Abstract
The default mode network (DMN) is a set of functionally connected brain regions which shows deactivation (task induced deactivation, TID) during a cognitive task. Evidence shows an age-related decline in task-load-related modulation of the activity within the DMN during cognitive tasks. However, the effect of age on the functional coupling within the DMN and their relation to cognitive performance has hitherto been unexplored. Using functional magnetic resonance imaging, we investigated functional connectivity within the DMN in older and younger subjects during a working memory task with increasing task load. Older adults showed decreased connectivity and ability to suppress low frequency oscillations of the DMN. Additionally, the strength of the functional coupling of posterior cingulate (pCC) with medial prefrontal cortex (PFC) correlated positively with performance and was lower in older adults. pCC was also negatively coupled with task-related regions, namely the dorsolateral PFC and cingulate regions. Our results show that in addition to changes in canonical task-related brain regions, normal aging is also associated with alterations in the activity and connectivity of brain regions within the DMN. These changes may be a reflection of a deficit in cognitive control associated with advancing age that results in deficient resource allocation to the task at hand.
Keywords: functional magnetic resonance imaging, aging, default mode, connectivity, working memory
1. INTRODUCTION
Functional neuroimaging studies have traditionally investigated brain functioning by studying task-dependent increases in neural activity, typically called “activation”. Recently, much interest has been focused on task-induced deactivation (TID). TID is a decrease in brain activity that occurs during the performance of an experimental task relative to rest (Raichle, et al. 2001) or a low demanding baseline condition (Greicius and Menon 2004). One set of regions showing TID are thought to reflect a task-negative network, the so-called “default mode of brain function network” (DMN) that is active during rest and suppressed during an active task (Mazoyer, et al. 2001; Raichle, et al. 2001). The strong anticorrelation of the task-positive and the task-negative networks (Fransson 2005; 2006), suggests antagonistic psychological functions on these two systems (Fox, et al. 2005). Accumulating evidence indeed suggests a role of the DMN in “stimulus independent thoughts”, ranging from internal and external monitoring (Gusnard and Raichle 2001), to mind wandering (Mason, et al. 2007).
The brain regions that consistently show TID irrespective of the task being performed include midline areas such as the medial prefrontal cortex (mPFC), posterior cingulate cortex (pCC), precuneus, and bilateral inferior parietal cortex (IPC). This group of brain regions is consistent across the lifespan (Grady, et al. 2006). Nevertheless, numerous studies have consistently shown an age-related modulation of the spatial extent and the magnitude of TID within this network. Lustig et al. (2003) found decreased deactivation in older adults relative to younger subjects in both mPFC and pCC and parieto-temporal regions during a semantic classification task compared with rest. Older adults also showed a flattened and slowed time course of blood oxygen level-dependent (BOLD) fMRI response when compared to younger subjects. These results were further confirmed by Grady et al. (2006) who investigated a population spanning from 20 to 87 years and found an age-related linear increase in BOLD activity during a fixation baseline relative to a task in medial DMN regions, namely medial frontal gyrus, pCC as well as in bilateral temporal regions. Indeed, older adults showed an increase in activity of brain regions not traditionally recruited during the performance of the task such as the mPFC and pCC, whereas they showed decreased activation in task-relevant areas when compared to younger subjects. Persson et al. (2007) using a verb generation task showed greater deactivation, but only at levels of the task that required a higher selection demand. More interestingly within the DMN, posterior midline regions including pCC/precuneus show significantly smaller and slower deactivations in older adults when compared to their younger counterparts (Grady, et al. 2006; Lustig, et al. 2003). Furthermore, there is evidence that deactivation in these brain regions is even more severely reduced in patients with mild cognitive impairment (Rombouts, et al. 2005) and Alzheimer’s disease (AD; Greicius, et al. 2004; Lustig, et al. 2003).
Recent evidence also suggests that cognitive load can also modulate the degree of TID. McKiernan et al. (2003) demonstrated a linear increase in deactivation of the midline DMN regions with increasing task difficulty during an auditory detection task. Consistent with this, Tomasi et al. (2006) and Esposito et al. (2006) found a load-related general increase of TID in the DMN, during working memory (WM) tasks. These findings suggest reallocation of attentional resources from DMN to the brain regions actively involved in the ongoing tasks. Although, attentional impairment is a commonly reported age-related cognitive change (Gazzaley, et al. 2007; West 1999), studies on the effects of aging on load-related changes in DMN are relatively few and inconclusive. Gould et al. (2006) using a visuospatial paired associate learning task did not find any difference in deactivations with increasing task load between older and young subjects. Conversely, Persson et al. (2007) found an interaction of age-by-task-load in mPFC, pCC as well as in lateral parietal cortex, showing relatively decreased load-related deactivation in the older adults when compared to younger subjects.
The brain regions in the DMN show strong functional coupling (Fransson 2005; Greicius, et al. 2003) as revealed by synchronous low frequency oscillations (LFOs, <0.1 Hz) of BOLD signal at rest (Biswal, et al. 1995; Greicius, et al. 2003; Xiong, et al. 1999). Connectivity patterns tend to be consistent albeit attenuated during a cognitive task (Fransson 2006). Of further note, the strength of the functional coupling within DMN regions during a WM task correlates positively with performance on the task (Gilbert, et al. 2006; Hampson, et al. 2006), thus suggesting a facilitating (Gilbert, et al. 2006) or monitoring role of DMN regions. Thus far none of the studies that explored an age-by-task-load interaction looked at the functional coupling among the DMN brain regions or their relationship to cognitive performance.
In the current study, we explored age-related changes in the DMN focusing not only on changes in single brain regions but also on the functional coupling among these regions, and the impact of these changes on WM performance. In order to address both global and regional changes in functional connectivity we used a WM task with increasing difficulty and both univariate and multivariate statistical approaches. Independent component analysis (ICA) is a model-independent multivariate statistical analysis technique designed to extract spatially independent and temporally synchronous activity patterns in brain regions, thus yielding functional covariance in brain regions. On the other hand, the general linear model (GLM) analyses uses univariate statistics based on a priori defined task design and hemodynamic response function (HRF) as predictors of modulations in the BOLD signal. The combination of these two statistical techniques has already proven to be effective to explore different aspects of brain activity (Esposito, et al. 2006), for e.g. GLM analysis is more sensitive to detect functional specificity, whereas ICA is better suited to define functional connectivity. Based on evidence to date (vide supra), our hypotheses were that: first, older adults would show decreased extent of the deactivation network and an altered functional connectivity within the DMN regions when compared to young subjects. Second, an increase in task load would increase deactivation in DMN in both age groups, although to a smaller degree in older adults. Third, these differences in fMRI measures would predict differences in task performances.
2. METHODS
2.1. Subjects
Fifty-seven right-handed Caucasian subjects, 29 young subjects and 28 older adults participated in this study (Table 1). All subjects had normal or corrected to normal visual acuity. Handedness was assessed with the Edinburgh Questionnaire (Oldfield 1971). Exclusion criteria included past history or the presence of any medical, neurological or psychiatric disorders according to DSM-IV (following a Structured Clinical Interview, SCID-IV, First, et al. 1996), drug treatment (except birth control pills in young women and hormonal substitution therapy in postmenopausal women), past head trauma with loss of consciousness. Older subjects underwent a thorough neuropsychological assessment to evaluate cognitive status and exclude cognitive decline (see Table 2). All of them gave written informed consent to take part in the experiment, which was approved by the Intramural Review Board of the National Institute of Mental Health. As previously described by Rombouts et al. (2005), we classified the education level of the participants into three levels according to completed years of education: low (<12 years, primary school), middle (13–16 years, college/university), high (17–22 years, postgraduate).
Table 1.
Demographics
| Old | Young | Difference | |
|---|---|---|---|
| N | 28 | 29 | |
| Male:female ratio | 13:15 | 15:14 | n.s. |
| Age (M±S.D., years) | 64.2±8.3 | 27.5±5.1 | p<0.001 |
| Age range (years) | 55–90 | 21–35 | |
| Handedness (% right) | 100 | 100 | n.s |
| Education (M±S.D.) | 2.46±0.58 | 2.17±0.60 | n.s. |
Education level was determined on a discrete scale with three levels: low = 1, middle= 2, high = 3. n.s, non significant difference
Table 2.
