Abstract
Normal endometrial function requires a balance of progesterone (P4) and estrogen (E2) effects. E2 acts to stimulate the proliferation of uterine epithelial cells, while P4 action inhibits E2-mediated proliferation of the epithelium. P4 through its cognate receptor, the P4 receptor (Pgr), has important roles in the establishment and maintenance of pregnancy. In previous studies, we have identified ERBB receptor feedback inhibitor 1 (Errfi1) as a downstream target of Pgr action in the uterus. Herein, we show that Errfi1 mRNA expression was significantly increased in the uterus after Day 2.5 of gestation. Its expression is also induced in the uterus by acute E2 treatment, and this induction is synergistically induced by chronic E2 and P4 treatment. Although it is known that conditional ablation of Errfi1 in the Pgr-positive cells (Errfi1d/d) results in infertility, the function of Errfi1 in reproductive biology has remained elusive. Using Errfi1d/d mice, we have identified Errfi1 as an important mediator of uterine implantation. Epithelial ESR1 and target genes were significantly increased in the uteri of Errfi1d/d mice. Our results identify a new signaling paradigm of steroid hormone regulation in female reproductive biology that adds insight into the underlying dysregulation of hormonal signaling in human reproductive disorders such as endometriosis and endometrial cancer.
Keywords: Errfi1, estradiol receptor, estrogen, estrogen receptor, female reproductive tract, implantation, steroid hormones, uterus
Mice with conditional ablation of Errfi1 in the uterus, reveal that Errfi1 is important for embryo implantation. Epithelial estrogen receptors and target genes were significantly increased in the uterus of Errfi1d/d mice.
INTRODUCTION
The uterus consists of heterogeneous cell types that undergo dynamic changes to support embryo development and implantation. These phenomena are primarily dependent on coordinated interactions mediated by the ovarian steroid hormones progesterone (P4) and estrogen (E2). E2 stimulates proliferation of both the uterine epithelial and stromal cells in neonatal mice. However, this proliferative action of E2 is restricted to epithelial cells in the adult mouse uterus [1, 2]. In contrast, while P4 is inhibitory to E2-mediated proliferation of the luminal and glandular epithelial cells, P4 (alone or in conjunction with E2) leads to uterine stromal cell proliferation [2–4].
P4 is an essential regulator in the uterus during pregnancy [5, 6]. The physiological effects of P4 are mediated through the P4 receptor (Pgr), which exists as two isoforms, PR-A and PR-B, arising from alternate transcriptional start sites on the same gene [7, 8]. Pgr is a transcription factor that belongs to the nuclear receptor superfamily [9]. The fertility defects exhibited by the Pgr knockout (PgrKO) mice unequivocally demonstrated the critical importance of P4 and its receptor in the establishment and maintenance of pregnancy [6, 10].
Although the effect of the P4-Pgr axis on murine uterine function has been extensively investigated, there are several P4-Pgr-regulated genes that have been identified. These include genes encoding amphiregulin (Areg) [11], histidine decarboxylase (Hdc) [12], homeobox A10 (Hoxa10) and homeobox A11 (Hoxa11) [13], calcitonin (Calca) [14, 15], calbindin-D9K (S100g) [16], Indian hedgehog (Ihh) [17], hypoxia-inducible factor 1 (Hif1a) [18], and immune-responsive gene 1 (Irg1) [19]. These target genes have been identified by testing candidate genes [11], by differential library screening [14], and by DNA microarray approaches [17, 19]. The advent of the latter high-density DNA microarray technology has immensely improved the ability to identify Pgr-regulated genes in the uterus.
We recently used oligonucleotide microarrays to identify the genes whose expression is regulated by the P4-Pgr axis in the mouse uterus [20, 21]. Using this method, we identified several genes that were regulated by Pgr in the uterus in response to P4 [21]. Notably, we found that the expression of ERBB receptor feedback inhibitor 1 (Errfi1, Mig-6, RALT, or gene 33) was induced significantly by P4 in a Pgr-dependent manner [20, 21]. Errfi1 is an immediate early response gene that can be induced by various mitogens and commonly occurring chronic stress stimuli [22–25]. It has also been shown to act as a negative feedback inhibitor of epidermal growth factor receptor (EGFR) signaling through direct interaction with the EGFR family [26–29]. Errfi1 is an adaptor molecule containing a Cdc42/Rac interactive binding (CRIB) [22] domain, Src homology 3 (SH3) binding domain, and 14-3-3 binding domain [22, 30, 31]. Sustained Errfi1 expression is thought to trigger cells to initiate hypertrophy in chronic pathological conditions such as diabetes and hypertension through stress-activated protein kinase/c-Jun NH2-terminal kinase activation by binding between its CRIB domain and CDC42 [22, 32]. Ablation of Errfi1 in mice leads to the development of animals with epithelial hyperplasia, adenoma, and adenocarcinomas in organs such as the lung, gallbladder, and bile duct [33–35]. ERRFI1 is located on human chromosome 1p36, a locus frequently associated with human cancer [36] and endometriosis. Down-regulated expression of the ERRFI1 gene is observed in human breast carcinomas, which correlates with reduced overall survival of patients with breast cancer [33, 37]. The ERRFI1 gene is mutated in human non-small cell lung cancer cell lines NCI-H226 and NCI-H 322M, as well as in one primary human lung cancer [35]. Recently, altered ERRFI1 expression has been observed in endometrial RNA isolated from women with endometriosis and endometrial cancer [38, 39]. These data point to ERRFI1 as a tumor suppressor gene in both mice and humans.
Absence of Errfi1 in mice results in the inability of P4 to inhibit E2-induced uterine weight gain and expression of E2-responsive target genes [20]. Errfi1d/d mice develop hyperplasia and endometrial cancer dependent on E2 and P4. In addition, the observation that endometrial carcinomas from women have a significant reduction in ERRFI1 expression provides compelling support for an important growth regulatory role for ERRFI1 in the uterus of both humans and mice [20]. This demonstrates that ERRFI1 is a critical regulator in the tumorigenesis of endometrial cancer. However, the mechanism of ERRFI1 action in the uterus is uncertain.
