Abstract
Natural killer (NK) cells expressing inhibitory receptors that bind to self-MHC class I are “licensed” or rendered functionally more responsive to stimulation, whereas “unlicensed” NK cells lacking receptors for self-MHC class I are hyporesponsive. Here we show that, contrary to the licensing hypothesis, unlicensed NK cells were the primary mediators of NK cell-mediated control of mouse cytomegalovirus infection in vivo. Depletion of unlicensed, but not licensed, NK cells impaired control of viral titers. Transfer of unlicensed NK cells was more protective than licensed NK cells. SHP-1 signaling limited proliferation of licensed, but not unlicensed NK cells during infection. Thus, “unlicensed” NK cells are critical for protection against viral infection.
Natural killer (NK) cells mediate resistance to certain viral infections and play an important role in the rejection of some tumors1. NK cells possess an extensive repertoire of activating and inhibitory receptors, many of which are expressed in a stochastic fashion resulting in subsets of NK cells defined by their receptor expression2. Several families of NK cell receptors, such as activating Killer cell Immunoglobulin-like Receptors (KIR) in humans, activating Ly49 receptors in rodents, NKG2D, the natural cytotoxicity receptors (NKp30, NKp44 and NKp46), and CD16 drive NK cell activation. Most activating NK receptors lack an intracellular signaling domain and instead associate non-covalently with immunoreceptor tyrosine-based activation motif (ITAM)-containing adaptor proteins DAP12, CD3ζ or FcεRIγ or the YINM motif-containing adaptor DAP102. Downstream signaling from these adaptors results in cytoskeletal rearrangements, proliferation and secretion of lytic granules and cytokines. The inhibitory Ly49 and KIR recognize polymorphic MHC class I ligands, and MHC class I engagement of these inhibitory receptors prevents NK cells from attacking self2. Upon MHC class I ligation the immunoreceptor tyrosine-based inhibitory motifs (ITIM) in the intracellular domains of these inhibitory receptors become phosphorylated, leading to the recruitment and activation of the tyrosine or lipid phosphatases SHP-1, SHP-2, and SHIP3–6. SHP-1 dephopshorylates Vav-1, a critical molecule in the signaling downstream of NK activating receptors6. In addition, c-Abl phosphorylates Crk upon ligation of inhibitory receptors. Although the exact mechanism of how this prevents NK cell function is unclear, phosphorylation of Crk may disrupt the Cbl-Crk-p130CAS-C3G activation complex7. Thus, NK cell function is determined by the integration of signals arising from the engagement of both activating and inhibitory receptors with their ligands on potential target cells.
NK cells from MHC class I-deficient mice, resulting from deletion of either the H- 2K and H-2D MHC class I heavy chains or the β2-microglobulin subunit, are relatively hyporesponsive when stimulated by antibodies to several activating receptors8. NK cell responsiveness was restored by the reintroduction of MHC class I, but only occurred in the NK cell subsets that expressed inhibitory receptors for the particular MHC class I allele reintroduced. Although the molecular mechanisms responsible for self-MHC class I reactive inhibitory receptors conferring responsiveness are unknown, a functional ITIM in the inhibitory NK receptor is required, but both SHP-1 and SHIP are dispensable8. These observations may explain why NK cells from MHC class I-deficient animals are developmentally mature, yet do not exert overt autoimmunity9. In normal mice, a significant number of phenotypically mature NK cells lack inhibitory receptors for self-MHC class I. These cells are hyporesponsive when assayed in vitro by engaging their activating receptors and are unable to acutely reject MHC class I-deficient bone marrow10. Similarly, human NK cells that express inhibitory KIRs recognizing self-HLA are more responsive than NK cells that lack self-reactive inhibitory KIRs when stimulated in vitro with antibodies against their activating receptors11–14. Thus, NK cells expressing an inhibitory receptor for self-MHC class I have been deemed “armed” or “licensed” 15. NK cells expressing a greater number of self-reactive inhibitory receptors have an increased responsive potential indicating that licensing is a quantitative event 16–18. Thus, inhibitory receptors recognizing self-MHC class I play a paradoxical role in enhancing NK cell responsiveness. An alternate “disarming” hypothesis proposes that lack of MHC class I inhibition renders these NK cells anergic or exhausted due to chronic stimulation19. Here we will simply refer to NK cells expressing an inhibitory receptor for self-MHC class I as “licensed”, without implying a preference for one or the other hypotheses. Unlicensed cells become as responsive as licensed cells when stimulated in vitro with high doses of IL-12 and IL-18 or with PMA and ionomycin or when cultured in IL-2 8, 15. In addition, after acute infection with Listeria monocytogenes both licensed and unlicensed NK cells produce IFN-γ equivalently, suggesting that under inflammatory conditions licensing may not be an important factor in NK cell function10. Although implicated in the control of many viral and bacterial infections, NK cells are perhaps most important in the control of herpesviruses such as mouse cytomegalovirus (MCMV)20, 21. The MCMV glycoproteins m152 and m06 inhibit MHC class I expression in infected cells, presumably to evade detection by CD8+ T cells22, 23, 24. By down-regulating surface MHC class I on infected cells, MCMV should be sensitive to control by licensed NK cells which may normally be restrained by their self-MHC class I reactive inhibitory receptors. Several mouse strains express NK cell activating receptors that recognize MCMV-infected cells. Ma/My mice (H-2k), express the Ly49P receptor, which recognizes MCMV-infected cells expressing the viral protein m04 and host H-2Dk; PWK mice express an as yet unidentified receptor encoded by the Cmv4 locus within the NK complex on mouse chromosome 6 that is necessary for NK cell control of MCMV infection; C57BL/6 (B6) mice express the Ly49H receptor, which recognizes the viral m157 glycoprotein25–29. Engagement of Ly49H by m157 on infected cells results in NK cell-mediated cytotoxicity, secretion of IFN-γ, extensive proliferation, and control of MCMV infection25, 26, 30, 31. In naïve B6 mice, only a subset of the Ly49H+ NK cells expresses an inhibitory Ly49 that recognizes self-MHC class I and are thus licensed, as defined by their ability to degranulate or produce cytokines efficiently after activating receptor cross-linking in vitro16, 32. Here we have examined how licensed and unlicensed NK cells expressing the MCMV-specific Ly49H receptor behave in vivo in response to infection with MCMV.
