Abstract
The Spag16L gene codes for a protein that is localized to the central apparatus which is essential for normal sperm motility and male fertility. Sperm from mice homozygous for a targeted deletion of the Spag16L gene were examined to assess their flagellar motor functions compared with age- and strain-matched control sperm. Sperm were also demembranated with Triton X-100 and examined for their ability to respond to free calcium, as well as for their ability to undergo microtubule sliding driven by dynein action. In addition, the passive flagella, inhibited by sodium metavanadate to disable the dyneins, were examined for mechanical abnormalities. Live Spag16L-null sperm exhibited much less bending of the flagellum during the beat. The amount of microtubule sliding in the R-bend direction of the beat was selectively restricted, which suggests that there is limited activation of the dyneins on one side of the axoneme in the live cells. This is corroborated by the results on detergent-extracted sperm models. The flagellar response to calcium is greatly reduced. The calcium response requires the activation of the dyneins on outer doublets 1, 2, 3, and 4. These are the same dyneins required for R-bend formation. In axonemes prepared to disintegrate by microtubule sliding, we observed little or no extrusion of doublets 1 and 2, consistent with a reduced activity of their dyneins. This deficit in motor function, and an increased rigidity of the midpiece region which we detected in the passive flagella, together can explain the observed motility characteristics of the Spag16L-null sperm.
Keywords: axoneme, calcium, central pair, ciliopathies, dynein, hyperactivation
Mouse sperm lacking SPAG16L have altered motility and a reduced response to calcium.
INTRODUCTION
Sperm motility in mammals is based on the functioning of the sperm tail, which is a modified eukaryotic flagellum. The cilia and flagella of eukaryotic cells serve many functions in both the plant and animal kingdoms. They also exhibit remarkable conservation of both structure and molecular composition in widely divergent eukaryotic organisms. So dramatic is this conservation that many of the component proteins of mammalian cilia and flagella are being identified and assigned probable functions by studying their orthologues in the flagellated green algae Chlamydomonas reinhardtii. Notable examples of this are the sperm-associated antigens (SPAGs) SPAG6 and SPAG16, which are the orthologues of Chlamydomonas proteins PF16 and PF20, respectively [1–3]. These proteins are crucial for motility in Chlamydomonas and also are important for the motile functioning of cilia and flagella in mice. In Chlamydomonas, it was demonstrated that the proteins are components of the central pair (cp) apparatus of the flagellum [4, 5], information that has since been confirmed for the corresponding proteins in mouse and human sperm [6, 7].
Both the Spag6 and Spag16 genes are required for normal fertility in mice and, when disrupted, result in males that are infertile. The infertility is accompanied by gross disorganization of the flagellar axoneme in the case of Spag6 disruption [8], but only mild structural abnormalities in the case of Spag16L [3]. In Chlamydomonas, PF20 is localized to the interdoublet bridge of the cp and is a single protein. In mice, there are two gene products of Spag16, controlled by separate promoters, and only one of the two gene products (SPAG16L) is localized to the flagella; the other protein (SPAG16S) associates with the spermatid nucleus. When only the Spag16L gene is disrupted, the mice express a milder phenotype that mainly affects sperm motility and axonemal instability in response to severe stress [3, 7].
The Spag16L-null mice (official allele symbol Spag16tm2Jfs), therefore, presented us with the opportunity to look at the changes in flagellar functioning caused by a rather specific and well-localized single-gene/single-protein anomaly. The sperm that carry this mutation are fairly well formed and possess an ultrastructurally normal-looking flagellum that has an interesting and characteristic pattern of motility [3]. The motility is distinctly different enough from wild type (WT) that we sought to ascertain what specific functional properties of the axoneme are most affected by the mutation. In this study, we identify some changes in underlying motor function that result from the deletion of the cp element coded by Spag16L. Specifically, there is a significant reduction in the activation of microtubule (MT) sliding between certain doublet MTs, and this leads to a greatly reduced responsiveness to Ca2+. These changes in motor function are expressed in the beat as a marked reduction in the bend development, especially in the R-bend direction, and reduced amplitude of beating. These observations establish a critical role for SPAG16L in mediating the flagellar response to calcium as well as the mechanical features of the sperm midpiece.
MATERIALS AND METHODS
Animals
Animals were created and maintained at the University of Pennsylvania (Philadelphia, PA) prior to the maintenance and housing of the colony at Virginia Commonwealth University or the housing at Oakland University. Establishment of the targeted disruption of the Spag16L gene in mice is described in detail elsewhere [3]. Additional information about this mutation and these mice was previously published [1, 3, 9].
All care and use of animals was conducted following standard ethical guidelines and approval of each investigator's Institutional Animal Care and Use Committees; Oakland University (06041), Virginia Commonwealth University (2-92407), and University of Pennsylvania (436501).
Sperm Collection and Sperm Stock Solution
To create a stock sperm solution, cells were recovered from the cauda epididymis of CO2-asphyxiated, sexually mature male mice. For most experiments, fresh epididymides were nicked with a razor blade, and sperm was obtained by expressing them onto a 60 × 15 mm Falcon (no. 351007) plastic Petri dish (Becton Dickinson, Franklin Lakes, NJ). The cells were immediately covered with 0.9% NaCl solution for all reactivations and MT sliding assays or with modified Krebs-Ringer bicarbonate (MKRB) medium (see Table 1 in Neil et al., [10]) for all other experiments. Both solutions were at room temperature (23°C–25°C) prior to use. The percent motility and vanadate experiments used a variation of the sperm recovery method described. Nicked epididymides were placed directly into microcentrifuge tubes containing a small volume (1–2.5 ml) of MKRB media. Sperm were given a minimum of 15 min to “swim up” into solution at room temperature.
Table 1.
Percent motility, beat frequency and maximum curvature development in live WT and Spag16L−/− mouse sperm.
Unless stated otherwise, all chemicals used in the experiments were obtained from Sigma-Aldrich (St. Louis, MO) and were of the highest purity available.
