Abstract
Obesity and hypertension are the two major risk factors that contribute to the progression of end-stage renal disease. To examine whether hypertension further exacerbates oxidative stress and vascular dysfunction and inflammation in obese rats, four groups of male Sprague Dawley rats were fed either normal (7% fat) or high fat (36% fat) diet for 6 weeks and osmotic pumps were implanted to deliver angiotensin II (ANG) or vehicle for four additional weeks. High fat diet treatment did not alter ANG-induced hypertension compared to normal diet (174±6 vs. 170±5 mmHg, respectively). High fat diet treatment increased body weight gain and plasma leptin levels and induced insulin resistance in normotensive and ANG hypertensive rats. Plasma TBARs, a measure of oxidative stress, was elevated in high fat diet fed rats compared to control (11.2±1 vs. 8.4±1 nmol/ml, respectively) and was further increased in ANG hypertensive rats fed high fat diet (18.8±2.2 nmol/ml). Urinary nitrite excretion was also decreased in rats fed high fat diet with or without ANG infusion compared to control. Afferent arteriolar relaxation to acetylcholine was impaired in high fat fed rats with or without ANG infusion. Renal cortical TNF-α, COX-2, and phospho-IKK expression increased in high fat diet compared to normal diet fed rats. The increases in phospho-IKK and COX-2 expression were further elevated in ANG hypertensive rats fed high fat diet. These data suggest that ANG-induced hypertension exacerbates oxidative stress and renal inflammation without further impairment in vascular dysfunction in high fat diet-induced obesity.
Keywords: Obesity, oxidative stress, inflammation, hypertension, vascular dysfunction
Introduction
Obesity is one of the biggest global health problems nowadays [1]. However, the etiology of obesity remains unclear. The danger of obesity over time is that the increase in body weight will cause insulin resistance, diabetes, dyslipidemia, atherosclerosis, and hypertension, what is also referred to as metabolic syndrome [2, 3]. Obesity and hypertension have been identified as independent risk factors for the development of vascular dysfunction and renal disease [4]. Interestingly, hypertension and diabetes account for seventy percent of patients with end-stage renal disease [4, 5]. Studies have shown that obese Zucker and obese spontaneous hypertensive rats develop renal inflammation and endothelial dysfunction [6–8]; however, the mechanisms of vascular dysfunction and renal inflammation are not clear and require further elucidation.
Oxidative stress plays a major role in the pathogenesis of endothelial dysfunction and inflammation in cardiovascular disease [9]. The increase in oxidative stress and its impact on NO metabolism in rats with diet-induced metabolic syndrome are well established [10, 11]. Previous studies have shown that NADPH oxidase is the major source of superoxide production in high fat diet fed rats [10, 12]. Oxidative stress is also an important trigger for insulin resistance [13]. Increased superoxide production decreases NO bioavailability and activates inflammatory responses in obese animal models that could contribute to the incidence of renal injury [14–16]. Inflammatory cytokines also play a critical role in the incidence of insulin resistance in obese subjects [17]. For example, a positive correlation has been found between serum tumor necrosis factor-alpha (TNF-α) concentration and both systolic blood pressure and insulin resistance in subjects with a wide range of adiposity [18]. TNF-α expression is increased in patients with weight gain and is reduced following weight loss [19]. Likewise, neutralizing TNF-α in obese mice improved insulin sensitivity suggesting that elevated TNF-α levels in obesity induce vascular insulin resistance by impairing insulin signaling [20, 21]. TNF-α has been shown to activate the nuclear transcription factor kappa B (NFκB) [22, 23] which then translocates to the nucleus to activate downstream inflammatory signaling such as COX-2 gene perturbing the inflammatory signal and this could be a possible mechanism for obesity-induced vascular dysfunction and renal inflammation. It is not clear whether the increase in blood pressure or the increase in body weight gain is the main cause of obesity-induced vascular dysfunction and inflammation. Thus, we hypothesized that obese hypertensive rats have exacerbated vascular dysfunction and inflammation than obese or hypertensive rats alone. Therefore, in this study we investigated the effect of chronic high fat diet treatment on vascular function in control and ANG hypertension and examined the potential mechanisms involved in the development of vascular dysfunction in obesity and hypertension.
Methods
All animal studies were approved by the Medical College of Georgia institutional review committee according to the National Institutes of Health guidelines for care and use of laboratory animals. Ten-eleven week old male Sprague-Dawley rats (Charles River, Wilmington, MA) were used in this study. Rats were fed either normal rodent chow diet (Bio-Serv #F3028, Frenchtown, NJ) or high fat diet (Bio-Serv #F2685, Frenchtown, NJ) for 10 weeks. The normal chow diet contains 11.9% kcal as fat and a total of 3.3 kcal/g and the high fat diet contains 58.3% kcal as fat and a total of 5.4 kcal/g. After 6 weeks of a normal or high fat diet treatment, osmotic mini-pumps (Alzet, model 2004, Cupertino, CA) were used to deliver angiotensin II (60 ng/min, Phoenix, AZ) subcutaneously for the last 4 weeks of experiment and rats were maintained on either a normal or high fat diet until the end of the experimental period. Four groups of rats (n=6–8) were used in this study as follows: normal, normal/angiotensin II (ANG), high fat, and high fat/ANG. Body weight gain, blood glucose levels from a tail vein using a glucometer, and systolic blood pressure using tail-cuff plethysmography were determined weekly in all rat groups. Rats were placed in metabolic cages (Nalgene Corp. Rochester, NY) for 24 hour urine collection at the end of the 10 week experiment. After 10 weeks, rats were terminated and plasma was separated and used to determine lipid profiles and other hormonal and metabolite levels. For example, plasma insulin (Mercodia, Winston Salem, NC), cholesterol (Wako Chemicals, Richmond, VA), triglycerides (Wako Chemicals), LDL (Wako Chemicals), leptin (Linco Research), and thiobarbituric acid reactive substances (TBARs, Zeptometrix, Franklin, MA) and urinary nitrite excretion (Cayman Chemical, Ann Arbor, MI) were determined. Renal cortical tissues were dissected and snap frozen in liquid nitrogen for western blotting.
Insulin Resistance Measurement
The development of insulin resistance was identified in the four rat groups (n=3–4) using the hyperinsulinemic euglycemic clamp method as previously described [8]. Briefly, rats were anesthetized and placed on a heating pad to maintain body temperature at 37°C. Jugular and femoral veins were catheterized and used as i.v injection lines. Carotid artery was catheterized for blood collection. Before starting the clamp, two arterial blood samples were taken for blood glucose determination. Insulin was then infused at a rate of 0.01U/min. Blood glucose readings were taken every five minutes for 20 minutes. A glucose solution (10%) was infused and the rate was adjusted until blood glucose readings average 125 mg/dl for the last 30 minutes. The rate of glucose infusion during the last 20 minutes determines insulin sensitivity.