Herein, we have used conditional Errfi1 knockout mice to understand the role of Errfi1 in uterus. From this study, we have identified Errfi1 as a critical mediator of embryo implantation. We observed increased E2 signaling in the Errfi1d/d uterus that resulted in an implantation defect. Thus, we demonstrate a critical role for Errfi1 in the preparation of the uterus for the incoming embryo as a mediator of P4 signaling via inhibition of E2 signaling.
MATERIALS AND METHODS
Animals and Tissue Collection
Mice were maintained in the designated animal care facility at Baylor College of Medicine according to the institutional guideline for the care and use of laboratory animals. For the study of steroid hormone regulation, wild-type C57BL/6 mice at age 6 wk were ovariectomized. Two weeks later, ovariectomized wild-type mice were injected with one of the following: vehicle (sesame oil), P4 (1 mg/mouse), E2 (0.1 μg/mouse), or P4 plus E2. Three mice from each group were injected with one of the treatments, and uteri were collected at 4 h, 16 h, or 40 h. The injections were repeated every 12 h for the 16-h and 40-h samples. The mice were anesthetized with Avertin (2,2,-tibromoethyl alcohol; Sigma-Aldrich, St. Louis, MO) and euthanized by cervical dislocation under anesthetic at 4 h, 16 h, or 40 h (4 h after the fourth injection) to collect the uteri. To get uterine samples from early pregnancy mice, wild-type C57BL/6 mice at age 8 wk were mated with wild-type male mice, and different days of pregnant uterine samples were obtained; the morning of vaginal plug was designated as Day 0.5. Uterine tissues were flash frozen at the time of dissection and stored at −80°C for RNA extraction or fixed with 10% (vol/vol) formalin for in situ hybridization. In the implantation study, 8-wk-old female Errfi1d/d and Errfi1f/f mice were mated with intact wild-type male mice, and 0.1 ml of 1% Chicago Sky Blue 6B (Sigma-Aldrich) was intravenously injected to mice at 5.5 days postcoitus (dpc) of pregnancy before dissection. The number of implantation sites was identified.
Quantitative Real-Time PCR
RNA was extracted from the uterine tissues using the RNeasy total RNA isolation kit (Qiagen, Valencia, CA). Expression levels of Errfi1, E2 receptor α (Esr1), its target genes, and Pgr mRNA were measured by real-time RT-PCR TaqMan analysis using the ABI Prism 7700 Sequence Detector System according to the manufacturer's instructions (Applied Biosystems, Foster City, CA). Prevalidated probes and primers for Errfi1 (Mm00505292_m1), Esr1 (Mm00433149_m1), C3 (Mm01232779_m1), Ltf (Mm00434787_m1), Muc1 (Mm00449604_m1), Clca3 (Mm00489959_m1), Pgr (Mm00435628_m1), and 18S rRNA (4319413E) were purchased from Applied Biosystems. The cDNA was produced from 1 μg of total RNA using random hexamers and MMLV RT (Invitrogen Corp., Carlsbad, CA). The RT-PCR was performed using RT-PCR Universal Master Mix reagent (Applied Biosystems) according to the manufacturer's instructions. All real-time PCR was performed using six independent RNA sets. The mRNA quantities were normalized against 18S rRNA using ABI rRNA control reagents. Statistical analyses were performed using one-way ANOVA analysis, followed by Tukey post hoc multiple range test or Student t-test using the Instat package from GraphPad (San Diego, CA).
In Situ Hybridization
The spatial expression of Errfi1 mRNA in uterine tissue sections was determined by in situ hybridization as described previously by Simmons et al. [40]. Briefly, uterine tissues were fixed in 10% (vol/vol) formalin. After overnight fixation at room temperature, tissues were dehydrated through a series of ethanol and then processed for paraffin embedding. Paraffin sections were mounted onto poly-l-lysine-coated slides (VWR Scientific Products, West Chester, PA) and then used for in situ hybridization. The Errfi1 templates for antisense and sense cRNA probes were amplified using PCR primer containing the T7 polymerase promoter sequence flanking the antisense and sense nucleotide primer sequence, respectively. The riboprobes were generated by in vitro transcription (Promega, Madison, WI) of 0.1 μg of amplified DNA products using [35S] uridine triphosphate (Amersham Biosciences, Piscataway, NJ). Slides were incubated for 7 min at room temperature in proteinase K (20 μg/ml) in a buffer containing 50 mM Tris and 5 mM edetic acid (EDTA) (pH 8.0). Slides were then acetylated with acetic anhydride, dehydrated, and exposed to either denatured antisense or sense probes (5 × 106 cpm/slide) in hybridization buffer (50% [vol/vol] formamide, 10% [wt/vol] dextran sulfate, 5× Denhardt solution, 300 mM NaCl, 5 mM EDTA [pH 8], 20 mM Tris [pH 8.0], and 0.05 mg/ml of yeast tRNA). Hybridization was performed at 55°C overnight in a humidity chamber containing 5× saline and sodium citrate (SSC) and 50% (vol/vol) formamide. Coverslips were floated off the slides by placement in 5× SSC and 10 mM β-mercaptoethanol (βME) for 30 min at 55°C, and remaining coverslips were gently removed with a pair of forceps. Sections were then washed as follows: 50% formamide, double-strength SSC, and 50 mM βME for 20 min at 65°C; single-strength TEN (0.05 M NaCl, 10 mM Tris [pH 8], and 5 M EDTA) for 10 min at room temperature; and then three times in single-strength TEN for 10 min at 37°C. Hybridized slides were exposed to 20 μg/ml of RNase A for 30 min at 37°C. Slides were washed in 50% (vol/vol) formamide, 2× SSC, and 100 mM 2-mercaptoethanol, followed by 2× SSC at 55°C for 30 min; dehydrated in a graded series of ethanol in 0.3 M ammonium acetate; and exposed to Biomax MR film overnight (Kodak, Rochester, NY). The following morning, slides were dipped in autoradiography emulsion (GE Healthcare, Piscataway, NJ) and placed at 4°C in a lightproof box for several days. Following development, slides were counterstained with hematoxylin.
