Abstract
Immunotherapy holds great promise for Alzheimer's disease (AD) and other conformational disorders but certain adverse reactions need to be overcome. Prior to the side effects in the first Elan/Wyeth AD vaccine trial, we proposed using amyloid-β (Aβ) derivatives as a safer approach. The route of administration may also affect vaccine safety. To assess the feasibility of oral immunization that promotes mucosal immunity, Tg2576 AD model mice were treated prophylactically three times over 6 weeks starting at 3−5 months of age with a Salmonella vaccine expressing K6Aβ1−30. At 22−24 months of age, cortical Aβ plaque burden and total Aβ40/42 levels were reduced by 48−75% in the immunized mice compared to controls, which received unmodified Salmonella. Plaque clearance was not associated with increased microglial activation which may be explained by the long treatment period. Furthermore, cerebral microhemorrhages were not increased in the treated mice in contrast to several passive Aβ antibody studies. These results further support our findings with this immunogen delivered subcutaneously, and demonstrate its efficacy when given orally which may provide added benefits for human use.
Keywords: Amyloid-β, Transgenic mice, Salmonella, Vaccine, Oral, Immunization, Microhemorrhages
INTRODUCTION
Immune modulation to clear Aβ is a promising therapy for AD, and is based primarily on studies showing that immunization with aggregated Aβ1−42 reduces Aβ plaque burden and associated pathology in mouse brains [58]. Prior and subsequent studies indicated that this effect was likely to be antibody-mediated [6,7,15,16,38,64,66,67], and resulted in cognitive improvements [17,30,36,45]. Following and during these promising mouse studies, clinical trials were initiated using aggregated Aβ1−42 along with QS-21 adjuvant that promotes cytotoxic T-cell responses [31]. These trials were subsequently halted because of meningoencephalitis observed in a small subset of patients [50,57]. The clinical symptoms, when they occurred, and subsequent histopathological analysis in two patients indicated that the encephalitis was T-cell mediated directly related to the vaccination, caused by the antigen and/or adjuvant and probably not related to the Aβ antibodies per se [18,49,50]. However, positive preliminary findings have emerged from this trial, and refinement of this approach is currently underway. Four autopsies from the trial have shown plaque clearance but vascular amyloid and tau pathology remained [18,43,48,49]. Two of the four autopsy subjects did not develop encephalitis, indicating that reduced amyloid burden is not a consequence of brain inflammation. Regarding cognitive improvements, in the Zurich cohort there was a positive correlation between the presence of antibodies that recognized Aβ in tissue sections [26] and a less pronounced cognitive decline [27]. Also, a report from the Phase I study of AN-1792 showed less decline in a cognitive test compared to untreated age-matched controls [8]. In the larger Phase IIa trial, z-score analyses across the neuropsychological test battery indicated that the antibody responders differed from the placebo subjects [22]. However, a recent report on additional subjects from the Phase I trial indicated that substantial or complete removal of plaques did not prevent progression to a severe end-stage dementia at the time of death [28]. Overall, these preliminary findings on cognitive effects and Aβ clearance in the human trials suggest that targeting Aβ for clearance may have limited effect once cognitive impairments are evident. However, prophylactic treatment to clear Aβ prior to irreversible neuronal damage is likely to be more efficacious. Furthermore, as the other major hallmark of AD, pathological tau protein, correlates better with the degree of dementia than Aβ deposition [3,74], targeting it may provide more benefits at later stages of the disease [4,63].
Prior to the side effects in the AN-1792 trial, we raised concerns about administering full-length Aβ1−42 in humans, and we advocated the use of adjuvants that favor a Th2 response promoting antibody production instead of a Th1 response which mediates a cytotoxic T-cell response [66]. The primary objective in designing our Aβ derivatives was to maintain antibody epitopes while reducing their β-sheet content compared to Aβ to eliminate direct toxicity and amyloid seeding potential. These modifications also altered or removed potential T-cell epitopes. Interestingly, recent findings in the prion field indicate also that immune responses to β-helical structures appear to involve more the Th2 pathway whereas β-sheet conformation favors Th1 activation [32]. Our initial report was on K6Aβ1−30 which contains 6 lysines to increase immunogenicity and reduce β-sheet propensity. This peptide elicited a similar antibody response as Aβ1−42 in mice which resulted in a comparable therapeutic efficacy [66]. Our subsequent findings with this and other Aβ derivatives that elicit a variable antibody response indicate that a robust immune response towards Aβ is not needed to improve cognition [5,59,64]. We have now observed that these Aβ homologs are safe in lemur primates (Microcebus murinus), and we are currently evaluating the efficacy of K6Aβ1−30 in older lemurs in preparation for future human trials [70,71].
The immune response also depends on the route of administration which may affect vaccine safety. Salmonella-based vaccines contain various strains of attenuated Salmonella that are well tolerated, can be administered orally to promote mucosal immunity [41,44,51], and are being assessed in humans [20,40,47,68]. We have previously reported on the effectiveness of a Salmonella-PrP-based vaccine to prevent prion infection [23,24]. Here we report that oral administration of a different strain of Salmonella vaccine, that expresses 4 copies of the non-fibrillogenic Aβ derivative K6Aβ1−30, prevented cognitive decline and reduced brain amyloid burden in Tg2576 AD model mice. These results confirm our previous findings with this and related immunogens delivered subcutaneously [5,64,66], and demonstrate its efficacy when given orally prophylactically which may be beneficial for human use.
MATERIALS AND METHODS
Peptide
K6Aβ1−30-NH2 and Aβ1−40 were synthesized at the Keck Foundation at Yale University, as described previously [66]. This Aβ derivative maintains the two major immunogenic sites of the Aβ peptide, which are residues 1−11 and 22−28 of Aβ1−42 [29]. The peptide is amidated on the C-terminus to maintain the immunogenicity of that epitope. The 6 lysyl residues on the N-terminus were added to enhance immunogenicity and further reduce β-sheet content. Both peptides were used for coating ELISA plates to determine antibody response towards the vaccine.
Salmonella Vaccine Construct
The construction of the SL3261 strain of the Salmonella typhimurium vaccine was performed in a similar manner as has been previously described for Salmonella typhimurium aroC LVR01 by Chabalgoity et al. [12,13]. Plasmid pTECH2 was used as has been described [34]. It allows the expression of multiple tandem copies of a foreign antigen as a C-terminal fusion to the non-toxic fragment C of tetanus toxin (TetC). The construction of the K6Aβ1−30 expression vector was as follows. The full length coding sequence of K6Aβ1−30 was custom synthesized (Sigma Genosys, Woodlands, TX). Forward and reverse primers were tailored with BamHI and SpeI respectively to allow directional cloning into pTECH2. The construction of TetC fusions comprising two tandem copies of K6Aβ1−30 was done as previously described for a different immunogen [11]. Briefly, aliquots of the recombinant fusion vector were simultaneously digested with both XbaI and PstI, or with SpeI and PstI. Each digest generated two restriction fragments from which the fragment containing the Aβ peptide sequence was purified. The overhangs generated by XbaI and SpeI are compatible, but the recognition sites for both of these enzymes are destroyed upon ligation. Thus, the XbaI and SpeI sites flanking the Aβ peptide sequence remain unique and the procedure can be serially repeated, doubling the copy number of the peptide with each cycle. The plasmid constructs encoding four copies of K6Aβ1−30 was introduced into the SL3261 strain of Salmonella typhimurium by electroporation. Increasing the copy number increases the immune response to the expressed protein [11,33]. The expression of K6Aβ1−30 by the Salmonella strain was assessed by SDS-PAGE and Western blotting using anti-Aβ monoclonal 6E10 (courtesy of Richard Ksacsak, IBRDD, Staten Island) and standard procedures.
