Abstract
A PET study of patients with Alzheimer's disease (AD) engaged in a delayed match-to-sample face recognition task revealed that performance declines as a function of increasing delay, a pattern accompanied by reduced functional connectivity of prefrontal cortex but increased connectivity of the left amygdala. Here, we characterize the changes in interactions within this amygdalar circuit across the memory delays using structural equation modeling. The magnitude of effective connections was found to be much greater in the patients than in the controls, notably from the left amygdala to left inferior prefrontal cortex, which, in turn, influenced its right homologue. The influence from the amygdala to the left hippocampus, in contrast, was not strong in either group. We interpret this pattern of interactions as possibly reflecting the compensatory recruitment of a dynamic neural network, perhaps involved in implicit emotional processing, in the context of a faulty executive maintenance and retrieval system.
Keywords: aging, structural equation modeling, amygdala, hippocampus, inferior prefrontal cortex, plasticity
1. Introduction
The existence of dynamic and complex interactions between cognition and emotion within local convergent sites is receiving greater attention (Dalgleish, 2004; LaBar & Cabeza, 2006; Phelps, 2004; Stuss & Levine, 2002) but remains poorly understood at the level of distributed brain systems and the nature of their communication. Disruption to one or more of these local sites and their connections, as in the case of Alzheimer's disease (AD), necessarily influences the functional milieu of the remaining network1. A likely consequence of this breakdown is a decline in performance, such as the behavior changes seen on tests of short-term episodic memory. At the same time, there is the possibility of compensatory changes that allow performance to remain above what would be expected given the extent of structural damage to the hippocampus and related regions (Braak et al., 1993; Perry & Hodges, 1996). For example, memory networks may be redirected towards more primitive circuitry involving regions implicated in emotional processing that are known to be relatively spared in early AD (Hamann, 2001; Kazui et al., 2000; LaBar et al., 2000; Padovan et al., 2002; Winograd et al., 1999). The present study examines the neural basis of such a network as a possible compensatory mechanism in AD patients who show short-term episodic memory decline, likely corresponding to known loss of integrity in medial temporal lobe (MTL) structures.
That such functional reorganization in AD is possible is indicated by an investigation of interregional covariances of activity underlying short-term episodic memory for faces under varying delay intervals between initial exposure and recognition (Grady et al., 2001). Results showed that activity in right prefrontal cortex (PFC) in AD patients and controls, together with left amygdala activity only in the patients, increased with better recognition at the longest delays, though only the former of the two structures is relatively preserved until later stages of disease progression (Chase et al., 1987; Grady et al., 1990). Nonetheless, correlations of right PFC activation were limited to other right PFC regions in the patients and did not include the hippocampus or face-specific perceptual regions found to be correlated with right PFC in controls. Presumably, this rerouting in the patients accounts for the interference with normal face recognition, but performance in the patients was not at chance, suggesting the presence of a mechanism to offset any further decline. Accordingly, unlike PFC, which is often engaged more in older than younger adults during memory tasks (Cabeza et al., 1997; Grady et al., 1994), the amygdala is not normally recruited to any greater extent, though increased activity within this region was found to be associated with better task performance during longer memory delays in the patients. The compensatory value of this structure may be better understood in the context of other emotion-related regions that are anatomically connected (Amaral et al., 1992) and with which activity covaried in the patients, namely inferior PFC, anterior cingulate, and insula, in contrast to a less extensive network of temporal and occipital correlations in the controls (Grady et al., 2001).
These findings raise the possibility that the amygdala and its connectivity with related brain structures may serve as a buffer for episodic memory decline following degeneration of other MTL regions, either directly by influencing other MTL regions, or indirectly. The goal of the present study was to characterize the strength and direction of effective connections associated with short-term memory for faces in healthy aging and AD, using structural equation modeling (SEM) to specify the effective connectivity among regions within the emotion processing network identified previously (Grady et al., 2001). With this approach, we sought to explore how the brain is able to support some short-term episodic memory despite local damage to structures believed to be necessary, which may be achieved through the emergence of unique, though perhaps suboptimal, modes of interaction among regions within the remaining system. We therefore expected greater utilization of a limbic network associated with incidental emotional processing of faces with increasing delay in AD patients, but not in controls, to compensate for weaker functional links between anterior and posterior memory regions. The hippocampus, which is implicated in emotional memory through interactions with the amygdala but is not considered an emotion processing structure on its own (Bechara et al., 1995; Hamann, 2000), also was included in the analysis. Demonstration that the hippocampus is not modulated directly by activity in emotion-related structures would be suggestive of an implicit or indirect emotional strategy underlying improved performance in the patients.
