Abstract
Maternal hyperglycemia is believed to be the metabolic derangement associated with both early pregnancy loss and congenital malformations in a diabetic pregnancy. Using an in vitro model of embryo exposure to hyperglycemia, this study questioned if increased flux through the hexosamine signaling pathway (HSP), which results in increased embryonic O-linked glycosylation (O-GlcNAcylation), underlies the glucotoxic effects of hyperglycemia during early embryogenesis. Mouse zygotes were randomly allocated to culture treatment groups that included no glucose (no flux through HSP), hyperglycemia (27 mM glucose, excess flux), 0.2 mM glucosamine (GlcN) in the absence of glucose (HSP flux alone), and O-GlcNAcylation levels monitored immunohistochemically. The impact of HSP manipulation on the first differentiation in development, blastocyst formation, was assessed, as were apoptosis and cell number in individual embryos. The enzymes regulating O-GlcNAcylation, and therefore hexosamine signaling, are the beta-linked-O-GlcNAc transferase (OGT) and an O-GlcNAc-selective beta-N-acetylglucosaminidase (O-GlcNAcase). Inhibition of these enzymes has a negative impact on blastocyst formation, demonstrating the importance of this signaling system to developmental potential. The ability of the OGT inhibitor benzyl-2-acetamido-2-deoxy-alpha-D-galactopyranoside (BADGP) to reverse the glucotoxic effects of hyperglycemia on these parameters was also sought. Excess HSP flux arising from a hyperglycemic environment or glucosamine supplementation reduced cell proliferation and blastocyst formation, confirming the criticality of this signaling pathway during early embryogenesis. Inhibition of OGT using BADGP blocked the negative impact of hyperglycemia on blastocyst formation, cell number, and apoptosis. Our results suggest that dysregulation of HSP and O-GlcNAcylation is the mechanism by which the embryotoxic effects of hyperglycemia are manifested during preimplantation development.
Keywords: apoptosis, early development, embryo, embryo metabolism, glucose signaling
Dysregulation of the hexosamine signaling pathway and excess O-linked glycosylation is reponsible for embryotoxic impact of hyperglycemia during preimplantation development in the mouse.
INTRODUCTION
Diabetes-induced hyperglycemia has diverse effects on somatic metabolism, growth, and fertility, with the frequency of fetal developmental defects and early pregnancy complications much higher in diabetic pregnancies. Studies of both chemically induced diabetic mice [1, 2] and spontaneously diabetic mice [3] demonstrate that developmental delays are apparent as early as the preimplantation period in development. These are manifested by reduced rates of proliferation and metabolic capacity [1] as well as increased cell death [4]. In addition to effects on preimplantation embryo cell survival, exposure to hyperglycemia in utero may increase birth weight and the chances of obesity later in life through metabolic programming of the fetus. In light of recent studies demonstrating that metabolic programming events may be determined prior to implantation [5, 6], the mechanisms by which the embryo senses and responds to its nutrient environment are of significant interest.
We have recently identified the hexosamine biosynthetic pathway (HBP) as an essential glucose-sensing mechanism involved in blastocyst formation and metabolic differentiation [7]. Approximately 1%–3% of glucose entering the cell is shunted down this pathway by the rate-limiting enzyme fructose-6-phosphate aminotransferase (GFPT) [8], resulting in the formation of the acetylated amino-sugar nucleotide uridine 5′-diphospho-N-acetylglucosamine (UDP-GlcNAc), which acts as a donor substrate for a number of biosynthetic reactions, including GPI lipid anchor biosynthesis, sugar nucleotide, glycoside, and ganglioside biosynthesis. Additionally, UDP-GlcNAc acts as a precursor used to post-translationally modify regulatory proteins in the cytosol and nucleus through O-linked glycosylation with N-acetylglucosamine (O-GlcNAcylation). This novel metabolic signaling arm of the HBP, named the hexosamine signaling pathway (HSP), utilizes many of the early steps of the HBP but diverges at the level of UDP-GlcNAc [9]. The terminal step in the HSP, O-GlcNAcylation, involves a dynamic cycle of addition and removal of O-linked N-acetylglucosamine (O-GlcNAc) at serine and threonine residues of a multitude of cellular proteins. This modification is functionally reciprocal to phosphorylation at these same sites and can therefore potentially alter the activity and or stability of numerous cellular proteins in response to glucose availability [10, 11].
The enzymes involved in the terminal transfer of the GlcNAc moiety to and from target proteins, respectively, are the X-linked O-linked N-acetylglucosaminyltransferase (OGT) and the β-selective N-acetylglucosaminidase (O-GlcNAcase) [11, 12]. These form a single cooperatively regulated enzyme complex whose activity is exquisitely sensitive to nutrient supply. Levels of UDP-GlcNAc (the precursor substrate for O-GlcNAcylation) respond directly to nutrient excess to modulate OGT affinity for different peptides and thus regulate O-GlcNAcylation [13].
We propose that dysregulation of the HSP and O-GlcNAcylation in the early embryo represents the underlying mechanism of the “glucotoxic” effects of hyperglycemia on developmental potential. Therefore, this study aimed to explore the role of the HSP and O-GlcNAcylation in the early mouse embryo by manipulating flux through the HSP and the activity of the OGT/O-GlcNAcase enzyme complex involved in the terminal transfer/removal of the GlcNAc moiety to target proteins.
MATERIALS AND METHODS
Ethics
The Animal Ethics and Experimentation Committees of the University of Queensland, Australia, approved all experiments on mice. These committees are approved by the National Health and Medical Research Council of Australia.