Mice and Vaccine Administration
Animal experimentation was performed in accord with institutional guidelines under an IACUC approved protocol. Tg2576 AD model mice were vaccinated by oral gavage at the age of 3−5 months with SL3261 Salmonella vaccine strain that contained the pTECH plasmid that expressed 4 copies of K6Aβ1−30 (11 females and 10 males). Control mice received the unmodified Salmonella strain (11 females and 10 males). Age-matched wild-type mice served as additional controls (2 females and 12 males). A second and third inoculation was administered 2 and 6 weeks after the first vaccination. During the course of the experiment, 12 treated Tg and 15 control Tg died and their brains could not be collected for analysis. In contrast, all the wild-type mice survived until the end of the study. At 22−24 months of age, the animals were perfused and their brains removed for analysis (Tg controls: 6 females; Tg vaccinated: 8 females and 1 male). Additional controls were wild-type littermates (n=14, controls: 4 males; immunized: 8 males and 2 females).
Antibody Response
The mice were bled prior to vaccination (T0), one week after the third inoculation (T1) and at the end of the study (Tfinal). IgG, IgM and IgA antibody levels were determined in plasma at 1:200 dilution by using ELISA as we have described previously [66], in which Aβ1−40 or its derivative K6Aβ1−30 were coated overnight at 4°C onto microtiter wells (0.5 μg/100 μl/well in TBS with 0.1% Tween-20 (TBS-T); Immulon 2HB, Thermo Electron Corp., Milford, MA). Additionally, IgG and IgA antibody response against Salmonella typhimurium lipopolysaccharides (LPS) was determined in plasma at 1:50 dilution (in 0.1% BSA in PBS-T) as we have described previously [23], in which plates were coated with S. typhimurium LPS (Sigma Aldrich, St. Louis, MO) in Reggiardo's buffer with 0.1% deoxycholate (0.5 μg/50 μl/well overnight at 37°C in a moist chamber). The antibodies were detected by a goat anti-mouse IgG (Amersham biosciences, Piscataway, NJ), goat anti-mouse IgM (u-chain specific, Sigma-Aldrich), or goat anti-mouse IgA (α-chain specific, Sigma Aldrich) all linked to a horseradish peroxidase, and tetramethyl benzidine (TMB; Pierce, Rockford, IL) was the substrate. Data is presented for all the transgenic mice that survived until the end of the study (5 controls and 9 vaccinated). The mice that died during the study had a similar immune response (data not shown) as those that lived.
Western blot
For assessment of constructs, aliquots of Salmonella containing the constructs – pTECH-(K6Aβ1−30) ×2 or ×4 were loaded onto gel, electrophoresed and electroblotted onto a nitrocellulose membrane. The membrane was then blocked with 5% nonfat dried milk in 50 mM phosphate/150 mM NaCl/0.1% Tween 20 pH 7.2 (PBS-T), and then incubated overnight at 4°C with 1:1500 6E10 (mouse monoclonal IgG anti-Aβ) in PBS-T. Subsequently, the membranes were incubated for 2 h with 1:3000 horseradish-peroxidase (HRP) conjugated sheep anti-mouse antibody (Amersham) or 1:2000 HRP-goat anti-rabbit antibody (Amersham), and developed (ECL, Amersham).
Histology
The mice were anesthetized with ketamine/xylazine (250 mg/50 mg per kg body weight, i.p.), perfused transaortically with phosphate buffered saline, and the brains processed as described previously [62,65]. The brain was immersion-fixed in 2% periodate-lysine-paraformaldehyde. Serial coronal sections (40 μm) were cut, and every fifth section (30−40 sections in total) was stained with 6E10, a monoclonal antibody that recognizes Aβ and stains both pre-amyloid and Aβ plaques [35]. Staining was performed as described previously [56,62,64]. Every tenth section (15−20 sections in total) was stained with tomato lectin (Vector Laboratories, Burlingame, CA) or with Perl's iron stain. Tomato lectin binds to poly-N-acetyl lactosamine residues and in neural tissue it has specific affinity for microglial cells [1]. Those cells are associated with Aβ deposits. Perl's iron stain allows detection of cerebral bleeding.
Immunohistochemistry
Immunostaining was performed as described previously [62,66]. Briefly, sections were incubated in 6E10 at a 1:1000 dilution for 3 h. A mouse-on-mouse immunodetection kit (Vector Laboratories, Burlingame, CA) was used, with the biotinylated anti-mouse IgG secondary antibody reacted for 1 h at a 1:2000 dilution. The avidin-peroxidase complex was subsequently reacted for 30 min at the same dilution. The same procedure without primary antibody was used to assess the presence of IgG in Aβ plaques. Tomato lectin staining was performed as described [66] with a 2 h incubation (biotinylated tomato lectin: 10 μg/ml PBS; Vector) followed by 1 h reaction in avidin-horseradish peroxidase (Vector). The sections were reacted in 3,3-diaminobenzidine tetrahydrochloride (Sigma) with nickel ammonium sulfate (Ni; Mallinckrodt, Paris, KY) intensification.
Iron Staining
Perl's iron stain was performed to detect cerebral bleeding by placing defatted and hydrated sections in a solution containing 5% potassium ferrocyanide and 10% hydrochloric acid for 30 min as we have described previously [5]. The slides were then rinsed in distilled water, and the sections were dehydrated, cleared in Hemo-De, and coverslipped. This same method was used in three previous reports that showed that passive immunization against Aβ increased the frequency of microhemorrhages in AD model mice [53,54,73]. Diamino benzidine intensification of the iron staining, which is useful for detecting low levels of iron in Aβ plaques, did not appear to improve sensitivity for detecting the microhemorrhages and was, therefore, not employed. To verify that our methodology would allow us to detect increases in hemorrhages, positive controls were used. These mice had brain hemorrhages that were caused by intracerebral cannula placement. These hemorrhages were more extensive than the microhemorrhages observed in the Tg mice.
Image analysis
Immunohistochemistry of tissue sections was quantified with a Bioquant image analysis system (BIOQUANT Image Analysis Corporation, Nashville, TN), and unbiased sampling was used [72], as we have described [5,64,66]. All procedures were performed by an individual blinded to the experimental condition of the study. The cortical area analyzed was dorsomedial from the cingulate cortex and extended ventrolaterally to the rhinal fissure within the right hemisphere. The area of the grid was 800 μm2 × 800 μm2, and Aβ deposit load was measured in 20 cortical frames per mouse (640 × 480 μm2 each) chosen randomly. The Aβ burden is defined as the percentage of area in the measurement field occupied by reaction product. For determination of plaque sizes, the numbers in each category (small: 0.1−50 μm2; medium: 50.01−1000 μm2; large >1000 μm2) are totals from the 20 frames analyzed.