In-vitro Juxtamedullary Nephron Preparation
This technique was used to evaluate endothelial function in normal, normal/ANG, high fat, and high fat/ANG rats (n=5) as previously described [8]. Rats were anesthetized with pentobarbital (40 mg/kg body weight i.p.). The right kidney was isolated and after a midline laparotomy, the right renal artery was cannulated through the superior mesenteric artery. The kidney was immediately perfused with a Tyrode’s solution containing 6% albumin and a mixture o f L-amino acids. After the microdissection procedures were completed, the renal artery perfusion pressure was set to 100 mm Hg. The tissue surface was continuously superfused with a Tyrode’s solution containing 1% albumin. After a 20-minute equilibration period, an afferent arteriole was chosen for study, and baseline diameter was measured. After the control period, the afferent arteriole was constricted with phenylephrine and the endothelium-dependent relaxation was assessed using increasing concentrations of acetylcholine (0.01–10 µm). The afferent arteriole diameter changes to acetylcholine were monitored for 3 minutes at each concentration. Steady-state diameter to acetylcholine was attained by the end of the second minute, and the average diameter at the third minute was used for statistical analysis. Endothelial-independent relaxation was also assessed at the end of experiment using sodium nitroprusside.
Renal cortical TNF-α, COX-2 and IKKα/B expression
Frozen kidney cortex was sliced very thinly and homogenized in complete lysis buffer for preparation of whole cell extract using a nuclear extract kit (Active Motif, Carlsbad, CA). Homogenate was centrifugated at 9000 g for 10 minutes and then supernatant was aliquoted and stored at −80°C. Protein concentration was determined using bicinchoninic acid protein assay kit (Pierce, Rockford, IL). 50 µg protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 10% Tris-glycine gel, and proteins were transferred electrophoretically to a PVDF membrane. Non-specific binding sites were blocked by incubating the blots overnight at 4°C in a Tris-NaCl buffer (TBS) containing 5% non fat dry milk and 0.1% Tween 20 in addition to the primary antibody. The primary antibodies used were rabbit TNF-α, phospho-IKKα (ser180)/IKKβ (ser181), IKK, and COX-2 (Cell signaling technology, Danvers, MA). The blots were then washed in a TBS-0.1% Tween 20 and incubated with the secondary antibody (goat anti-rabbit 1:5000) conjugated to horseradish peroxidase for 1 hour at room temperature. Detection was accomplished using enhanced chemiluminescence western blotting and band intensity was measured densitometrically and the values were factored for β-actin. For IKK, band intensity for both phospho-IKKα and phospho-IKKβ was also measured densitometrically and the values were factored for IKK.
NFκB transcription factor assay
Twenty µg of cortical whole-cell extract was used for the determination of NFκB activity using the TransAM NFκB p65 transcription factor assay kit (Active Motif, Carlsbad, CA).
Pair fed experiment
Initially, average daily food consumption was measured in male Sprague Dawley rats and total caloric intake was calculated. Rats were then divided into two groups (n=4) and fed either a normal diet or pair fed a high fat diet (limit amount of food intake of high fat diet to the same caloric intake of normal diet) for 10 weeks. The amount of food intake for the two groups was adjusted each week to have the same caloric intake. Body weight gain and blood glucose were determined weekly in the two rat groups. Rats were terminated after 10 weeks and plasma insulin levels were determined. Afferent arteriolar relaxation to acetylcholine was also assessed.
Statistical analysis
All data are presented as mean ± SEM. Systolic blood pressure, % body weight gain, and % afferent arteriolar relaxation data were analyzed using analysis of variance (ANOVA) for repeated measurements. All other data were analyzed using one-way (ANOVA) followed by Tukey’s post-hoc test for multiple group comparisons. Differences were considered statistically significant with p < 0.05 compared to the control. Analyses were performed using GraphPad Prism Version 4.0 software (GraphPad Software Inc, La Jolla, CA).
Results
Blood Pressure and Body Weight Gain
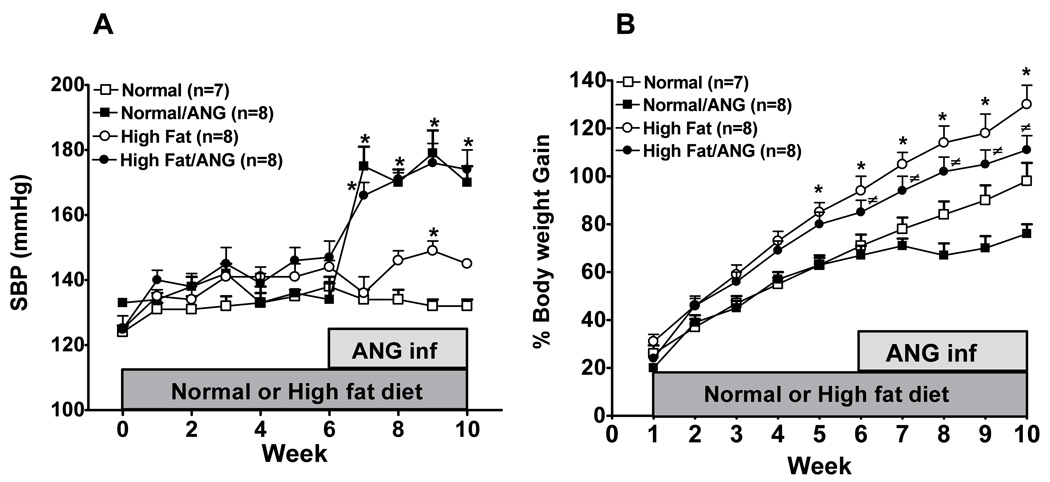
Figure 1A shows the effect of long-term high fat diet treatment on blood pressure. Systolic blood pressure mildly increased in high fat diet fed rats compared to normal diet fed rats and this increase was only significant at the end of the 9-week treatment (149±3 vs. 132±2 mmHg). ANG infusion did not potentiate the increase in blood pressure in high fat/ANG compared to normal/ANG (179±7 vs. 176±6 mmHg) suggesting that high fat diet did not exacerbate ANG-induced hypertension. Figure 1B shows that body weight gain significantly increased in rats fed high fat diet and in high fat/ANG groups compared to normal diet and there was no difference in body weight gain in high fat fed rats and high fat/ANG. However, ANG treated rats fed normal or high fat diet did not gain as much weight as rats fed normal or high fat diet alone. In fact, rats fed normal diet started to lose weight after ANG infusion that could be attributed to the decrease in food intake during ANG infusion. The average food intake for rats fed normal diet and high fat diet was 30.4±1.5 g/day and 20±1 g/day, respectively. Infusion of ANG resulted in a 14% decrease in food intake in rats fed normal diet and a 20% decrease in rats fed high fat diet at the end of 10 weeks treatment.
Figure 1.
A: Systolic blood pressure and B: % Body weight gain in rats fed a normal or high fat diet for 10 weeks with or without angiotensin II (ANG) infusion in the last 4 weeks of the treatment. Values are mean ±SEM (n=7–8). *P < 0.05 high fat vs. normal diet and #P < 0.05 high fat/ANG vs. normal/ANG.
