Abstract
Background
Polyphenols, the most abundant dietary antioxidants, also possess many other anticarcinogenic activities. Urinary metabolites of polyphenols could complement dietary assessment of the bioavailability of these nutrients.
Methods
We conducted a study of 353 incident breast cancer cases and 701 individually-matched controls nested within the Shanghai Women's Health Study cohort of women aged 40–70 years at baseline. Liquid chromatography photo-diode array electrospray mass spectrometry was used to measure tea polyphenols (epicatechin, epigallocatechin, and their metabolites) and flavonols (e.g. quercetin and kaempferol). Multivariate conditional logistic regression analyses were used to assess associations between breast cancer risk and urinary excretion rates of polyphenols.
Results
Urinary excretion of tea polyphenols increased with increasing tea leaves consumed among controls, but not among breast cancer cases. Compared with cases, controls had higher levels of urinary total polyphenols and tea polyphenols, particularly epicatechin. In contrast, we did not find any dose-response relationship between urinary polyphenols and breast cancer risk. Urinary excretion of epicatechin was inversely associated with breast cancer risk [Odds Ratio (OR) and 95 % Confidence Interval (CI) of 0.59 (0.39–0.88) for the intermediate tertile]. In spline regression, we found an overall dose-response relationship between epicatechin level and risk of breast cancer, although it was not apparent in low and middle urinary excretion range. In conclusion, high epicatechin may be related to a reduced risk of breast cancer. Further studies are warranted to confirm our findings.
Keywords: breast cancer, urinary polyphenols, flavanols, flavonols
Introduction
Migration studies have suggested the importance of changing lifestyle in the etiology of breast cancer[1]. With a similar structure to estrogens, soy isoflavones, a specific group of polyphenols consumed in large quantities in East Asian populations with traditionally low breast cancer risk, have been considered as a potential protective factor [2–6]. However, growing evidence indicates that soy consumption alone is unlikely to explain the observed low rates of breast cancer in China and Japan [7–9].
In addition to soyfoods, East Asian women also consume high levels of green tea, allium vegetables, and other fruits and vegetables, all of which contain high concentrations of polyphenols, particularly flavanols and flavonols. In addition to potent antioxidant activity, flavanols and flavonols also possess other effects, including inhibition of aromatase [10–12] and inhibition or induction of phase I bioactivating enzymes and phase II detoxifying enzymes, and anti-inflammatory and anti-telomerase activities [13–23]. Furthermore, with a catechol ring, flavanols and flavonols are the only two common groups of polyphenols possessing a similar structure to catechol estrogen metabolites. Hence, flavanols and/or flavonols may play an important role in the etiology of breast cancer by competitively inhibiting production or metabolism of catechol estrogen metabolites [24].
Polyphenols are the most abundant antioxidants in our diets. Most polyphenols exist primarily in the outer sections of fruits and vegetables [25–27] and the culinary preparation, such as peeling and heating may lead to substantial loss of polyphenols [26, 27]. The absorption rate of individual polyphenols differs greatly from 0.3%–1.4% for flavonol quercetin to 3–26% for flavanols and isoflavones [27]. Therefore, it is difficult to estimate polyphenols from dietary intake using a food-frequency questionnaire (FFQ). In fact, many polyphenols can be absorbed only after metabolism by gut microflora and a dramatic inter-person variation in the bioavailability of polyphenols has been observed after identical intake of polyphenols [27–31]. Therefore, urinary metabolites of these polyphenols may be a more accurate measure of bioavailability than dietary assessment. Although urinary excretion of polyphenol metabolites may reflect intake levels of these compounds primarily over the past 24 to 96 hours [28, 32], personal dietary preference, such as allium vegetables, tea drinking and intakes of soy foods, are likely to be relatively stable over time, particularly in adults [33].
No prospective study has directly examined the association of urinary flavonols and flavanols with breast cancer risk. In a nested case-control study within the Shanghai Women's Health Study (SWHS) cohort, we investigated the associations between urinary excretions of flavonols and flavanols and risk of breast cancer.
Materials and Methods
The Shanghai Women's Health Study
Details on the establishment of the SWHS have been reported elsewhere [34]. In brief, at the baseline from March 1997 to May 2000, a cohort of 74,942 Chinese women between 40 and 70 years of age was formed with a 92% participation rate in Shanghai. Participants were interviewed by trained interviewers to elicit information on demographic characteristics, medical history, anthropometrics, usual dietary habits, physical activities, and other lifestyle factors. Two measurements were performed at the end of the in-person interview for weight, height, and circumferences of the waist and hips. A third measurement was conducted if the difference between the first two measurements was larger than 1 kg for weight, 1 cm for height, and 0.5 cm for circumferences. The study was approved by all relevant institutional review boards in China and the United States. All participants provided informed written consent.
Cohort follow-up and outcome ascertainment
The SWHS participants were tracked for the occurrence of cancer by a combination of active surveys conducted every two years and annual record linkage of the study population to cancer case data collected by the population-based Shanghai Cancer Registry and death certificates collected by the Shanghai Municipal Center for Disease Control and Prevention. Nearly all cohort members were successfully followed. The response rates were 99.8% (2000–2002) for the first in-person follow-up survey, 98.7% (2002–2004) for the second, and 96.7% (2004–2007) for the third follow up. All possible incident cancer cases were verified by home visits and medical record review.
Sample collection, storage and processing
A spot urine sample was collected into a sterilized 100-ml cup containing 125 mg of ascorbic acid. The collected samples were kept in a portable, insulated bag with ice packs (at about 0–4°C) [34]. The urine samples were processed within 6 hours of collection, and the urine specimens were immediately stored at −80°C.
Nested case-control design
The nested case-control study was conducted among women who donated a urine sample (65,754 (87.7%) of the cohort) at baseline or during first follow-up (approximately two years later). Among them, 436 cases were indentified from Nov 1997 to Sep 2004 (all of the urine samples were collected before cancer diagnosis). Controls were selected among the study participants who were cancer free at the time of cancer diagnosis of the matched cases. Two controls were randomly selected and matched with each case based on age at baseline (± 2 years), date at study enrollment (≤ 30 days), time (morning or afternoon) of urine collection, interval since last meal (≤ 2 hours), menopausal status (pre- or post-), and antibiotic use (yes/no) in the past week. The first 353 identified cases (with an average 6.1 years of follow-up) and their individually-matched controls were included in the present study. The remaining 83 cases and their matched controls were excluded due to changes in laboratory instruments and conditions. Two controls were successfully matched with each of 349 cases, while 3 cases were matched with only one control each. One case was not matched with a control, and was therefore excluded. In total, 352 cases and 701 controls were available for this study.
Exposure Measurement
Tea consumption
At the baseline survey, every participant was asked whether she ever drank tea three times or more per week for at least 6 months. If the participant answered `yes', she was considered a regular tea drinker. She was then asked whether currently she still drank tea regularly, the age she started, and the type of tea she mainly drank, as well as the amount of dry tea leaves she consumed each month. If she was not a current regular tea drinker, she was asked at what age she stopped regularly consuming tea.