Neuropsychological status of the older adults
| M (S.D.) | |
|---|---|
| Cognitive Status | |
| Mini-mental state examination (MMSE) | 30 |
| Clinical dementia rating (CDR) | 0 |
| Executive function | |
| Trail making test B (s) | 52.7 (10.2)a |
| Word fluency test (letters) | 50.0 (11.1)a |
| Category fluency test (animals) | 54.5 (8.8)a |
| Letter and number sequencing | 12.9 (2.6)b |
| WAIS picture completion | 11.5 (2.3) c |
| WAIS arithmetic | 11.9 (2.9) c |
| WAIS similarity | 14.4 (4.1) c |
| Memory | |
| WMS logical memory immediate recall | 12.9 (2.3)d |
| WMS logical memory delayed recall | 14.15 (2.2)e |
| Processing Speed | |
| Trail making test A (s) | 50.9 (12.3)a |
| WAIS digit symbol test | 16.0 (10.4) c |
This information was available on
23,
21,
27,
22,
20 subjects.
2.2. Procedure
2.2.1. Task
Subjects performed an n-back WM task as described elsewhere (Callicott, et al. 1999). Briefly, n-back refers to the number of previous stimuli that the subject had to recall. The stimuli presented in all the conditions consisted of numbers (1–4) shown in random sequence and displayed at the corners of a diamond-shaped box. A non-memory guided control condition (0-back) that required subjects to identify the stimulus currently seen, alternated with the WM condition. The WM condition required the recollection of a stimulus seen one stimulus (1-back) or two stimuli (2-back) previously while continuing to encode additionally incoming stimuli. Performance data were recorded as the number of correct responses (accuracy) and as reaction time.
The stimuli were arranged in a two-run block-design, and each run consisted of eight 30-second blocks: four blocks of control condition (0-back) alternated with four blocks of the WM condition (n=1 or n= 2). The order of the task combinations was counter-balanced across subjects. The 2-back reaction time data for one of the older adults were lost due to a computer glitch.
2.2.2. Image acquisition
Each subject was scanned on a GE Signa (Milwaukee, WI) 3T scanner. A gradient echo BOLD-EPI pulse sequence was used to acquire 120 images per run. Each functional image consisted of 24 6-mm-thick axial slices covering the entire cerebrum and most of the cerebellum (TR= 2000 ms; TE= 30 ms; field of view= 24 cm; flip angle= 90).
2.3. Data analysis
2.3.1. Demographics and behavioral data
Two sample t-tests were used to compare demographics, and behavioral data of continuous variables. Chi-square was used to compare categorical variables. Correlation analyses were performed with Pearson’s r test
2.3.2. Image Analysis
Following preprocessing (A) all the data were analyzed using both independent component analyses (B) and general linear model analysis approaches. ICA was carried out to study the functional covariance patterns in the spatial (B1) as well as temporal (B2) domain, identifying spatial components as well as estimated time courses for each task load. GLM analyses (C) were performed to confirm the spatial ICA results and compare TIDs as well as task related activations across different task loads between the older and younger adults. Additionally, functional connectivity analysis (D) was performed to assess functional coupling between regions within the DMN and its relationship to behavioral performance.
A. Preprocessing
Data were pre-processed and analyzed using Statistical Parametrical Mapping (SPM 2, Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk; Friston, et al. 1995; Worsley and Friston 1995) (see Supplementary materials for details).
B. Independent Component Analysis
A spatial ICA was performed using the Group ICA of fMRI Toolbox [GIFT; http://icatb.sourceforge.net (Calhoun, et al. 2001; Correa, et al. 2005)] (see Supplementary materials for details). The 20 individual ICs consisting of individual spatial independent maps and time courses were sorted using the two components that showed the greatest absolute spatial correlation with a canonical DMN map (1-back: Anterior r=0.20, posterior r=0.37; 2-back: anterior r=0.20, posterior r=0.28).
Each spatial voxel of the IC map was expressed as a t-statistic, which was converted to a z-statistic. Each subject’s components of interest (COI) for each task load were entered into SPM2 and analyzed using a second level random effects analyses. One-sample t-tests were used to calculate within group maps for each task load. ANOVAs, masked by the combination of the main effects maps of both age groups for each task load (p<0.001), were eventually used to compare the activations between age groups within each age load.
B.1. Spatial extent
To explore the spatial magnitude of COIs in specific brain regions within the DMN template in regions of interest (ROIs: mPFC, pCC) and a control region (anterior cingulate cortex, aCC) previously identified using WFU Pickatlas, we calculated the percent of activated voxels within the two selected DMN COIs for each task load and for both older and young subjects using MarsBar toolbox within SPM2 (Brett, et al. 2002) with a significance threshold of p<0.001. These data were eventually entered into a one-way factorial ANOVA (age-by-task load) for each ROI.
B.2. Power Spectral Density (PSD)
To explore the global time course of the deactivations, we computed the power spectral density (PSD) using the Welch method of spectral estimation (Welch 1967), available in the MATLAB Signal Processing Toolbox (Mathworks, Natick, MA), for the COI that showed age-related differences (see Supplementary materials for details). Each individual periodogram was divided into five frequency bins (0.03, 0.08, 013, 0.24, 0.3). The spectral density of each bin was entered into repeated measures ANOVA with task load as within subject factor and age group as between subject factor. In the bin that showed significant differences between the two age groups, we eventually performed a repeated measure ANOVA to compare the PSD change (difference between activation and baseline condition) between the two groups.
C. General Linear Model (GLM) analyses (see supplementary materials)
D. Functional connectivity (fcMRI)
We performed a functional connectivity (fcMRI) analysis as in previous reports (Andrews-Hanna, et al. 2007; Bertolino, et al. 2006). Because of our strong a priori evidence of the involvement of pCC region which was also confirmed by our ICA and GLM findings, we set the seed for the analysis on a 10mm-radius sphere centered on the coordinates of pCC published in previous studies (Bluhm, et al. 2007; Fransson 2005; Greicius, et al. 2003; McKiernan, et al. 2003).
The median of the time course of pCC activity was then extracted for all participants, mean-centered and used as a covariate in a subsequent single-subject analysis of covariance to identify voxels whose activity showed significant covariation, positive or negative, with pCC BOLD signal. To remove the confounding effects of other sources of variance on the connectivity measures, calculations were performed after applying a high pass filter (f=1/120 Hz) and covaring out the estimated effects of the following variables: 1) six head movement parameters (three translational and three rotational) calculated during realignment; 2) mean brain signal; 3) mean CSF signal computed from the lateral ventricles (anatomical ROI from WFU Pickatlas); 4) mean white matter signal estimated from two ROIs (two 5-mm-radius spheres located in the anterior part of corona radiata, centered on x=26, y=24, z=19, and x=−26, y=24, z=19, respectively); 5) two covariates, one for 0-back condition and the other for the WM condition (1-back or 2-back) to covary out the effect of task. Using SPM 5 software, effects at each voxel were estimated according to the general linear model, and regionally specific effects were computed by analysis of covariance identifying brain pCC functional connectivity for each subject separately. Fisher’s r to z’ transformation was applied to the correlation maps thus producing nearly normal distribution (Fisher 1921). Finally, we entered the subject-specific transformed connectivity maps into a second-level random effects analysis (ANOVA) to compare regional coupling differences with the pCC between the two age groups and across task loads.
Correlation analyses were then performed using simple regression with accuracy as covariate of interest and individual connectivity maps as random effects variables for both 1-back and 2-back tasks.
3. RESULTS
3.1. Behavioral performance
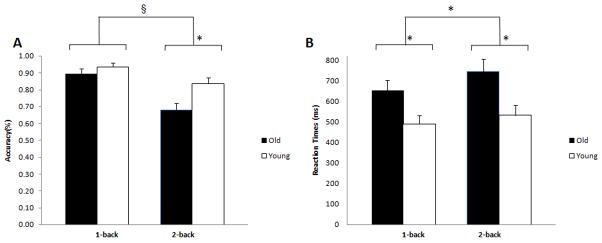
We found a significant effect of age on accuracy [Fig. 1A; F(1, 55)= 6.467, p=.01] with older adults performing worse relative to younger subjects, as well as an effect of task load [F(1, 55)= 42.867, p=.00001] with lower accuracy at higher task loads. There was a significant age-by-task-load interaction, with older adults showing a greater decline in performance with increasing task load [F(1, 55)= 5.702, p=.02]. While the performance was similar between the two groups at 1-back, older adults performed significantly worse than young subjects at 2-back [A; t(55)= 2.991, p=0.004].
Figure 1. Behavioral results. Older adults performed worse than younger subjects at higher task load (2-Back) (A), and were slower than younger subjects at both 1-back and 2-back (B).
Accuracy is indicated as mean % correct responses. Error bars indicate standard error of the mean. *, p<0.05; §, p<0.01.
