Abstract
Both insulin alone and the somatostatin analogue octreotide alone facilitate memory in patients with Alzheimer's disease (AD). Since octreotide inhibits endogenous insulin secretion, the cognitive effects of insulin and octreotide may not be independent. This study tested the individual and interactive effects of insulin and octreotide on memory and plasma growth hormone (GH) levels in older adults. Participants were 16 memory-impaired (AD=7, amnestic mild cognitive impairment=9; apolipoprotein E [APOE] ε4- [no ε4 alleles]=9, ε4+ [1-2 ε4 alleles]=7) and 19 cognitively-intact older adults (APOE ε4-=17, ε4+=1). On separate days, fasting participants received counterbalanced infusions of (1) insulin (1 mU·kg-1·min-1) and dextrose to maintain euglycemia, (2) octreotide (150 μg/h), (3) insulin, dextrose, and octreotide, or (4) saline. Story recall was the principal endpoint. Insulin alone facilitated delayed recall for ε4-patients, relative to ε4+ patients (P=0.0012). Furthermore, ε4- patients with higher Mattis Dementia Rating Scale (DRS) scores had greater octreotide-induced memory facilitation (P=0.0298). For healthy adults, octreotide facilitated memory (P=0.0122). Unexpectedly, hyperinsulinemia with euglycemia increased GH levels in healthy controls (P=0.0299). Thus, insulin and octreotide appear to regulate memory in older adults. APOE ε4 genotype modulates responses to insulin and octreotide. Finally, insulin may regulate GH levels during euglycemia.
Keywords: Alzheimer's disease (AD), apolipoprotein E (APOE), acetylcholine, growth hormone (GH), insulin, memory, mild cognitive impairment (MCI), octreotide, somatostatin
1. Introduction
Alzheimer's disease (AD) has been associated with abnormalities in the insulin and somatostatin systems [1,2], both of which influence memory. Increasing insulin levels facilitates memory in patients with AD [3,4] and healthy older adults [4]. Somatostatin, expressed in the cerebral cortex and hippocampus [5], contributes to memory in part via effects on long-term potentiation and synaptic plasticity [6,7]. Notably, PS1xAPP mice, an AD model, have decreased levels of somatostatin in brain and somatostatin immuno-positive neurons [8]. Other somatostatinergic changes related to AD include a decline in somatostatin type 2 receptors (SSTR2) in cortex and hippocampus [1,9]. Consequently, agents that enhance insulin or somatostatin activity constitute potential therapeutic strategies for cognitive impairment in patients with AD [7,10].
Since octreotide inhibits endogenous secretion of insulin and growth hormone (GH) [11], cognitive effects of insulin and octreotide may not be independent. Amassing evidence supports the notion that the effects of insulin on memory are centrally mediated [12]. Likewise, octreotide's effects may be centrally mediated, since octreotide binds primarily to SSTR2 receptors in brain; however, in vitro work suggests that octreotide has low penetration across the intact blood-brain barrier [13]. Even so, microvascular damage may increase brain penetration and enhance central effects of octreotide in patients with AD [14]. Alternatively, octreotide-induced memory facilitation may reflect peripheral changes, such as inhibition of pro-inflammatory cytokine secretion [15], which appears to impair memory [16].
Administration of insulin (while maintaining normoglycemia) or octreotide (a long-acting cyclic octapeptide somatostatin analogue) facilitates memory [3], but their interactive effects have not been tested. Furthermore, the potential role of apolipoprotein E (APOE) genotype should be considered. This established risk factor for AD modulates insulin-induced changes in memory and plasma amyloid precursor protein levels [4]. Similarly, APOE genotype may influence somatostatin loss in AD [17]. The present study tested the individual and interactive effects of insulin and octreotide on cognition for patients with AD. We predicted that both insulin and octreotide would facilitate memory and that APOE genotype would moderate the cognitive effects of insulin and octreotide.
2. Materials and Methods
2.1. Subjects
The University of Washington Human Subjects Review Committee approved this study; all subjects gave written informed consent before participation. Subjects were 16 memory-impaired patients with mild-to-moderate AD (n=7) or amnestic mild cognitive impairment (MCI, n=9), widely believed to represent a prodromal stage of AD [18], and 19 older adults with intact cognition. An expert panel of neurologists, psychiatrists, and neuropsychologists diagnosed probable AD (NINCDS/ADRDA criteria [19]) or MCI (Petersen's criteria for amnestic MCI or multiple domain MCI with amnestic features [18]). Patients scored lower on the Mini Mental State Examination (MMSE) and Mattis Dementia Rating Scale (DRS) and had fewer individuals without an APOE ε4 allele (ε4-=no alleles, ε4+=1-2 alleles); otherwise, patient and control groups had similar baseline characteristics (Table 1). Subjects were screened by history, physical examination, electrocardiogram, and clinical chemistry and hematology, and were in good general health and free from psychiatric and neurological disorders other than AD or MCI. Three memory-impaired patients were taking stable doses of a cholinesterase inhibitor; otherwise, no subjects were taking medications known to affect cognition, CNS functions, or glucose regulation.
