Abstract
The increasing prevalence of metabolic syndrome (MS) with age in older men has been linked with decreasing testosterone levels. Interestingly, while testosterone levels decline with age, estradiol (E2) levels remain relatively stable resulting in a decreased testosterone/estradiol ratio. Because E2 levels tend to be elevated in morbid obesity, insulin resistance and diabetes, it is reasonable to hypothesize that high E2 levels are associated with MS in older men.
We studied the relationship of total and free E2 with MS after adjustment for multiple confounders including age, BMI, smoking, alcohol consumption, physical activity, interleukin-6 (IL-6), fasting insulin and testosterone.
452 men 65 yr or older (age range 65–96) had complete data on estradiol, testosterone, fasting insulin, sex hormone binding globulin, interleukin-6 (IL-6), and albumin. Concentrations of free estradiol and free testosterone were calculated using the mass action equations. MS was defined according to ATPIII criteria.
Participants with MS had significantly higher serum free and total E2 (p<.001) (p=0.003). After adjusting for confounders, including age, smoking, alcohol consumption, physical activity, log (IL-6), log (insulin), participants with higher log (total E2) (OR: 2.31, 95 % CI 1.39–4.70, p=0.02) and higher log (free E2) (OR: 2.69, 1.38–5.24, p<0.001) had an increased risk of having MS. Log (free E2) (p=0.04) maintained significant correlation with MS even after further adjustment for BMI.
In older men high E2 is independently associated with MS. Whether confirmed in other studies, assessment of E2 should be also considered in older men. Whether changes in this hormonal pattern play a role in the development of MS should be further tested in longitudinal studies.
Keywords: estradiol, metabolic syndrome, older men
Introduction
The Metabolic syndrome (MS) is a cluster of metabolic alterations associated with high risk of developing diabetes mellitus and cardiac disease (1–3). The prevalence of MS increases with age and it is generally believed that its pathogenesis is multifactorial, especially in older persons (4–7). The age-related increasing prevalence of MS has been linked with the parallel decline in testosterone levels (8–11), perhaps due to increased conversion of testosterone into estradiol (E2) in obese individuals (12–13). It has been proposed that the relative increase of circulating levels of E2 derived from conversion of testosterone in the adipose tissue inhibits the hypothalamic-pituitary unit (13) resulting in a reduction of testosterone/estradiol ratio and leading to the vicious cycle of “obese estrogenic hypogonadism” (14–15). Previous studies have shown that E2 levels are 2 fold higher in obese men (16) and are positively associated with inflammation (17) and diabetes (18), suggesting that overproduction of E2 may be one of the missing links between obesity and coronary artery disease and stroke (19–21). However, to the best of our knowledge no previous study has tested the association between total and free E2 and MS in older men.
Aim of the Study
To test the hypothesis that E2 levels are higher in older men with MS versus those without MS.
Methods
Study Population
The InCHIANTI is an epidemiological study conducted on a representative sample of the population living in the Tuscany Region of Italy.
Overall 1260 persons (543 men and 726 women) aged 65 years and older were randomly selected from the population registry, and were eligible for the study. Of these, 1154 consented to participate in the InCHIANTI Study and 1055 donated a blood sample.
This analysis is limited to 459 male participants. Of these, 452 (83 % of the 534 men who donated blood sample) (age range 65–96) had complete data on estradiol, testosterone, SHBG, fasting insulin, interleukin-6 (IL-6), albumin and complete set of parameters for the diagnosis of MS (7). The Italian National Institute of Research and Care of Aging Institutional Review Board ratified the study protocol (22) and all participants received a full description of the study and consented to participate.
Definition of Metabolic Syndrome
In accordance with the National Cholesterol Education Program’s Adult Treatment Panel III (ATP-III) criteria, the diagnosis of MS was established as the presence of three or more of the following: fasting blood glucose levels ≥ 126 mg/dl, fasting serum triglycerides ≥ 150 mg/dl, serum HDL-Cholesterol <40 mg/dl, blood pressure ≥ 130/85 mmHg (or the use of anti-hypertensive medications) and waist circumference > 102 cm (1).
Components of Metabolic Syndrome
Waist circumference was measured at the midpoint between the lower rib margin and the iliac crest (normally umbilical level). Weight and height were measured using standard techniques. Body mass index (BMI) was calculated as weight (in kilograms) divided by height (in meters2). Baseline blood pressure was recorded using a standard mercury sphygmomanometer. All blood pressure measurements were performed with the participant in a supine position on three occasions separated by intervals of 2 minutes and the average of the last two measures was used in the analysis.