Reaction times showed an age effect, older adults being slower than younger subjects [Fig 1B; F(1, 55)= 8.4641, p=0.005], as well as an effect of load [F(1, 55)= 5.880, p=.02], with longer reaction times at 2-back.
3.2. ICA
3.2.1. DMN independent components
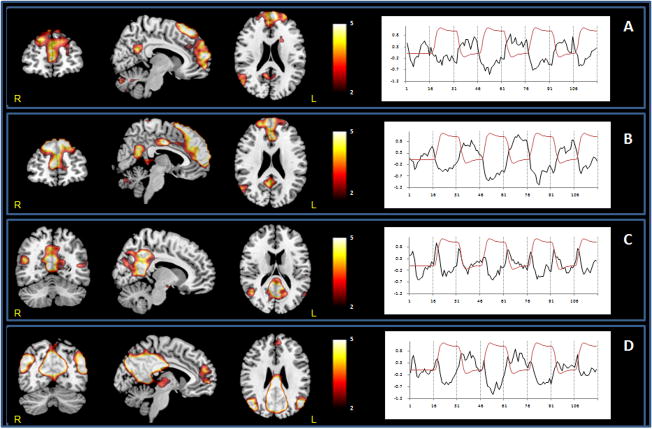
Two COIs that spanned across the DMN regions were identified for each task load (Fig. 2 and Fig. 3). The first COI showed regions that were more anterior (1-back-ANT, Fig. 2A and B; 2-back-ANT, Fig. 3A and B) and included the midline mPFC (BA9/10), aCC (BA24/32), precuneus, right middle and superior temporal gyrus and bilateral ventro lateral-PFC (BA45/47). The second COI showed regions that were more posterior (1-back-POST, Fig. 2C and D; 2-back-POST, Fig. 3C and D) and included pCC (BA31), precuneus (BA5/7), posterior parietal (BA7), BA10, bilateral posterior hippocampus (1-back only) and posterior parietal cortices. The one-sample t-test maps within each group showed a similar pattern of midline DMN regions in both older and young subjects for each task load.
Figure 2. Default mode network ICs at 1-back across groups. Two components were identified: 1-back-ANT (A, Old; B, Young) and 1-back-POST (C, Old; D, Young). The anterior component included mPFC, and right superior parietal regions, whereas the posterior component encompassed pCC, precunes and bilateral posterior parietal regions.
Coronal, sagittal and axial sections illllustrating the spatial pattern of DMN activity across groups. Sagittal and axial slices are overlaid on a T1 template. The color bar indicates T-values. Time courses represent the temporal profile of each component across groups (black) overlaid on the paradigm “box-car” design (red).
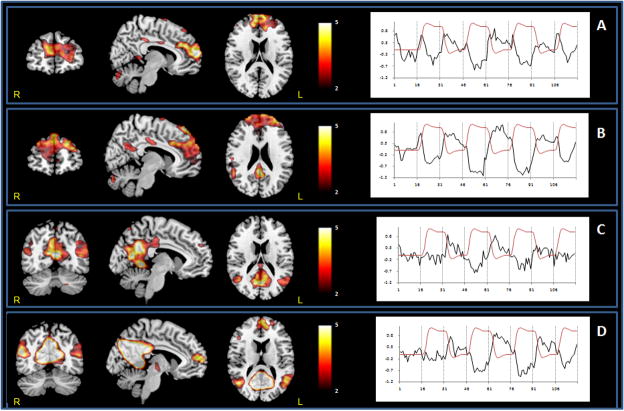
Figure 3. Default mode network ICs at 2-back across groups. Two components were identified: 2-back-ANT (A, Old; B, Young) and 2-back-POST (C, Old; D, Young). Anterior components included mPFC and aCC. Posterior components included pCC, precunes and bilateral posterior parietal regions.
Coronal, sagittal and axial sections display the spatial pattern of DMN activity across groups. Sagittal and axial slices are overlaid on a T1 template. The color bar indicates T-values. Time courses represent the temporal profile of each component across group (black) overlaid on the paradigm “box-car” design (red).
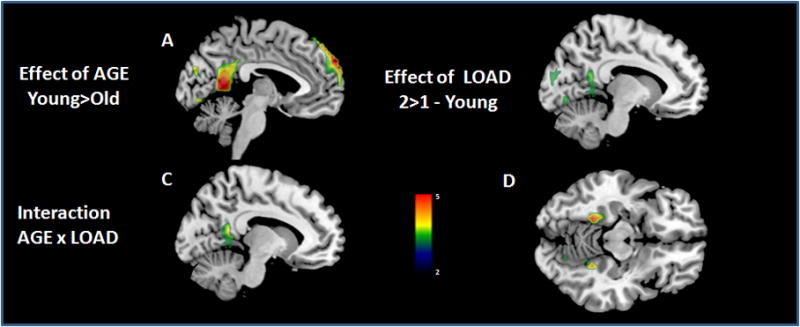
3.2.2. Between groups analyses
At 1-back, young subjects showed relatively greater activity in mPFC (BA9/BA10) in 1-back ANT, and in a large cluster (cluster extent in voxel, k=603) that encompassed the pCC (BA31), precuneus (BA7) and BA10 in 1-back-POST when compared to older adults (see Table 3).
Table 3.
Default Mode Network differences identified with Independent Component Analysis
| Task | COI | Comparison | x | y | z | k | Z |
|---|---|---|---|---|---|---|---|
| 1-back | |||||||
| Anterior | |||||||
| Young>Old | |||||||
| BA9/10 | −8 | −56 | 46 | 12 | 3.36* | ||
| Posterior | |||||||
| Young>Old | |||||||
| pCC (BA31) | 11 | −49 | 30 | 603 | 5.15 | ||
| Precuneus (BA7) | 15 | −67 | 30 | 4.85 | |||
| BA10 | 4 | 60 | 4 | 7 | 3.37 | ||
| 2-back | |||||||
| Posterior | |||||||
| Young>Old | |||||||
| precuneus/pCC (BA31) | −11 | −67 | 15 | 594 | 5.57 | ||
| pCC (BA29) | 8 | −56 | 8 | 5.42 | |||
| pCC (BA30) | 8 | −49 | 4 | 5.19 | |||
| Right angular gyrus (BA39) | −49 | −71 | 34 | 46 | 3.86 | ||
| Left superior temporal gyrus (BA41) | −52 | −26 | 8 | 8 | 3.74 | ||
k, number of voxels within the cluster; x,y,z, MNI coordinates of the centre of mass; Z= z-score;
small volume corrected (SVC) for mPFC. Subclusters are italicized
At 2-back there was greater activity in a large cluster (k=594) encompassing the precuneus/pCC (BA31), pCC (BA29/BA30), right angular gyrus (BA39) and left superior temporal gyrus in younger subjects relative to older adults (see Table 3).
There were no significant differences between the young and older adults on 2-back ANT.
3.2.3. Time-course Comparison: PSD Analyses
In order to compare the time courses of the DMN COIs between the two age groups across different task loads, we ran a PSD analysis (Welch 1967) in the components that showed the most significant age effects, namely the posterior COIs. PSD analyses revealed a main effect of age [F(1,55)= 10.457; p=0.002], with an overall decrease of low frequency oscillations (LFOs, 0.03–0.08 Hz) in older adults relative to younger subjects, as well as an effect of load [F(1,55)= 5.142; p=0.027] with decreased power spectral density in the LFOs bin at the lower task loads. Fisher’s post hoc analyses confirmed the age effect in the LFO bin at both 1-back (p= 0.05) and 2-back (p=0.01). No significant differences were found between the two groups at both task loads in the other frequency bins.
A repeated measure ANOVA (Fig. 4) was used to address the relationship between the power density in the DMN in the LFO frequency bin and the task conditions. The LFO power attenuation associated with a shift from baseline to a more demanding cognitive condition was significantly decreased in older adults when compared to younger subjects [effect of age: F(1,55)= 9.359; p=0.003]. There was a significant effect of task load with increasing LFO power attenuation with increasing task load [effect of load: F(1,55)= 3.734; p=0.058 2-back >1-back]. A Fisher’s post hoc analysis confirmed a significant decreased attenuation in older adults relative to younger subjects at both 1-back (p=0.04) and 2-back (p=0.02).
Figure 4. Percent power spectral density (PSD) mean change in the low frequency bin (0.03–0.08 Hz) at 1-back and 2-back in the Posterior COIs within the DMN. Older adults showed significantly decreased power attenuation in the low frequency domain relative to younger subjects when switching from the task condition (On) to the control task condition (Off).