Blood Glucose Levels and Insulin Sensitivity
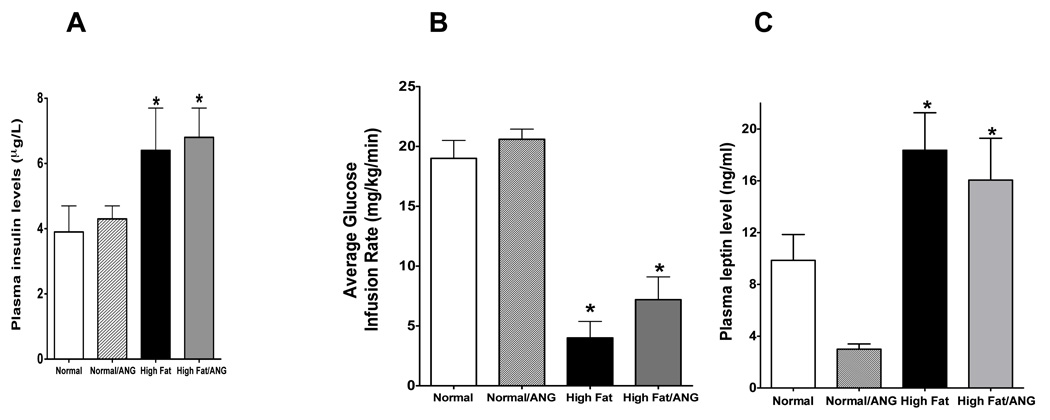
Random blood glucose levels, plasma insulin, and leptin levels, and insulin sensitivity were assessed in rats fed normal and high fat diet for 10 weeks with or without ANG infusion. Although there were no differences in blood glucose levels during the high fat diet treatment or at the end of the 10 weeks study period between all rat groups (Table 1), plasma insulin significantly increased approximately 65% in rats fed high fat diet and in high fat/ANG compared to rats fed normal diet (Figure 2A). Hyperinsulinemic-euglycemic clamp experiments were carried out to monitor peripheral insulin sensitivity in normal and high fat diet with or without ANG treatment for 10 weeks (Figure 2B). Rats fed high fat diet for 10 weeks with or without ANG showed a 60–70 % reduction in glucose infusion rate compared with normal diet fed rats, indicating the development of insulin resistance (P < 0.05). Plasma leptin levels were also significantly increased in both high fat and high fat/ANG groups compared to normal diet fed rats and ANG infusion decreased plasma leptin levels in rats fed normal diet (Figure 2C).
Table 1.
Plasma lipid profile, in normal, normal/ANG, high fat diet, high fat diet/ANG treated rats. Values are mean ±SEM (n=6–8).
| Plasma | Normal | Normal/ANG | High Fat | High Fat/ANG |
|---|---|---|---|---|
| Cholesterol (mg/dl) | 67±6 | 58±2 | 100±8* | 76±4# |
| Triglyceride (mg/dl) | 83±7 | 71±10 | 108±13 | 81±10 |
| LDL (mg/dl) | 22±2 | 19±1 | 30±3* | 31±5# |
| Blood glucose (mg/dl) | 110±5 | 100±2 | 124±5 | 115±3 |
P<0.05 high fat vs. normal diet
P<0.05 high fat/ANG vs. normal/ANG.
Figure 2.
A: Plasma insulin levels in rats fed a normal or high fat diet for 10 weeks with or without ANG infusion in the last 4 weeks of the treatment (n=6–8). B: Glucose infusion rates from hyperinsulinemic, euglycemic clamp experiments in normal, normal/ANG, high fat diet, and high fat diet/ANG treated rats (n=3–4). C: Plasma leptin concentrations in normal, normal/ANG, high fat, and high fat/ANG treated rats (n=6–8). Values are mean ±SEM. *P < 0.05 high fat vs. normal diet.
Afferent Arteriole Endothelial Function
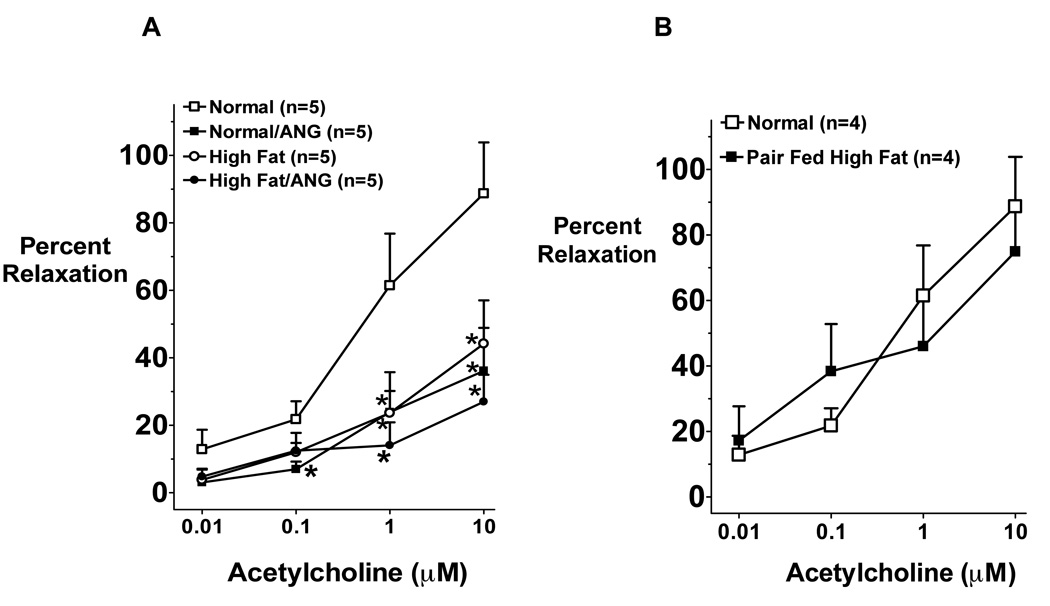
Afferent arteriole endothelial function was assessed using the in vitro juxtamedullary preparation and the results are depicted in Figure 3A. Afferent arteriole relaxation to acetylcholine was significantly impaired in normal/ANG, high fat, and high fat/ANG treated rats compared to normal diet fed rats (P < 0.05). Although high fat diet showed some tendency to exacerbate this impairment in ANG treated rats, this effect did not reach a statistical difference (Figure 3A). Endothelial-independent relaxation to sodium nitroprusside was not different between rat groups (data not shown) indicating that only the endothelial dependent dilatory response is impaired by high fat and/or ANG in this model.
Figure 3.
A: Afferent arteriole vascular response to acetylcholine (n=5) in rats fed a normal or high fat diet for 10 weeks with or without ANG infusion in the last 4 weeks of the treatment. Values are mean ±SEM. *P < 0.05 ANG or high fat with or without ANG vs. normal diet. B: Afferent arteriole vascular response to acetylcholine in rats fed a normal or caloric restricted high fat diet to the same amount of calories in a normal diet for 10 weeks treatment period. Values are mean ±SEM (n=4).