Table 1.
Baseline subject characteristics.
| Memory Impaired | Healthy Control | |
|---|---|---|
| Age (yr) | 76.1 (5.2) | 73.5 (7.2) |
| Gender (F/M) | 5/11 | 8/11 |
| Education (yr) | 14.4 (3.2) | 14.9 (1.8) |
| MMSE | 24.4 (3.8) | 28.1 (1.9)** |
| Mattis DRS | 125.6 (13.7) | 140.0 (3.6)** |
| APOE (ε4-/ε4+) | 9/7 | 17/1* |
| BMI (kg/m2) | 24.8 (2.7) | 25.9 (3.7) |
| FPG | 92.8 (16.5) | 98.7 (13.4) |
Note. MMSE=Mini Mental State Examination, Mattis DRS=Mattis Dementia Rating Scale, BMI=body mass index, FPG=fasting plasma glucose. Age, education, MMSE, Mattis DRS, BMI, and FPG are expressed as mean (SD).
p<0.01,
p<0.001.
2.2. Procedure
2.2.1. Infusion Protocol
Subjects made four study visits separated by one-to-six weeks and received four randomized metabolic conditions in counterbalanced order: (1) Insulin infusion (1 mU·kg-1·min-1) to raise plasma levels to ∼80 μU/mL and variable dextrose infusion (20%) to maintain plasma glucose levels at ∼100 mg/dL; (2) Octreotide infusion (150 μg/h; Sandostatin®, Novartis, East Hanover, NJ, USA); (3) Insulin and octreotide infusions (as in conditions 1 and 2); and (4) Saline infusion (placebo) to maintain fasting insulin and glucose levels. Fasting subjects arrived at ∼0800 h, and a research nurse inserted intravenous (IV) catheters for infusions and blood sampling. Subjects rested for 15 min before the nurse acquired a blood sample and started infusions. After 90 min of infusion, a second blood sample was acquired, and subjects completed a 45-min cognitive battery. Infusions persisted through cognitive testing. The nurse assessed plasma glucose levels with a Beckman glucose analyzer (Beckman Instruments, Fullerton, CA, USA) every 5-10 min and adjusted the dextrose infusion rate to maintain euglycemia. Medical staff that administered infusions were aware of metabolic conditions; subjects and study personnel who administered and scored cognitive tests and performed assays were blinded to condition.
2.2.2. Cognitive Protocol
Four parallel forms, administered in randomized and counterbalanced order, assessed verbal memory (story recall, Buschke selective reminding test) and complex attention (Stroop color-word interference test, self-ordered pointing task [SOPT]). For story recall, subjects heard a brief story twice and recalled as many details as possible immediately following each presentation and following a 10-minute delay. Accurate responses were summed to compute immediate and delayed recall scores [4,20]. For the Buschke, subjects received 8 learning trials of a 12-item word list. On trial 1, an examiner simultaneously read and displayed individual words; on trials 2-8, only items missed on the previous trial were presented. Recall was elicited after each learning trial and after a delay. Correct responses were summed to compute immediate and delayed recall scores [10]. The Stroop test consisted of 3 conditions: word reading, color naming, and inhibition of an automatic word-reading response in favor of a color-naming response. We recorded completion time and errors for each condition [4]. For the SOPT, an array of 10 or 12 abstract designs appeared on a computer touch screen, and subjects were instructed to touch any design. The screen cleared, the array reappeared in a different order, and subjects were instructed to touch a different design. The number of trials equaled the number of designs. Errors were recorded [21].
2.3. Assays and APOE Genotyping
Plasma insulin and GH levels were ascertained in samples acquired after 90 min of infusion (immediately before cognitive testing). Plasma insulin levels were measured by radioimmunoassay as previously described [22]. GH levels were measured using a commercially available IRMA kit (Diagnostic Systems Laboratories, Inc., Webster, Texas). The lowest detection limit was 0.02 μg/l. The intra-assay precision was determined from the mean of 12 replicates each with three human serum samples, and the resulting CVs were 3.1%, 3.9%, and 5.4%. DNA was derived and APOE genotypes were determined as previously described [4].
2.4. Statistical Analyses
Cognitive scores were subjected to a multivariate analysis of variance (MANOVA). Covariates were age, DRS score, body mass index (BMI), and APOE ε4 genotype (ε4-, ε4+). Following a significant Wilks' λ for condition (P<0.05), we conducted planned comparisons to detect differences between the placebo and active conditions. Graphs depict data as adjusted means and standard errors. To test the relationships between covariates and metabolic effects on cognitive measures, we calculated cognitive change scores as the percentage of baseline (i.e., score during an active metabolic condition divided by the corresponding score during saline) and computed Spearman rank order correlations. We replaced missing data (∼5%) with the average of three known cells when only one of four conditions for an individual was missing; otherwise, cells were left empty. Average blood glucose levels were computed for a 30-min interval prior to cognitive testing. Insulin and average blood glucose levels were subjected to MANOVA without covariates. GH levels were very low during the octreotide infusions, and they are reported both as means (SD), calculated from the detectable samples, and as the proportion of samples that reached the limit of detection (Table 2). Baseline demographic variables were subjected to t-tests or chi square tests.