Image Acquisition, Analysis, and Processing
All experiments were visualized using phase-contrast imaging on a Nikon TE2000U inverted microscope (Melville, NY) with a diffuser lens in the light path. Individual images or video sequences of each experiment were captured using an HP xw8400 Workstation (Hewlett-Packard, Palo Alto, CA), Matrox Imaging Solios eCL/XCL digital frame grabber, Inspector 8.0 software (Dorval, QC, Canada), and a JAI Pulnix Inc. (San Jose, CA) TM-1402CL camera. Three Nikon objectives were used: 20× Plan Fluor (0.45 N.A.; resolution 0.235 μm per pixel); 40× Plan Fluor (0.60 N.A.; resolution 0.119 μm per pixel); and 40× Plan Apochromat (0.95 N.A.; resolution 0.116 μm per pixel). A 1.5 magnification changer was sometimes combined with the 20× Plan Fluor objective and resulted in the resolution increasing to 0.158 μm per pixel. The speed of image capture was approximately 30 frames per second. All experimental preparations were contained in either a custom-made Plexiglas imaging chamber with a glass coverslip bottom (imaging chamber) or Falcon plastic Petri dishes. Photoshop CS2, CS3, or CS4 (Adobe Systems Inc., San Jose, CA) was used to minimally adjust levels, tonal curves, brightness, or contrast of individual images for viewing ease.
Percent Motility
A sperm dilution was achieved by combining 30–420 μl of the swim-up sperm stock with 70–1900 μl of the MKRB medium (23°C) to achieve final concentrations that ranged from 5 × 104 to 31 × 104 cells/ml. Diluted sperm were incubated in multiple Petri dishes at 37°C in a humidified Thermolyne 5000 series incubator (ThermoScientific, Waltham, MA) with 5% CO2/95% air. For each n value, percent motility was determined using a hemacytometer. Motile cells were counted first, followed by the number of total cells. A total of 20 μl of the incubated sample was added to each side of the hemacytometer. Within the grid, three squares on each side of the hemacytometer were used to calculate the percent of the total motile cells counted. The hemacytometer was cleaned, filled with a 20 μl of the sample on only one side of the hemacytometer, and counted. For each of the three separate counts, a mean of 25 cells were evaluated for motility and the results averaged.
Beat Frequency
Beat frequencies were hand calculated from AVI files of live, free-swimming sperm in MKRB medium at room temperature (23°C–24°C). For each of the 20 cells analyzed from both groups, the mean was determined from five individually calculated frequencies.
Curvature Analysis
Live, free-swimming WT and Spag16L-null sperm in MKRB media (23°C–24°C) were observed for motility in Petri dishes. Each cell chosen for analysis exhibited motility and had at least the first 50 μm of its flagellum, if not the entire flagellum, clearly visible in the plane of focus. Because of the nearly straight configuration of the midpiece region of the Spag16L-null sperm, curvature development was measured for both groups by calculating the change in shear angle over the change in distance at both 15 μm and 30 μm from the head tail junction using full-size printed images. The midline of the head axis was drawn on the print and angles measured with a protractor. Curvature measurements were converted to radians per meter.
Shear Angle Plots
The MKRB stock sperm solution was diluted 1:1 with additional ambient temperature (25°C) MKRB medium. Diluted sperm stock (6 μl) was placed in an imaging chamber and covered with a piece of glass coverslip for viewing and recording motility.
Analyzed sequences were recorded at 1.3 and 1 h, WT and Spag16L-null, respectively, after sperm recovery from the animal. Sperm were maintained at room temperature this entire time period. Cells were chosen that were representative of the observed motility, and the entire flagellum was visible in the plane of focus. Shear angle development for one complete beat cycle was measured relative to the midline of the head axis every 5 μm for the entire length of the flagellum for each image. Raw shear angle (rad) data were plotted as a simple spline curve as a function of flagellar length for a complete beat cycle using Sigma Plot 11.0 (San Jose, CA). Because of the inability to clearly see the entire flagellum in the plane of the beat, one frame was omitted for the WT plot and three frames for the Spag16L−/− plot.
Response to Ca2+
A stock sperm solution was centrifuged for 10 min at 540 × g, and the supernatant was decanted to form sperm pellets. To each sperm pellet 0.5 ml of a reactivation solution was added to initiate motility. The reactivation solution (pH 7.8) contained the following: 0.024 M potassium glutamate, 0.132 M sucrose, 0.02 M Tris-HCl, 1 mM dithiothreitol (DTT), 2 mM MgCl2, 0.1% Triton X-100 (ThermoScientific, Rockford, IL), 0.5 mM ethylene glycol tetraacetic acid (EGTA), 0.3 mM ATP, 3 μM cAMP, and 1 mM CaCl2 (10−5 to 10−4 M free Ca2+) [11]. Cells were incubated for 7–22 min at room temperature. Cells were deemed to have responded to Ca2+ if the curvature of the flagellum changed from the normal “C” shape of untreated WT sperm (non-hook/curlicue direction) to the opposite direction (Ca2+-hook/curlicue response).
To quantify the curvature response to Ca2+, individual cells were selected for analysis from the reactivation that were adhered by their head to the bottom of an imaging chamber with the entire length of the flagellum free to move and presenting a clear view of the basal portion of the flagellum. The radius of curvature in the first 20 μm from the head-tail junction of the flagellum was measured on 24 separate cells from each group by matching circles of known radius to the curve of the flagellum using Matrox Inspector. Curvature measurements were converted to radians per meter.
Mitochondrial Sheath Extraction
To remove the mitochondrial sheath, a disintegration solution was added to each sperm reactivation to induce MT sliding. To achieve this, 4.5 ml of a disintegration solution (34°C) was added to the 0.5 ml of reactivated sperm pellet preparation described previously. The disintegration solution (pH 9.4–9.5) included the following: 0.1 M potassium glutamate, 2 mM DTT, 0.02 M Tris, 5 mM MgCl2, 0.5% Triton X-100, 0.5 mM EGTA, 1 mM ATP, and 1.0 mM CaCl2 (10−5 to 10−4 M free Ca2+). Samples were incubated at 34°C in a Haake E52 circulating water heater (Berlin, Germany) for 5–10 min. To assess MT sliding, a 0.5-ml sample was viewed in an imaging chamber, or a 15-μl sample was viewed on an inverted microscope slide with a glass coverslip.
