the use of a small mirror fixed in the magnet directly above the head of the subject. Filling of an annulus instructed an extension of the corresponding digit. The annuli were filled, one at a time (0.75 Hz), in either a randomized sequence (unpredictable task) or a regular left-to-right repeating sequence (predictable task). The reaction time and error rate were recorded during each motor response. The data during the performance of each task were pooled for the current analysis (see Statistical Data Analysis below); task-related changes in functional activation will be reported separately. Both the direction and the degree of handedness for each subject were determined on the basis of the Edinburgh inventory (13), which enabled us to assign a laterality quotient in the range of −100 to +100 for which −100 indicated extreme left handedness, 0 indicated ambidexterity, and +100 indicated extreme right handedness. The absolute value of the laterality quotient was used to determine degree of handedness in both RH and LH subjects.
Experimental Design.
The behavioral tasks were presented in 60-s blocks. The presentation of the tasks was randomized for each hand, and within each hand the order of tasks was randomized. Each task period was bracketed by two 60-s visual control periods (the whole comprising one 180-s experimental period) during which appropriate control visual stimuli (predictable or unpredictable) were shown and the subjects were instructed to attend to the stimulus but not to produce a motor response. Each 180-s experimental period was followed by a rest interval of ≈120 s. There was one experimental period for each condition (hand/task) after which the sequence of conditions was repeated.
MRI.
Magnetic resonance images were obtained in a 4 Tesla whole body system equipped with an actively shielded head gradient insert and a quadrature head coil (SIS, Palo Alto, CA, and Siemens, Erlangen, Germany). Anatomical T1-weighted images of the whole brain [multislice turboFLASH, echo time (TE) = 3 ms, repetition time (TR) = 7 ms, 128 × 128 voxels, field of view (FOV) = 24 × 24 cm2, 5-mm slice thickness] were first obtained in coronal, sagittal, and transverse planes to allow identification of the anterior and posterior commissures and to determine the appropriate volume for the subsequent functional images. This volume subsequently was imaged in the transverse plane with T1-weighted echo planar imaging (EPI) [4-segment EPI, TE = 8 ms, TR = 42 ms/segment and 3 s/image, inversion time = 1.2 s, 128 × 128 voxels, FOV = 24 × 24 cm2, 5-mm slice thickness] to provide for accurate overlay of the functional images (14). Blood oxygen level-dependent (BOLD)-based functional MR images in the transverse plane (TE = 25 ms, TR = 50 ms, 64 × 64 voxels, FOV = 24 × 24 cm2) were obtained with blipped EPI, with the total imaged volume extending from the superior pole of the cortex to a depth of 50 mm in 10 slices. Functional images had an in-plane resolution of 3.75 × 3.75 mm2 and a thickness of 5 mm and were collected every 3 s during each 180-s experiment, with 20 images collected in each of the control and task periods.
Functional Image Analysis.
Functional images were first screened for movement artifact by inspecting the images and examining head motion data that simultaneously were monitored by using a pressure sensor; contaminated images were not analyzed further. The functional data were zero-filled to 128 × 128 pixels and then Fourier-transformed to yield a resultant nominal in-plane resolution of 1.875 × 1.875 mm2. Voxels with signal intensities having a coefficient of variation >2.5% during the task control periods were masked to eliminate large vessel contributions (15). For each remaining voxel, two separate Student’s t tests were performed for each of the two repetitions of the hand/task condition: one to test for differences between the pretask control and the task and the other to test for differences between the posttask control and the task periods. The independent t test has been shown to be both sensitive and specific for the detection of functional activation in MRIs (16). Only those voxels that showed significant differences in all four t tests, each at a significance level of P < 0.05 (comparison-wise), were included in the analysis. For each individual voxel, the experiment-wise error rate was P = 0.000144, calculated using a Monte Carlo simulation. Voxels activated using these criteria formed a functional activation map, which was overlaid onto the EPI anatomical images.
Statistical Data Analysis.
The primary datum was the number of activated voxels calculated separately for each area of interest contralateral and ipsilateral to the hand used to perform the task. Before statistical analysis, the volume of activated voxels was transformed using a square root transformation for counts to stabilize the variance and normalize the distribution (17). There were no significant interactions between task (unpredictable/predictable) and hand (dominant/nondominant), task and handedness (RH/LH), or task, hand, and handedness. Therefore, the results for both behavioral tasks were pooled in this analysis; main effects of task will be reported separately. Main effects and interactions were tested using a repeated measures ANOVA.
Anatomical Boundaries.