Table 2.
Neuroendocrine response to metabolic conditions.
| Placebo | Octreotide | Ins + Octreotide | Insulin | |
|---|---|---|---|---|
| Patients | ||||
| Insulin (pmol/L) | 82 (43) | 32 (17)** | 656 (256)** | 617 (228)** |
| Glucose (30-min average, mmol/L) | 5.3 (0.3) | 5.7 (0.9) | 5.6 (0.4)* | 5.5 (0.7) |
| GH (μg/L) | 0.136 (0.112) | 0.065 (0.007) | 0.093 (0.076) | 0.910 (1.905) |
| GH: detection rate (%)*** | 50 | 14 | 21 | 50 |
| Healthy Controls | ||||
| Insulin (pmol/L) | 89 (48) | 35 (18)** | 438 (373)** | 626 (322)** |
| Glucose (30-min average, mmol/L) | 5.7 (0,8) | 5.8 (0.7) | 5.9 (0.9) | 5.7 (0.6) |
| GH (μg/L) | 0.29 (0.502) | 0.04 (NA) | 0.090 (0.122) | 0.911 (1.050)* |
| GH: detection rate (%)*** | 67 | 6 | 17 | 56 |
Note. Values are expressed as mean (SD). Significantly different from placebo,
p<0.05,
p<0.001.
Percent of GH values above the assay detection limit.
3. Results
3.1. Cognitive Measures
Our primary endpoint was delayed story recall. The effects of diagnostic status (normal or memory-impaired) on this measure were overwhelming (F[1.26]=13.65, P=0.0010). Across infusion conditions, adults with intact cognition recalled approximately three times more information than did adults with impaired cognition. Therefore, results of cognitive testing were analyzed separately for diagnostic groups.
3.1.1 Memory-Impaired Adults
The amount of story information recalled differed by infusion condition (λ=0.3931, F[3,8]=4.12, p=0.0486), and the effects of infusion condition on story recall were moderated by APOE genotype n (λ=0.2506, F[3,8]=7.97, p =0.0087). Subsequent analyses used planned contrast statements to determine which specific infusion condition(s) had an effect on story recall. Insulin facilitated delayed story recall irrespective of APOE genotype (F[1,10]=8.83, p =0.0140, Fig. 1A), and the effect was moderated by APOE genotype (F[1,10]=20.04, p =0.0012, Fig. 1B);. Specifically, insulin improved delayed recall only for ε4- patients (F[1,5]=10.61, p =0.0225). The other infusion conditions did not significantly influence story recall, although Fig. 1A suggests that insulin and octreotide together facilitated story recall nearly to the same degree as did insulin alone. This observation may reflect both our small sample size (n=16) and the stronger relationship between baseline recall and recall facilitated by insulin alone (Spearman r=0.91, p<0.0001) versus otreotde+insulin-facilitated recall (Spearman r=0.69, p=0.0048).
Fig. 1.