Immunohistochemistry
Uterine sections from paraffin-embedded tissue were cut at 5 μm and mounted on silane-coated slides, deparaffinized, and rehydrated in a graded alcohol series. Sections were preincubated with 10% normal serum in PBS (pH 7.5) and then incubated with primary antibody diluted in 10% normal serum in PBS (pH 7.5) overnight at 4°C at the following dilutions: 1:500 for PGR (rabbit polyclonal anti-human PGR; DAKO Corp., Carpinteria, CA), 1:500 for ESR1 (mouse monoclonal anti-human ESR1; DAKO Corp.), 1:500 for phospho-ESR1 (rabbit monoclonal anti-human phospho-ESR1; Abcam, Cambridge, MA), 1:800 for MUC1 (rabbit polyclonal anti-human MUC1; Abcam), and 1:800 for LTF (rabbit polyclonal anti-human LTF; Millipore, Bedford, MA). Rabbit IgG isotype control was used for a negative control. On the following day, sections were washed in PBS and incubated with secondary antibody diluted 1:200 (biotinylated anti-rabbit IgG anti-rabbit IgG for anti-PR, anti-phospho-ESR1, MUC1, and LTF and biotinylated anti-mouse IgG for anti-ESR1; Vector Laboratories, Burlingame, CA) for 1 h at room temperature and subsequently washed three times with PBS. Horseradish peroxidase-conjugated streptavidin (Vector Laboratories) at a dilution of 1:1000 was added to the slides and incubated for 30 min. Immunoreactivity was detected using the Vectastain Elite ABC kit (Vector Laboratories). After this, the nuclei were stained with hematoxylin for 30 sec. The slides were subsequently washed in water and increasing gradients of ethanol, then placed in xylene before mounting in a xylene-based mounting solution, and observed under a microscope.
RESULTS
Expression of Errfi1 During Early Pregnancy
We examined the expression profile of Errfi1 mRNA in mouse uteri during the preimplantation, periimplantation, and postimplantation periods by performing real-time RT-PCR to measure levels of Errfi1. The initiation of pregnancy was marked by the presence of the postcoital vaginal plug (0.5 dpc). As shown in Figure 1A, the expression of Errfi1 was detected on 0.5 dpc, which gradually increased until 5.5 dpc, reaching statistical significance after 2.5 dpc in the uterus. Next, the spatial expression profile of Errfi1 mRNA was assayed during early pregnancy using in situ hybridization. The uterine sections were cut from the tissues, fixed, and embedded as described in the In Situ Hybridization subsection of Materials and Methods. In situ hybridization analysis of uterine cross sections revealed increased expression of Errfi1 at 2.5 dpc and 4.5 dpc compared with 0.5 dpc in the stroma, luminal epithelium, and glandular epithelium. The expression of Errfi1 mRNA was also evident in the decidua on 6.5 dpc.
FIG. 1.
The expression of Errfi1 by real-time RT-PCR and in situ hybridization in pseudopregnancy. A) The expression level of Errfi1 was measured in uteri of pseudopregnancy. Total RNA used for the RT-PCR assays was prepared from pseudopregnant uteri. The results represent the mean ± SEM of three independent RNA sets. **P < 0.01 and ***P < 0.001. B) The localization pattern of Errfi1 mRNA by in situ hybridization during early pregnancy. Nuclei are counterstained with hematoxylin. E, embryonic day; EM, embryo; LE, luminal epithelium; GE, glandular epithelium; PDZ, primary decidual zone; SDZ, secondary decidual zone; ST, stroma cells; MYO, myometrium; bar = 500 μm. Arrowheads indicate luminal and glandular epithelium.
Steroid Hormone Regulation of Errfi1
We previously identified Errfi1 as a P4- and Pgr-regulated gene in the murine uterus using DNA microarray analysis [21, 41]. In this study, the effect of E2 and E2 plus P4 on Errfi1 expression was examined. Ovariectomized wild-type female mice were daily injected with one of the following: vehicle (sesame oil), P4 (1 mg/mouse), E2 (0.1 μg/mouse), or P4 plus E2 (1 mg of P4 and 0.1 μg of E2 per mouse). Mice were euthanized at 4 h, 16 h, and 40 h after the initial hormone treatment (n = 5 mice per treatment) and subjected to real-time PCR to characterize the effect of steroid hormone treatment on the relative expression of Errfi1. As shown in Figure 2, P4 induced Errfi1 mRNA expression at 4 h but not 16 h and 40 h after treatment, as in our previous microarray results [20, 21]. The expression of Errfi1 mRNA was significantly increased at 4 h, 16 h, and 40 h after E2 treatment. The results suggest that E2 induces the expression of Errfi1 in the uterus. Figure 2 also shows that treatment of mice with E2 plus P4 significantly increased the induction of Errfi1 at 16 h and 40 h. These results indicate that the expression of Errfi1 was induced by acute E2 or P4 treatment and that Errfi1 was synergistically induced by chronic E2 plus P4 treatment in the uterus.
FIG. 2.
The expression pattern of Errfi1 by E2 and P4 in the uterus. Total RNA used for the RT-PCR assays was prepared from wild-type mice that were treated with P4, E2, E2 plus P4, or vehicle (sesame oil [Veh]) for 4 h, 16 h, and 40 h. The results represent the mean ± SEM of three independent RNA sets. *P < 0.05 and **P < 0.01.