Rating of microgliosis
The assessment of the tomato lectin (microglia) stained sections was based on a semi-quantitative analysis of the extent of microgliosis associated with the Aβ deposits (0, a few resting microglia; 1+, a few ramified and/or phagocytic microglia; 2+, moderate number of ramified/phagocytic microglia; 3+, numerous ramified/phagocytic microglia; see [5] for representative images of this rating scale).
Aβ Levels
Extraction of Aβ from brain homogenate was performed as we have described previously in detail [5]. The ELISA procedure was performed as described by the ELISA kit manufacturer (Invitrogen; formerly Biosource International).
Statistical Analysis
The data was analyzed by Graph Pad Prism 4.03 (San Diego, CA). Aβ deposit burden and Aβ levels were analyzed by Student's t-test or Mann-Whitney, its nonparametric equivalent. Analysis of brain microhemorrhages was performed by Kruskal Wallis non-parametric test (as the data failed Bartlett's test for equal variances). The tests were one-tailed except the analysis of microhemorrhages that was two-tailed as it could be expected to be increased (treated Tg) or decreased (wild-type). Correlation was determined by calculating the Pearson r correlation coefficient.
RESULTS
Prior to vaccination, expression of 4 copies of K6Aβ1−30 in SL3261 attenuated Salmonella typhimurium was confirmed on Western blots with 6E10 anti-Aβ antibody (data not shown).
As commonly observed in this Tg2576 strain, numerous animals died over the course of the study as indicated in the Method section in which group assignments are detailed. Of the transgenic (Tg) animals, 3 treated females and 9 treated males as well as 6 control females and 9 control males died during the course of the study, and their brains could not be analyzed. In contrast, all the 14 wild-type mice survived the study, 10 of which received the control Salmonella. Importantly, the immunization or the attenuated Salmonella strain per se were not associated with death as similar numbers of treated and control Tg mice perished during the study, and all the wild-type mice survived the control inoculation.
Antibody Response
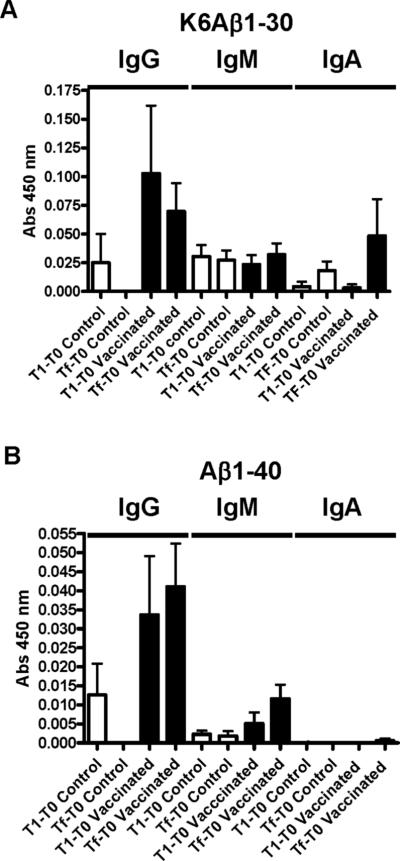
Low plasma levels of antibodies that recognized K6Aβ1−30 or Aβ1−40 were generated in response to the vaccine (Figure 1). As expected, IgG levels were higher than IgM and IgA levels and those antibodies preferentially recognized the immunogen K6Aβ1−30 but cross-reacted with Aβ1−40 to some extent. As detailed in Methods, 4 copies of K6Aβ1−30 were expressed in the Salmonella as a C-terminal fusion to the non-toxic fragment C of tetanus toxin (TetC). Random sampling indicated that the mouse immune system was adequately exposed to the vaccine construct as high levels of IgA antibodies against Salmonella typhimurium lipopolysaccharides were observed in plasma [Abs. at 450 nm: 1.12 ± 0.10 (1:50 dilution, arbitrary absorbance value: average ± SEM)]. Random sampling from wild-type mice gave similar results with respect to Aβ and LPS (data not shown).
Figure 1. Weak antibody response is generated towards K6Aβ1−30 expressed in the Salmonella.
As expected, IgG levels were higher than IgM and IgA levels and those antibodies preferentially recognized the immunogen K6Aβ1−30 (A) but cross-reacted with Aβ1−40 to some extent except for IgA (B). Tg controls: n=5; Tg vaccinated: n=9.
Histology and Aβ Levels
Aβ Plaque Burden
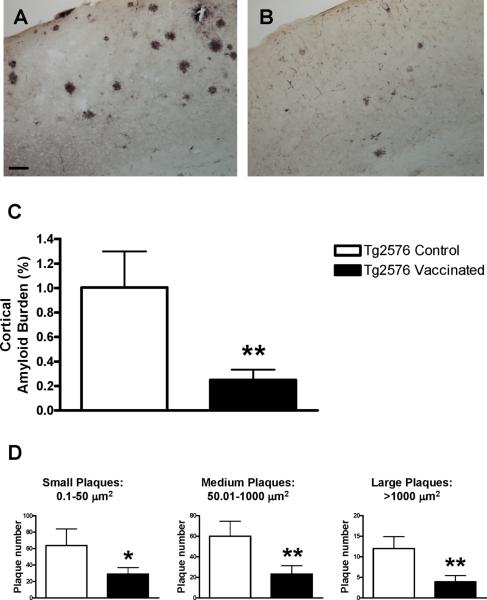
Quantitative analysis of cortical Aβ plaque burden at 22−24 months of age, as assessed by the 6E10 antibody, revealed a 75% reduction in the immunized Tg mice compared to Tg controls (Figure 2A-C; p<0.01). Plaques of different sizes were reduced to a similar degree in the vaccinated mice (Figure 2D; 0.1−50 μm2: 54% reduction, p<0.05; 50.01−1000 μm2: 61% reduction, p<0.01; >1000 μm2, p<0.01, 68% reduction). Amount of vascular Aβ deposits appeared to be comparable between the groups.
Figure 2. Prophylactic oral vaccination against Aβ leads to diminished Aβ plaque deposition.
(A, B) Representative coronal sections stained with Aβ antibody 6E10 through the cortex of a control Tg2576 mouse (A) compared to a vaccinated mouse (B). More amyloid deposits are observed in the control mouse. Bar in A is 100 μm.
(C) Quantitative analysis of the cortical amyloid burden revealed a 75% reduction in the immunized Tg mice (n=9) compared to Tg controls (n=6; p<0.01).
(D) Similar reduction was observed in plaques of different sizes in the treated mice (54% - (small), 61% - (medium) and 68% reduction (large)).
