Abstract
We have recently reported reduced telomere length in T lymphocytes of individuals with Down syndrome (DS) and dementia due to Alzheimer’s disease (AD). We have now replicated and extended that study by finding that people with DS and mild cognitive impairment (MCI-DS) also have shorter telomeres than people with DS without MCI-DS. Additional new findings demonstrated that light intensity measurements from chromosome 21 alone, or in concert with chromosomes 1, 2, and 16, exhibited shorter telomeres in adults with DS and with either dementia or MCI-DS compared to aging per se. Chromosome 21 measurements appeared to be especially promising for use as a biomarker because there was no overlap in the distribution of light intensity measurement scores between demented or MCI-DS and non-demented participants. Given that early clinical symptoms of AD can be very difficult to recognize in this population of adults due to their pre-existing cognitive impairments, a valid biomarker would be of great value. Early detection is especially important because it would allow treatments to begin before significant damage to the central nervous system has occurred. Our findings suggest that it may be feasible to use telomere shortening as a biomarker for accurately inferring dementia status.
Keywords: Down syndrome, PNA, FISH, Dementia, Mild cognitive impairment, Telomere shortening, Metaphase, Interphase, Individual chromosomes 21, 1, 2, 16
1. Introduction
Telomeres, chromosome ends consisting of highly conserved TTAGGG repeats, become shorter with every cell division, and increased telomere shortening has been associated with a variety of conditions that include replicative cellular senescence, apoptosis (Allsopp et al., 1992; Hao et al., 2004), neoplastic transformation (Plentz et al., 2004), in vivo cellular aging (Hastie et al., 1990; Lindsey et al., 1991; Ahmed et al., 2001; Flanary et al., 2003), stress (Epel et al., 2004; Damjanovic et al., 2007), heart disease (Samani et al., 2001; Benetos et al., 2004), osteoporosis (Valdes et al., 2007), obesity (Valdes et al., 2005), dyskeratosis congenita (Vulliamy and Dokal, 2007), Alzheimer’s disease (AD) (Panossian et al., 2003), and Down syndrome (DS) (Jenkins et al., 2006). The recent discovery of UUAGGG-repeat telomeric RNAs may provide better understanding of the role of the telomere in the above conditions (Schoeftner and Blasco, 2007).
We have previously reported that telomeres were shorter in metaphase and interphase preparations from short-term whole blood cultures of nine women with DS and dementia compared to age-matched women with DS without dementia (Jenkins et al., 2006). We have replicated this result in another six individuals with DS and dementia, including two males aged 58 and 62, and their matched non-demented controls. We also examined whether individual chromosomes may be used alone (separately from whole metaphases or interphases) to distinguish between individuals with DS with and without dementia, and for this purpose we selected chromosomes 1, 2, 16 and 21 as examples that span the full spectrum of chromosome length. Chromosome 21 was selected explicitly because of its pivotal role in DS and the influence of triplication of the gene for amyloid precursor protein (APP) in the high risk for AD observed among adults with DS. Additionally, we have expanded our analysis to include individuals with DS who were exhibiting declines in cognition analogous to mild cognitive impairment (MCI) in the general population (MCI-DS) to see if their telomeres were reduced in size compared to sex- and age-matched peers without MCI.
MCI is defined clinically as a stage that is intermediate between the declines in cognition typical of brain aging, per se, and the deficits that characterize dementia, during which activities of daily living are largely unaffected (e.g., Gauthier et al., 2006; Petersen et al., 1999; Winblad et al., 2004). Very importantly, observed declines are of insufficient severity to meet the diagnostic criteria for dementia (Petersen et al., 1999). Individuals with MCI, especially those with decline in memory functions, are more likely to convert to dementia than their peers without MCI (Petersen et al., 1999). As with the diagnosis of dementia, identifying MCI in adults with DS is complicated by the presence of lifelong cognitive deficits and substantial interindividual variability in baseline abilities. However, longitudinal assessments of cognition can be used to document changes suggestive of MCI within this population, and that was the procedure employed herein (Devenny et al, 2000; Silverman et al, 2004).