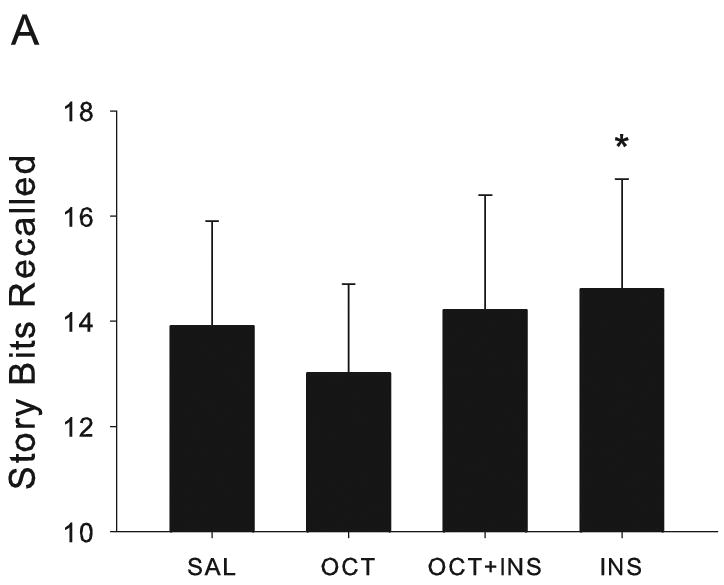
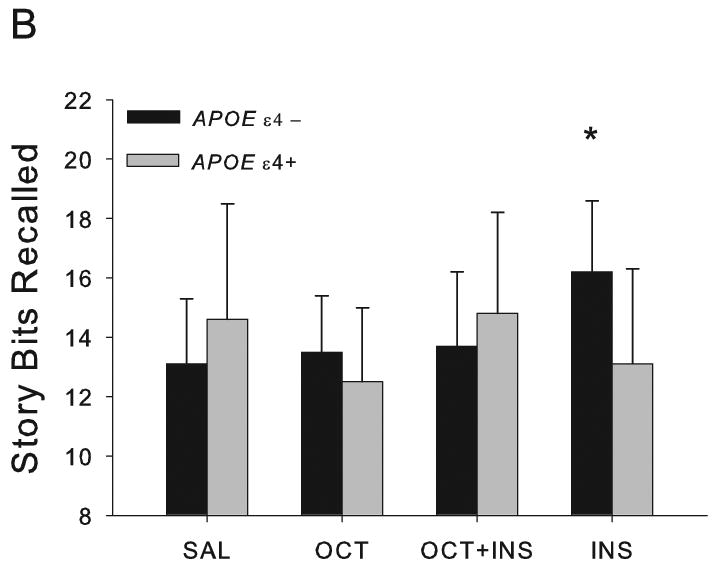
Metabolic effects on delayed story recall in memory-impaired patients. (A) Insulin facilitated delayed story recall for patients with AD or amnestic MCI (p=0.0140). (B) Insulin's effects on delayed recall were limited to patients without an APOE ε4 allele (p=0.0012). In Fig. 1, 3, and 4, data are expressed as adjusted means (SEM).
For memory-impaired adults, DRS scores may serve as an index of disease severity Interestingly, DRS scores had a significant effect on story recall (F[1,10]=11.45, p=0.0070), suggesting that disease severity may modulate the effects of octreotide and/or insulin on story recall; however, follow up analyses did not reveal a significant relationship between DRS scores and recall in any of the active conditions. Therefore, we divided-memory-impaired subjects into carriers and non-carriers of APOE ε4, and we calculated the percent change from baseline scores for each of the three active conditions. Then, we correlated these change scores with DRS scores, both for the total memory impaired sample and for memory-impaired patients by APOE genotype. For the total sample, the correlations between DRS scores and percent change in story recall were not significant. In contrast, the presence or absence of an APOE ε4 allele modulated the effects of octreotide on story recall. When subjects did not have an APOE ε4 allele, higher DRS scores were associated with a greater degree of octreotide-induced memory facilitation (Spearman r=0.7167, n=9, p =0.0298; Fig. 2). In contrast, ε4+ patients with higher DRS scores tended to have lower octreotide-induced memory facilitation (Spearman r=-0.7537, n=6, p=0.0835). (Although these Spearman p-values are not as strong as we would like, the associated correlation coefficients reveal large effects in this small sample.)
Fig. 2.
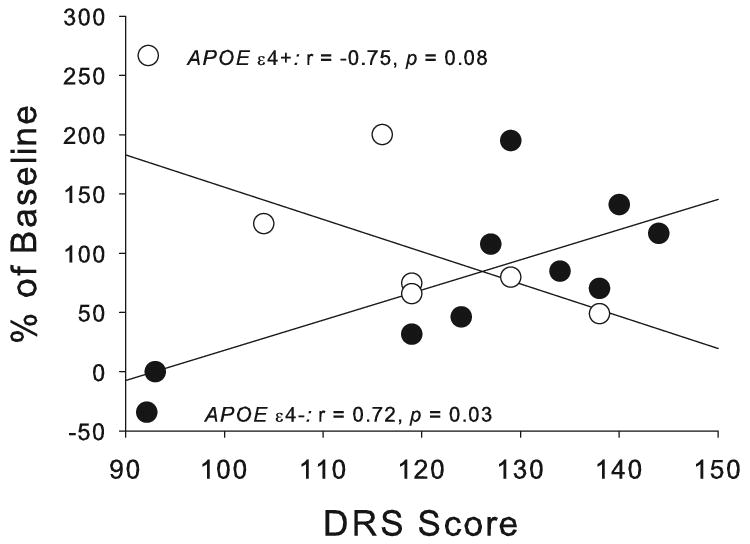
Octreotide's effects on delayed story recall in memory-impaired patients. Delayed recall scores are presented as percentage of saline performance: >100% represents octreotide-induced memory enhancement, and <100% represents octreotide-induced memory decline. APOE genotype and DRS score modulated octreotide's effects on delayed recall. For ε4- patients, individuals with the highest DRS scores also had the greatest octreotide-induced memory facilitation (Spearman r=0.7167, p=0.0298). In contrast, ε4+ patients showed a trend for higher DRS scores to be associated with lower octreotide-induced memory facilitation (Spearman r=-0.7537, p=0.0835).
