Abstract
Krüppel-associated box-zinc finger proteins (KRAB-ZFPs) are the largest class of transcriptional regulators in mammals, yet few have been assigned biological roles. Cloning the genes underlying the regulator of sex-limitation (rsl) phenotype, in which the normally male-specific sex-limited protein (SLP) is expressed in female mice, identified two KRAB-ZFPs, Rsl1 and Rsl2, as influencing sexually dimorphic liver gene expression. Combined absence of both repressors in rsl mice leads to increased expression in female liver of major urinary proteins (MUPs) and certain enzymes of steroid metabolism, as well as SLP. We hypothesized that this altered gene expression might affect reproductive physiology in rsl females. Urinary MUP (uMUP) concentration varied with the estrous cycle in both wt and rsl females but was consistently higher in rsl urine. A behavioral odor test revealed that wild-type (wt) males preferred rsl to wt females, possibly due to elevated uMUPs providing greater pheromone presentation. To ascribe activity to Rsl1, Rsl2, or both, the genes were individually expressed as liver-specific transgenes. RSL2 overexpression accentuated uMUP fluctuations across the estrous cycle, whereas RSL1 overexpression did not. In addition, puberty onset, as indicated by vaginal opening (VO), occurred 2 days earlier in rsl females, and excess RSL2, but not RSL1, restored VO timing to wt. Hence, transcriptional repression by RSL in liver modifies female mouse reproduction via targets that likely impact both hormonal and pheromonal cues. The large and rapidly diversifying KRAB-ZFP family may modulate biological processes, including reproduction, to confer individual differences that may isolate populations and ultimately lead to speciation.
Keywords: gene regulation, major urinary protein, mechanisms of hormone action, pheromones, pubertal timing, puberty, regulator of sex-limitation, sexual dimorphism, sexually dimorphic gene expression
The KRAB-zinc finger transcriptional repressors, regulator of sex-limitation (RSL) 1 and RSL2, influence timing of female puberty and MUP levels through estrous by effects on liver gene expression.
INTRODUCTION
Sex-specific gene expression is widespread and occurs in tissues and organs not directly involved in reproduction, such as liver, kidney, and muscle [1]. In liver, for instance, over 70% of active genes show some degree of sex-biased expression, and nearly 400 transcripts differ by 1.5-fold or more in abundance between males and females [1, 2]. The maintenance of sexually dimorphic gene expression in adults relies on sex steroids and their receptors as well as on multiple transcription factors, many of which are not restricted by sex. In the rodent liver, for example, certain members of the cytochrome P450 (CYP), sulfotransferase (SULT), and organic transporter (SLC) families are more highly expressed in one sex or the other, and these patterns depend largely on the non-dimorphic signal transducer and activator of transcription 5b (STAT5B) [3–6]. In mouse liver, major urinary protein (MUP) pheromone carriers and sex-limited protein (SLP) are also male-predominant, with transcription rates dependent on multiple factors [7, 8].
Slp has been an invaluable tool for examining male-specific gene expression in the mouse [8]. In some inbred strains, however, Slp is expressed in females at levels nearly equivalent to those of wild-type (wt) males and is further elevated in males of these variant strains [9, 10]. This phenotype was named regulator of sex-limitation (rsl). We identified the genes responsible, Rsl1 and Rsl2, by positional cloning and verified their function by BAC transgene rescue [11]. The Rsl genes encode paralogous Krüppel-associated box-zinc finger proteins (KRAB-ZFPs), which are potent repressors and the largest family of transcriptional regulators in mammalian genomes [12, 13]. KRAB-ZFPs interact with KAP1 to recruit HP1 and associated methylases, thereby targeting chromatin modification to specific genomic sites [14]. Extensive cross-regulation among KRAB-ZFPs has been found by genomewide analysis [15]. Despite a detailed understanding of their biochemical mechanism [16, 17], biological roles for most KRAB-ZFPs remain largely unknown. Intriguingly, the KRAB motif is recent in evolution, and KRAB-ZFP genes often occur in clusters and diverge between species, suggesting some of their functions may be species specific [12, 18].
Some sexually dimorphic genes in addition to Slp are elevated in livers of rsl mice [2, 10, 11]. Many encode enzymes of steroid biotransformation, sulfate modification, and transport (e.g., CYPs, SULTs, and SLCs) that contribute to the clearance of circulating steroid hormones [19]. MUPs, also targets of RSL, bind pheromones to facilitate their passage in urine and slow their dissipation from scent marks [20]. Furthermore, Rsl affects numerous genes that are not sexually dimorphic, but sometimes modulates these targets only in one sex [2]. Recent microarray analysis showed that over 1000 genes in liver are affected by Rsl, with a large number involved in cholesterol and lipid metabolism [2]. Overall, more genes are affected by Rsl in males than females, but the effect on specific targets is generally more pronounced in females [2]. Though altered expression of these genes due to the absence of Rsl does not appear to be grossly detrimental to the health of rsl mice, the hormonal milieu and/or physiology may be perturbed in ways that may affect reproduction.
Because RSL affects a broad array of genes to incur sex differences in a hormone-independent manner, and some of these differences themselves impact hormone metabolism, we hypothesized rsl females would vary in reproductive physiology. We tested this in wt, Rsl-null (rsl), and transgenic (tg) mice that overexpress Rsl1 or Rsl2 in the liver. Here we report that Rsl affects estrous-associated patterns of urinary MUP (uMUP) excretion, mate choice, and puberty timing. Aspects of each of these complex traits show differential effects of Rsl1 and Rsl2. Our results suggest that the KRAB-ZFP transcription factors RSL1 and RSL2 influence reproductive phenotypes by modulating expression patterns of probably multiple target genes in liver.