2. Methods
Data for the present study were from an earlier study by Grady et al. (2001). Details of the experimental design are provided in the original study and described briefly here.
2.1. Subjects
The study included 32 participants: 11 patients diagnosed with Alzheimer-type dementia (3 possible and 8 probable; 4 women and 7 men; mean age = 67.9 ± 11 yrs., range = 59-84 yrs.; IQ = 109 ± 14; MMSE = 24 ± 4) and 21 healthy, age-matched controls (9 women and 12 men; mean age = 66.8 ± 5.6 yrs., range = 57-81 yrs.; IQ = 129 ± 7). All participants had a mean of 16 years of education and, other than AD in the patients, none suffered from neurological or psychiatric impairment. None of the patients reported feelings of increased arousal during task performance.
2.2. Data Acquisition
PET scans were performed on all participants with injections of 37.5 mCi of H215O each, separated by 12 minutes on a Scanditronix PC2048-15B tomograph (Uppsala, Sweden; reconstructed resolution of 6.5 mm in both transverse and axial planes). A more detailed description of the scanning procedure is provided in Grady et al. (2001). Informed written consent was obtained from all participants, and experimental procedures followed the guidelines on ethical conduct for research with human subjects as prescribed by the National Institute on Aging in accordance with the Declaration of Helsinki.
2.3. Task Design
Participants were administered a two-alternative, forced-choice recognition task of unfamiliar faces selected from a high school yearbook across 4 delays ranging from 1 to 16 sec. between study and choice arrays, presented for 4 sec. each (Haxby et al., 1995). The study array contained the to-be-remembered ‘sample’ face centered in the top half of the screen. This was followed by a blank array presented during the delay period, and then a choice array with two faces appearing side by side in the bottom half of the screen, with one of the faces matching the sample face. Participants were instructed to determine as quickly and accurately as possible the photograph that depicted the same face as a studied target with a corresponding button press. Alternating right and left button presses to noise patterns with visual complexity similar to that of the faces and placed in the same positions as in the stimulus array served as a sensorimotor control task. The focus of the current analysis is only on the shortest (1 sec.) and longest (16 sec.) delay conditions, in which the difference in performance between groups with increasing delay was most pronounced. Specifically, recognition accuracy in the patients decreased significantly from 92% at the 1-sec. delay (SD, 8.08; Range, 76-100%) to 72% at the 16-sec. delay (SD, 14.33; Range, 54-100%). In contrast, accuracy remained above 90% in the older controls, decreasing from 97% at the 1-sec. delay (SD, 6.69; Range, 74-100) to 94% at the 16-sec. delay (SD, 8.44; Range, 75-100). Inclusion of effective connectivity analysis at the 1-sec. delay, when performance of the AD patients was indistinguishable from controls, was to serve as a contrast to the 16-sec. delay condition, when performance differed significantly, to show that the network of emotion-related structures comes online as performance changes.
2.4. Data Analysis
Images were registered, normalized into standard Talairach and Tournoux space, and smoothed with a Gaussian filter of 10 mm using statistical parametric mapping (SPM95; Wellcome Department of Cognitive Neurology, London, UK). In the prior analysis, we determined the brain regions with delay-related changes in rCBF for each group and how brain activity correlated with behavioural performance (Grady et al., 2001). A region of the left amygdala was identified that showed task and behaviour-related activity in the patients, and an additional analysis identified the brain areas where activity was correlated with the amygdala. The regions chosen for the current analysis were obtained from these analyses as described below.
