Abstract
Rationale
Pathological neovascularization is a critical component of diseases such as proliferative retinopathies, cancer and rheumatoid arthritis, yet much remains to be learned about the underlying causes. Previous studies showed that VEGF-A activates the membrane-anchored metalloproteinase ADAM17 in endothelial cells, thereby stimulating crosstalk between VEGF-A and ERK. These findings raised important questions about the role of ADAM17 in angiogenesis and neovascularization in vivo.
Objective
The objective of this study was to inactivate ADAM17 in endothelial cells or in pericytes to determine how this affects developmental angiogenesis, pathological retinal neovascularization and heterotopic tumor growth.
Methods and Results
We generated animals in which floxed ADAM17 was removed by Tie2-Cre in endothelial cells, or by α-smooth muscle actin (αsma) Cre in smooth muscle cells and pericytes. There were no evident developmental defects in either conditional knockout strain, but pathological retinal neovascularization and growth of heterotopically injected tumor cells was reduced in Adam17flox/flox/Tie2-Cre mice, although not in Adam17flox/flox/αsma-Cre mice. Moreover, lack of ADAM17 in endothelial cells decreased ex vivo tube formation, and this could be largely restored by addition of the ADAM17 substrate HB-EGF. Finally we found that ADAM17 is important for the VEGF-A stimulated processing of several receptors with known functions in endothelial cell biology.
Conclusions
These results provide the first evidence for a role for ADAM17 in pathological neovascularization in vivo. Since ADAM17 does not appear to be required for normal developmental angiogenesis or vascular homeostasis, it could emerge as a good target for treatment of pathological neovascularization.
Subject codes: Angiogenesis, Animal models of human disease, Vascular Biology
Keywords: ADAMs, Metalloproteinase-disintegrins, TNFα-convertase, proliferative retinopathy, pathological neovascularization
Introduction
Pathological neovascularization has a critical role in diseases such as cancer 1, 2, rheumatoid arthritis 3 and proliferative retinopathies, including retinopathy of prematurity, diabetic retinopathy and the wet form of macular degeneration 4, 5. Therefore molecules with roles in pathological neovascularization are considered potential targets for treatment of these conditions. Previous studies have identified a role for the cell surface metalloproteinase ADAM17 (a disintegrin and metalloproteinase 17, also referred to as TNFα converting enzyme, (TACE)) in crosstalk between the VEGFR2 and ERK1/2 in endothelial cells, and in processing several receptors with key functions in angiogenesis, including the VEGFR2 and Tie2 6. The goal of the present study was to determine whether ADAM17 has a role in angiogenesis or pathological neovascularization in vivo by subjecting conditional knockout mice carrying floxed alleles of ADAM17 7 and a Cre-recombinase expressed either in endothelial cells (Tie2-Cre) or in smooth muscle cells and pericytes (α-smooth muscle actin (αsma) Cre) to mouse models of pathological neovascularization.
ADAM17 was first discovered as the converting enzyme for TNFα 8, 9, a potent pro-inflammatory cytokine that is a causative factor in autoimmune diseases such as rheumatoid arthritis and Crohn’s disease as well as in septic shock in mice 10. Once mice lacking ADAM17 were generated, it became clear that ADAM17 is also critical for EGF-receptor (EGFR) signaling, via the proteolytic release of several ligands of the EGFR 11. Mice lacking ADAM17 die shortly after birth with defects resembling those in animals lacking TGFα (wavy whiskers and open eyes), HB-EGF (thickened and misshapen heart valves), or the EGFR 11, 12. Further studies of ADAM17 demonstrated that it is responsible for the stimulated release of numerous additional membrane-anchored proteins, including molecules with important functions in endothelial cells, such as the VEGFR2 and Tie2 6, 13, 14. Moreover ADAM17-dependent shedding of several of its substrates, including EGFR-ligands, can be stimulated by VEGF-A in endothelial cells 6. The activation of ADAM17 by VEGF-A is responsible for crosstalk between the VEGFR2 and ERK1/2, most likely because EGFR-ligands shed from VEGF-A-stimulated endothelial cells activate the EGFR 6.
The ability of ADAM17 to release endothelial cell membrane proteins upon stimulation with VEGF-A raised questions about what role ADAM17 has during developmental angiogenesis and in pathological neovascularization in adult animals. Although mice lacking ADAM17 die perinatally, most likely as a consequence of their severe heart valve defects 11, 12, there have been no reports of defects in developmental angiogenesis in these animals. To address whether ADAM17 has a role in angiogenesis or pathological neovascularization or both, we conditionally inactivated ADAM17 in endothelial cells or in α-smooth muscle expressing cells such as pericytes, and then determined how lack of ADAM17 affects two mouse models for pathological neovascularization, the oxygen induced retinopathy model for retinopathy of prematurity, and growth of heterotopically injected tumor cells. Moreover, we assessed proliferation and tube formation of endothelial cells lacking ADAM17, and evaluated the role of ADAM17 in the proteolytic release of membrane proteins with known roles in angiogenesis and pathological neovascularization.
Materials and Methods
Reagents, Cell lines
Porcine aortic endothelial cells expressing VEGFR2/KDR (PAE/KDR cells) and mouse embryonic fibroblasts (mEFs) lacking ADAM17 have been described previously 6, 15. Reagents were from Sigma, unless indicated otherwise. VEGF-A and HB-EGF were from R&DSystems, and antibodies against PECAM, NG2, eNOS and αsma were from BD Pharmingen.
Mouse lines
To generate mice lacking ADAM17 in endothelial cells, we crossed Adam17flox/flox mice 7 with Tie2-Cre mice 16 (kindly provided by Dr. Tom Sato) or αsma-Cre mice (Jackson labs; Tg(TagIn-cre) 1Her/J). Expression of Cre was monitored using Rosa26-Lac-Z reporter (R26R) mice (Jackson labs; B6.129S4-Gt(ROSA)26Sortm1Sor/J).
Oxygen-induced retinopathy, heterotopic tumor injection and evaluation of retinal vascular development
The analysis of postnatal retinal vascular development, the oxygen-induced retinopathy model and heterotopic injection of B16F0 melanoma cells have been described elsewhere 17, 18 (see online materials and methods for details).
Shedding assays
Protein ectodomain shedding assays using alkaline phosphatase (AP)-tagged substrates in mouse embryonic fibroblasts and PAE/KDR cells were performed as described 6, 15.
Endothelial cell assays
Primary endothelial cells from lungs and hearts of 9 – 12 day-old mice were prepared as described 19. Proliferation of primary endothelial cells was measured with the Celltiter proliferation assay from Promega. In vitro endothelial cell tube formation was performed using a kit from Cell Biolabs Inc. (San Diego, CA).