* p<0.05; ** p<0.01
Aβ Levels
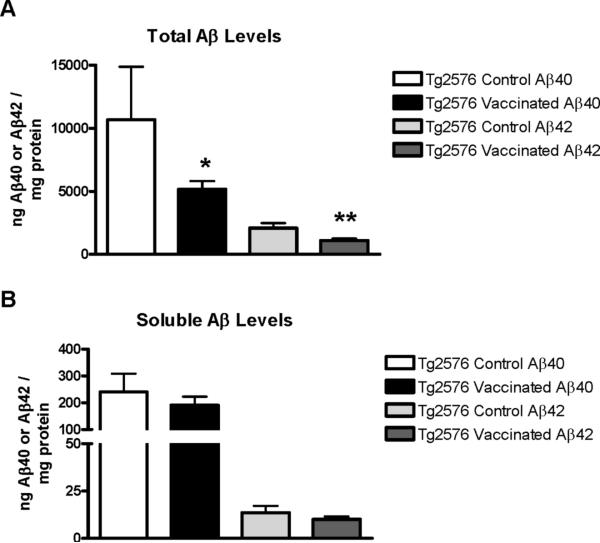
Similar treatment effect was observed in total Aβ levels (Figure 3; Aβ40, 52% reduction, p=0.03; Aβ42, 48% reduction, p<0.01), but soluble Aβ levels were not significantly altered. Aβ deposit burden and Aβ levels correlated well (total Aβ40, p<0.01; total Aβ42, p=0.01; soluble Aβ40, p=0.07; soluble Aβ42, p<0.01). Furthermore, total and soluble Aβ42 levels correlated very well (p<0.0001), whereas total and soluble Aβ40 levels did not correlate significantly.
Figure 3. Therapy-induced reduction in total Aβ levels.
(A) Quantitative analysis of total Aβ levels revealed a 52% reduction in total Aβ40 (p=0.05), and a 48% reduction in total Aβ42 (p<0.01) in the immunized Tg mice (n=9) compared to Tg controls (n=5). One Tg control mouse brain could not be used for biochemistry. That mouse died a few hours before scheduled euthanasia and its brain was fixed in the skull to preserve the integrity of the tissue.
(B) Levels of soluble Aβ were not significantly reduced in the immunized mice.
Microglial Activation
Semiquantitative analysis (rating scale of 0−3+) of microgliosis associated with the Aβ deposits did not reveal any significant changes between the treated [2.7± 0.2 (average ± SEM)] and control groups [2.8±0.1]. The plaques were strongly infiltrated by tomatolectin-positive microglia as we routinely observe in this model (data not shown, [5]). Also, IgG was not detected in the plaques in either group as assessed by staining with an anti-IgG antibody.
Microhemorrhages
The immunization-induced clearance of Aβ plaques was not associated with increase in brain microhemorrhages [iron positive profiles per section: Tg2576 controls = 0.48 ± 0.17 (average ± SEM); Tg2576 vaccinated = 0.39 ± 0.10; Wild-type = 0.09 ± 0.03]. As we have previously observed [5], the Tg2576 mice had more iron-positive profiles per brain section than wild-type animals (Kruskal Wallis, p=0.07), although in the current study this difference was not quite significant because of variance in the Tg mice.
DISCUSSION
Our present findings indicate that oral administration of K6Aβ1−30 expressed in attenuated Salmonella vaccine construct reduces Aβ plaque burden and Aβ levels in Tg2576 mice. Interestingly, the mice received only 3 inoculations of the vaccine over a 6 week period, starting at 3−5 months of age and the effectiveness of the vaccine was observed when the animals were at 2 years of age. As oral vaccines such as those based on attenuated Salmonella strains usually require only a few inoculations, it was feasible to assess if early prophylactic therapy may prevent or delay the accumulation of Aβ aggregates at an old age. This approach appears to have been successful and future studies will determine the efficacy of this type of immunotherapy when initiated at the cusp of or following the onset of pathology.
Importantly, the levels of antibodies in plasma were very low compared to other adjuvants and/or different routes of administration (subcutaneous) with this same K6Aβ1−30 immunogen [5,66]. This limited immune response appears to be sufficient to provide lasting therapeutic benefit which may provide added benefit for human use as stronger immune response is likely to be associated with more adverse reactions. This issue is of a particular concern when self-antigens like Aβ are being targeted. In our initial study with this immunogen, the first report on an Aβ derivative vaccine, K6Aβ1−30 in Freund's adjuvant reduced Aβ plaque burden in Tg2576 mice by 81−89% and soluble Aβ1−42 by 57% when administered from 11−18 months of age [66]. There we suggested that the weaker alum adjuvants should be employed in future clinical trials as those promote primarily antibody response (Th2) rather than a cytotoxic T-cell response (Th1). The latter type of immune activation is less appropriate when self antigens are being targeted. In our subsequent study employing K6Aβ1−30 in alum adjuvant, we observed 30−37% reduction in Aβ deposit burden and levels in Tg2576 mice treated from 11−19 months of age [5]. Furthermore, those immunized animals were cognitively superior to the Tg controls and performed at a similar level as their wild-type littermates. In the current study, 48−75% reduction in plaque burden and Aβ levels was obtained in the orally inoculated mice although antibody levels in plasma were very low. However, the prophylactic oral immunotherapy was initiated at a young age (3−5 months), which is several months before Aβ deposition occurs in the Tg2576 mice (9−12 months), the age at which therapy started in the previous two studies with this immunogen [5,66]. A major benefit of the Tg2576 model is its relatively slow rate of Aβ deposition that mirrors the presumed age of onset in AD, but it is also its disadvantage. As we detail here and often goes unreported, a large percentage of the animals, including controls, does not survive up to the 18 to 24 months of age that are needed for robust plaque deposition, and subsequent analysis.
The degree of microgliosis associated with the Aβ deposits was comparable in the treated and control Tg groups. This finding is as expected because of the long period between inoculation (at 3−5 months) and tissue analysis (at 22−24 months), considering as well the modest immune response against Aβ. Under these conditions, microglia-mediated removal of Aβ deposits should be very gradual which is preferable. In support of this observation, IgG was not detected in the plaques. The oral vaccination reduced the number of plaques of different sizes to a similar degree as we have observed previously with a related but different immunogen, K6Aβ1−30[E18E19] administered subcutaneously with Freund's adjuvant [64]. In line with gradual removal of plaques, qualitative assessment indicated that the amount of vascular Aβ deposits was comparable in the groups. This observation is in agreement with our previous findings which indicated that active immunization with K6Aβ1−30 in alum adjuvant reduced plaque burden but the amount of vascular Aβ deposits did not differ between treated and controls [5]. Acute phagocytic removal of Aβ-antibody complexes and subsequent increase in deposited vascular Aβ such as can be envisioned after passive immunization with high affinity monoclonal anti-Aβ antibodies is more likely to be associated with adverse reactions, such as microhemorrhages.