Blood Assays
Fasting blood samples were drawn between 7:00 and 8:00 AM and were stored at −80°C until analysis. Estradiol was measured by ultrasensitive RIA (DSL-4800 Diagnostic Systems Laboratories, Webster, TX) and in same batch with a minimum detectable concentration (MDC) of 2.2 pg/mL. Intra-assay coefficients of variation (CVs) and means for four different concentrations were 8.9 % (5.3 pg/mL), 6.5% (24.9 pg/mL), 7.6% (40.4 pg/mL) and 6.9% (92.6 pg/mL). The inter-assay CVs and correspondent means were 7.5 % (5.3 pg/mL), 9.7% (28.0 pg/mL), 8.0 % (42.3 pg/mL) and 12.2% (108.7 pg/mL), respectively. Total testosterone and dehydroepiandrosterone sulphate (DHEA-S) were assayed using commercial kits (Diagnostic Systems Laboratories, Webster, TX). For testosterone, MDC was 0.03 nmol/L; intra-assay and inter-assay CVs for 3 different concentrations were less than 9.6 and 9.1%, respectively. For DHEAS, MDC was 1.7 μg/dl; intra- and interassay CVs for three different concentrations ranged between 4.1 and 5.3% and between 4.6 and 7.0%, respectively. Sex hormone-binding globulin (SHBG) was measured by a radioimmunoassay (Diagnostic Products Corporation, Los Angeles, CA) with a MDC of 0.04 nmol/L and inter-assay and intra-assay CVs for 3 concentrations less than 6.9%, and 3.6%, respectively. Concentrations of free estradiol were calculated using the mass action equations described by Sodergard et al. (23). Concentration of free testosterone was calculated using the Vermeulen formula (24). Plasma insulin level was determined with a double-antibody, solid-phase radioimmunoassay (intra-assay CV= 3.1 + 0.3%; Sorin Biomedica, Milan, Italy). Cross-reactivity with human proinsulin was 0.3% (7). Serum glucose level was determined by using an enzymatic colorimetric assay (Roche Diagnostics, Mannheim, Germany) and a Roche-Hitachi 917 analyzer. Serum IL-6 was measured by high sensitivity ELISA (BIOSOURCE, Camarillo, California). A commercial enzymatic test was used to measure serum HDL cholesterol and triglycerides concentration (Roche Diagnostics). The inter-assay CV was less than 3.8% for HDL cholesterol and less than 2.5 % for triglycerides (10).
Assessment of Covariates
Information on physical activity was collected by a modified version of a standard questionnaire and coded as hours per week (25). Daily alcohol (g) intake was estimated by the European Prospective Investigation Into Cancer and Nutrition Food Frequency Questionnaire (26). Smoking was assessed by self-report and expressed as pack-years (packs smoked per day)*(years of smoking). Social demographic variables included educational level.
Statistical Analysis
Because of skewed distributions, log-transformed values for total and free E2, SHBG, IL-6, DHEAS, free testosterone and insulin were used in the analyses. Differences in hormonal levels and other parameters among participants with and without MS were tested using age-adjusted linear regression models and Mantel-Haenszel Chi-Square Tests, when appropriate. Free E2 levels were also divided into quartiles to better describe their relationship with MS. Differences in the prevalence of the metabolic syndrome according to specific free E2 quartiles were formally tested by Pearson chi-square tests.
A fully adjusted logistic regression analysis was used to test the hypothesis that higher free and total E2 level were associated with a significantly higher probability of having the MS, after adjusting for potential confounders (age, smoking, alcohol consumption, physical activity, log IL-6, log insulin) (model 1). To evaluate the association of E2 and metabolic syndrome independently of other hormones precursors of E2 and body composition measurements, we additionally adjusted logistic regression models for total testosterone, DHEAS, SHBG (model 2) and then for BMI (model 3). Logistic regression analysis was also used to test the relationship between log (total E2) and log (free E2) (predictors) and each component of MS (outcome) after adjusting for all the covariates used in Model 1.
The SAS 8.2 statistical package (SAS Institute Inc, Cary, North Carolina) was used for all analyses.
RESULTS
Characteristics of Study population according to MS
Table 1 shows the general characteristics of the study population, according to the presence or absence of the MS criteria. Overall, 73 participants (15.8 %) had 3 or more recognized features of MS. Participants with MS had higher fasting insulin levels and BMI. As expected, subjects with MS compared to those without MS had lower HDL cholesterol, higher triglycerides, higher glucose values, wider waist circumference. However, blood pressure did not significantly differ between the two groups (Table 1).
Table 1.
Characteristics of General Population According to Presence of Metabolic Syndrome.