Regions of interest were delineated in the EPI anatomical images by using anatomical landmarks in the brains of the individual subjects. “Primary motor cortex” was defined as the volume of cortex that included the posterior half of the precentral gyrus (including the anterior bank of the central sulcus). “Premotor cortex” included the anterior half of the precentral gyrus as well as the anterior bank of the precentral sulcus. “Supplementary motor area” (SMA) was limited to the cortex on the medial wall of the hemisphere, extending from the superior pole to the depth of the cingulate sulcus, including the dorsal bank of the cingulate sulcus (18, 19); the posterior boundary was halfway between the extension of the central and precentral sulci onto the medial surface, and the anterior boundary was defined by the vertical line drawn through the anterior commissure (VCA) line at the level of the anterior commissure. “Presupplementary motor area” was the extension of the SMA rostral to the vertical line drawn through the anterior commissure (VCA) line to include sector D in Talairach space (20), from the superior pole to the cingulate sulcus (including the dorsal bank). “Cingulate motor area” was contained within the cingulate gyrus inferior to SMA. “Superior parietal lobule” extended anteriorly to the postcentral sulcus, laterally to the intraparietal sulcus, posteriorly to the parieto-occipital sulcus, and medially to the parieto-occipital and cingulate sulci.
RESULTS
Functional images of the brain were taken in 13 subjects during visually instructed sequences of finger movements performed with the right and left hands separately. We calculated the number of activated voxels in six cortical motor areas (motor cortex, premotor cortex, SMA, pre-SMA, cingulate motor area, superior parietal lobule) contralateral and ipsilateral to the hand movement. We tested the relation between hand dominance and the volume of functional activation using a repeated measures ANOVA, examining the effect of handedness (RH/LH) and the hand (dominant/nondominant) used in the task on the volume of contralateral functional activation. There was a significant main effect of hand on activation in the contralateral motor cortex (P = 0.027) but not in the other contralateral motor areas either individually or on the average. There was no main effect of handedness nor was there an interaction effect (Fig. 1). These results suggest that use of the dominant hand is associated with greater activation (compared with the nondominant hand) in the contralateral motor cortex and that this increase is similar in RH and LH subjects. These findings cannot be attributed to differences in the performance of the two hands because neither response time (dominant = 414.8 ± 11.7 ms, nondominant = 430.4 ± 12.9 ms) nor error rate (dominant = 1.8 ± 0.4%, nondominant = 1.2 ± 0.3%) differed.
Figure 1.
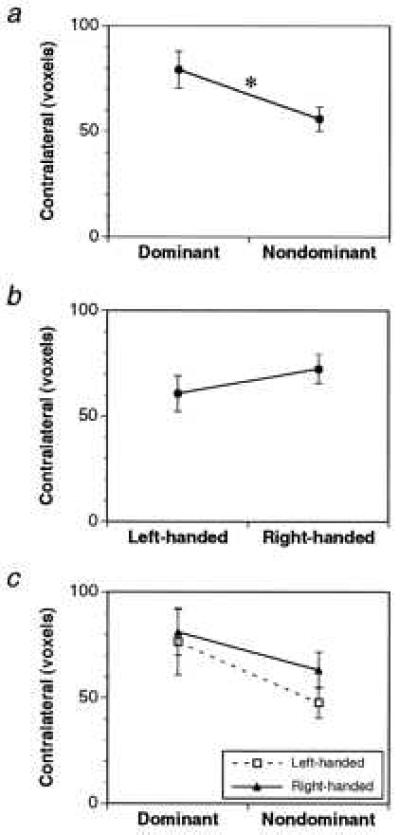
Effects of hand (a), handedness (b), and their interaction (c) on the volume of contralateral functional activation in the motor cortex during movement of the hand. Only the effect of hand reached statistical significance (P = 0.027, indicated by the ∗).
It occurred to us that the level of functional activation contralateral to the dominant hand also may reflect the degree of handedness in individual subjects. We examined this issue by relating the contralateral activation to a quantitative measure of handedness. The degree of handedness was determined on the basis of the Edinburgh inventory questionnaire (ref. 13 and see Methods). We found no significant correlation between the degree of handedness and contralateral activation. However, there was a negative correlation (Figs. 2 and 3) between the degree of handedness and activation ipsilateral to the dominant hand, which only reached significance in the motor cortex (r = −0.601, P = 0.03). This finding suggested that those subjects who had the strongest hand preference had the least amount of activation ipsilateral to the preferred hand during movement. Nevertheless, the highest correlation (r = 0.694, P = 0.008) between the activation of the motor cortex and the degree of hand preference was seen when we took both the contralateral and ipsilateral activations into account in a lateralization index [defined as the volume of contralateral activation expressed as a percentage of the total activation, i.e., contra/(contra + ipsi)]. In general, a high degree of handedness was associated with greater lateralization of functional activation during use of the dominant hand in both the RH and LH subjects. Therefore, although ipsilateral activation seems to be relatively more important than contralateral activation in its contribution to the correlation, its relation to the degree of handedness is not as strong as that of the lateralization index that takes both ipsilateral and contralateral activations into account. Although the correlation between extent of handedness and lateralization was not significant in any of the other five cortical motor areas individually, the correlation did reach significance on the average across all five areas (r = 0.584, P = 0.036), suggesting a weaker but similar trend in these nonprimary motor areas as that seen in the motor cortex.