Embryo Collection and Culture
Zygotes were collected from 6- to 8-wk old superovulated and mated female Quakenbush Swiss albino mice (own colony) 18 h post-hCG in Hepes-buffered KSOM medium [14] in the absence of glucose (H-KSOM-Glu). Cumulus-oocyte complexes were dispersed with hyaluronidase (1 g/L) (Sigma, St. Louis, MO), and the cumulus-free oocytes were transferred at a density of one embryo per microliter to microdroplets of KSOM medium (modified according to the experimental design) and cultured at 37°C under liquid paraffin oil in a humidified atmosphere of 5% CO2/5% O2/90% N2 for up to 90 h post-hCG or as per experimental design below.
Experimental Design
To assess the impact of O-GlcNAcylation on early embryonic development in vitro, flux through the HSP (Fig. 1) was manipulated using substrates such as glucose, glucosamine (which enters the HSP downstream of the rate-limiting enzyme GFPT and thus provides high flux), and inhibitors of the terminal transfer/removal of the O-GlcNAc moiety. Benzyl-2-acetamido-2-deoxy-α-D-galactopyranoside (BADGP; Sigma) inhibits OGT, and [O-(2-acetamido-2-deoxy-D-glucopyranosylidene) amino-N-phenylcarbamate] (PUGNAc) is a potent inhibitor of the O-GlcNAcase (Toronto Research Chemicals, North York, ON, Canada; Fig. 1). Zygotes (18 h post-hCG) were randomly allocated to culture treatment groups (20/20-μl microdroplet cultured as above) that included: no glucose and hence no flux through HSP, hyperglycemia (27 mM glucose, excess flux), and 0.2 mM glucosamine (GlcN) in the absence of glucose (HSP flux alone). Controls included culture in standard KSOM medium (with 0.2 mM glucose and glutamine, but without other amino acids in order to eliminate the potentially confounding impact of gluconeogenesis), KSOM supplemented with 26.8 mM sucrose (osmotic control for hyperglycemia), or dimethyl sulfoxide (DMSO; solvent control for inhibitors). See Table 1 for treatment summary and anticipated effects on levels of nuclear glycocylation.
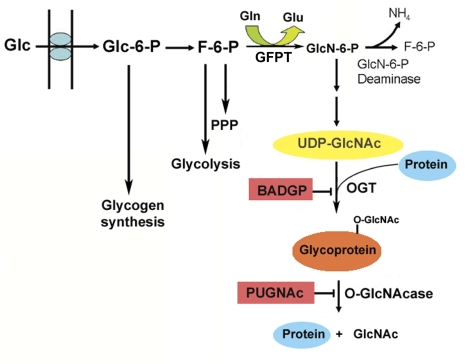
FIG. 1.
Glucose is phosphorylated upon cellular entry and isomerized to fructose-6-phosphate (F-6-P) prior to utilization through glycolysis and pentose phosphate pathway (PPP). Up to 5% of F-6-P, however, is converted to glucosamine-6-phosphate (GlcN-6-P) by the rate-limiting enzyme GFPT and subsequently acetylated and charged with UDP for use as a donor substrate in multiple biosynthetic reactions, including O-GlcNAcylation. Addition of GlcNAc to ser/thr residues of target proteins is catalyzed by OGT and its removal by an O-GlcNAcase, which together form a single complex. These reactions can be manipulated using specific inhibitors, BADGP and PUGNAc, allowing for the systematic evaluation of the role of O-linked GlcNAcylation in our system.
TABLE 1.
Experimental culture manipulations and anticipated effects on nuclear levels of O-GlcNAcylation.
For dose-response studies, zygotes were cultured in KSOM supplemented with increasing levels of either BADGP or PUGNAc, and levels of blastocyst formation were assessed following 72-h culture. The effect of DMSO at the relevant concentrations was also assessed. To assess the impact of these inhibitors on their above-specified targets, the level of O-GlcNAcylation was assessed immunologically in morulae at 68 h post-hCG (below) since previous observations had indicated that under some conditions, namely when there is no glucose flux through the hexosamine biosynthetic pathway and thus HSP activity, embryos fail to reach the blastocyst stage and degenerate as morulae [7].
The antiserum RL2, which specifically recognizes the β-O-GlcNAc linkage [15], was used to confirm that levels of O-GlcNAc were modified in response to these treatments as anticipated. Its specificity has been thoroughly investigated using a combination of Western blotting and immunoadsorption, glycosylation competition experiments, and hexoseaminidase and pronase digestion experiments [15]. The impact of HSP manipulation on the first differentiation in development, blastocyst formation, was assessed by observing the proportion of zygotes that had developed a blastocoel cavity at 90 h post-hCG. The impact on apoptosis and cell number was also assessed in individual embryos (see below).
Assessment of Nuclear Levels of O-GlcNAcylation by RL2 Immunofluorescence
Embryos were fixed in 2% paraformaldehyde in PBS (150 mM NaCl, 7.5 mM Na2HPO4, 2.6 mM NaH2PO4; pH 7.4) for 20 min at room temperature (RT) and washed twice in PBS prior to mounting upon Cell-Tak-coated cover slips (Collaborative, Biomedical Products, Bedfort, MA) for further processing as previously described [16, 17]. Following permeabilization and neutralization, embryos were blocked with 10% normal goat serum (NGS; Sigma), 4 g/L bovine serum albumin (BSA; Morgate Biotech, Bulimba, Australia), and 1% Tween-20 (ICN Biomedicals Inc., Aurora, OH) in PBS for 1 h at RT. Embryos were exposed to RL2 antiserum (Abcam Ltd., Cambridge, U.K.) at a 1:200 dilution in 5% NGS, 2 g/L BSA, and 0.5% Tween-20 in PBS overnight at 4°C and then extensively washed in PBT (4 g/L BSA, 1% Tween-20 in PBS) prior to exposure to Texas Red-conjugated secondary antibody (Calbiochem-Novachem, Alexandra, NSW, Australia) diluted 1:100 in 5% NGS, 2 g/l BSA, and 0.5% Tween-20 in PBS for 1 h at RT. Coverslips with adherent embryos were then mounted in glycerol following extensive washing in PBT and exposure to 25%, 50%, and 75% (v/v) glycerol in PBS. RL2 immunoreactivity was assessed using a Bio-Rad MRC 1024 laser-scanning confocal microscope (Bio-Rad, Reagents Park, Australia) and quantified by image analysis using the Image J software program (http://rsb.info.nih.gov/ij/). For assessment of nuclear O-GlcNAc levels, the mean gray scale intensity of two nuclei in the same optical plane for each embryo was quantified and averaged to yield one value per embryo. This was done at the same optical depth from the coverslip for each adherent embryo in each treatment per experiment to minimize any confounding impact that variable cellular depth may have on fluorescence intensity. At least five embryos per treatment in each of three experiments were assayed in this way, and data were statistically analyzed using ANOVA to determine inter- and intra-experimental variation.