For patients, octreotide alone increased SOPT errors (F[1,9]=11.91, p=0.0073, Fig. 3), whereas, insulin alone or together with octreotide did not affect errors. We did not observe metabolic effects for memory-impaired adults on the Buschke or Stroop tests.
Fig. 3.
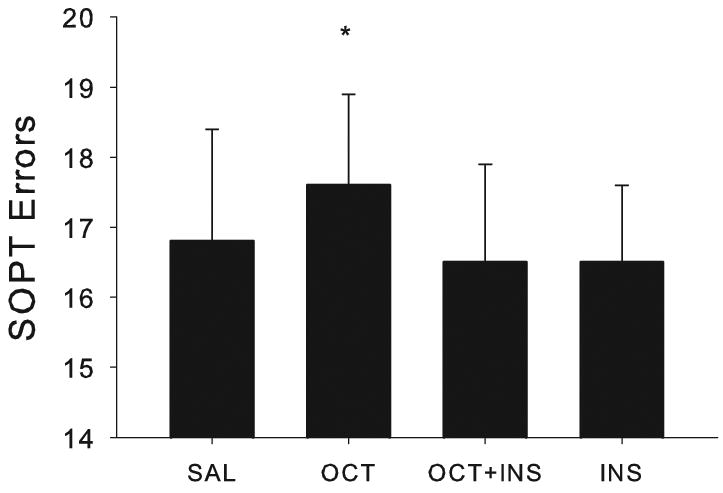
For patients, octreotide increased SOPT errors (p=0.0073).
3.1.2. Cognitively Intact Adults
Among adults without memory impairments, the amount of story information recalled differed by infusion condition (λ=0.4961, F[3,8]=3.72, p=0.0454). Therefore, we examined the effects of each active condition, relative to saline, In contrast to patients, healthy adults demonstrated memory facilitation with octreotide (F[1,13]=8.47, p=0.0122, Fig. 4), but not with insulin alone or insulin in combination with octreotide. Visual inspection of the data (Fig. 4) raises a difficult issue: the (non-significant) effect of octreotide+insulin appears to be greater than the (significant) effect of octreotide alone. We wanted to clarify this issue with a simple, straight forward approach to the data. Therefore, we computed the percent change from baseline (%recall = [active condition recall – saline recall] / saline recall × 100). The %recall score associated with octreotide alone (M[SEM]=9.1 [5.3]) was significantly larger (about 50%) than the %recall score associated with octreotide+insulin (M[SEM]=6.2 [5.5]; λ=0.7262, F[1,14]=5.28, p=0.0375). Thus, two converging tests support the notion that octreotide alone facilitated memory in intact adults. A larger sample might have provided the power to detect memory facilitation by octreotide plus insulin, as well as by octreotide alone. Metabolic effects were not observed on other cognitive measures for intact adults.
Fig. 4.
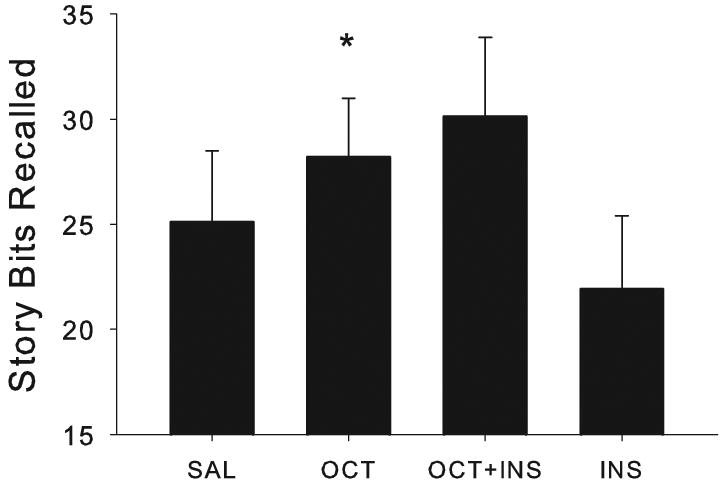
Octreotide facilitated delayed story recall for healthy older adults (p=0.0122). Neither insulin alone nor in combination with octreotide had a significant effect on memory.
3.2. Neuroendocrine Measures
In the combined (memory-impaired and intact sample, insulin administration achieved target plasma insulin levels, and octreotide administration suppressed endogenous insulin release (Table 2). Notably, octreotide together with insulin tended to lower insulin levels for healthy controls, relative to patients (F[1,29]=3.72, p=0.0636), possibly due to more efficient insulin clearance in control subjects. Unexpectedly, hyperinsulinemia in the absence of hypoglycemia significantly increased GH levels in healthy controls (t[7]=2.72, p=0.0299).