Figure 2.
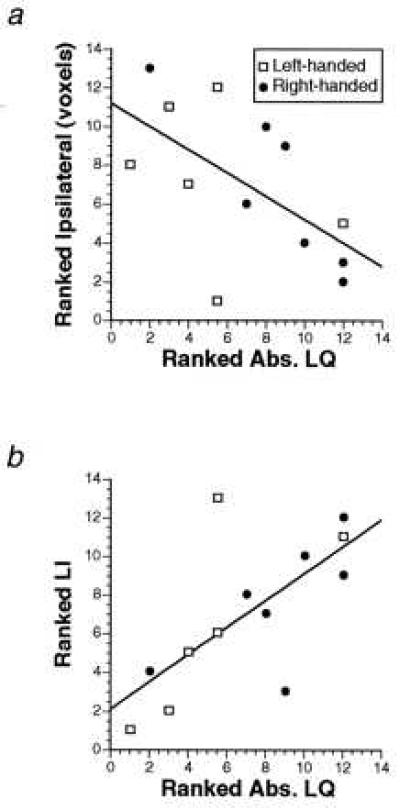
(a) Ranked correlation between ipsilateral activation in the motor cortex and absolute laterality quotient (Abs. LQ) during use of the dominant hand in RH and LH subjects (r = −0.601, P = 0.03). (b) Ranked correlation between the lateralization index (LI) in the motor cortex and absolute laterality quotient (Abs. LQ) during use of the dominant hand in RH and LH subjects (r = 0.694, P = 0.008). In a and b, the absolute value of the laterality quotient scores was taken, and the subjects were ranked according to the strength of handedness (irrespective of hand preference). The ranking on both axes is from lowest to highest.
Figure 3.
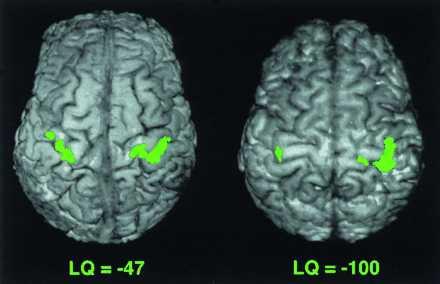
Functional activation in the motor cortex during movement of the left hand in two LH subjects with different LQ. Subject with LQ of −47 has LI of 0.69, and subject with LQ of −100 has LI of 0.94. The lateralization index is greater with the larger LQ. In these images, the anterior portion of the brain is on the top, and the right hemisphere is on the right.
DISCUSSION
In summary, we found a clear association between the behavioral lateralization manifested by handedness and functional activation. The volume of activation in the contralateral motor cortex of the RH and LH subjects was consistently greater during movements of the dominant compared with those of the nondominant hand. The activation therefore indicated the direction of handedness in these subjects. We also documented a correlation between the degree of handedness and the lateralization of cortical activation during use of the dominant hand. Activation in the motor cortex was more lateralized with increasing degrees of handedness in both RH and LH subjects. To our knowledge, these relations among functional brain activation, direction of handedness, and degree of handedness have not been demonstrated previously using functional brain imaging.
Our results suggest that there is a high degree of association between functional activation of the brain and motor behavior. They are in general agreement with the results of a study in nonhuman primates that showed that hand preference in the squirrel monkey was correlated with the size of the area from which responses to microstimulation could be elicited in the contralateral hemisphere (21). The issue of whether such functional asymmetry is related to concomitant structural changes in the motor cortex has not been resolved. A recent study using in vivo magnetic resonance morphometry has shown an increase in the volume of motor cortex contralateral to the dominant hand (9). However this finding is at variance with the results of a study of autopsy brains that failed to show any consistent relation between the volume of motor cortex (area 4) and hand preference (10). In addition, in the latter study, the authors found no asymmetry at other levels of the sensorimotor system such as the medulla or spinal cord although such asymmetries have been reported by others (22–24). In an effort to account for the lack of structural asymmetry at the level of the motor cortex, White et al. (10) have suggested that structural changes in the primary motor cortex contralateral to the dominant hand may be balanced by structural changes associated with “less obvious capabilities” than handedness in the motor cortex contralateral to the nondominant hand. The issue of structural asymmetry in the sensorimotor systems of humans remains an open question. This does not, however, detract from our findings, which are quite clear with respect to functional asymmetry. The concordance between structure and function may indeed be elusive, as evidenced by the passage of over 1 hundred years between the localization of language to the left hemisphere (25) and the demonstration of an asymmetry in the planum temporale (26) between hemispheres.