Immunofluorescence, Western blot and FACS analysis
Immunofluorescence analysis for PECAM, isolectin B4, NG2 and αsma, Western blot analysis of retina extracts and FACS analysis was performed as described 17, 18, 20.
Results
Characterization of Adam17flox/flox/Tie2-Cre mice
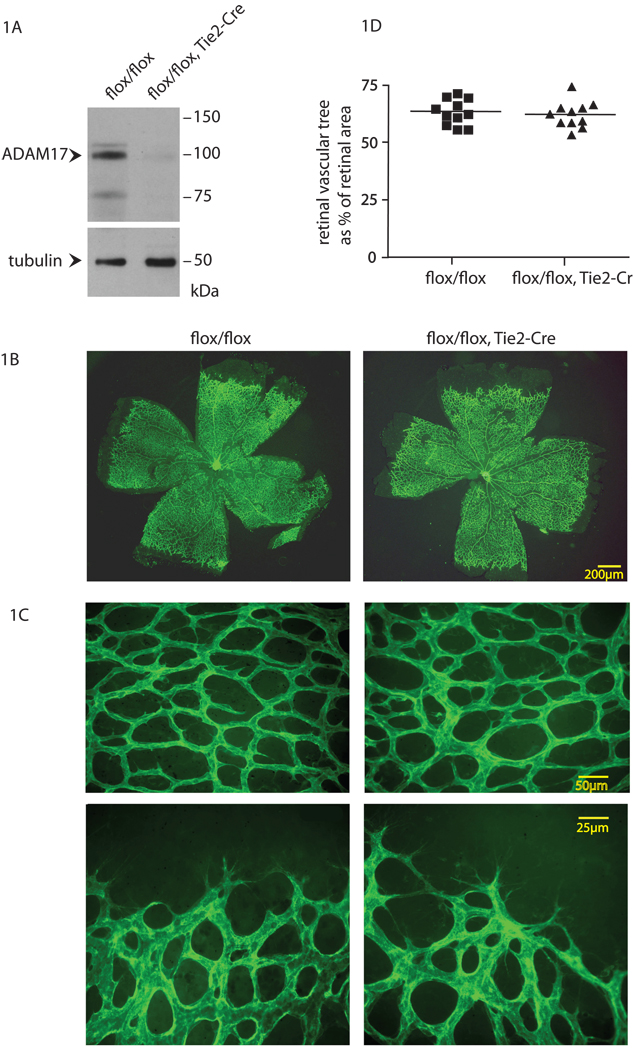
In order to assess whether ADAM17 has a role in pathological neovascularization, we generated mice carrying floxed alleles of ADAM17 and the Cre-recombinase expressed in endothelial cells under the Tie-2 promoter 16 (see materials and methods for details). Matings of Adam17flox/flox/Tie2-Cre with Adam17flox/flox mice gave rise to offspring of the expected Mendelian ratio (48% Adam17flox/flox, 52% Adam17flox/flox/Tie2-Cre, n=327). The efficient excision of ADAM17 in endothelial cells isolated from Adam17flox/flox/Tie2-Cre mice was confirmed by Western blot analysis (Fig. 1A). Adam17flox/flox/Tie2-Cre mice appeared normal during routine handling, and a complete necropsy and histopathological evaluation did not uncover any evident defects compared to littermate controls (Adam17flox/flox) (see materials and methods). Moreover, staining of histological sections of the aorta or a vessel in the heart with antibodies against the endothelial cell marker PECAM or the pericyte marker α-SMA did not reveal differences in the appearance or patterning of the stained structures from Adam17flox/flox/Tie2-Cre mice compared to Adam17flox/flox controls (Online Figure I). In order to determine whether the absence of ADAM17 affected the distribution of Tie2-Cre expressing cells, we performed X-gal staining on sections of the aorta, heart and lung of mice carrying Tie2-Cre and the ubiquitously expressed Cre-dependent Lac-Z reporter (Rosa26 Lac-Z reporter (R26R)) in the presence of either one or both floxed alleles of ADAM17 (Adam17flox/flox/Tie2-Cre/R26R or Adam17flox/+/Tie2-Cre/R26R). No difference in the distribution of X-gal stained cells in the presence or absence of ADAM17 was observed (Online Figure II). Moreover, the presence or absence of Tie2-Cre in Adam17flox/flox mice also did not affect the development of the retinal vascular tree with respect to its size relative that of the retina as well as the appearance of the vessels at postnatal day 6 (Fig. 1B–D). Thus conditional inactivation of ADAM17 in endothelial cells did not lead to evident defects in mouse development or adult homeostasis, or in the development of the retinal vasculature.
Figure 1. Evaluation of developmental retinal angiogenesis in Adam17flox/flox/Tie2-Cre mice.
(A) Western blot analysis shows a strong reduction of ADAM17 protein levels in primary endothelial cells isolated from an Adam17flox/flox/Tie2-Cre mouse compared to an Adam17flox/flox littermate control, with tubulin serving as a loading control (see materials and methods). (B,C) Whole-mount isolectin B4 staining of the developing retinal vasculature of 6 day-old Adam17flox/flox/Tie2-Cre and Adam17flox/flox littermates. Panel C shows higher magnification photomicrographs of the density of the vascular web (top panels) and the leading edge of the vascular tree (lower panels). (D) The ratio of the surface area of the developing vascular tree over that of the retina showed no significant difference (WMW test, p=0.76) in the extent of developmental retinal angiogenesis in Adam17flox/flox/Tie2-Cre mice (n=11) compared to Adam17flox/flox controls (n=11).
Conditional inactivation of ADAM17 in endothelial cells reduces oxygen-induced retinopathy
In order to assess whether ADAM17 contributes to pathological retinal neovascularization, we subjected Adam17flox/flox/Tie2-Cre mice and Adam17flox/flox littermate controls to a model for retinopathy of prematurity, the oxygen induced retinopathy (OIR) model, (see materials and methods). At the completion of the OIR experiment at day p17, we found a significantly larger central avascular area in Adam17flox/flox/Tie2-Cre mice compared to controls (Fig. 2A,B). Moreover, there was a significant decrease in the number of endothelial cells that traversed the internal limiting membrane towards the vitreous body in Adam17flox/flox/Tie2-Cre mice compared to controls (Fig. 2C). X-gal staining of retinas from Adam17flox/flox/Tie2-Cre/R26R mice corroborated that Tie2-Cre is active in endothelial cells throughout the retinal vasculature (Online Figure IIIA) and in pathological neovascular tufts (Online Figure IIIB). When we subjected mice carrying one wild type and one floxed allele of ADAM17 in the presence or absence of Tie2-Cre to the OIR model (Adam17flox/+/Tie2-Cre mice or Adam17flox/+ controls), we found that Tie2-Cre did not significantly affect the outcome of this model when ADAM17 was present. This control corroborates that the decreased response of Adam17flox/flox/Tie2-Cre mice to the OIR model is due to deletion of floxed ADAM17, but not the expression of Tie2-Cre (Online Figure IIIC). An immunofluorescence analysis of the expression of the endothelial cell marker isolectin B4 or the pericyte marker NG2 in pathological neovascular tufts showed a similar staining pattern in the tufts that developed in Adam17flox/flox/Tie2-Cre mice compared to Adam17flox/flox controls (Online Figure IIID). Finally, a Western blot analysis of retina extracts from wild type or Adam17flox/flox/Tie2-Cre mice subjected to the OIR model showed expression of ADAM17 at all stages after return to room air at P12 in wild type mice (Online Figure IIIE), and comparable expression at P12, 14 and 17 in Adam17flox/flox/Tie2-Cre mice (Online Figure IIIF), so deletion of the widely expressed ADAM17 in endothelial cells does not noticeably alter ADAM17 levels in extracts of whole retinas.