2. Materials and methods
2.1
Adult participants with DS were recruited using an Institutional Review Board-approved protocol with informed consent provided by correspondents and participant assent. A community-based sample of 234 women (45–78 years of age) and 124 men with DS (45–73 years of age) was ascertained through the New York State Developmental Disability Service System or direct contacts with provider agencies in neighboring states. These individuals have been participating in a longitudinal study examining changes in functioning associated with aging and dementia in adults with intellectual disability and have been evaluated at 14–18 month intervals to determine cognitive, functional and health status (Silverman et al., 2004). To examine telomere length from short-term T lymphocyte cultures, we obtained whole blood samples from 13 pairings of females and two pairings of males who were of the same sex and age and whose blood was drawn at the same time. All samples were from individuals with complete trisomy 21. The matched pair study design allowed us to assay paired samples together. Because individuals with MCI or dementia are generally older than those who are free of dementia, the number of possible pairings constrained our sample size severely. However, matching closely on participant age, sex and time of blood draw provided controls on conditions that we felt necessary for these initial studies. Each pair consisted of age- and sex-matched individuals, one of whom had a diagnosis of dementia or MCI-DS, while the other exhibited no signs of AD-related functional and/or cognitive decline. This sample includes three of the nine pairings described in our previous publication (Jenkins et al., 2006). Samples from females have been more available than males because of an ongoing project focused exclusively on women’s health [Schupf et al., 2003].
The dementia status of each participant was determined at a consensus conference which included all senior staff members participating in our longitudinal studies and research assistants who had direct contact with the participants under consideration (Silverman et al., 2004; Zigman et al., 2004). Participants were classified based upon consideration of all information available from a detailed review of clinical records, informant interviews, and direct assessments of selected cognitive functions, including evidence of decline over the 14- to 20-month period between assessments. Dementia status was classified consistent with diagnostic guidelines recommended by the AAMR-IASSID Working Group for the Establishment of the Criteria for the Diagnosis of Dementia in Individuals with Developmental Disability (Aylward et al., 1997; Burt & Aylward, 2000), that were based upon current ICD-10 criteria (WHO, 1992). Each case was classified as: (a) non-demented, indicating with reasonable certainty that significant age-associated impairment was absent; (b) MCI-DS status, indicating that there was substantial uncertainty regarding dementia status, with some indication of mild cognitive and/or functional decline but importantly, the observed change(s) did not meet dementia criteria; (c) possible dementia, indicating that some signs and symptoms of dementia were present, but declines over time were not judged to be totally convincing, although there was more certainty than for the MCI-DS status classification; (d) definite dementia, indicating with reasonable confidence that dementia was present based upon substantial decline over time and absence of other conditions that might mimic dementia (e.g., untreated hypothyroidism). For each participant who was rated as having possible or definite dementia, findings were reviewed to establish a differential diagnosis. These were either AD or AD in combination with possible other cause(s) (e.g., Parkinson’s disease), given the substantial AD neuropathology characteristic of DS at these ages. Participants could also be categorized as (e) status uncertain due to complications, indicating that the criteria for possible dementia had been met, but symptoms might be caused by some other substantial concern, usually a medical condition unrelated to a dementing disorder (e.g., severe sensory loss, poorly resolved hip fracture, psychiatric diagnosis), and (f) indeterminable, indicating that the preexisting disability was of such severity that detection of decline indicative of dementia was not possible (e.g., profound ID with multiple handicaps). Participants in these latter two categories were not included in the present study.
2.2. Telomere length measurements
Telomere length was determined by measuring changes in fluorescence intensity using an FITC-labeled peptide nucleic acid (PNA) probe (Applied Biosystems) and Applied Imaging software similar to that previously reported (Lansdorp et al., 1996; Londoño-Vallejo et al., 2001). Analyses of metaphase and interphase preparations from short-term whole blood cultures were carried out using quantitative PNA FISH technology blind to dementia status. Light intensities from individual chromosomes 21 and 1 were determined for Studies 1–15, while similar analyses for chromosomes 2 and 16 were carried out in only a subset of pairs, Studies 3–6, 8–10, and 13. For each individual, 20 cells were assessed for each analysis and the mean and standard deviation of the readings were computed and compared statistically within each pair of specimens from individuals with DS with and without dementia or MCI-DS, using the Student’s t-test. *
2.3 Apolipoprotein E (APOE) genotypes
APOE genotyping was carried out as described in a previous study [Schupf et al., 1996] by PCR/RFLP analysis using HhaI (CfoI) digestion of an APOE genomic PCR product spanning the polymorphic (cys/arg) sites at codons 112 and 158, followed by acrylamide gel electrophoresis to document the restriction fragment sizes [Hixson & Vernier 1990]. Participants were classified according to the presence or absence of an APOE ε4 allele.