2.5. Structural Equation Modeling
SEM combines known anatomical pathways with interregional covariances among selected brain regions to quantify the influence of each region on another (i.e., effective connectivity; McIntosh & Gonzalez-Lima, 1994). The first step in SEM is to determine the anatomical model. We constructed this model based on the results from the prior analysis, which suggested differential recruitment of a network of brain regions, each represented by a single voxel2, that covaried across delay with the left amygdala in the AD group. In addition to the left amygdala, the model included bilateral inferior PFC, anterior cingulate, and left insula, as they are anatomically interconnected and displayed strong positive correlations with the amygdala in the patients but not in the controls in the original report (Grady et al., 2001). These regions are all known to play a role in emotional processing of stimuli, including faces (Dolan et al., 1996; Hornak et al., 1996; Morris et al., 1998; Phillips et al., 1997; Whalen et al., 1998). The left hippocampus was also entered as a node as an added test to determine if the amygdala modulates activity of this region in either group. The coordinates of regions included in the model are presented in Table 1.
Table 1.
Coordinates of Regions Included in the Model
| Region | BA | Hemi | Alzheimer's Patients | Older Adults | ||||
|---|---|---|---|---|---|---|---|---|
| X | y | z | x | y | z | |||
| Inferior Prefrontal | 47 | R | 34 | 32 | -8 | 30 | 26 | -4 |
| 47 | L | -34 | 20 | -16 | -34 | 20 | -16 | |
| Anterior Cingulate | 24 | M | 6 | -12 | 36 | 12 | 14 | 28 |
| Insula | L | -34 | -22 | 4 | -30 | -4 | -16 | |
| Amygdala | L | -24 | -6 | -12 | -24 | -12 | -12 | |
| Hippocampus | L | -36 | -26 | -12 | -28 | -26 | -8 | |
Note. Functional connectivity and task analyses reported for the two groups (see Grady et al., 2001) served as the source for all coordinates of regions included in the current SEM analysis. Coordinates in bold refer to the location of the left amygdala reference region (seed) with which activity in the other regions was found to covary. BA, Brodmann area; Hemi, Hemisphere; R, Right; L, Left.
Following the selection of regions, neuroanatomical networks of afferent, or feedforward, connections between regions were defined based on the existing non-human primate literature to determine the causal structure of the model (Amaral et al., 1992; Petrides & Pandya, 1994; Suzuki & Amaral, 1994; Van Hoesen et al., 1993). For simplicity, anatomically based feedback connections as well as interhemispheric homologous connections were included in the model only if the modification index suggested that these connections would contribute substantially to the model (McIntosh & Gonzalez-Lima, 1994).
Correlation matrices of activity between regions were calculated for each group, and estimates of path coefficients (numerical weights) representing the magnitude and nature (i.e., excitatory or inhibitory; Nyberg et al., 1996) of each neural connection were then defined for each connection using LISREL (Joreskog & Sorbom, 1999). An omnibus test of the difference in path coefficients between the two groups was first calculated using a stacked model. The difference between chi-square goodness-of-fit values for null and alternative models, in which path coefficients were set to be equal or allowed to vary between groups, respectively, was calculated to determine if the models differed significantly. Differences in individual paths were then tested with a hierarchical model. Connections were set to be equal across groups and were allowed to vary in a step-wise fashion, and only those connections found to differ significantly across group were left unconstrained as the analysis progressed to other connections. A more detailed account of this method has been described previously (McIntosh & Gonzalez-Lima, 1994; Nyberg et al., 1996).