Results
MHC class I-mediated inhibition overrides licensing
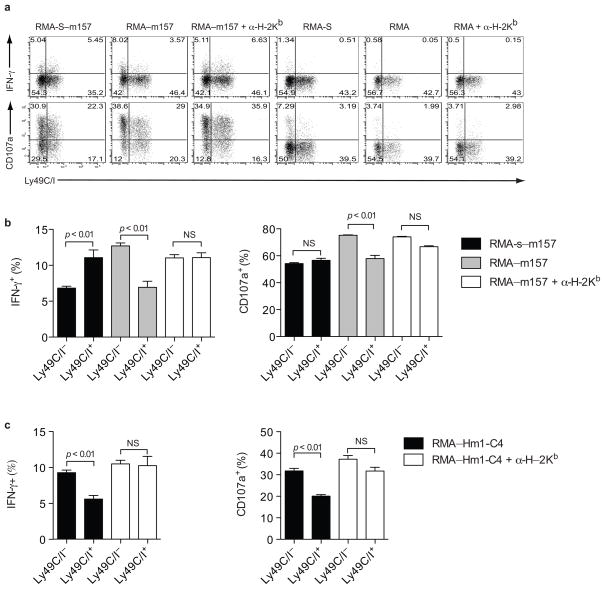
NK cells in B6 (H-2b) mice express four well-characterized inhibitory Ly49 receptors, Ly49A, Ly49C, Ly49G2, and Ly49I, which are present on overlapping NK cells subsets. NK cells expressing Ly49C and/or Ly49I bind to H-2Kb and are licensed in H-2b mice, whereas NK cells expressing only Ly49G2 or Ly49A, which do not efficiently bind to H-2b, are not licensed16, 32. To examine whether NK cells bearing Ly49H are licensed similar to NK cells expressing other activating receptors, we transduced the MCMV m157 gene, which encodes the natural ligand for the Ly49H activating receptor, into MHC class I-deficient RMA-S cells. In agreement with prior findings, licensed naïve B6 Ly49H+ NK cells expressing Ly49C and/or I (as detected by the 5E6 monoclonal antibody that binds both Ly49C and Ly49I) produced IFN-γ more readily than the unlicensed Ly49H+Ly49C/I− NK cells upon co-culture with RMA-S-m157 targets (Fig. 1a, b). Degranulation, as measured by surface expression of CD107a, was only slightly increased in the Ly49C/I+ Ly49H+ NK cell subset compared to the Ly49H+Ly49C/I− NK cells; however, this difference was reproducible over several experiments (Fig. 1a, b). Thus, expression of the self-MHC class I receptors Ly49C and/or Ly49I enhanced the responsiveness of NK cells, particularly for IFN-γ production.
Figure 1. MHC class I inhibition overrides NK cell licensing.
(a) IFN-γproduction and degranulation by naïve Ly49H+ B6 NK cells stimulated ex vivo with RMA, RMA-S, RMA-S-m157, or RMA-m157 targets in the presence or absence of blocking antibody to H-2Kb. Plots are gated on Ly49H+ NK1.1+ cells. (b) Average IFN-γproduction and degranulation by Ly49H+ B6 NK cells from 4 mice stimulated with RMA-S-m157 or RMA-m157 targets in the presence or absence of blocking antibody to H-2Kb. Error bars indicate the standard error of the mean. (c) Average IFN-γproduction and degranulation by Ly49D+ B6 NK cells from 4 mice stimulated with RMA-Hm1-C4 targets in the presence or absence of blocking antibody to H-2Kb. Error bars indicate the standard error of the mean. Data are representative of five experiments with 3–4 mice per experiment
In these experiments, the Ly49H receptor was engaged by its ligand m157 on target cells lacking MHC class I. To determine whether licensing still confers a responsive advantage when MHC class I is expressed on target cells we transduced m157 into MHC class I-sufficient RMA (RMA-m157) cells. A greater percentage of Ly49H+Ly49C/I− NK cells produced IFN-γ and degranulated compared to the Ly49H+Ly49C/I+ NK cells after RMA-m157 stimulation, indicating that MHC class I-mediated inhibition superseded the responsive advantage conferred by licensing (Fig. 1a, b). Addition of a H-2Kb blocking antibody partially abrogated the Ly49C/I-mediated inhibition of NK cell responses, indicating that MHC class I interactions limit the responsiveness of licensed NK cells (Fig. 1a, b). The incomplete restoration of responsiveness in Ly49C/I+Ly49H+ NK cells following antibody treatment may be due to incomplete blockade of Ly49C/I-H-2Kb interactions or to residual inhibitory signals induced by H-2Db, which is expressed endogenously on RMA targets. Antibody blockade of H-2Kb did not affect the responsive capacity of the Ly49C/I− subset of Ly49H+ NK cells to RMA-m157 stimulation, indicating that only the Ly49C/I+ subset of Ly49H+ NK cells was inhibited by H-2Kb (Fig. 1a, b). RMA transduced cells exhibited higher m157 expression than the RMA-S transduced cells, likely accounting for the differences in responsiveness of the Ly49C/I− Ly49H+ NK cells to these different targets. Ly49H+ NK cells stimulated with either parental RMA-S or RMA treated with blocking anti-H-2Kb antibody did not appreciably respond under these assay conditions, thus absence of MHC class I alone was not sufficient to activate NK cells (Fig. 1a). Although Ly49C/I expression renders B6 NK cells more responsive to MHC class I-deficient targets, target cells bearing MHC class I elicited more vigorous responses from unlicensed NK cells than licensed NK cells. Thus, licensing is insufficient to overcome Ly49C/I-mediated inhibition upon ligation with H-2Kb.
To determine whether this observation is unique to the Ly49H receptor, we examined Ly49D+ NK cell responses to RMA targets transduced with the cognate ligand hamster MHC class I (Hm1-C4)33. Licensed NK cells have been shown to be more responsive than unlicensed NK cells when stimulated with anti-Ly49D antibody8, 10. However, similar to Ly49H+ NK cells stimulated with RMA-m157, Ly49D+Ly49C/I− NK cells produced IFN-γ and degranulated more frequently than their Ly49D+Ly49C/I+ counterparts when stimulated with MHC class I-expressing target cells (Fig. 1c). Blocking H-2Kb abrogated this difference, thus confirming that MHC I interactions with the inhibitory Ly49C/I receptors override the responsive advantage gained by licensing.