Transmission Electron Microscopy
Samples for transmission electron microscopy (TEM) were treated using one of two methods. For the first method (n = 1), the disintegrated sperm sample was centrifuged for 10 min at 10 000 rpm. The pellet was fixed overnight at room temperature with a 2% formaldehyde, 0.02% picric acid fixative in 0.1 M sodium phosphate buffer, pH 7.2, (product no. 15956; Electron Microscopy Sciences, Hatfield, PA) supplemented with 1% glutaraldehyde. The pellet was washed in 7% sucrose-PBS (pH 7.4), stained with 1% osmium tetroxide in phosphate buffer (pH 7.4) for 1 h on ice. The pellet was further processed through washes, dehydration, embedding in PolyBed 812 (Electron Microscopy Sciences), sectioning, and staining with lead and uranyl acetate using standard methods. For the second method (n = 2), samples were rapidly fixed with 25% glutaraldehyde and centrifuged at 2000 rpm for 10 min. The supernatant was decanted and replaced with 2.5% glutaraldehyde and 1% tannic acid in a 0.5 mM sucrose, 10 mM NaH2PO4 buffer and stored at room temperature for 1 h. This was followed by a brief rinse with 50% ethanol and a 2-h en bloc 1% uranyl acetate stain. Samples were subsequently dehydrated, embedded in PolyBed 812, sectioned, and stained with lead and uranyl acetate. For data collection, lower-magnification field views (≤30 000×) were printed full size onto an 8 × 10 sheet of paper. Each clearly visible axoneme cross-section or free doublet MT(s) was categorized. “Intact” cells showed no signs of doublet sliding. “Slid” cells showed a loss of one or more MTs, but it was impossible to determine which of the nine was missing. Cells that showed half of the axoneme missing MTs were classified as extruding MTs from the “front,” which corresponds to doublets 9, 1, and 2, or from the “back,” which corresponds to doublets 4, 5, 6, and 7. Cells were deemed to have slid MTs from “both” sides of the axoneme if all MTs were missing or if three or fewer MTs were present and showed no distinct organization that enabled classification as coming from one side or the other.
Vanadate Treatment
Reactivated sperm samples were treated with 50 μM sodium metavanadate (prepared fresh) to inactivate the dynein motor proteins [12].
Statistical Analysis
Sigma Plot 11.0 was used to determine statistical significance using the Mann-Whitney rank sum test on square root-transformed data.
RESULTS
Characterization of Sperm Motility Defects: Reduced Flagellar Bending and Restricted MT Sliding in Sperm Lacking SPAG16L
The initial characterization of the motility of live sperm from Spag16L−/− mouse sperm was published in a previous report [3]. In that report, the percent motility of the null sperm compared with age- and strain-matched controls was 26% and 69%, respectively. Characterization of the motility of the Spag16L−/− mouse sperm using a visual count method and counting highly motile (progressive) cells gave lower numbers for percent motility but found a similar ratio between the null and control sperm, as is summarized in Table 1. The most notable additional differences we observed in the motility of the live cells were a greatly reduced curvature of bending and a higher beat frequency in the null sperm. The combination of reduced bend development and increased frequency gives the general appearance of vibratory motion in many of the cells, as opposed to orderly propagation of bending waves. Nonetheless, a considerable fraction (7%) of the cells makes forward progress and is quite active. The curvature data are also informative in another regard. Although the curvature of bending is significantly less in the null cells compared with WT cells at both 15 and 30 microns along the flagellum (P < 0.001), it is severely reduced at the 15-μm position, which corresponds to the mitochondrial middle piece. This gives the impression that the middle piece is rigid.
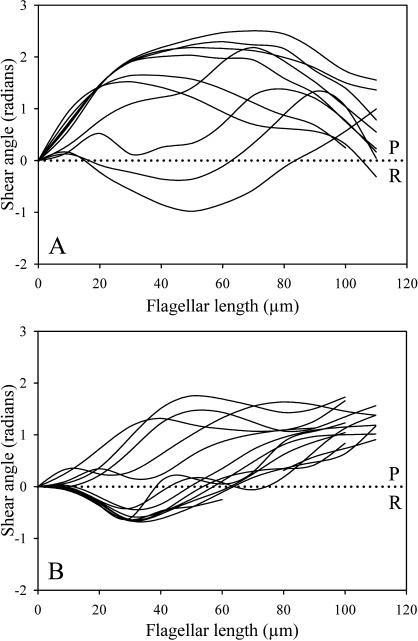
To examine the dynamics of the beat cycle in more detail, shear angle development over the course of one beat cycle in live, free-swimming sperm was measured as a function of flagellar position for both Spag16L−/− and age- and strain-matched WT sperm (Fig. 1). Cells were chosen for optimal motility and visibility. A Spag16L−/− cell is compared directly to a plot measured from a matched WT control. Shear angle is the change in flagellar angle as measured from the base of the flagellum to the local point of measurement on the flagellum. It is a useful measure because the amount of interdoublet shear that develops during the beat may be found by multiplying the shear angle times the effective interdoublet spacing in the beat plane [13, 14]. Consequently, shear angle is indicative of the extent of interdoublet sliding. Figure 1 illustrates quite clearly that the interdoublet shear that develops in the beat of the null mutant sperm is considerably reduced compared with control sperm. The beat has much less excursion in one sliding direction, which we call the reverse-bend (R-bend) direction because it corresponds to the subdominant bending direction in the control mouse sperm.
FIG. 1.
Shear angle development of WT and Spag16L−/− mouse sperm during one beat cycle. Shear angles were measured on live, free-swimming mouse sperm in MKRB media at 24°C and are plotted as a function of flagellar length (in micrometers) from the head-tail junction. The WT sperm (A) exhibit a greater development of shear in both the principal and reverse directions of the beat compared with Spag16L−/− mouse sperm (B). The largest difference between the two plots is in the shear excursion in the R-bend direction (downward deflection) of the Spag16L−/− sperm. Tracings are at approximately 33-ms intervals. Principal bend (P) and reverse bend (R) directions of the beat are identified.