4. Discussion
As predicted, both insulin and octreotide modulated verbal declarative memory. Insulin facilitated delayed memory for APOE ε4- memory-impaired patients, but not for ε4+ patients or healthy older adults, consistent with our previous work [20,23]. Octreotide's effect on delayed memory in patients was dependent both on APOE genotype and severity of cognitive impairment. Specifically, ε4- patients with the least cognitive impairment showed the greatest octreotide-induced memory facilitation, while the opposite relationship tended to characterize ε4+ patients. We have reported that octreotide facilitated memory for patients with AD [3]; here, octreotide facilitated delayed memory for healthy older adults. Thus, the present study extended our previous findings by demonstrating the effects of octreotide on memory in healthy older adults and suggesting APOE genotype is a potential modulator of octreotide's effects on memory. Furthermore, the absence of interactive effects for co-administration of insulin and octreotide suggests that these agents facilitate memory by different mechanisms.
APOE genotype modulates insulin's effects on selective attention and memory for AD patients and healthy older adults [4]. This is the first study, to our knowledge, to show that APOE genotype modulates octreotide's effects on memory, although other investigators have shown that APOE genotype can influence AD-related changes in the somatostatinergic system. Grouselle et al. characterized the relationship of APOE genotype and somatostatin-like immunoreactivity in the frontal cortex of AD patients and cognitively intact controls [17]. Somatostatin concentrations decreased 63% more in ε4+ than in ε4- patients, but were equivalent for intact controls. Thus, possession of an APOE ε4 allele may accelerate the rate of AD-related somatostatin loss in brain and CSF [24,25]. Our findings emphasize the need to determine mechanisms that underlie the potential relationship among APOE genotype, somatostatinergic systems, and AD.
APOE-dependent effects of octreotide on memory may reflect the reciprocal relationship between the somatostatinergic and cholinergic systems [26], both of which are altered in AD. Adequate acetylcholine levels are essential for memory formation, and somatostatin contributes to memory, in part, through cholinergic activity [27]. Somatostatin activates the muscarinic cholinergic system [26], regulates the release of endogenous acetylcholine in the hippocampus [28], and enhances long-term potentiation, a model of synaptic plasticity and learning [29]. In rats, somatostatin reverses memory deficits associated with the anticholinergic compound scopolamine, as well as deficits associated with cysteamine-induced somatostatin depletion [27]. Notably, several investigators have reported cholinergic deficits related to APOE genotype, including a severe loss of choline acetyltransferase activity [30]. Given the reciprocal relationship between somatostatinergic and cholinergic systems, changes in the cholinergic system related to APOE genotype could modulate somatostatinergic effects on learning and memory.
Finally, we observed that insulin increased plasma GH levels when plasma glucose levels were held in normal range. While insulin-induced hypoglycemia invokes GH release in healthy young adults [31] and older adults [32], hyperinsulinemia in the absence of hypoglycemia reportedly has limited effects on plasma GH levels [31,32]. In contrast, Galassetti and Davis concluded that higher insulin levels (with similar levels of glucose) enhance GH secretion [33]. Although limited by the small sample size and modest GH detection limit, our observation of insulin-induced elevation of GH levels suggests that the relationship of insulin per se and GH deserves further examination.
In summary, abnormalities in both the somatostatinergic system and insulin-related energy metabolism have been associated with AD. The present study corroborates prior reports that insulin and octreotide modulate memory in patients with AD and identifies octreotide-induced memory enhancement in healthy older adults. To our knowledge, this is the first study to demonstrate that APOE genotype can modulate the cognitive effects of octreotide. Additional studies are needed to elucidate mechanisms that explain the cognitive and pathophysiological relationships among somatostatin, APOE genotype, and AD. Finally, future work is needed to confirm that insulin contributes to the regulation of GH levels, independent of hypoglycemia.
Acknowledgments
This work was supported by the Department of Veterans Affairs and by National Institutes of Health grants 5 R37 AG010880 (SC) and 1 K01 AG023650 (GSW). Dr. Craft and Dr. Watson had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. No author as a relevant conflict of interest to disclose. The authors thank the following persons for their excellent technical help: Pam Asberry, Darla Chapman, Donna Davis, Karen Enstrom, Karen Hyde, Tracia Clark, Dana Belongia, Jaime Tdwell, Michelle Keeling, Amy Morgan, Margaret Pagoria, and Monica Kletke.