Rats fed caloric restricted high fat diet for 10 weeks gained as much weight as rats fed normal diet (body weight was 540±30 vs. 543±24 g, respectively). The impairment in afferent arteriolar relaxation to acetylcholine that was previously seen upon high fat diet was abolished when rats were fed caloric restricted high fat diet (figure 3B). There was no difference in plasma insulin levels in rats fed caloric restricted high fat diet compared to normal diet fed rats (3.6 ± 0.5 vs. 4.3 ± 0.1 µg/L, respectively). Together these data suggest that the impairment in renal endothelial function and the induction of insulin resistance is due to obesity and it is not due to the high fat diet content.
Plasma Lipid Levels
Table (1) shows plasma lipid profile in rats fed normal or high fat diet with or without ANG infusion. There was a 50 % increase in plasma cholesterol, a 30% increase in plasma triglyceride and a 35% increase in plasma LDL levels in rats fed high fat diet compared to rats fed normal diet. The same trend was also shown in high fat/ANG compared to normal/ANG (Table 1). However, plasma cholesterol and triglyceride levels were lower in high fat/ANG rats compared to high fat group and again this could be due to the decrease in food intake as high fat/ANG rats consumed 20% less daily food than high fat diet fed rats.
Oxidative Stress and Inflammatory Markers
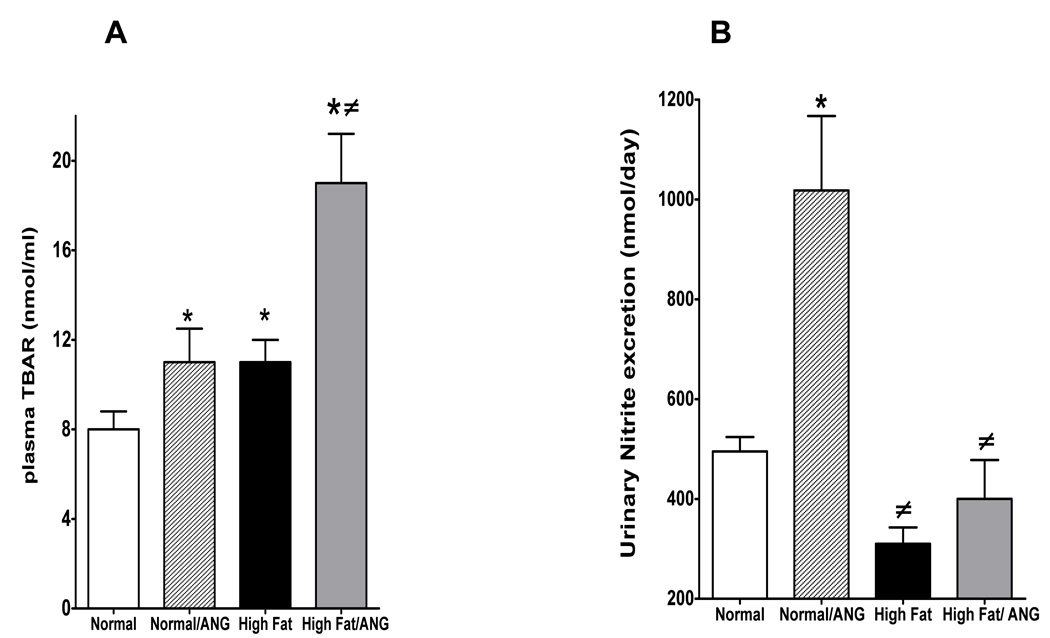
Plasma TBARs were assessed as a measure of oxidative stress, in rats fed normal or high fat diet. High fat diet increased plasma TBARs compared to normal diet (11±1 vs. 8±0.8 nmol/ml) and the increase in plasma TBARs were further exacerbated in high fat/ANG treated rats (19±2.2 nmol/ml) (Figure 4A). ANG infusion increased urinary nitrite excretion in rats fed a normal diet; however, urinary nitrite excretion was decreased in high fat diet fed rats and in high fat/ANG compared to normal and normal/ANG treatment (Figure 4B). These data suggest that high fat diet treatment increases oxidative stress and subsequently decreases NO bioavailability in obese rats.
Figure 4.
A: Plasma TBARs and B: urinary nitrite excretion in rat fed a normal or high fat diet for 10 weeks with or without ANG infusion in the last 4 weeks of the treatment. Values are mean ±SEM (n=6–8). *P < 0.05 high fat vs. normal diet and #P < 0.05 high fat with or without ANG vs. normal/ANG.
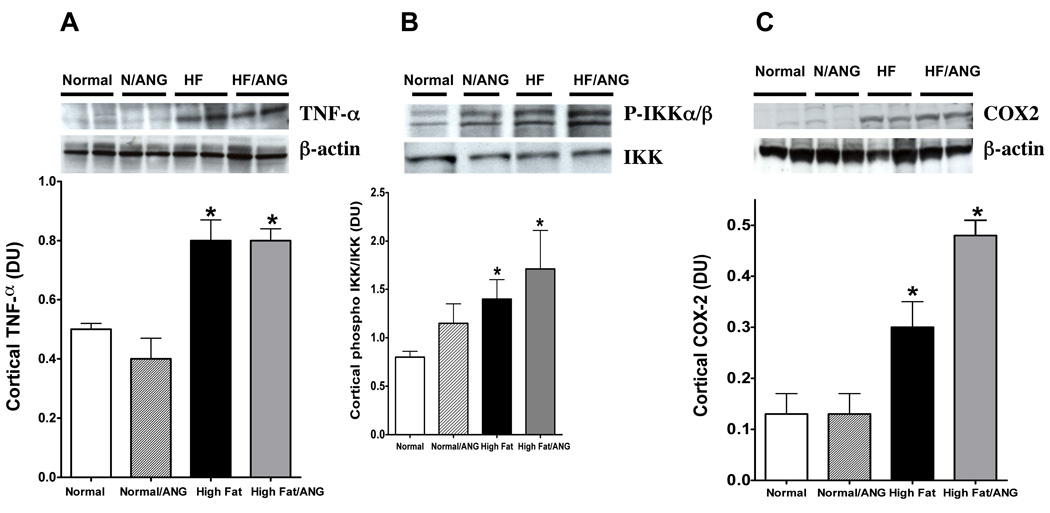
We next assessed protein expression levels of some inflammatory cytokines in the kidney cortex of rats fed normal or high fat diet with and without ANG infusion. Renal cortical TNF-α expression significantly increased in high fat diet and high fat/ANG groups compared to normal diet fed rats (Figure 5A). Renal cortical phospho-IKKα/β was significantly elevated in high fat diet fed rats compared to normal diet fed rats and was further increased in high fat/ANG (Figure 5B). We then verified the activation of NFκB by measuring renal cortical p65-NFκB. High fat diet alone did not significantly change renal cortical p65-NFκB compared to control (0.06±0.008 vs. 0.1±0.03 pg/mg, respectively; however, cortical p65-NFκB was significantly elevated in high fat/ANG compared to normal or normal/ANG rats (0.2±0.03 vs. 0.1±0.03 or 0.05±0.006 pg/mg, respectively). Likewise, cortical COX-2 expression significantly increased in rats fed high fat compared to control and this increase was further exacerbated in ANG/high fat compared to high fat alone.