3. Results
Fig. 1 shows the path coefficients for each group for the 1-sec. and 16-sec. delays. The omnibus comparison revealed a significant difference in path coefficients between groups at each delay (χ2diff(13) = 46.28, p < 10-5). Testing of individual paths indicated that there was little engagement of the network at the 1-sec. delay in either group. Both healthy controls and AD patients showed a strong positive influence of left inferior PFC on right inferior PFC. Controls showed additional, relatively weak positive influences of the left amygdala on left hippocampus and anterior cingulate on right inferior PFC. The reverse pattern was present in the patients, such that the left amygdala had a weak negative influence on left hippocampus and a stronger negative influence of anterior cingulate on right inferior PFC. At the 16-sec. delay, path coefficients were generally of greater strength in the patients than in the controls, particularly with respect to positive influences from left hippocampus and left insula on anterior cingulate, and from hippocampus and amygdala on inferior PFC in the left hemisphere, which also received negative input from left insula but culminated in a positive influence on its right homologue. Direction of interactions also differed across groups such that the patients showed additional positive influences of anterior cingulate on left amygdala, a negative influence of the anterior cingulate on right PFC and from right to left inferior PFC. In contrast, no strong direct input to the left hippocampus from the left amygdala was identified in either group at the 16-sec delay.
Fig. 1.
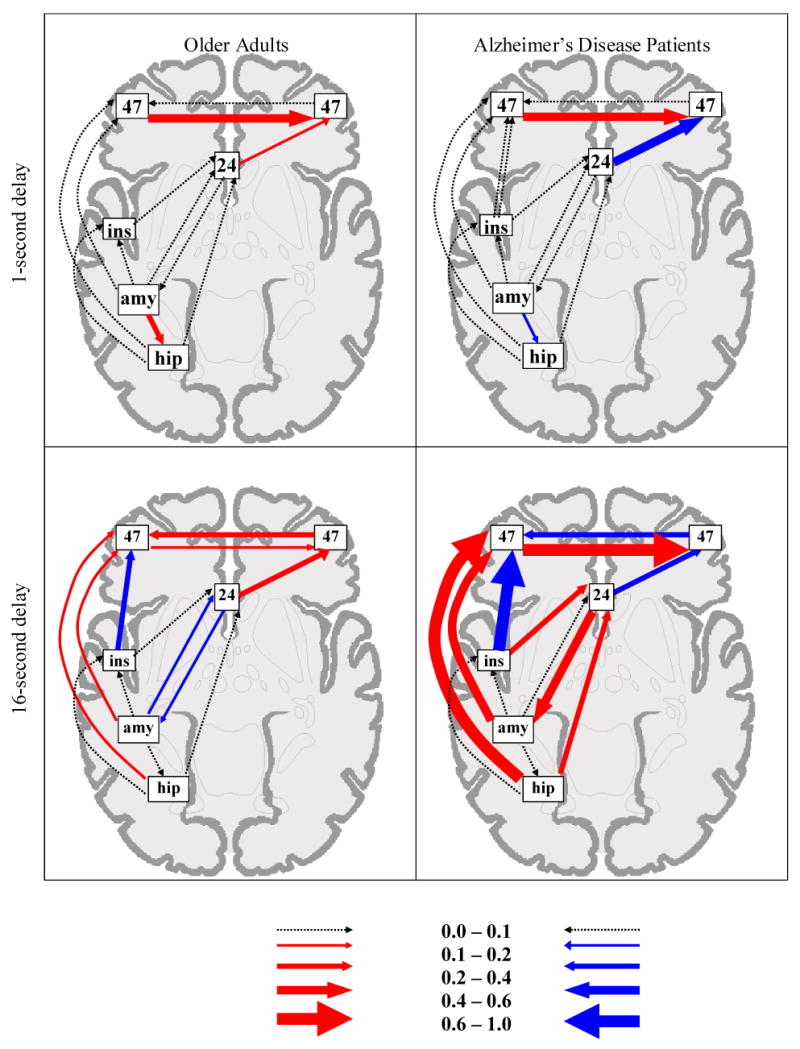
Graphic representation of the functional networks relating to the left amygdala reference region (seed) for each group. The arrow width for each path represents the magnitude of each connection. Values for the width gradient are provided in the legend at the bottom. Positive path coefficients are represented as red arrows, negative coefficients as blue arrows, and coefficients that did not differ across group as gray arrows. To maintain figure clarity, paths where the coefficient was at or near zero are represented by dotted lines, and the relative locations of brain regions are distorted.