Assessment of Apoptosis-TUNEL Assay
Embryos were fixed and permeabilized as above and apoptosis determined by TUNEL assay using the In Situ Death Detection Kit (Roche Diagnostics) as previously described [7]. Briefly, following permeabilization, embryos were washed thrice in PBS containing 5 mg/ml polyvinyl-pyrrolidone (PVP; Sigma) in PBS (PVP/PBS) and incubated in 15-μl drops containing 10 U/μl terminal deoxynucleotidyl transferase (DNTT), 20 μM fluorescein-conjugated dUTP, and reaction buffer for 1 h at 37°C in the dark. Positive controls were incubated with DNase I (100 U/ml; Sigma), which cleaves all DNA, for 20 min at 37°C before TUNEL. Negative controls were incubated in fluorescein-dUTP in the absence of DNTT. After TUNEL, embryos were washed three times with PVP/PBS, counterstained with 0.5 μg/ml propidium iodide (Sigma) after RNase A treatment (50 μg/ml, 60 min, 37°C), and mounted in hanging drops of glycerol prior to examination. The proportion of apoptotic nuclei per embryo was assessed in a minimum of five embryos per treatment in each of three independent experiments.
Statistics
GraphPad Prism (version 3; GraphPad Software, Inc., La Jolla, CA) was used for factorial ANOVA and subsequent means range testing using the Tukey method to determine the difference between individual treatments. Proportional data were arcsine transformed prior to ANOVA.
RESULTS
Enzymatic Inhibition of Reversible O-GlcNAcylation
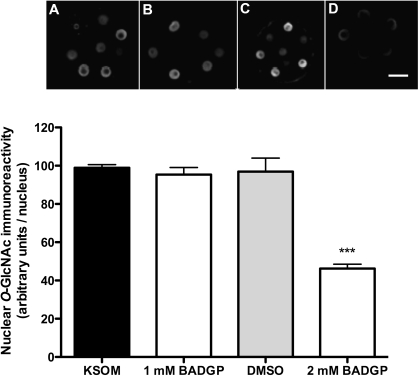
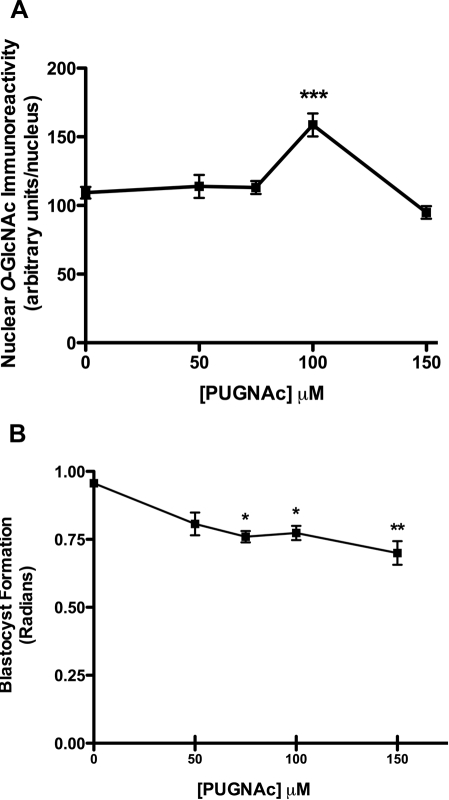
Using an antiserum that specifically targets the β-O-GlcNAc linkage (RL2), we find O-GlcNAcylation to be concentrated in nuclei rather than elsewhere in the cell (Fig. 2). Moreover, 2 mM BADGP decreases nucleoplasmic O-GlcNAc immunoreactivity by about 55% (P < 0.001) as anticipated, relative to nuclear O-GlcNAc immunoreactivity in control (KSOM) embryos (Fig. 2). Concentrations of BADGP lower than 2 mM were ineffective on both glycosylation and blastocyst formation (not shown), but though higher concentrations did show dose-dependent inhibition, this was confounded by the cytotoxic and nonspecific effects of DMSO used as a solvent. Consequently, 2 mM BADGP was used for subsequent experiments consistent with levels used to inhibit O-GlcNAcylation in bovine oocytes [18]. Conversely and as expected, inhibition of the O-GlcNAcase with 100 μM PUGNAc increased nucleoplasmic O-GlcNAcylation by 50% (Fig. 3A). Higher or lower doses were ineffective on O-GlcNAcylation but did decrease blastocyst formation (Fig. 3B).
FIG. 2.
Effect of OGT inhibition using BADGP on levels of embryonic O-GlcNAcylation assessed at 68 h post-hCG. Representative confocal immunofluorescent images of embryos immunolabeled with RL2 antiserum following culture in KSOM (0.2 mM glucose; A) or KSOM supplemented with OGT inhibitor BADGP at 1 and 2 mM (B and D, respectively). Immunoreactivity of RL2 in the presence of DMSO (solvent control) was also assessed (C). Original magnification ×250; bar = 25 μm. The mean gray scale intensity of at least two nuclei in the same optical plane for each embryo was quantified using Image J and averaged to yield one value per embryo. For each treatment in each of two experiments, at least five embryos were analyzed, and data are presented graphically as mean nuclear O-GlcNAc immunoreactivity ± SEM. Factorial ANOVA revealed no interexperimental variation or interaction and a significant decrease in RL2 immunoreactivity in response to 2 mM BADGP (Tukey post hoc test, ***P < 0.001).