Effect of Errfi1 Ablation on Implantation
Because Errfi1 ablation resulted in numerous pathologies and decreased longevity [34, 42–44], we generated conditional Errfi1 ablation (PRcre/+Errfi1f/f and Errfi1d/d) in the reproductive tract by breeding Errfi1f/f mice and PRCre (official allele symbol Pgrtm2(cre)Lyd) mice to effectively investigate the role of Errfi1 in the regulation of uterine function [20, 45, 46]. Female Errfi1d/d mice were infertile [22]. This defect was not due to an ovarian phenotype, as these mice exhibited normal ovarian morphology, steroidogenesis, and function [20]. Uterine function was first assessed by examining the ability of Errfi1d/d mice to undergo a decidual response. Female Errfi1d/d mice exhibited a normal decidual response. Thus, our previous results suggest that the fertility defect of the Errfi1d/d mice occurred either before implantation or after decidualization.
To dissect the cause of infertility, 8-wk-old female Errfi1d/d and Errfi1f/f control mice were placed with intact wild-type male mice. Mice were euthanized at 5.5 dpc of pregnancy, and the number of implantation sites was identified. We dissected mice on the morning of 5.5 dpc and counted the number of implantation sites by injecting Chicago Sky Blue dye. Implantation sites were not detected in the mutant uterine horns (n = 7), while normal implantation sites (mean ± SEM, 7.25 ± 0.48) were scored in the controls (n = 4) (Fig. 3). This result demonstrates that the fertility defect of the Errfi1d/d mice occurs in the preimplantation period.
FIG. 3.
Implantation defect in Errfi1d/d mice. Mice were euthanized at 5.5 dpc of pregnancy, and the number of implantation sites was counted by injecting Chicago Sky Blue dye. Implantation sites were not detected in the mutant uterine horns (n = 7), while normal implantation sites (mean ± SEM, 7.25 ± 0.48) were scored in the controls (n = 4). Bar = 1 cm.
E2 Receptor Activity Is Enhanced in the Uteri of Errfi1d/d Mice
One of the major roles of P4 is to down-regulate Esr1 activity in the uterine luminal epithelium, which consequently opens the uterine receptivity window [47, 48]. Because Errfi1d/d mice have an implantation defect, we investigated whether Errfi1 is a mediator of P4′s suppression of ESR1 activity during the preimplantation period. To address this, the expression level of E2-responsive genes, chloride channel calcium-activated 3 (Clca3), mucin 1 transmembrane (Muc1), complement component 3 (C3), and lactotransferrin (Ltf) was examined by quantitative real-time RT-PCR analysis. The expression of Clca3, Muc-1, C3, and Ltf was significantly increased in the Errfi1d/d mice compared with the Errfi1f/f mice (Fig. 4A). Immunohistological staining detected high LTF and MUC1 protein expression in the epithelium of the Errfi1d/d mice compared with the Errfi1f/f mice (Fig. 4B). These results demonstrate that E2 activity is indeed enhanced in the uterine epithelial compartment of the Errfi1d/d mice.
FIG. 4.
An increase in expression of Esr1-regulated genes in Errfi1d/d mice. A) Real-time RT-PCR analysis of C3, Ltf, Muc1, and Clca3 was performed on uteri of Errfi1f/f and Errfi1d/d mice at 3.5 dpc. The results represent the mean ± SEM of six independent mouse sets. *P < 0.05 and ***P < 0.001. B) Immunohistochemical analysis of LTF (a and b) and MUC1 (c and d) in uteri of Errfi1f/f (a and c) and Errfi1d/d (b and d) mice at 3.5 dpc. Bar = 200 μm.
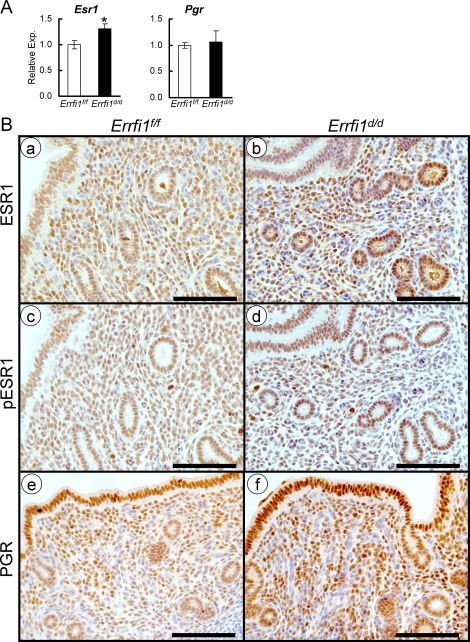
In an attempt to further dissect the molecular mechanism of enhanced E2 activity in the mutant uterine epithelium, we examined whether uterine Esr1 and Pgr levels are altered in the Errfi1d/d mice. The expression of Esr1 mRNA was significantly increased in the whole-uterine tissues of Errfi1d/d mice by real-time RT-PCR (Fig. 5A). Because the uterus consists of heterogeneous cell types that undergo dynamic changes to support embryo development and implantation, the spatial expression of ESR1 was examined by immunohistological staining using an ESR1-specific antibody. The results showed increased expression of ESR1 in the epithelial compartment of Errfi1d/d mice (Fig. 5B). To further determine whether these receptors were activated or not, we examined the Ser-122 phosphorylation status of ESR1 using antiphosphorylated ESR1 antibody and observed increased phosphorylation of ESR1 in the uterine epithelium of Errfi1d/d mice (Fig. 5B).
FIG. 5.
An increase in E2 receptor activity in the uterine of Errfi1d/d mice. A) Real-time RT-PCR analysis of Esr1 and Pgr was performed on uteri of Errfi1f/f and Errfi1d/d mice at 3.5 dpc. The results represent the mean ± SEM of six independent mouse sets. *P < 0.05. B) Immunohistochemical analysis of ESR1 (a and b), phospho-ESR1 (c and d), and PGR (e and f) in uteri of Errfi1f/f and Errfi1d/d mice at 3.5 dpc. Bar = 200 μm.