Figure 5.
Cortical Protein expression of A: TNF-α relative to β-actin, B: Phospho-IKKα/β relative to IKK, and C: COX-2 expression relative to β-actin in normal, normal /ANG, high fat, high fat/ANG treated rats. Values are mean ±SEM (n=6–8). *P < 0.05 high fat with or without ANG vs. normal diet and #P < 0.05 high fat/ANG vs. high fat.
Discussion
The incidence of obesity dramatically increased in the United States over the past two decades [24]. Studies have demonstrated a close association between obesity, hypertension, and inflammation although the exact mechanisms remain to be elucidated [4]. In this study, we tested whether hypertension exacerbates vascular dysfunction, oxidative stress, and inflammation in rats fed high fat diet. Although ANG infusion did not potentiate the elevation in blood pressure and impairment in vascular function in high fat diet vs. normal diet fed rats, it exacerbated the increase in plasma TBARs in high fat diet fed rats. High fat diet increased renal TNF-α, phospho-IKK, and COX-2 expressions and COX-2 expression was further increased in ANG hypertensive rats fed high fat diet. These data suggest that ANG-induced hypertension exacerbates oxidative stress and inflammation without worsening vascular dysfunction in high fat diet-induced obesity.
Consistent with our previous findings [8], body weight gain was significantly higher in rats fed high fat diet than those fed normal diet. Rats fed a normal diet tended to lose weight during ANG infusion. This could be attributed to the decrease in food intake in rats fed normal diet during ANG infusion. Our data are in agreement with previously published data [25–27]. For example, Brink et al. reported that ANG infused hypertensive rats lost 18–26% of body weight after week one compared to sham control and pair-feeding experiments indicated that 74% of this loss was due to a reduction in food intake [26].
Although studies have demonstrated that long-term high fat diet increased blood pressure in rats [28–30], our study showed that there was only a mild increase in blood pressure in rats fed high fat diet and this increase was only significant after 9 weeks of high fat diet treatment. Because rats fed a high fat diet for 10 weeks did not develop hypertension, we postulated that ANG infusion would potentiate the elevation in blood pressure in high fat diet fed rats. Surprisingly, there was no difference in blood pressure between ANG hypertensive rats fed high fat diet vs. normal diet. These data are in agreement with our previous findings in normotensive Wistar Kyoto (WKY) and spontaneously hypertensive rats (SHR). High fat diet treatment for 10 weeks did not alter systolic blood pressure but increased monocyte chemoattractant protein-1 (MCP-1) excretion, a marker of renal inflammation in WKY and SHR; however, high fat only increased microalbuminuria and protein excretion, markers of renal injury, in SHR [8]. The increase in renal injury without changing blood pressure suggests that high fat diet impairs renal function via pressure-independent mechanisms. We also recently published that there was a difference in systolic blood pressure between obese (db/db) mice and lean (db/m) mice and obesity did not potentiate the elevation in blood pressure upon DOCA-salt treatment [31]. Collectively, these data suggest that high fat diet treatment for 10 weeks increases body weight gain without inducing hypertension or exacerbating ANG-induced hypertension.
Interestingly, we observed significant endothelial dysfunction after 10 weeks of high fat diet in both normotensive and ANG hypertensive rats as well as in ANG hypertensive rats fed normal diet. The endothelial independent relaxation to sodium nitroprusside was not different among groups suggesting that the impairment in afferent arteriolar response to acetylcholine was a result of endothelial and not smooth muscle dysfunction. These data are consistent with our previous findings that afferent arteriolar responses were impaired in SHR vs. WKY rats and high fat diet treatment for 10 weeks also induced impairment in afferent arteriolar responses to acetylcholine in both WKY and SHR without affecting smooth muscle function [8]. To test the possibility that the incidence of endothelial dysfunction is due to the diet content, we fed rats either normal or caloric restricted high fat diet for 10 weeks. Pair feeding rat caloric restricted high fat diet abolished the increase in body weight gain and the impairment in afferent arteriolar relaxation to acetylcholine. These data suggest that the incidence of endothelial dysfunction is due to obesity or hypertension and is not linked to the diet content and ANG-induced hypertension did not further increase the impairment in endothelial function in high fat fed rats.
Previous studies have shown that high fat diet or ANG infusion increased oxidative stress and the increase in superoxide levels could result in endothelial dysfunction via scavenging of NO and decreasing NO bioavailability [10, 28, 32–34]. However, superoxide-induced endothelial dysfunction may not be the only mechanism for decreased NO bioavailability. Chalupsky & Cai demonstrated that during oxidative stress, uncoupling of NO synthase (NOS) occurs through oxidation of tertahydrobiopterin (BH4), an important cofactor in NO synthesis, and hence NO synthase produces superoxide rather than NO [35]. We have recently demonstrated that obesity is the main trigger for increased oxidative stress and renal injury in db/db mice and the increase in oxidative stress is exaggerated with the coexistence of DOCA-salt hypertension in db/db obese mice [31]. In the current study, high fat diet increased plasma TBARs that was further exacerbated in high fat/ANG hypertensive rats. NO is metabolized via intermediates to nitrate and nitrite, which are excreted in the urine. Therefore, the excretion of NO2 and NO3 has been used as an index of NO generation [36]. In agreement with the previous findings of Dobrian et al. which demonstrated that long term high fat diet increased oxidative stress and decreased urinary and plasma nitrate/nitrite [28], increased oxidative stress upon high fat diet treatment was parallel by a decrease in nitrite excretion. Surprisingly, nitrite excretion significantly increased in ANG hypertensive rats fed normal diet although plasma TBARs was elevated compared to control. Deng et al. demonstrated that acute ANG infusion increased nitrite/nitrate excretion in rats and this could be attributed to increased shear stress. Nitrite/nitrate excretion was also higher in rats chronically infused with ANG although these changes was not significant suggesting that shear stress–induced increases in renal NO generation are lost during prolonged ANG infusions [36]. Niwanthi et al. have also shown that plasma nitrate/nitrite increased in ANG infused rat and this increase was further exacerbated in the presence of exogenous L-arginine [37]. Based on these data, we postulated that the increase in nitrite excretion after ANG infusion is due to compensatory upregulation of the NO system in ANG hypertensive rats fed a normal diet and this effect was abolished during high fat diet treatment. This could also explain why a high fat diet failed to exacerbate the blood pressure elevation and vascular dysfunction in ANG hypertensive rats even though oxidative stress was further elevated in high fat/ANG rats. In addition, ANG infusion could have maximized superoxide–induced blood pressure increase and hence ANG infusion failed to produce any further elevation in blood pressure when combined with high fat diet treatment. Collectively, these data suggest that increased oxidative stress could be a potential mechanism for high fat diet-induced hypertension and vascular dysfunction and ANG infusion only exacerbates oxidative stress without further impairment in vascular dysfunction in high fat diet-induced obesity.