| Criteria for the Metabolic Syndrome | |||
|---|---|---|---|
| <3 (N= 389) | ≥3 (N=73) | P value* | |
| Age (years) | 75 ± 7 | 74 ± 6 | 0.74 |
| Body Mass Index (kg/m2) | 26 ± 3 | 30 ± 3 | <0.001 |
| Interleukin–6 (pg/ml) median (IQR)# | 1.6 (1.7) | 1.3 (1.1) | 0.3 |
| Insulin (mIU/L) median (IQR)# | 9.4 (7.1) | 11.1(9.5) | <0.001 |
| Physical Activity (hours/week) | 1.9±0.5 | 1.89±0.4 | 0.55 |
| Smoking (packs/year) | 24±24 | 30±28 | 0.06 |
| Alcohol intake (g/day) | 24 ± 19 | 22±20 | 0.32 |
| Formal education (years) | 6±4 | 6±3 | 0.32 |
| Components of Metabolic Syndrome | |||
| HDL Cholesterol (mg/dL) | 53 ± 13 | 39 ± 8 | <0.001 |
| Triglycerides, mg/dL, mean ± SD | 115 ± 57 | 208± 101 | <.01 |
| Blood Glucose (mg/dL) | 94 ± 25 | 115 ± 34 | 0.04 |
| Waist Circumference (cm) | 93 ± 8.3 | 103.4 ± 8.2 | <0.001 |
| Waist Circumference >102 cm N (%) | 41 (11) | 45 (63) | 0.45 |
| Blood Pressure >130/85 mmHg N (%) | 195 (51) | 64 (87) | 0.13 |
Values expressed as means ± standard deviations unless indicated otherwise.
IQR= interquartile range.
Differences in parameters among patients with and without MS, were tested using age-adjusted linear regression model and Mantel-Haenszel Chi-Square Test, when appropriate.
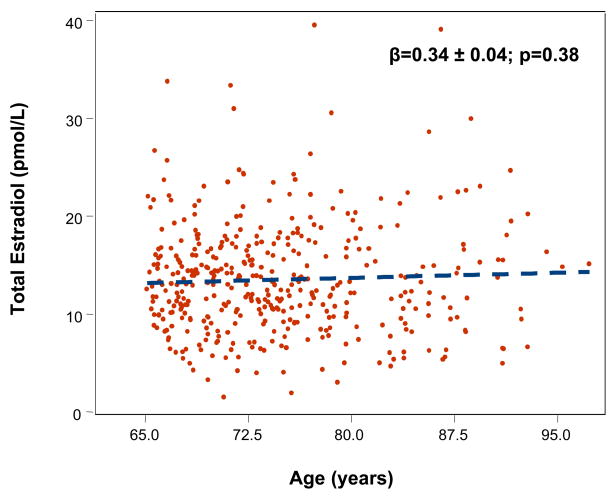
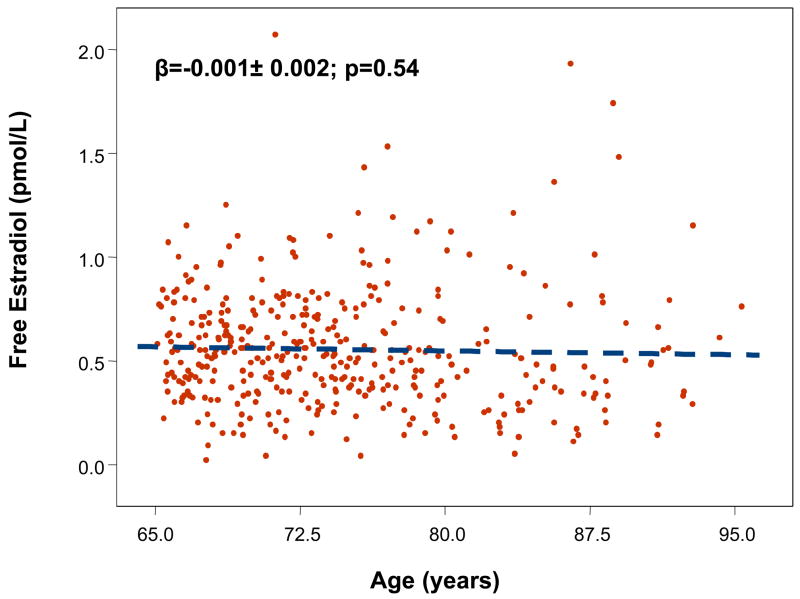
Mean age of the sample was 75 (range 65–96) years. Total and free E2 were not significantly affected by age (Fig. 1–2), while testosterone was significantly lower with older age (data not shown). There was no significant effect of age in participants with MS for both total E2 (β ± SE: −0.002 ± 0.11, p=0.9) and free E2 (−0.002 ±0.007, p=0.73). The age trend for total E2 (0.044 ±0.04, p=0.30) and free E2 (−0.01 ±0.002, p=0.62) were not statistically significant also in participants without MS.
Figure 1.
Relationship between total estradiol (pmol/L) and age (years).
Figure 2.
Relationship between free estradiol (pmol/L) and age (years).
Hormones according to presence of MS
Participants with MS had age-adjusted lower levels of total testosterone, but higher levels of total and free E2 (Table 2). In the age adjusted analysis, log (total E2) and log (Free E2) were significantly associated with MS (Table 3). After further adjustment for smoking, alcohol intake, physical activity, log (IL-6), log (insulin), the association between log (E2) and MS did not substantially change (Table 3). In the multivariate logistic regression model including all the above-mentioned confounders, BMI and log (DHEAS), total testosterone, log (SHBG), log (total E2) and log (free E2) remained significantly associated with MS, (P = 0.05) and (P=0.04) (Table 3).
Table 2.