MATERIALS AND METHODS
Mice
B10.D2 (wt) mice were purchased from The Jackson Laboratory (Bar Harbor, ME; Stock #000463). Congenic B10.D2.PL (rsl) mice were initially provided by Dr. Ray Miller [21] and possess ∼2.2 cM of Chromosome 13 of PL/J mice (Stock #000680; The Jackson Laboratory, Bar Harbor, ME) on the B10.D2 genetic background. Transgenic mice were created by the University of Michigan Transgenic Animal Model Core facility. Fertilized oocytes from B10.D2 mice were injected with pTTR1ExV5/RSL1 or pTTR1ExV5/RSL2, plasmids encoding Rsl1 or Rsl2 cDNAs, respectively, driven by the strong liver-specific transthyretin promoter [2, 22]. Transgenic founders were backcrossed for two generations to the B10.D2.PL strain to place the transgenes on an rsl background. F2 mice were weaned and segregated by sex at 21 days and housed in fresh cages in groups of two to four mice, regardless of genotype. Females from three independent lines for each Rsl transgene were used for uMUP and vaginal opening (VO) assessment (see Urine Collection and MUP, VO, and Estrous Cycle Assessment). Data were segregated by Rsl transgene but pooled across lines to increase the number of individuals per genotype. Although transgene copy number varied from one to three, no significant differences were noted between lines. All animal experimentation protocols were approved by the University of Michigan Committee on Use and Care of Animals.
Odor Preference Tests
A Y-maze was assembled from plastic pipe based on the design of Kavaliers and Kinsella [23], with chambers containing stimulant odors (soiled bedding) at the ends of two arms. Test mice were placed in the third arm of the maze and watched until they emerged at one of the two chambers. The stimulant odors were frequently exchanged, in a random patt, between arms of the maze to overcome any potential “sidedness” in the test mice. Adult (>9 wk) male wt mice were placed in the maze 10 times per test. Three tests were conducted over three consecutive days for each stimulant odor arrangement. The apparatus was washed with Alconox and dried with 70% ethanol between test days. Five days separated each series of tests. Stimulant odors were generated by placing mice (n = 3) in a cage of clean bedding for 1 h, and the resulting soiled bedding was used immediately in the Y-maze.
Urine Collection and MUP, VO, and Estrous Cycle Assessment
Urine was obtained by placing mice on plastic wrap and gently massaging their abdomens. Voided urine was transferred into 1.5-ml microfuge tubes. Urine samples were placed on ice for 10 min and centrifuged at 4°C for 5 min. Protein concentration was determined using the Protein Assay Reagent (Bio-Rad, Hercules, CA) according to the manufacturer's instructions, and relative concentrations were confirmed by running 0.1 μl of urine on 20% SDS-PAGE gels and staining with Coomassie Blue.
VO was determined by gently probing the genital region with a disposable plastic pipet tip and visual inspection of the vaginal tissue beneath a drop of 0.9% sodium chloride solution to magnify the view. Vaginal smears were collected by lavage with PBS. Recovered cells were stained with methylene blue (Wright's Stain; VWR, West Chester, PA) and analyzed under a light microscope. Phases of the estrous cycle were determined according to Rugh [24].
Genotyping and RT-PCR
Genotyping was performed by PCR on mouse genomic DNA as follows: 35 cycles of 94°C for 15 sec, 62°C for 30 sec, and 72°C for 90 sec. Marker D13Dmr14 (Forward Primer: 5′-GGAAACTGGAATGGGGCTAT-3′, Reverse Primer: 5′-GGGGGTGCACCTAGAAGAA-3′) identifies the Rsl locus, with the B10.D2 allele yielding a 390-bp product and the B10.D2.PL allele a 327-bp product. Transgenes were detected with vector-specific forward primer TTR1Intron1Seq2-F: 5′-CAAGCCGGTTTACTCTGACC-3′ and Rsl1-specific reverse primer Rslcan9spp-R: 5′-TCTGTTGACACATTGTTCATGCT-3′ or Rsl2-specific reverse primer Rslcan4spp-R: 5′-GAAATCTTTTGACACATTCTTCATACA-3′.
Transgene expression was detected by RT-PCR of total liver RNA. RNA was isolated by the guanidinium isothiocyanate method [25], and 2 μg was reverse transcribed with Superscript II Reverse Transcriptase (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Semiquantitative PCR was performed with reverse primer Rslcan9spp-R or Rslcan4spp-R and radiolabeled forward primers (Rsl1-specific Can9E1-F: 5′-GGTCTGTACTCGTGCGTCTTT-3′ or Rsl2-specific Can4E1-F: 5′-TGCCTCTTCCTCAAAGTTGG-3′), end-labeled with [γ-32P]ATP (MP Biomedicals, Solon, OH), and T4 polynucleotide kinase (New England Biolabs [NEB], Ipswich, MA) prior to PCR, according to instructions from NEB. PCR conditions were as described above, with cycles reduced to 25 to allow semiquantitative comparisons. β-actin (GenBank Accession #NM_007393) was used as an internal control (PCR for 20 cycles with MusβAct-F: 5′-CGGGATCCCAGCTTCTTTGCAGCTCCTT-3′ and MusβAct-R: 5′-GGAATTCAGTCCGCCTAGAAGCACTTG-3′). Products were separated on 5% polyacrylamide per 7 M urea sequencing gels followed by autoradiography and PhosphorImager analysis.