Ly49C/I limits proliferation during MCMV infection
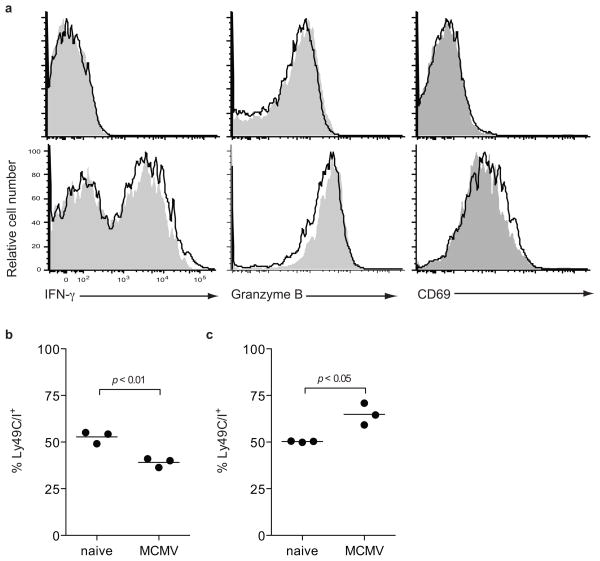
To examine the aggregate impact of Ly49C/I expression (both licensing and inhibition) on NK cell responses during infection we compared Ly49C/I+ and Ly49C/I− subsets of Ly49H+ NK cells during MCMV infection in vivo. Thirty-six hours after infection both populations were similarly activated as evinced by upregulation of IFN-γ, granzyme B, and CD69 (Fig. 2a). At this early time-point activation of NK cells is dependent on the cytokine milieu and independent of Ly49H31. Thus, both Ly49C/I+ and Ly49C/I− NK cells are activated by the pro-inflammatory cytokines induced during MCMV infection. A similar activation of both licensed and unlicensed NK cells was also noted during Listeria infection and is thus likely to be a general characteristic of NK cell responses to infection, cytokines, and/or inflammation10.
Figure 2. Licensed NK cells become under-represented during MCMV infection.
(a) Unlicensed Ly49C/I− (black line)and licensed Ly49C/I+ (grey fill) Ly49H+ NK cells from naïve (top row) or 36 hour MCMV-infected (bottom row). B6 mice were analyzed for CD69 upregulation and ex vivo IFN-γand granzyme B expression. Ly49C/Iexpression was analyzed on Ly49H+ NK cells from (b) B6 wild-type mice and (c) H2KbDb−/− animals prior to infection and 5 days after MCMV infection. Data are representative of three experiments with 3–4 mice per group.
During MCMV infection, Ly49H+ NK cells undergo a robust and specific proliferation between days 2 and 7 post-infection31. By four days post-infection the frequency of Ly49H+ NK cells expressing Ly49C/I decreased from 53%±2% to 40%±1% (Fig 2b). Conversely, in mice lacking MHC class I (H-2KbDb−/− mice) the frequency of Ly49H+ NK cells expressing Ly49C/I increased from 50%±1% to 65%±3%, indicating that the preferential loss of Ly49C/I-expressing Ly49H+ NK cells in wild-type B6 mice required interaction with MHC class I (Fig. 2c). B2m−/− B6 mice did not exhibit a decrease in the frequency of Ly49C/I+Ly49H+ NK cells after infection (data not shown). A similar decrease in the frequency of Ly49C/I+ NK cells has been observed in other viral infections in B6 mice, such as LCMV, vaccinia, and MHV30.
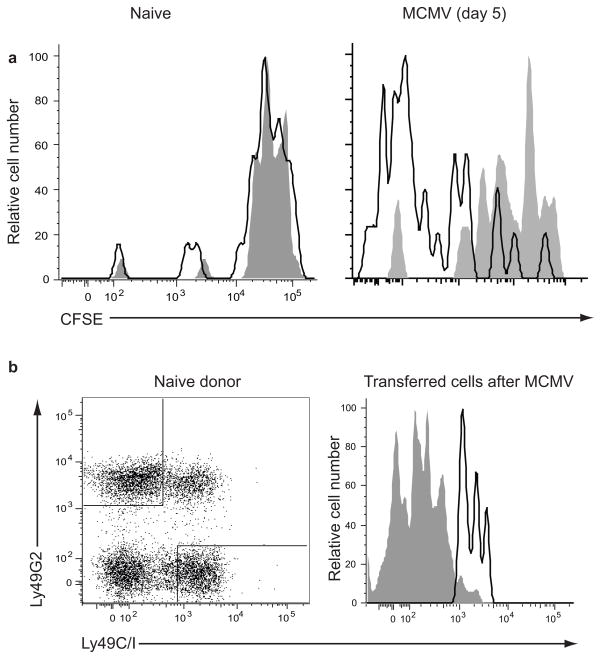
The decrease in frequency of Ly49H+ NK cells expressing Ly49C/I during MCMV infection could have been caused by impaired proliferation of Ly49H+Ly49C/I+ NK cells or by loss of expression of Ly49C/I on cells. To determine whether Ly49C/I limits Ly49H-driven proliferation, we adoptively transferred CFSE-labeled NK cells into congenically marked wild-type hosts and infected them with MCMV. Only the Ly49H+ subset of NK cells proliferates extensively after MCMV infection and this is dependent on the expression of the cognate ligand, MCMV m15734. By day 5 post-infection, the Ly49C/I− Ly49H+ NK subset had undergone more rounds of division compared to the Ly49C/I+ Ly49H+ subset, confirming that Ly49C/I limits NK cell proliferation induced by MCMV infection (Fig. 3a). Thus, licensed Ly49C/I+ NK cells are impaired in their ability to proliferate during MCMV infection in the presence of MHC class I compared with the unlicensed Ly49C/INK cells. The difference in proliferation as measured by CFSE dilution was more pronounced than the change in the frequency of NK cells expressing Ly49C/I (Fig. 2b), perhaps due to increased activation-induced cell death in the Ly49C/I− subset that is not restrained by MHC class I engagement. To examine the possibility of Ly49C/I expression loss on individual NK cells upon infection, we sorted NK cells as Ly49C/I+Ly49G2− or Ly49C/I−Ly49G2+ and then transferred each subset into CD45-congenic recipients. After MCMV infection, Ly49C/I+ donor NK cells maintained expression of Ly49C/I, whereas Ly49G2+Ly49C/I− donor NK cells remained Ly49C/I− after infection, indicating that the decreased frequency of Ly49C/I+ cells among Ly49H+ NK cells during infection cannot be explained by loss of Ly49C/I expression (Fig. 3b).
Figure 3. Ly49C/I is stably expressed and limits proliferation of NK cells during MCMV infection.
(a) CFSE dilution by Ly49C/I+Ly49H+ (gray fill) and Ly49C/I− Ly49H+ (black line) NK cells in naïve recipients and 5 days after MCMV infection. Data are representative of five experiments with 3–4 animals each. (b) Ly49C/I expression on NK cells sorted as either Ly49G2+Ly49C/I− (grey fill) or Ly49G2− Ly49C/I+ (black line), transferred into naïve recipients, and analyzed for Ly49C/I expression six days after MCMV infection. Data are representative of two experiments.