4. Discussion
The present study aimed to uncover the nature of interactions within a network of structures that are associated with emotion processing and that may help to sustain performance on a delayed match-to-sample face recognition task in AD patients but not in healthy older adults. The set of interactions relating to left amygdala activity identified in the AD patients was found to be distinct from that utilized by the controls. In particular, the results indicated strong, positive increases in left amygdala input and output at the 16-sec. delay in the patients relative to controls. Strong, positive output from the left amygdala and from the left hippocampus to left and then right inferior PFC was identified. However, the left hippocampus was not modulated directly by the left amygdala in either group at the 16-sec. delay, and received only weak input from the amygdala in the control group at the 1-sec. delay. These findings suggest that an implicit emotional mechanism, mediated by amygdala connectivity with emotion-related regions, may underlie performance in the patients.
It is unlikely that brain regions that remain structurally intact are functionally insulated from the effects of damage elsewhere in the brain, whether resulting in the disruption or maintenance of performance (Price & Friston, 2002). Neurodegenerative diseases, such as AD, may differentially affect functional integrity within individual regions that are structurally compromised, such as the hippocampus and amygdala, as well as within networks of regions, and these changes are not always readily captured with univariate approaches that assess activity within each region separately. The few studies that have applied network analysis to understanding AD have reported degraded interactions between the PFC and posterior regions, including the hippocampus during short-term episodic memory (Grady et al., 2001), visual regions during face perception (Bokde et al., 2006; Horwitz et al., 1995), and parietal regions in the resting state (Azari et al., 1992; Horwitz et al., 1987; Wang et al., 2007). In contrast, successful performance on tests of explicit memory in AD patients appears to rely on interactions among a set of PFC regions in isolation of more posterior regions (Grady et al., 2003; Stern et al., 2000). Here we show that increased PFC connectivity may not be the only route by which AD patients compensate for cognitive loss due to neural degeneration early in the course of the disease.
Multiple, reciprocal connections among limbic and paralimbic systems with sensory, perceptual, memory, executive, and action systems form an intricate circuitry by which thought is coloured by valence and arousal. Emotion, in turn, is modulated by the ability to comprehend, attend to, maintain, and strategically encode and retrieve information. The network of influences identified in the AD patients but not in the controls may reflect this interplay, allowing for the emergence of an anterior-posterior circuit of primarily left-hemisphere ‘emotional’ regions to offset the earlier finding of a disconnection between ‘cognitive’ dorsolateral PFC and hippocampus (Grady et al., 2001). Correlated activity with the amygdala, but not hippocampus, was found to underlie better performance in the patients, even though the hippocampus and amygdala are both affected in early stages of AD. The current study extends this finding by showing that the two structures maintain different patterns of connectivity with other structures.
The MTL memory system, though not considered emotional on its own, is known to be strongly influenced by the emotional content of stimuli during explicit encoding and retrieval via its strong reciprocal connections with the amygdala (LaBar & Cabeza, 2006). Using SEM, Kilpatrick and Cahill (2003) provided direct evidence for such an effect: increased activity in the right amygdala led to increased activity in right parahippocampus and right inferior PFC during encoding of emotional film clips in healthy young adults. We found a similar influence of left amygdala activity on left inferior PFC activity, which in turn led to an increase in right inferior PFC activity, but no such influence on the left hippocampus was found, suggesting that an explicit emotional memory strategy in the present study was unlikely.