PGR in the stroma has been implicated as a critical modulator of Esr1 activity in the epithelium [6, 7]. Because the activity of Esr1 is enhanced in the Errfi1d/d mice, we wanted to determine if ablation of Errfi1 altered the expression of stromal PGR. To address this possibility, we used quantitative PCR and PGR-specific immunostaining to assess the expression of PGR in the uterus of Errfi1d/d mice. The results show that the expression level of Pgr mRNA was not changed in the whole uterus and that the level of PGR protein was not reduced in any compartment of the Errfi1d/d mice (Fig. 5, A and B). These results suggest that Errfi1 mediates epithelial E2 activity by regulating ESR1 levels and activation.
DISCUSSION
P4, acting through its cognate nuclear receptors, is critical for normal uterine functions associated with the establishment and maintenance of pregnancy [7, 49, 50]. Studies [6, 10] of the mouse model utilizing a null mutation of the Pgr gene (PgrKO) demonstrated the essential roles that these receptors have in P4-mediated response. Thus, the identification of P4-regulated pathways in the uterus is crucial for understanding the impairments that underlie the complex phenotype of the PgrKO mice. In previous studies [20, 21], we have identified Errfi1 as a Pgr-regulated gene. Herein, we show that the expression of Errfi1 was increased from 0.5 dpc to 5.5 dpc, reaching statistical significance after 2.5 dpc (Fig. 1), which correlates with elevated P4 levels also seen on 2.5 dpc. Together, these results validate our previous findings that Errfi1 is a Pgr target, as its expression correlates with both an increase in serum P4 levels and Pgr expression [20, 21]. We also showed that acute E2 treatment up-regulated Errfi1 expression (Fig. 2). Notably, we observed synergistic induction of Errfi1 mRNA expression upon chronic E2 and P4 treatment in the uterus. Recently, DNA microarray analysis has been used to compare the global expression pattern of endometrial genes in the eutopic endometrium of women with and without endometriosis [38]. Comparative gene expression analysis of P4-regulated genes in secretory-phase endometrium confirmed the observation of attenuated P4 response in endometriosis. This microarray analysis showed that Errfi1 is induced in the secretory phase when P4 is high and that the expression of Errfi1 is reduced in the endometrium of women with endometriosis [38]. These results suggest that Errfi1 is also a P4-regulated gene expressed in the human endometrium and that its expression is altered in endometriosis.
Because the PRCre (Pgrtm2(cre)Lyd) mouse shows Cre recombinase activity in the pituitary, ovary, uterus, and mammary gland, the cause of infertility in these mice may be due to a defect in any of these tissues [45]. However, Errfi1d/d mice did not show any alterations in ovarian morphology, ovulation, or fertilization and exhibited a normal estrous cycle and normal levels of serum P4 and E2 [20]. These results show that ovarian morphology, steroidogenesis, and function were unaffected in the Errfi1d/d females. These data suggest that the defects observed in the Errfi1d/d mice are inherent to the uterus. However, Errfi1d/d mice did not show any decidualization defect [20] but have an implantation defect. Implantation requires interactions between the embryo and the uterus. The PRCre (Pgrtm2(cre)Lyd) mouse does not show Cre recombinase activity in the embryo. However, to exclude any possible role for the lack of Errfi1 in the embryo, we transferred wild-type embryos to the uteri of Errfi1f/f and Errfi1d/d mice. The embryo-transferred Errfi1d/d mice also showed implantation defects compared with Errfi1f/f mice (data not shown). These data suggest that the implantation defect in the Errfi1d/d mice is due to Errfi1 ablation in the uterus and not in the embryo.
PgrKO mice [6] and conditional null mutant mice of Ihh [51] and Nr2f2 (COUP-TFII) [52] are infertile. Ihh and Nr2f2 are essential mediators of Pgr action in the uterus. Conditional Ihh and Nr2f2 knockout mice are infertile because of implantation failure [51, 52]. The ablation of Nr2f2 enhanced epithelial E2 activity, which impedes the maturation of receptive uterus [52]. Our finding that Errfi1 suppresses Esr1 action is intriguing. Esr1 has been shown to regulate the expression of many glycoproteins during the periimplantation period [53, 54]. Muc1 is an E2 target encoding an epithelial glycoprotein, and its expression is decreased at the time of implantation to facilitate epithelial remodeling [53, 55]. Persistent expression of Muc1 during the periimplantation period prevents uterine receptivity and embryo attachment [55]. Real-time PCR showed high expression levels of Muc1 in Errfi1d/d mice (Fig. 4A). In addition, immunohistochemistry detected high expression levels of MUC1 in the apical surface of mutant luminal epithelia (Fig. 4B). Clca3 is a gene important for the overproduction of mucus protein [56] and a known E2 target gene in the uterine epithelium [57]. Clca3 is also shown to be highly up-regulated in Errfi1d/d mice (Fig. 4). Using tissue recombination technique, Buchanan et al. [58] showed that epithelial Ltf expression is not only regulated by epithelial ESR1 but also regulated by stromal ESR1. Ltf was also elevated in the Errfi1d/d mice (Fig. 4A), indicating that E2 activity is up-regulated in the uterine luminal epithelium of Errfi1d/d mice. Errfi1 was highly expressed in both stroma and epithelium. This raises the possibility that both stromal Errfi1 and epithelial Errfi1 are required to suppress epithelial Esr1 function. Therefore, up-regulation of Esr1 target genes suggests that Errfi1 is essential for the Pgr-mediated suppression of Esr1 activity in the epithelium, which is necessary for induction of the window of receptivity. These results suggest that abnormally increased E2 activity might be the underlying cause of the uterine receptivity defect displayed in Errfi1d/d mice.