Analysis of Demembranated Sperm Confirms Restricted MT Sliding in Sperm Lacking SPAG16L
Triton X-100-extracted sperm models have been used extensively to investigate the role of Ca2+ on motor functioning in mammalian sperm [11, 15, 16]. We used this approach to examine the Ca2+ response of the mouse sperm from Spag16L-null mice compared with matched controls. The difference in the response to Ca2+ was very dramatic, as can be seen in Figure 2. Counts of the numbers of cells responding and the severity of the response as measured by the curvature of the middle piece are summarized in Table 2. Triton X-100-extracted mouse sperm show a very extreme response to elevated free Ca2+ that has been described previously as a curlicue [17]. The curlicue response involves the development of a strong curvature along the entire length of the flagellum and renders the flagellum into a tight spiral. This can only be accomplished by the uniform activation of the dyneins on the doublets numbered 2-3-4, which work to bend the flagellum in this direction. The response of the Spag16L-null sperm is greatly reduced, as can be seen in the numerical data in Table 2.
FIG. 2.
Response of Triton X-100-extracted mouse sperm reactivated at room temperature with 0.3 mM Mg-ATP, 0.5 mM EGTA, and 1 mM CaCl2. A) Sperm from WT mice exhibit the typical curlicue response to CaCl2 which is a characterized by a strong development of curvature over the entire length of the flagellum that can result in two, or even three complete 360° coils. B) Spag16L−/− sperm do not exhibit the same strong curlicue response to 1 mM CaCl2. Although curvature development of the flagellum is changed to the opposite direction of normal, untreated cells (which is a characteristic “C” shape in the same direction as the pointed sperm head), the response is drastically reduced compared with wild-type cells, with Spag16L−/−cells developing a slight change of curvature. Circled areas were selected for the magnified inset views. Arrow indicates the commonly found kinking at the midpiece-principal piece junction. Bars = 30 μm; inset bars = 10 μm.
Table 2.
Reactivated WT and Spag16L−/− mouse sperm response to 1 mM CaCl2 at room temperature.
Deficits in the Response to Ca2+ in Sperm Lacking SPAG16L
The doublets that power the Ca2+ response are the doublets that bend the flagellum, in a sense, opposite to the curve of the sperm head (which may be used as a marker of the polarity of the axoneme/flagellum in mouse sperm). This is also the side we have designated as the R-bend direction in the shear angle tracings of Figure 1. Consequently, the absence of a strong Ca2+ response in the modeled sperm corresponds to a deficiency in the activation by Ca2+ of the same dyneins that are responsible for the development of the R-bends in rhythmic motility.
Rigidity of the Midpiece in Sperm Lacking SPAG16L
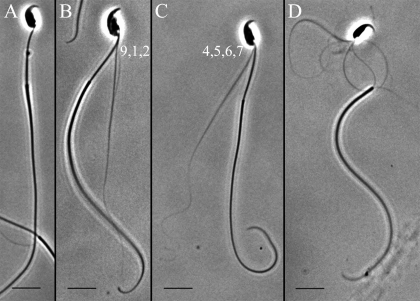
We have devised a protocol to effectively remove the mitochondrial sheath of mouse sperm, as presented in detail in Materials and Methods. Sperm subjected to this protocol undergo disintegration of the axoneme by interdoublet sliding when Mg-ATP is added to the suspension. Extrusion of doublets with their attached outer dense fibers (ODFs) provides a way to assay the activity of the dyneins on the two sides of the axoneme. As can be seen in Figure 3, there are three patterns of disintegration that are observed. A disintegrating sperm can extrude a bundle of fibers from the “front,” which is the same side as the acrosome tip (Fig. 3B), from the “back,” which is the side opposite the acrosome tip (Fig. 3C), or from both sides of the axoneme (Fig. 3D).
FIG. 3.
Phase-contrast imaging of MT disintegration patterns observed in mouse sperm after reactivation with 1 mM CaCl2 and 0.3 mM Mg-ATP and subsequent removal of the mitochondrial sheath. After preparing the sperm sample as detailed in Materials and Methods, four basic patterns of MT extrusion from the flagellum are observed relative to the polarity defined by the sperm head. Some sperm remain intact and do not extrude MTs, as shown in A. Other sperm extrude only doublet fibers 9, 1, and 2, as in B (front), or 4, 5, 6, and 7, as in C (back), or from both sides of the axoneme, as in D. Bars = 10 μm.
In the predominant pattern of sliding, fibers emerged from the side opposite the acrosomal tip. This pattern is expected to result from the sliding of doublets 7, 8, and 9. It is the action of the dyneins on doublets 6 and 7 that can push the doublets 5 and 6 baseward and out of the fibrous sheath [18]. Because doublets 5 and 6 are permanently bridged in mammals [19], these two doublets with their very large ODFs emerge together and are quite recognizable on TEM images. Visual counts from captured light-microscopic images suggested that the disintegrations that required the action of the dyneins on doublets 2-3-4 are greatly reduced in the Spag16L-null sperm.
The light-microscopic images are suggestive of a reduction in motor function of a specific subset of dyneins in the Spag16L-null mice. We conducted a series of TEM examinations of disintegrated and fixed specimens to see whether this result could be confirmed under conditions where the identity of the emerging doublets could be unambiguously verified and quantified. As can be seen in Figures 4–6, the absence of the two large ODFs that are linked to doublets 5 and 6 and the appearance of these linked doublets outside of the sheath can be used to identify events where these elements have been extruded from the sheath. Figures 4 and 5 provide examples of the disintegration products that were counted from the collection of TEM images for three separate experiments. Figure 6 provides the reader with a broader field image showing that the extent of disintegration was noticeably reduced in the Spag16L-null samples. This is evident in the numerical tallies on both the light-microscopic samples and from the TEM image counts that are presented in Figure 7. As can be seen from the summarized data in Figure 7, both the light-microscopic data and the TEM data indicate a marked deficiency in the extrusion of doublet 1 and its attached ODF. This is consistent with a reduced motor function of the dyneins on the 2-3-4 side of the axoneme in the null sperm. It is these dyneins that must activate to extrude doublet 1. It is noteworthy that these are also the dyneins that must activate to produce a Ca2+ response and which are responsible for bending the flagellum in the R-bend direction [18].
FIG. 4.