Figure 2. Oxygen induced retinopathy.
(A) Whole-mount analysis of the retinal vasculature of Adam17flox/flox/Tie2-Cre mice or Adam17flox/flox controls after exposure to the oxygen induced retinopathy model (see materials and methods). The size of the central avascular area at p17 (five days after removal from 75% oxygen) is outlined in yellow. (B) The size of the central avascular area relative to that of the entire retina (expressed in %) was significantly larger in Adam17flox/flox/Tie2-Cre mice (n=22) compared to Adam17flox/flox controls (n=26) subjected to the OIR model (WMW p<0.001). (C) Quantification of the endothelial cells that had crossed the internal limiting membrane to form pathological neovascular tufts showed a significantly decreased number in Adam17flox/flox/Tie2-Cre mice (n=22) compared to littermate Adam17flox/flox controls (n=25, p<0.001).
Heterotopic tumor injection model
Because the results of the OIR model suggested that ADAM17 in endothelial cells has a role in pathological neovascularization, we subjected Adam17flox/flox/Tie2-Cre mice and Adam17flox/flox controls to a heterotopic tumor injection model, which provides information on the contribution of host-derived factors and cells, such as endothelial cells, to tumor growth. After subcutaneous injection of B16F0 melanoma cells, tumor growth was monitored for two to three weeks. In three separate experiments, tumor growth was significantly reduced in Adam17flox/flox/Tie2-Cre mice compared to controls (Fig. 3A). Sections of tumors from Adam17flox/flox/Tie2-Cre mice and controls did not display significant differences in the distribution or appearance of PECAM-stained tumor vessels (Fig. 3B,C). When we compared heterotopic tumor growth in mice with one wild type allele of ADAM17 in the presence or absence of Tie2-Cre (Adam17flox/+/Tie2-Cre or Adam17flox/+ mice), we found no difference in tumor growth, arguing against an effect of the Tie2-Cre alone on this heterotopic tumor model (Online Figure IV). These experiments are consistent with a role for ADAM17 in pathological neovascularization or in generation of host-derived factors from endothelial cells that contribute to tumor growth.
Figure 3. Heterotopic injection of B16F0 melanoma cells.
(A) Size of tumors that developed from 106 heterotopically injected B16F0 melanoma cells in Adam17flox/flox/Tie2-Cre mice (n=20) or Adam17flox/flox controls (n=24; three separate experiments; 6 to 10 mice per experiment). All animals in a given experiment were injected with the same suspension of cells. A significant decrease in the growth of B16F0 cells was seen in Adam17flox/flox/Tie2-Cre mice compared to Adam17flox/flox controls (WMW p<0.001) (B) Staining of representative tumor sections from a Adam17flox/flox/Tie2-Cre mouse and Adam17flox/flox control with the endothelial cell marker PECAM showed comparable appearance and spacing of tumor vessels. (C) Quantification of vessel spacing and density on tumor sections from Adam17flox/flox/Tie2-Cre mice (n=9) or Adam17flox/flox controls (n=9) showed no significant difference (WMW p=0.25)
Inactivation of ADAM17 in αsma-expressing cells does not detectably affect pathological neovascularization
Pericytes represent another major cell type in the vasculature besides endothelial cells. In order to determine whether ADAM17 in pericytes is important for angiogenesis or pathological neovascularization, we generated mice carrying floxed ADAM17 and a Cre-recombinase expressed under the control of the αsma promoter (Adam17flox/flox/αsma-Cre mice). The Adam17flox/flox/αsma-Cre animals were born at the expected Mendelian ratio (52% Adam17flox/flox/αsma-Cre, 48% Adam17flox/flox, n=101), and developed normally, with no evident pathological changes compared to littermate Adam17flox/flox controls (see materials and methods). A Western blot analysis of vascular smooth muscle cells (VSMC) cultured from aortae of Adam17flox/flox/αsma-Cre mice and Adam17flox/flox controls by the explant method (see materials and methods) corroborated the efficient excision of floxed ADAM17 by αsma-Cre in VSMCs (Online Figure V). Moreover, an analysis of several tissues and organs containing αsma-expressing cells (aorta, heart, small intestine) did not uncover any evident defects in Adam17flox/flox/αsma-Cre mice compared to Adam17flox/flox controls (Online Figure VIA). In order to determine whether the presence or absence of ADAM17 affected the distribution of αsma-Cre expressing cells, we performed X-gal staining on sections of aortae and hearts of mice carrying αsma-Cre and the Rosa26 lac-Z reporter in the presence of either one or both floxed alleles of ADAM17 (Adam17flox/flox/αsma-Cre/R26R or Adam17flox/+/αsma-Cre/R26R). No difference in the distribution of X-gal stained cells in the presence or absence of ADAM17 was observed (Online Figure VIB). In addition, there was no difference in the development of the retinal vascular tree at P6 in Adam17flox/flox mice in the presence or absence of αsma-Cre (Online Figure VIIA,B). After exposure to the OIR model, the size of the central avascular area (Online Figure VIIIA,B) and the number of endothelial cells that crossed the internal limiting membrane were comparable between Adam17flox/flox/αsma-Cre mice and Adam17flox/flox controls (Online Figure VIIIC, please note that due to the relatively low numbers of endothelial cells in Adam17flox/flox controls, we can not rule out subtle effects of the lack of ADAM17 in αsma-expressing cells in the OIR model). X-gal staining of retinal sections from αsma-Cre/R26R mice corroborated the expression of αsma-Cre in neovascular tufts (Online Figure VIIID). Finally, heterotopically injected B16F0 melanoma cells gave rise to tumors of similar weight in Adam17flox/flox/αsma-Cre mice and Adam17flox/flox controls (Online Figure IX). Thus, we found no evidence for a contribution of ADAM17 in αsma-expressing cells to developmental retinal angiogenesis, pathological retinal neovascularization or to a heterotopic tumor model.