References
- 1.Beal MF, Mazurek MF, Tran VT, Chattha G, Bird ED, Martin JB. Reduced numbers of somatostatin receptors in the cerebral cortex in Alzheimer's disease. Science. 1985;229:289–291. doi: 10.1126/science.2861661. [DOI] [PubMed] [Google Scholar]
- 2.Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 2004;3:169–178. doi: 10.1016/S1474-4422(04)00681-7. [DOI] [PubMed] [Google Scholar]
- 3.Craft S, Asthana S, Newcomer JW, Wilkinson CW, Matos IT, Baker LD, Cherrier M, Lofgreen C, Latendresse S, Petrova A, Plymate S, Raskind M, Grimwood K, Veith RC. Enhancement of memory in Alzheimer disease with insulin and somatostatin, but not glucose. Arch Gen Psychiatry. 1999;56:1135–1140. doi: 10.1001/archpsyc.56.12.1135. [DOI] [PubMed] [Google Scholar]
- 4.Craft S, Asthana S, Cook DG, Baker LD, Cherrier M, Purganan K, Wait C, Petrova A, Latendresse S, Watson GS, Newcomer JW, Schellenberg GD, Krohn AJ. Insulin dose-response effects on memory and plasma amyloid precursor protein in Alzheimer's disease: interactions with apolipoprotein E genotype. Psychoneuroendocrinology. 2003;28:809–822. doi: 10.1016/s0306-4530(02)00087-2. [DOI] [PubMed] [Google Scholar]
- 5.Chan-Palay V. Somatostatin immunoreactive neurons in the human hippocampus and cortex shown by immunogold/silver intensification on vibratome sections: coexistence with neuropeptide Y neurons, and effects in Alzheimer-type dementia. J Comp Neurol. 1987;260:201–223. doi: 10.1002/cne.902600205. [DOI] [PubMed] [Google Scholar]
- 6.Dutar P, Vaillend C, Viollet C, Billard JM, Potier B, Carlo AS, Ungerer A, Epelbaum J. Spatial learning and synaptic hippocampal plasticity in type 2 somatostatin receptor knock-out mice. Neuroscience. 2002;112:455–466. doi: 10.1016/s0306-4522(02)00074-x. [DOI] [PubMed] [Google Scholar]
- 7.Tokita K, Inoue T, Yamazaki S, Wang F, Yamaji T, Matsuoka N, Mutoh S. FK962, a novel enhancer of somatostatin release, exerts cognitive-enhancing actions in rats. Eur J Pharmacol. 2005;527:111–120. doi: 10.1016/j.ejphar.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 8.Ramos B, Baglietto-Vargas D, Rio JC, Moreno-Gonzalez I, Santa-Maria C, Jimenez S, Caballero C, Lopez-Tellez JF, Khan ZU, Ruano D, Gutierrez A, Vitorica J. Early neuropathology of somatostatin/NPY GABAergic cells in the hippocampus of a PS1xAPP transgenic model of Alzheimer's disease. Neurobiol Aging. 2005 doi: 10.1016/j.neurobiolaging.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 9.Kumar U. Expression of somatostatin receptor subtypes (SSTR1-5) in Alzheimer's disease brain: an immunohistochemical analysis. Neuroscience. 2005;134:525–538. doi: 10.1016/j.neuroscience.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Watson GS, Cholerton BA, Reger MA, Baker LD, Plymate SR, Asthana S, Fishel MA, Kulstad JJ, Green PS, Cook DG, Kahn SE, Keeling ML, Craft S. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry. 2005;13:950–958. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- 11.Krentz AJ, Boyle PJ, Macdonald LM, Schade DS. Octreotide: a long-acting inhibitor of endogenous hormone secretion for human metabolic investigations. Metabolism. 1994;43:24–31. doi: 10.1016/0026-0495(94)90153-8. [DOI] [PubMed] [Google Scholar]
- 12.Watson GS, Craft S. Modulation of memory by insulin and glucose: neuropsychological observations in Alzheimer's disease. Eur J Pharmacol. 2004;490:97–113. doi: 10.1016/j.ejphar.2004.02.048. [DOI] [PubMed] [Google Scholar]
- 13.Jaehde U, Masereeuw R, De Boer AG, Fricker G, Nagelkerke JF, Vonderscher J, Breimer DD. Quantification and visualization of the transport of octreotide, a somatostatin analogue, across monolayers of cerebrovascular endothelial cells. Pharm Res. 1994;11:442–448. doi: 10.1023/a:1018929508018. [DOI] [PubMed] [Google Scholar]
- 14.Zipser BD, Johanson CE, Gonzalez L, Berzin TM, Tavares R, Hulette CM, Vitek MP, Hovanesian V, Stopa EG. Microvascular injury and blood-brain barrier leakage in Alzheimer's disease. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Karalis K, Mastorakos G, Chrousos GP, Tolis G. Somatostatin analogues suppress the inflammatory reaction in vivo. J Clin Invest. 1994;93:2000–2006. doi: 10.1172/JCI117193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 17.Grouselle D, Winsky-Sommerer R, David JP, Delacourte A, Dournaud P, Epelbaum J. Loss of somatostatin-like immunoreactivity in the frontal cortex of Alzheimer patients carrying the apolipoprotein epsilon 4 allele. Neurosci Lett. 1998;255:21–24. doi: 10.1016/s0304-3940(98)00698-3. [DOI] [PubMed] [Google Scholar]