The extent of brain microhemorrhages was not increased in the immunized Tg mice compared to Tg controls, similar to our previous report with the same immunogen, K6Aβ1−30, not expressed in Salmonella but administered instead with an alum adjuvant [5]. As we have discussed previously [5], several studies on passive Aβ immunotherapy have reported increased microhemorrhages that can colocalize with vascular amyloid. This bleeding may be related to rapid removal of parenchymal Aβ via the vasculature and subsequent vascular Aβ deposition, as well as caused by a direct immune response to Aβ laden vessels. As our studies have been prophylactic in nature, and thereby prevented plaque deposition, those cannot be directly compared to the passive studies that have attempted removal of Aβ deposits in parenchyma and vasculature. The microhemorrhages in the old mice in the present study may have been caused by the continuous accumulation of Aβ in the vessel wall as soluble Aβ was being cleared from the parenchyma. It is also conceivable that vascular amyloid was not affected by the antibodies although plaque burden was reduced. Initial autopsy data from the clinical AN1792 trial suggested that Aβ deposited in the vasculature was not being cleared and the degree of microhemorrhages may have been increased [18,43,49]. More recently, a larger study focusing on this issue in 20 cases from the trial suggests that Aβ1−42 immunization caused a transient increase in cerebral congophilic angiopathy and microhemorrhages [9]. It is not surprising that gentler forms of immunization and prophylactic measures such as ours that should result in a more gradual clearance of Aβ would not increase the likelihood of this side effect.
We have previously demonstrated the effectiveness of oral Salmonella-based vaccines in prion disease [23,24], and this approach is the most effective active vaccination paradigm described to date to prevent the onset of that disease characterized by spongiform encephalopathy. In those studies, we used the LVR01 vaccine strain which is confined to the gut mucosa whereas SL3261 that we used in the current study spreads systemically. The latter strain is more appropriate to use in AD models as those do not involve entry of infectious agent through the intestines as in the prion models we employed. Salmonella vaccine strains have been extensively used in mice to deliver foreign antigens and elicit a mucosal immune response [40,41,44,51]. This approach has also been successfully used in humans [47,68].
As in our prion studies with the LVR01 strain expressing PrP, the SL3261 strain expressing K6Aβ1−30 elicited a very low antibody response towards the immunogen. However, in all the studies, high levels of antibodies were detected against the lipopolysaccharides in the Salmonella vaccine. Together, these findings indicate that the attenuated Salmonella indicating that the attenuated Salmonella gained entry through the gut mucosa and it and its expressed construct were presented to the immune system. We have observed in our previous studies with this and other Aβ derivatives that a modest antibody response towards Aβ is sufficient to prevent cognitive decline in AD mouse models [5,64]. Similar findings have been observed by other investigators. For example, mice have been vaccinated with a phage displaying amino acids 3 to 6 of the Aβ peptide which resulted in a weak antibody response, promoted clearance of amyloid deposits [19], and improved cognition [37]. Several additional promising studies on unaltered Aβ fragments have been described that have the objective to promote antibody production without cytotoxic T-cell response [2,14,19,21,25,37,39,42,60,61,77]. Phase I clinical trials, recently initiated on one of these approaches, was halted temporarily because of skin rashes observed in one individual [69]. It is unclear whether this reaction was related to the immunogen and/or adjuvant or neither. Other forms of immunotherapies targeting Aβ that may have less side effects, compared to full length Aβ, include infusions of anti-Aβ antibodies [7], or the use of proteolytic antibodies that can cleave Aβ [52]. In addition, IVIg that contains some anti-Aβ antibodies has shown promise in Phase I trials but its mechanism of action may at least in part be due to its known anti-inflammatory effects [55]. Currently ongoing immunization trials that target Aβ are three active Phase I trials and five passive trials ranging from Phase I to III [10,75].
The effectiveness of other types of oral vaccines expressing Aβ or its fragment has been reported. One of these vaccines consists of recombinant adeno-associated virus (AAV) expressing a fusion protein of cholera toxin B subunit and Aβ1−42 [76]. A single administration of this vaccine induced a modest increase in anti-Aβ IgG in serum of Tg APP/V717I mice that was reduced to low levels over 12 month period, and was associated with diminished cognitive decline, clearance of Aβ plaques and reduced astrocytosis. A similar approach employed AVV vector encoding cDNA for Aβ1−43 or Aβ1−21 which also resulted in elevated anti-Aβ antibodies that diminished over time but remained slightly increased over control values 25 weeks later [25]. This vaccine resulted in reduced Aβ burden, and slower progression of cognitive impairments [25,46]. Together, these and our findings indicate that a prophylactic short-term therapy at a young age can substantially diminish Aβ pathology at an old age in AD mouse models. The advantage of the Salmonella-based oral vaccination approach over the viral vectors is that the former may be safer as it does not involve an irreversible incorporation of foreign genetic material that may affect normal gene expression and has been shown to cause cancer in certain individuals in clinical trials. Overall, our findings support the feasibility of delivering orally a vaccine targeting Aβ that may prove useful as a prophylactic measure to prevent or slow the progression of AD.
Acknowledgements
Supported by NIH/NIA grants AG20197, AG20245, AG05891, AG28187, the Alzheimer's Association and Intellect Neurosciences.
References
- 1.Acarin L, Vela JM, Gonzalez B, Castellano B. Demonstration of poly-N-acetyl lactosamine residues in ameboid and ramified microglial cells in rat brain by tomato lectin binding. J. Histochem. Cytochem. 1994;42:1033–1041. doi: 10.1177/42.8.8027523. [DOI] [PubMed] [Google Scholar]
- 2.Agadjanyan MG, Ghochikyan A, Petrushina I, Vasilevko V, Movsesyan N, Mkrtichyan M, Saing T, Cribbs DH. Prototype Alzheimer's disease vaccine using the immunodominant B cell epitope from β-amyloid and promiscuous T cell epitope pan HLA DR-binding peptide. J Immunol. 2005;174:1580–1586. doi: 10.4049/jimmunol.174.3.1580. [DOI] [PubMed] [Google Scholar]