FIG. 3.
Effect of inhibition of O-GlcNAcase with PUGNAc on RL2 immunoreactivity (A) and blastocyst formation (B). Zygotes (18 h post-hCG) were cultured in KSOM (0.2 mM glucose), with increasing concentrations of PUGNAc to 96 h post-hCG, and blastocyst formation was assessed. A) A subset taken at 68 h post-hCG was immunolabeled with RL2 antiserum as described in Materials and Methods. Nuclear immunoreactivity was quantified using Image J software as described. For each treatment in each of three experiments, at least five embryos were analyzed, and data are presented graphically as mean nuclear O-GlcNAc immunoreactivity ± SEM. Two-way ANOVA revealed no interexperimental variation and a significant increase in RL2 immunoreactivity in response to 100 μM PUGNAc (***P < 0.01 by Tukey post hoc test, relative to control). B) Blastocyst formation was significantly decreased by increasing concentrations of PUGNAc. Each point represents the mean ± SEM from three separate experiments, each with a minimum of 10 embryos per treatment (*P < 0.05, **P < 0.01, relative to control by ANOVA and Tukey post hoc test).
Effect of HSP Flux Perturbation on O-GlcNAcylation
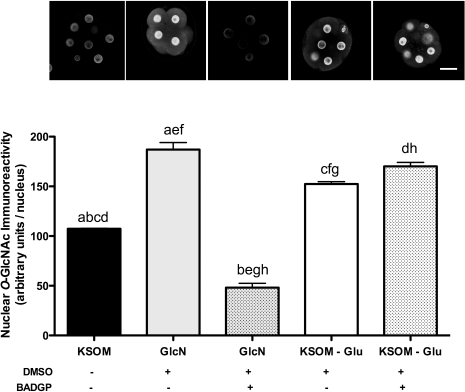
Consistent with our hypothesis, increasing flux through the HSP using 0.2 mM glucosamine, which feeds into the pathway downstream of GFPT, increased nuclear immunoreactive O-GlcNAc by about 70% (P < 0.001) as anticipated (Fig. 4). Surprisingly, embryos cultured in the absence of glucose (no flux) or in the presence of 0.2 mM glucosamine demonstrated elevated nuclear levels of O-GlcNAcylation relative to control-cultured embryos in KSOM medium (P < 0.01; Fig. 4). In order to confirm that the increased nuclear O-GlcNAcylation observed in embryos cultured in the absence of glucose and 0.2 mM glucosamine was due to increased flux through HSP and subsequent OGT activity, zygotes were cultured with KSOM in the absence of glucose but with 0.2 mM glucosamine in the presence and absence of the OGT inhibitor BADGP. Inhibition of OGT had no effect on the glucose deprivation-induced increase in nuclear O-GlcNAcylation, whereas it completely abolished the 0.2 mM glucosamine-induced increase, resulting in levels that were approximately 40% lower than control (KSOM) levels (P < 0.001; Fig. 4).
FIG. 4.
Effect of BADGP on RL2 immunoreactivity in embryos cultured under conditions that elevate levels of RL2 immunoreactivity. Zygotes (16–18 h post-hCG) were cultured in the presence and absence of 0.2 mM glucose (Glu) or 0.2 mM glucosamine (GlcN) and in the presence and absence of 2 mM BADGP. DMSO in KSOM at concentration equivalent to 2 mM BADGP was also included as solvent control. At 68 h post-hCG, the effect of these treatments on nuclear levels of RL2 immunoreactivity was assessed as previously described in each of two experiments each with at least five embryos per treatment. Each bar represents the mean (± SEM) nuclear immunoreactivity per embryo. Factorial ANOVA determined no experimental variation and no interaction. Means with the same lowercase letters are significantly different (c: P < 0.01; a, b, d–h: P < 0.001; Tukey post hoc test). Representative confocal immunofluorescent images of embryos in each treatment are also presented above each bar. Original magnification ×250; bar = 25 μm.
Effects of Perturbed HSP Flux on Blastocyst Formation
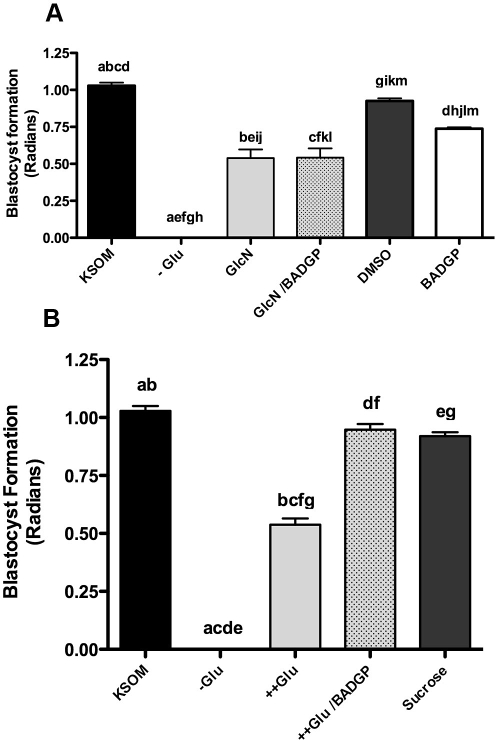
Zygotes were cultured from 18 to 90 h post-hCG in a modified glucose/glucosamine environment in the presence of the OGT inhibitor BADGP, and the impact of this on blastocyst formation was assessed. In the complete absence of glucose (-Glu), blastocyst formation is completely blocked (P < 0.001; Fig. 5). Though low levels (0.2 mM) of glucosamine in the absence of glucose may facilitate some development (Fig. 5A), this rate is significantly lower than control (P < 0.01; Fig. 5A). Moreover, embryos cultured in 5 mM glucosamine degenerated prior to 72 h post-hCG and failed to form blastocysts (not shown). BADGP was unable to ameliorate the negative effect of glucosamine on blastocyst formation (P > 0.05; Fig. 5A), suggesting that glucosamine action may have an impact on pathways other than HSP, which affects development despite significantly elevated levels of O-GlcNAcylation (Fig. 4). In contrast to this, inhibition of OGT with BADGP completely reversed the negative impact of 27 mM glucose on blastocyst formation (Fig. 5B), in support of the hypothesis that O-GlcNAcylation may be the mechanism by which maternal hyperglycemia affects blastocyst formation. The rate of blastocyst formation in the presence of 26.8 mM sucrose was not significantly different to KSOM (Fig. 5B), eliminating the possibility that hyperglycemic effects may derive from increased osmolarity of the culture medium due to elevated glucose.