ADAM17 has a role in tube formation of endothelial cells
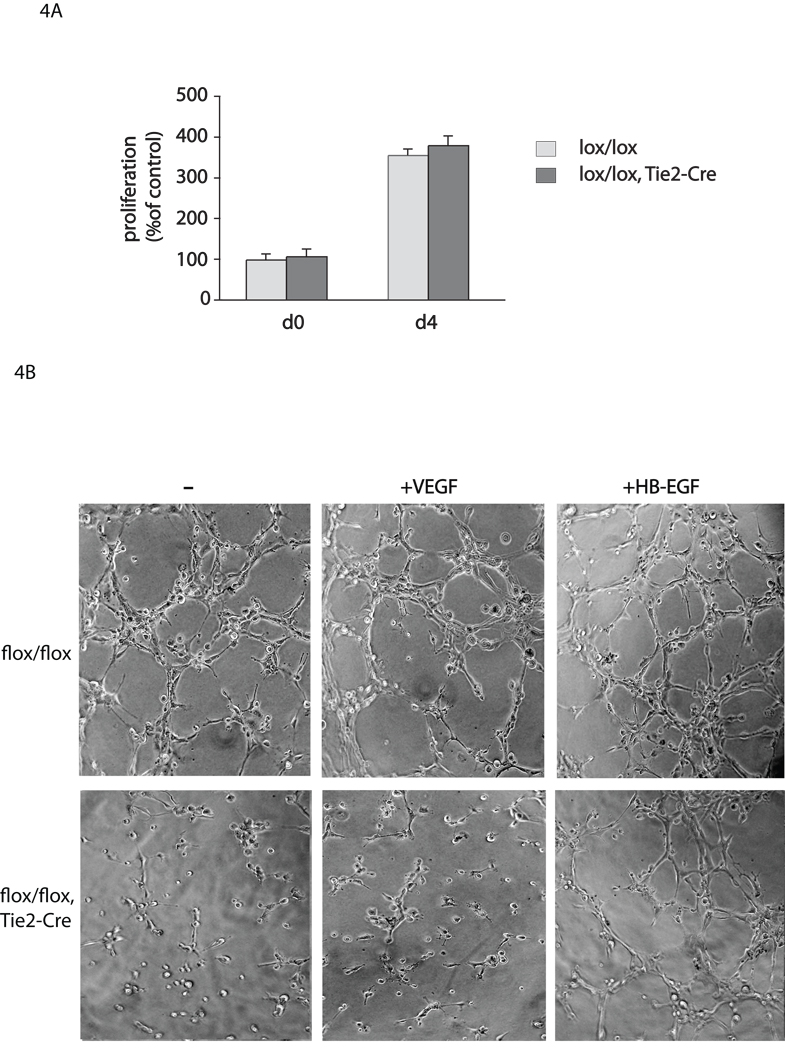
To explore the contribution of ADAM17 to ex vivo endothelial cell assays, we isolated endothelial cells from Adam17flox/flox/Tie2-Cre mice and Adam17flox/flox controls, and assessed proliferation and tube formation in the presence or absence of VEGF-A o r H B-EGF. We found no difference in proliferation of Adam17flox/flox/Tie2-Cre endothelial cells compared to controls (Fig. 4A). However, there was a substantial decrease in tube formation in endothelial cells from Adam17flox/flox/Tie2-Cre mice that were treated with or without VEGF-A compared to controls (Fig. 4B). This defect in tube formation in Adam17flox/flox/Tie2-Cre endothelial cells could be largely rescued by addition of soluble HB-EGF, an EGFR-ligand that is a substrate of ADAM17 (Fig. 4B).
Figure 4. Endothelial cell proliferation and tube formation assays.
(A) Proliferation assay and (B) tube formation assay with endothelial cells isolated from Adam17flox/flox/Tie2-Cre mice or Adam17flox/flox controls (see materials and methods). There was no significant difference in proliferation at day 4 (d4) of the culture plated a comparable density on day 0 (d0) (A), but a clear decrease in tube formation was seen in endothelial cells from Adam17flox/flox/Tie2-Cre mice compared to controls (B), which could be largely rescued by treatment with 5ng/ml HB-EGF, but not with 5ng/ml VEGF-A.
ADAM17 is involved in shedding a variety of membrane proteins with roles in angiogenesis and neovascularization
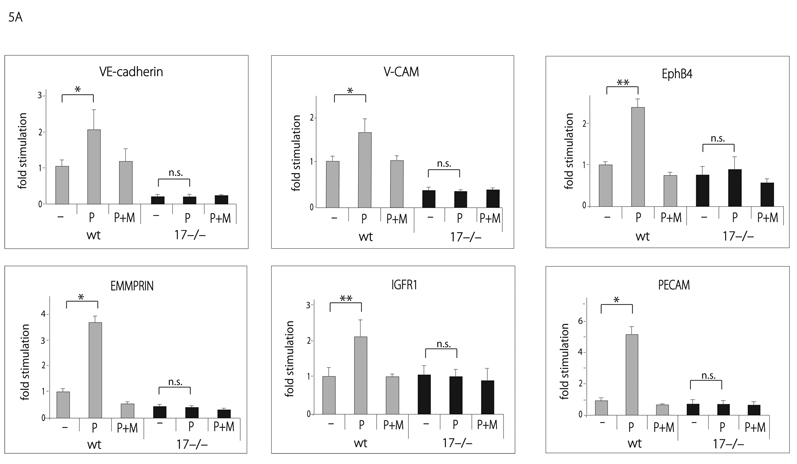
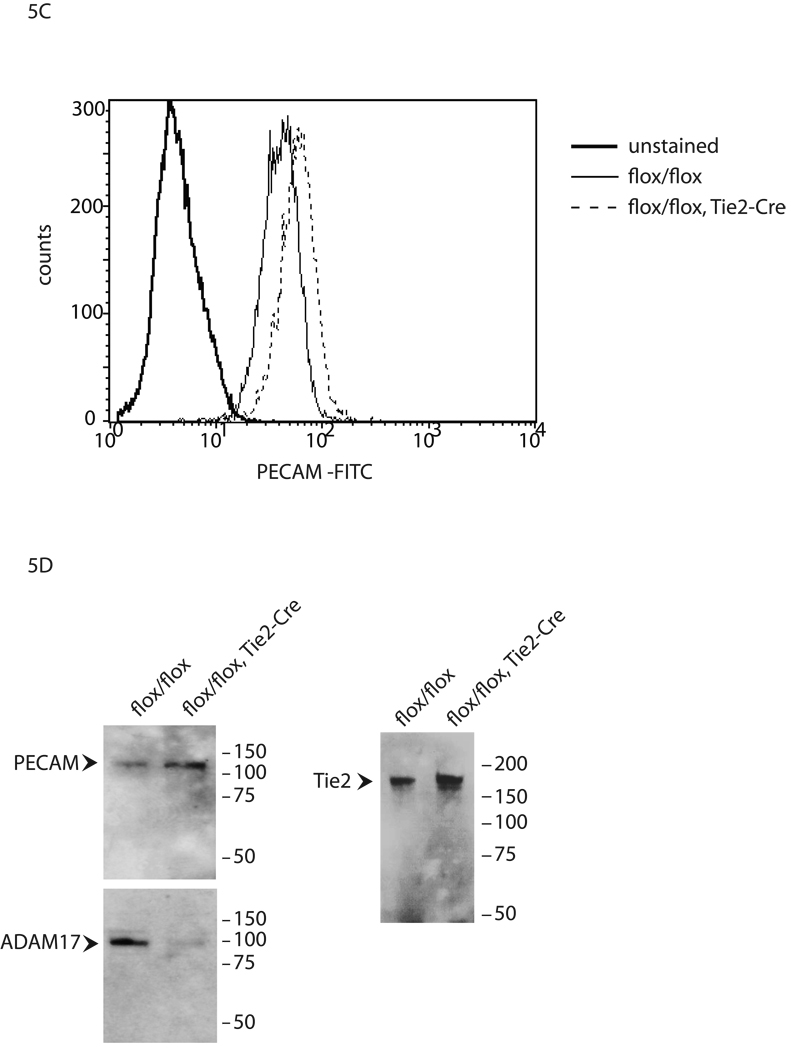
Previous studies have implicated ADAM17 in the proteolytic release of several membrane-anchored proteins, including molecules with known roles in angiogenesis, such as VEGFR2, ICAM-1, Tie2 and CD40 6. To test whether ADAM17 is involved in the shedding of additional membrane-proteins with known functions in endothelial cell biology, we transfected AP-tagged VE-cadherin, V-CAM, EphB4, EMMPRIN, IGFR1, or PECAM into wild type or Adam17−/− mouse embryonic fibroblasts (mEFs). We found a PMA-dependent increase in the shedding of the ectodomains of these membrane proteins in wild type mEFs, which could be prevented by incubation with the hydroxamic acid-type metalloproteinase inhibitor marimastat (Fig. 5A). The PMA-stimulated component for each of these substrates was abolished in Adam17−/− mEFs, and for VE-cadherin, V-CAM and EMMPRIN, constitutive shedding was also reduced. Moreover, we found that shedding of the ADAM17 substrates VE-cadherin, V-CAM, EphB4, EMMPRIN, IGFR1 or PECAM from pig aortic endothelial cells expressing the VEGFR2 (PAE-KDR cells) was stimulated by addition of VEGF-A, whereas shedding of the ADAM10 substrates EGF and betacellulin was not (Fig. 5B). Finally, FACS analysis showed an approximately 40% increase in PECAM on the surface of endothelial cells from Adam17flox/flox/Tie2-Cre mice compared to Adam17flox/flox controls (Fig. 5C). This was further corroborated by Western blot analysis of the sorted cells, where increased levels of PECAM and Tie2 correlated with strongly decreased ADAM17 in Adam17flox/flox/Tie2-Cre endothelial cells compared to Adam17flox/flox controls (Fig. 5D). These results confirm that ADAM17 regulates the levels of endogenous PECAM and Tie2 in primary endothelial cells.