Urinary excretion of tea polyphenols
Measurements of tea polyphenols were carried out by liquid chromatography electrospray mass spectrometry (LC/ESI-MS) as established recently in Dr. Franke's laboratory [35]. Quercetin, kaempferol, epicatechin (EC), epigallocatechin (EGC), and their respective metabilites 5-(3',4',5'-trihydroxyphenyl)-γ-valerolactone (M4) and 5-(3',4',-dihydroxyphenyl)-γ-valerolactone (M6) as well as 4'-MeEGC, the methylated form of EGC, were measured by HPLC with electrospray ionization (negative mode) high resolution tandem mass spectrometry (model TSQ, Thermo, San Jose, CA) similar to earlier reports [36–38]. 2H2 labeled enterolactone (University of Helsinki, Finland) was added as internal standard to each urine specimen hydrolyzed with glucuronidase and sulfatase (Roche Applied Sciences, Indianapolis, IN) for one hour at 37 °C followed by phase separation with ethylacetate [39]. The organic phase was dried under nitrogen at room temperature and redissolved in a 1:1 mixture of acetonitrile/sodium acetate buffer (0.2M, pH5). In each aforementioned step 0.1% aq. ascorbic acid and 0.1% aq. EDTA was also present to preserve the catechins. 25 μL of this extract were separated on a Beta Basic-8 column (100 mm × 2.1 mm i.d., 3 μm) coupled to a BetaBasic-8 precolumn (10 mm × 2.1 mm i.d., 3 μm; both from Thermo Corp). All other HPLC and MS conditions were used as previously [38]. Multiple reaction monitoring was performed using transitions (collision energies) for quercetin from m/z 301.0 to m/z 178.9 (27 eV),150.9 (31 eV), and 107.1 (37 eV); for kaempferol from m/z 285.0 to m/z 239.2 (35 eV), 117.0 (60 eV), and 93.0 (51 eV); for EC from m/z 289.0 to m/z 245.0 (25 eV), m/z 203.0 (29 eV), and m/z 122.9 (51 eV); for EGC from m/z 305.0 to m/z 219.0 (21 eV), m/z167,1 (31 eV), and m/z 124.9 (27 eV); for 4'-MeEGC from m/z 319.0 to m/z 179.2 (25 eV) and m/z 137.9 25 eV); M4 from m/z 223.2 to m/z 178.7 (25 eV) and m/z 137.8 (25 eV); and for M6 from m/z 207.0 to m/z 163.0 (25 eV), m/z 122.0 (25 eV), and m/z 121.0 (25 eV). Post-column addition of 0.25% aq. ammonia at 40 μL/min as dopant did not significantly improve signal intensity. The limits of detection for EC, EGC, kaempferol and quercetin are 1nM, while the limits of detection for M4, M6, 4M_EGC are 100nM. Final results were expressed after adjusting for creatinine concentrations (ng/mg creatinine). Creatinine levels were determined with a test kit (kit 555l; Sigma Co., St. Louis, MO), based on the Jaffe reaction [6], and mean coefficients of variation for intra- and inter-assay variability were found to be 4.1 and 6.7%, respectively.
Statistical Analysis
Baseline covariates were compared between cases and controls to evaluate the potential confounding factors. Paired Wilcoxon signed rank test was used to compare the urinary excretion of polyphenols between cases and controls. Except for M4, urinary levels of polyphenols were categorized based on the tertile distribution in controls. The M4 level was under the minimal detectable limit for about 75% of participants. Thus, participants with undetectable values were classified as reference group and median was used as cutpoint for those with detectable levels. Conditional logistic regression was used to analyze the association between urinary excretion levels of polyphenols and the risk of breast cancer. The models were adjusted for age alone (model 1) or for multiple covariates that might confound the relation between polyphenols and breast cancer (model 2). Potential confounding factors included in final regression model (model 2) were listed in the footnotes of relevant tables. Stratified analyses by menopausal status, body mass index, status of regular tea drinking, years of tea drinking, and plasma concentrations of carotenoids at baseline as well as urinary levels of M4 were performed to evaluate whether risk of breast cancer differed according to these factors. We utilized the body mass index (BMI) cut points for Asian populations (23 for overweight and 27.5 for obesity), recommended by World Health Organization [40]. Because this is a matched case-control design, stratum-specific ORs were derived from conditional regression with the inclusion of terms for main effect along with interaction terms (e.g. dummy variables for the middle or highest levels of polyphenols with dichotomous variable for stratified variable). By adding these interaction terms, case-control pairs were not broken, and all of the subjects were included in the model building. Based on the values (0, 1) of the stratified variable, the stratum-specific ORs were calculated [41]. Likelihood ratio tests were conducted to test for potential interactions. For the association between tea consumption and urinary excretion of tea polyphenols, test for nonlinearity was conducted using restricted cubic spline; and if it was not significant (e.g. likely linear association), we further conducted tests for linear trend. Tests for trend were performed by entering the categorical variables as continuous variables in the model using median value. The above-mentioned statistical data analysis was performed with SAS 9.1 software (SAS Institute, Cary, NC). All of the reported P values were two-tailed, and statistical significance was set at 0.05.
We also utilized the restricted cubic spline function models to examine the dose-response association of urinary polyphenols with risk of breast cancer and tea consumption [42] using R package version 2.8.0 (http://cran.r-project.org/).
Results
Shown in Table 1 are the comparisons of potential confounding factors between cases and controls at the time of urine collection. The median age of cases at diagnosis was 52.8 years. Approximately half of the cases and controls were postmenopausal at baseline. Compared to controls, cases were more likely to have higher educational achievement, earlier age at menarche, later age at first live birth, a family history of breast cancer, and a history of breast fibroadenoma. Controls were more likely to smoke cigarettes, breastfeed longer, and have a higher intake of red meat and soy isoflavones compared with cases.
Table 1.