Ly49C/I inhibits NK cell responses via SHP-1
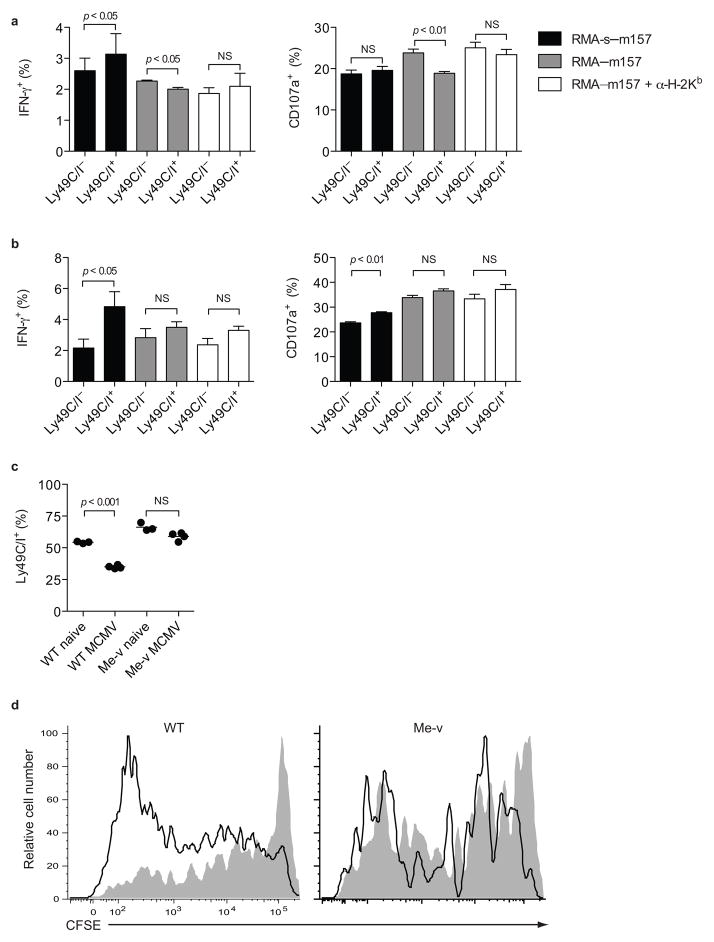
NK cell inhibitory receptors use several signaling pathways to inhibit cell function, including the activation of phosphatase SHP-1. SHP-1 dephosphorylates Vav-1, a critical signaling molecule downstream of ITAM-associated receptor activation6. Motheaten viable (Me-v) mice express a hypomorphic SHP-1, display a hyper-inflammatory phenotype, and have reduced lifespans5. To examine whether SHP-1 is acting intrinsically downstream of Ly49C/I to suppress Ly49H-induced proliferation of NK cells during MCMV infection, we generated bone marrow chimeras by mixing wild-type (CD45.1) and SHP-1Me-v (CD45.2) bone marrow. The wild-type NK cells in the chimeras responded to RMA-m157 and RMA-S-m157 stimulation similarly to NK cells from normal wild-type mice. However, the response was of lower magnitude, possibly due to the inflammatory environment stemming from the SHP-1Me-v hematopoietic cells (Fig. 1 and 4a). Similar to wild-type NK cells, Ly49H+Ly49C/I+ Me-v NK cells in the mixed bone marrow chimeras were more responsive than the Ly49H+Ly49C/I− Me-v NK cells upon stimulation with RMA-S-m157, indicating that Ly49C/I-mediated licensing does not require fully functional SHP-1 (Fig. 4a, b), in agreement with a prior report8. However, unlike wild-type cells, MHC class I on RMA-m157 target cells did not inhibit the responses of Ly49H+Ly49C/I+ Me-v NK cells in the chimeras, indicating that Ly49C/I-mediated inhibition requires functional SHP-1. Moreover, Me-v Ly49H+Ly49C/I+ NK cell responses to RMA-m157 were not affected by blocking H-2Kb, whereas wild-type Ly49H+Ly49C/I+ NK cell responses were enhanced by this treatment (Fig. 4a, b). Therefore, licensing of NK cells does not depend on functional SHP-1, but functional SHP-1 was required for Ly49C/I-mediated inhibition of NK cell function upon engagement with MHC class I. Thus, Ly49C/I-mediated licensing and inhibition are separable functions.
Figure 4. Ly49C/I-mediated inhibition requires functional SHP-1.
IFN-γ and degranulation by mixed bone marrow chimeric (a) Ly49H+ wild-type NK cells and (b) Ly49H+ SHP-1Me-v NK cells stimulated with RMA-S-m157 or RMA-m157 targets in the presence or absence of blocking antibody to H-2Kb. Data are representative of three experiments with 3–4 mice per experiment. (c) Ly49C/Iexpression was analyzed on mixed bone marrow chimeric Ly49H+ wild-type and SHP-1Me-v NK cells prior to infection and 5 days after MCMV infection. Data are representative of three experiments with 3–4 mice per group. (d) CFSE dilution by Ly49C/I+ (gray fill) and Ly49C/I− (black line) mixed bone marrow chimeric Ly49H+ wild-type and SHP-1Me-v NK cells 5 days after MCMV infection. Data are representative of three experiments with 2–3 animals each.
As seen in normal wild-type animals, MCMV infection of mixed chimeras resulted in decreased frequency of Ly49H+Ly49C/I+ wild-type NK cells, whereas the frequency of Ly49C/I+ cells did not change in the Ly49H+ Me-v NK cell population, suggesting that Ly49C/I impairs NK cell proliferation via SHP-1 (Fig. 4c). Transfer of CFSE-labeled NK cells from the mixed chimera into a wild-type host confirmed this finding, because Ly49H+Ly49C/I+ and Ly49H+Ly49C/I− Me-v NK cells diluted CFSE to a similar extent, whereas Ly49H+Ly49C/I+ wild-type NK cells underwent fewer rounds of division than Ly49H+Ly49C/I− wild-type cells (Fig. 4d). Thus, Ly49C/I signaling through SHP-1 restrains Ly49H+ NK cell proliferation during MCMV infection. Me-v NK cells demonstrated a general proliferation defect compared to wild-type cells, which was due to a cell intrinsic defect in SHP-1. This may be due to developmental alterations stemming from SHP-1 deficiency independent of Ly49C/I.