Hormonal Parameters According to Presence of Metabolic Syndrome in Older Men.
| Criteria for the Metabolic Syndrome | |||
|---|---|---|---|
| Hormones | < 3 (N=389) | ≥3 (N=73) | P value* |
| Total Testosterone (ng/dL) means±SD | 433 ± 129 | 399 ± 139 | 0.03 |
| Free Testosterone (ng/dL) | 3.8 (2.2) | 4.2 (1.9) | 0.22 |
| Dehydroepiandrosterone sulphate (μg/dL) | 66.4 (66.5) | 71.1 (78.4) | .98 |
| Sex hormone binding globulin (nmoL/L) | 104.0 (62.2) | 83.6 (41.6) | <0.001 |
| Total Estradiol (pmol/L) | 12.3 (6.5) | 14.6 (6.8) | 0.003 |
| Free Estradiol (pmol/L) | 0.54 (0.27) | 0.72 (0.33) | <0.001 |
Values expressed as medians and interquartile range unless indicated otherwise.
Differences in parameters among patients with and without MS were tested using age-adjusted linear regression model.
Table 3.
Logistic Regression Models Evaluating The Relationship Between Circulating Levels of Estradiol (Total and Free) (Predictors) and Metabolic Syndrome (Outcome) in Older Men.
| Variable | O.R. [95%CI] | P value* |
|---|---|---|
| Age-adjusted Log (Total Estradiol) (pmol/L) | 2.69 [1.38–5.24] | 0.004 |
| Age-adjusted Log (Free Estradiol) (pmol/L) | 3.35 [1.84–6.07] | <.0001 |
| Model 1* | ||
| Log (Total Estradiol) (pmol/L) | 2.31 [1.39–4.70] | 0.02 |
| Log (Free Estradiol) (pmol/L) | 2.69 [1.38–5.24] | <0.001 |
| Model 2 (Model 1+BMI + log (SHBG)+ total testosterone +log (DHEAS)) | ||
| Log (Total Estradiol) (pmol/L) | 2.61 [0.96–6.85] | 0.053 |
| Log (Free Estradiol) (pmol/L) | 2.59 [1.01–6.66] | 0.048 |
| Model 3 (Model 1+ BMI) | ||
| Log (total Estradiol) (pmol/L) | 1.79 [0.77–4.09] | 0.18 |
| Log (Free Estradiol) (pmol/L) | 2.11 [1.03–4.33] | 0.041 |
Model 1: each line refers the results of a separate model adjusted for age, smoking, alcohol, physical activity, log (IL-6) and log (Insulin).
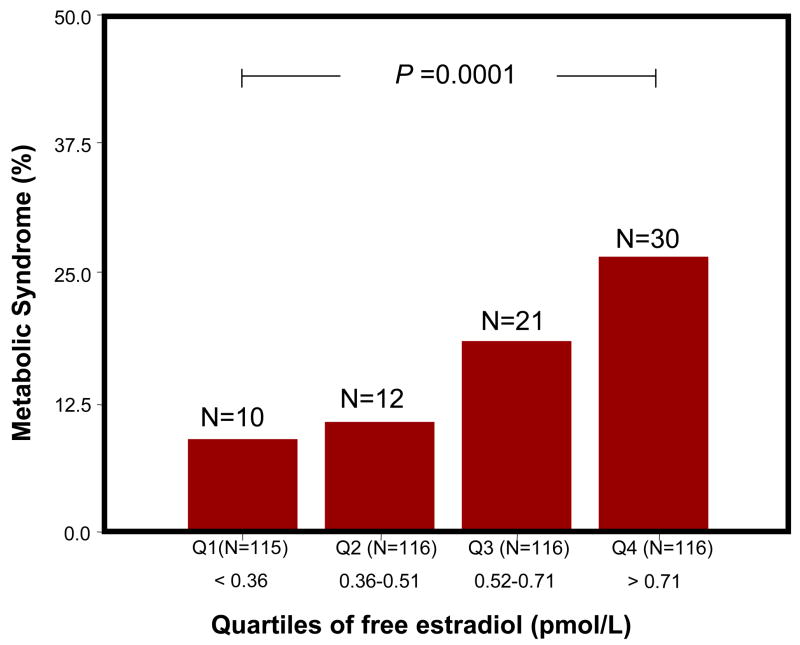
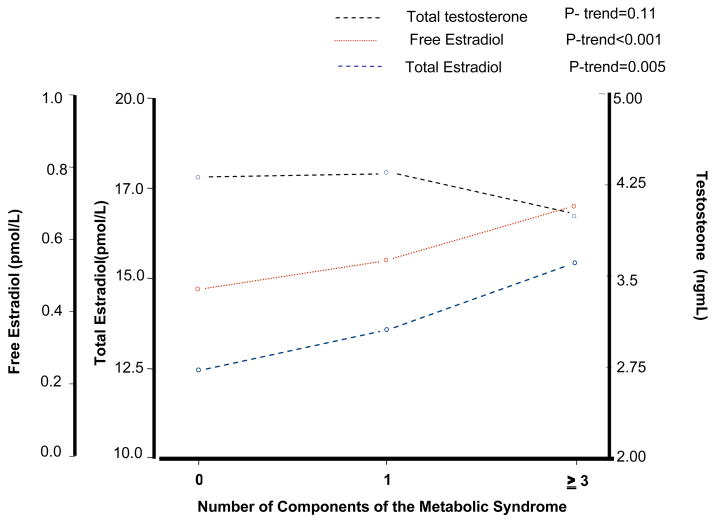
Figure 3 shows the number and percentage of participants with and without MS according to free E2 quartiles. The percentage of participants with MS was progressively and significantly higher across quartiles of free E2 (P for trend <0.001). Similar results were found with total E2 (P for trend =0.002) (data not shown). Figure 4 shows age adjusted levels of sex hormones for categories of number of ATP-III MS criteria (0, 1 and 3 or more). Interestingly, total and free E2 increased with increasing number of MS criteria mirroring changes in testosterone.