3. Results
Figure 1 illustrates a metaphase and interphase from a short-term whole blood culture, hybridized with an FITC-labeled PNA probe such that telomeres are labeled at each chromosome end and within the interphase nucleus. An initial set of analyses replicated our earlier studies of overall telomere shortening associated with dementia in six adults with DS. In all cases t-tests revealed significantly shorter telomere lengths for the individual with dementia, ps <.001 for metaphase analyses and ps<.02 for interphase analyses (data available but not shown).
Fig. 1.
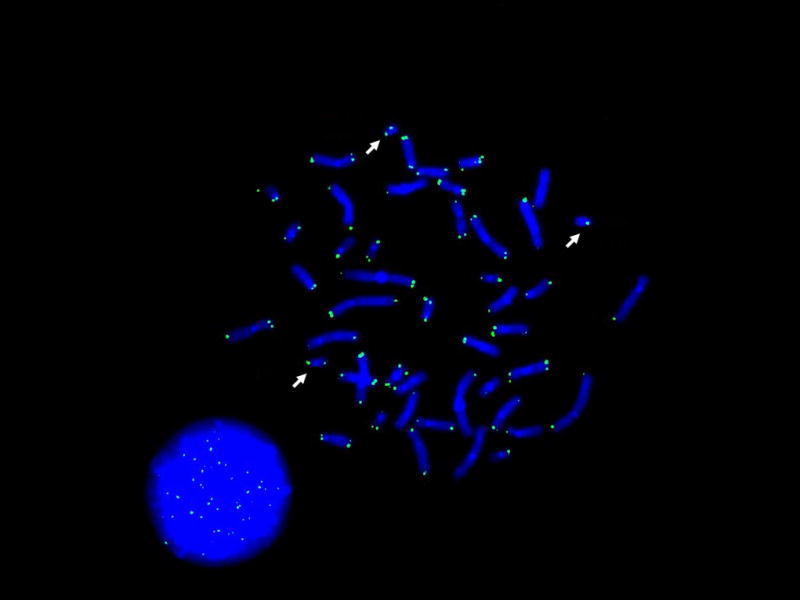
Telomeres in metaphase and interphase shown by an FITC-labeled PNA probe with DAPI counterstaining. Arrows indicate chromosome 21.
The age, sex and dementia status of the study participants are given in Table 1. Results extending our findings to individuals with MCI-DS are presented in Table 2. Each of six pairs of individuals was compared for both metaphase and interphase measures, and in all cases MCI-DS was associated with significantly shorter telomeres than those of their age-matched peers without MCI-DS or dementia. Tables 3 and 4 summarize our studies of individual chromosomes 21, 1, 2, and 16. Here again, both dementia and MCI-DS were associated with telomere shortening in every case, although the p value for the chromosome 1 comparison in Study 1 only reached significance at the one-tailed level. The number of matched pairs for Chromosomes 2 and 16 is not complete at this time due to the departure of a member of our research team. However, the subset of completed individuals should be representative of the larger group.
Table 1.
Age, sex and dementia status of 30 individuals with trisomy 21
| Study No. | Age (yrs) | Sex | Dementia Status |
|---|---|---|---|
| 1 | 58.31 | F | PD2 |
| 1 | 60.3 | F | ND |
| 2 | 53.8 | F | DD |
| 2 | 57 | F | ND |
| 3 | 58.8 | F | PD |
| 3 | 60.6 | F | ND |
| 4 | 62.5 | M | DD |
| 4 | 63.2 | M | ND |
| 5 | 58 | M | PD |
| 5 | 64.5 | M | ND |
| 6 | 59 | F | DD |
| 6 | 65.4 | F | ND |
| 7 | 66.9 | F | PD |
| 7 | 69.8 | F | ND |
| 8 | 57 | F | DD |
| 8 | 58 | F | ND |
| 9 | 55.8 | F | DD |
| 9 | 57.8 | F | ND |
| 10 | 49.8 | F | MCI |
| 10 | 50.2 | F | ND |
| 11 | 54.2 | F | MCI |
| 11 | 54.6 | F | ND |
| 12 | 51.5 | F | MCI |
| 12 | 53 | F | ND |
| 13 | 53 | F | MCI |
| 13 | 53.3 | F | ND |
| 14 | 55.9 | F | MCI |
| 14 | 56.8 | F | ND |
| 15 | 55.0 | F | MCI |
| 15 | 59.9 | F | ND |
At time of short-term whole blood culture
DD, PD, ND = definite, probable, no dementia; MCI = mild cognitive impairment
Table 2.