The TEM examination of Ca2+-treated, demembranated, and disintegrated WT sperm samples. The images shown are representative of the types of MT extrusion patterns/examples that we counted for an ultrastructural analysis of disintegrated WT sperm and confirmation of our optical microscopy results. Intact axonemes are shown in A and B. An image of MT extrusion from both sides of the axoneme is shown in C. Images of the sliding of MTs 5 and 6 from the axoneme are shown in D and E. Individual 5–6 MTs and their associated ODFs completely extruded from the axoneme are shown in F–H. Wild-type sperm respond to Ca2+ by extruding a significantly greater number of MTs from the 9, 1, 2 side of the axoneme. Examples of free MTs 1 and 2 or the MT 1 alone (and corresponding ODFs) are shown in I–L. The standard numerical assignments of the ODFs associated with their MT doublets are provided. Bar = 500 nm.
FIG. 6.
A typical field-view micrograph of demembranated and disintegrated WT and Spag16L-null sperm treated with 1 mM CaCl2. A) Wild-type sperm characteristically slide a larger number of individual fibers from the axoneme compared with Spag16L-null sperm, which is evident by the four individual 5–6 doublet pairs (and corresponding ODFs) observed. B) More intact axonemes are identified from Spag16L−/− sperm after disintegration compared with WT sperm. Additionally, only a very small percentage of cells extrude doublet fibers from the 9, 1, 2 side of the axoneme. Bar = 500 nm.
FIG. 5.
The TEM examination of Ca2+-treated, demembranated, and disintegrated Spag16L-null sperm samples. Images shown represent the most common types of MT extrusion patterns found in electron micrographs of Spag16L-null sperm. Compared with wild-type sperm, null sperm remain intact more often, as shown in A–C, G, and H. Null sperm most frequently extrude fibers from only the side of the axoneme that corresponds to activation of doublets numbered 4, 5, 6, and 7. The reduced number of images where the doublet 1 and ODF were extruded implicates a reduced response to Ca2+. Examples of this pattern of disintegration are shown in D, I, and J. Examples of free 5–6 doublet pairs (and the corresponding ODFs) are found in F, J, L, and M. In a number of instances it was impossible to determine which side of the axoneme extruded MTs, even though sliding was evident, as in E and K. The extrusion of doublets 1 and 2 requires the action of the same dyneins that bend the flagellum in the direction of the Ca2+ response. An individual doublet 1 was found, a rare occurrence, as in J. It is notable that we could not find any comparable images of the 9, 1, 2 group as was found for WT. The standard numerical assignments of the ODFs associated with their MT doublets are provided. Bar = 500 nm.
FIG. 7.
The mean frequency (percent sliding of total sperm count) ± SEM of the disintegration patterns of WT and Spag16L−/− mouse sperm treated with 1 mM CaCl2. A) Eleven separate experiments were examined by light microscopy to determine the frequency of MT disintegration patterns (as shown in Fig. 3) after treatment with 1 mM CaCl2. The mean percent frequency ± SEM is plotted as a function of the disintegration pattern observed for WT (black bars) and Spag16L−/− (white bars) sperm cells. The frequency of sliding of the 9, 1, 2 group of MTs in WT mouse sperm is statistically significantly different from Spag16L−/− mouse sperm (aP = 0.003; bP = 0.007). B) Three of the MT sliding experiments were subsequently processed for TEM to confirm the results. The mean percent ± SEM of each sliding pattern observed from TEM processing of both sperm types is plotted in B. Although not enough experimental repeats were performed to establish statistical significance, the frequency of sliding in the 9, 1, 2 group of MTs from both WT and Spag16L−/− mouse sperm follows the trend observed at the light-microscopy level.
It is notable that an experiment run to induce hyperactivity in WT and Spag16L-null sperm found a greatly reduced hyperactivation response compared with WT cells (data not shown). Hyperactivation is a motility pattern that is known to have a critical dependence on Ca2+ [20].
Mechanical Abnormalities in the Passive Flagellum of Sperm Lacking SPAG16L
We also examined the mechanical behavior of the sperm when the dyneins were disabled by 50 μM sodium metavanadate. We did find that the passive flagellum of the Spag16L-null sperm appears to have a structural irregularity that is not found in the matched controls. It is much more difficult to impose a smooth bend in the proximal portion of the flagellum with a microprobe in the null sperm. It was our intention to directly measure the stiffness of the flagellum and compare it with the stiffness of the matched controls. However, this requires manipulating the flagellum into a smooth bend that can be measured for curvature while simultaneously measuring the force imposed by the microprobe. We encountered immense difficulty applying the measurement protocol we previously used to measure stiffness of sperm because of the odd mechanical behavior of the null sperm. The sperm were uniformly difficult to bend in the mitochondrial midpiece portion of the flagellum, but they showed a discontinuity of stiffness at the midpiece-principal piece junction that lead to sharp bending at that position along the flagellum. This finding is illustrated by the image in Figure 8 and Supplemental Movies S1 and S2 (available online at www.biolreprod.org). This unexpected mechanical behavior of the passive flagella was a consistent finding and suggests that the midpiece may be unusually stiff in these cells, that there is a structural weakness at the midpiece-principal piece junction, or both.
FIG. 8.
Response to manipulation in vanadate-treated sperm cells. Wild-type (A and B) and Spag16L−/− (C and D) Triton X-100-extracted sperm were treated with 50 μM vanadate and manipulated with a glass microprobe at room temperature. Wild-type cells exhibit a typical response, which is the smooth development of curvature in the midpiece region when bent in both directions. Spag16L−/− cells do not display this same response; rather, they are quite stiff in the midpiece region, and smooth curvature development occurs only after the midpiece-principal junction (arrowheads). See Supplemental Movies S1 and S2 to view the manipulation of WT and Spag16L−/− cells, respectively. Bar = 20 μm.
Live cells from the Spag16L-null mice show a higher incidence of kinking at the midpiece-principal piece junction (Fig. 2B). As noted above, the data in Table 1 show that there is very little curvature and bend development in the middle piece of live sperm with the Spag16L-null mutation. This is also consistent with a stiff midpiece region and might explain why bending in both the principal-bend and R-bend directions is reduced in the null sperm in the region near the base.