Figure 3.
Free Estradiol and Metabolic Syndrome. Percent distribution of the participants (vertical axis) with metabolic syndrome (red bars) according to quartiles of free estradiol (horizontal axis). The number of participants with metabolic syndrome according to quartiles of free estradiol are indicated in parentheses above the bar graphs.
Figure 4.
Age adjusted means of sex hormones according to number of ATP III criteria for the metabolic syndrome.
Free and total Estradiol and components of MS
In the age-adjusted analysis, log (total E2) and log (free E2) were positively associated with triglycerides (OR: 2.11, 95 % CI 1.24–3.60, p=0.006 and OR: 2.63, 95 % CI 1.64–4.21, p<0.0001), hypertension (OR: 1.64., 95 % CI 1.04–2.57, p=0.03 and OR: 1.51, 95 % CI 1.05–2.19, p=0.02) and waist circumference (OR: 1.93., 95 % CI 1.03–3.64, p=0.04 and OR: 2.24, 95 % CI 1.29–3.87, p=0.004). In the age-adjusted analysis, log (total E2) and log (free E2) were not associated with HDL-cholesterol (OR: 0.93., 95 % CI 0.52–1.67, p=0.81 and OR: 0.79, 95 % CI 0.49–1.27, p=0.33) and fasting glucose (OR: 0.79, 95 % CI 0.44–1.41, p=0.41 and OR: 0.79, 95 % CI 0.49–1.27, p=0.33).
After adjustment for age, total E2 levels were significantly and positively associated with BMI (Age-adjusted: b: 0.20, SE: 0.088; p=0.0225).
In addition total and free E2 levels were significantly higher in obese than non obese men. Total E2 levels were 9.2 ± 6.2 pmol/L (mean±SD) in non obese and 10.1 ± 6.2 pmol/L (mean±SD) in obese older men (age-adjusted p value=0.0479). Free E2 levels were 0.37±0.43 pmol/L (mean±SD) in non obese and 0.43± 0.32 pmol/L (mean±SD) in obese older men (age-adjusted p value=0.042). In the fully-adjusted analysis, log (total E2) and log (free E2) were still positively associated with triglycerides (OR: 1.77, 95 % CI 1.02–3.07, p=0.04 and OR: 2.33, 95 % CI 1.43–3.78, p=0.0007); log (free E2) (OR: 2.00, 95 % CI 1.12–3.58, p=0.02) but not log (total E2) (OR: 1.65, 95 % CI 0.84–3.22, p=0.14) was still associated with waist circumference, and both log (total E2) and log (free E2) were almost associated with hypertension (OR: 1.55, 95 % CI 0.96–2.50, p=0.07 and OR: 1.43, 95 % CI 0.97–2.11, p=0.07). Both log (total E2) and log (free E2) were not associated with HDL-cholesterol (p=0.81; p=0.33) and fasting glucose (p=0.17; p=0.25) in the multivariate analysis.
DISCUSSION
In a representative sample of older Italian men, we found a positive independent relationship of total and free E2 with MS.
Estradiol and age
In contrast to some studies (14, 27–31), but in accordance with others (32–33) both total and free fractions of E2 did not significantly change with age irrespective of MS.
Estradiol and presence of MS
Since this is the first study to our knowledge to evaluate the relationship between E2 and MS in a population of older men, our results cannot be compared with other studies in the literature. In contrast to our study, Muller et al failed to detect a significant association between E2 and MS in adult men, although E2 levels were positively associated with central obesity and triglycerides. However, the younger age of the study population (mean age=60 years) and lack of information on the free fraction of E2 makes that study hardly comparable with our study (8). Kiel et al found strong relationships of total and free E2 with total and HDL-cholesterol. However, fasting insulin, alcohol consumption and physical activity (all factors known to influence E2 levels) were not included as confounders in their multivariate analysis (35).
The role of E2 in men and its influence on features of MS are still unclear. Conditions of estrogen deficiency, such as congenital aromatase deficiency, are associated with impaired glucose and lipid metabolism (36). On the contrary, in accordance with other studies we found that obese have higher total and free E2 levels than non obese men (16). The discrepancy between these two different conditions remains unclear.