It has been suggested that other motor structures, particularly the premotor cortex and the SMA, are more strongly related to hand preference than the motor cortex (27). Our results do not support this suggestion; among the six motor areas we studied, only the motor cortex showed a significant relation to either the direction or degree of handedness. This is consistent with the primary role of the motor cortex in motor output: It has the highest concentration of corticospinal projection neurons (28) and is the only cortical area with monosynaptic connections to spinal motoneurons (29). The motor cortex also has been shown to reflect changes in hand use such as may occur during the acquisition of a motor skill (30, 31) and thus might be expected to reflect the behavioral asymmetry manifested by handedness.
The relation between lateralization in the motor cortex and the degree of handedness raises a number of interesting issues. The first concerns the nature of handedness itself, whether it is a categorical variable with RH and LH and perhaps including another category for ambidextrous subjects or, alternatively, whether handedness is a continuous variable as suggested by Woo and Pearson (32) on the basis of data compiled by Galton and as used in different handedness rating scales (13). We have documented a continuous relation between the degree of handedness and functional activation (see Fig. 2), which suggests that handedness is not categorical as far as brain activation is concerned. The second issue concerns the validity and significance of ipsilateral activation of the motor cortex during hand movement, which has been a point of controversy in the literature (33). Although the most significant relation between degree of handedness and functional activation is found for lateralization (which includes both contralateral and ipsilateral activity) in the motor cortex, the essence of this relation is encapsulated in the ipsilateral activation alone. We found an inverse relation between the degree of handedness and the extent of ipsilateral activation in the motor cortex. The corollary of this is that a high degree of handedness is subserved by activation of the motor cortex, which is almost exclusively contralateral to the movement. Our findings in relation to the degree of handedness may be relevant in predicting the outcome of motor rehabilitation in human subjects with stroke. We would predict that patients with a high degree of handedness would be more incapacitated in the use of the dominant hand after damage to the contralateral motor areas than those with a low degree of handedness because the latter are likely to have greater residual input from the ipsilateral motor areas. Although studies have examined the extent of motor impairment related to damage to a particular hemisphere (34, 35), none has addressed impairment related to the degree of handedness.
Acknowledgments
We thank J. Bullis for help with data processing, S. M. Lewis and A. P. Georgopoulos for constructive comments on the manuscript, and A. Verbanov and G. Oehlert for helpful discussion. This work was supported by the National Institutes of Health Grant NS 32437, the Charles A. Dana Foundation, the Department of Veterans Affairs, and the American Legion chair in Brain Sciences.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: RH, right-handed; LH, left-handed; SMA, supplementary motor area; EPI, echo planar imaging; LQ, laterality quotient.
However, some neurophysiological studies indicate that the volume of cortex devoted to hand may not be proportionately larger than that devoted to proximal muscles in monkeys (7).