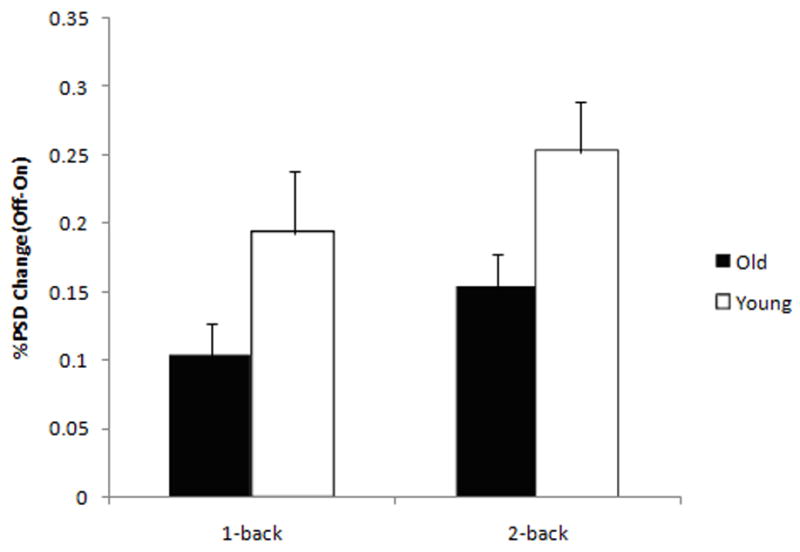
Error bars indicate standard error of the mean. *, p<0.05; §, p<0.01.
3.2.4. Spatial Comparison
To explore if there was differential recruitment of regions within the DMN components across age and task load, we computed the percent of activated voxels within ROIs (the most significantly activated regions within the DMN, i.e., mPFC, pCC and a control region, aCC) in both components for each task load for each age group, and performed random effects ANOVAs (age-by-task-load, Fig. 5). The ANOVA on the pCC showed a main effect of age [older adults showed a smaller extent: F(1,55)= 7.610, p=0.007], and an age-by-task-load interaction [F(1,55)= 3.635, p=0.056], with older adults showing a decrease in the spatial extent within this ROI with increasing task load, which was in the opposite direction to the load-related change observed in young subjects. The extent of recruitment of mPFC in the DMN showed a main effect of age [F(1,55)= 4.662, p= 0.03] with older adults showing decreased engagement relative to younger subjects. The spatial extent of aCC (BA24) did show a trend for the effect of age [F(1,55)= 3.0106; p=0.08] with older adults showing a greater engagement of this area and no effect of task load.
Figure 5. Age and task load effects on spatial extent within the DMN regions. Older adults showed decreased recruitment of pCC in the DMN, and a decreased task load–related engagement of this area relative to younger subjects, who instead showed increased recruitment. Younger subjects showed greater recruitment of mPFC at both task loads.
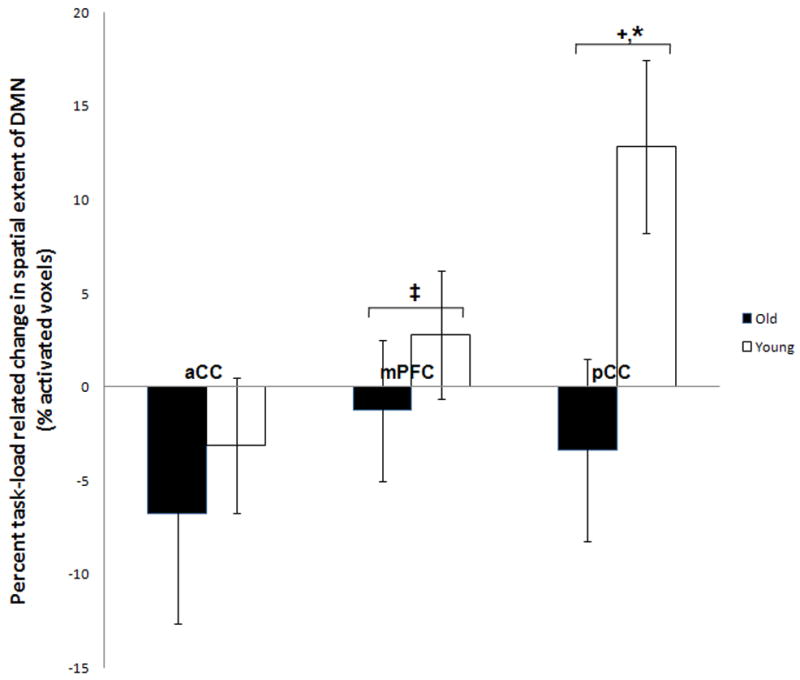
The differences shown are calculated as (2-back – 1-back) percent voxel extent differences for all the DMN ICs for each age group. +, effect of age p= 0.007; *, effect of interaction p=0.05; ‡, effect of age, p= 0.03.
The ICA findings show decreased modulation in DMN in older adults relative to younger subjects at both 1-back and 2-back. These results are confirmed by the PSD results that suggest an age-related decrease in power of the LFOs correlated with the DMN. Furthermore, the ROI extent findings suggest a differential effect of age across the task load in pCC, with older adults showing decreased modulation of this COI with increasing task load.
3.3. GLM
To further explore the effect of task load on deactivations across lifespan, we also ran a GLM analysis (Table S1; Fig. 6).
Figure 6. General linear model analysis of task-induced-deactivations (TIDs) in young and older adults across different task loads. Older adults showed smaller extent of TIDs relative to young subjects in both mPFC and posterior midline regions (A). Subjects showed greater deactivations at 2-back in middle dorsal cingulate and BA10 (B). There was a significant age-by-task-load interaction in the retrosplenial pCC (C) and posterior parahippocampus (D), with older adults showing relatively decreased deactivation with increasing task load (see also Figure 7) when compared to younger subjects (see Supplementary materials for details).
Middlesagittal (x=0, A, B, C, F, G) and axial sections (z=−9, D) of the T1 template with overlaid deactivation t-maps. The color bar indicates T-values.
Consistent with results obtained using ICA, GLM analyses (see Supplementary materials) also revealed decreased TIDs in older adults irrespective of the task load. Increase in task load resulted in a greater extent of TIDs in younger subjects. The most caudal part of pCC (retrosplenial pCC) and posterior parahippocampus showed a significant age-by-task-load interaction; older adults failed to show a relative decrease in deactivation with increasing task load as opposed to their younger counterparts (Fig. 7).
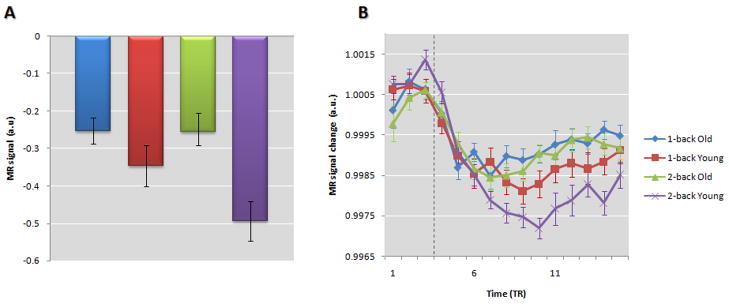
Figure 7. Timecourse of deactivations in older and young subjects across different task loads in pCC. Older adults showed significantly decreased deactivations in these regions relative to younger subjects [F(1,55)=8.841, p=0.004]. TIDs increased with task load [F(1,55)= 5.590, p=0.021], and this change was greater in younger subjects than in older adults [F(1,55)= 5.026, p=0.029]. (A) shows the mean signal across all the task in the pCC; the bars represent the standard error of the mean. (B) shows the mean timecourse in the first two blocks of the task (the baseline and the activation condition, 1-back and 2-back) in the pCC scaled to the mean (see Supplementary materials for details).
The vertical dashed line represents the beginning [a 6-sec delay was added to account for the HRF lag time (Glover 1999)] during the activation condition of the task. Time is measured in units of TR, MR signal in arbitrary units; the bars represent the standard error of the mean.
3.3.1. Correlations of TIDs with performance
In order to explore the role of the DMN in the allocation of attentional resources, and ultimately in the performance of the task, we investigated which areas of TIDs within DMN showed a significant correlation to behavioral performance.
We found a positive correlation (Fig. 8A) between performance and TIDs at 2-back in mPFC (BA9/8; x=−4, y=45, z=57, k=123, r=0.5, q-FDR=0.003), left posterior parietal cortex (x=−41, y=−67, z=23, k=35, q-FDR=0.016), and pCC (x=−4, y=−52, z=11, k=5, p=0.003). Both older (r= 0.47) and young (r= 0.42) subjects showed similar extent of correlation in mPFC. We also found a negative correlation (Fig. 8B) between deactivation in aCC (BA24/32; x=0, y=30, z=8, k=8, p<0.0001) and performance during the 2-back condition.