Estradiol and presence of MS: the role of BMI
Total E2 levels were significantly and positively associated with BMI, independent of age. After adjusting for all confounders including BMI, a rough measure of obesity and body composition, free E2 was still associated with MS, suggesting that relationship between E2 and MS is only partially explained by BMI and by increased conversion from testosterone via aromatization in adipose tissue. In previous studies low total testosterone was associated or was a predictor of MS in men (8–11). Whether low testosterone levels are linked to increased conversion to E2 levels in older men with MS it is not clear, although it has been shown that E2 may operate in reducing testosterone levels through a negative feedback at the hypothalamic and pituitary levels (13).
The relative condition of hypogonadotropic hypogonadism of obese male subjects was reported several years ago and confirmed recently in large studies (37–38).
Estradiol and presence of MS: the role of the androgens
In the final model including all the covariates and the hormones, such as DHEAS, testosterone and SHBG, the association between total or free E2 and MS was still significant, suggesting that the role of E2 in MS is not accounted by the biological effects of its precursors.
Free and total Estradiol and components of MS
One of the most interesting findings is the association with increasing number of criteria for metabolic syndrome. This association suggests a dose response effect of E2 on MetS. In the adjusted analysis, total and free E2 levels were associated with three components of MS, namely hypertension, triglycerides and waist circumference, which may also explain the mechanisms by which E2 may have an impact on diabetes and cardiovascular diseases (18–20). In addition in this older male population we found a positive association between E2 and IL-6 (data not shown) which can explain another factor mediating the relationship between E2 and MetS. E2 was found to be an independent predictor of the progression of carotid intimae-media thickness in middle-aged men (39). Moreover, in older men in the Honolulu-Asia Aging Study with a negative history for coronary artery disease and cancer, E2 level, but not testosterone or SHBG, was a strong predictor of stroke (21). However the presence of MS was not investigated in any of these studies.
Limitations and Strengths of the Study
Our study has limitations. The main limitation of our study is the cross-sectional design, which does not allow any inference on the causal role of E2 on MS. A mechanism of reverse causality, i.e. that MS, classically associated with central obesity and inflammatory status, positively influences E2 levels cannot be excluded. In this regard there is evidence that inflammatory cytokines, also produced in adipose tissue, stimulate aromatase expression (40). Secondly, we did not directly measure free estradiol, instead we estimated its levels from calculations (20), and lacked information on E2 receptors which may have provided more details on the E2 pathway (32).
Estradiol assays were performed 8 years later after blood drawing in stored plasma samples and more recently than Testosterone, SHBG and DHEAS. However, the samples used to measure E2 were maintained at −80°C and never thawed before the analysis. Also even if some decoy of the E2 molecule observed, it is likely that the relative values between individuals maintain the same rank order. Therefore we believe that in spite of this limitation, our findings maintain their validity.
However, these limitations are offset by important strengths. This is the first study to evaluate the relationship between E2, including the free fraction, and MS in a large representative sample of older men with complete information on ATP III criteria for MS; the participants were screened for multiple confounders, such as inflammatory markers, smoking, physical activity and alcohol intake and fasting insulin levels. Furthermore, we evaluated E2 with ultrasensitive methods. Although weakened by the cross-sectional nature, the results of this study have important clinical and conceptual implications.
Clinical implications
Since previous epidemiologic studies in older men focused on testosterone rather than estradiol, we suggest that further studies should test the relationship between this parameter and MS in older men. Given the opposite relationship of testosterone and E2 with MS, future studies should also look at the role of testosterone/estradiol ratio in MS. The findings, once confirmed in other populations, raise the possibility that aromatase inhibitor therapy might be a good therapeutic option in older male participants affected by MS.
In older men, high E2 levels are associated with MS, independent of potential confounders. Whether changes in this hormonal pattern play a role in the development of MS should be further tested in longitudinal studies.
Acknowledgments
Financial Disclosure: The InCHIANTI Study was supported as a “targeted project” (ICS 110.1/RS97.71) by the Italian Ministry of Health and in part by the U.S. National Institute on Aging (Contracts N01-AG-916413 and N01-AG-821336) and by the Intramural Research Program of the U.S. National Institute on Aging (Contracts 263 MD 9164 13 and 263 MD 821336).
Sponsor’s Role: The funding institutes had no role in the design, methods, subject recruitment, data collection, analysis, and preparation of manuscript or in the decision to submit the manuscript for publication.
Footnotes
Conflict of interests: The authors declare that they have no conflict of interest to disclose concerning this manuscript.