Tissue recombinants composed of uterine tissues from PgrKO and wild-type mice were also used to demonstrate an important role for stromal Pgr in the inhibition of uterine epithelial proliferation [59]. Decreased expression of stromal Pgr is observed in Errfi1d/d mice treated with E2 plus P4 for 3 days [20]. However, we observed no decrease in Pgr expression at 3.5 dpc in Errfi1d/d mice. The administration of E2 plus P4 treatment for 3 days mimics a later stage in pregnancy seen by increased stromal Pgr, whereas 3.5 dpc is during early pregnancy, which sees increased epithelial Pgr expression; therefore, the two conditions may not correlate. Although a detailed regulatory mechanism of this interdependence has yet to be defined, our results suggest a regulatory loop in which Errfi1 acts as a mediator of Pgr function during periimplantation. Errfi1 function then acts to repress E2 target genes, which may provide feedback and affect Pgr expression in the stroma.
We also showed that the expression of ESR1 and Ser-122 phosphorylated ESR1 is increased in the Errfi1d/d mice (Fig. 5). Enhanced expression of these molecules likely contributes to the observed increase in Esr1 activity and the subsequent activation of the downstream Esr1 targets. Increased phosphorylation of ESR1 appears proportional to the increase in expression levels seen in ESR1. The phosphorylation of ESR1 has been shown to couple with growth factor signaling and may be controlled through a paracrine mechanism and not likely through autophosphorylation; therefore, this finding supports the notion that the observed phenomena seen in the Errfi1d/d mice uterus are by dysregulation in epithelial-stromal communication. It is more likely that stromal Errfi1 regulates Pgr to control an unknown paracrine signal, which acts through its epithelial receptor to suppress epithelial Esr1 activity, as well as Esr1 expression. Therefore, P4 suppression of epithelial E2 activity is initiated from the stromal compartment via Errfi1 through a complex epithelial-stromal cross communication pathway. However, the compartment-specific functions of Errfi1 and epithelial-stromal cross talk need to be addressed using tissue recombination techniques or compartment-specific ablation of Errfi1 in the uterus.
In conclusion, Errfi1 suppresses Esr1 activity in the uterine epithelium, an event that is required for successful embryo implantation. Based on our previous observation that Errfi1 is a mediator of the Pgr, these data suggest that Errfi1 mediates P4′s suppression of E2 signaling during embryo implantation. Because increased E2 signaling and/or decreased P4 signaling is a hallmark of uterine diseases, including infertility, endometriosis, and endometrial cancer, understanding the mechanism by which Errfi1 regulates the balance between these steroid hormone signaling pathways may provide valuable insight into these diseases.
Acknowledgments
We thank Jinghua Li for technical assistance and Cory A. Rubel, MS, and Michael J. Large for manuscript preparation.
Footnotes
Supported by National Institutes of Health (NIH) grant R01HD057873 (to J.W.J.) and NIH grant R01CA77530 (to J.P.L.).
REFERENCES
- Martin L, Finn CA, Trinder G.Hypertrophy and hyperplasia in the mouse uterus after oestrogen treatment: an autoradiographic study. J Endocrinol 1973; 56: 133–144. [DOI] [PubMed] [Google Scholar]
- Huet-Hudson YM, Andrews GK, Dey SK.Cell type-specific localization of c-myc protein in the mouse uterus: modulation by steroid hormones and analysis of the periimplantation period. Endocrinology 1989; 125: 1683–1690. [DOI] [PubMed] [Google Scholar]
- Martin L, Das RM, Finn CA.The inhibition by progesterone of uterine epithelial proliferation in the mouse. J Endocrinol 1973; 57: 549–554. [DOI] [PubMed] [Google Scholar]
- Paria BC, Huet-Hudson YM, Dey SK.Blastocyst's state of activity determines the “window” of implantation in the receptive mouse uterus. Proc Natl Acad Sci U S A 1993; 90: 10159–10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke CL, Sutherland RL.Progestin regulation of cellular proliferation. Endocr Rev 1990; 11: 266–301. [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O'Malley BW.Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 1995; 9: 2266–2278. [DOI] [PubMed] [Google Scholar]
- Conneely OM, Mulac-Jericevic B, DeMayo F, Lydon JP, O'Malley BW.Reproductive functions of progesterone receptors. Recent Prog Horm Res 2002; 57: 339–355. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM.Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science 2000; 289: 1751–1754. [DOI] [PubMed] [Google Scholar]
- Tsai MJ, O'Malley BW.Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 1994; 63: 451–486. [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Conneely OM, O'Malley BW.Reproductive phenotypes of the progesterone receptor null mutant mouse. J Steroid Biochem Mol Biol 1996; 56: 67–77. [DOI] [PubMed] [Google Scholar]
- Das SK, Chakraborty I, Paria BC, Wang XN, Plowman G, Dey SK.Amphiregulin is an implantation-specific and progesterone-regulated gene in the mouse uterus. Mol Endocrinol 1995; 9: 691–705. [DOI] [PubMed] [Google Scholar]
- Paria BC, Das N, Das SK, Zhao X, Dileepan KN, Dey SK.Histidine decarboxylase gene in the mouse uterus is regulated by progesterone and correlates with uterine differentiation for blastocyst implantation. Endocrinology 1998; 139: 3958–3966. [DOI] [PubMed] [Google Scholar]