Figure 5. Evaluation of the role of ADAM17 in ectodomain shedding of membrane proteins with roles in angiogenesis.
(A) To identify potential ADAM17 substrates in endothelial cells, AP-tagged fusion proteins for membrane proteins with roles in angiogenesis were transfected into wild type or Adam17−/− mouse embryonic fibroblasts (mEFs). Shedding of VE-cadherin, VCAM, EphB4, EMMPRIN, IGFR1 and PECAM from transfected wild type mouse embryonic fibroblasts was stimulated by short-term treatment with 25 ng/ml PMA, which activates ADAM17, but not ADAM10 15, 20, 40. The PMAdependent and constitutive shedding of VE-cadherin, V-CAM, EMMPRIN and PECAM was reduced in Adam17−/− mEFs, as was the PMA-dependent, but not the constitutive shedding of IGFR1 and EphB4, corroborating that ADAM17 is responsible for the stimulated shedding of these proteins in mEF cells. (B) When the AP-tagged receptors were transfected into porcine aortic endothelial cells expressing VEGFR2/KDR (PAE/KDR cells), treatment with VEGF-A, which activates ADAM17, but not ADAM10 6, increased the shedding of VE-cadherin, VCAM, EphB4, EMMPRIN, IGFR1 and PECAM, but not of the ADAM10 substrates EGF and betacellulin (BTC) (WMW test, * indicates P=0.04, ** indicates P=0.02). (C) A FACS analysis of primary endothelial cells gated for the endothelial cell markers eNOS and ICAM2 showed increased PECAM levels (+41.2% +/−6.4) on cells from Adam17flox/flox/Tie2-Cre mice compared to controls (D). A Western blot of FACS-sorted ICAM-positive primary endothelial cells shows increased levels of PECAM/CD31 (top left panel) and Tie2 (top right panel) and strongly decreased levels of ADAM17 (bottom panel, same blot as top panel, stripped and re-probed with anti-ADAM17) in cells from Adam17flox/flox/Tie2-Cre mice compared to Adam17flox/flox controls (see materials and methods for details).
Discussion
The main objective of this study was to evaluate the role of the membrane-anchored metalloproteinase ADAM17 in angiogenesis and pathological neovascularization. We found that inactivation of ADAM17 in endothelial cells had no evident effect on developmental angiogenesis, whereas it significantly reduced pathological neovascularization in a mouse model for retinopathy of prematurity, and affected the growth of heterotopically injected tumor cells. Moreover, tube formation in ADAM17-deficient endothelial cells was strongly reduced compared to controls, and could be partially rescued by addition of the EGFR-ligand and ADAM17 substrate HB-EGF, which is expressed on endothelial cells 12, 15, 21. On the other hand, inactivation of ADAM17 in αsma-expressing cells had no evident effect on retinal angiogenesis, the outcome of the OIR model or on the growth of heterotopically injected tumor cells.