Comparison of breast cancer cases and controls by selected baseline demographic and risk factors, a nested case-control study within the Shanghai Women's Health Study (SWHS), 1997-2006
| Subject Characteristic | Cases (n=352) | Controls (n=701) | P valuea |
|---|---|---|---|
| Age (years), mean ± SD | 52.8± 8.8 | 52.9 ± 8.9 | 0.84 |
| Income, % | |||
| Low | 26.6 | 29.0 | |
| Middle | 38.0 | 37.9 | |
| High | 35.4 | 33.1 | 0.66 |
| Education, % | |||
| Elementary school or below | 13.6 | 21.5 | |
| Middle or high school | 68.8 | 67.2 | |
| College or above | 17.6 | 11.3 | <0.01 |
| Breast cancer in first-degree relative, % | 4.6 | 1.4 | <0.01 |
| Ever had breast fibroadenoma, % | 7.2 | 4.2 | 0.04 |
| Age at menarche (years) | 14.8 ± 1.9 | 15.0 ± 1.8 | 0.06 |
| Age at first live birth (years), mean ± SDb | 26.3 ± 4.1 | 25.6 ± 4.1 | <0.01 |
| Months of breastfeeding (month), mean ± SDb | 13.7 ± 15.8 | 15.5 ± 16.2 | 0.08 |
| Post-menopausal, % | 48.7 | 49.6 | 0.79 |
| Age at menopause (years), mean ± SD | 49.7 ± 4.8 | 48.9 ± 4.0 | 0.06 |
| Use of hormone replacement therapy, % | 5.1 | 3.4 | 0.19 |
| Physically active past 5 years, % | 33.2 | 34.2 | 0.76 |
| BMI (kg/m2), mean ± SD | 24.3 ± 3.5 | 24.4 ± 3.4 | 0.60 |
| Waist-to-hip ratio, mean ± SD | 0.82 ± 0.06 | 0.82 ± 0.05 | 0.97 |
| Ever smoke regularly, % | 0.8 | 2.7 | 0.046 |
| Ever exposure to passive smoking, % | 80.2 | 83.5 | 0.19 |
| Ever drink alcohol regularly, % | 1.7 | 3.0 | 0.21 |
| Ever drink tea regularly, % | 31.7 | 29.5 | 0.46 |
| Amount of tea drunk (monthly 50 grams) | 7.8 ± 15.5 | 7.6 ± 16.2 | 0.84 |
| Use of ginseng regularly, % | 29.5 | 26.5 | 0.32 |
| Dietary factors | |||
| Daily energy intake (kcal), mean ± SD | 1658.7 ± 355.3 | 1698.7 ± 393.9 | 0.11 |
| Daily intake of fish, mean ± SD | 49.5 ± 38.8 | 53.6 ± 50.6 | 0.18 |
| Daily intake of red meat (g), mean ± SD | 46.90 ± 31.1 | 52.3 ± 35.7 | 0.02 |
| Daily intake of vegetables, mean ± SD | 297.6 ± 158.9 | 303.8 ± 170.8 | 0.57 |
| Daily intake of fruits, mean ± SD | 265.9 ± 163.0 | 269.7 ± 175.0 | 0.73 |
| Daily intake of isoflavones (mg), mean ± SD | 29.6 ± 20.2 | 32.5 ± 25.4 | 0.07 |
For χ2 test (categorical variables) or Cochran Mantel Haenzel χ2 test (ordinal categorical variables) or t test (continuous variables)
Among parous women only
Urinary excretions of five tea polyphenols were statistically significantly correlated with pair-wise correlation coefficients ranging from 0.09 to 0.76 among controls (Data not shown). We compared the amount of tea leaves consumed, as derived from questionnaire data, with the urinary excretion levels of tea polyphenols. In controls, except for 4'-MeEGC, urinary excretion of tea polyphenols, including EGC, EC, and their metabolites M6 and M4 (nmol/mg creatinine), significantly increased with the amount of tea leaves consumed in a linear dose-response manner (Table 2). In breast cancer cases, however, we found no significant linear dose-response relationship of urinary levels of tea polyphenols with the amount of tea consumed (Table 2). The patterns were similar in the sensitivity analyses after excluding cases diagnosed within three years from baseline (Data not shown).
Table 2.
the association of log-transformed urinary polyphenols with green tea intake amount among controls or cases after adjusting for age, within the Shanghai Women's Health Study (SWHS), 1997-2006
| Geometric level of urinary polyphenols (nmol/mg creatinine) a by amount of green tea intake (g/day) |
|||||||
|---|---|---|---|---|---|---|---|
| Green Tea Drinking | The amount of tea leaves | EC | EGC | EGC_4M | M4d | M6 | |
| n | Controls (n=701) | ||||||
| Non-drinkers | None | 494 | 0.020(0.015~0.026) | 0.052(0.040~0.068) | 0.002(0.002~0.003) | 1.349(1.008~1.804) | 1.492(1.152~1.932) |
| Regular Drinkers | ≤ 1.7 | 56 | 0.032(0.015~0.070) | 0.068(0.031~0.154) | 0.005(0.002~0.012) | 2.254(0.954~5.329) | 3.615(1.683~7.765) |
| ≤ 3.3 | 76 | 0.028(0.014~0.055) | 0.106(0.053~0.213) | 0.007(0.003~0.015) | 3.483(1.658~7.316) | 2.421(1.252~4.682) | |
| ≤ 5.0 | 28 | 0.146(0.048~0.443) | 0.173(0.055~0.545) | 0.023(0.006~0.089) | 4.105(1.212~13.91) | 2.075(0.702~6.137) | |
| > 5.0 | 42 | 0.200(0.081~0.494) | 0.246(0.097~0.626) | 0.011(0.004~0.032) | 12.00(4.438~32.47) | 5.879(2.429~14.230) | |
| P for nonlinearity b | 0.272 | 0.059 | 0.004 | 0.099 | 0.683 | ||
| P for trend c | <0.001 | <0.001 | <0.001 | 0.0035 | |||
| Cases(n=352) | |||||||
| Non-drinkers | None | 241 | 0.023(0.016~0.033) | 0.040(0.027~0.059) | 0.003(0.002~0.004) | 1.046(0.711~1.540) | 1.493(1.070~2.084) |
| Regular Drinkers | ≤ 1.7 | 36 | 0.012(0.004~0.031) | 0.085(0.032~0.228) | 0.002(0.001~0.005) | 3.126(1.150~8.494) | 1.899(0.802~4.499) |
| ≤ 3.3 | 31 | 0.100(0.035~0.287) | 0.133(0.046~0.386) | 0.004(0.001~0.013) | 20.38(6.940~59.881) | 9.896(3.906~25.069) | |
| ≤ 5.0 | 16 | 0.023(0.005~0.101) | 0.378(0.085~1.675) | 0.028(0.005~0.162) | 24.78(5.480~112.03) | 6.207(1.689~22.812) | |
| > 5.0 | 25 | 0.023(0.007~0.073) | 0.203(0.062~0.664) | 0.009(0.002~0.037) | 1.767(0.533~5.865) | 2.450(0.871~6.895) | |
| P for nonlinearity b | 0.774 | 0.021 | 0.295 | 0.001 | 0.002 | ||
| P for trend c | 0.444 | 0.009 | |||||
Geometric means and 95% confidence interval ± standard deviation for urinary polyphenols
Obtained from the restricted cubic spline function models (significance indicating non-linear association).
Calculated using linear regression model
Presented as×10−5
The urinary excretion rates of polyphenols were compared between cases and controls using the paired Wilcoxon signed rank test (Table 3). The detectable rate of EC, EGC, EGC_4M, M4, M6, K and Q were 52.4%, 79.5%, 52.7%, 29.1%, 93.2%, 84.7% and 87.3%, respectively. In paired signed rank tests, we found except for quercertin, urinary excretion rates of all other polyphenols were significantly lower among cases than those among controls. However, we found no significant linear associations of breast cancer risk with levels of any polyphenols in a dose-response manner (Table 4). The only significant association was a reduced risk (OR=0.59 (0.40–0.89)) for the intermediate tertile of EC, but the OR for the highest tertile level was not reduced. After adjusting for tea consumption, the results did not change substantially. In a separate analysis based on quintile distribution, the ORs (95%CI) for EC were 1.00 (reference), 0.53 (0.34–0.86), 0.79 (0.49–1.26), 1.24 (0.80–1.91) and 0.87 (0.55–1.36) from the lowest to the highest quintile levels. In the restricted cubic spline function model with nonlinear terms, overall, the risk of breast cancer decreased with urinary level of EC in a dose-response manner, particularly in high urinary excretion range (Fig. 1). We also found the dose-response association was non-linear (p=0.06). We found a much weaker dose-response curve for urinary level of EGC (Fig. 2). Stratified analyses showed that menopausal status, body mass index and plasma concentrations of total carotenoids and tocopherols did not significantly modify the associations between urinary polyphenols and breast cancer risk (data not shown).