- 18.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 19.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 20.Craft S, Asthana S, Schellenberg G, Cherrier M, Baker LD, Newcomer J, Plymate S, Latendresse S, Petrova A, Raskind M, Peskind E, Lofgreen C, Grimwood K. Insulin metabolism in Alzheimer's disease differs according to apolipoprotein E genotype and gender. Neuroendocrinology. 1999;70:146–152. doi: 10.1159/000054469. [DOI] [PubMed] [Google Scholar]
- 21.Petrides M, Milner B. Deficits on subject-ordered tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia. 1982;20:249–262. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- 22.Kahn SE, Leonetti DL, Prigeon RL, Boyko EJ, Bergstrom RW, Fujimoto WY. Relationship of proinsulin and insulin with noninsulin-dependent diabetes mellitus and coronary heart disease in Japanese-American men: impact of obesity--clinical research center study. J Clin Endocrinol Metab. 1995;80:1399–1406. doi: 10.1210/jcem.80.4.7714116. [DOI] [PubMed] [Google Scholar]
- 23.Craft S, Asthana S, Schellenberg G, Baker L, Cherrier M, Boyt AA, Martins RN, Raskind M, Peskind E, Plymate S. Insulin effects on glucose metabolism, memory, and plasma amyloid precursor protein in Alzheimer's disease differ according to apolipoprotein-E genotype. Ann N Y Acad Sci. 2000;903:222–228. doi: 10.1111/j.1749-6632.2000.tb06371.x. [DOI] [PubMed] [Google Scholar]
- 24.Heilig M, Sjogren M, Blennow K, Ekman R, Wallin A. Cerebrospinal fluid neuropeptides in Alzheimer's disease and vascular dementia. Biol Psychiatry. 1995;38:210–216. doi: 10.1016/0006-3223(94)00239-Y. [DOI] [PubMed] [Google Scholar]
- 25.Reinikainen KJ, Riekkinen PJ, Jolkkonen J, Kosma VM, Soininen H. Decreased somatostatin-like immunoreactivity in cerebral cortex and cerebrospinal fluid in Alzheimer's disease. Brain Res. 1987;402:103–108. doi: 10.1016/0006-8993(87)91052-3. [DOI] [PubMed] [Google Scholar]
- 26.Nakata A, Saito H, Nishiyama N. Facilitatory role of somatostatin via muscarinic cholinergic system in the generation of long-term potentiation in the rat dentate gyrus in vivo. Brain Res. 1996;723:135–140. doi: 10.1016/0006-8993(96)00233-8. [DOI] [PubMed] [Google Scholar]
- 27.Matsuoka N, Maeda N, Yamaguchi I, Satoh M. Possible involvement of brain somatostatin in the memory formation of rats and the cognitive enhancing action of FR121196 in passive avoidance task. Brain Res. 1994;642:11–19. doi: 10.1016/0006-8993(94)90900-8. [DOI] [PubMed] [Google Scholar]
- 28.Araujo DM, Lapchak PA, Collier B, Quirion R. Evidence that somatostatin enhances endogenous acetylcholine release in the rat hippocampus. J Neurochem. 1990;55:1546–1555. doi: 10.1111/j.1471-4159.1990.tb04937.x. [DOI] [PubMed] [Google Scholar]
- 29.Matsuoka N, Kaneko S, Satoh M. Somatostatin augments long-term potentiation of the mossy fiber-CA3 system in guinea-pig hippocampal slices. Brain Res. 1991;553:188–194. doi: 10.1016/0006-8993(91)90823-e. [DOI] [PubMed] [Google Scholar]
- 30.Poirier J, Delisle MC, Quirion R, Aubert I, Farlow M, Lahiri D, Hui S, Bertrand P, Nalbantoglu J, Gilfix BM, Gauthier S. Apolipoprotein E4 allele as a predictor of cholinergic deficits and treatment outcome in Alzheimer disease. Proc Natl Acad Sci U S A. 1995;92:12260–12264. doi: 10.1073/pnas.92.26.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Degn KB, Brock B, Juhl CB, Djurhuus CB, Grubert J, Kim D, Han J, Taylor K, Fineman M, Schmitz O. Effect of intravenous infusion of exenatide (synthetic exendin-4) on glucose-dependent insulin secretion and counterregulation during hypoglycemia. Diabetes. 2004;53:2397–2403. doi: 10.2337/diabetes.53.9.2397. [DOI] [PubMed] [Google Scholar]
- 32.Meneilly GS, Cheung E, Tuokko H. Altered responses to hypoglycemia of healthy elderly people. J Clin Endocrinol Metab. 1994;78:1341–1348. doi: 10.1210/jcem.78.6.8200936. [DOI] [PubMed] [Google Scholar]
- 33.Galassetti P, Davis SN. Effects of insulin per se on neuroendocrine and metabolic counter-regulatory responses to hypoglycaemia. Clin Sci (Lond) 2000;99:351–362. [PubMed] [Google Scholar]