The observation that the inactivation of ADAM17 in endothelial cells reduces pathological neovascularization provides the first direct evidence for a role of this cellular sheddase in endothelial cells in vivo. Moreover, the ability of HB-EGF to largely rescue the decreased tube formation of ADAM17-deficient endothelial cells suggests that the underlying mechanism involves EGFR-signaling stimulated by HB-EGF or related EGFR-ligands released by ADAM17 from endothelial cells. This is consistent with previous studies that have implicated HB-EGF and its proteolytic release in angiogenesis, although the identity of the responsible enzyme was not determined 22–26. Moreover, our finding that HB-EGF partially rescues tube formation in ADAM17-deficient endothelial cells, whereas VEGF does not, suggests that HB-EGF affects endothelial cells directly instead of via production of VEGF, as proposed by Hollborn et al 27. The apparently normal pericyte ensheathment of endothelial cells in the absence of ADAM17 suggests that release of HB-EGF by ADAM17 is not essential for this process 21. However, addition of HB-EGF to ADAM17-deficient endothelial cells did not completely restore tube formation, so other substrates of ADAM17 that are important for its role in neovascularization are likely to exist. Indeed, we also show that ADAM17 is able to process several receptors with important functions in endothelial cells, and that their shedding can be activated by VEGF (see also 6), and so defects in the processing of one or more additional substrates of ADAM17 could also be partially responsible for its role in neovascularization. In principle, processing could inactivate membrane receptors, although ectodomain shedding can also activate receptors such as Notch 28.
Given the substantial number of currently known substrates for ADAM17 and the dearth of information on how shedding affects the function of individual receptors, it is currently not possible to predict which additional shedding events besides the release of HB-EGF account for the role of ADAM17 in neovascularization. In order to address this question, it will be necessary to study how the processing of individual receptors affects their function in cell-based assays and in vivo, for example by “knocking-in” mutations that inactivate their cleavage site. Nevertheless, the ability of HB-EGF to largely rescue tube formation in ADAM17-deficient endothelial cells suggests that activation of the EGFR is an important component of the mechanism underlying the role of ADAM17 in neovascularization. So, even though ADAM17 can, in principle, process many membrane proteins on the surface of endothelial cells, our results suggest that HB-EGF and possibly also other EGFR-ligands that are shed by ADAM17 are likely to be the functionally dominant substrates of ADAM17 in the context of pathological neovascularization. Perhaps the increase in surface levels of membrane proteins such as Tie2 and PECAM in ADAM17-deficient endothelial cells is less relevant to angiogenesis and neovascularization than the regulation of the bio-availability of EGFR-ligands, which is also the functionally dominant activity of ADAM17 during mouse development. ADAM17 has also been implicated in processing Notch 29. However, mice lacking Notch1 and 4 die very early during embryogenesis 28, 30, and ADAM10-deficient mice resemble mice that lack Notch1 and 4 31, whereas mice lacking ADAM17 die at birth 11. Therefore ADAM17 does not appear to be essential for activating Notch during mouse development. Finally, it should be noted that ADAMs are modular proteins that also contain a disintegrin domain, cysteine-rich region and a cytoplasmic domain, so it is conceivable that functions of these ancillary domains that are not related to the catalytic activity of ADAM17 could also be important for its role in pathological neovascularization 13, 14.
Taken together, these results suggest that ADAM17 could be an attractive target for treatment of proliferative retinopathies and potentially also for preventing other diseases that depend on pathological neovascularization, such as tumor growth and rheumatoid arthritis. An appealing feature of ADAM17 in the context of pathological neovascularization is that it does not have an evident role in normal developmental angiogenesis or in the maintenance of the vasculature in adult mice. ADAM17 is currently considered as a target for treatment of rheumatoid arthritis because of its role in generating soluble TNFα 32, and for treatment of ErbB-dependent tumors, because of its critical role in activating EGFR-ligands 33. Interestingly, TIMP3, which is tightly associated with ADAM17 in extracts from endothelial cells and inhibits ADAM17 and other metalloproteinases 34–36, reduces pathological neovascularization in an OIR mouse model 37. Moreover, abnormal choroidal neovascularization as well as an increased angiogenic response has been observed in Timp3−/− mice 38. Since conditional inactivation of ADAM17 in endothelial cells has a similar effect in the mouse OIR model as intravitreal injection of TIMP3-expressing adeno-associated viral vectors 37, ADAM17 is likely a functionally relevant target of TIMP3 during pathological neovascularization.
In summary, the conditional inactivation of ADAM17 in endothelial cells provides the first evidence for a critical role of ADAM17 during pathological neovascularization in mice in vivo. Moreover, the ability of HB-EGF to rescue tube formation in endothelial cells lacking ADAM17 is consistent with the previously established essential role for ADAM17 in activating ligands of the EGFR, including HB-EGF 11–13, 15, 39. Based on these results, it will now be interesting to test how conditional inactivation of the EGFR in endothelial cells or pericytes affects the outcome of the models for pathological neovascularization presented here. Our results raise the possibility that selective inhibition of ADAM17 could be beneficial for treatment of pathological neovascularization in the context of proliferative retinopathies, rheumatoid arthritis and cancer.
Novelty and Significance
What is known?
The cell surface metalloproteinase ADAM17 (a disintegrin and metalloproteinase 17, also referred to as TNFα-converting enzyme, TACE) regulates the bio-availability and function of several ligands of the EGF receptor, including HB-EGF, TGFα.
Mice lacking ADAM17 die at birth, with developmental defects that resemble those observed in knockout mice for the EGF receptor, or its ligands TGFα (open eyes at birth, skin defects) and HB-EGF (heart valve defects).
What new information does this article contribute?
This study establishes a role for ADAM17 on the vasculature that could be of significant clinical relevance.
We show that inactivation of ADAM17 in endothelial cells in mice reduces pathological neovascularization in a model for proliferative retinopathies and impedes the growth of injected tumor cells, without detectably affecting the development of a normal vasculature.
Studies with isolated endothelial cells lacking ADAM17 uncover defects in chord formation that can be rescued by addition of the EGF receptor ligand HB-EGF.
Taken together, our results provide the first evidence for a role of ADAM17 in pathological neovascularization, and suggest that this is caused by a defect in the functional activation of ligands of the EGF receptor.
Summary
ADAM17 is a cell surface metalloproteinase with critical roles in EGF receptor signaling and processing the pro-inflammatory cytokine TNFα. Mice lacking ADAM17 die at birth because of severe skin and heart valve defects, so it has not been possible to study the role of ADAM17 in the adult vasculature. The main goal of this study was to evaluate how inactivation of ADAM17 in vascular cells affects physiological and pathological vascular functions. We found that deletion of ADAM17 in endothelial cells offers significant protection from oxygen-induced retinopathy and slows the growth of injected tumor cells in mice. Moreover, cell culture experiments suggest that ADAM17 is important for EGF receptor signaling in endothelial cells. These results show for the first time that ADAM17 has a critical role in pathological neovascularization, even though we found no evidence for a contribution to normal vascular development. The implications of this study for clinical medicine are that inhibitors of ADAM17, currently in development for treatment of cancer and rheumatoid arthritis, could potentially offer previously unanticipated benefits by preventing pathological neovascularization in rapidly growing tumors, rheumatoid arthritis and proliferative retinopathies such as retinopathy of prematurity, diabetic retinopathy and the wet form of macular degeneration.