Another potential mechanism for high fat diet-induced vascular dysfunction is the incidence of insulin resistance. Previous studies suggested that increased oxidative stress is an important trigger for insulin resistance [13]. For example, Park et al. have recently shown a positive correlation between oxidative stress biomarkers and insulin resistance in non diabetic young adults [38]. Matsuzawa-Nagata et al. have also demonstrated that increased oxidative stress preceded the onset of high fat diet-induced obesity and insulin resistance in high fat diet fed mice [39]. In the current study, plasma insulin levels were significantly higher in rats fed high fat diet compared to normal diet suggesting that long-term high fat diet treatment induced insulin resistance. The incidence of insulin resistance was confirmed using the euglycemic hyperinsulinemic clamp where a reduction in peripheral insulin sensitivity after 10 weeks of high fat diet treatment was observed. These data are consistent with our recent findings where 10 weeks of high fat diet treatment also increased plasma insulin levels in WKY and SHR [8]. Insulin resistance has been shown to contribute to the development of endothelial dysfunction [24, 40] as high insulin levels induce renal hemodynamic changes, glomerular hypertrophy, and mesangial cell proliferation [41, 42]. Together, these data suggest that high fat diet induced insulin resistance and ANG hypertension did not potentiate this effect.
Recent studies suggest a clear link between visceral obesity and the development of insulin resistance and inflammation via increasing inflammatory cytokines and adipokines such as leptin [4, 8, 43, 44]. Cytokines produced by adipose tissue in obesity, such as TNF-α and MCP-1, have been associated with the progression of vascular dysfunction and inflammation [45–47]. We have previously observed that endothelial dysfunction precedes renal injury in WKY and SHR fed high fat diet and hypertension combined with obesity induced powerful inflammatory responses and disruption of renal filtration barrier [8]. Plasma leptin levels also increased in WKY and SHR fed high fat diet for 10 weeks and this increase was significantly higher in WKY vs. SHR [8]. Consistent with our previous finding, plasma leptin levels were elevated in rats fed a high fat diet with or without ANG infusion compared to control in the current study. In contrast with the previously published data of Kim et al. [48] which showed that ANG increased leptin secretion from cultured adipose cell lines and human adipose tissue in-vitro, ANG infusion in vivo decreased plasma leptin levels in our study. Studies have demonstrated that high leptin levels reduced acetylcholine dilatory responses and this could be attributed to the ability of leptin to increase oxidative stress and decrease NO bioavailability [49, 50]. Renal TNF-α expression was also elevated in rats fed high fat diet, which may have contributed to the development of vascular dysfunction observed as a result of high fat diet treatment. TNF-α is a proinflammatory adipokine that is secreted by the adipose tissue. TNF-α plays a primary role in stimulating the production of leptin and other inflammatory cytokines [51–53]. Plasma TNF-α increased in obese patient [54] and expression of TNF-α increased with weight gain and decrease with weight loss [19]. Neutralizing TNF-α in obese mice improved insulin sensitivity suggesting that elevated TNF-α levels in obesity induce vascular insulin resistance [20, 21]. Collectively, our study suggests that the incidence of insulin resistance and the increase in leptin and TNF-α upon high fat diet treatment could be potential mechanisms for the induction of renal dysfunction and inflammation and ANG induced hypertension did not exacerbate the increase in leptin and TNF-α in obese rats.
The increase in oxidative stress and/or TNF-α also activates the intracellular transcription factor NFκB, which then translocates from cytoplasm to nucleus to activate downstream inflammatory cytokines perturbing the inflammatory cycle [4, 55, 56]. Normally, NFκB proteins are composed of two subunits that are usually present in the cytoplasm as inactive heterodimers bind to the inhibitory protein IκB. The main enzyme responsible for NFκB activation is the IKK kinase that phosphorylates IκB subunit where IκB then degrades [22, 23]. This will allow the activation of NFκB in cytoplasm and its translocation to the nucleus to regulate the transcription of many inflammatory genes such as COX-2 and MCP-1 [8, 23]. Thus, NFκB promotes synthesis and release of inflammatory cytokines that recruit monocyte and macrophage to vessel wall and this could be another potential mechanism for endothelial dysfunction. Our previous findings demonstrated that 10 weeks of high fat diet in combination with hypertension resulted in marked inflammatory responses manifested by increased MCP-1 excretion in WKY and SHR [8]. This combination also strongly stimulated the upregulation of inflammatory cytokine mRNA compared with high fat diet or hypertension alone. In the current study, renal cortical phospho-IKK increased in high fat diet fed compared to normal diet fed rats and was further elevated in ANG hypertensive rats fed high fat diet. Renal p65-NFκB activity was also elevated in ANG hypertensive rats fed high fat diet vs. normal diet rats. Renal COX-2 expression increased in rats fed high fat diet compared to those fed normal diet and ANG infusion exacerbated this increase. These data suggest that inflammatory cytokines could play a role in the incidence of endothelial dysfunction upon high fat diet treatment and the coincidence of hypertension with obesity does exacerbate the increase in inflammation but not the vascular dysfunction in obese rats.
In summary, ANG induced hypertension did not exacerbate insulin resistance or vascular dysfunction; however, it potentiated oxidative stress and inflammation in obese rats. Oxidative stress and inflammation are clearly associated with vascular dysfunction in obesity. The incidence of blood pressure elevation cannot be overstated, yet the growing percentage increase in hypertension worldwide and its impact on renal function especially with the coexistence with obesity will need further elucidation. Future study will address the use of anti-oxidant and anti-inflammatory drugs in slowing the progression of vascular dysfunction in obese rats.
Acknowledgments
Sources of Funding
This work was supported by Advancing a Healthier Wisconsin and National Institute of Health grant HL59699.