FIG. 5.
A) Effect of glucosamine (GlcN) in the presence and absence of 2 mM BADGP on blastocyst formation. Zygotes were cultured from 18 to 96 h post-hCG in control KSOM (0.2 mM glucose; KSOM), KSOM-glucose (-Glu), KSOM-glucose supplemented with 0.2 mM GlcN (GlcN), 0.2 mM GlcN in the presence of 2 mM BADGP (GlcN/BADGP). A subset of zygotes were also cultured in KSOM supplemented with either 2 mM BADGP (BADGP) or DMSO. Bars represent mean ± SEM from three separate experiments each with 30 zygotes per treatment. Factorial ANOVA determined no interexperimental variation and no interaction (P > 0.05). Means were further analyzed to determine differences between different treatments. Means with the same lowercase letters are statistically different (a–i, k: P < 0.01; j, l, m: P < 0.05; Tukey post hoc test). B) Effect of hyperglycemia in the presence of 2 mM BADGP on blastocyst formation. Zygotes were cultured from 18 to 96 h post-hCG in control KSOM (KSOM), KSOM-glucose (-Glu), KSOM supplemented with 27 mM glucose (++Glu), or 27 mM glucose supplemented with 2 mM BADGP (++Glu/BADGP). 26.8 mM sucrose in KSOM (containing 0.2 mM glucose; Sucrose) was used as an osmotic control. Bars represent mean ± SEM from three separate experiments each with 30 zygotes per treatment. Factorial ANOVA determined no interexperimental variation and no interaction (P > 0.05). Means with the same lowercase letters are statistically different (P < 0.001; Tukey post hoc test).
It should be noted that both 27 mM glucose and 0.2 mM glucosamine caused a similar reduction in blastocyst formation (62% and 63%, respectively; P < 0.001; Fig. 6), highlighting the relative potency of the two HSP substrates. Moreover, as previously indicated, 2 mM BADGP significantly reduced blastocyst formation by 34% (P < 0.01; Fig. 5A) relative to control KSOM- and vehicle control (DMSO)-cultured embryos (P < 0.05; Fig. 5A), indicating that optimal OGT activity and O-GlcNAcylation are required for maximum developmental potential.
FIG. 6.
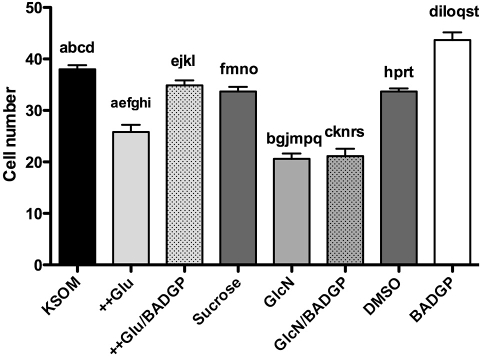
Effects of culture treatment on cell number at 90 h post-hCG. Zygotes were cultured from 18 to 90 h post-hCG in control KSOM (KSOM), KSOM supplemented with 27 mM glucose (++Glu) , KSOM with 27 mM glucose supplemented with 2 mM BADGP (++Glu/BADGP), KSOM supplemented with 26.8 mM sucrose as osmolarity control (Sucrose), 0.2 mM GlcN (GlcN), 0.2 mM GlcN supplemented with 2 mM BADGP (GlcN/BADGP), KSOM with DMSO equivalent to 2 mM BADGP (DMSO), and KSOM supplemented with 2 mM BADGP (BADGP). Embryos were fixed at 90 h post-hCG, and cell nuclei were stained with propidium iodide. Bars represent mean number of cells per embryo ± SEM from three separate experiments each with 30 zygotes per treatment. Two-way ANOVA determined no interexperimental variation (P > 0.05). Means were further analyzed to determine differences between different treatments. Means with the same lowercase letters are statistically different (a–c, e, f, h–t: P < 0.001; d, g: P < 0.05; Tukey post hoc test).
Effects of Perturbed HSP Flux on Cell Number
Surprisingly, though 2 mM BADGP reduced blastocyst formation (P < 0.01; Fig. 5A), it increased cell number by approximately 16% relative to control-cultured embryos (P < 0.01; Fig. 6). Increased flux through the HSP as a result of both hyperglycemia (27 mM glucose) and glucosamine supplementation reduced cell number at 90 h post-hCG by approximately 22% (P < 0.001) and 46% (P < 0.001), respectively (Fig. 6). Once again, glucosamine appeared more potent, causing a reduction in cell number that was significantly lower (P < 0.01) than was observed in embryos exposed to hyperglycemia. Indeed, embryos cultured in the presence of 5 mM glucosamine degenerated prior to 72 h post-hCG; hence, cell number and apoptosis could not be assessed in these embryos. BADGP was able to completely reverse the effects of hyperglycemia on cell number, suggesting that O-GlcNAcylation is the mechanism by which hyperglycemia negatively affects cellular proliferation in the early embryo. Consistent with observations on blastocyst formation (Fig. 5), BADGP was not effective in reversing the impact of glucosamine on cell number, though it did reduce the difference relative to hyperglycemia-treated embryos to nonsignificance (Fig. 6).