DISCUSSION
Our current understanding of ciliary and flagellar functioning is at a most interesting juncture. It has been known for some time that radial spoke and cp-defective mutants of the green algae Chlamydomonas are most often paralyzed [21]. This has led to speculation that the main control of the flagellar beat is in some way governed by the cp-radial spoke linkage. Support for this idea has grown as successive components of a regulatory pathway have been identified in the cp, spokes, and dynein regulatory complex (for a recent overview see Wirschell et al. [22]). The Spag16L-null mice we examined here as well as the SPAG6 and hydin knockouts all target proteins known to make up a portion of the cp complex [7, 23, 24]. Spag16-, Spag6-, and Hydin-null mice display ciliopathies. It appears that all of these cp proteins are part of a regulatory mechanism that controls ciliary and flagellar beating. However, the specific functional defects in the flagellar motor mechanism need to be examined before the cause of ciliary dysfunction can be assigned and the purpose of the regulatory mechanism elucidated.
In this study, we have examined the specific motility defects imparted by disruption of the Spag16L gene in mouse sperm. This targeted disruption has a minimal effect on the flagellar ultrastructure and does not incapacitate all of the cilia of the animal, but it does impair sperm motility [3]. Because the flagella appear structurally close to normal and retain some motility, it allowed us to examine the motility to see what functions are affected by the specific deletion. Our examination of the basic motor functions in these flagella provides some useful information about the functional nature of this cp-mediated control defect.
Most of the significant differences we identified between Spag16L-null sperm and matched controls indicate that the dyneins on one side of the axoneme are less functional in the Spag16L-null sperm. This result could be caused by a specific subpopulation of dyneins being completely nonfunctional as a result of the deletion, or it could result from incomplete activation of the dyneins that contribute to R-bend development. One thing we can say for certain is that the affected dyneins are the same ones that contribute to the Ca2+ response of the sperm flagella. We are also able to identify the specific doublets that have decreased motor function as those on the 1-2-3-4 side of the axonemal ring. These are the same set of dyneins that previously were found to be selectively sensitive to inhibition with Ni2+ and Cd2+ ion [25] and mediate the response to Ca2+ [11, 24].
It is well established that the Ca2+ response of the sperm flagellum is integral to the hyperactivated mode of motility [11, 14, 26–28]. Therefore, the nature of this functional defect makes hyperactivated motility all but impossible. We did not observe anything resembling normal hyperactivated motility in the live Spag16L-null sperm samples we prepared. The inability to hyperactivate may be contributory to the loss of fertility in the mice that carry this genetic defect, because some sperm from Spag16L-null mice do make sufficient forward progress and presumably should be able to swim to the site of fertilization.
The central apparatus of the flagellar and ciliary axoneme must play an important role in normal flagellar functioning because many of the single-protein defects that target this structure result in immotile flagella or reduced motility. It was suggested that regulation through the cp and radial spokes is the main control of the beat cycle [22, 29]. There is an alternate possibility that is suggested by some recent results, including the ones presented in this study. The control of motor function of the dyneins in most cilia and flagella is responsive to Ca2+ ion (for review, see Lindemann and Kanous [30] and Dymek and Smith [31]). There is recent evidence that at least a portion of the control that makes the Ca2+ response possible is mediated through a cp-radial spoke interaction [32–34].
Our own findings on Spag16L-null sperm also strongly implicate that the Ca2+ response mechanism is severely impaired by the deletion of the central apparatus protein SPAG16L. The mechanism of this effect appears to be by disabling the dyneins on specific doublets. These are the same dyneins and doublets which must activate to bring about a Ca2+ response. Rendering a subset of the dyneins dysfunctional would also be expected to disrupt the normal reciprocation of switching necessary for a normal beat cycle. This is what is observed. The selective effects on specific outer doublets may be explained by the asymmetrical distribution of proteins in the central apparatus. This is well documented in the case of the Chlamydomonas cp, but it has not been definitively established for the mammalian 9+2 axoneme. If the mammalian axoneme is constructed with the asymmetry of the Chlamydomonas central apparatus, then a model of interacting molecules can be constructed that might account for the selective impact of the absence of SPAG16L on MT sliding. We have previously shown that SPAG16L, the orthologue of Chlamydomonas PF20, which resides on the C2 MT, interacts with SPAG6, the mammalian orthologue of PF16, which is located on the C1 MT in the Chlamydomonas central apparatus. SPAG6, in turn, interacts with SPAG17, the mammalian orthologue of Chlamydomonas PF6, which is part of a calmodulin-containing complex that is thought to affect the dynein activity of the outer doublets [33]. Our findings suggest that the absence of SPAG16L impairs the transduction of the calcium signal, perhaps by altering the function of SPAG6, which fails to engage SPAG17. This is a model that should be examined experimentally.
The other significant finding we made in addition to the motor defect is that the flagellum appears to be more rigid in the midpiece region of the Spag16L-null sperm. This finding may also play a contributory role to the motility phenotype by restricting the amplitude of the flagellar beat in the midpiece region and also by resisting the response to Ca2+ in the proximal region of the sperm flagellum. Our result supports the work of Suarez et al. [35], which suggested that there is a compartmentalization of flagellar function between the midpiece and the principal piece that work together and independently in wave production and propagation. It is somewhat surprising to discover another anomaly that seems to be unrelated to motor function and that is structurally distant from the cp apparatus. It is interesting that other gene defects have been reported to have a similar effect on the midpiece stiffness in mammalian sperm [35–37].
It may be that the greater stiffness is attributable to a maturation defect. Woolley et al. [37] provide evidence that as sperm mature, the ODFs start out mechanically connected to the mitochondrial sheath and dissociate from the sheath as sperm initiate motile function. In the genetic defect they studied—mouse sperm missing a dynein heavy chain—the maturation process was not completed, resulting in midpiece rigidity. If the same maturation defect is common to many flagellar mutations it might explain the widespread nature of this phenotypic trait.
In support of this view, it is instructive to note that the class of defects that delete cp components are known to have a powerful impact on flagellar assembly [37, 38]. The orthologous defect to SPAG16L in Chlamydomonas results in a complete disruption of the assembly of the entire cp apparatus. The closely related Spag6 gene (the two proteins are known to interact [1]) results in gross flagellar assembly defects in mouse sperm [8].