- 3.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 4.Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J. Neurosci. 2007;27:9115–9129. doi: 10.1523/JNEUROSCI.2361-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asuni AA, Boutajangout A, Scholtzova H, Knudsen E, Li YS, Quartermain D, Frangione B, Wisniewski T, Sigurdsson EM. Vaccination of Alzheimer's model mice with Aβ derivative in alum adjuvant reduces Aβ burden without microhemorrhages. Eur. J Neurosci. 2006;24:2530–2542. doi: 10.1111/j.1460-9568.2006.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bacskai BJ, Kajdasz ST, McLellan ME, Games D, Seubert P, Schenk D, Hyman BT. Non-Fc-mediated mechanisms are involved in clearance of amyloid-β in vivo by immunotherapy. J. Neurosci. 2002;22:7873–7878. doi: 10.1523/JNEUROSCI.22-18-07873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 8.Bayer AJ, Bullock R, Jones RW, Wilkinson D, Paterson KR, Jenkins L, Millais SB, Donoghue S. Evaluation of the safety and immunogenicity of synthetic Aβ42 (AN1792) in patients with AD. Neurology. 2005;64:94–101. doi: 10.1212/01.WNL.0000148604.77591.67. [DOI] [PubMed] [Google Scholar]
- 9.Boche D, Zotova E, Weller RO, Love S, Neal JW, Pickering RM, Wilkinson D, Holmes C, Nicoll JAR. Consequence of Aβ immunization on the vasculature of human Alzheimers disease brain. Brain. 2008;131:3299–3310. doi: 10.1093/brain/awn261. [DOI] [PubMed] [Google Scholar]
- 10.Brody DL, Holtzman DM. Active and passive immunotherapy for neurodegenerative diseases. Annu. Rev. Neurosci. 2008;31:175–193. doi: 10.1146/annurev.neuro.31.060407.125529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chabalgoity JA, Khan CM, Nash AA, Hormaeche CE. A Salmonella typhimurium htrA live vaccine expressing multiple copies of a peptide comprising amino acids 8−23 of herpes simplex virus glycoprotein D as a genetic fusion to tetanus toxin fragment C protects mice from herpes simplex virus infection. Mol. Microbiol. 1996;19:791–801. doi: 10.1046/j.1365-2958.1996.426965.x. [DOI] [PubMed] [Google Scholar]
- 12.Chabalgoity JA, Moreno M, Carol H, Dougan G, Hormaeche CE. Salmonella typhimurium as a basis for a live oral Echinococcus granulosus vaccine. Vaccine. 2000;19:460–469. doi: 10.1016/s0264-410x(00)00197-3. [DOI] [PubMed] [Google Scholar]
- 13.Chabalgoity JA, Villareal-Ramos B, Khan CM, Chatfield SN, de Hormaeche RD, Hormaeche CE. Influence of preimmunization with tetanus toxoid on immune responses to tetanus toxin fragment C-guest antigen fusions in a Salmonella vaccine carrier. Infect. Immun. 1995;63:2564–2569. doi: 10.1128/iai.63.7.2564-2569.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cribbs DH, Ghochikyan A, Vasilevko V, Tran M, Petrushina I, Sadzikava N, Babikyan D, Kesslak P, Kieber-Emmons T, Cotman CW, Agadjanyan MG. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with β-amyloid. Int. Immunol. 2003;15:505–514. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das P, Howard V, Loosbrock N, Dickson D, Murphy MP, Golde TE. Amyloid-β immunization effectively reduces amyloid deposition in FcRgamma−/− knock-out mice. J. Neurosci. 2003;23:8532–8538. doi: 10.1523/JNEUROSCI.23-24-08532.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-Aβ antibody alters CNS and plasma Aβ clearance and decreases brain Aβ burden in a mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holtzman DM, Paul SM. Immunization reverses memory deficits without reducing brain Aβ burden in Alzheimer’s disease model. Nat. Neurosci. 2002;5:452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- 18.Ferrer I, Boada RM, Sanchez Guerra ML, Rey MJ, Costa-Jussa F. Neuropathology and pathogenesis of encephalitis following amyloid-β immunization in Alzheimer's disease. Brain Pathol. 2004;14:11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frenkel D, Dewachter I, Van Leuven F, Solomon B. Reduction of β-amyloid plaques in brain of transgenic mouse model of Alzheimer's disease by EFRH-phage immunization. Vaccine. 2003;21:1060–1065. doi: 10.1016/s0264-410x(02)00609-6. [DOI] [PubMed] [Google Scholar]
- 20.Garmory HS, Brown KA, Titball RW. Salmonella vaccines for use in humans: Present and future perspectives. FEMS Microbiology Reviews. 2002;26:339–353. doi: 10.1111/j.1574-6976.2002.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 21.Ghochikyan A, Petrushina I, Lees A, Vasilevko V, Movsesyan N, Karapetyan A, Agadjanyan MG, Cribbs DH. Aβ-immunotherapy for Alzheimer's disease using mannan-amyloid-β peptide immunoconjugates. Dna and Cell Biology. 2006;25:571–580. doi: 10.1089/dna.2006.25.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, Eisner L, Kirby L, Boada RM, Forette F, Orgogozo JM. Clinical effects of Aβ immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 23.Goni F, Knudsen E, Schreiber F, Scholtzova H, Pankiewicz J, Carp R, Meeker HC, Rubenstein R, Brown DR, Sy MS, Chabalgoity JA, Sigurdsson EM, Wisniewski T. Mucosal vaccination delays or prevents prion infection via an oral route. Neuroscience. 2005;133:413–421. doi: 10.1016/j.neuroscience.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 24.Goni F, Prelli F, Schreiber F, Scholtzova H, Chung E, Kascsak R, Kascsak R, Brown DR, Sigurdsson EM, Chabalgoity JA, Wisniewski T. High titers of mucosal and systemic anti-PrP antibodies abrogate oral prion infection in mucosal-vaccinated mice. Neuroscience. 2008;153:679–686. doi: 10.1016/j.neuroscience.2008.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hara H, Monsonego A, Yuasa K, Adachi K, Xiao X, Takeda S, Takahashi K, Weiner HL, Tabira T. Development of a safe oral Aβ vaccine using recombinant adeno-associated virus vector for Alzheimer's disease. J Alzheimers. Dis. 2004;6:483–488. doi: 10.3233/jad-2004-6504. [DOI] [PubMed] [Google Scholar]
- 26.Hock C, Konietzko U, Papassotiropoulos A, Wollmer A, Streffer J, Von Rotz RC, Davey G, Moritz E, Nitsch RM. Generation of antibodies specific for β-amyloid by vaccination of patients with Alzheimer disease. Nat. Med. 2002;8:1270–1275. doi: 10.1038/nm783. [DOI] [PubMed] [Google Scholar]
- 27.Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Muller-Tillmanns B, Lemke U, Henke K, Moritz E, Garcia E, Wollmer MA, Umbricht D, de Quervain DJ, Hofmann M, Maddalena A, Papassotiropoulos A, Nitsch RM. Antibodies against β-amyloid slow cognitive decline in Alzheimer's disease. Neuron. 2003;38:547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- 28.Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, Zotova E, Nicoll JAR. Long-term effects of Aβ(42) immunisation in Alzheimer's disease: Follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 29.Jameson BA, Wolf H. The antigenic index: A novel algorithm for predicting antigenic determinants. Comput Appl Biosci. 1988;4:181–186. doi: 10.1093/bioinformatics/4.1.181. [DOI] [PubMed] [Google Scholar]