Mean light intensity units (×104) are shown from within pairs for whole metaphases and interphases matched specimens from individuals with DS with and without MCI-DS.
| Study | Dementia Status | Metaphase (SD)* | Interphase (SD) |
|---|---|---|---|
| 10 | MCI-DS | 13.0 (2.7) | 17.5 (3.4) |
| 10 | ND | 19.4 (4.4) | 22.1 (3.2) |
| 11 | MCI-DS | 21.6 (3.6) | 19.6 (2.5) |
| 11 | ND | 26.1 (3.1) | 22.9 (2.8) |
| 12 | MCI-DS | 18.7 (2.1) | 16.7 (2.3) |
| 12 | ND | 22.9 (4.2) | 18.7 (3.3) |
| 13 | MCI-DS | 16.8 (2.8) | 15.2 (2.0) |
| 13 | ND | 21.9 (3.4) | 17.5 (3.0) |
| 14 | MCI-DS | 15.0 (3.2) | 16.4 (4.1) |
| 14 | ND | 22.3 (3.8) | 22.3 (2.8) |
| 15 | MCI-DS | 17.3 (3.1) | 19.0 (2.7) |
| 15 | ND | 33.7 (6.6) | 25.1 (4.0) |
SD (standard deviation); raw data, based on light intensity measurements of 20 metaphases and interphases per subject are available but not shown; p values for metaphase and inter-phase comparisons ranged from p<.0003–<.000001 and p<.04–<.000005, respectively.
Table 3.
Mean light intensity units (×103) for chromo-somes (C) 21 and 1.
| Study | Dementia Status | C21 | C1 |
|---|---|---|---|
| 1 | PD | 5.6 (1.9)* | 4.1 (2.0) |
| 1 | ND | 10.3 (2.8) | 5.5 (2.8) |
| 2 | DD | 7.0 (2.2) | 6.6 (1.7) |
| 2 | ND | 11.4 (3.8) | 9.7 (3.3) |
| 3 | PD | 7.2 (2.4) | 5.5 (2.5) |
| 3 | ND | 11.0 (2.7) | 9.9 (3.2) |
| 4 | DD | 7.7 (2.4) | 6.3 (2.6) |
| 4 | ND | 13.0 (4.0) | 9.5 (3.3) |
| 5 | PD | 5.0 (2.2) | 4.6 (2.1) |
| 5 | ND | 9.8 (2.2) | 7.3 (1.9) |
| 6 | DD | 7.7 (2.7) | 7.3 (2.2) |
| 6 | ND | 10.1 (2.7) | 9.5 (2.5) |
| 7 | PD | 5.4 (1.6) | 5.7 (2.6) |
| 7 | ND | 11.0 (2.8) | 9.3 (3.1) |
| 8 | DD | 8.1 (2.8) | 7.1 (1.8) |
| 8 | ND | 13.0 (3.2) | 11.2 (3.2) |
| 9 | DD | 6.4 (3.6) | 4.7 (3.0) |
| 9 | ND | 14.7 (5.7) | 10.6 (4.0) |
| Subtotal n=9 | PD/DD | 6.7 (1.1) | 5.8 (1.1) |
| Subtotal n=9 | ND | 11.6 (1.6) | 9.2 (1.7) |
| 10 | MCI-DS | 5.7 (2.3) | 5.4 (1.8) |
| 10 | ND | 10.6 (3.4) | 7.5 (3.3) |
| 11 | MCI-DS | 8.1 (2.2) | 7.9 (1.6) |
| 11 | ND | 15.6 (2.5) | 12.4 (2.6) |
| 12 | MCI-DS | 9.3 (2.4) | 8.3 (1.3) |
| 12 | ND | 13.5 (3.7) | 10.0 (3.0) |
| 13 | MCI-DS | 7.5 (2.6) | 7.5 (2.8) |
| 13 | ND | 12.6 (3.4) | 10.3 (2.9) |
| 14 | MCI-DS | 6.1 (2.6) | 4.9 (2.5) |
| 14 | ND | 12.4 (3.6) | 7.7 (3.5) |
| 15 | MCI-DS | 9.0 (2.4) | 7.1 (2.4) |
| 15 | ND | 16.8 (5.1) | 14.9 (4.4) |
| Subtotal n=6 | MCI-DS | 7.6 (1.5) | 6.9 (1.4) |
| Subtotal n=6 | ND | 13.6 (2.3) | 10.5 (2.8) |
| Total n=15 | DD/PD/MCI | 7.1 (1.3)** | 6.2 (1.3) |
| Total n=15 | ND | 12.4 (2.1) | 9.7 (2.2) |
mean light intensity is 5.6 with a standard deviation of (1.9); p values, for chromosomes 21 and 1, ranged from <.008–<.000000 and <.031–<.000000, respectively, except for study 1, C1, that reached only one-tailed significance.
overall mean light intensity and standard deviation for chromosome 21 from individuals with DS and Definite or probable dementia (DD or PD) or Mild cognitive impairment (MCI); values for ND as well as those for chromosomes 1, 2, and 16 are also given.