The network of interactions revealed may instead reflect incidental processing of the emotional content of the faces, which included neutral and happy expressions. The amygdala, anterior cingulate, insula, and inferior PFC have all been associated with emotional face perception and memory (Hornak et al., 1996; Morris et al., 1998; Phillips et al., 1997), even when emotional processing is covert (Critchley et al., 2000; Dolan et al., 1996; Whalen et al., 1998). Left amygdala and inferior PFC function have been associated with a wide range of emotions, including processing of neutral and happy facial expressions (Fitzgerald et al., 2006). Also consistent with the current results are earlier reports of bilateral amygdala, anterior cingulate, and inferior PFC recruitment during nonconscious or incidental perception of happy faces (Gorno-Tempini et al., 2001; Killgore & Yurgelun-Todd, 2004; Williams et al., 2004). A related possibility is that the AD patients recruited an inhibitory system to suppress any emotional response elicited by the faces that is irrelevant to task performance. This is suggested by recent evidence that the role of left inferior PFC in inhibitory processing extends to control of emotional distraction during the delay period of a short-term memory task for neutral faces (Dolcos et al., 2006; Dolcos & McCarthy, 2006). Importantly, activity in this region was correlated with that of the amygdala (Dolcos & McCarthy, 2006). These findings suggest that the amygdala sends input to inferior PFC to signal the presence of emotional distraction, a result that is consistent with the pattern of connectivity seen in our model. It is also possible that the network revealed in the current study reflects differences in the emotional response of the patients and controls (Ressler & Mayberg, 2007). We view this explanation as unlikely, however, as none of the participants had a history of anxiety or mood disorder, and there was no suggestion of increased arousal in either group during task performance. Future work is needed to differentiate among these alternatives and to determine whether this altered connectivity directly supports short-term memory in AD.
To conclude, a core network of influences within an emotional circuit was more pronounced in AD patients than in healthy older participants during a delayed match-to-sample face recognition task. The direction of influences from left amygdala and left hippocampus on left and then right inferior PFC, in the absence of direct amygdala-hippocampal interactions, may reflect an implicit signaling of emotional content in the faces to increase their memorability or the need to diminish emotional distraction. To our knowledge, this is one of only two studies to apply effective connectivity analysis to neuroimaging of AD (see Horwitz et al., 1987), and the first to use this approach to characterize the functional interactions that facilitate memory performance. The shift to “hot” emotional processing to achieve what would normally be under the guidance of “cold” cognitive processing may inform intervention strategies for AD patients in early stages of the disease.
Acknowledgments
This work was supported by a grant from the Canadian Institutes of Health Research (CIHR MOP 14036). R.S.R. is supported by a CIHR New Investigator Award and the Natural Sciences and Engineering Research Council, and C.L.G. is supported by the Canada Research Chairs program. B.H. acknowledges the support of the NIDCD Intramural Research Program.
Footnotes
The term ‘network’ is intended to include specified brain structures as well as the white matter connecting those structures, both of which are likely compromised in AD.
A single voxel is representative of a larger region due to spatial smoothing of PET data.
Disclosure Statement: The authors declare that they have no conflict of interest, financial or otherwise, related to the present work.
Informed written consent was obtained from all participants, and experimental procedures followed the guidelines on ethical conduct for research with human subjects as prescribed by the National Institute on Aging in accordance with the Declaration of Helsinki.
References
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Wiley Liss; New York: 1992. pp. 1–66. [Google Scholar]