Table 3.
Comparison of urinary excretion rates of polyphenols between breast cancer cases and controls, a nested case-control study within the Shanghai Women's Health Study (SWHS), 1997–2006
| Urinary excretion rate (ng/mg creatinine) | Cases (n=352) | Controls (n=701) | paired differencea | P value b |
|---|---|---|---|---|
| Total polyphenols | 20.99 ± 48.79 | 32.87 ± 168.12 | −12.11 ± 128.64 | <0.001 |
| Tea polyphenols (flavanols) | 20.03 ± 48.60 | 31.87 ± 167.88 | −12.07 ± 127.43 | <0.001 |
| Parents (EGC+EC) | 2.62 ± 9.96 | 4.22 ± 19.44 | −1.57 ± 17.09 | <0.001 |
| EC | 0.79 ± 2.77 | 1.60 ± 10.35 | −0.79 ± 7.79 | <0.001 |
| EGC | 1.83 ± 8.43 | 2.62 ± 13.60 | −0.78 ± 12.89 | <0.001 |
| Metabolites (M4+M6) | 17.20 ± 45.96 | 27.44 ± 163.88 | −10.49 ± 124.58 | 0.001 |
| M4 | 0.03 ± 0.18 | 0.10 ± 0.82 | −0.07 ± 0.59 | <0.001 |
| M6 | 17.17 ± 45.91 | 27.33 ± 163.24 | −10.42 ± 124.15 | <0.001 |
| EGC_4M | 0.21 ± 1.02 | 0.22 ± 0.84 | −0.01 ± 1.20 | <0.001 |
| flavonols (K+Q) | 0.96 ± 2.40 | 1.00 ± 2.62 | −0.03 ± 2.78 | 0.155 |
| kaempferol | 0.49 ± 1.16 | 0.61 ± 2.04 | −0.11 ± 1.82 | 0.035 |
| quercetin | 0.47 ± 1.93 | 0.39 ± 1.01 | 0.08 ± 1.73 | 0.123 |
The mean ± standard deviation for the difference between case and the average of matched control
Paired Wilcoxon signed rank test for cases and the average of two matched controls
Table 4.
Odds ratios (ORs) and 95% confidence intervals (CI) for risk of breast cancer associated with urinary excretion rate of polyphenols, a nested case-contro study within the Shanghai Women's Health Study (SWHS), 1997–2006
| Urinary excretion rate (ng/mg creatinine) | Urinary excretion rate of polyphenols by tertile | |||||
|---|---|---|---|---|---|---|
| T1(Low Tertile) a | T2 | T3 | P for Trend | |||
| Total polyphenols | Model 1 | 1.00 | 1.26(0.92–1.72) | 1.09(0.78–1.53) | 0.316 | |
| Model2 | 1.00 | 1.48(1.05–2.10) | 1.12(0.77–1.61) | 0.171 | ||
| Model3 | 1.00 | 1.48(1.04–2.09) | 1.08(0.75–1.57) | 0.200 | ||
| Tea polyphenols (flavanols) | Model1 | 1.00 | 1.35(0.97–1.87) | 1.14(0.81–1.60) | 0.221 | |
| Model2 | 1.00 | 1.52(1.06–2.19) | 1.12(0.77–1.63) | 0.210 | ||
| Model3 | 1.00 | 1.52(1.05–2.19) | 1.09(0.74–1.59) | 0.246 | ||
| Parent Flavanols (EGC+EC) | Model1 | 1.00 | 0.94(0.68–1.28) | 0.99(0.71–1.38) | 0.848 | |
| Model2 | 1.00 | 0.90(0.64–1.27) | 0.99(0.69–1.42) | 0.788 | ||
| Model3 | 1.00 | 0.91(0.64–1.28) | 0.97(0.68–1.40) | 0.736 | ||
| EC | Model1 | 1.00 | 0.65(0.45–0.94) | 1.01(0.75–1.37) | 0.637 | |
| Model2 | 1.00 | 0.59(0.40–0.89) | 1.01(0.72–1.40) | 0.564 | ||
| Model3 | 1.00 | 0.60(0.40–0.89) | 1.00(0.71–1.39) | 0.517 | ||
| EGC | Model1 | 1.00 | 0.94(0.69–1.30) | 0.90(0.66–1.24) | 0.535 | |
| Model2 | 1.00 | 0.81(0.57–1.15) | 0.88(0.62–1.26) | 0.344 | ||
| Model3 | 1.00 | 0.81(0.57–1.16) | 0.88(0.61–1.25) | 0.336 | ||
| Metabolites (M4+M6), | Model1 | 1.00 | 1.07(0.78–1.49) | 1.09(0.78–1.51) | 0.590 | |
| Model2 | 1.00 | 1.16(0.81–1.66) | 1.04(0.72–1.49) | 0.659 | ||
| Model3 | 1.00 | 1.13(0.77–1.65) | 0.99(0.71–1.40) | 0.733 | ||
| M6 | Model1 | 1.00 | 1.07(0.78–1.49) | 1.09(0.78–1.51) | 0.590 | |
| Model2 | 1.00 | 1.16(0.81–1.66) | 1.04(0.72–1.49) | 0.659 | ||
| Model3 | 1.00 | 1.15(0.80–1.65) | 1.02(0.71–1.47) | 0.733 | ||
| EGC_4M | Model1 | 1.00 | 1.09(0.77–1.54) | 1.04(0.76–1.42) | 0.719 | |
| Model2 | 1.00 | 1.11(0.76–1.63) | 1.01(0.72–1.42) | 0.823 | ||
| Model3 | 1.00 | 1.13(0.77–1.65) | 0.99(0.71–1.40) | 0.881 | ||
| Flavonols (K+Q) | Model1 | 1.00 | 1.29(0.94–1.76) | 1.11(0.80–1.54) | 0.238 | |
| Model2 | 1.00 | 1.14(0.81–1.61) | 1.04(0.73–1.48) | 0.605 | ||
| Model3 | 1.00 | 1.15(0.81–1.62) | 1.03(0.72–1.48) | 0.613 | ||
| Kaempferol (K) | Model1 | 1.00 | 1.22(0.89–1.68) | 1.17(0.83–1.63) | 0.254 | |
| Model2 | 1.00 | 1.15(0.82–1.63) | 1.11(0.77–1.60) | 0.463 | ||
| Model3 | 1.00 | 1.15(0.81–1.63) | 1.10(0.76–1.58) | 0.491 | ||
| Quercetin (Q) | Model1 | 1.00 | 0.89(0.64–1.23) | 1.02(0.74–1.41) | 0.812 | |
| Model2 | 1.00 | 0.86(0.60–1.24) | 1.01(0.71–1.43) | 0.740 | ||
| Model3 | 1.00 | 0.86(0.60–1.23) | 1.00(0.70–1.41) | 0.694 | ||
33.3th and 66.7th percentiles for each polyphenols.
b participants with undetectable values were classified as low tertile and median was used as cutpoint for T2 and T3.