Real-time quantitative RT-PCR (Q-RT-PCR) was performed by first synthesizing cDNA using 2.5 μg total liver RNA and the High Capacity cDNA Archive Kit (Applied Biosystems, Warrington, U.K.) according to the manufacturer's instructions. PCR was performed in the Applied Biosystems 7500 thermocycler with SYBR Green (Applied Biosystems) reagent according to the manufacturer's instructions. Primers specific to each gene were designed using the Primer3 program (http://frodo.wi.mit.edu) and the NCBI mouse genome sequence (Build 36.1). Q-RT-PCR primer sequences for Mup genes are as follows: for Mup1 (NM_031188.1) forward: 5′-AAGGTTTGCACAACTATGTGAGA-3′, reverse: 5′-TCCTGGTGAAAAGTCTCCACTC-3′; for Mup2 (NM_008647.2) forward: 5′-CGGGAAGGAACTTTAATGTACAAA-3′, reverse: 5′-TTGTCAGCAACCATAGATAATTCG-3′; for Mup3 (NM_001039544.1) forward: 5′-GAAAATATCATTGACCTAACCAATGT-3′; reverse: 5′-TCCTGGTGAAAAGTCTCCACTC-3′. 18S rRNA amplified with the following primers served as a reference for normalization: forward: 5′-CGCCGCTAGAGGTGAAATTC-3′, reverse: 5′-CCAGTCGGCATCGTTTATCG-3′. Cycle threshold (Ct) values were converted to relative expression levels using the 2-ΔΔCt method [26].
Statistics
Odor preference test data analysis involved the chi-squared statistic with a 50:50 outcome as the null hypothesis. All other graphs reflect the mean values (±SEM). Student t-tests were used to determine differences between wt and rsl mice. Multiple genotypes were analyzed by single-factor ANOVA followed by post hoc pairwise comparisons with modified Bonferroni correction.
RESULTS
Oscillation of Urinary MUPs Across the Estrous Cycle
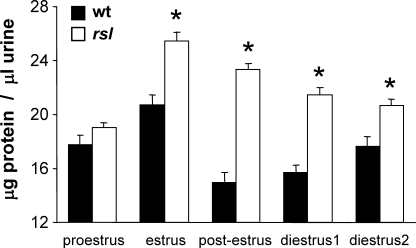
Previously we observed that, on average, Mup gene expression is higher in rsl than wt females [10, 11], although individual variation covers a wide range. To determine if a similar variation occurred in MUP concentration in urine and whether it correlated with the estrous cycle, urine was collected each day for two consecutive cycles from adult (>10-wk-old) wt and rsl females. Urinary MUP levels were estimated from total protein concentrations because MUP represents the overwhelming majority of protein in urine [27]. Vaginal smears were concurrently obtained to monitor cycle progression. There were no genotype-dependent differences in the length of the estrous cycles (wt = 5.1 days ± 1.2; rsl = 5.7 days ± 0.8; P = 0.273). However, the relative concentration of MUP in urine was significantly greater for rsl than wt females at each stage of the cycle, except at proestrus (Fig. 1). Furthermore, wt and rsl females each expressed the maximum level of MUP during estrus. After estrus, uMUP levels dropped sharply in wt mice and then gradually increased throughout the remainder of the cycle, whereas uMUPs declined gradually after estrus in rsl mice, but rose quickly in the transition from proestrus to estrus.
FIG. 1.
Urinary MUP concentration varies with the estrous cycle in female mice. Vaginal smears and urine were obtained from female mice over 14 consecutive days. Smears were examined to monitor phase of estrous cycle. Total protein in the urine was determined spectrophotometrically as a representative of uMUP levels. Each bar represents the mean ± SEM. wt, n = 12; rsl, n = 14; *, means are significantly different (P < 0.05).
The Rsl Locus Affects Odor Preference
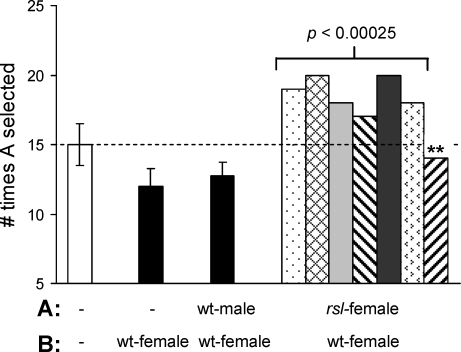
Urinary MUPs facilitate pheromone communication [20] and are about 3- to 8-fold higher in males than females [27]. Because uMUPs are also higher in rsl than wt mice [10, 11], we speculated that the ability of rsl females to attract mates might be affected. Differences between wt and rsl female urinary pheromone signals were examined using an odor preference assay. Sexually experienced wt test males were placed individually into a Y-maze that ended in two chambers. The chambers were charged with stimulant odors from wt females, wt males, or rsl females. The cumulative results from three independent trials are shown in Figure 2. Despite some individual variation, six out of seven test males responded similarly, being more attracted to chambers with bedding soiled by rsl females than wt females. Interestingly, one test male, who showed little bias toward either chamber (Fig. 2, asterisks), failed to produce copulation plugs when housed with sexually mature females. To challenge the possibility that test males were responding to communal odors or aggressive urges rather than mate choice, four of the test males were given a choice between clean bedding and wt females, or bedding soiled by wt males or wt females. On average, test males chose chambers with females more often than either clean bedding or males, suggesting they were motivated to a greater extent by female pheromones. In contrast, a separate cohort of sexually naive test males showed no odor preference (data not shown). Together, these results suggest that the Rsl locus influences reproductive behavior, likely due to elevated pheromone presentation in rsl females as a consequence of elevated uMUP excretion.
FIG. 2.
Males select rsl females in odor preference tests. Labels A and B refer to the two chambers at the ends of the maze and the corresponding stimulant odor in each set of tests; –, clean bedding. Each male was tested 10 times over 3 days for a total of 30 trials. The three bars on the left are the mean ± SEM of four test males. Bars on the right are individual results for seven males tested for urinary odor preference of rsl females to wt females. The P-value indicates the “goodness of fit” using the chi-square statistic with a 50:50 outcome as the null hypothesis. Asterisks highlight one male who was omitted from statistical analysis due to his failure to produce copulatory plugs when housed with females. The dashed line highlights the expected mean if the chamber preference was unbiased. Details of the assay are given in Materials and Methods.