Figure 8. Correlation of TIDs with behavioral performance at 2-back. Accuracy correlated positively (A) with deactivation in the mPFC and PCC during 2-back, and negatively (B) with deactivation in aCC and posterior parietal cortex.
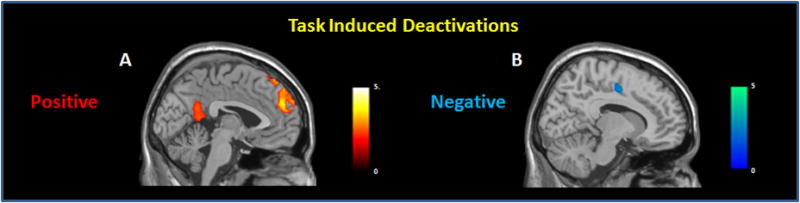
Correlation t-maps are overlaid T1 template, and are showed on the middlesagittal plane. The color bar indicates T-values.
3.4. fcMRI
Given our convergent ICA and GLM results, and our a priori hypothesis of a role of pCC, we wanted to address age-related alterations in local connectivity of this region within the DMN. To this end, we examined the fcMRI differences within the DMN between young and older adults and its relationship to behavioral differences. In these analyses, we set a seed in pCC and explored the voxel-wise correlation during the activation (both 1-back and 2-back) conditions (see Supplementary materials for main effects).
There was a main effect of load with greater negative coupling between pCC and bilateral DLPFC (BA46) and BA10 at 2-back relative to 1-back (Fig. 9B). There was a main effect of age with the older adults showing significantly decreased negative coupling (q-FDR <0.001, Fig. 9A) relative to younger subjects between pCC and a network of brain regions including VLPFC (BA44/47), DLPFC (BA9/46), inferior parietal lobules, and superior temporal gyri.
Figure 9. Functional connectivity with pCC: negative (A, B) and positive (C) coupling. DLPFC, VLPFC and posterior parietal cortices showed a decreased negative coupling with pCC in older as compared to younger subjects (A). All subjects showed a greater negative coupling in DLPFC with increasing task load (B).Older adults showed decreased positive functional coupling relative to younger subjects between pCC and mPFC (C).
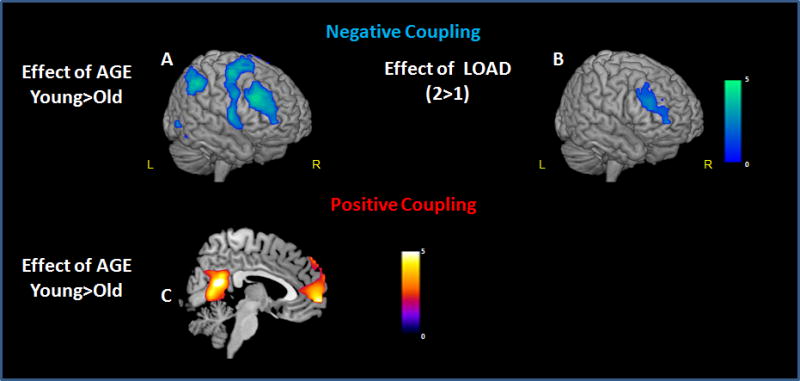
Maps of voxel-wise connectivity of the pCC during the n-back task overlaid on an T1 template, and shown in the mid-sagittal plane (C), or rendered on the surface of the cortex (A, B). The color bar indicates T-values.
The comparison of positive functional coupling between older and young subjects showed a main effect of age with older adults showing decreased positive functional coupling between the pCC and the mPFC (BA10, k=95; x=0, y=60, z=−4, Z=4.72, q-FDR= 0.02 SVC; BA9, k=23; x=0, y=56, z=42, Z=4.89, q-FDR= 0.02 SVC Fig. 9C) when compared to the younger subjects.
The fcMRI analyses showed decreased positive coupling of the mPFC with the pCC in older adults. The positive coupling with the aCC decreased with increasing task load. While the negative coupling of the pCC with PFC increased with task load in both groups, this change was significantly reduced in the older adults.
3.4.1. Correlation of functional coupling between regions within the DMN and performance
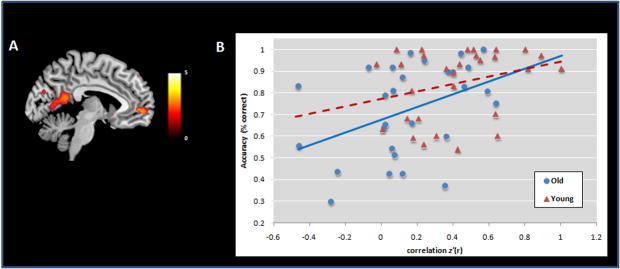
In order to understand the impact of functional connectivity between the pCC and the rest of the DMN on task performance (accuracy) we performed a simple correlation analyses. Since the behavioral performance at 1-back showed a ceiling effect in both groups, we tested this hypothesis just on the 2-back data. Behavioral performance was positively correlated (r=0.44) with the strength of the functional coupling measure between pCC and mPFC at 2-back during the activation condition in mPFC (BA10, k=5, x=4, y=56, z=−4, q-FDR= 0.06, Fig. 10A). Although the coupling with mPFC was smaller, this correlation was greater but not significant in the older adults (r= 0.41, Fig. 10B) relative to younger subjects (r= 0.29, Fig. 10B).
Figure 10. Accuracy correlated (r=0.44) with positive coupling between mPFC (BA10) and pCC both (A, B) at 2-back task. Though not statistically significant, older adults showed higher correlation (r=0.41) between performance and positive coupling between mPFC and pCC when compared to younger subjects (r=0.29).
Midsagittal slice of the correlation t-maps overlaid on a T1 template. The scatterplot (B) reflects accuracy (measured as percent correct) and functional coupling represented as correlation [z′(r)] values for old (circles, continuous line) and young (triangles, dashed line) subjects. The color bar indicates T-values.
In order to confirm that this correlation was not related to the activation condition, we ran the same analysis on the control condition. We again found a positive correlation (r=.42) with the coupling between pCC and mPFC (BA10, R, k=28, x=0, y=56, z=−4, q-FDR=0.04, SVC).
All together, these results show a crucial role of the functional coupling between the pCC and the mPFC on performance of a WM task. This is further corroborated by decreased coupling between the pCC and the mPFC and its relation to performance in older adults.
4. DISCUSSION
The present study shows significant age- and WM load-related changes in the activity and connectivity of DMN regions, and their impact on performance of a WM task. We obtained converging results in age-related changes in functional connectivity and local deactivations using different statistical techniques, namely ICA and GLM. First, we found a significantly decreased extent and magnitude of deactivations (Figures 6, 7 and Table 3) as well as a decreased coupling between specific regions (Figure 9) of the DMN in older adults when compared to younger subjects. Additionally, older adults showed a decrease in LOFs in the DMN and a decrease in task-related suppression of these signal oscillations (Figure 4). Second, analyzing the spatial extent (Figure 5) and the time courses (Figure 4) of the COIs, and the magnitude of the deactivations we demonstrated an age-by-task-load interaction in the pCC and posterior hippocampus, where older adults failed to show a load-related increase in deactivation when compared to the young subjects. Third, we also observed an age-related decrease in DMN connectivity together with a decline in task performance. This to our knowledge has not been reported previously. We found that irrespective of age, the stronger the positive functional coupling between the pCC and mPFC was, the better was the WM performance, i.e. the more the pCC deactivates, the more the mPFC deactivates and this was associated with improved performance (Figure 10). Most interestingly, younger subjects, who performed better at the 2-back task, showed stronger positive coupling within the DMN as compared to the older adults. Additionally, we also found greater negative functional coupling between pCC and the task-related regions in young subjects, namely the dorsal and ventral PFC.