References
- 1.Coren S, Porac C. Science. 1977;198:631–632. doi: 10.1126/science.335510. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert A N, Wysocki C S. Neuropsychologica. 1992;30:601–608. doi: 10.1016/0028-3932(92)90065-t. [DOI] [PubMed] [Google Scholar]
- 3.Goodglass H, Quadfasal F A. Brain. 1954;77:521–548. doi: 10.1093/brain/77.4.521. [DOI] [PubMed] [Google Scholar]
- 4.Satz P. Science. 1970;203:1131–1133. doi: 10.1126/science.424744. [DOI] [PubMed] [Google Scholar]
- 5.Levy J, Nagylaki T. Genetics. 1972;72:117–128. doi: 10.1093/genetics/72.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Penfield W, Boldrey E. Brain. 1937;60:389–343. [Google Scholar]
- 7.Gould H J, III, Cusick C G, Pons T P, Kaas J H. J Comp Neurol. 1986;247:297–325. doi: 10.1002/cne.902470303. [DOI] [PubMed] [Google Scholar]
- 8.Woolsey T A, Van der Loos H. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- 9.Amunts K, Schlaug G, Schleicher A, Steinmetz H, Dabringhaus A, Roland P E, Zilles K. Neuroimage. 1996;4:216–222. doi: 10.1006/nimg.1996.0073. [DOI] [PubMed] [Google Scholar]
- 10.White L E, Andrews T J, Hulette C, Andrews R, Groelle M, Paydarfar J, Parves D. Cereb Cortex. 1997;7:31–47. doi: 10.1093/cercor/7.1.31. [DOI] [PubMed] [Google Scholar]
- 11.Kim S-G, Ashe J, Hendrich K, Ellermann J M, Merkle H, Uǧurbil K, Georgopoulos A P. Science. 1993;261:615–617. doi: 10.1126/science.8342027. [DOI] [PubMed] [Google Scholar]
- 12.Volkmann J, Schnitzler A, Witte O W, Freund H-J. Neuroimage. 1997;5:S239. [Google Scholar]
- 13.Oldfield R C. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. (abstr.). [DOI] [PubMed] [Google Scholar]
- 14.Kim S-G, Hu X, Adriany G, Uǧurbil K. Magn Reson Med. 1995;35:895–902. doi: 10.1002/mrm.1910350618. [DOI] [PubMed] [Google Scholar]
- 15.Kim S-G, Hendrich K, Merkle H, Uǧurbil K. NMR Biomed. 1994;7:69–74. doi: 10.1002/nbm.1940070111. [DOI] [PubMed] [Google Scholar]
- 16.Xiong J H, Gao J H, Lancaster J L, Fox P T. Hum Brain Mapp. 1996;4:153–167. doi: 10.1002/(SICI)1097-0193(1996)4:3<153::AID-HBM1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Snedecor G W, Cochran W G. Statistical Methods. Ames, IA: Iowa State Univ. Press; 1989. [Google Scholar]
- 18.Barbas H, Pandya D N. J Comp Neurol. 1987;256:211–228. doi: 10.1002/cne.902560203. [DOI] [PubMed] [Google Scholar]
- 19.Vogt B A, Pandya D N, Rosene D L. J Comp Neurol. 1987;262:256–270. doi: 10.1002/cne.902620207. [DOI] [PubMed] [Google Scholar]
- 20.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 21.Nudo R J, Jenkins W M, Merzenich M M, Prejean T, Grenda R. J Neurosci. 1991;12:2918–2947. doi: 10.1523/JNEUROSCI.12-08-02918.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flechsig P E. Die Lietungsbahnen im Gehrin und Rückenmark des Menschen auf Grund Entwickelungsgeschichlicher Untersuchungen. Leipzig, Germany: Engelmann; 1876. [Google Scholar]
- 23.Yalovlev P, Rakic P. Trans Am Neurol Assoc. 1966;91:366–367. [Google Scholar]
- 24.Nathan P W, Smith M C, Deacon P. Brain. 1990;113:303–324. doi: 10.1093/brain/113.2.303. [DOI] [PubMed] [Google Scholar]
- 25.Broca P. Bull Soc Anat Paris. 1865;6:398–407. [Google Scholar]
- 26.Geshwind N, Levitsky W. Science. 1968;161:186–187. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- 27.Peters M. CIBA Found Symp. 1991;162:300–311. [PubMed] [Google Scholar]
- 28.Dum R P, Strick P L. J Neurosci. 1991;11:667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porter R, Lemon R. Corticospinal Function and Voluntary Movement. New York: Oxford Univ. Press; 1993. [Google Scholar]
- 30.Karni A, Meyer G, Jezzard P, Adams M M, Turner R, Ungerleider L G. Nature (London) 1995;377:155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- 31.Pascual-Leone A, Nguyet D, Cohen L G, Brasil-Neto J P, Cammarota A, Hallett M. J Neurophysiol. 1995;74:1037–1045. doi: 10.1152/jn.1995.74.3.1037. [DOI] [PubMed] [Google Scholar]
- 32.Woo T L, Pearson K. Biometrika. 1927;19:165–199. [Google Scholar]
- 33.Roland P E, Zilles K. Curr Opin Neurobiol. 1996;6:773–781. doi: 10.1016/s0959-4388(96)80027-4. [DOI] [PubMed] [Google Scholar]
- 34.Haaland K Y, Harrington D L. Brain Cogn. 1994;24:104–122. doi: 10.1006/brcg.1994.1006. [DOI] [PubMed] [Google Scholar]
- 35.Harrington D L, Haaland K Y. Brain. 1992;115:857–874. doi: 10.1093/brain/115.3.857. [DOI] [PubMed] [Google Scholar]