References
- 1.Executive Summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;12:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 3.Scuteri A, Najjar SS, Muller DC, Andres R, Hougaku H, Metter EJ, Lakatta E. Metabolic syndrome amplifies the age-associated increases in vascular thickness and stiffness. J Am Coll Cardiol. 2004;43:1388–1395. doi: 10.1016/j.jacc.2003.10.061. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: Findings from the third national health and nutrition examination survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 5.Noale M, Maggi S, Marzari C, Limongi F, Gallina P, Bianchi D, Crepaldi G ILSA Working Group. Components of the metabolic syndrome and incidence of diabetes in elderly Italians: the Italian Longitudinal Study on Aging. Atherosclerosis. 2006;187:385–392. doi: 10.1016/j.atherosclerosis.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Morley JE. The metabolic syndrome and aging. J Gerontol A Biol Sci Med Sci. 2004;59:139–142. doi: 10.1093/gerona/59.2.m139. [DOI] [PubMed] [Google Scholar]
- 7.Maggi S, Noale M, Gallina P, Bianchi D, Marzari C, Limongi F, Crepaldi G ILSA Working Group. Metabolic syndrome, diabetes, and cardiovascular disease in an elderly Caucasian cohort: the Italian Longitudinal Study on Aging. J Gerontol A Biol Sci Med Sci. 2006;61:505–510. doi: 10.1093/gerona/61.5.505. [DOI] [PubMed] [Google Scholar]
- 8.Muller M, Grobbee DE, den Tonkelaar I, Lamberts SW, van der Schouw YT. Endogenous sex hormones and metabolic syndrome in aging men. J Clin Endocrinol Metab. 2005;90:2618–2623. doi: 10.1210/jc.2004-1158. [DOI] [PubMed] [Google Scholar]
- 9.Laaksonen DE, Niskanen L, Punnonen K, Nyyssönen K, Tuomainen TP, Valkonen VP, Salonen R, Salonen JT. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27:1036–1041. doi: 10.2337/diacare.27.5.1036. [DOI] [PubMed] [Google Scholar]
- 10.Maggio M, Lauretani F, Ceda GP, Bandinelli S, Basaria S, Ble A, Egan J, Paolisso G, Najjar S, Jeffrey Metter E, Valenti G, Guralnik JM, Ferrucci L. Association between hormones and metabolic syndrome in older Italian men. J Am Geriatr Soc. 2006;54:1832–1838. doi: 10.1111/j.1532-5415.2006.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez A, Muller DC, Metter EJ, Maggio M, Harman SM, Blackman MR, Andres R. Aging, androgens and the metabolic syndrome in a longitudinal study of aging. J Clin Endocrin Metab. 2007;92:3568–3572. doi: 10.1210/jc.2006-2764. [DOI] [PubMed] [Google Scholar]
- 12.Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, Amarneh B, Ito Y, Fisher CR, Michael MD, Mendelson CR, Bulun SE. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocrin Rev. 1994;15:342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- 13.Hayes FJ, Seminara SB, Decruz S, Boepple PA, Crowley WF., Jr Aromatase inhibition in the human male reveals a hypothalamic site of estrogen feedback. J Clin Endocrinol Metab. 2000;85:3027–3035. doi: 10.1210/jcem.85.9.6795. [DOI] [PubMed] [Google Scholar]
- 14.Vermeulen A, Kaufman JM, Goemaere S, van Pottelberg I. Estradiol in elderly men. Aging Male. 2002;5:98–102. [PubMed] [Google Scholar]
- 15.Cohen P. Obesity in men: The hypogonadal-estrogen receptor relationship and its effect on glucose homeostasis. Med Hypotheses. 2008;70:358–360. doi: 10.1016/j.mehy.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 16.Schneider G, Kirschner MA, Berkowitz R, Ertel NH. Increased estrogen production in obese men. J Clin Endocrinol Metab. 1979;48:633–638. doi: 10.1210/jcem-48-4-633. [DOI] [PubMed] [Google Scholar]
- 17.Nakhai Pour HR, Grobbee DE, Muller M, van der Schouw YT. Association of endogenous sex hormone with C-reactive protein levels in middle-aged and elderly men. Clinical Endocrinology. 2007;66:394–398. doi: 10.1111/j.1365-2265.2007.02745.x. [DOI] [PubMed] [Google Scholar]
- 18.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes. A systematic review and meta-analysis. JAMA. 2006;295:1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 19.Phillips GB, Pinkernell BH, Jing TY. The association of hyperestrogenemia with coronary thrombosis in men. Arterioscler Thromb Vasc Biol. 1996;16:1383–1387. doi: 10.1161/01.atv.16.11.1383. [DOI] [PubMed] [Google Scholar]
- 20.Barrett-Connor E, Khaw KT. Endogenous sex hormones and cardiovascular disease in men: a prospective population based study. Circulation. 1988;78:539–545. doi: 10.1161/01.cir.78.3.539. [DOI] [PubMed] [Google Scholar]