Consistent with previous reports (Grady, et al. 2006; Lustig, et al. 2003; Persson, et al. 2007), our data show an age-related modulation in the response of the DMN. Although, both age groups showed a TID pattern in the DMN regions, we found a relatively decreased deactivation in older adults in mPFC as well as in pCC regions. In keeping with Schroeter et al. (2004) who reported a reduction of LFOs with increasing age during rest and functional stimulation of the human visual cortex, we found a decrease of LFOs in older adults. LFOs are thought to arise from fluctuations in metabolic processes and have been suggested to reflect synchronicity among functionally correlated regions (Biswal, et al. 1995). A decrease of LFOs therefore may be suggestive of a decline of DMN connectivity in older adults. More interestingly, we also found that older adults showed a decrease in ability to suppress LFOs of the DMN with increasing task load (Figure 4). Taken together these results suggest that persistent DMN activity, particularly in pCC, in the older adults (as reflected by decreased deactivation) during the WM task along with decreased negative coupling of pCC with the cognitive control regions (DLPFC and aCC) may be indicative of fluctuation in sustained attention with a consequent decline in task performance (default mode interference hypothesis, Sonuga-Barke and Castellanos 2007). Consistent with this notion, Chee and Choo (2004) showed that inadequate suppression of the activity within the DMN along with decreased activation in task-related regions following sleep deprivation was associated with worse cognitive performance. Based on these observations they concluded that both adequate suppression of activity within the DMN as well as activation in task-related regions are critical for allocation of the attentional resources necessary for the performance of a cognitive task. A similar mechanism has been proposed to explain cognitive deficits in patients with attention deficit hyperactivity disorder (Sonuga-Barke and Castellanos 2007) as well as in social deficits in patients with autism (Kennedy, et al. 2006).
Interestingly, we also found an age-by-task-load interaction in the magnitude and spatial extent of deactivation in the pCC reflected as an absence of load-related increase in deactivation in older adults compared to the younger subjects. Consistent with this, several neuroimaging studies have previously shown decreased deactivation in pCC in older adults relative to younger subjects (Grady, et al. 2006; Lustig, et al. 2003; Persson, et al. 2007), an observation that becomes more significant with increasing task demands (Hugenschmidt, et al. 2007; Persson, et al. 2007). As highlighted above, Chee and Choo (2004) report that this pattern of changes in deactivation is similar to those observed when young subjects perform similar tasks following sleep deprivation. Gusnard and Raichle et al. (2001) suggest that the pCC is involved in visuo-spatial and emotional processing and may play an adaptive role in the detection of predators, tonically gathering external as well as internal information and modulating the allocation of attentional resources. Numerous connections (Baleydier and Mauguiere 1980; Greicius, et al. 2007; Morris, et al. 1999) link pCC with mPFC and enthorhinal cortex (Goldman-Rakic, et al. 1984; Kobayashi and Amaral 2003; Morris, et al. 1999), thus conferring the pCC a crucial role of an interface between these regions that have no direct connections. pCC may also contribute to the transition from short to long-term memory (Kobayashi and Amaral 2003; Woodard, et al. 2007), by playing a direct role in output monitoring, encoding (Fletcher, et al. 1995; Moscovitch 1992) or in retrieval strategies (Moscovitch 1992). The importance of pCC in cognitive function is also supported by fMRI studies that report a decrease in deactivation in this region as well as in other DMN regions in patients with mild cognitive impairment (MCI; Rombouts, et al. 2005) and patients with AD (Greicius, et al. 2004; He, et al. 2007; Lustig, et al. 2003; Rombouts, et al. 2005). Additionally, Mosconi et al (2007) using FDG-PET have shown a reduction in glucose metabolism in these regions to predict cognitive decline in normal older adults to MCI and AD (Mosconi, et al. 2007). More recently, Andrews-Hanna et al. using DTI and fcMRI reported decreased connectivity between pCC and mPFC in older adults which correlated with a decline in neuropsychological measures of executive function, memory, and processing speed (Andrews-Hanna, et al. 2007). This is consistent with the observations of Hampson et al. (2006) who showed that the strength of the positive coupling between pCC and mPFC positively correlated with performance during a WM task. Consistent with these observations, we also found an age-related reduction in functional coupling between pCC and mPFC which significantly correlated with a decline in WM task performance.
Age-related functional changes in DMN may either reflect a compensatory process or a deficit in cognitive control with deficient resource allocation (from the DMN) to the task at hand. Although an important role of compensatory processes has been proposed as a possible explanation for the age-related changes in task-related activation studies (Cabeza, et al. 2002; Mattay, et al. 2006; Reuter-Lorenz, et al. 2000), such compensation seems to be unlikely here. The positive correlation between deactivation and connectivity within the DMN and task performance, as well as the negative correlation of the DMN connectivity with cognitive control regions, namely the DLPFC and cingulate are more in support of the deficient resource allocation concept. Consistent with this notion, the age-related increase of prefrontal activations may be compensatory to the decreased functioning of the DMN in older adults (Persson, et al. 2007).
Alternatively, an age-related impairment in cognitive control may lead to decreased deactivations in the DMN with a resultant decrease in task performance. Through top-down control of attention, cognitive control allows an active selection of contents of consciousness. This is a result of excitatory processes which enhance the information related to the task at hand, and inhibitory processes which suppress interfering information (Hasher and Zacks 1988). At a system functioning level, a malfunction of this integration process may result in an inadequate transition from the baseline DMN activity to an active cognitive demanding task-related network (Sonuga-Barke and Castellanos 2007). At the psychological level, a failure of the dynamic integration of these processes results in less focused attention and increased distractibility. Indeed, older adults show difficulty in inhibiting irrelevant processes on a variety of cognitive tasks including reading (Tun, et al. 2002), attention (Cohn, et al. 1984; Zeef, et al. 1996), memory (Gazzaley, et al. 2005; Rowe, et al. 2006) and visual search tasks (Scialfa, et al. 1998) with a consequent impairment in behavioral performance.
The age-related reduction of dopaminergic innervation of PFC might affect the modulation and synchronization of default mode and task-related networks, thus resulting in decreased DMN deactivation and “intrusion” of spontaneous LFOs. Studies measuring event-related potentials (Chao and Knight 1997) and fMRI (Gazzaley, et al. 2005) demonstrate an age-related impairment in PFC functioning together with deficits in inhibiting processes. PFC is richly innervated by the dopaminergic system and plays an important role in attention as well as in WM. More specifically, dopamine modulates firing of pyramidal neurons and of the surrounding GABA inhibitory interneurons within the PFC through its effect on dopamine receptors in these neurons. Through these mechanisms dopamine is thought to focus and stabilize the response of PFC to the task at hand (Seamans, et al. 1997; Seamans and Yang 2004). Furthermore, dopamine modulates network synchronization of cortical oscillation (Breakspear, et al. 2003; Li, et al. 2000; Seamans and Yang 2004) which may represent a way to increase synaptic gain and amplify task-related signals (Fries, et al. 2001) or inhibit irrelevant information (Tiesinga, et al. 2004). Several post-mortem (Allard and Marcusson 1989; Ma, et al. 1999; Seeman, et al. 1987) as well as imaging studies (Ichise, et al. 1998; Rinne, et al. 1998; Van Dyck, et al. 1995) have shown a significant age-related decrease in dopaminergic markers (Reeves, et al. 2002). The decreased functionality of dopaminergic neurotransmission in older adults has been associated with a decline in cognitive performance (Bäckman, et al. 2000, 2006; Erixon-Lindroth, et al. 2005; Mozley, et al. 1999; Volkow, et al. 1998). The increase of LFOs and the instability of DMN network can affect the availability of attentional resources for the task at hand and as a consequence result in poorer WM performance when the resources are limited (higher task load and aging). Of interest, a recent study (Bluhm, et al. 2007) found an intriguing correlation between a decrease in LFOs of the network connecting pCC with PFC and brain stem and negative symptoms in schizophrenia that have been linked to alteration in the dopaminergic circuits. Future studies using pharmacological manipulations with dopaminergic drugs or functional polymorphisms in dopamine genes (Sambataro, et al. 2005) may shed more light on these mechanisms.
There are some limitations of this study. First, we used a simple low demanding control condition and not a pure resting state condition. Greicius et al. (2004) using an ICA analysis approach showed intrinsic activity within the default mode is affected little by attending to a simple stimulus. Moreover, since our primary goal was to look at age- and load-related differences in DMN, a more constrained but still low demanding baseline condition allowed better between- and- within group comparisons. The convergence of ICA and GLM results further confirms the reliability of the design we used. Second, we cannot exclude whether the difference in LFOs between the younger and older adults is not a result of the changes in the microvascular smooth muscular tissues which lead to increased arterial stiffness in older adults (Lundberg and Crow 1999). Although, as a consequence of this the older adults may show an increased time-to-peak in the BOLD response of task relevant areas (Taoka, et al. 1998), it is very unlikely that this neurovascular change would result in the persistence of DMN activity during the cognitive condition of the task. Furthermore, ICA does not assume any specific HRF shape and therefore any age-related alteration in neurovascular coupling would not affect these results. Third, the block design paradigm we used in this study does not allow the isolation of the brain response associated with either correct or incorrect trials only. Therefore, we cannot exclude the possibility that the decrease in functional connectivity we observed is a not an epiphenomenon of decreased performance. However, prior anatomical (Burke and Barnes 2006) and neuroimaging studies (Cook, et al. 2007) have shown decreased connectivity in older adults. Furthermore, we found an age-related decline in functional connectivity during the control condition as well as during the 1-back task (data not shown) during which both groups performed similarly.