Therefore, it is likely that the absence of SPAG16L is also accompanied by some assembly defects in the construction of the flagellar apparatus. Such a phenomenon has been reported before, where a single-protein deletion of a dynein arm heavy chain caused multiple, seemingly unrelated defects in mouse sperm [17, 37, 39]. SPAG16L is known to anchor serine/threonine kinases, including TSSK2 and STK36, and in the absence of the SPAG16L scaffold, assembly of flagellar and axonemal components may be impaired [40]. The fact that sperm from humans and mice with heterozygous mutations in the SPAG16 gene are less stable to physical stress is consistent with this idea [7, 41].
Puzzling results such as these remind us that the flagellum is not only an engine of motility, but it is one of the most amazing self-assembly systems that nature has created. Philosophically, it may be noteworthy that the remarkable conserved nature of the flagellum may rest on the requirements for competent self-assembly. That is to say, evolutionary loss of a component that is part of the self-assembly sequence cannot be tolerated, because of assembly competence requirements. This might help to explain why SPAG16L has a recognizable orthologue in a pond scum organism.
Acknowledgments
The authors thank Loan Dang of the Eye Research Institute at Oakland University for assistance with the electron microscopy. We also thank Benjamin Dionne, Anetra Knowles, and Anissa Knowles for help with data collection. Transmission electron microscopy was performed in the Imaging Core of Virginia Commonwealth University (5P30NS047463).
Footnotes
Supported by National Science Foundation grant MCB-0516181 to C.B.L. and National Institutes of Health grant HD037416 to J.F.S.
REFERENCES
- Zhang Z, Sapiro R, Kapfhamer D, Bucan M, Bray J, Chennathukuzhi V, McNamara P, Curtis A, Zhang M, Blanchette-Mackie EJ, Strauss JF., IIIA sperm-associated WD repeat protein orthologous to Chlamydomonas PF20 associates with SPAG6, the mammalian orthologue of Chlamydomonas PF16. Mol Cell Biol 2002; 22: 7993–8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Jones BH, Tang W, Moss SB, Wei Z, Ho C, Pollack M, Horowitz E, Bennet J, Baker ME, Strauss JF., IIIDissecting the axoneme interactome: the mammalian orthologue of Chlamydomonas PF6 interacts with sperm-associated antigen 6, the mammalian orthologue of Chlamydomonas PF16. Mol Cell Proteomics 2005; 4: 914–923. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Kostetskii I, Tang W, Haig-Ladewig L, Sapiro R, Wei Z, Patel AM, Bennet J, Gerton GL, Moss SB, Radice GL, Strauss JF., IIIDeficiency of SPAG16L causes male infertility associated with impaired sperm motility. Biol Reprod 2006; 74: 751–759. [DOI] [PubMed] [Google Scholar]
- Smith EF, Lefebvre PA.PF16 encodes a protein with armadillo repeats and localizes to a single microtubule of the central apparatus in Chlamydomonas flagella. J Cell Biol 1996; 132: 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EF, Lefebvre PA.PF20 gene product contains WD repeats and localizes to the intermicrotubule bridges in Chlamydomonas flagella. Mol Biol Cell 1997; 8: 455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapiro R, Tarantino LM, Velazquez F, Kiriakidou M, Hecht NB, Bucan M, Strauss JF., IIISperm antigen 6 is the murine homologue of the Chlamydomonas reinhardtii central apparatus protein encoded by the PF16 locus. Biol Reprod 2000; 62: 511–518. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zariwala MA, Mahadevan MM, Caballero-Campo P, Shen X, Escudier E, Duriez B, Bridoux AM, Leigh M, Gerton GL, Kennedy M, Amselem S, et al. A heterozygous mutation disrupting the SPAG16 gene results in biochemical instability of central apparatus components of the human sperm axoneme. Biol Reprod 2007; 77: 864–871. [DOI] [PubMed] [Google Scholar]
- Sapiro R, Kostetskii I, Olds-Clarke P, Gerton GL, Radice GL, Strauss JF., IIIMale infertility, impaired sperm motility, and hydrocephalus in mice deficient in sperm-associated antigen 6. Mol Cell Biol 2002; 22: 6298–6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Kostetskii I, Moss SB, Jones BH, Ho C, Wang H, Kishida T, Gerton GL, Radice GL, Strauss JF., IIIHaploinsufficiency for the murine orthologue of Chlamydomonas PF20 disrupts spermatogenesis. Proc Natl Acad Sci U S A 2004; 101: 12946–12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill JM, Olds-Clarke P.A computer-assisted assay for mouse sperm hyperactivation demonstrates that bicarbonate but not bovine serum albumin is required. Gamete Res 1987; 18: 121–140. [DOI] [PubMed] [Google Scholar]
- Lindemann CB, Goltz JS.Calcium regulation of flagellar curvature and swimming pattern in Triton X-100-extracted rat sperm. Cell Motil Cytoskeleton 1988; 10: 420–431. [DOI] [PubMed] [Google Scholar]
- Schmitz-Lesich KA, Lindemann CB.Direct measurement of the passive stiffness of rat sperm and implications to the mechanism of the calcium response. Cell Motil Cytoskeleton 2004; 59: 169–179. [DOI] [PubMed] [Google Scholar]
- Lindemann CB.Functional significance of the outer dense fibers of mammalian sperm examined by computer simulation with the geometric clutch model. Cell Motil Cytoskeleton 1996; 34: 258–270. [DOI] [PubMed] [Google Scholar]
- Lindemann CB, Macauley LJ, Lesich KA.The counterbend phenomenon in dynein-disabled rat sperm flagella and what it reveals about interdoublet elasticity. Biophys J 2005; 89: 1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann CB, Goltz JS, Kanous KS.Regulation of activation state and flagellar wave form in epididymal rat sperm: evidence for the involvement of both Ca2+ and cAMP. Cell Motil Cytoskeleton 1987; 8: 324–332. [DOI] [PubMed] [Google Scholar]