Model 1: Conditional logistic regression model adjusting for age only.
Model 2: Conditional logistic regression model adjusting for age, education, age at menarche (continuous), age at 1st live birth (continuous), months of breast fibroadenoma (yes/no), first-degree family cancer history (yes/no), ever smoke (never/ever), total intake of red meat and isoflavones, and use of hormone replacement therapy.
Model 3: Conditional logistic regression model adjusting for covariates of model 2 and tea intakes
Fig. 1.
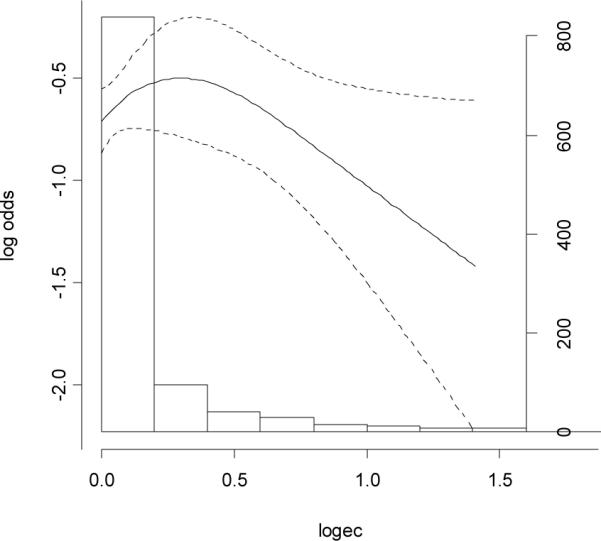
Association between baseline levels of urinary excretion rates epicatechin (ng/mg creatinine, log transformed by 10) and risk of breast cancer
Fig. 2.
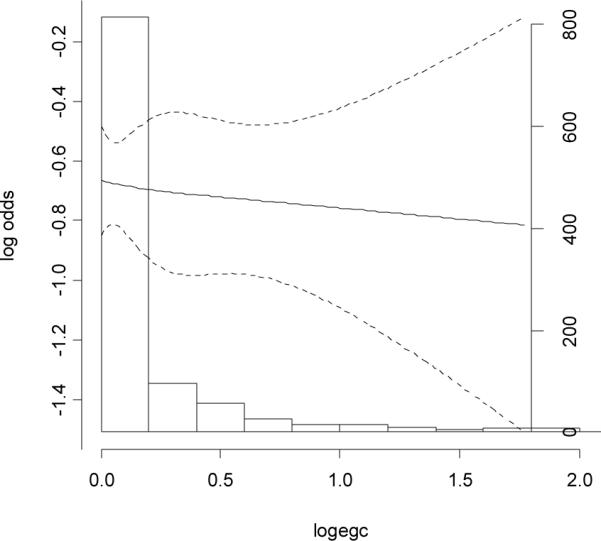
Association between baseline levels of urinary excretion rates of epigallocatechin (ng/mg creatinine, log transformed by 10) and risk of breast cancer
Discussion
To our knowledge, this is the first prospective study examining the association between urinary levels of polyphenol flavanols and flavonols and risk of breast cancer. Strikingly, we found urinary excretion of tea polyphenols increased with increasing tea leaves consumed among controls, but not breast cancer cases. We found levels of polyphenol EC and M4 to be significantly higher among controls than their matched cases. However, no statistically significant linear dose-response associations were found between urinary excretion of flavanols and flavonols and risk of breast cancer in categorized analyses. In the restricted cubic spline function model with nonlinear terms, we found an overall non-linear dose-response relationship between level of EC and risk of breast cancer, although it was not apparent in low urinary excretion range.
We found in the current study that the dose-response associations between tea drinking amount and urinary tea polyphenols were observed only among healthy controls, which is consistent with a previous study [43]. We found the dose-response relationship with EC was not as strong as that for EGC in controls. Unlike EGC, EC could also be derived from certain other sources of fruits and vegetables, such as apples. On the other hand, no clear dose-response relationships were seen among breast cancer cases. It is possible that breast cancer patients have a different hormone metabolism pattern from healthy subjects. For example, previous studies found that tea polyphenols and estrogens were metabolized by the same enzymes such as catechol-o-methyltransferase (COMT) [44, 45] whereas polymorphisms in the COMT gene may be associated with breast cancer risk [46–49] . Therefore, the differing associations between tea drinking amount and urinary tea polyphenols by case-control status may be because polyphenols are metabolized differently in cases and controls. This finding, if confirmed, has very important implications as it can potentially be utilized to develop tests in indentify high-risk women.
Three previous case-control studies evaluated the associations between dietary intakes of flavonols and flavanols and risk of breast cancer and found inconsistent results [50, 51] [52]. Very recently, a cohort study found dietary intakes of flavonols and flavones not related to the breast cancer risk [53]. Epidemiologic studies have also generated discordant findings for the association between tea drinking (abundant in flavonols, particularly flavanols) and breast cancer. In a recent meta-analysis of epidemiologic studies, black tea consumption was inversely associated with breast cancer risk in eight case-control studies, whereas in five cohort studies a moderately increased risk was observed [54]. In accordance with an earlier meta-analysis, a non-significant inverse association was observed between green tea and breast cancer risk in three cohort studies, and only in one case-control study the inverse association reached statistical significance[54, 55]. We found in a population-based case-control study conducted among Chinese women in Shanghai that regular green tea drinking was related to a 12% reduction in risk of breast cancer [56], in consistent with another case-control study conducted among Chinese women [57]. In the current prospective study, we did not find an overall association for green tea drinking [58]. In addition, previous studies also generated inconclusive results on the associations between dietary intake of allium vegetables (rich in flavonols) and risk of breast cancer [32, 59–62].
Possible explanations for the inconsistencies across previous studies include 1) food preparation processes which may vary by population were not collected in these previous studies; and 2) substantial inter-person variation in absorption of polyphenols have not been considered [26, 27, 63]. In contrast to the observed significant difference in mean level of EC between cases and controls, we did not find a significant linear dose-response relationship in categorical analyses (by tertile). This may be due to the fact that in the restricted cubic spline function model with nonlinear terms, we also found the dose-response association was non-linear. Furthermore, in the spline curve, the urinary level of EC for most of cases and controls was in the low range, in which we did not find an apparent dose-response association. However, when urinary level of EC further increased, the risk of breast cancer more appreciably reduced. The stronger inverse association observed for EC than EGC and other tea polyphenols is biologically plausible because previous in vitro and in vivo studies found EC was more potent than EGC in inhibiting breast cancer cell lines and breast cancer progression [64, 65]. We, however, cannot exclude the possibility that this finding is solely due to chance or other underlying confounding factors.