Unlicensed NK cells control MCMV infection
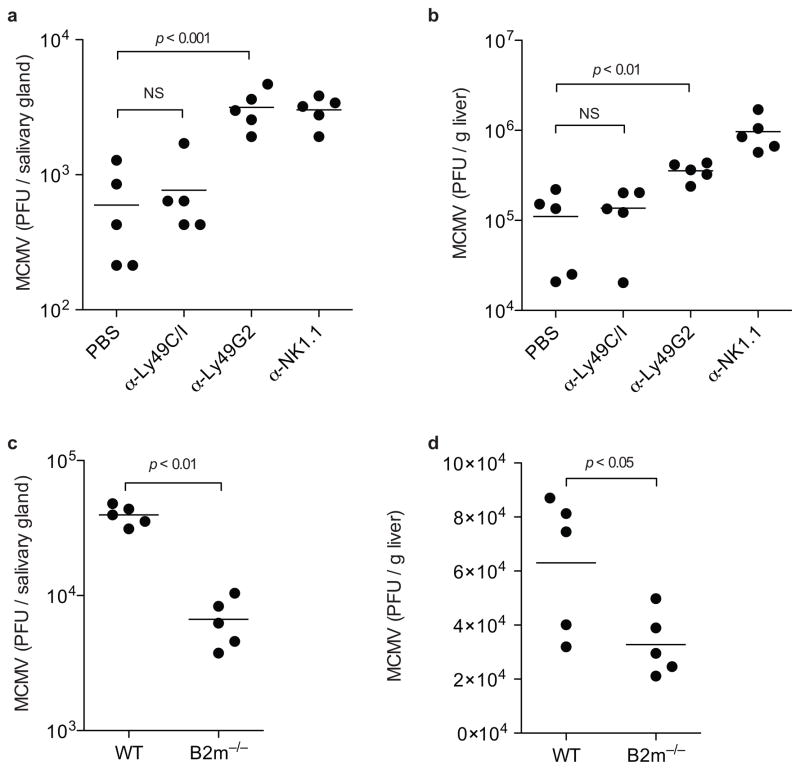
NK cell control of MCMV infection in the salivary glands is at least partially dependent on robust NK cell proliferation34. To examine the role of licensed NK cells in controlling viral replication, we depleted Ly49C/I+ NK cells (licensed), Ly49G2+ NK cells (containing both licensed and unlicensed), or all NK cells and determined the viral titers in the salivary glands and livers seven days post-infection. Ly49G2 is expressed on a similar number of Ly49H+ NK cells as Ly49C/I (Fig. 3b), but does not confer licensing in B6 mice and thus depletion of this subset controls for absolute numbers of NK cells after subset depletion. Depletion of the Ly49C/I+ licensed NK cells resulted in a modest 1.3-fold increase in viral titers compared to undepleted controls, whereas depleting a similar number of NK cells using the anti-Ly49G2 mAb increased the viral titers more than 5-fold in the salivary gland, to a level comparable to complete NK cell depletion (Fig. 5a). Similarly, depletion of Ly49C/I+ NK cells had only a minimal impact on MCMV titers in the liver, whereas Ly49G2 depletion increased titers in the liver more than 3-fold (Fig. 5b). This indicates that licensed Ly49C/I+ NK cells make only a modest contribution to controlling MCMV. To confirm that treatment with the depleting anti-Ly49C/I antibody 5E6 efficiently eliminated licensed NK cells we stained cells from treated mice with an anti-Ly49I antibody and an anti-Ly49C antibody, neither of which are blocked by the 5E6 antibody. We observed complete loss of NK cells expressing Ly49I and/or Ly49C, indicating that 5E6 mAb treatment resulted in depletion of NK cells (data not shown).
Figure 5. Unlicensed NK cells control MCMV infection.
MCMV titers in the (a) salivary glands and (b) liver one-week post infection in B6 wild-type mice either untreated or depleted of Ly49C/I+ NK cells, Ly49G2+ NK cells, or all NK cells. Data are representative of two experiments with five mice per group. MCMV titers in the (c) salivary glands and (d) liver one-week post infection of B6 wild-type mice and B2m−/− mice, both depleted of CD8+ T cells. Data are representative of two experiments with five mice per group.
Cells lacking β2-microglobulin (B2m) are unable to stably express MHC class I on their surface, and NK cells from mice lacking β2-microglobulin are unlicensed due to the lack of MHC class I expression8. To confirm that expression of an inhibitory receptor reactive to self-MHC class I, the definition of a “licensed” cell, impairs control of MCMV, we compared viral titers in wild-type and B2m−/− B6 mice. Because B2m−/− mice also lack CD8+ T cells, which play a role in controlling MCMV, we treated both sets of animals with a depleting anti-CD8 antibody35. One week post-infection the B2m−/− mice, containing only unlicensed NK cells, controlled infection better than wild-type mice, which contain both licensed and unlicensed NK cells, in both the salivary glands and liver (Fig. 5c,d). A previous report found that MCMV titers were not different between wild-type and B2m−/− mice at three days post-infection36. Thus, limiting NK cell proliferation may be the major mechanism by which interactions with MHC class I impedes NK cell control of MCMV. Collectively, these findings indicate that licensed NK cells are not required to control viral replication, rather expression of an inhibitory receptor that recognizes self-MHC class I limits the ability of NK cells to control the infection.
Licensed NK cells fail to protect neonates from MCMV
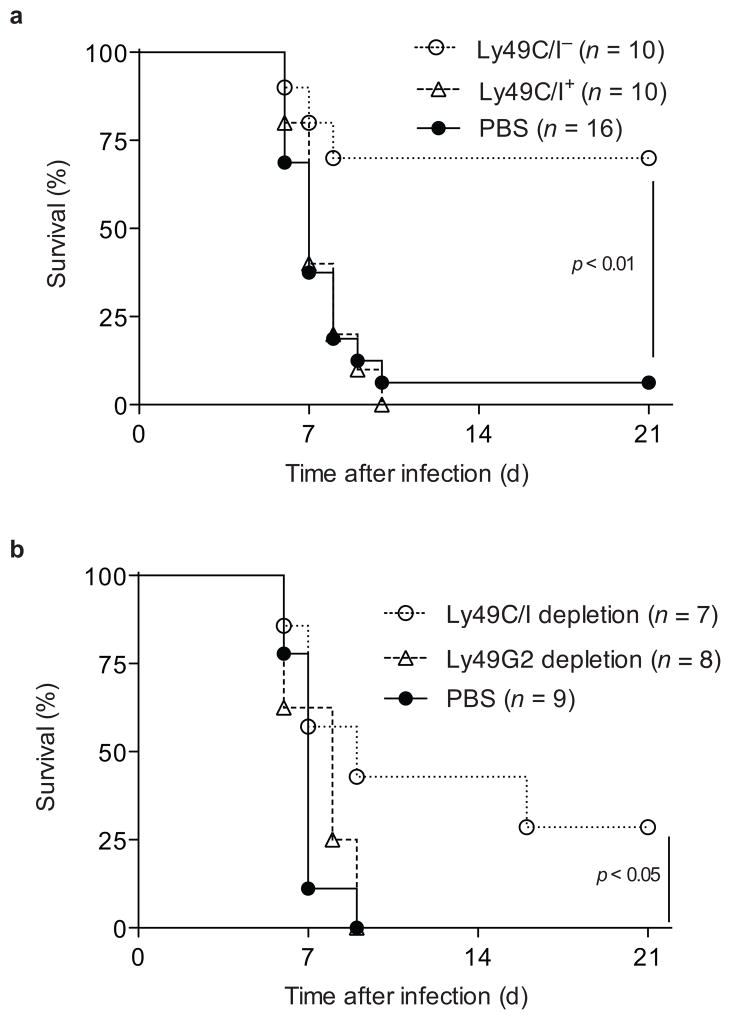
Transfer of adult Ly49H+ NK cells into neonatal mice is sufficient to protect against MCMV infection37. To further examine if Ly49C/I-mediated inhibition impedes NK cell control of MCMV, we sorted naïve NK cells as either Ly49C/I+ or Ly49C/I− Ly49G2+, transferred equal numbers of Ly49H+ cells from either group into neonatal Ly49h−/− mice, and challenged with MCMV. Seven of ten neonates receiving Ly49C/I− NK cells survived, whereas all ten of the neonates receiving Ly49C/I+ NK cells succumbed to infection, similar to mice that did not receive NK cells (Fig. 6a). Transfer of Ly49H+ NK cells from adult mice pre-depleted of licensed Ly49C/I+ NK cells protected neonatal recipients better than transfer of an equal number of Ly49H+ NK cells from adult mice pre-depleted of Ly49G2+ NK cells and thus containing both licensed and unlicensed NK cells (Fig. 6b). These findings indicate that unlicensed NK cells are more protective than the total NK cell population containing both licensed and unlicensed cells. Thus, expression of self-MHC class I inhibitory receptors dampens the capacity of licensed NK cells to protect against MCMV.