- Lim H, Ma L, Ma WG, Maas RL, Dey SK.Hoxa-10 regulates uterine stromal cell responsiveness to progesterone during implantation and decidualization in the mouse. Mol Endocrinol 1999; 13: 1005–1017. [DOI] [PubMed] [Google Scholar]
- Zhu LJ, Cullinan-Bove K, Polihronis M, Bagchi MK, Bagchi IC.Calcitonin is a progesterone-regulated marker that forecasts the receptive state of endometrium during implantation. Endocrinology 1998; 139: 3923–3934. [DOI] [PubMed] [Google Scholar]
- Kumar S, Zhu LJ, Polihronis M, Cameron ST, Baird DT, Schatz F, Dua A, Ying YK, Bagchi MK, Bagchi IC.Progesterone induces calcitonin gene expression in human endometrium within the putative window of implantation. J Clin Endocrinol Metab 1998; 83: 4443–4450. [DOI] [PubMed] [Google Scholar]
- Nie GY, Li Y, Wang J, Minoura H, Findlay JK, Salamonsen LA.Complex regulation of calcium-binding protein D9k (calbindin-D(9k)) in the mouse uterus during early pregnancy and at the site of embryo implantation. Biol Reprod 2000; 62: 27–36. [DOI] [PubMed] [Google Scholar]
- Takamoto N, Zhao B, Tsai SY, DeMayo FJ.Identification of Indian hedgehog as a progesterone-responsive gene in the murine uterus. Mol Endocrinol 2002; 16: 2338–2348. [DOI] [PubMed] [Google Scholar]
- Daikoku T, Matsumoto H, Gupta RA, Das SK, Gassmann M, DuBois RN, Dey SK.Expression of hypoxia-inducible factors in the peri-implantation mouse uterus is regulated in a cell-specific and ovarian steroid hormone-dependent manner: evidence for differential function of HIFs during early pregnancy. J Biol Chem 2003; 278: 7683–7691. [DOI] [PubMed] [Google Scholar]
- Cheon YP, Xu X, Bagchi MK, Bagchi IC.Immune-responsive gene 1 (Irg1) is a novel target of progesterone receptor and plays a critical role during implantation in the mouse. Endocrinology 2003; 144: 5623–5630. [DOI] [PubMed] [Google Scholar]
- Jeong JW, Lee HS, Lee KY, White LD, Broaddus RR, Zhang YW, Vande Woude GF, Giudice LC, Young SL, Lessey BA, Tsai SY, Lydon JP, et al. Mig-6 modulates uterine steroid hormone responsiveness and exhibits altered expression in endometrial disease. Proc Natl Acad Sci U S A 2009; 106: 8677–8682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JW, Lee KY, Kwak I, White LD, Hilsenbeck SG, Lydon JP, DeMayo FJ.Identification of murine uterine genes regulated in a ligand-dependent manner by the progesterone receptor. Endocrinology 2005; 146: 3490–3505. [DOI] [PubMed] [Google Scholar]
- Makkinje A, Quinn DA, Chen A, Cadilla CL, Force T, Bonventre JV, Kyriakis JM.Gene 33/Mig-6, a transcriptionally inducible adapter protein that binds GTP-Cdc42 and activates SAPK/JNK: a potential marker transcript for chronic pathologic conditions, such as diabetic nephropathy: possible role in the response to persistent stress. J Biol Chem 2000; 275: 17838–17847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarikoski ST, Rivera SP, Hankinson O.Mitogen-inducible gene 6 (MIG-6), adipophilin and tuftelin are inducible by hypoxia. FEBS Lett 2002; 530: 186–190. [DOI] [PubMed] [Google Scholar]
- van Laar T, Schouten T, van der Eb AJ, Terleth C.Induction of the SAPK activator MIG-6 by the alkylating agent methyl methanesulfonate. Mol Carcinog 2001; 31: 63–67. [DOI] [PubMed] [Google Scholar]
- Wick M, Burger C, Funk M, Muller R.Identification of a novel mitogen-inducible gene (mig-6): regulation during G1 progression and differentiation. Exp Cell Res 1995; 219: 527–535. [DOI] [PubMed] [Google Scholar]
- Anastasi S, Fiorentino L, Fiorini M, Fraioli R, Sala G, Castellani L, Alema S, Alimandi M, Segatto O.Feedback inhibition by RALT controls signal output by the ErbB network. Oncogene 2003; 22: 4221–4234. [DOI] [PubMed] [Google Scholar]
- Fiorentino L, Pertica C, Fiorini M, Talora C, Crescenzi M, Castellani L, Alema S, Benedetti P, Segatto O.Inhibition of ErbB-2 mitogenic and transforming activity by RALT, a mitogen-induced signal transducer which binds to the ErbB-2 kinase domain. Mol Cell Biol 2000; 20: 7735–7750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorini M, Ballaro C, Sala G, Falcone G, Alema S, Segatto O.Expression of RALT, a feedback inhibitor of ErbB receptors, is subjected to an integrated transcriptional and post-translational control. Oncogene 2002; 21: 6530–6539. [DOI] [PubMed] [Google Scholar]
- Hackel PO, Gishizky M, Ullrich A.Mig-6 is a negative regulator of the epidermal growth factor receptor signal. Biol Chem 2001; 382: 1649–1662. [DOI] [PubMed] [Google Scholar]
- Burbelo PD, Drechsel D, Hall A.A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. J Biol Chem 1995; 270: 29071–29074. [DOI] [PubMed] [Google Scholar]
- Pirone DM, Carter DE, Burbelo PD.Evolutionary expansion of CRIB-containing Cdc42 effector proteins. Trends Genet 2001; 17: 370–373. [DOI] [PubMed] [Google Scholar]
- Mahgoub MA, Abd-Elfattah AS.Diabetes mellitus and cardiac function. Mol Cell Biochem 1998; 180: 59–64. [PubMed] [Google Scholar]
- Anastasi S, Sala G, Huiping C, Caprini E, Russo G, Iacovelli S, Lucini F, Ingvarsson S, Segatto O.Loss of RALT/MIG-6 expression in ERBB2-amplified breast carcinomas enhances ErbB-2 oncogenic potency and favors resistance to Herceptin. Oncogene 2005; 24: 4540–4548. [DOI] [PubMed] [Google Scholar]
- Ferby I, Reschke M, Kudlacek O, Knyazev P, Pante G, Amann K, Sommergruber W, Kraut N, Ullrich A, Fassler R, Klein R.Mig6 is a negative regulator of EGF receptor-mediated skin morphogenesis and tumor formation. Nat Med 2006; 12: 568–573. [DOI] [PubMed] [Google Scholar]