The present study has several notable strengths. Levels of bioavailable polyphenols were measured together with its major metabolites using a sensitive method (LC/PDA/ESI-MS). The parent study, the population-based Shanghai Women's Health Study, had high rates for baseline participation and follow-up, which minimized selection bias. However, one spot urine was used to measure urinary levels of polyphenols, which may not reflect well the long-term exposure levels. Intra-person variation should lead to non-differential misclassification, which usually biases the result to the null. To the extent that intra-person variation levels of these polyphenols exist in our data, the true associations of EC and other tea polyphenol levels with breast cancer risk could be stronger than those we observed.
We found the relationship between tea consumption and urinary excretion levels of tea polyphenol differed in healthy subjects from breast cancer cases. If confirmed, this finding may be used to develop strategies to identify high-risk women. In addition, our finding suggests a possible link between urine EC level and a reduced risk of breast cancer. Future studies are necessary to confirm this finding, particularly longitudinal studies with multiple collections of biological samples at different time window.
Acknowledgements
The authors would like to thank the study participants. The authors also thank Laurie Custer for the skillful performance of LC/MS assays. We acknowledge the support by NIH grant S10 RR020890-01.
Sources of support: This study was supported by USPHS grant R01CA106591 as well as USPHS grant R01CA70867 and NIH intramural program (N02 CP1101066) for the parent study
References
- 1.Ziegler RG, Hoover RN, Pike MC, Hildesheim A, Nomura AM, West DW, Wu-Williams AH, Kolonel LN, Horn-Ross PL, Rosenthal JF, Hyer MB. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993;85:1819–1827. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- 2.Adlercreutz H, Mazur W. Phyto-oestrogens and Western diseases. Ann Med. 1997;29:95–120. doi: 10.3109/07853899709113696. [DOI] [PubMed] [Google Scholar]
- 3.Dai Q, Franke AA, Jin F, Shu XO, Hebert JR, Custer LJ, Cheng J, Gao YT, Zheng W. Urinary excretion of phytoestrogens and risk of breast cancer among Chinese women in Shanghai. Cancer Epidemiol Biomarkers Prev. 2002;11:815–821. [PubMed] [Google Scholar]
- 4.Dai Q, Franke AA, Yu H, Shu XO, Jin F, Hebert JR, Custer LJ, Gao YT, Zheng W. Urinary phytoestrogen excretion and breast cancer risk: evaluating potential effect modifiers endogenous estrogens and anthropometrics. Cancer Epidemiol Biomarkers Prev. 2003;12:497–502. [PubMed] [Google Scholar]
- 5.Dai Q, Shu XO, Jin F, Potter JD, Kushi LH, Teas J, Gao YT, Zheng W. Population-based case-control study of soyfood intake and breast cancer risk in Shanghai. Br J Cancer. 2001;85:372–378. doi: 10.1054/bjoc.2001.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng W, Dai Q, Custer LJ, Shu XO, Wen WQ, Jin F, Franke AA. Urinary excretion of isoflavonoids and the risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 1999;8:35–40. [PubMed] [Google Scholar]
- 7.Willett WC. Diet and breast cancer. J Intern Med. 2001;249:395–411. doi: 10.1046/j.1365-2796.2001.00822.x. [DOI] [PubMed] [Google Scholar]
- 8.Wu AH, Ursin G, Koh WP, Wang R, Yuan JM, Khoo KS, Yu MC. Green tea, soy, and mammographic density in singapore chinese women. Cancer Epidemiol Biomarkers Prev. 2008;17:3358–3365. doi: 10.1158/1055-9965.EPI-08-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Key TJ, Sharp GB, Appleby PN, Beral V, Goodman MT, Soda M, Mabuchi K. Soya foods and breast cancer risk: a prospective study in Hiroshima and Nagasaki, Japan. Br J Cancer. 1999;81:1248–1256. doi: 10.1038/sj.bjc.6690837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satoh K, Sakamoto Y, Ogata A, Nagai F, Mikuriya H, Numazawa M, Yamada K, Aoki N. Inhibition of aromatase activity by green tea extract catechins and their endocrinological effects of oral administration in rats. Food Chem Toxicol. 2002;40:925–933. doi: 10.1016/s0278-6915(02)00066-2. [DOI] [PubMed] [Google Scholar]
- 11.Monteiro R, Becker H, Azevedo I, Calhau C. Effect of hop (Humulus lupulus L.) flavonoids on aromatase (estrogen synthase) activity. J Agric Food Chem. 2006;54:2938–2943. doi: 10.1021/jf053162t. [DOI] [PubMed] [Google Scholar]
- 12.Monteiro R, Faria A, Mateus N, Calhau C, Azevedo I. Red wine interferes with oestrogen signalling in rat hippocampus. J Steroid Biochem Mol Biol. 2008;111:74–79. doi: 10.1016/j.jsbmb.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Hatono S, Jimenez A, Wargovich MJ. Chemopreventive effect of S-allylcysteine and its relationship to the detoxification enzyme glutathione S-transferase. Carcinogenesis. 1996;17:1041–1044. doi: 10.1093/carcin/17.5.1041. [DOI] [PubMed] [Google Scholar]
- 14.Le Marchand L. Cancer preventive effects of flavonoids--a review. Biomed Pharmacother. 2002;56:296–301. doi: 10.1016/s0753-3322(02)00186-5. [DOI] [PubMed] [Google Scholar]
- 15.Muto S, Fujita K, Yamazaki Y, Kamataki T. Inhibition by green tea catechins of metabolic activation of procarcinogens by human cytochrome P450. Mutat Res. 2001;479:197–206. doi: 10.1016/s0027-5107(01)00204-4. [DOI] [PubMed] [Google Scholar]
- 16.Singh A, Singh SP. Modulatory potential of smokeless tobacco on the garlic, mace or black mustard-altered hepatic detoxication system enzymes, sulfhydryl content and lipid peroxidation in murine system. Cancer Lett. 1997;118:109–114. doi: 10.1016/s0304-3835(97)00240-1. [DOI] [PubMed] [Google Scholar]
- 17.Steele VE, Kelloff GJ, Balentine D, Boone CW, Mehta R, Bagheri D, Sigman CC, Zhu S, Sharma S. Comparative chemopreventive mechanisms of green tea, black tea and selected polyphenol extracts measured by in vitro bioassays. Carcinogenesis. 2000;21:63–67. doi: 10.1093/carcin/21.1.63. [DOI] [PubMed] [Google Scholar]