Figure 6. Licensed NK cells do not protect neonates from MCMV infection.
Ly49h−/− neonates received (a) 1 × 105 Ly49H+ NK cells sorted as either Ly49C/I+ or Ly49G2+Ly49C/I− or PBS or (b) 7.5 × 104 Ly49H+ NK cells from donors pre-depleted of Ly49C/I+ or Ly49G2+ cells in vivo or PBS and challenged with 2 × 103 pfu of MCMV. Animals were monitored daily for morbidity.
Discussion
Expression of inhibitory receptors for self-MHC class I rendered NK cells more responsive when stimulated through their activating receptors in vitro. This suggested that licensing or arming of NK cells by interactions between inhibitory receptors and self-MHC class I proteins results in more functionally responsive NK cells. To better understand the significance of NK cell licensing, we compared the responses of licensed and unlicensed cells against target cells expressing or lacking MHC class I in vitro and during MCMV infection in vivo. Here we show that Ly49C/I interactions with H-2b on target cells limits NK cell degranulation and IFN-γ production, offsetting the advantage gained by licensing. Moreover, Ly49C/I self-MHC class I interactions on licensed NK cells restrain the proliferation of licensed MCMV-specific Ly49H+ NK cells in vivo.
Early during MCMV infection licensed and unlicensed NK cells expressed similar amounts of IFN-γ and granzyme B, likely due to cytokine-induced activation, which has been shown to obviate licensing; thus, both populations contribute to the inflammatory milieu. However, the combined suppression of effector functions and NK cell expansion by Ly49C/I had a profound impact on the ability of NK cells to control MCMV infection. Elimination of the NK cells expressing Ly49C/I, and are thus licensed, had a minimal impact on NK cell control of MCMV. Conversely, depletion of Ly49G2+ cells eliminated a similar number of NK cells, but resulted in a significant loss of control of MCMV infection. This is unlikely to be due to an intrinsic function of Ly49G2, which has no known ligand in the B6 mouse, but rather due to the elimination of a large number of Ly49C/I− Ly49G2+ unlicensed NK cells32. Similarly, the NK cells of B2m−/− mice, all of which are unlicensed, were better able to control MCMV infection than the NK cells of wild-type mice, many of which are licensed, but inhibited by Ly49C/I interacting with H-2b. Finally, transfer of unlicensed and uninhibited Ly49H+ NK cells was sufficient to protect neonates from MCMV challenge, whereas an equal number of licensed Ly49H+ NK cells were unable to protect from such a challenge. Thus, although better at responding to MHC class I-deficient targets, licensed NK cells appear impaired in their ability to protect against viral infection in normal MHC class I-intact animals, indicating that the advantage of licensing by expressing self-reactive inhibitory MHC class I receptors is relatively weak compared to the effect of inhibition mediated by these same receptors during MCMV infection.
The licensed NK cells were inhibited by interactions with MHC class I during MCMV infection despite the fact that MCMV-encoded proteins m152- and m06 inhibit expression of surface MHC class I by MCMV-infected cells23. m152 prevents the de novo expression of MHC class I by blocking the transport of MHC class I through the ERGIC/cis-Golgi compartments while m06 targets MHC class I proteins to lysosomes for degradation38, 39. Thus, infected cells are starved of new surface MHC class I and slowly lose expression due to normal turnover of pre-existing surface MHC class I. However, this is a relatively slow process. Total H-2Kb surface expression is only reduced by ~30% 12 h post-MCMV infection23. Expression of m157 is more rapid, with m157 transcripts detectable by 4 h post-infection and surface expression of m157 protein by 12 h40. Although MCMV impairs expression of surface MHC class I, our results suggest that the residual amounts of MHC class I on the surface of infected cells are sufficient to engage the inhibitory receptors expressed by licensed NK cells and impede NK cell activation via Ly49H recognition of m157. Therefore, it is the Ly49H+ NK cells lacking inhibitory receptors for self-MHC class I that are able to respond vigorously to MCMV infection.
NK cells from naïve mice expressing self-MHC class I reactive inhibitory receptors respond preferentially to activating receptor stimulation in vitro in the absence of MHC class I and are able to reject MHC class I-deficient bone marrow in vivo 10. The hyporesponsiveness of the unlicensed cells may have evolved to avoid auto-aggression. During infection or exposure to inflammatory conditions in vivo or after in vitro culture with high doses of IL-12 and IL-18 or IL-2, the responsive capacity of unlicensed NK cells is restored8, 15. During infections in which NK cell activities are not dependent on interactions with target cells expressing MHC class I, but rather act to amplify the inflammatory milieu by cytokine secretion (e.g. Listeria infection), both licensed and unlicensed NK cells may contribute equally to immunity10. However, during infections such as MCMV that require target cell contact for NK cells to confer protection, the ability to respond and eliminate MHC class I-sufficient targets is impaired by the inhibitory receptors that mediate licensing during homeostatic conditions, thus rendering licensed NK cells less effective effectors that uninhibited NK cells. Although not yet addressed experimentally, licensed NK cells might play an important role in vivo in eliminating transformed cells that down-regulate MHC class I expression in the absence of inflammation.