Effects of Perturbed HSP Flux on Apoptosis
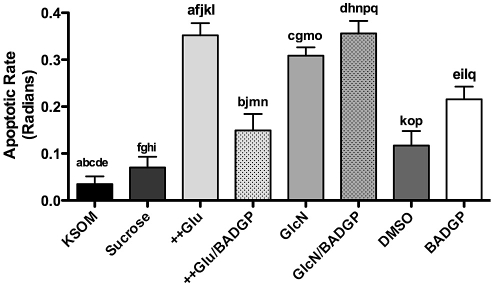
Though embryo exposure to BADGP increased cell number (Fig. 6), it also increased the proportion of cells undergoing apoptosis by more than 3-fold (P < 0.05; Fig. 7). Neither 26.8 mM sucrose nor DMSO had any significant effect on apoptosis as assessed at 90 h post-hCG, in contrast to both hyperglycemia and 0.2 mM glucosamine, which similarly and significantly increased apoptosis relative to control (Fig. 7). Consistent with previous observations, BADGP was able to limit the proapoptotic effects of hyperglycemia (P < 0.05; Fig. 7) to a level not significantly different from the DMSO control but nonetheless higher than control. Once again OGT inhibition did not reverse the apoptotic effects of glucosamine supplementation.
FIG. 7.
Impact of perturbed HSP flux on apoptosis in blastocysts at 90 h post-hCG. Zygotes (18 h post-hCG) were cultured in KSOM (KSOM), KSOM supplemented with 26.8 mM sucrose as osmolarity control (Sucrose), KSOM-glucose supplemented with 27 mM glucose (++Glu), KSOM with 27 mM glucose supplemented with 2 mM BADGP (++Glu/BADGP), 0.2 mM GlcN (GlcN), 0.2 mM glucosamine supplemented with 2 mM BADGP (GlcN/BADGP), KSOM with DMSO equivalent to 2 mM BADGP (DMSO), and KSOM with 2 mM BADGP (BADGP). The proportion of TUNEL-positive nuclei per embryo is presented as the rate of apoptosis in radians for statistical analysis by ANOVA. Bars represent means ± SEM from three separate experiments each with five embryos per treatment. Factorial ANOVA determined no interexperimental variation and no interaction (P > 0.05). Means with the same lowercase letters are statistically different (a, c–h, j, k, m–p: P < 0.001; i, l, q: P < 0.01; b: P < 0.05; Tukey post hoc test).
DISCUSSION
Maternal hyperglycemia is thought to be the metabolic derangement associated with both early pregnancy loss and congenital malformations in a diabetic pregnancy. Moreover, it has been proposed that hyperglycemia-induced apoptosis of the embryonic progenitor cells as early as the preimplantation period may contribute to subsequent spontaneous abortion and morphogenic defects [19]. The mechanisms by which the embryotoxic effects of hyperglycemia are manifested at this time of development are not known. We have found significant data to support our hypothesis that increased flux through the HSP resulting in the O-GlcNAcylation may underlie the glucotoxic effects of hyperglycemia on early embryo development.
Previous studies suggested that hyperglycemia triggers BAX-dependent apoptosis in the mouse blastocyst via a downregulation of, notably, SLC2A1 glucose transporter expression [20]. We believe this is secondary to dysregulation of the HSP and increased O-GlcNAcylation. Consistent with our hypothesis, all treatments that perturbed the level of O-GlcNAc have a negative impact on early development.
Our observations of predominantly nuclear O-GlcNAc immunoreactivity, rather than cytoplasmic or extracellular, support others that show the highest levels of O-GlcNAcylated proteins are concentrated in the nucleus [21]. This is also consistent with nuclear accumulation of 14C-label grains in embryos that were pulse-labeled with 14C-glucosamine [22]. Several hundred proteins are modified by O-GlcNAcylation, including transcription factors, kinases, phosphatases, cytoskeletal proteins, and nuclear hormone receptors. This reversible modification is mediated by the unique OGT, which unlike other protein glycosyltransferases is not found in the Golgi secretory pathway but is concentrated in the nucleoplasmic compartment [21], once again consistent with our observations of nuclear concentrated O-GlcNAcylation. Inhibition of the enzymes involved in the terminal transfer of the O-GlcNAc, OGT and O-GlcNAcase, causes, respectively, a decrease and an increase in the level of nucleoplasmic O-GlcNAcylation in early embryos as anticipated. More importantly, these perturbations of embryonic O-GlcNAcylation result in decreased blastocyst formation, suggesting that this equilibrium and level of O-GlcNAcylation is tightly regulated in embryos and critical for cellular homeostasis. Paradoxically, inhibition of OGT increased cell number despite decreased levels of blastocyst formation, suggesting that the level of glucose in the culture medium (0.2 mM) may not be optimal. The elevated level of apoptosis, however, does not support this proposal.
Consistent with previous observations [7], glucose deprivation completely ablated blastocyst formation. Intriguingly, this did not cause a reduction in nucleoplasmic O-GlcNAcylation as might be anticipated. Indeed the level of nucleoplasmic O-GlcNAc was increased significantly. That BADGP did not reverse this level of O-GlcNAcylation, despite its efficacy on glucosamine-induced O-GlcNAc upregulation, suggests that in the complete absence of glucose flux through this pathway and hence limited production of UDP-GlcNAc and hence OGT activity, O-GlcNAcase activity may also be inhibited, again reflecting the tight control of this equilibrium.