REFERENCES
- 1.Wylie-Rosett J. The diabetes epidemic: what can we do? J. Am. Diet. Assoc. 2009;109:1160–1162. doi: 10.1016/j.jada.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Jiamsripong P, Mookadam M, Alharthi MS, Khandheria BK, Mookadam F. The metabolic syndrome and cardiovascular disease: part 2. Prev. Cardiol. 2008;11:223–229. doi: 10.1111/j.1751-7141.2008.00002.x. [DOI] [PubMed] [Google Scholar]
- 3.Jiamsripong P, Mookadam M, Honda T, Khandheria BK, Mookadam F. The metabolic syndrome and cardiovascular disease: Part I. Prev. Cardiol. 2008;11:155–161. doi: 10.1111/j.1751-7141.2008.07809.x. [DOI] [PubMed] [Google Scholar]
- 4.Imig JD. Eicosanoids and renal damage in cardiometabolic syndrome. Expert. Opin. Drug. Metab. Toxicol. 2008;4:165–174. doi: 10.1517/17425255.4.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lea J, Cheek D, Thornley-Brown D, Appel L, Agodoa L, Contreras G, Gassman J, Lash J, Miller ER, 3rd, Randall O, Wang X, McClellan W. Metabolic syndrome, proteinuria, and the risk of progressive CKD in hypertensive African Americans. Am. J. Kidney. Dis. 2008;51:732–740. doi: 10.1053/j.ajkd.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Picchi A, Gao X, Belmadani S, Potter BJ, Focardi M, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ. Res. 2006;99:69–77. doi: 10.1161/01.RES.0000229685.37402.80. [DOI] [PubMed] [Google Scholar]
- 7.Nishimatsu H, Suzuki E, Takeda R, Takahashi M, Oba S, Kimura K, Nagano T, Hirata Y. Blockade of endogenous proinflammatory cytokines ameliorates endothelial dysfunction in obese Zucker rats. Hypertens. Res. 2008;31:737–743. doi: 10.1291/hypres.31.737. [DOI] [PubMed] [Google Scholar]
- 8.Knight SF, Quigley JE, Yuan J, Roy SS, Elmarakby A, Imig JD. Endothelial dysfunction and the development of renal injury in spontaneously hypertensive rats fed a high-fat diet. Hypertension. 2008;51:352–359. doi: 10.1161/HYPERTENSIONAHA.107.099499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cottone S, Lorito MC, Riccobene R, Nardi E, Mule G, Buscemi S, Geraci C, Guarneri M, Arsena R, Cerasola G. Oxidative stress, inflammation and cardiovascular disease in chronic renal failure. J. Nephrol. 2008;21:175–179. [PubMed] [Google Scholar]
- 10.Roberts CK, Barnard RJ, Sindhu RK, Jurczak M, Ehdaie A, Vaziri ND. Oxidative stress and dysregulation of NAD(P)H oxidase and antioxidant enzymes in diet-induced metabolic syndrome. Metabolism. 2006;55:928–934. doi: 10.1016/j.metabol.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Ebenezer PJ, Mariappan N, Elks CM, Haque M, Francis J. Diet-induced Renal Changes in Zucker Rats Are Ameliorated by the Superoxide Dismutase Mimetic TEMPOL. Obesity (Silver Spring) 2009 May 7; doi: 10.1038/oby.2009.137. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galili O, Versari D, Sattler KJ, Olson ML, Mannheim D, McConnell JP, Chade AR, Lerman LO, Lerman A. Early experimental obesity is associated with coronary endothelial dysfunction and oxidative stress. Am. J. Physiol. Heart. Circ. Physiol. 2007;292:H904–H911. doi: 10.1152/ajpheart.00628.2006. [DOI] [PubMed] [Google Scholar]
- 13.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 14.Chow FY, Nikolic-Paterson DJ, Ma FY, Ozols E, Rollins BJ, Tesch GH. Monocyte chemoattractant protein-1-induced tissue inflammation is critical for the development of renal injury but not type 2 diabetes in obese db/db mice. Diabetologia. 2007;50:471–480. doi: 10.1007/s00125-006-0497-8. [DOI] [PubMed] [Google Scholar]
- 15.Kim CH, Vaziri ND, Rodriguez-Iturbe B. Integrin expression and H2O2 production in circulating and splenic leukocytes of obese rats. Obesity (Silver Spring) 2007;15:2209–2216. doi: 10.1038/oby.2007.262. [DOI] [PubMed] [Google Scholar]
- 16.Kalantar-Zadeh K, Balakrishnan VS. The kidney disease wasting: inflammation, oxidative stress, and diet-gene interaction. Hemodial. Int. 2006;10:315–325. doi: 10.1111/j.1542-4758.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- 17.Mooradian AD, Albert SG, Haas MJ. Low serum high-density lipoprotein cholesterol in obese subjects with normal serum triglycerides: the role of insulin resistance and inflammatory cytokines. Diabetes. Obes. Metab. 2007;9:441–443. doi: 10.1111/j.1463-1326.2006.00636.x. [DOI] [PubMed] [Google Scholar]
- 18.Reinehr T, Stoffel-Wagner B, Roth CL, Andler W. High-sensitive C-reactive protein, tumor necrosis factor alpha, and cardiovascular risk factors before and after weight loss in obese children. Metabolism. 2005;54:1155–1161. doi: 10.1016/j.metabol.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 19.Keith ME, Norwich KH, Jeejeebhoy KN. Nutrition support affects the distribution and organ uptake of cachectin/tumor necrosis factor in rats. J. Parenter. Enteral. Nutr. 1995;19:341–350. doi: 10.1177/0148607195019005341. [DOI] [PubMed] [Google Scholar]
- 20.Kern PA. Potential role of TNFalpha and lipoprotein lipase as candidate genes for obesity. J. Nutr. 1997;127:1917S–1922S. doi: 10.1093/jn/127.9.1917S. [DOI] [PubMed] [Google Scholar]
- 21.Hube F, Hauner H. The role of TNF-alpha in human adipose tissue: prevention of weight gain at the expense of insulin resistance? Horm. Metab. Res. 1999;31:626–631. doi: 10.1055/s-2007-978810. [DOI] [PubMed] [Google Scholar]
- 22.Thompson WL, Van Eldik LJ. Inflammatory cytokines stimulate the chemokines CCL2/MCP-1 and CCL7/MCP-7 through NFkappaB and MAPK dependent pathways in rat astrocytes. Brain. Res. 2009;1287:47–57. doi: 10.1016/j.brainres.2009.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim YS, Ahn Y, Hong MH, Kim KH, Park HW, Hong YJ, Kim JH, Kim W, Jeong MH, Cho JG, Park JC, Kang JC. Rosuvastatin suppresses the inflammatory responses through inhibition of c-Jun N-terminal kinase and Nuclear Factor-kappaB in endothelial cells. J. Cardiovasc. Pharmacol. 2007;49:376–383. doi: 10.1097/FJC.0b013e31804a5e34. [DOI] [PubMed] [Google Scholar]
- 24.Belin de Chantemele EJ, Irfan Ali M, Mintz J, Stepp DW. Obesity induced-insulin resistance causes endothelial dysfunction without reducing the vascular response to hindlimb ischemia. Basic. Res. Cardiol. 2009 Jun 23; doi: 10.1007/s00395-009-0042-2. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004;44:223–229. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brink M, Wellen J, Delafontaine P. Angiotensin II causes weight loss and decreases circulating insulin-like growth factor I in rats through a pressor-independent mechanism. J. Clin. Invest. 1996;97:2509–2516. doi: 10.1172/JCI118698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension. 2001;37:1329–1335. doi: 10.1161/01.hyp.37.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dobrian AD, Davies MJ, Schriver SD, Lauterio TJ, Prewitt RL. Oxidative stress in a rat model of obesity-induced hypertension. Hypertension. 2001;37:554–560. doi: 10.1161/01.hyp.37.2.554. [DOI] [PubMed] [Google Scholar]