Supplementary Material
Acknowledgements
We thank Dr. Tom Sato for providing Tie2-Cre mice, and Elin Mogollon for excellent technical assistance.
Sources of funding: This work was supported by NIH EY015719 (CPB), HL076770 (SE), AHA-EIA 0740042N (SE), a minority supplement (VG), the Weill Cornell Vision training grant (KM), and was conducted in a facility constructed with support from the NIH Research Facilities Improvement Program Grant C06-RR12538-01.
Non-standard Abbreviations and Acronyms
- ADAMs
a disintegrin and metalloproteinase
- HB-EGF
heparin binding epidermal growth factor like growth factor
- TNFα
tumor necrosis factor α
- AP
alkaline phosphatase
- αsma
α smooth muscle actin
- OIR
oxygen induced retinopathy
- WMW
Wilcoxon-Mann-Whitney test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None.
References
- 1.Folkman J, Ingber D. Inhibition of angiogenesis. Semin. Cancer Biol. 1992;3:89–96. [PubMed] [Google Scholar]
- 2.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat. Rev. Mol. Cell. Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 3.Khong TL, Larsen H, Raatz Y, Paleolog E. Angiogenesis as a therapeutic target in arthritis: learning the lessons of the colorectal cancer experience. Angiogenesis. 2007;10:243–258. doi: 10.1007/s10456-007-9081-1. [DOI] [PubMed] [Google Scholar]
- 4.Bradley J, Ju M, Robinson GS. Combination therapy for the treatment of ocular neovascularization. Angiogenesis. 2007;10:141–148. doi: 10.1007/s10456-007-9069-x. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis. 2007;10:133–140. doi: 10.1007/s10456-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 6.Swendeman S, Mendelson K, Weskamp G, Horiuchi K, Deutsch U, Scherle P, Hooper A, Rafii S, Blobel CP. VEGF-A stimulates ADAM17-dependent shedding of VEGFR2 and crosstalk between VEGFR2 and ERK signaling. Circ Res. 2008;103:916–918. doi: 10.1161/CIRCRESAHA.108.184416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horiuchi K, Kimura T, Miyamoto T, Takaishi H, Okada Y, Toyama Y, Blobel CP. Cutting Edge: TNF-{alpha}-Converting Enzyme (TACE/ADAM17) Inactivation in Mouse Myeloid Cells Prevents Lethality from Endotoxin Shock. J Immunol. 2007;179:2686–2689. doi: 10.4049/jimmunol.179.5.2686. [DOI] [PubMed] [Google Scholar]
- 8.Black R, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloprotease disintegrin that releases tumour-necrosis factor-α from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 9.Moss ML, Jin S-LC, Milla ME, Burkhart W, Cartner HL, Chen W-J, Clay WC, Didsbury JR, Hassler D, Hoffman CR, Kost TA, Lambert MH, Lessnitzer MA, McCauley P, McGeehan G, Mitchell J, Moyer M, Pahel G, Rocque W, Overton LK, Schoenen F, Seaton T, Su J-L, Warner J, Willard D, Becherer JD. Cloning of a disintegrin metalloproteinase that processes precursor tumour-recrosis factor-α. Nature. 1997;385:733–736. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 11.Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russel WE, Castner BJ, Johnson RS, Fitzner JN, Boyce RW, Nelson N, Kozlosky CJ, Wolfson MF, Rauch CT, Cerretti DP, Paxton RJ, March CJ, Black RA. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 12.Jackson LF, Qiu TH, Sunnarborg SW, Chang A, Zhang C, Patterson C, Lee DC. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. Embo J. 2003;22:2704–2716. doi: 10.1093/emboj/cdg264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blobel CP. ADAMs: key players in EGFR-signaling, development and disease. Nat. Rev. Mol. Cell. Bio. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- 14.Murphy G. The ADAMs: signalling scissors in the tumour microenvironment. Nat. Rev. Cancer. 2008;12:929–941. doi: 10.1038/nrc2459. [DOI] [PubMed] [Google Scholar]
- 15.Sahin U, Weskamp G, Zhou HM, Higashiyama S, Peschon JJ, Hartmann D, Saftig P, Blobel CP. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR-ligands. J. Cell Biol. 2004;164:769–779. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 17.Horiuchi K, Weskamp G, Lum L, Hammes HP, Cai H, Brodie TA, Ludwig T, Chiusaroli R, Baron R, Preissner KT, Manova K, Blobel CP. Potential role for ADAM15 in pathological neovascularization in mice. Mol. Cell Biol. 2003;23:5614–5624. doi: 10.1128/MCB.23.16.5614-5624.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guaiquil V, Swendeman S, Yoshida T, Chavala S, Campochiaro P, Blobel CP. ADAM9 is involved in pathological retinal neovascularization. Mol Cell Biol. 2009;29:2694–2703. doi: 10.1128/MCB.01460-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim YC, Luscinskas FW. Isolation and culture of murine heart and lung endothelial cells for in vitro model systems. Methods Mol Biol. 2006;341:141–154. doi: 10.1385/1-59745-113-4:141. [DOI] [PubMed] [Google Scholar]
- 20.Le Gall S, Bobe P, Reiss K, Horiuchi K, Niu X-D, Lundell D, Gibb D, Conrad D, Saftig P, Blobel C. ADAMs 10 and 17 represent differentially regulated components of a general shedding machinery for membrane proteins such as TGFα, L-Selectin and TNFα. Mol Biol Cell. 2009;20:1785–1794. doi: 10.1091/mbc.E08-11-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iivanainen E, Nelimarkka L, Elenius V, Heikkinen SM, Junttila TT, Sihombing L, Sundvall M, Maatta JA, Laine VJ, Yla-Herttuala S, Higashiyama S, Alitalo K, Elenius K. Angiopoietin-regulated recruitment of vascular smooth muscle cells by endothelial-derived heparin binding EGF-like growth factor. Faseb J. 2003;17:1609–1621. doi: 10.1096/fj.02-0939com. [DOI] [PubMed] [Google Scholar]