- Azari NP, Rapoport SI, Grady CL, Schapiro MB, Salerno JA, Gonzales-Aviles A, Horwitz B. Patterns of interregional correlations of cerebral glucose metabolic rates in patients with dementia of the Alzheimer type. Neurodegeneration. 1992;1:101–111. [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Bokde AL, Lopez-Bayo P, Meindl T, Pechler S, Born C, Faltraco F, Teipel SJ, Moller HJ, Hampel H. Functional connectivity of the fusiform gyrus during a face-matching task in subjects with mild cognitive impairment. Brain. 2006;129:1113–1124. doi: 10.1093/brain/awl051. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. Eur Neurol. 1993;33:403–408. doi: 10.1159/000116984. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, Jennings JM, Houle S, Craik FIM. Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. J Neurosci. 1997;17:391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase TN, Burrows GH, Mohr E. Cortical glucose utilization patterns in primary degenerative dementias of the anterior and posterior types. Arch Gerontol Geriatr. 1987;6:289–297. doi: 10.1016/0167-4943(87)90028-8. [DOI] [PubMed] [Google Scholar]
- Critchley H, Daly E, Phillips M, Brammer M, Bullmore E, Williams S, Van Amelsvoort T, Robertson D, David A, Murphy D. Explicit and implicit neural mechanisms for processing of social information from facial expressions: a functional magnetic resonance imaging study. Hum Brain Mapp. 2000;9:93–105. doi: 10.1002/(SICI)1097-0193(200002)9:2<93::AID-HBM4>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish T. The emotional brain. Nat Rev Neurosci. 2004;5:582–589. doi: 10.1038/nrn1432. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Fletcher P, Morris J, Kapur N, Deakin JF, Frith CD. Neural activation during covert processing of positive emotional facial expressions. Neuroimage. 1996;4:194–200. doi: 10.1006/nimg.1996.0070. [DOI] [PubMed] [Google Scholar]
- Dolcos F, Kragel P, Wang L, McCarthy G. Role of the inferior frontal cortex in coping with distracting emotions. Neuroreport. 2006;17:1591–1594. doi: 10.1097/01.wnr.0000236860.24081.be. [DOI] [PubMed] [Google Scholar]
- Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. J Neurosci. 2006;26:2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL. Beyond threat: amygdala reactivity across multiple expressions of facial affect. NeuroImage. 2006;30:1441–1448. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Pradelli S, Serafini M, Pagnoni G, Baraldi P, Porro C, Nicoletti R, Umita C, Nichelli P. Explicit and incidental facial expression processing: an fMRI study. NeuroImage. 2001;14:465–473. doi: 10.1006/nimg.2001.0811. [DOI] [PubMed] [Google Scholar]
- Grady CL, Haxby JV, Schapiro MB, Gonzalez-Aviles A, Kumar A, Ball MJ, Heston L, Rapoport SI. Subgroups in dementia of the Alzheimer type identified using positron emission tomography. J Neuropsychiatry Clin Neurosci. 1990;2:373–384. doi: 10.1176/jnp.2.4.373. [DOI] [PubMed] [Google Scholar]
- Grady CL, Furey ML, Pietrini P, Horwitz B, Rapoport SI. Altered brain functional connectivity and impaired short-term memory in Alzheimer's disease. Brain. 2001;124:739–756. doi: 10.1093/brain/124.4.739. [DOI] [PubMed] [Google Scholar]
- Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, Pietrini P, Wagner E, Haxby JV. Age-related changes in cortical blood flow activation during visual processing of faces and location. J Neurosci. 1994;14:1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Beig S, Keightley ML, Burian H, Black SE. Evidence from functional neuroimaging of a compensatory prefrontal network in Alzheimer's disease. J Neurosci. 2003;23:986–993. doi: 10.1523/JNEUROSCI.23-03-00986.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann S. Cognitive and neural mechanisms of emotional memory. Trends Cogn Sci. 2001;5:394–400. doi: 10.1016/s1364-6613(00)01707-1. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Ungerleider LG, Horwitz B, Rapoport SI, Grady CL. Hemispheric differences in neural systems for face working memory: a PET-rCBF study. Hum Brain Mapp. 1995;3:68–82. [Google Scholar]
- Hornak J, Rolls ET, Wade D. Face and voice expression identification in patients with emotional and behavioural changes following ventral frontal lobe damage. Neuropsychologia. 1996;34:247–261. doi: 10.1016/0028-3932(95)00106-9. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Grady CL, Schlageter NL, Duara R, Rapoport SI. Intercorrelations of regional glucose metabolic rates in Alzheimer's disease. Brain Res. 1987;407:294–306. doi: 10.1016/0006-8993(87)91107-3. [DOI] [PubMed] [Google Scholar]
- Horwitz B, McIntosh AR, Haxby JV, Furey M, Salerno J, Schapiro MB, Rapoport SI, Grady CL. Network analysis of PET-mapped visual pathways in Alzheimer's type dementia. NeuroReport. 1995;6:2287–2292. doi: 10.1097/00001756-199511270-00005. [DOI] [PubMed] [Google Scholar]