Table 4.
Mean light intensity units (×103) for chromosomes (C) 2, and 16
| Study | Dementia Status | C2* | C16* |
|---|---|---|---|
| 3 | PD | 5.8 (2.6) | 5.0 (2.0) |
| 3 | ND | 11.0 (3.0) | 8.0 (2.4) |
| 4 | DD | 6.8 (3.0) | 5.8 (2.8) |
| 4 | ND | 9.2 (3.6) | 8.2 (4.2) |
| 5 | PD | 4.6 (2.5) | 3.7 (2.6) |
| 5 | ND | 7.4 (3.3) | 7.0 (2.8) |
| 6 | DD | 5.9 (2.6) | 5.1 (2.4) |
| 6 | ND | 8.1 (2.8) | 7.3 (2.6) |
| 8 | DD | 7.3 (2.0) | 5.3 (2.0) |
| 8 | ND | 11.4 (4.1) | 8.4 (2.7) |
| 9 | DD | 5.0 (2.4) | 3.4 (2.2) |
| 9 | ND | 10.2 (3.7) | 8.4 (4.3) |
| Subtotal n=6 | PD/DD | 5.9(1.0) | 4.7(.9) |
| Subtotal n=6 | ND | 9.6(1.6) | 7.9 (0.6) |
| 10 | MCI-DS | 5.2 (2.0) | 2.8 (1.7) |
| 10 | ND | 8.1 (3.2) | 5.9 (3.0) |
| 13 | MCI-DS | 6.3 (2.4) | 5.9 (2.6) |
| 13 | ND | 8.4 (1.8) | 7.9 (3.0) |
| Subtotal n=2 | MCI-DS | 5.8(0.8) | 4.4(2.2) |
| Subtotal n=2 | ND | 8.2(0.2) p<.05 | 6.9(1.4) p>.3 |
| Total n=8 | DD/PD/MCI | 5.9 (0.9) | 4.6 (1.2) |
| Total n=8 | ND | 9.2 (1.5) | 7.6 (0.9) |
p values, for chromosomes 2 and 16, ranged from <.046–<.000000 and <.04–<.000000, respectively.
To determine if the effects of dementia or MCI-DS differed among the selected chromosomes, an additional repeated measures multivariate analysis of variance was run using the General Linear Models module within Statistica 8.0 (Statsoft, 2007). Chromosome number (1, 2, 16 and 21) and Group (Dementia/MCI-DS versus Not Demented) were factors, and, as expected, fluorescence signal was greater for the Not Demented Group, F (1,7) = 83.8, p < .00005. In addition, a significant Chromosome by Group interaction was also present, F (3, 5) = 11.48, p < 0.02. Further analysis indicated that this interaction was due to a greater effect of dementia/MCI-DS on telomere shortening for Chromosome 21 compared to the other chromosomes. Given the presence of an extra Chromosome 21 within each cell, this analysis was repeated with the chromosome 21 values divided by 1.5 to determine if trisomy alone was responsible for the differential effect, and in fact the interaction no longer approached significance (with both multivariate and univariate Fs < 1.0). Although fluorescence signals were larger for the two longer chromosomes (1 & 2) compared to the two shorter chromosomes (16 & 21), F (3,5) = 18.6, p < .005, the impact of dementia/MCI-DS on this signal was comparable for the four chromosomes studied. Analyses were repeated omitting the MCI-DS cases and results were essentially unchanged.