Differential Affects of Rsl1 and Rsl2 on uMUP Excretion
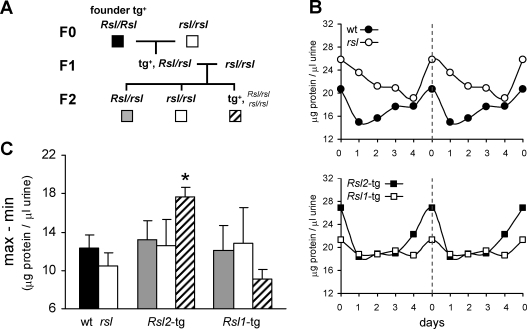
Previous analysis of liver RNAs from mice containing BAC transgenes reveals that Rsl1, but not Rsl2, represses Slp, and that Rsl2, but not Rsl1, represses Mup expression, despite near identity of these KRAB-ZFPs [11]. The presence of neighboring KRAB-ZFP genes within the BACs and the mixed genetic background of these mice complicates further analysis. Therefore, transgenic B10.D2 mice were created with the strong transthyretin promoter/enhancer driving expression of Rsl1 or Rsl2 cDNAs [2, 22] to distinguish additional actions of Rsl1 from Rsl2. RT-PCR and quantitative real time RT-PCR confirmed that the Rsl transgenes were expressed specifically in liver and at levels much higher than their endogenous counterparts [2]. Transgenic (tg) founders were backcrossed to rsl mice (Fig. 3A) to place the transgenes on a null background, resulting in high Rsl expression exclusively in liver, in contrast to wt mice in which Rsl is broadly expressed at low levels [28]. The resulting tg and non-tg F2 females were compared to determine the impact of liver RSL on indicators of reproductive physiology.
FIG. 3.
Urinary MUP concentration is influenced during estrous by Rsl transgenes. A) Pedigree of the two-generation backcross of the B10.D2 tg mice. Non-tg littermates served as age- and environmentally matched controls. Symbols beneath the pedigree genotypes correspond to the shading and striping in the graphs below. B) Means of the total urinary protein obtained over 14 consecutive days representing two complete estrous cycles per mouse. Vaginal smears and urine were obtained and analyzed as in Figure 1. The data is plotted here as two identical cycles for ease of pattern recognition. Day 0 on each graph is the midpoint of estrus. The dashed vertical line indicates where the patterns repeat. Results are from parental (top: wt, n = 12; rsl, n = 14) and F2 transgenic mice (bottom: Rsl2-tg, n = 11; Rsl1-tg, n = 7). C) Variance of uMUP concentration throughout the estrous cycle. To compare cyclicity of uMUP expression, maximum and minimum urinary protein concentration over 14 consecutive days was calculated for each mouse. The means of the differences were calculated and compared by genotype. The shading and striping are in accord with the genotypes indicated in A. Each bar represents the mean differential ± SEM. *, means are significantly different from rsl (P < 0.005).
The effects of Rsl1 and Rsl2 individually on uMUPs was determined by examining urine from tg females across the estrous cycle as described above. Rsl2-tg females displayed pronounced peaks of uMUP excretion, with a steep rise two days before estrus and a rapid decline shortly thereafter, a pattern more similar to wt than to rsl females (Fig. 3B). The non-tg mice from litters with Rsl2-tg cage mates showed cycles of gradually changing uMUP levels, with patterns intermediate to that of wt and rsl mice (data not shown). In contrast, Rsl1-tg females (Fig. 3B) as well as their non-tg littermates (data not shown) had uMUP levels that seemed to fluctuate more modestly across estrous, in part due to a greater degree of individual variation.
Examination of uMUP excretion profiles across the estrous cycle suggested the characteristic most influenced by excess Rsl is the differential between high and low levels (i.e., amplitude). Amplitudes were compared by plotting the means of the differences between the maximum and minimum values for each mouse within each genotype. Rsl influence was most evident around the estrus peak. Despite elevated uMUP levels in rsl compared to wt females, their amplitudes did not differ significantly (Fig. 3C). However, the Rsl2-tg females had amplitudes that were about 1.5-fold greater than any other genotype (Fig. 3C). Thus, overexpression of Rsl2, but not Rsl1, exaggerated the oscillation of uMUP excretion across the estrous cycle. Together, these findings provide further evidence that Rsl1 and Rsl2 regulation overlap but are not completely redundant.
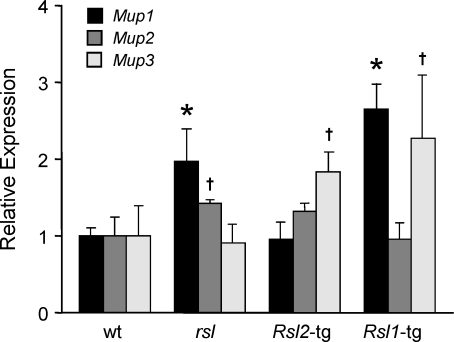
RSL Modulation of Expression of Individual Mup Genes in Liver
Mup expression appears to be directly repressed by RSL, a pattern summed over several distinct Mup genes [10, 11]. Q-RT-PCR was used to assess the individual expression levels of the most abundant liver Mup transcripts, Mup1, Mup2, and Mup3 [7, 27]. In wt females, Mup1 is most abundant, with levels approximately 100- and 35-fold greater than Mup2 and Mup3, respectively. Relative to wt females, transcripts for Mup1 were higher in rsl and Rsl1-tg mice, both of which lack Rsl2, thus implicating Rsl2 in control of MUP1 levels. Mup2 transcripts were nearly the same across genotypes, and Mup3 transcripts were higher only in the tg mice (Fig. 4). These results confirm and extend earlier findings that Rsl2 can repress total Mup gene expression, shown here to be largely accounted for by effects on Mup1, and that Rsl1 cannot [11]. Furthermore, excess of either Rsl1 or Rsl2 had no effect on Mup2 but affected Mup3. Since expression of Mup3 was higher with greater levels of RSL repressors, this regulation may be indirect, possibly due to RSL repression of other KRAB-ZFPs [2, 15].