- Joreskog KG, Sorbom D. LISREL 8.3: User's Reference Guide. Scientific Software Inc.; Mooresville: 1999. [Google Scholar]
- Kazui H, Mori E, Hashimoto M, Hirono N, Imamura T, Tanimukai S, Hanihara T, Cahill L. Impact of emotion on memory. Controlled study of the influence of emotionally charged material on declarative memory in Alzheimer's disease. Br J Psychiatry. 2000;177:343–347. doi: 10.1192/bjp.177.4.343. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Activation of the amygdala and anterior cingulate during nonconscious processing of sad versus happy faces. NeuroImage. 2004;21:1215–1223. doi: 10.1016/j.neuroimage.2003.12.033. [DOI] [PubMed] [Google Scholar]
- Kilpatrick L, Cahill L. Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. NeuroImage. 2003;20:2091–2099. doi: 10.1016/j.neuroimage.2003.08.006. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nat Rev Neurosci. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Mesulam M, Gitelman DR, Weintraub S. Emotional curiosity: modulation of visuospatial attention by arousal is preserved in aging and early-stage Alzheimer's disease. Neuropsychologia. 2000;38:1734–1740. doi: 10.1016/s0028-3932(00)00077-4. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez-Lima F. Structural equation modeling and its application to network analysis in functional brain imaging. Hum Brain Mapp. 1994;2:2–22. [Google Scholar]
- Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, Dolan RJ. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121:147–157. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Nyberg L, McIntosh AR, Cabeza R, Nilsson LG, Houle S, Habib R, Tulving E. Network analysis of positron emission tomography regional cerebral blood flow data: ensemble inhibition during episodic memory retrieval. J Neurosci. 1996;16:3753–3759. doi: 10.1523/JNEUROSCI.16-11-03753.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padovan C, Versace R, Thomas-Anterion C, Laurent B. Evidence for a selective deficit in automatic activation of positive information in patients with Alzheimer's disease in an affective priming paradigm. Neuropsychologia. 2002;40:335–339. doi: 10.1016/s0028-3932(01)00101-4. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Hodges JR. Spectrum of memory dysfunction in degenerative disease. Curr Opin Neurol. 1996;9:281–285. doi: 10.1097/00019052-199608000-00007. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Comparative architectonic analysis of the human and macaque frontal cortex. In: Boller F, Grafman J, editors. Handbook of Neuropsychology. Vol. 9. Elsevier; Amsterdam: 1994. pp. 17–57. [Google Scholar]
- Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, Bullmore ET, Perrett DI, Rowland D, Williams SC, Gray JA, David AS. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Functional imaging studies of neuropsychological patients: applications and limitations. Neurocase. 2002;8:345–354. doi: 10.1076/neur.8.4.345.16186. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Moeller JR, Anderson KE, Luber B, Zubin NR, DiMauro AA, Park A, Campbell CE, Marder K, Bell K, Van Heertum R, Sackeim HA. Different brain networks mediate task performance in normal aging and AD: defining compensation. Neurol. 2000;55:1291–1297. doi: 10.1212/wnl.55.9.1291. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Levine B. Adult clinical neuropsychology: Lessons from studies of the frontal lobes. Annu Rev Psychol. 2002;53:401–433. doi: 10.1146/annurev.psych.53.100901.135220. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Morecraft RJ, Vogt BA. Connections of the monkey cingulate cortex. In: Vogt BA, Gabriel M, editors. Neurobiology of Cingulate Cortex and Limbic Thalamus: A Comprehensive Handbook. Birkhauser; Boston: 1993. pp. 461–477. [Google Scholar]
- Wang K, Liang M, Wang L, Tian L, Zhang X, Li K, Jiang T. Altered functional connectivity in early Alzheimer's disease: A resting-state fMRI study. Hum Brain Mapp. 2007;28:967–978. doi: 10.1002/hbm.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MA, Morris AP, McGlone F, Abbott DF, Mattingley JB. Amygdala responses to fearful and happy facial expressions under conditions of binocular suppression. J Neurosci. 2004;24:2898–2904. doi: 10.1523/JNEUROSCI.4977-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winograd E, Goldstein FC, Monarch ES, Peluso JP, Goldman WP. The mere exposure effect in patients with Alzheimer's disease. Neuropsychology. 1999;13:41–46. doi: 10.1037//0894-4105.13.1.41. [DOI] [PubMed] [Google Scholar]