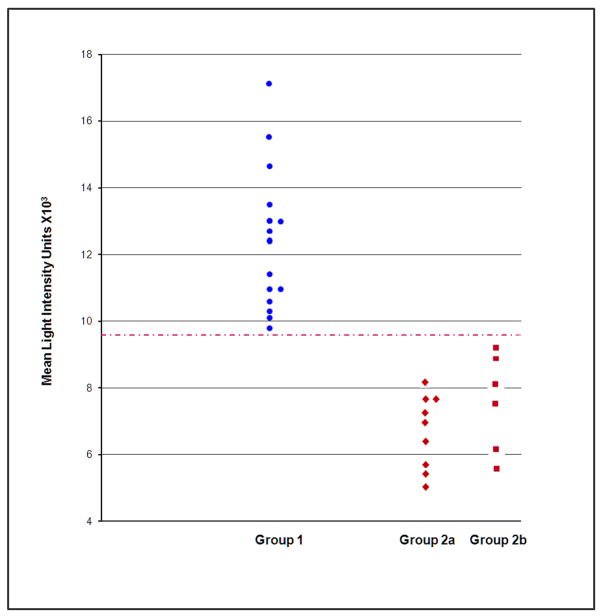
Detailed inspection of the results from our individual chromosome studies showed that there was no overlap in the distribution of scores between demented or MCI-DS and non-demented participants in the case of Chromosome 21, t (30) = 8.3, p<.000001. These data are illustrated in Figure 2. As indicated in Tables 3 and 4, there was also little to no overlap in the distributions of scores for Chromosomes 2 and 16, and modest overlap for Chromosome 1. Non-parametric group comparisons consistently showed that mean telomere lengths were significantly shorter for the Dementia/MCI-DS group. Wilcoxon Signed Ranks Test significance ranged from .005<ps<.05.
Fig. 2.
Chromosome 21 light intensities from 15 individuals with DS only (no dementia, no MCI) vs. those with DS and Dementia DS and MCI. Note there was no overlap in values between the groups labeled Dementia or MCI and the group labeled No Dementia, No MCI (although there was overlap within each group).
To determine if inferred telomere shortening was due to severity of cognitive impairment, fluorescence signals of individuals within the Dementia/MCI-DS group were related to both IQ (obtained prior to the onset of dementia signs and symptoms) and a measure of dementia severity (Silverman et al, 2004). None of these correlations approached significance, rs < .17, ps > 0.55, suggesting that effects of dementia/MCI-DS on fluorescence signal can be expected to be relatively consistent within this population. The apolipoprotein E (APOE) ε4 allele has been associated with increased risk for dementia (Schupf et al., 1996) and with early mortality (Zigman et al, 2005) in this population. Possible effects of APOE genotype were also examined by focusing on: (a) overall fluorescence signals within the Dementia/MCI-DS group (chromosomes 1 + 21; 1 + 2 + 16 + 21), (b) overall fluorescent signals within the comparison group, and (c) the difference between the Dementia/MCI and comparison groups. Analyses involving the Dementia/MCI-DS group were conducted twice, once including all cases and once excluding the MCI-DS cases to reduce any confound with stage of progression of Alzheimer’s disease. None of these analyses produced a significant effect.
4. Discussion
We have used a number of converging analyses to show that shorter telomeres occur in people with DS and AD or MCI-DS compared to unaffected individuals with DS. We have shown this in both whole metaphase and interphase preparations and have begun to investigate whether such differences can be detected with individual chromosomes or groups of chromosomes. Chromosome 21 findings appeared to be the most promising for clinical application given the evidence of high sensitivity and specificity provided by the lack of overlap in the distribution of telomere lengths between groups. There was no overlap in telomere length for Chromosome 21 between unaffected and affected individuals indicating both high sensitivity and high specificity. This suggests that it may not be necessary to collect and process samples from individuals with suspected dementia/MCI-DS and matched unaffected controls simultaneously which will greatly expand the utility of the assay as a biomarker of risk. However, it must be recognized that many other chromosomes (and combinations of chromosomes) were not examined here. While the present data provide proof of principle, additional research is needed to discover which fluorescence signals provide maximum separation in the distributions between patient populations and unaffected individuals at risk. Further additional research will be needed to discover the mechanisms underlying the relationship between telomere length and the emergence of age-associated impairments due to AD, as well as factors that might be contributing to individual differences in findings (e.g., Aβ levels in CSF or blood; APOE genotype).
Further work on unrelated samples from affected and unaffected individuals will be conducted to provide the foundation for a diagnostic procedure having both high sensitivity and specificity. Since there is as yet no clear biomarker for dementia/AD, a longitudinal study would be useful to determine whether increased telomere shortening in individuals with DS precedes clinical signs of dementia, and to determine the underlying mechanism responsible for this association. Recognition of pre-clinical and early dementia would be very useful in this population of adults who already have pre-existing cognitive impairments and who are at high risk for dementia. Early detection is especially important because it would contribute to treatment and intervention decisions at a point in time when damage to the central nervous system caused by this devastating and progressive disease could be minimized. Further, a valid biomarker would be of enormous value for differential diagnosis, given the variety of conditions that can cause behavioral or cognitive changes in older adults with DS.