- Zhang YW, Staal B, Su Y, Swiatek P, Zhao P, Cao B, Resau J, Sigler R, Bronson R, Vande Woude GF.Evidence that MIG-6 is a tumor-suppressor gene. Oncogene 2007; 26: 269–276. [DOI] [PubMed] [Google Scholar]
- Nomoto S, Haruki N, Kondo M, Konishi H, Takahashi T, Takahashi T, Takahashi T.Search for mutations and examination of allelic expression imbalance of the p73 gene at 1p36.33 in human lung cancers. Cancer Res 1998; 58: 1380–1383. [PubMed] [Google Scholar]
- Amatschek S, Koenig U, Auer H, Steinlein P, Pacher M, Gruenfelder A, Dekan G, Vogl S, Kubista E, Heider KH, Stratowa C, Schreiber M, et al. Tissue-wide expression profiling using cDNA subtraction and microarrays to identify tumor-specific genes. Cancer Res 2004; 64: 844–856. [DOI] [PubMed] [Google Scholar]
- Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC.Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 2007; 148: 3814–3826. [DOI] [PubMed] [Google Scholar]
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, Nayak NR, Giudice LC.Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology 2006; 147: 1097–1121. [DOI] [PubMed] [Google Scholar]
- Simmons DM, Arriza JL, Swanson LW.A complete protocol for in situ hybridization of messenger RNAs in brain and other tissues with radiolabeled single-stranded RNA probes. J Histotechnol 1989; 12: 169–181. [Google Scholar]
- Jeong JW, Lee HS, Franco HL, Broaddus RR, Taketo MM, Tsai SY, Lydon JP, DeMayo FJ.beta-Catenin mediates glandular formation and dysregulation of beta-catenin induces hyperplasia formation in the murine uterus. Oncogene 2009; 28: 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YW, Staal B, Su Y, Swiatek P, Zhao P, Cao B, Resau J, Sigler R, Bronson R, Vande Woude GF.Evidence that MIG-6 is a tumor-suppressor gene. Oncogene 2007; 26: 269–276. [DOI] [PubMed] [Google Scholar]
- Zhang YW, Su Y, Lanning N, Swiatek PJ, Bronson RT, Sigler R, Martin RW, Vande Woude GF.Targeted disruption of Mig-6 in the mouse genome leads to early onset degenerative joint disease. Proc Natl Acad Sci U S A 2005; 102: 11740–11745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YW, Vande Woude GF.Mig-6, signal transduction, stress response and cancer. Cell Cycle 2007; 6: 507–513. [DOI] [PubMed] [Google Scholar]
- Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ, Lydon JP.Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 2005; 41: 58–66. [DOI] [PubMed] [Google Scholar]
- Jin N, Gilbert JL, Broaddus RR, Demayo FJ, Jeong JW.Generation of a Mig-6 conditional null allele. Genesis 2007; 45: 716–721. [DOI] [PubMed] [Google Scholar]
- Franco HL, Jeong JW, Tsai SY, Lydon JP, DeMayo FJ.In vivo analysis of progesterone receptor action in the uterus during embryo implantation. Semin Cell Dev Biol 2008; 19: 178–186. [DOI] [PubMed] [Google Scholar]
- Lee KY, Jeong JW, Tsai SY, Lydon JP, DeMayo FJ.Mouse models of implantation. Trends Endocrinol Metab 2007; 18: 234–239. [DOI] [PubMed] [Google Scholar]
- Conneely OM, Jericevic BM.Progesterone regulation of reproductive function through functionally distinct progesterone receptor isoforms. Rev Endocr Metab Disord 2002; 3: 201–209. [DOI] [PubMed] [Google Scholar]
- Conneely OM, Lydon JP.Progesterone receptors in reproduction: functional impact of the A and B isoforms. Steroids 2000; 65: 571–577. [DOI] [PubMed] [Google Scholar]
- Lee K, Jeong J, Kwak I, Yu CT, Lanske B, Soegiarto DW, Toftgard R, Tsai MJ, Tsai S, Lydon JP, DeMayo FJ.Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet 2006; 38: 1204–1209. [DOI] [PubMed] [Google Scholar]
- Kurihara I, Lee DK, Petit FG, Jeong J, Lee K, Lydon JP, DeMayo FJ, Tsai MJ, Tsai SY.COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet 2007; 3: e102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surveyor GA, Gendler SJ, Pemberton L, Das SK, Chakraborty I, Julian J, Pimental RA, Wegner CC, Dey SK, Carson DD.Expression and steroid hormonal control of Muc-1 in the mouse uterus. Endocrinology 1995; 136: 3639–3647. [DOI] [PubMed] [Google Scholar]
- Liu Y, Teng CT.Estrogen response module of the mouse lactoferrin gene contains overlapping chicken ovalbumin upstream promoter transcription factor and estrogen receptor-binding elements. Mol Endocrinol 1992; 6: 355–364. [DOI] [PubMed] [Google Scholar]
- Lagow E, DeSouza MM, Carson DD.Mammalian reproductive tract mucins. Hum Reprod Update 1999; 5: 280–292. [DOI] [PubMed] [Google Scholar]
- Nakanishi A, Morita S, Iwashita H, Sagiya Y, Ashida Y, Shirafuji H, Fujisawa Y, Nishimura O, Fujino M.Role of gob-5 in mucus overproduction and airway hyperresponsiveness in asthma. Proc Natl Acad Sci U S A 2001; 98: 5175–5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JW, Lee KY, Lydon JP, DeMayo FJ.Steroid hormone regulation of Clca3 expression in the murine uterus. J Endocrinol 2006; 189: 473–484. [DOI] [PubMed] [Google Scholar]
- Buchanan DL, Setiawan T, Lubahn DB, Taylor JA, Kurita T, Cunha GR, Cooke PS.Tissue compartment-specific estrogen receptor-alpha participation in the mouse uterine epithelial secretory response. Endocrinology 1999; 140: 484–491. [DOI] [PubMed] [Google Scholar]
- Kurita T, Young P, Brody JR, Lydon JP, O'Malley BW, Cunha GR.Stromal progesterone receptors mediate the inhibitory effects of progesterone on estrogen-induced uterine epithelial cell deoxyribonucleic acid synthesis. Endocrinology 1998; 139: 4708–4713. [DOI] [PubMed] [Google Scholar]