FIG. 4.
Mup genes are regulated by RSL in mouse liver. Real time Q-RT-PCR results for Mup1, Mup2, and Mup3 in liver RNA from adult wt and rsl females as well as Rsl-tg females in the mutant rsl background. Each bar represents the mean ± SEM from four or more mice from each genotype. Relative RNA quantities were normalized to a “calibrator” wt female, and equivalent amounts of cDNA (0.25 ng) were used in the PCR to compare the relative abundance of each Mup gene. *, means are significantly different from wt (P < 0.05); †, means are different from wt (P < 0.1).
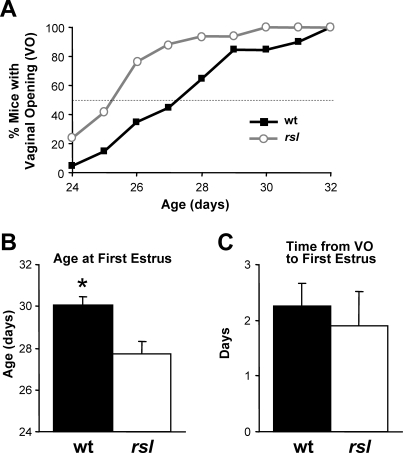
Earlier VO in rsl than in wt Mice
Increased uMUP excretion, Mup gene expression, and attractiveness to males in rsl females are consistent with altered pheromone signaling. Puberty timing in mice is an additional marker of reproductive physiology that is also sensitive to pheromones as well as other modifying genetic and environmental factors [29]. To determine whether RSL affects puberty timing, VO was examined as a marker of puberty onset [30] in wt and rsl females. Females were examined daily starting at 24 days of age. Approximately 50% of wt females arrived at puberty by Postnatal Day 28, whereas 50% of rsl females reached puberty two days earlier (Fig. 5A). This two-day differential was also evident by scoring for age at first estrus (∼30 days for wt, ∼28 days for rsl), determined by the cell composition of vaginal smears (Fig. 5B). Despite puberty initiating two days earlier in rsl females, the time from VO to first estrus did not differ significantly for the mice that could be assessed (Fig. 5C).
FIG. 5.
Puberty occurs 2 days earlier in rsl females than in wt females. Female mice were monitored daily for VO. After VO, vaginal smears were used to identify first estrus. A) Percent of mice with VO from Postnatal Day 24 to 32. B) Age of mice at first estrus. Each bar represents the mean ± SEM. wt, n = 20; rsl, n = 17; *, means are significantly different (P < 0.05). C) Time from VO to first estrus. Each bar represents the mean ± SEM. wt, n = 19; rsl, n = 13.
Differential Affects of Rsl1 and Rsl2 on Sexual Maturation
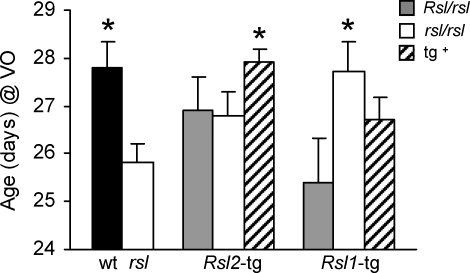
In order to determine the influence of Rsl1 or Rsl2 individually on puberty onset, VO was measured in the tg mice and their non-tg cage mates. The Rsl2-tg females developed VO at 28 days (Fig. 6, striped bar, middle group), similar to wt, indicating rescue of this mutant phenotype by RSL2 overexpression. Their non-tg cage mates (Fig. 6, first two bars, middle group) did not differ significantly from mutant, developing VO at intermediate ages between wt and rsl mice. VO in Rsl1-tg mice varied more than in the Rsl2-tg mice but occurred largely between 26 and 27 days, failing to differ significantly from the age of either wt or rsl (Fig. 6, striped bar, right group). Surprisingly, the non-tg heterozygous cage mates of the Rsl1-tg mice (stippled bar, right group) developed VO at ages similar to rsl (∼26 days), whereas those homozygous for rsl were more similar to wt (∼28 days). It is possible that pheromonal influences derived from Rsl1-overexpressing females. However, this analysis did not account for variable pheromone exposure due to the presence in cages of sires and male litter mates prior to weaning, as well as female cage mates varying in RSL expression. Nevertheless, Rsl2, unlike Rsl1, clearly influences VO in animals in which it is overexpressed, and this may play a part in the VO of cage mates.
FIG. 6.
Puberty timing is restored to wt in Rsl2-tg mice. Age of mice at VO. Each bar represents the mean ± SEM. Black bars are homozygous wt (n = 19), white bars are homozygous rsl (n = 13), and non-tg F2 mice in the mutant rsl background. Gray bars are heterozygous (Rsl/rsl) mice. Stripes indicate mice that are positive for the transgene indicated below the x-axis. The non-transgenic results (solid bars) were from the non-transgenic littermates (cage mates) of Rsl1 and Rsl2 transgenic mice, respectively. (Rsl2 non-tg – Rsl/rsl, n = 12; Rsl2 non-tg – rsl/rsl, n = 16; Rsl2 tg, n = 25; Rsl1 non-tg – Rsl/rsl, n = 5; Rsl1 non-tg – rsl/rsl, n = 11; Rsl1 tg – rsl/rsl, n = 20). *, means are significantly different from rsl (P < 0.005).