Acknowledgments
Thanks are due the research participants and various cooperating agencies as well as all staff involved in this project. We also thank G. Y. Wen, Ph.D., and W. Ted Brown, M.D., Ph.D., for constructive comments during manuscript preparation, Ezzat El-Akkad of our Institute’s Graphics Resources and Multimedia Services (GRAMS) and Lawrence Black, Institute Librarian. This work was supported in part by the New York State Office of Mental Retardation and Developmental Disabilities, Alzheimer’s Association grants IRG-07-60558, IIRG-99-1598 and IIRG-96-077; NIH grants PO1-HD35897, RO1-HD37425, and RO1-AG014673.
Footnotes
Disclosure Statement
There are no actual or potential conflicts of interest among the authors of this article.
References
- Ahmed A, Tollefsbol T. Telomeres and telomerase: basic science implications for aging. J Am Geriatr Soc. 2001;49:1105–9. doi: 10.1046/j.1532-5415.2001.49217.x. [DOI] [PubMed] [Google Scholar]
- Allsopp RC, Caziri H, Patterson C, Boldsten S, Younglai EV, Futcher AB, Greider CW, Harley CB. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci USA. 1992;89:10114–8. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward E, Burt D, Thorpe L, Lai F, Dalton A. Diagnosis of dementia in individuals with intellectual disability. J Intellect Disabil Res. 1997;41:162–64. doi: 10.1111/j.1365-2788.1997.tb00692.x. [DOI] [PubMed] [Google Scholar]
- Benetos A, Gardner JP, Zureik M, Labat C, Xiaobin L, Adamopoulos C, Temmar M, Bean KE, Thomas F, Aviv A. Short telomeres are associated with increased carotid atherosclerosis in hypertensive subjects. Hypertension. 2004;43(20):182–5. doi: 10.1161/01.HYP.0000113081.42868.f4. [DOI] [PubMed] [Google Scholar]
- Burt DB, Aylward E. Test battery for the diagnosis of dementia in individuals with intellectual disability. J Intellect Disabil Res. 2000;44:262–70. doi: 10.1046/j.1365-2788.2000.00264.x. [DOI] [PubMed] [Google Scholar]
- Damjanovic AK, Yang Y, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, Zou U, Beversdorf DQ, Weng NP. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease patients. J Immunol. 2007;179:4249–54. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenny DA, Krinsky-McHale SJ, Sersen G, Silverman WP. Sequence of cognitive decline in dementia in adults with Down’ syndrome. J Intellect Disabil Res. 2000;44 ( Pt 6):654–65. doi: 10.1046/j.1365-2788.2000.00305.x. [DOI] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. PNAS. 2004;101(49):17312–15. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanary BE, Streit WJ. Telomeres shorten with age in rat cerebellum and cortex in vivo. J Anti- Aging Med. 2003;6:299–308. doi: 10.1089/109454503323028894. [DOI] [PubMed] [Google Scholar]
- Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, Bellevil H, Bennett D, Chertkow H, Cummings JL, de Leon M, Feldman H, Ganguli M, Scheltens P, Tierney MC, Whitehouse P, Winblad B. Mild cognitive impairment. Lancet. 2006;367(9518):1262–70. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- Hao LY, Strong MA, Greider CW. Phosphorylation of H2AX at short telomeres in T cells and fibroblasts. J Biol Chem. 2004;279(43):45148–54. doi: 10.1074/jbc.M403924200. [DOI] [PubMed] [Google Scholar]
- Plentz RR, Caselitz M, Bleck JS, Gebel M, Flemming P, Kubicka S, Manns MP, Rudolph KL. Hepatocellular telomere shortening correlates with chromosomal instability and the development of human hepatoma. Hepatology. 2004;40(1):80–6. doi: 10.1002/hep.20271. [DOI] [PubMed] [Google Scholar]
- Hastie ND, Dempster M, Dunlop MG, Thompson AM, Green DK, Allshire RC. Telomere reduction in human colorectal carcinomal and with ageing. Nature. 1990;346(6287):866–8. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31(3):545–8. [PubMed] [Google Scholar]