- 22.Blotnick S, Peoples GE, Freeman MR, Eberlein TJ, Klagsbrun M. T lymphocytes synthesize and export heparin-binding epidermal growth factor-like growth factor and basic fibroblast growth factor, mitogens for vascular cells and fibroblasts: differential production and release by CD4+ and CD8+ T cells. Proc Natl Acad Sci U S A. 1994;91:2890–2894. doi: 10.1073/pnas.91.8.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta VB, Besner GE. HB-EGF promotes angiogenesis in endothelial cells via PI3-kinase and MAPK signaling pathways. Growth Factors. 2007;25:253–263. doi: 10.1080/08977190701773070. [DOI] [PubMed] [Google Scholar]
- 24.Clarke PA, Dickson JH, Harris JC, Grabowska A, Watson SA. Gastrin enhances the angiogenic potential of endothelial cells via modulation of heparin-binding epidermal-like growth factor. Cancer Res. 2006;66:3504–3512. doi: 10.1158/0008-5472.CAN-05-0280. [DOI] [PubMed] [Google Scholar]
- 25.Ongusaha PP, Kwak JC, Zwible AJ, Macip S, Higashiyama S, Taniguchi N, Fang L, Lee SW. HB-EGF is a potent inducer of tumor growth and angiogenesis. Cancer Res. 2004;64:5283–5290. doi: 10.1158/0008-5472.CAN-04-0925. [DOI] [PubMed] [Google Scholar]
- 26.Fujiyama S, Matsubara H, Nozawa Y, Maruyama K, Mori Y, Tsutsumi Y, Masaki H, Uchiyama Y, Koyama Y, Nose A, Iba O, Tateishi E, Ogata N, Jyo N, Higashiyama S, Iwasaka T. Angiotensin AT(1) and AT(2) receptors differentially regulate angiopoietin-2 and vascular endothelial growth factor expression and angiogenesis by modulating heparin binding-epidermal growth factor (EGF)-mediated EGF receptor transactivation. Circ Res. 2001;88:22–29. doi: 10.1161/01.res.88.1.22. [DOI] [PubMed] [Google Scholar]
- 27.Hollborn M, Iandiev I, Seifert M, Schnurrbusch UE, Wolf S, Wiedemann P, Bringmann A, Kohen L. Expression of HB-EGF by retinal pigment epithelial cells in vitreoretinal proliferative disease. Curr Eye Res. 2006;31:863–874. doi: 10.1080/02713680600888807. [DOI] [PubMed] [Google Scholar]
- 28.Gridley T. Notch signaling in vascular development and physiology. Development. 2007;134:2709–2718. doi: 10.1242/dev.004184. [DOI] [PubMed] [Google Scholar]
- 29.Brou C, Logeat F, Gupta N, Bessia C, LeBail O, Doedens JR, Cumano A, Roux P, Black RA, Israel A. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol. Cell. 2000;5:207–216. doi: 10.1016/s1097-2765(00)80417-7. [DOI] [PubMed] [Google Scholar]
- 30.Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, Smith GH, Stark KL, Gridley T. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- 31.Hartmann D, de Strooper B, Serneels L, Craessaerts K, Herreman A, Annaert W, Umans L, Lubke T, Lena Illert A, von Figura K, Saftig P. The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for alpha-secretase activity in fibroblasts. Hum. Mol. Genet. 2002;11:2615–2624. doi: 10.1093/hmg/11.21.2615. [DOI] [PubMed] [Google Scholar]
- 32.Feldmann M, Maini SR. Role of cytokines in rheumatoid arthritis: an education in pathophysiology and therapeutics. Immunol Rev. 2008;223:7–19. doi: 10.1111/j.1600-065X.2008.00626.x. [DOI] [PubMed] [Google Scholar]
- 33.Zhou BB, Peyton M, He B, Liu C, Girard L, Caudler E, Lo Y, Baribaud F, Mikami I, Reguart N, Yang G, Li Y, Yao W, Vaddi K, Gazdar AF, Friedman SM, Jablons DM, Newton RC, Fridman JS, Minna JD, Scherle PA. Targeting ADAM-mediated ligand cleavage to inhibit HER3 and EGFR pathways in non-small cell lung cancer. Cancer Cell. 2006;10:39–50. doi: 10.1016/j.ccr.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohammed FF, Smookler DS, Taylor SE, Fingleton B, Kassiri Z, Sanchez OH, English JL, Matrisian LM, Au B, Yeh WC, Khokha R. Abnormal TNF activity in Timp3−/− mice leads to chronic hepatic inflammation and failure of liver regeneration. Nat Genet. 2004;36:969–977. doi: 10.1038/ng1413. [DOI] [PubMed] [Google Scholar]
- 35.Mahmoodi M, Sahebjam S, Smookler D, Khokha R, Mort JS. Lack of tissue inhibitor of metalloproteinases-3 results in an enhanced inflammatory response in antigen-induced arthritis. Am J Pathol. 2005;166:1733–1740. doi: 10.1016/S0002-9440(10)62483-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwak HI, Mendoza EA, Bayless KJ. ADAM17 co-purifies with TIMP-3 and modulates endothelial invasion responses in three-dimensional collagen matrices. Matrix Biol. 2009;28:470–479. doi: 10.1016/j.matbio.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Auricchio A, Behling KC, Maguire AM, O'Connor EM, Bennett J, Wilson JM, Tolentino MJ. Inhibition of retinal neovascularization by intraocular viral-mediated delivery of anti-angiogenic agents. Mol Ther. 2002;6:490–494. doi: 10.1006/mthe.2002.0702. [DOI] [PubMed] [Google Scholar]
- 38.Janssen A, Hoellenriegel J, Fogarasi M, Schrewe H, Seeliger M, Tamm E, Ohlmann A, May CA, Weber BH, Stohr H. Abnormal vessel formation in the choroid of mice lacking tissue inhibitor of metalloprotease-3. Invest Ophthalmol Vis Sci. 2008;49:2812–2822. doi: 10.1167/iovs.07-1444. [DOI] [PubMed] [Google Scholar]
- 39.Sunnarborg SW, Hinkle CL, Stevenson M, Russell WE, Raska CS, Peschon JJ, Castner BJ, Gerhart MJ, Paxton RJ, Black RA, Lee DC. Tumor necrosis factor-alpha converting enzyme (TACE) regulates epidermal growth factor receptor ligand availability. J. Biol. Chem. 2002;277:12838–12845. doi: 10.1074/jbc.M112050200. [DOI] [PubMed] [Google Scholar]
- 40.Horiuchi K, Le Gall S, Schulte M, Yamaguchi T, Reiss K, Murphy G, Toyama Y, Hartmann D, Saftig P, Blobel C. Substrate Selectivity of EGF-Receptor Ligand Sheddases and Their Regulation by Phorbol Esters and Calcium Influx. Mol. Biol. Cell. 2007;18:176–188. doi: 10.1091/mbc.E06-01-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.