- 30.Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Heslin D, French J, Mount HT, Nixon RA, Mercken M, Bergeron C, Fraser PE, George-Hyslop P, Westaway D. Aβ peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 31.Kensil CR, Wu JY, Soltysik S. Structural and immunological characterization of the vaccine adjuvant QS-21. Pharm. Biotechnol. 1995;6:525–541. doi: 10.1007/978-1-4615-1823-5_22. [DOI] [PubMed] [Google Scholar]
- 32.Khalili-Shirazi A, Quaratino S, Londei M, Summers L, Tayebi M, Clarke AR, Hawke SH, Jackson GS, Collinge J. Protein conformation significantly influences immune responses to prion protein. J Immunol. 2005;174:3256–3263. doi: 10.4049/jimmunol.174.6.3256. [DOI] [PubMed] [Google Scholar]
- 33.Khan CM, Villarreal-Ramos B, Pierce RJ, Demarco dH, McNeill H, Ali T, Chatfield S, Capron A, Dougan G, Hormaeche CE. Construction, expression, and immunogenicity of multiple tandem copies of the Schistosoma mansoni peptide 115−131 of the P28 glutathione S-transferase expressed as C-terminal fusions to tetanus toxin fragment C in a live aro-attenuated vaccine strain of Salmonella. J. Immunol. 1994;153:5634–5642. [PubMed] [Google Scholar]
- 34.Khan CM, Villarreal-Ramos B, Pierce RJ, Riveau G, Demarco dH, McNeill H, Ali T, Fairweather N, Chatfield S, Capron A. Construction, expression, and immunogenicity of the Schistosoma mansoni P28 glutathione S-transferase as a genetic fusion to tetanus toxin fragment C in a live Aro attenuated vaccine strain of Salmonella. Proc. Natl. Acad. Sci. U. S. A. 1994;91:11261–11265. doi: 10.1073/pnas.91.23.11261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim KS, Wen GY, Bancher C, Chen CMJ, Sapienza V, Hong H, Wisniewski HM. Detection and quantification of amyloid β-peptide with 2 monoclonal antibodies. Neurosci Res Comm. 1990;7:113–122. [Google Scholar]
- 36.Kotilinek LA, Bacskai B, Westerman M, Kawarabayashi T, Younkin L, Hyman BT, Younkin S, Ashe KH. Reversible memory loss in a mouse transgenic model of Alzheimer's disease. J. Neurosci. 2002;22:6331–6335. doi: 10.1523/JNEUROSCI.22-15-06331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lavie V, Becker M, Cohen-Kupiec R, Yacoby I, Koppel R, Wedenig M, Hutter-Paier B, Solomon B. EFRH-phage immunization of Alzheimer's disease animal model improves behavioral performance in Morris water maze trials. J Mol. Neurosci. 2004;24:105–113. doi: 10.1385/JMN:24:1:105. [DOI] [PubMed] [Google Scholar]
- 38.Lemere CA, Spooner ET, LaFrancois J, Malester B, Mori C, Leverone JF, Matsuoka Y, Taylor JW, DeMattos RB, Holtzman DM, Clements JD, Selkoe DJ, Duff KE. Evidence for peripheral clearance of cerebral Aβ protein following chronic, active Aβ immunization in PSAPP mice. Neurobiol. Dis. 2003;14:10–18. doi: 10.1016/s0969-9961(03)00044-5. [DOI] [PubMed] [Google Scholar]
- 39.Leverone JF, Spooner ET, Lehman HK, Clements JD, Lemere CA. Aβ1−15 is less immunogenic than Aβ1−40/42 for intranasal immunization of wild-type mice but may be effective for “boosting”. Vaccine. 2003;21:2197–2206. doi: 10.1016/s0264-410x(02)00754-5. [DOI] [PubMed] [Google Scholar]
- 40.Levine MM, Galen J, Barry E, Noriega F, Chatfield S, Sztein M, Dougan G, Tacket C. Attenuated Salmonella as live oral vaccines against typhoid fever and as live vectors. J. Biotechnol. 1996;44:193–196. doi: 10.1016/0168-1656(95)00094-1. [DOI] [PubMed] [Google Scholar]
- 41.Lillard JW, Jr., Boyaka PN, Singh S, McGhee JR. Salmonella-mediated mucosal cell-mediated immunity. Cell Mol. Biol. (Noisy. -le-grand) 2001;47:1115–1120. [PubMed] [Google Scholar]
- 42.Maier M, Seabrook TJ, Lazo ND, Jiang LY, Das P, Janus C, Lemere CA. Short amyloid-β (Aβ) immunogens reduce cerebral Aβ load and learning deficits in an Alzheimer's disease mouse model in the absence of an Aβ-specific cellular immune response. J. Neurosci. 2006;26:4717–4728. doi: 10.1523/JNEUROSCI.0381-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masliah E, Hansen L, Adame A, Crews L, Bard F, Lee C, Seubert P, Games D, Kirby L, Schenk D. Aβ vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology. 2005;64:129–131. doi: 10.1212/01.WNL.0000148590.39911.DF. [DOI] [PubMed] [Google Scholar]
- 44.Mastroeni P, Chabalgoity JA, Dunstan SJ, Maskell DJ, Dougan G. Salmonella: Immune responses and vaccines. Vet. J. 2001;161:132–164. doi: 10.1053/tvjl.2000.0502. [DOI] [PubMed] [Google Scholar]
- 45.Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW. Aβ peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 46.Mouri A, Noda Y, Hara H, Mizoguchi H, Tabira T, Nabeshima T. Oral vaccination with a viral vector containing Aβ cDNA attenuates age-related Aβ accumulation and memory deficits without causing inflammation in a mouse Alzheimer model. Faseb Journal. 2007;21:2135–2148. doi: 10.1096/fj.06-7685com. [DOI] [PubMed] [Google Scholar]
- 47.Nardelli-Haefliger D, Kraehenbuhl JP, Curtiss R, III, Schodel F, Potts A, Kelly S, De Grandi P. Oral and rectal immunization of adult female volunteers with a recombinant attenuated Salmonella typhi vaccine strain. Infect. Immun. 1996;64:5219–5224. doi: 10.1128/iai.64.12.5219-5224.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nicoll JA, Barton E, Boche D, Neal JW, Ferrer I, Thompson P, Vlachouli C, Wilkinson D, Bayer A, Games D, Seubert P, Schenk D, Holmes C. Aβ species removal after Aβ42 immunization. J Neuropathol. Exp. Neurol. 2006;65:1040–1048. doi: 10.1097/01.jnen.0000240466.10758.ce. [DOI] [PubMed] [Google Scholar]
- 49.Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-β peptide: A case report. Nat. Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 50.Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, Jouanny P, Dubois B, Eisner L, Flitman S, Michel BF, Boada M, Frank A, Hock C. Subacute meningoencephalitis in a subset of patients with AD after Aβ42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 51.Pasetti MF, Anderson RJ, Noriega FR, Levine MM, Sztein MB. Attenuated deltaguaBA Salmonella typhi vaccine strain CVD 915 as a live vector utilizing prokaryotic or eukaryotic expression systems to deliver foreign antigens and elicit immune responses. Clin. Immunol. 1999;92:76–89. doi: 10.1006/clim.1999.4733. [DOI] [PubMed] [Google Scholar]
- 52.Paul S, Nishiyama Y, Planque S, Karle S, Taguchi H, Hanson C, Weksler ME. Antibodies as defensive enzymes. Springer Semin. Immunopathol. 2005;26:485–503. doi: 10.1007/s00281-004-0191-1. [DOI] [PubMed] [Google Scholar]
- 53.Pfeifer M, Boncristiano S, Bondolfi L, Stalder A, Deller T, Staufenbiel M, Mathews PM, Jucker M. Cerebral hemorrhage after passive anti-Aβ immunotherapy. Science. 2002;298:1379. doi: 10.1126/science.1078259. [DOI] [PubMed] [Google Scholar]