DISCUSSION
rsl was first identified as a modifier of male-specific Slp expression in the liver. Both sexes of rsl mice express more SLP than wt and from an earlier age [10]. More recently, we showed that the Rsl KRAB-ZFPs modulate a broad array of liver genes, including those encoding pheromone carriers (Mups) and enzymes of steroid (Cyps) and lipid metabolism [2, 11]. MUPs are effectors of pheromone signaling, CYPs affect steroid hormone levels, and lipid metabolism affects energy storage, all of which contribute to reproductive physiology [6, 29, 31–33]. We therefore hypothesized that Rsl might impact reproduction and tested that idea in female mice.
Mup gene expression and uMUP levels are higher in rsl than wt mice [10, 11], but variability among females regardless of genotype suggests estrous might play a role. Although uMUP excretion is not known to be regulated by ovulatory hormones, pheromone concentration in urine and female pheromone signaling increase during estrus [31, 34, 35]. Here we show that uMUP levels also peak at estrus and are higher at each phase in rsl than wt females (Fig. 1). In addition, the excretion profiles across the cycle differ depending on genotype—uMUP concentrations decline in rsl females at a time when they are increasing in wt mice (Figs. 1 and 3B). That this variation in uMUPs is in accord with circulating estrogen levels suggests Rsl in some manner may influence, or be influenced by, ovarian hormone regulation.
Urinary MUPs function as pheromone carriers and differ between males and females primarily in quantity, with the pheromones themselves being sex-specific [29, 36]. Although the chemical composition of murine pheromones is not well known, their biological activities in urine are well documented and include the ability to hasten puberty onset, influence mate choice, and synchronize estrous cycles [29, 34, 37]. uMUPs dictate the strength of the pheromone signal by allowing the complex to pass unfiltered through the kidney and by slowing pheromone dissipation from urinary scent marks [20, 29, 38]. Because male urine has MUP levels several-fold higher than females [27], elevated uMUPs in rsl females might more effectively present (female) pheromone and thereby affect the ability to attract mates. Finding that wt males prefer urinary odors from rsl females (Fig. 2) suggests that a higher level of uMUPs contributes to greater pheromone signaling.
The individual effects of the highly homologous RSL proteins are evident in uMUP excretion profiles from tg mice that overexpress cDNAs for Rsl1 or Rsl2 specifically in the liver. Excess Rsl2 exaggerates the wt suppression of uMUPs before and after estrus (Fig. 3B), when ovarian hormones in wt are below peak levels. Suppression of uMUPs is overcome coincident with the hormonal surge preceding estrus. Following estrus, uMUP excretion again rapidly declines in Rsl2-tg females. This profile resembles the wt, suggesting Rsl2 may in part oppose, but at estrus succumb to, ovarian hormone action in uMUP excretion. Interestingly, median uMUP levels are not significantly less in Rsl2-tg mice, as might be expected due to high repressor levels, but instead the range from maximum to minimum level is exaggerated (Fig. 3C). In contrast, Rsl1 excess seems to reduce changes in uMUP levels even during estrus, apparently dampening the effects of fluctuating ovarian hormones (Fig. 3).
Modest levels of Rsl2 from a BAC transgene can restore uMUP excretion and Mup1/2 transcript levels to that of wt mice [11]. Here, liver-restricted overexpression of Rsl2, but not Rsl1, also rescues (i.e., suppresses) the otherwise elevated levels of the predominant Mup1 (Fig. 4). However, other Mup family members are either not greatly affected by Rsl (e.g., Mup2) or are influenced indirectly since expression patterns indicate activation rather than repression (e.g., Mup3). Regardless of the direction, the effects of Rsl on Mup genes are modest (<3-fold differences). More dramatic differences occur for female-predominant steroid metabolizing enzymes [2]. In particular, in gene expression profiles of wt, rsl, and Rsl-tg liver, enzymes in all three phases of steroid hormone metabolism (i.e., biotransformation [e.g., CYP3A41], sulfate modification [e.g., SULT3A1], and transport [e.g., SLC20A22]) are Rsl responsive. Expression patterns for other genes encoding enzymes of steroid and lipid metabolism also respond to Rsl (e.g., Sult2a2, Fmo3, Acot3, Cyp17a1) [2]. Moreover, these genes appear to be direct targets of RSL repression, exhibiting elevated expression in rsl females that is greatly decreased by tg overexpression. Thus, Rsl excess or absence could affect steroid levels in serum via metabolism and clearance in the liver, with subsequent effects on endocrine or pheromone signaling. Furthermore, Rsl affects several other transcription factor and KRAB-ZFP genes that could form regulatory cascades influencing these reproductive phenotypes [2, 15].
Earlier VO in rsl females suggests RSL participates in puberty timing; however, as with uMUP excretion, the precise mode of regulation appears to be complex. Puberty onset in mice occurs in response to multiple intrinsic and extrinsic cues and can be modified by hormones, pheromones, and nutrition [29, 33]. As discussed above, Rsl may impact pheromone signaling via MUP levels and affect endocrine signaling via enzymes of steroid metabolism. Rsl also affects nutrition since rsl mice are leaner than wt [2], although leanness is more often associated with delayed rather than precocious puberty [32]. MUP1 may also impact nutrition directly since its elevation in circulation improves glucose and lipid metabolism in diabetic mice [39]. Given the broad array of genes affected, numbering more than 800 in females [2], Rsl most likely impacts sexual maturation through multiple pathways.