This is not surprising given that the activity of the O-GlcNAcase, which also incorporates a histone acetyl transferase domain [23], may be modulated as a result of its association into a single O-GlcNAc modifying multi-enzyme complex with OGT [23, 24]. Further illustrating the complex regulation of the OGT/O-GlcNAcase equilibrium, OGT levels of expression are upregulated, whereas expression of O-GlcNAcase is downregulated by glucose deprivation and thus reduced UDP-GlcNAc levels [24, 25]. The failure of BADGP to inhibit the glucose deprivation-induced increase in O-GlcNAcylation, as it did for glucosamine, suggests that the effects on O-GlcNAcase activity predominate in glucose-deprived embryos.
The functional outcome of this equilibrium perturbation by the absence of glucose is that blastocyst formation is completely inhibited. The impact of PUGNAc as a transient increase in RL2 immunoreactivity also supports a model of cooperative activity between the two enzymes, as does other data that demonstrate that basal OGT activity is modulated by its own intrinsic O-glycosylation/phosphorylation status [25]. Ultimately, this cooperativity would result in a finely balanced equilibrium wherein even small deviations in O-GlcNAcylation would affect cellular signaling activities and, therefore, cellular homeostasis in response to a nutrient signal.
An interesting observation was that addition of glucosamine is able to partially overcome the block to blastocyst formation caused by glucose deprivation. Either the level of HSP flux is not optimal at this concentration of glucosamine or alternate pathways modulated by glucosamine affect developmental potential. Glucosamine is a potent stimulator of O-GlcNAcylation because even at 0.2 mM, glucosamine increased O-GlcNAcylation by 72% and BADGP inhibition of OGT dramatically reduced this hyper O-GlcNAcylation well below control levels. Glucosamine, which specifically feeds through the HSP downstream of GFPT (Fig. 1) to increase O-GlcNAcylation, was also more potent in inhibiting/blocking blastocyst formation than hyperglycemia. Indeed, 0.2 mM glucosamine reduced blastocyst formation by approximately the same degree as 27 mM glucose, and 5 mM glucosamine inhibited blastocyst formation completely. The relative potency of glucose to glucosamine may arise from differential negative feedback on hexokinase by the phosphorylated products to limit further phosphorylation and hence uptake of glucose by facilitative diffusion. Glucose-6-phosphate is a potent inhibitor of hexokinase, whereas GlcN-6-P is a relatively weak inhibitor [26]. This would result in the rapid accumulation of GlcN-6-P and significantly higher HSP flux.
Though embryo development is much more sensitive to glucosamine, this impact is not ameliorated by OGT inhibition despite a reduction in nuclear immunoreactive O-GlcNAc levels. Perhaps glucosamine affects additional pathways in order to exert these morphological effects in the early embryo. This is in sharp contrast to embryonic exposure to hyperglycemia in vitro and to studies of pancreatic β-cells in which BADGP reversed the apoptotic effects of glucosamine [27]. The difference may be because we used glucosamine in the absence of glucose, whereas the latter study of pancreatic cells used the medium (M199 containing 5.5 mM glucose) supplemented with glucosamine. The absence of glucose may have widespread effects in embryos, such as increases in reactive oxygen species as recently demonstrated [28].
So though glucosamine enables embryonic metabolic differentiation as previously reported [7], in the absence of glucose additional stress response pathways may be activated as a result of oxidative stress, independently of its effects through O-GlcNAcylation. Glucosamine enters cells and is readily phosphorylated to GlcN-6-P, which enters the HSP downstream of entry into the PPP (Fig. 1). In the absence of glucose, flux through the PPP is attenuated, reducing NADPH production and increasing oxidative stress [29]. Moreover, GlcN-6-P is a potent inhibitor of the first enzyme of the PPP glucose-6-P-dehydrogenase [29]; therefore, this high GlcN-6-P arising from glucosamine supplementation may also limit PPP flux arising from any gluconeogenic activity. The sum result is compromised development and increased cell death that cannot be reversed by OGT inhibition. Although such metabolism has not been reported in preimplantation embryos, administration of glucosamine to pregnant (Day 7.5) mice inhibits PPP relative to glucose and increases oxidative stress more potently than hyperglycemia within 3 h [30] in support of our hypothesis.
Nonetheless, the key finding is that the toxicity of maternal hyperglycemia in a number of morphological parameters of early development is indeed either reversed or ameliorated (apoptosis) by inhibition of OGT, supporting the hypothesis that the HSP does indeed represent a mechanism by which hyperglycemia affects early embryo development and survival. The central mechanism implicated in the regulation of embryonic cellular proliferation and survival is the PI 3-kinase/Akt pathway [31]. In the early embryo, this survival signaling pathway is normally activated by trophic growth factor signals [32] to reduce apoptosis and enhance cellular proliferation by activating downstream pathways of cellular growth, proliferation, and survival. Additionally, it regulates glucose transporter recycling at the plasma membrane, allowing the embryo to coordinate nutrient transport and protein synthetic activities with the modified metabolic requirements of stimulated proliferation [33]. Impaired PI 3-kinase activation and reduced Akt activity in response to increased O-GlcNAcylation provides an explanation for decreased survival in response to hyperglycemia, defective glucose transporter recycling, and stimulated cell proliferation, and hence the insulin/growth factor resistance feature of diabetes. Though PI 3-kinase has been identified as an OGT substrate [10], it is not clear whether this is so for Akt. However, in pancreatic islet cells, increased flux through HSP impairs the PI 3-kinase/Akt survival signaling pathway and can be overcome by OGT inhibition [27]. Moreover, inhibition of the O-GlcNAcase with PUGNAc also inhibits phosphorylation of both Akt and GSK-3β [34], suggesting that O-GlcNAcylation has a direct impact on Akt activity and hence cell survival, consistent with our proposal. The results suggest that in a clinical situation where a diabetic woman seeks to become pregnant, extremely close management of metabolism during the conception and preimplantation phases is warranted to maximize fertility and reduce the potential for an impact on health of the offspring.
In conclusion, we have shown that the HSP and O-GlcNAcylation are the mechanisms by which the embryotoxic effects of hyperglycemia are manifested during the earliest stages of development. As in somatic cells, this glucose-sensing pathway provides the early embryo with a mechanism with which to tightly couple cellular physiology with nutrient availability.