- 54.Racke MM, Boone LI, Hepburn DL, Parsadainian M, Bryan MT, Ness DK, Piroozi KS, Jordan WH, Brown DD, Hoffman WP, Holtzman DM, Bales KR, Gitter BD, May PC, Paul SM, DeMattos RB. Exacerbation of cerebral amyloid angiopathy-associated microhemorrhage in amyloid precursor protein transgenic mice by immunotherapy is dependent on antibody recognition of deposited forms of amyloid β. J Neurosci. 2005;25:629–636. doi: 10.1523/JNEUROSCI.4337-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Relkin NR, Szabo P, Adamiak B, Burgut T, Monthe C, Lent RW, Younkin S, Younkin L, Schiff R, Weksler ME. 18-Month study of intravenous immunoglobulin for treatment of mild Alzheimer disease. Neurobiol. Aging. 2008 Feb. 20 doi: 10.1016/j.neurobiolaging.2007.12.021. In Press. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 56.Sadowski M, Pankiewicz J, Scholtzova H, Ripellino JA, Li Y, Schmidt SD, Mathews PM, Fryer JD, Holtzman DM, Sigurdsson EM, Wisniewski T. A synthetic peptide blocking the apolipoprotein E/β-amyloid binding mitigates β-amyloid toxicity and fibril formation in vitro and reduces β-amyloid plaques in transgenic mice. Am. J Pathol. 2004;165:937–948. doi: 10.1016/s0002-9440(10)63355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schenk D. Amyloid-β immunotherapy for Alzheimer's disease: The end of the beginning. Nat. Rev. Neurosci. 2002;3:824–828. doi: 10.1038/nrn938. [DOI] [PubMed] [Google Scholar]
- 58.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 59.Scholtzova H, Wisniewski T, Ahlawat S, Watanabe M, Quartermain D, Frangione B, Sigurdsson EM. Safety of potential vaccines for Alzheimer's disease. Soc. Neurosci. Abstr. 2002:227.1. [Google Scholar]
- 60.Seabrook TJ, Jiang L, Thomas KE, Lemere CA. Boosting with intranasal dendrimeric Aβ1−15 but not Aβ1−15 peptide leads to an effective immune response following a single injection of Aβ1−40/42 in APP-tg mice. J Neuroinflammation. 2006;3:14. doi: 10.1186/1742-2094-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seabrook TJ, Thomas K, Jiang L, Bloom J, Spooner E, Maier M, Bitan G, Lemere CA. Dendrimeric Aβ1−15 is an effective immunogen in wildtype and APP-tg mice. Neurobiol Aging. 2007;28:813–823. doi: 10.1016/j.neurobiolaging.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 62.Sigurdsson EM. Histological staining of amyloid-β in mouse brains. Methods Mol. Biol. 2005;299:299–308. doi: 10.1385/1-59259-874-9:299. [DOI] [PubMed] [Google Scholar]
- 63.Sigurdsson EM. Immunotherapy targeting pathological tau protein in Alzheimer's disease and related tauopathies. Journal of Alzheimer's Disease. 2008;15:157–168. doi: 10.3233/jad-2008-15202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sigurdsson EM, Knudsen E, Asuni A, Fitzer-Attas C, Sage D, Quartermain D, Goni F, Frangione B, Wisniewski T. An attenuated immune response is sufficient to enhance cognition in an Alzheimer's disease mouse model immunized with amyloid-β derivatives. J Neurosci. 2004;24:6277–6282. doi: 10.1523/JNEUROSCI.1344-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sigurdsson EM, Lorens SA, Hejna MJ, Dong XW, Lee JM. Local and distant histopathological effects of unilateral amyloid-β 25−35 injections into the amygdala of young F344 rats. Neurobiol. Aging. 1996;17:893–901. doi: 10.1016/s0197-4580(96)00169-8. [DOI] [PubMed] [Google Scholar]
- 66.Sigurdsson EM, Scholtzova H, Mehta PD, Frangione B, Wisniewski T. Immunization with a non-toxic/non-fibrillar amyloid-β homologous peptide reduces Alzheimer's disease associated pathology in transgenic mice. Am. J. Pathol. 2001;159:439–447. doi: 10.1016/s0002-9440(10)61715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Solomon B, Koppel R, Frankel D, Hanan-Aharon E. Disaggregation of Alzheimer β-amyloid by site-directed mAb. Proc. Natl. Acad. Sci. U. S. A. 1997;94:4109–4112. doi: 10.1073/pnas.94.8.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tacket CO, Sztein MB, Losonsky GA, Wasserman SS, Nataro JP, Edelman R, Pickard D, Dougan G, Chatfield SN, Levine MM. Safety of live oral Salmonella typhi vaccine strains with deletions in htrA and aroC aroD and immune response in humans. Infect. Immun. 1997;65:452–456. doi: 10.1128/iai.65.2.452-456.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tom Fagan, Trial Troika - Immunotherapy Interrupted, Lipitor Lags, Dimebon Delivers. [April 28, 2008];Alzheimer Research Forum. 2008 http:www.alzforum.org/new/detail.asp?id=1807, Posted April 25, 2008.
- 70.Trouche SG, Asuni A, Boutajangout A, Frangione B, Wisniewski T, Rouland S, Verdier JM, Sigurdsson EM, Mestre-Frances N. Neuropathological evaluation of the nonhuman primate Microcebus murinus immunized with K6Aβ1−30, an Aβ derivative peptide. Alzheimer's & Dementia. 2008;4:T211. [Google Scholar]
- 71.Trouche SG, Asuni A, Rouland S, Wisniewski T, Frangione B, Verdier JM, Sigurdsson EM, Mestre-Frances N. Antibody response and plasma Aβ1−40 levels in young Microcebus murinus primates immunized with Aβ 1−42 and its derivatives. Vaccine. 2009;27:957–964. doi: 10.1016/j.vaccine.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.West MJ. Stereological methods for estimating the total number of neurons and synapses: Issues of precision and bias. Trends Neurosci. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- 73.Wilcock DM, Rojiani A, Rosenthal A, Subbarao S, Freeman MJ, Gordon MN, Morgan D. Passive immunotherapy against Aβ in aged APP-transgenic mice reverses cognitive deficits and depletes parenchymal amyloid deposits in spite of increased vascular amyloid and microhemorrhage. J Neuroinflammation. 2004;1:24. doi: 10.1186/1742-2094-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilcock GK, Esiri MM. Plaques, tangles and dementia: A quantitative study. Journal of the Neurological Sciences. 1982;56:343–356. doi: 10.1016/0022-510x(82)90155-1. [DOI] [PubMed] [Google Scholar]
- 75.Wisniewski T, Konietzko U. Amyloid-β immunisation for Alzheimer's disease. Lancet Neurology. 2008;7:805–811. doi: 10.1016/S1474-4422(08)70170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang J, Wu X, Qin C, Qi J, Ma S, Zhang H, Kong Q, Chen D, Ba D, He W. A novel recombinant adeno-associated virus vaccine reduces behavioral impairment and β-amyloid plaques in a mouse model of Alzheimer's disease. Neurobiol Dis. 2003;14:365–379. doi: 10.1016/j.nbd.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 77.Zhou J, Fonseca MI, Kayed R, Hernandez I, Webster SD, Yazan O, Cribbs DH, Glabe CG, Tenner AJ. Novel Aβ peptide immunogens modulate plaque pathology and inflammation in a murine model of Alzheimer's disease. J Neuroinflammation. 2005;2:28. doi: 10.1186/1742-2094-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]