During a viral infection, these activated NK cells lacking inhibitory receptors for self-MHC class I could potentially attack healthy, uninfected cells in the host, in addition to killing virus-infected cells. Indeed, further studies are warranted to determine whether these activated, uninhibited NK cells mediate collateral damage during an infection. However, prior studies have shown that NK cells can tolerate normal, healthy cells lacking MHC class I, as in mixed bone marrow chimeras repopulated with bone marrow from wild-type and B2m−/− mice41. In this case, in the absence of infection, hematopoietic cells lacking MHC class I are tolerated by the wild-type NK cells by unknown mechanisms, whereas after activation by viral infection the tolerance is broken and unlicensed NK cells indeed attack and eliminate healthy B2m−/− hematopoietic cells42. The tolerance break during viral infection is likely due to cytokine-induced activation of NK cells. Because in these chimeras the targeted cells lacked surface MHC class I, it is possible that both Ly49C/I+ and Ly49C/I− NK cells participate in this aggression. During a viral infection in a normal, MHC class I-sufficient host, unlicensed NK cells might initially attack normal, healthy cells, but this process might be self-limiting due to exhaustion or anergy, as proposed by the disarming hypothesis19. For example, we and others have demonstrated that continuous exposure of Ly49H+ NK cells to m157 expressed in transgenic mice or transduced into bone marrow cells results in anergic NK cells43, 44. A similar process might limit autoreactivity of NK cells lacking self-MHC class I inhibitory receptors. Although these cells may initially respond quite vigorously to self, they may become anergic due to an overwhelming number of targets and a lack of inhibitory receptor-induced restraint. Alternatively, these unlicensed NK cells may be prevented from attacking normal self by inhibitory receptors, such as KLRG1, 2B4, or LAIR-1, which recognize non-MHC self ligands. Clearly, tolerance in NK cells is an important issue that remains incompletely understood.
Our findings may have particular bearing on the clinical use of NK cells. In leukemia patients receiving allogeneic hematopoietic stem cell transplants, adoptive transfer of donor-derived NK cells lacking inhibitory KIR for the recipient HLA class I might allow killing of residual leukemia cells and prevent relapses45. Cultured and expanded donor NK cells infused into the recipients post-transplantation with the goal of killing any residual leukemia cells has been attempted already45. Our results predict that to achieve graft-versus-leukemia in patients, NK cells uninhibited by recipient HLA, regardless of licensing status might be more effective than licensed NK cells that are inhibited by the recipient HLA. In the context of HLA-matched transplants, hematopoietic stem cell recipients receiving cells from donors possessing inhibitory KIRs that were not responsive to the common HLA type (i.e. containing cells that were unlicensed in reference to the donor and uninhibited in reference to the recipient) had a higher frequency of survival and a decreased incidence of leukemic relapse than patients receiving NK cells that did not contain unlicensed NK cells46–50. Importantly, positive outcomes increased with the number of inhibitory KIR HLA mismatches and these unlicensed NK cells were shown to be functional after transplantation50. These findings suggest that, similar to MCMV infection, NK cells lacking self-MHC I inhibitory receptors are more effective in eliminating leukemias. Thus, although NK cells lacking self-MHC class I inhibitory receptors are hyporesponsive during non-inflammatory conditions, they may be critical for NK cell immunity against pathogens and tumors.
Supplementary Material
Acknowledgments
We thank J. Jarjoura for assistance with cell sorting, H. Consengco for assistance with retroviral transduction, H. Robson MacDonald for the RMA-Hm1-C4 and J. Beilke and D. Hesslein for critical reading of the manuscript. M.T.O. is an Irvington Postdoctoral Fellow of the Cancer Research Institute. This study was supported by NIH grant AI066897 and L.L.L. is an American Cancer Society Professor.
Footnotes
Author Contributions
M.T.O. planned and performed experiments and wrote the manuscript, W.J.M. contributed to experimental design and provide essential reagents, and L.L.L. contributed to experimental design, data evaluation, and writing of the manuscript. The authors have no conflicting financial interests.
References
- 1.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 2.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binstadt BA, et al. Sequential involvement of Lck and SHP-1 with MHC-recognizing receptors on NK cells inhibits FcR-initiated tyrosine kinase activation. Immunity. 1996;5:629–638. doi: 10.1016/s1074-7613(00)80276-9. [DOI] [PubMed] [Google Scholar]
- 4.Long EO. Negative signaling by inhibitory receptors: the NK cell paradigm. Immunol Rev. 2008;224:70–84. doi: 10.1111/j.1600-065X.2008.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura MC, et al. Mouse Ly-49A interrupts early signaling events in natural killer cell cytotoxicity and functionally associates with the SHP-1 tyrosine phosphatase. J Exp Med. 1997;185:673–684. doi: 10.1084/jem.185.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stebbins CC, et al. Vav1 dephosphorylation by the tyrosine phosphatase SHP-1 as a mechanism for inhibition of cellular cytotoxicity. Mol Cell Biol. 2003;23:6291–6299. doi: 10.1128/MCB.23.17.6291-6299.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peterson ME, Long EO. Inhibitory receptor signaling via tyrosine phosphorylation of the adaptor Crk. Immunity. 2008;29:578–588. doi: 10.1016/j.immuni.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim S, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 9.Bix M, et al. Rejection of class I MHC-deficient haemopoietic cells by irradiated MHC-matched mice. Nature. 1991;349:329–331. doi: 10.1038/349329a0. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez NC, et al. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105:4416–4423. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anfossi N, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Kim S, et al. HLA alleles determine differences in human natural killer cell responsiveness and potency. Proc Natl Acad Sci U S A. 2008;105:3053–3058. doi: 10.1073/pnas.0712229105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yawata M, et al. MHC class I-specific inhibitory receptors and their ligands structure diverse human NK-cell repertoires toward a balance of missing self-response. Blood. 2008;112:2369–2380. doi: 10.1182/blood-2008-03-143727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu J, et al. Hierarchy of the human natural killer cell response is determined by class and quantity of inhibitory receptors for self-HLA-B and HLA-C ligands. J Immunol. 2007;179:5977–5989. doi: 10.4049/jimmunol.179.9.5977. [DOI] [PubMed] [Google Scholar]
- 15.Yokoyama WM, Kim S. Licensing of natural killer cells by self-major histocompatibility complex class I. Immunol Rev. 2006;214:143–154. doi: 10.1111/j.1600-065X.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 16.Brodin P, Lakshmikanth T, Johansson S, Karre K, Hoglund P. The strength of inhibitory input during education quantitatively tunes the functional responsiveness of individual natural killer cells. Blood. 2009;113:2434–2441. doi: 10.1182/blood-2008-05-156836. [DOI] [PubMed] [Google Scholar]