- 18.Weisburger JH, Nagao M, Wakabayashi K, Oguri A. Prevention of heterocyclic amine formation by tea and tea polyphenols. Cancer Lett. 1994;83:143–147. doi: 10.1016/0304-3835(94)90311-5. [DOI] [PubMed] [Google Scholar]
- 19.Ju YH, Doerge DR, Woodling KA, Hartman JA, Kwak J, Helferich WG. Dietary genistein negates the inhibitory effect of letrozole on the growth of aromatase-expressing estrogen-dependent human breast cancer cells (MCF-7Ca) in vivo. Carcinogenesis. 2008;29:2162–2168. doi: 10.1093/carcin/bgn161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beltz LA, Bayer DK, Moss AL, Simet IM. Mechanisms of cancer prevention by green and black tea polyphenols. Anticancer Agents Med Chem. 2006;6:389–406. doi: 10.2174/187152006778226468. [DOI] [PubMed] [Google Scholar]
- 21.Berletch JB, Liu C, Love WK, Andrews LG, Katiyar SK, Tollefsbol TO. Epigenetic and genetic mechanisms contribute to telomerase inhibition by EGCG. J Cell Biochem. 2008;103:509–519. doi: 10.1002/jcb.21417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang CS, Sang S, Lambert JD, Hou Z, Ju J, Lu G. Possible mechanisms of the cancer-preventive activities of green tea. Mol Nutr Food Res. 2006;50:170–175. doi: 10.1002/mnfr.200500105. [DOI] [PubMed] [Google Scholar]
- 23.Yokoyama M, Noguchi M, Nakao Y, Ysunaga M, Yamasaki F, Iwasaka T. Antiproliferative effects of the major tea polyphenol, (-)-epigallocatechin gallate and retinoic acid in cervical adenocarcinoma. Gynecol Oncol. 2008;108:326–331. doi: 10.1016/j.ygyno.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Collins-Burow BM, Burow ME, Duong BN, McLachlan JA. Estrogenic and antiestrogenic activities of flavonoid phytochemicals through estrogen receptor binding-dependent and -independent mechanisms. Nutr Cancer. 2000;38:229–244. doi: 10.1207/S15327914NC382_13. [DOI] [PubMed] [Google Scholar]
- 25.Bazemore R, Rouseff R, Naim M. Linalool in orange juice: origin and thermal stability. J Agric Food Chem. 2003;51:196–199. doi: 10.1021/jf0257291. [DOI] [PubMed] [Google Scholar]
- 26.Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 27.Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130:2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 28.Franke AA, Custer LJ. High-performance liquid chromatographic assay of isoflavonoids and coumestrol from human urine. J Chromatogr B Biomed Appl. 1994;662:47–60. doi: 10.1016/0378-4347(94)00390-4. [DOI] [PubMed] [Google Scholar]
- 29.Fuhr U, Kummert AL. The fate of naringin in humans: a key to grapefruit juice-drug interactions? Clin Pharmacol Ther. 1995;58:365–373. doi: 10.1016/0009-9236(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 30.Rowland IR, Wiseman H, Sanders TA, Adlercreutz H, Bowey EA. Interindividual variation in metabolism of soy isoflavones and lignans: influence of habitual diet on equol production by the gut microflora. Nutr Cancer. 2000;36:27–32. doi: 10.1207/S15327914NC3601_5. [DOI] [PubMed] [Google Scholar]
- 31.Kilkkinen A, Stumpf K, Pietinen P, Valsta LM, Tapanainen H, Adlercreutz H. Determinants of serum enterolactone concentration. Am J Clin Nutr. 2001;73:1094–1100. doi: 10.1093/ajcn/73.6.1094. [DOI] [PubMed] [Google Scholar]
- 32.Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiol Biomarkers Prev. 1998;7:1091–1100. [PubMed] [Google Scholar]
- 33.Chen Z, Zheng W, Custer LJ, Dai Q, Shu XO, Jin F, Franke AA. Usual dietary consumption of soy foods and its correlation with the excretion rate of isoflavonoids in overnight urine samples among Chinese women in Shanghai. Nutr Cancer. 1999;33:82–87. doi: 10.1080/01635589909514752. [DOI] [PubMed] [Google Scholar]
- 34.Zheng W, Chow WH, Yang G, Jin F, Rothman N, Blair A, Li HL, Wen W, Ji BT, Li Q, Shu XO, Gao YT. The Shanghai Women's Health Study: rationale, study design, and baseline characteristics. Am J Epidemiol. 2005;162:1123–1131. doi: 10.1093/aje/kwi322. [DOI] [PubMed] [Google Scholar]
- 35.Maskarinec G, Watts K, Kagihara J, Hebshi SM, Franke AA. Urinary isoflavonoid excretion is similar after consuming soya milk and miso soup in Japanese-American women. Br J Nutr. 2008;100:424–429. doi: 10.1017/S0007114508898686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franke AA, Custer LJ, Wilkens LR, Le Marchand LL, Nomura AM, Goodman MT, Kolonel LN. Liquid chromatographic-photodiode array mass spectrometric analysis of dietary phytoestrogens from human urine and blood. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;777:45–59. doi: 10.1016/s1570-0232(02)00216-7. [DOI] [PubMed] [Google Scholar]
- 37.Blair RM, Appt SE, Franke AA, Clarkson TB. Treatment with antibiotics reduces plasma equol concentration in cynomolgus monkeys (Macaca fascicularis) J Nutr. 2003;133:2262–2267. doi: 10.1093/jn/133.7.2262. [DOI] [PubMed] [Google Scholar]
- 38.Maskarinec G, Hebshi S, Custer L, Franke AA. The relation of soy intake and isoflavone levels in nipple aspirate fluid. Eur J Cancer Prev. 2008;17:67–70. doi: 10.1097/CEJ.0b013e3281108101. [DOI] [PubMed] [Google Scholar]
- 39.Franke AA, Custer LJ, Wang W, Shi CY. HPLC analysis of isoflavonoids and other phenolic agents from foods and from human fluids. Proc Soc Exp Biol Med. 1998;217:263–273. doi: 10.3181/00379727-217-44231. [DOI] [PubMed] [Google Scholar]
- 40.Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 41.Dai Q, Gao YT, Shu XO, Yang G, Milne G, Cai Q, Wen W, Rothman N, Cai H, Li H, Xiang Y, Chow WH, Zheng W. Oxidative stress, obesity, and breast cancer risk: results from the Shanghai Women's Health Study. J Clin Oncol. 2009;27:2482–2488. doi: 10.1200/JCO.2008.19.7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harrell FEJ. Regression Modeling Strategies : with application to linear models, logistic regression and survival analysis. Springer; New York, NY: 2001. [Google Scholar]
- 43.Yuan JM, Gao YT, Yang CS, Yu MC. Urinary biomarkers of tea polyphenols and risk of colorectal cancer in the Shanghai Cohort Study. Int J Cancer. 2007;120:1344–1350. doi: 10.1002/ijc.22460. [DOI] [PubMed] [Google Scholar]