Finally, given the well documented evidence of age-related changes in brain structure, the question arises if any anatomical/volumetric differences exist between groups in ROIs selected as part of the DMN and if so how this would affect the functional connectivity results. Damoiseaux et al (2007) have previously shown that age-related decline of functional brain connectivity within the DMN remains significant after correcting for age-related changes in gray matter volume (total and regional). While we did not have structural data obtained with similar pulse sequences in both groups to do a similar analysis, we used an alternate approach using CSF and white matter signal variance in the BOLD fMRI time series as covariates. This suggests that increased atrophy and thereby increased CSF, if any, did not affect the reported age-related changes in the functional connectivity of brain regions within the DMN that we observed.
In conclusion, our findings show that normal aging is associated with not only changes in activity patterns of canonical task-related brain regions but also with alterations in the activity and connectivity of brain regions within the DMN.
Supplementary Material
Acknowledgments
We thank Prof. Alessandro Bertolino, MD, PhD, Annabella Di Giorgio, MD, PhD, and Herve Lemaitre, PhD for helpful discussions and Jennifer Brook, BA and Matt Emery, BS for assistance in data analysis. This work was supported by the National Institute of Mental Health Intramural Research Program.
Footnotes
Disclosure statement: None of the authors have any actual or potential conflicts of interest.
The study protocol was approved by the Intramural Review Board of the National Institute of Mental Health and appropriate procedures were used concerning the human subjects involved.
References
- Allard P, Marcusson JO. Age-correlated loss of dopamine uptake sites labeled with [3H]GBR-12935 in human putamen. Neurobiol of Aging. 1989;10:661–4. doi: 10.1016/0197-4580(89)90001-8. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–35. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckman L, Ginovart N, Dixon RA, Wahlin TBR, Wahlin A, Halldin C, Farde L. Age-related cognitive deficits mediated by changes in the striatal dopamine system. Am J Psychiatry. 2000;157:635–7. doi: 10.1176/ajp.157.4.635. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Nyberg L, Lindenberger U, Li SC, Farde L. The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neurosci Biobehav Rev. 2006;30:791–807. doi: 10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Baleydier C, Mauguiere F. The duality of the cingulate gyrus in monkey. Neuroanatomical study and functional hypothesis. Brain. 1980;103:525–54. doi: 10.1093/brain/103.3.525. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Rubino V, Sambataro F, Blasi G, Latorre V, Fazio L, Caforio G, Petruzzella V, Kolachana B, Hariri A, Meyer-Lindenberg A, Nardini M, Weinberger DR, Scarabino T. Prefrontal-hippocampal coupling during memory processing is modulated by COMT val158met genotype. Biol Psychiatry. 2006;60:1250–8. doi: 10.1016/j.biopsych.2006.03.078. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RW, Theberge J, Schaefer B, Williamson P. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33:1004–12. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breakspear M, Terry JR, Friston KJ. Modulation of excitatory synaptic coupling facilitates synchronization and complex dynamics in a biophysical model of neuronal dynamics. Network. 2003;14:703–32. [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. Proceedings of the 8th International Conference on Functional Mapping of the Human Brain; 2002. Neuroimage 16, abstract 497. [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–51. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, Goldberg TE, Weinberger DR. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9:20–6. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- Chao LL, Knight RT. Prefrontal deficits in attention and inhibitory control with aging. Cereb Cortex. 1997;7:63–9. doi: 10.1093/cercor/7.1.63. [DOI] [PubMed] [Google Scholar]
- Chee MWL, Choo WC. Functional imaging of working memory after 24 hr of total sleep deprivation. J Neurosci. 2004;24:4560–7. doi: 10.1523/JNEUROSCI.0007-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn NB, Dustman RE, Bradford DC. Age-related decrements in Stroop Color Test performance. J Clin Psychol. 1984;40:1244–50. doi: 10.1002/1097-4679(198409)40:5<1244::aid-jclp2270400521>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Cook IA, Bookheimer SY, Mickes L, Leuchter AF, Kumar A. Aging and brain activation with working memory tasks: an fMRI study of connectivity. Int J Geriatr Psychiatry. 2007;22:332–42. doi: 10.1002/gps.1678. [DOI] [PubMed] [Google Scholar]
- Correa N, Adali T, Yi-Ou L, Calhoun VD. Comparison of blind source separation algorithms for FMRI using a new Matlab toolbox: GIFT. IEEE International Conference on Acoustics, Speech, and Signal Processing. 2005;5:401– 4. [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA. Reduced resting-state brain activity in the "default network" in normal aging. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Erixon-Lindroth N, Farde L, Wahlin TBR, Sovago J, Halldin C, Bäckman L. The role of the striatal dopamine transporter in cognitive aging. Psychiatry Res. 2005;138:1–12. doi: 10.1016/j.pscychresns.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Esposito F, Bertolino A, Scarabino T, Latorre V, Blasi G, Popolizio T, Tedeschi G, Cirillo S, Goebel R, Di Salle F. Independent component model of the default-mode brain function: Assessing the impact of active thinking. Brain Res Bull. 2006;70:263–9. doi: 10.1016/j.brainresbull.2006.06.012. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. Biometric Research Department; New York: 1996. User’s guide for the structured clinical interview for DSM IV Axis I disorders (SCID-I, Research version) [Google Scholar]
- Fisher RA. On the probable error of a coefficient of correlation deduced from a small sample. Metron. 1921;1:3–32. [Google Scholar]
- Fletcher PC, Frith CD, Grasby PM, Shallice T, Frackowiak RS, Dolan RJ. Brain systems for encoding and retrieval of auditory-verbal memory. An in vivo study in humans. Brain. 1995;118(Pt 2):401–16. doi: 10.1093/brain/118.2.401. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836–45. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: An fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–3. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KP, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D'Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci. 2005;8:1298–300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Sheridan MA, Cooney JW, D'Esposito M. Age-related deficits in component processes of working memory. Neuropsychology. 2007;21:532–9. doi: 10.1037/0894-4105.21.5.532. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Simons JS, Frith CD, Burgess PW. Performance-related activity in medial rostral prefrontal cortex (area 10) during low-demand tasks. J Exp Psychol Hum Percept Perform. 2006;32:45–58. doi: 10.1037/0096-1523.32.1.45. [DOI] [PubMed] [Google Scholar]
- Glover GH. Deconvolution of impulse response in event-related BOLD fMRI. Neuroimage. 1999;9:416–29. doi: 10.1006/nimg.1998.0419. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12:719–43. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Gould RL, Brown RG, Owen AM, Bullmore ET, Howard RJ. Task-induced deactivations during successful paired associates learning: an effect of age but not Alzheimer's disease. Neuroimage. 2006;31:818–31. doi: 10.1016/j.neuroimage.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. Age-related changes in brain activity across the adult lifespan. J Cogn Neurosci. 2006;18:227–41. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J Cogn Neurosci. 2004;16:1484–92. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–42. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Dougherty R. Functional Connectivity Reflects Structural Connectivity in a Human Memory Network. Proceedings of the Thirteenth Annual Meeting of the Organization for Human Brain Mapping (HBM'07); 2007. CD-ROM paper no. 336 TH-PM., 94 W-PM. [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–94. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26:13338–43. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: A review and a new view. In: Bower GH, editor. The psychology of learning and motivation: Advances in research and theory. Oxford University Press; New York: 1988. pp. 193–225. [Google Scholar]