- 29.Wang MH, Smith A, Zhou Y, Chang HH, Lin S, Zhao X, Imig JD, Dorrance AM. Downregulation of renal CYP-derived eicosanoid synthesis in rats with diet-induced hypertension. Hypertension. 2003;42:594–599. doi: 10.1161/01.HYP.0000090123.55365.BA. [DOI] [PubMed] [Google Scholar]
- 30.Deutsch C, Portik-Dobos V, Smith AD, Ergul A, Dorrance AM. Diet-induced obesity causes cerebral vessel remodeling and increases the damage caused by ischemic stroke. Microvasc. Res. 2009;78:100–106. doi: 10.1016/j.mvr.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quigley JE, Elmarakby AA, Knight SF, Manhiani MM, Stepp DW, Olearzcyk JJ, Imig JD. Obesity-Induced Renal Oxidative Stress Contributes to Renal Injury in Salt-Sensitive Hypertension. Clin. Exp. Pharmacol. Physiol. 2009;36:724–728. doi: 10.1111/j.1440-1681.2009.05139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou MS, Schulman IH, Raij L. Role of angiotensin II and oxidative stress in vascular insulin resistance linked to hypertension. Am. J. Physiol. Heart. Circ. Physiol. 2009;296:H833–H839. doi: 10.1152/ajpheart.01096.2008. [DOI] [PubMed] [Google Scholar]
- 33.Smith AD, Brands MW, Wang MH, Dorrance AM. Obesity-induced hypertension develops in young rats independently of the renin-angiotensin-aldosterone system. Exp. Biol. Med. (Maywood) 2006;231:282–287. doi: 10.1177/153537020623100307. [DOI] [PubMed] [Google Scholar]
- 34.Dobrian AD, Schriver SD, Prewitt RL. Role of angiotensin II and free radicals in blood pressure regulation in a rat model of renal hypertension. Hypertension. 2001;38:361–366. doi: 10.1161/01.hyp.38.3.361. [DOI] [PubMed] [Google Scholar]
- 35.Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. 2005;102:9056–9061. doi: 10.1073/pnas.0409594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng X, Welch WJ, Wilcox CS. Role of nitric oxide in short-term and prolonged effects of angiotensin II on renal hemodynamics. Hypertension. 1996;27:1173–1179. doi: 10.1161/01.hyp.27.5.1173. [DOI] [PubMed] [Google Scholar]
- 37.Rajapakse NW, De Miguel C, Das S, Mattson DL. Exogenous L-arginine ameliorates angiotensin II-induced hypertension and renal damage in rats. Hypertension. 2008;52:1084–1090. doi: 10.1161/HYPERTENSIONAHA.108.114298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park K, Steffes M, Lee DH, Himes JH, Jacobs DR., Jr Association of inflammation with worsening HOMA-insulin resistance. Diabetologia. 2009 Aug 13; doi: 10.1007/s00125-009-1486-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuzawa-Nagata N, Takamura T, Ando H, Nakamura S, Kurita S, Misu H, Ota T, Yokoyama M, Honda M, Miyamoto K, Kaneko S. Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metabolism. 2008;57:1071–1077. doi: 10.1016/j.metabol.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 40.Romanko OP, Ali MI, Mintz JD, Stepp DW. Insulin resistance impairs endothelial function but not adrenergic reactivity or vascular structure in fructose-fed rats. Microcirculation. 2009;16:414–423. doi: 10.1080/10739680902832795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hall JE, Brands MW, Dixon WN, Smith MJ., Jr Obesity-induced hypertension. Renal function and systemic hemodynamics. Hypertension. 1993;22:292–299. doi: 10.1161/01.hyp.22.3.292. [DOI] [PubMed] [Google Scholar]
- 42.Sarafidis PA, Ruilope LM. Insulin resistance, hyperinsulinemia, and renal injury: mechanisms and implications. Am. J. Nephrol. 2006;26:232–244. doi: 10.1159/000093632. [DOI] [PubMed] [Google Scholar]
- 43.Koh KK, Han SH, Quon MJ. Inflammatory markers and the metabolic syndrome: insights from therapeutic interventions. J. Am. Coll. Cardiol. 2005;46:1978–1985. doi: 10.1016/j.jacc.2005.06.082. [DOI] [PubMed] [Google Scholar]
- 44.Neels JG, Olefsky JM. Inflamed fat: what starts the fire? J. Clin. Invest. 2006;116:33–35. doi: 10.1172/JCI27280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chudek J, Adamczak M, Nieszporek T, Wiecek A. The adipose tissue as an endocrine organ--a nephrologists' perspective. Contrib. Nephrol. 2006;151:70–90. doi: 10.1159/000095320. [DOI] [PubMed] [Google Scholar]
- 46.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J. Clin. Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baron AD, Steinberg HO. Endothelial function, insulin sensitivity, and hypertension. Circulation. 1997;96:725–726. [PubMed] [Google Scholar]
- 48.Kim S, Whelan J, Claycombe K, Reath DB, Moustaid-Moussa N. Angiotensin II increases leptin secretion by 3T3-L1 and human adipocytes via a prostaglandin-independent mechanism. J. Nutr. 2002;132:1135–1140. doi: 10.1093/jn/132.6.1135. [DOI] [PubMed] [Google Scholar]
- 49.Beltowski J, Jamroz-Wisniewska A, Wojcicka G, Lowicka E, Wojtak A. Renal antioxidant enzymes and glutathione redox status in leptin-induced hypertension. Mol. Cell. Biochem. 2008;319:163–174. doi: 10.1007/s11010-008-9889-z. [DOI] [PubMed] [Google Scholar]
- 50.Wojcicka G, Jamroz-Wisniewska A, Widomska S, Ksiazek M, Beltowski J. Role of extracellular signal-regulated kinases (ERK) in leptin-induced hypertension. Life. Sci. 2008;82:402–412. doi: 10.1016/j.lfs.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 51.Schreyer SA, Chua SC, Jr, LeBoeuf RC. Obesity and diabetes in TNF-alpha receptor-deficient mice. J. Clin. Invest. 1998;102:402–411. doi: 10.1172/JCI2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schreyer SA, Wilson DL, LeBoeuf RC. C57BL/6 mice fed high fat diets as models for diabetes-accelerated atherosclerosis. Atherosclerosis. 1998;136:17–24. doi: 10.1016/s0021-9150(97)00165-2. [DOI] [PubMed] [Google Scholar]
- 53.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 54.Lechleitner M, Herold M, Dzien-Bischinger C, Hoppichler F, Dzien A. Tumour necrosis factor-alpha plasma levels in elderly patients with Type 2 diabetes mellitus-observations over 2 years. Diabet. Med. 2002;19:949–953. doi: 10.1046/j.1464-5491.2002.00846.x. [DOI] [PubMed] [Google Scholar]
- 55.Chandramohan G, Bai Y, Norris K, Rodriguez-Iturbe B, Vaziri ND. Effects of dietary salt on intrarenal angiotensin system, NAD(P)H oxidase, COX-2, MCP-1 and PAI-1 expressions and NF-kappaB activity in salt-sensitive and -resistant rat kidneys. Am. J. Nephrol. 2008;28:158–167. doi: 10.1159/000110021. [DOI] [PubMed] [Google Scholar]
- 56.Tian N, Moore RS, Braddy S, Rose RA, Gu JW, Hughson MD, Manning RD., Jr Interactions between oxidative stress and inflammation in salt-sensitive hypertension. Am. J. Physiol. Heart. Circ. Physiol. 2007;293:H3388–H3395. doi: 10.1152/ajpheart.00981.2007. [DOI] [PubMed] [Google Scholar]