- 21.Abbott RD, Launer LJ, Rodriguez BL, Ross GW, Wilson PW, Masaki KH, Strozyk D, Curb JD, Yano K, Popper JS, Petrovitch H. Serum estradiol and risk of stroke in elderly men. Neurology. 2007;68:563–568. doi: 10.1212/01.wnl.0000254473.88647.ca. [DOI] [PubMed] [Google Scholar]
- 22.Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, Guralnik JM. Subsystems contributing to the decline in ability to walk: Bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 23.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 24.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 25.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr, Montoye HJ, Sallis JF, Paffenbarger RS., Jr Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25 (1):71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol. 1997;26:S152–S160. doi: 10.1093/ije/26.suppl_1.s152. [DOI] [PubMed] [Google Scholar]
- 27.Bjornerem A, Straume B, Midtby M, Fønnebø V, Sundsfjord J, Svartberg J, Acharya G, Oian P, Berntsen GK. Endogenous sex hormones in relation to age, sex, lifestyle factors, and chronic diseases in a general population: the Tromso Study. J Clin Endocrinol Metab. 2004;89:6039–6047. doi: 10.1210/jc.2004-0735. [DOI] [PubMed] [Google Scholar]
- 28.Simon D, Preziosi P, Barrett-Connor E, Roger M, Saint-Paul M, Nahoul K, Papoz L. The influence of aging on plasma sex hormones in men: the Telecom study. Am J Epidemiol. 1992;135:783–791. doi: 10.1093/oxfordjournals.aje.a116365. [DOI] [PubMed] [Google Scholar]
- 29.Ferrini R, Barrett-Connor E. Sex hormones and age: a cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. Am J Epidemiol. 1998;147:750–754. doi: 10.1093/oxfordjournals.aje.a009519. [DOI] [PubMed] [Google Scholar]
- 30.Van den Beld AW, de Jong FH, Grobbee DE, Pols HA, Lamberts SW. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab. 2000;85:3276–3282. doi: 10.1210/jcem.85.9.6825. [DOI] [PubMed] [Google Scholar]
- 31.Orwoll E, Lambert LC, Marshall L, Phipps K, Blank J, Barrett-Connor E, Cauley J, Ensrud K, Cummings S. Testosterone and estradiol among older men. J Clin Endocrinol Metab. 2006;91:1336–1344. doi: 10.1210/jc.2005-1830. [DOI] [PubMed] [Google Scholar]
- 32.Muller M, den Tonkelaar I, Thijssen JH, Grobbee DE, van der Schouw YT. Endogenous sex hormones in men aged 40–80 years. Eur J Endocrinol. 2003;149:583–589. doi: 10.1530/eje.0.1490583. [DOI] [PubMed] [Google Scholar]
- 33.Belanger A, Candas B, Dupont A, Cusan L, Diamond P, Gomez JL, Labrie F. Changes in serum concentrations of conjugated and unconjugated steroids in 40- to 80-year-old men. J Clin Endocrinol Metab. 1994;79:1086–1090. doi: 10.1210/jcem.79.4.7962278. [DOI] [PubMed] [Google Scholar]
- 34.Gray A, Feldman HA, McKinlay JB, Longcope C. Age, disease and changing sex hormone levels in middle-aged men: results of the Massachusetts male aging study. J Clin Endocrinol Metab. 1991;73:1016–1025. doi: 10.1210/jcem-73-5-1016. [DOI] [PubMed] [Google Scholar]
- 35.Kiel DP, Baron JA, Plymate SR, Chute CG. Sex hormones and lipoproteins in men. Am J Med. 1989;87:35–39. doi: 10.1016/s0002-9343(89)80480-2. [DOI] [PubMed] [Google Scholar]
- 36.Goodman-Gruen D, Barrett-Connor E. Sex differences in the association of endogenous sex hormone levels and glucose tolerance status in older men and women. Diabetes Care. 2000;23:912–918. doi: 10.2337/diacare.23.7.912. [DOI] [PubMed] [Google Scholar]
- 37.Vermeulen A, Kaufman JM, Deslypere JP, Thomas G. Attenuated luteinizing hormone (LH) pulse amplitude but normal LH pulse frequency, and its relation to plasma androgens in hypogonadism of obese men. J Clin Endocrinol Metab. 1993;76:1140–1146. doi: 10.1210/jcem.76.5.8496304. [DOI] [PubMed] [Google Scholar]
- 38.Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, O’Neill TW, Bartfai G, Casanueva F, Forti G, Giwercman A, Huhtaniemi IT, Kula K, Punab M, Boonen S, Vanderschueren D European Male Aging Study Group. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93:2737–2745. doi: 10.1210/jc.2007-1972. [DOI] [PubMed] [Google Scholar]
- 39.Muller M, van den Beld AW, Bots ML, Grobbee DE, Lamberts SW, van der Schouw YT. Endogenous sex hormones and progression of carotid atherosclerosis in elderly men. Circulation. 2004;109:2074–2079. doi: 10.1161/01.CIR.0000125854.51637.06. [DOI] [PubMed] [Google Scholar]
- 40.Zhao Y, Nichols JE, Valdez R, Mendelson CR, Simpson ER. Tumor necrosis factor-alpha stimulates aromatase gene expression in human adipose stromal cells through use of an activating protein-1 binding site upstream of promoter 1.4. Mol Endocrinol. 1996;10:1350–1357. doi: 10.1210/mend.10.11.8923461. [DOI] [PubMed] [Google Scholar]