- 17.Johansson S, et al. Natural killer cell education in mice with single or multiple major histocompatibility complex class I molecules. J Exp Med. 2005;201:1145–1155. doi: 10.1084/jem.20050167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joncker NT, Fernandez NC, Treiner E, Vivier E, Raulet DH. NK cell responsiveness is tuned commensurate with the number of inhibitory receptors for self-MHC class I: the rheostat model. J Immunol. 2009;182:4572–4580. doi: 10.4049/jimmunol.0803900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raulet DH, Vance RE. Self-tolerance of natural killer cells. Nat Rev Immunol. 2006;6:520–531. doi: 10.1038/nri1863. [DOI] [PubMed] [Google Scholar]
- 20.Lodoen MB, Lanier LL. Natural killer cells as an initial defense against pathogens. Curr Opin Immunol. 2006;18:391–398. doi: 10.1016/j.coi.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 22.Lodoen MB, Lanier LL. Viral modulation of NK cell immunity. Nat Rev Microbiol. 2005;3:59–69. doi: 10.1038/nrmicro1066. [DOI] [PubMed] [Google Scholar]
- 23.Wagner M, Gutermann A, Podlech J, Reddehase MJ, Koszinowski UH. Major histocompatibility complex class I allele-specific cooperative and competitive interactions between immune evasion proteins of cytomegalovirus. J Exp Med. 2002;196:805–816. doi: 10.1084/jem.20020811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinto AK, Munks MW, Koszinowski UH, Hill AB. Coordinated function of murine cytomegalovirus genes completely inhibits CTL lysis. J Immunol. 2006;177:3225–3234. doi: 10.4049/jimmunol.177.5.3225. [DOI] [PubMed] [Google Scholar]
- 25.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 26.Smith HR, et al. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Natl Acad Sci U S A. 2002;99:8826–8831. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adam SG, et al. Cmv4, a new locus linked to the NK cell gene complex, controls innate resistance to cytomegalovirus in wild-derived mice. J Immunol. 2006;176:5478–5485. doi: 10.4049/jimmunol.176.9.5478. [DOI] [PubMed] [Google Scholar]
- 28.Desrosiers MP, et al. Epistasis between mouse Klra and major histocompatibility complex class I loci is associated with a new mechanism of natural killer cell-mediated innate resistance to cytomegalovirus infection. Nat Genet. 2005;37:593–599. doi: 10.1038/ng1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kielczewska A, et al. Ly49P recognition of cytomegalovirus-infected cells expressing H2-Dk and CMV-encoded m04 correlates with the NK cell antiviral response. J Exp Med. 2009;206:515–523. doi: 10.1084/jem.20080954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daniels KA, et al. Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to Ly49H. J Exp Med. 2001;194:29–44. doi: 10.1084/jem.194.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dokun AO, et al. Specific and nonspecific NK cell activation during virus infection. Nat Immunol. 2001;2:951–956. doi: 10.1038/ni714. [DOI] [PubMed] [Google Scholar]
- 32.Hanke T, et al. Direct assessment of MHC class I binding by seven Ly49 inhibitory NK cell receptors. Immunity. 1999;11:67–77. doi: 10.1016/s1074-7613(00)80082-5. [DOI] [PubMed] [Google Scholar]
- 33.Merck E, Voyle RB, MacDonald HR. Ly49D engagement on T lymphocytes induces TCR-independent activation and CD8 effector functions that control tumor growth. J Immunol. 2009;182:183–192. doi: 10.4049/jimmunol.182.1.183. [DOI] [PubMed] [Google Scholar]
- 34.Orr MT, et al. Ly49H signaling through DAP10 is essential for optimal natural killer cell responses to mouse cytomegalovirus infection. J Exp Med. 2009;206:807–817. doi: 10.1084/jem.20090168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koszinowski UH, Del Val M, Reddehase MJ. Cellular and molecular basis of the protective immune response to cytomegalovirus infection. Curr Top Microbiol Immunol. 1990;154:189–220. doi: 10.1007/978-3-642-74980-3_8. [DOI] [PubMed] [Google Scholar]
- 36.Tay CH, Welsh RM, Brutkiewicz RR. NK cell response to viral infections in beta 2-microglobulin-deficient mice. J Immunol. 1995;154:780–789. [PubMed] [Google Scholar]
- 37.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ziegler H, et al. A mouse cytomegalovirus glycoprotein retains MHC class I complexes in the ERGIC/cis-Golgi compartments. Immunity. 1997;6:57–66. doi: 10.1016/s1074-7613(00)80242-3. [DOI] [PubMed] [Google Scholar]
- 39.Reusch U, et al. A cytomegalovirus glycoprotein re-routes MHC class I complexes to lysosomes for degradation. EMBO J. 1999;18:1081–1091. doi: 10.1093/emboj/18.4.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tripathy SK, Smith HR, Holroyd EA, Pingel JT, Yokoyama WM. Expression of m157, a murine cytomegalovirus-encoded putative major histocompatibility class I (MHC-I)-like protein, is independent of viral regulation of host MHC-I. J Virol. 2006;80:545–550. doi: 10.1128/JVI.80.1.545-550.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu MF, Raulet DH. Class I-deficient hemopoietic cells and nonhemopoietic cells dominantly induce unresponsiveness of natural killer cells to class I-deficient bone marrow cell grafts. J Immunol. 1997;158:1628–1633. [PubMed] [Google Scholar]
- 42.Sun JC, Lanier LL. Cutting edge: viral infection breaks NK cell tolerance to “missing self”. J Immunol. 2008;181:7453–7457. doi: 10.4049/jimmunol.181.11.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun JC, Lanier LL. Tolerance of NK cells encountering their viral ligand during development. J Exp Med. 2008;205:1819–1828. doi: 10.1084/jem.20072448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tripathy SK, et al. Continuous engagement of a self-specific activation receptor induces NK cell tolerance. J Exp Med. 2008;205:1829–1841. doi: 10.1084/jem.20072446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Velardi A. Role of KIRs and KIR ligands in hematopoietic transplantation. Curr Opin Immunol. 2008;20:581–587. doi: 10.1016/j.coi.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Clausen J, et al. Impact of natural killer cell dose and donor killer-cell immunoglobulin-like receptor (KIR) genotype on outcome following human leucocyte antigen-identical haematopoietic stem cell transplantation. Clin Exp Immunol. 2007;148:520–528. doi: 10.1111/j.1365-2249.2007.03360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsu KC, et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105:4878–4884. doi: 10.1182/blood-2004-12-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller JS, et al. Missing KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood. 2007;109:5058–5061. doi: 10.1182/blood-2007-01-065383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sobecks RM, et al. Survival of AML patients receiving HLA-matched sibling donor allogeneic bone marrow transplantation correlates with HLA-Cw ligand groups for killer immunoglobulin-like receptors. Bone Marrow Transplant. 2007;39:417–424. doi: 10.1038/sj.bmt.1705609. [DOI] [PubMed] [Google Scholar]
- 50.Yu J, et al. Breaking tolerance to self, circulating natural killer cells expressing inhibitory KIR for non-self HLA exhibit effector function after T cell-depleted allogeneic hematopoietic cell transplantation. Blood. 2009;113:3875–3884. doi: 10.1182/blood-2008-09-177055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.