- Lindemann CB, Goltz JS, Kanous KS, Gardner TK, Olds-Clarke P.Evidence for an increased sensitivity to Ca2+ in the flagellum of sperm from tw32/+ mice. Mol Reprod Dev 1990; 26: 69–77. [DOI] [PubMed] [Google Scholar]
- Samant SA, Ogunkua OO, Hui L, Lu J, Han Y, Orth JM, Pilder SH.The mouse t complex distorter/sterility candidate, Dnahc8, expresses a gamma-type axonemal dynein heavy chain isoform confined to the principal piece of the sperm tail. Dev Biol 2005; 285: 57–69. [DOI] [PubMed] [Google Scholar]
- Lindemann CB, Orlando A, Kanous KS.The flagellar beat of rat sperm is organized by the interaction of two functionally distinct populations of dynein bridges with a stable central axonemal partition. J Cell Sci 1992; 102: 249–260. [DOI] [PubMed] [Google Scholar]
- Lindemann CB, Gibbons IR.Adenosine triphosphate-induced motility and sliding of filaments in mammalian sperm extracted with Triton X-100. J Cell Biol 1975; 65: 147–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann CB, Kanous KS.Regulation of mammalian sperm motility. Arch Androl 1989; 23: 1–22. [DOI] [PubMed] [Google Scholar]
- Witman GB, Plummer J, Sander G.Chlamydomonas mutants lacking radial spokes and central tubules. J Cell Biol 1978; 76: 729–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirschell M, Hendrickson T, Sale WS.Keeping an eye on I1: I1 dynein as a model for flagellar dynein assembly and regulation. Cell Motil Cytoskeleton 2007; 64: 569–579. [DOI] [PubMed] [Google Scholar]
- Lechtreck KF, Witman GB.Chlamydomonas reinhardtii hydin is a central pair protein required for flagellar motility. J Cell Biol 2007; 176: 473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechtreck KF, Delmotte P, Robinson ML, Sanderson MJ, Witman GB.Mutations in Hydin impair ciliary motility in mice. J Cell Biol 2008; 180: 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanous KS, Casey C, Lindemann CB.Inhibition of microtubule sliding by Ni2+ and Cd2+: evidence for a differential response of certain microtubule pairs within the bovine sperm axoneme. Cell Motil Cytoskeleton 1993; 26: 66–76. [DOI] [PubMed] [Google Scholar]
- Fraser LR.Minimum and maximum extracellular Ca2+ requirements during mouse sperm capacitation and fertilization in vitro. J Reprod Fertil 1987; 81: 77–89. [DOI] [PubMed] [Google Scholar]
- Suarez SS, Vincenti L, Ceglia MW.Hyperactivated motility induced in mouse sperm by calcium ionophore A23187 is reversible. J Exp Zool 1987; 244: 331–336. [DOI] [PubMed] [Google Scholar]
- White DR, Aitken RJ.Relationship between calcium, cAMP, ATP, and intracellular pH and the capacity of hamster spermatozoa to express hyperactivated motility. Gamete Res 1989; 22: 163–177. [DOI] [PubMed] [Google Scholar]
- Omoto CK, Gibbons IR, Kamiya R, Shingyoji C, Takahashi K, Witman GB.Rotation of the central pair microtubules in eukaryotic flagella. Mol Biol Cell 1999; 10: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann CB, Kanous KS.A model for flagellar motility. Int Rev Cytol 1997; 173: 1–72. [DOI] [PubMed] [Google Scholar]
- Dymek EE, Smith EF.A conserved CaM- and radial spoke associated complex mediates regulation of flagellar dynein activity. J Cell Biol 2007; 179: 515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano I, Kobayashi T, Yoshimura M, Shingyoji C.Central-pair-linked regulation of microtubule sliding by calcium in flagellar axonemes. J Cell Sci 2003; 116: 1627–1636. [DOI] [PubMed] [Google Scholar]
- Wargo MJ, Dymek EE, Smith EF.Calmodulin and PF6 are components of the complex that localizes to the C1 microtubule of the flagellar central apparatus. J Cell Sci 2005; 118: 4655–4665. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Shingyoji C.Bending-induced switching of dynein activity in elastase-treated axonemes of sea urchin sperm—roles of Ca2+ and ADP. Cell Motil Cytoskeleton 2009; 66: 292–301. [DOI] [PubMed] [Google Scholar]
- Suarez SS, Marquez B, Harris TP, Schimenti JC.Different regulatory systems operate in the midpiece and principal piece of the mammalian sperm flagellum. Soc Reprod Fertil Suppl 2007; 65: 331–334. [PubMed] [Google Scholar]
- Harris T, Marquez B, Suarez S, Schimenti J.Sperm motility defects and infertility in male mice with a mutation in Nsun7, a member of the sun domain-containing family of putative RNA methyltransferases. Biol Reprod 2007; 77: 376–382. [DOI] [PubMed] [Google Scholar]
- Woolley DM, Neesen J, Vernon GG.Further studies on knockout mice lacking a functional dynein heavy chain (MDHC7). 2. A developmental explanation for the asthenozoospermia. Cell Motil Cytoskeleton 2005; 61: 74–82. [DOI] [PubMed] [Google Scholar]
- Smith EF, Lefebvre PA.The role of central apparatus components in flagellar motility and microtubule assembly. Cell Motil Cytoskeleton 1997; 38: 1–8. [DOI] [PubMed] [Google Scholar]
- Samant SA, Ogunkua O, Hui L, Fossella J, Pilder S.The t-complex distorter 2 candidate gene, Dnahc8, encodes at least two testis-specific axonemal dynein heavy chains that differ extensively at their amino and carboxyl termini. Dev Biol 2002; 250: 24–43. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Shen X, Jones BH, Xu B, Herr JC, Strauss JF., IIIPhosphorylation of mouse sperm axoneme central apparatus protein SPAG16L by a testis-specific kinase, TSSK2. Biol Reprod 2008; 79: 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CW, Nguyen CT, Chen MH, Yang JH, Gacayan R, Huang J, Chen JN, Chuang PT.Fused has evolved divergent roles in vertebrate Hedgehog signalling and motile ciliogenesis. Nature 2009; 459: 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]