- Jenkins EC, Velinov MT, Ye L, Gu H, Li S, Jenkins EC, Jr, Brooks SS, Pang D, Devenny DA, Zigman WB, Schupf N, Silverman WP. Telomere shortening in T lymphocytes of older individuals with Down syndrome and dementia. Neurobiol Aging. 2006;27:41–5. doi: 10.1016/j.neurobiolaging.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Lansdorp PM, Verwoerd NP, van de Rijke FM, Dragowska V, Little M-T, Dirks RW, Raap AK, Tanke HJ. Heterogeneity in telomere length of human chromosomes. Hum Mol Genet. 1996;5:685–91. doi: 10.1093/hmg/5.5.685. [DOI] [PubMed] [Google Scholar]
- Lindsey J, McGill NI, Linsey LA, Gren DK, Cooke HJ. In vivo loss of telomeric repeats with age in humans. Mutat Res. 1991;256(1):45–8. doi: 10.1016/0921-8734(91)90032-7. [DOI] [PubMed] [Google Scholar]
- Londoño-Vallejo JA, DerSarkissian H, Cazes L, Thomas G. Differences in telomere length between homologous chromosomes in humans. Nucleic Acids Res. 2001;29:3164–71. doi: 10.1093/nar/29.15.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panossian LA, Porter VR, Valenzuela HF, Znhu X, Reback E, Masterman D, Cummings JL, Effros RB. Telomere shortening in T cells correlates with Alzheimer’s disease status. Neurobiol Aging. 2003;24:77–84. doi: 10.1016/s0197-4580(02)00043-x. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Plentz RR, Caselitz M, Bleck JS, Gebel M, Flemming P, Kubicka S, Manns MP, Lenhard Rudolph K. Hepatocellular telomere shortening correlates with chromosomal instability and the development of human hepatoma. Hepatology. 2004;40:80–86. doi: 10.1002/hep.20271. [DOI] [PubMed] [Google Scholar]
- Samani JJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening and atherosclerosis. Lancet. 2001;358(9280):472–3. doi: 10.1016/S0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
- Schoeftner S, Blasco MA. Developmentally regulated transcription of mammalian telomeres by DNA-dependent RNA polymerase II. Nat Cell Biol. 2008;10(2):228–36. doi: 10.1038/ncb1685. [DOI] [PubMed] [Google Scholar]
- Schupf N, Kapell D, Lee JH, Zigman W, Canto B, Tycko B, Mayeux R. Onset of dementia is associated with apolipoprotein E epsilon4 in Down’ syndrome. Ann Neurol. 1996;40(5):799–801. doi: 10.1002/ana.410400518. here. [DOI] [PubMed] [Google Scholar]
- Schupf N, Pang D, Patel BN, Silverman W, Schubert R, Lai F, Kline JK, Stern Y, Ferin M, Tycko B. Onset of dementia is associated with age at menopause in women with Down syndrome. Ann Neurol. 2003;54:433–8. doi: 10.1002/ana.10677. [DOI] [PubMed] [Google Scholar]
- Silverman W, Schupf N, Zigman W, Devenny D, Miezejeski C, Schubert R, Ryan R. Dementia in adults with mental retardation: assessment at a single point in time. Am J Ment Retard. 2004;109(2):111–25. doi: 10.1352/0895-8017(2004)109<111:DIAWMR>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. Obesity, cigarette smoking, and telomere length in women. Lancet. 2007;366(9486):662–4. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- Valdes AM, Richards JB, Gardner JP, Swaminathan R, Kimura M, Xiaobin L, Aviv A, Spector TD. Telomere length correlates with bone mineral density and is shorter in women with osteoporosis. Osteoporosis Int. 2007;18(9):1203–10. doi: 10.1007/s00198-007-0357-5. [DOI] [PubMed] [Google Scholar]
- Vulliamy TJ, Dokal I. The diverse clinical presentation of mutations in the telomerase complex. Biochimie. 2007;90(1):122–30. doi: 10.1016/j.biochi.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Zigman WB, Schupf N, Devenny DA, Miezejeski C, Ryan R, Urv TK, Schubert R, Silverman W. Incidence and prevalence of dementia in elderly adults with mental retardation without Down syndrome. Am J Ment Retard. 2004;109(2):126–41. doi: 10.1352/0895-8017(2004)109<126:IAPODI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Zigman WB, Jenkins EC, Tycko B, Schupf N, Silverman W. Mortality is associated with Apolipoprotein E in nondemented adults with Down syndrome. Neurosci Lett. 2005;390(2):93–97. doi: 10.1016/j.neulet.2005.08.002. [DOI] [PubMed] [Google Scholar]