- He Y, Wang L, Zang Y, Tian L, Zhang X, Li K, Jiang T. Regional coherence changes in the early stages of Alzheimer's disease: a combined structural and resting-state functional MRI study. Neuroimage. 2007;35:488–500. doi: 10.1016/j.neuroimage.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Hugenschmidt CE, Peiffer AM, Casanova R, Maldjian JA, Burdette JH, Hayasaka S, Laurienti PJ. Preservation of default mode functioning in healthy aging adults. Proceedings of the Thirteenth Annual Meeting of the Organization for Human Brain Mapping (HBM'07); 2007. CD-ROM paper no. 336 TH-PM., 49 TH-AM. [Google Scholar]
- Ichise M, Ballinger JR, Tanaka F, Moscovitch M, St George-Hyslop PH, Raphael D, Freedman M. Age-related changes in D2 receptor binding with iodine-123- iodobenzofuran SPECT. J Nucl Med. 1998;39:1511–8. [PubMed] [Google Scholar]
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proc Natl Acad Sci U S A. 2006;103:8275–80. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: II. Cortical afferents. J Comp Neurol. 2003;466:48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- Li SJ, Biswal B, Li Z, Risinger R, Rainey C, Cho JK, Salmeron BJ, Stein EA. Cocaine administration decreases functional connectivity in human primary visual and motor cortex as detected by functional MRI. Magn Reson Med. 2000;43:45–51. doi: 10.1002/(sici)1522-2594(200001)43:1<45::aid-mrm6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Lundberg MS, Crow MT. Age-related changes in the signaling and function of vascular smooth muscle cells. Exp Gerontol. 1999;34:549–57. doi: 10.1016/s0531-5565(99)00036-4. [DOI] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O'Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci U S A. 2003;100:14504–9. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SY, Ciliax BJ, Stebbins G, Jaffar S, Joyce JN, Cochran EJ, Kordower JH, Mash DC, Levey AI, Mufson EJ. Dopamine transporter-immunoreactive neurons decrease with age in the human substantia nigra. J Comp Neurol. 1999;409:25–37. doi: 10.1002/(sici)1096-9861(19990621)409:1<25::aid-cne3>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–5. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Berman KF, Das S, Meyer-Lindenberg A, Goldberg TE, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in working memory capacity. Neurosci Lett. 2006;392:32–7. doi: 10.1016/j.neulet.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–98. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Morris R, Petrides M, Pandya DN. Architecture and connections of retrosplenial area 30 in the rhesus monkey (Macaca mulatta) Eur J Neurosci. 1999;11:2506–18. doi: 10.1046/j.1460-9568.1999.00672.x. [DOI] [PubMed] [Google Scholar]
- Mosconi L, Brys M, Glodzik-Sobanska L, De Santi S, Rusinek H, de Leon MJ. Early detection of Alzheimer's disease using neuroimaging. Exp Gerontol. 2007;42:129–38. doi: 10.1016/j.exger.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Memory and Working-with-Memory - a Component Process Model Based on Modules and Central Systems. J Cogn Neurosci. 1992;4:257–67. doi: 10.1162/jocn.1992.4.3.257. [DOI] [PubMed] [Google Scholar]
- Mozley PD, Acton PD, Barraclough ED, Plössl K, Gur RC, Alavi A, Mathur A, Saffer J, Kung HF. Effects of age on dopamine transporters in healthy humans. J Nucl Med. 1999;40:1812–7. [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Persson J, Lustig C, Nelson JK, Reuter-Lorenz PA. Age differences in deactivation: a link to cognitive control? J Cogn Neurosci. 2007;19:1021–32. doi: 10.1162/jocn.2007.19.6.1021. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves S, Bench C, Howard R. Ageing and the nigrostriatal dopaminergic system. Int J Geriatr Psychiatry. 2002;17:359–70. doi: 10.1002/gps.606. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci. 2000;12:174–87. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Sahlberg N, Ruottinen H, Någren K, Lehikoinen P. Striatal uptake of the dopamine reuptake ligand [11C]beta-CFT is reduced in Alzheimer's disease assessed by positron emission tomography. Neurology. 1998;50:152–6. doi: 10.1212/wnl.50.1.152. [DOI] [PubMed] [Google Scholar]
- Rombouts SARB, Barkhof F, Goekoop R, Stam CJ, Scheltens P. Altered resting state networks in mild cognitive impairment and mild Alzheimer's disease: an fMRI study. Hum Brain Mapp. 2005;26:231–9. doi: 10.1002/hbm.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe G, Valderrama S, Hasher L, Lenartowicz A. Attentional disregulation: a benefit for implicit memory. Psychol Aging. 2006;21:826–30. doi: 10.1037/0882-7974.21.4.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambataro F, Kingshott E, Das S, Kolachana BS, Russo C, Mirsky AF, Goldberg TE, Egan MF, Meyer-Lindenberg A, Callicott JH, Weinberger DR, Mattay VS. Effect of Catechol-O-Methyl Transferase val158met Polymorphism on Information Processing in the Prefrontal Cortex during Working Memory in the Elderly; December 11–15, 2005; Waikoloa, Hawaii. 2005. p. 81. Neuropsychopharmacology. [Google Scholar]
- Schroeter ML, Schmiedel O, von Cramon DY. Spontaneous low-frequency oscillations decline in the aging brain. J Cereb Blood Flow Metab. 2004;24:1183–91. doi: 10.1097/01.WCB.0000135231.90164.40. [DOI] [PubMed] [Google Scholar]
- Scialfa CT, Esau SP, Joffe KM. Age, target-distractor similarity, and visual search. Exp Aging Res. 1998;24:337–58. doi: 10.1080/036107398244184. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Gorelova NA, Yang CR. Contributions of voltage-gated Ca2+ channels in the proximal versus distal dendrites to synaptic integration in prefrontal cortical neurons. J Neurosci. 1997;17:5936–48. doi: 10.1523/JNEUROSCI.17-15-05936.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Seeman P, Bzowej NH, Guan HC, Bergeron C, Becker LE, Reynolds GP, Bird ED, Riederer P, Jellinger K, Watanabe S. Human brain dopamine receptors in children and aging adults. Synapse. 1987;1:399–404. doi: 10.1002/syn.890010503. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: A neurobiological hypothesis. Neurosci Biobehav Rev. 2007;31:977–86. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Taoka T, Iwasaki S, Uchida H, Fukusumi A, Nakagawa H, Kichikawa K, Takayama K, Yoshioka T, Takewa M, Ohishi H. Age correlation of the time lag in signal change on EPI-fMRI. J Comput Assist Tomogr. 1998;22:514–7. doi: 10.1097/00004728-199807000-00002. [DOI] [PubMed] [Google Scholar]
- Tiesinga PHE, Fellous JM, Salinas E, Jose JV, Sejnowski TJ. Synchronization as a mechanism for attentional gain modulation. Neurocomputing. 2004;58–60:641–6. doi: 10.1016/j.neucom.2004.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Ernst T, Caparelli EC, Chang L. Common deactivation patterns during working memory and visual attention tasks: an intra-subject fMRI study at 4 Tesla. Hum Brain Mapp. 2006;27:694–705. doi: 10.1002/hbm.20211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun PA, O'Kane G, Wingfield A. Distraction by competing speech in young and older adult listeners. Psychol Aging. 2002;17:453–67. doi: 10.1037//0882-7974.17.3.453. [DOI] [PubMed] [Google Scholar]
- Van Dyck CH, Seibyl JP, Malison RT, Laruelle M, Wallace E, Zoghbi SS, Zea-Ponce Y, Baldwin RM, Charney DS, Hoffer PB, Innis RB. Age-related decline in striatal dopamine transporter binding with iodine- 123-beta-CIT SPECT. J Nucl Med. 1995;36:1175–81. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Ding YS, Gur RC, Gatley J, Logan J, Moberg PJ, Hitzemann R, Smith G, Pappas N. Parallel loss of presynaptic and postsynaptic dopamine markers in normal aging. Ann Neurol. 1998;44:143–7. doi: 10.1002/ana.410440125. [DOI] [PubMed] [Google Scholar]
- Welch PD. The Use of Fast Fourier Transform for the Estimation of Power Spectra: A Method Based on Time Averaging Over Short, Modified Periodograms. IEEE Trans Audio Electroacoust. 1967:70–3. [Google Scholar]
- West R. Visual distraction, working memory, and aging. Mem Cognit. 1999;27:1064–72. doi: 10.3758/bf03201235. [DOI] [PubMed] [Google Scholar]
- Woodard JL, Seidenberg M, Nielson KA, Miller SK, Franczak M, Antuono P, Douville KL, Rao SM. Temporally graded activation of neocortical regions in response to memories of different ages. J Cogn Neurosci. 2007;19:1113–24. doi: 10.1162/jocn.2007.19.7.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited--again. Neuroimage. 1995;2:173–81. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Xiong J, Parsons LM, Gao JH, Fox PT. Interregional connectivity to primary motor cortex revealed using MRI resting state images. Hum Brain Mapp. 1999;8:151–6. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<151::AID-HBM13>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeef EJ, Sonke CJ, Kok A, Buiten MM, Kenemans JL. Perceptual factors affecting age-related differences in focused attention: performance and psychophysiological analyses. Psychophysiology. 1996;33:555–65. doi: 10.1111/j.1469-8986.1996.tb02432.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.