As with uMUP excretion profiles, distinct liver-intrinsic functions of Rsl1 and Rsl2 on sexual maturation are revealed by overexpression in tg mice. Excess Rsl2 in liver restores puberty timing to that of wt females (i.e., 28 days; Fig. 6). That puberty is not delayed beyond 28 days suggests Rsl2 controls a discrete event rather than an effect dependent on gene dosage. The results with the Rsl2-tg mice are consistent with RSL2 both repressing Mup expression to wt levels (Fig. 4) and delaying puberty onset relative to rsl mice. It is tempting to speculate that these two effects of RSL2 are causally linked via pheromone signaling. The slight increase in age at VO of the rsl non-tg cage mates (∼27 days; Fig. 6) might reflect unaccounted-for pheromone signaling from sires and male siblings before weaning and differential Mup expression among sisters after weaning. However, Rsl1-tg mice reveal further complexity, since while Rsl1 is unable to restore puberty timing, sexual maturation of non-tg cage mates is altered, and in directions counter to expectations based on genotype. This may be due to varied environments on a cage-by-cage basis, or may hint at other factors controlled differentially by RSL1 and RSL2, perhaps including pheromone synthesis as well as presentation. It may also be that unexpected effects are due to gross overexpression of the transgenes and off-target actions. Future experiments will determine the extent to which RSL modulation of VO operates via hormonal or pheromonal control.
Mutations in liver transcriptional regulators, including Rsl, can cause subtle changes in pheromone and endocrine signaling pathways and thereby impact reproduction and breeding patterns. Such variations may have distinct advantages in different environments, leading to changes in population composition. In support of this, it is interesting that mutations in Rsl have arisen multiple times in inbred strains, perhaps having been selected due to earlier breeding of such variant mice [18]. In general, the recently expanded KRAB-ZFP family provides a wealth of genetic variation capable of modulating phenotypes via effects on diverse downstream targets. RSL was one of the first KRAB-ZFPs to have a biological role assigned, but increasingly these regulators are being linked to physiological functions. As examples, Zfp57 is involved in imprinting in mice and neonatal diabetes in man [40, 41], and Zfp568 controls polarized cell movements in embryos [42]. As more functions for individual KRAB-ZFPs are identified, it is notable that many appear to have species-specific features. By modulating aspects of reproduction, as RSL does, a global role for KRAB-ZFPs may be to enhance phenotypic diversity among populations and thus drive species evolution.
Acknowledgments
We thank the University of Michigan Transgenic Animal Model Core Facility for help in making the transgenic mice. We also thank Sarah Mendelowitz and Anna Cacciaglia for technical assistance, Lori Raetzman for advice on assessing puberty timing, Nicole Scott for suggestions on statistical analysis, and Sally Camper for comments on the manuscript.
Footnotes
Supported by NIDDK RO1-053998 (D.M.R.) and the University of Michigan Diabetes Research and Training Center (NIDDK grant 5P60DK-20572).
REFERENCES
- Yang X, Schadt EE, Wang S, Wang H, Arnold AP, Ingram-Drake L, Drake TA, Lusis AJ.Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res 2006; 16: 995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs CJ, Khan S, MacDonald JW, Sorenson M, Robins DM.Regulator of sex-limitation KRAB zinc finger proteins modulate sex-dependent and -independent liver metabolism. Physiol Genomics 2009; 38: 16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson JA, Mode A, Norstedt G, Skett P.Sex steroid induced changes in hepatic enzymes. Annu Rev Physiol 1983; 45: 51–60. [DOI] [PubMed] [Google Scholar]
- Shapiro BH, Agrawal AK, Pampori NA.Gender differences in drug metabolism regulated by growth hormone. Int J Biochem Cell Biol 1995; 27: 9–20. [DOI] [PubMed] [Google Scholar]
- Nelson DR, Zeldin DC, Hoffman SM, Maltais LJ, Wain HM, Nebert DW.Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics 2004; 14: 1–18. [DOI] [PubMed] [Google Scholar]
- Mode A, Gustafsson JA.Sex and the liver—a journey through five decades. Drug Metab Rev 2006; 38: 197–207. [DOI] [PubMed] [Google Scholar]
- Knopf JL, Gallagher JF, Held WA.Differential, multihormonal regulation of the mouse major urinary protein gene family in the liver. Mol Cell Biol 1983; 3: 2232–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins DM.Multiple mechanisms of male-specific gene expression: lessons from the mouse sex-limited protein (Slp) gene. Prog Nucleic Acid Res Mol Biol 2004; 78: 1–36. [DOI] [PubMed] [Google Scholar]
- Brown LJ, Shreffler DC.Female expression of the H-2-linked sex-limited protein (Slp) due to non-H-2 genes. Immunogenetics 1980; 10: 19–29. [DOI] [PubMed] [Google Scholar]
- Tullis KM, Krebs CJ, Leung JY, Robins DM.The regulator of sex-limitation gene, rsl, enforces male-specific liver gene expression by negative regulation. Endocrinology 2003; 144: 1854–1860. [DOI] [PubMed] [Google Scholar]
- Krebs CJ, Larkins LK, Price R, Tullis KM, Miller RD, Robins DM.Regulator of sex-limitation (Rsl) encodes a pair of KRAB zinc-finger genes that control sexually dimorphic liver gene expression. Genes Dev 2003; 17: 2664–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley S, Baggott DM, Hamilton AT, Tran-Gyamfi M, Yang S, Kim J, Gordon L, Branscomb E, Stubbs L.A comprehensive catalog of human KRAB-associated zinc finger genes: insights into the evolutionary history of a large family of transcriptional repressors. Genome Res 2006; 16: 669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrutia R.KRAB-containing zinc-finger repressor proteins. Genome Biol 2003; 4: 231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz DC, Ayyanathan K, Negorev D, Maul GG, Rauscher FJ., IIISETDB1: a novel KAP-1-associated histone H3, lysine 9-specific methyltransferase that contributes to HP1-mediated silencing of euchromatic genes by KRAB zinc-finger proteins. Genes Dev 2002; 16: 919–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Geen H, Squazzo SL, Iyengar S, Blahnik K, Rinn JL, Chang HY, Green R, Farnham PJ.Genome-wide analysis of KAP1 binding suggests autoregulation of KRAB-ZNFs. PLoS Genet 2007; 3: e89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JR, Fredericks WJ, Jensen DE, Speicher DW, Huang XP, Neilson EG, Rauscher FJ., IIIKAP-1, a novel corepressor for the highly conserved KRAB repression domain. Genes Dev 1996; 10: 2067–2078. [DOI] [PubMed] [Google Scholar]