- 44.Wu AH, Tseng CC, Van Den Berg D, Yu MC. Tea intake, COMT genotype, and breast cancer in Asian-American women. Cancer Res. 2003;63:7526–7529. [PubMed] [Google Scholar]
- 45.Zhu BT, Conney AH. Is 2-methoxyestradiol an endogenous estrogen metabolite that inhibits mammary carcinogenesis? Cancer Res. 1998;58:2269–2277. [PubMed] [Google Scholar]
- 46.Dias Pereira P, Lopes CC, Matos AJ, Pinto D, Gartner F, Lopes C, Medeiros R. Estrogens metabolism associated with polymorphisms: influence of COMT G482a genotype on age at onset of canine mammary tumors. Vet Pathol. 2008;45:124–130. doi: 10.1354/vp.45-2-124. [DOI] [PubMed] [Google Scholar]
- 47.Hamaguchi M, Nishio M, Toyama T, Sugiura H, Kondo N, Fujii Y, Yamashita H. Possible difference in frequencies of genetic polymorphisms of estrogen receptor alpha, estrogen metabolism and P53 genes between estrogen receptor-positive and -negative breast cancers. Jpn J Clin Oncol. 2008;38:734–742. doi: 10.1093/jjco/hyn097. [DOI] [PubMed] [Google Scholar]
- 48.Ji Y, Olson J, Zhang J, Hildebrandt M, Wang L, Ingle J, Fredericksen Z, Sellers T, Miller W, Dixon JM, Brauch H, Eichelbaum M, Justenhoven C, Hamann U, Ko Y, Bruning T, Chang-Claude J, Wang-Gohrke S, Schaid D, Weinshilboum R. Breast cancer risk reduction and membrane-bound catechol O-methyltransferase genetic polymorphisms. Cancer Res. 2008;68:5997–6005. doi: 10.1158/0008-5472.CAN-08-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson PA, Shields PG, Freudenheim JL, Stone A, Vena JE, Marshall JR, Graham S, Laughlin R, Nemoto T, Kadlubar FF, Ambrosone CB. Genetic polymorphisms in catechol-O-methyltransferase, menopausal status, and breast cancer risk. Cancer Res. 1998;58:2107–2110. [PubMed] [Google Scholar]
- 50.Bosetti C, Spertini L, Parpinel M, Gnagnarella P, Lagiou P, Negri E, Franceschi S, Montella M, Peterson J, Dwyer J, Giacosa A, La Vecchia C. Flavonoids and breast cancer risk in Italy. Cancer Epidemiol Biomarkers Prev. 2005;14:805–808. doi: 10.1158/1055-9965.EPI-04-0838. [DOI] [PubMed] [Google Scholar]
- 51.Peterson J, Lagiou P, Samoli E, Lagiou A, Katsouyanni K, La Vecchia C, Dwyer J, Trichopoulos D. Flavonoid intake and breast cancer risk: a case--control study in Greece. Br J Cancer. 2003;89:1255–1259. doi: 10.1038/sj.bjc.6601271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fink BN, Steck SE, Wolff MS, Britton JA, Kabat GC, Schroeder JC, Teitelbaum SL, Neugut AI, Gammon MD. Dietary flavonoid intake and breast cancer risk among women on Long Island. Am J Epidemiol. 2007;165:514–523. doi: 10.1093/aje/kwk033. [DOI] [PubMed] [Google Scholar]
- 53.Wang L, Lee IM, Zhang SM, Blumberg JB, Buring JE, Sesso HD. Dietary intake of selected flavonols, flavones, and flavonoid-rich foods and risk of cancer in middle-aged and older women. Am J Clin Nutr. 2009 doi: 10.3945/ajcn.2008.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun CL, Yuan JM, Koh WP, Yu MC. Green tea, black tea and breast cancer risk: a meta-analysis of epidemiological studies. Carcinogenesis. 2006;27:1310–1315. doi: 10.1093/carcin/bgi276. [DOI] [PubMed] [Google Scholar]
- 55.Seely D, Mills EJ, Wu P, Verma S, Guyatt GH. The effects of green tea consumption on incidence of breast cancer and recurrence of breast cancer: a systematic review and meta-analysis. Integr Cancer Ther. 2005;4:144–155. doi: 10.1177/1534735405276420. [DOI] [PubMed] [Google Scholar]
- 56.Shrubsole MJ, Lu W, Chen Z, Shu XO, Zheng Y, Dai Q, Cai Q, Gu K, Ruan ZX, Gao YT, Zheng W. Drinking green tea modestly reduces breast cancer risk. J Nutr. 2009;139:310–316. doi: 10.3945/jn.108.098699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang M, Huang J, Xie X, Holman CD. Dietary intakes of mushrooms and green tea combine to reduce the risk of breast cancer in Chinese women. Int J Cancer. 2009;124:1404–1408. doi: 10.1002/ijc.24047. [DOI] [PubMed] [Google Scholar]
- 58.Dai Q, Shu XO, Li HGY, Shrubsole Martha J, Hui Cai, Butian Ji, Wanqing Wen, Adrian Franke, Yu-Tang Gao, Wei Zheng. Is Green Tea Drinking Associated With a Later Onset of Breast Cancer? 2009 doi: 10.1016/j.annepidem.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fleischauer AT, Arab L. Garlic and cancer: a critical review of the epidemiologic literature. J Nutr. 2001;131:1032S–1040S. doi: 10.1093/jn/131.3.1032S. [DOI] [PubMed] [Google Scholar]
- 60.Torres-Sanchez L, Lopez-Carrillo L, Lopez-Cervantes M, Rueda-Neria C, Wolff MS. Food sources of phytoestrogens and breast cancer risk in Mexican women. Nutr Cancer. 2000;37:134–139. doi: 10.1207/S15327914NC372_3. [DOI] [PubMed] [Google Scholar]
- 61.Kim JY, Kwon O. Garlic intake and cancer risk: an analysis using the Food and Drug Administration's evidence-based review system for the scientific evaluation of health claims. Am J Clin Nutr. 2009;89:257–264. doi: 10.3945/ajcn.2008.26142. [DOI] [PubMed] [Google Scholar]
- 62.Malin AS, Qi D, Shu XO, Gao YT, Friedmann JM, Jin F, Zheng W. Intake of fruits, vegetables and selected micronutrients in relation to the risk of breast cancer. Int J Cancer. 2003;105:413–418. doi: 10.1002/ijc.11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang CS, Sang S, Lambert JD, Lee MJ. Bioavailability issues in studying the health effects of plant polyphenolic compounds. Mol Nutr Food Res. 2008;52(Suppl 1):S139–151. doi: 10.1002/mnfr.200700234. [DOI] [PubMed] [Google Scholar]
- 64.Friedman M, Mackey BE, Kim HJ, Lee IS, Lee KR, Lee SU, Kozukue E, Kozukue N. Structure-activity relationships of tea compounds against human cancer cells. J Agric Food Chem. 2007;55:243–253. doi: 10.1021/jf062276h. [DOI] [PubMed] [Google Scholar]
- 65.Nagarajan S, Nagarajan R, Braunhut SJ, Bruno F, McIntosh D, Samuelson L, Kumar J. Biocatalytically oligomerized epicatechin with potent and specific anti-proliferative activity for human breast cancer cells. Molecules. 2008;13:2704–2716. doi: 10.3390/molecules13112704. [DOI] [PMC free article] [PubMed] [Google Scholar]