- Ryan RF, Schultz DC, Ayyanathan K, Singh PB, Friedman JR, Fredericks WJ, Rauscher FJ., IIIKAP-1 corepressor protein interacts and colocalizes with heterochromatic and euchromatic HP1 proteins: a potential role for Kruppel-associated box-zinc finger proteins in heterochromatin-mediated gene silencing. Mol Cell Biol 1999; 19: 4366–4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs CJ, Larkins LK, Khan SM, Robins DM.Expansion and diversification of KRAB zinc-finger genes within a cluster including Regulator of sex-limitation 1 and 2. Genomics 2005; 85: 752–761. [DOI] [PubMed] [Google Scholar]
- You L.Steroid hormone biotransformation and xenobiotic induction of hepatic steroid metabolizing enzymes. Chem Biol Interact 2004; 147: 233–246. [DOI] [PubMed] [Google Scholar]
- Hurst JL, Robertson DHL, Tolladay U, Beynon RJ.Proteins in urine scent marks of male house mice extend the longevity of olfactory signals. Anim Behav 1998; 55: 1289–1297. [DOI] [PubMed] [Google Scholar]
- Jiang PP, Frederick K, Hansen TH, Miller RD.Localization of the mouse gene releasing sex-limited expression of Slp. Proc Natl Acad Sci U S A 1996; 93: 913–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs CJ, Khan SM, Mollard B, Robins DM.Adapter annealing to engineer restriction enzyme sites at cloning junctions. Anal Biochem 2006; 350: 313–315. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Kinsella DM.Male preference for the odors of estrous female mice is reduced by the neurosteroid pregnenolone sulfate. Brain Res 1995; 682: 222–226. [DOI] [PubMed] [Google Scholar]
- Rugh R. The Mouse: Its Reproduction and Development. New York:: Oxford University Press;; 1994. [Google Scholar]
- Chomczynski P, Sacchi N.Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987; 162: 156–159. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW.A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29: e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham SA, Smith AL, Armstrong SD, Beynon RJ, Hurst JL.Limited variation in the major urinary proteins of laboratory mice. Physiol Behav 2009; 96: 253–261. [DOI] [PubMed] [Google Scholar]
- Polanco JC, Wilhelm D, Mizusaki H, Jackson A, Browne C, Davidson T, Harley V, Sinclair A, Koopman P.Functional analysis of the SRY-KRAB interaction in mouse sex determination. Biol Cell 2009; 101: 55–67. [DOI] [PubMed] [Google Scholar]
- Novotny MV, Ma W, Wiesler D, Zidek L.Positive identification of the puberty-accelerating pheromone of the house mouse: the volatile ligands associating with the major urinary protein. Proc Biol Sci 1999; 266: 2017–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JF, Karelus K, Felicio LS, Johnson TE.Genetic influences on the timing of puberty in mice. Biol Reprod 1990; 42: 649–655. [DOI] [PubMed] [Google Scholar]
- Andreolini F, Jemiolo B, Novotny M.Dynamics of excretion of urinary chemosignals in the house mouse (Mus musculus) during the natural estrous cycle. Experientia 1987; 43: 998–1002. [DOI] [PubMed] [Google Scholar]
- Cunningham MJ, Clifton DK, Steiner RA.Leptin's actions on the reproductive axis: perspectives and mechanisms. Biol Reprod 1999; 60: 216–222. [DOI] [PubMed] [Google Scholar]
- Ebling FJ.The neuroendocrine timing of puberty. Reproduction 2005; 129: 675–683. [DOI] [PubMed] [Google Scholar]
- Ingersoll DW, Weinhold LL.Modulation of male mouse sniff, attack, and mount behaviors by estrous cycle-dependent urinary cues. Behav Neural Biol 1987; 48: 24–42. [DOI] [PubMed] [Google Scholar]
- Achiraman S, Archunan G.1-Iodo-2methylundecane, a putative estrus-specific urinary chemo-signal of female mouse (Mus musculus). Theriogenology 2006; 66: 1913–1920. [DOI] [PubMed] [Google Scholar]
- Singer AG, Clancy AN, Macrides F, Agosta WC, Bronson FH.Chemical properties of a female mouse pheromone that stimulates gonadotropin secretion in males. Biol Reprod 1988; 38: 193–199. [DOI] [PubMed] [Google Scholar]
- Whitten WK, Bronson FH, Greenstein JA.Estrus-inducing pheromone of male mice: transport by movement of air. Science 1968; 161: 584–585. [DOI] [PubMed] [Google Scholar]
- Sharrow SD, Vaughn JL, Zidek L, Novotny MV, Stone MJ.Pheromone binding by polymorphic mouse major urinary proteins. Protein Sci 2002; 11: 2247–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Jiang L, Rui L.Identification of MUP1 as a regulator for glucose and lipid metabolism in mice. J Biol Chem 2009; 284: 11152–11159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ito M, Zhou F, Youngson N, Zuo X, Leder P, Ferguson-Smith AC.A maternal-zygotic effect gene, Zfp57, maintains both maternal and paternal imprints. Dev Cell 2008; 15: 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay DJ, Callaway JL, Marks SM, White HE, Acerini CL, Boonen SE, Dayanikli P, Firth HV, Goodship JA, Haemers AP, Hahnemann JM, Kordonouri O, et al. Hypomethylation of multiple imprinted loci in individuals with transient neonatal diabetes is associated with mutations in ZFP57. Nat Genet 2008; 40: 949–951. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia MJ, Shibata M, Anderson KV.Chato, a KRAB zinc-finger protein, regulates convergent extension in the mouse embryo. Development 2008; 135: 3053–3062. [DOI] [PMC free article] [PubMed] [Google Scholar]