Footnotes
Supported by the NICHD National Cooperative Program on Female Health and Egg Quality under cooperative agreement U01 HD044644.
REFERENCES
- Beebe LF, Kaye PL.Preimplantation development in the streptozotocin-induced diabetic mouse. Reprod Fertil Dev 1990; 2: 407–412. [DOI] [PubMed] [Google Scholar]
- Diamond MP, Moley KH, Pellicer A, Vaughn WK, DeCherney AH.Effects of streptozotocin- and alloxan-induced diabetes mellitus on mouse follicular and early embryo development. J Reprod Fertil 1989; 86: 1–10. [DOI] [PubMed] [Google Scholar]
- Moley KH, Vaughn WK, DeCherney AH, Diamond MP.Effect of diabetes mellitus on mouse pre-implantation embryo development. J Reprod Fertil 1991; 93: 325–332. [DOI] [PubMed] [Google Scholar]
- Moley KH, Chi MM, Knudson CM, Korsmeyer SJ, Mueckler MM.Hyperglycemia induces apoptosis in pre-implantation embryos through cell death effector pathways. Nat Med 1998; 4: 1421–1424. [DOI] [PubMed] [Google Scholar]
- Fleming TP, Kwong WY, Porter R, Ursell E, Fesenko I, Wilkins A, Miller DJ, Watkins AJ, Eckert JJ.The embryo and its future. Biol Reprod 2004; 71: 1046–1054. [DOI] [PubMed] [Google Scholar]
- Watkins AJ, Ursell E, Panton R, Papenbrock T, Hollis L, Cunningham C, Wilkins A, Perry VH, Sheth B, Kwong WY, Eckert JJ, Wild AE, et al. Adaptive responses by mouse early embryos to maternal diet protect fetal growth but predispose to adult onset disease. Biol Reprod 2008; 78: 299–306. [DOI] [PubMed] [Google Scholar]
- Pantaleon M, Scott J, Kaye PL.Nutrient sensing by the early mouse embryo: hexosamine biosynthesis and glucose signaling during preimplantation development. Biol Reprod 2008; 78: 595–600. [DOI] [PubMed] [Google Scholar]
- Sayeski PP, Kudlow JE.Glucose metabolism to glucosamine is necessary for glucose stimulation of transforming growth factor-alpha gene transcription. J Biol Chem 1996; 271: 15237–15243. [DOI] [PubMed] [Google Scholar]
- Marshall S, Rumberger J.The hexosamine signaling pathway: role in glucose sensing and integration of cellular metabolism. Walker MBP, Rizza RA.The Diabetes Annual, vol. 13 New York:Elselvier;2000: 97–112. [Google Scholar]
- Love DC, Hanover JA.The hexosamine signaling pathway: deciphering the “O-GlcNAc code”. Sci STKE 2005; 312: re13 [DOI] [PubMed] [Google Scholar]
- Zachara NE, Cheung WD, Hart GW.Nucleocytoplasmic glycosylation, O-GlcNAc: identification and site mapping. Methods Mol Biol 2004; 284: 175–194. [DOI] [PubMed] [Google Scholar]
- Wells L, Hart GW.O-GlcNAc turns twenty: functional implications for post-translational modification of nuclear and cytosolic proteins with a sugar. FEBS Lett 2003; 546: 154–158. [DOI] [PubMed] [Google Scholar]
- Kreppel LK, Hart GW.Regulation of a cytosolic and nuclear O-GlcNAc transferase. Role of the tetratricopeptide repeats. J Biol Chem 1999; 274: 32015–32022. [DOI] [PubMed] [Google Scholar]
- Lawitts JA, Biggers JD.Culture of preimplantation embryos. Methods Enzymol 1993; 225: 153–164. [DOI] [PubMed] [Google Scholar]
- Holt GD, Snow CM, Senior A, Haltiwanger RS, Gerace L, Hart GW.Nuclear pore complex glycoproteins contain cytoplasmically disposed O-linked N-acetylglucosamine. J Cell Biol 1987; 104: 1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleon M, Jericho H, Rabnott G, Kaye PL.The role of insulin-like growth factor II and its receptor in mouse preimplantation development. Reprod Fertil Dev 2003; 15: 37–45. [DOI] [PubMed] [Google Scholar]
- Pantaleon M, Kanai-Azuma M, Mattick JS, Kaibuchi K, Kaye PL, Wood SA.FAM deubiquitylating enzyme is essential for preimplantation mouse embryo development. Mech Dev 2001; 109: 151–160. [DOI] [PubMed] [Google Scholar]
- Sutton-McDowall ML, Mitchell M, Cetica P, Dalvit G, Pantaleon M, Lane M, Gilchrist RB, Thompson JG.Glucosamine supplementation during in vitro maturation inhibits subsequent embryo development: possible role of the hexosamine pathway as a regulator of developmental competence. Biol Reprod 2006; 74: 881–888. [DOI] [PubMed] [Google Scholar]
- Moley KH.Hyperglycemia and apoptosis: mechanisms for congenital malformations and pregnancy loss in diabetic women. Trends Endocrinol Metab 2001; 12: 78–82. [DOI] [PubMed] [Google Scholar]
- Chi MM, Pingsterhaus J, Carayannopoulos M, Moley KH.Decreased glucose transporter expression triggers BAX-dependent apoptosis in the murine blastocyst. J Biol Chem 2000; 275: 40252–40257. [DOI] [PubMed] [Google Scholar]
- Holt GD, Hart GW.The subcellular distribution of terminal N-acetylglucosamine moieties. Localization of a novel protein-saccharide linkage, O-linked GlcNAc. J Biol Chem 1986; 261: 8049–8057. [PubMed] [Google Scholar]
- Acey R, Hazlett L, Dabich D.Mouse blastocysts pulse labeled with 14C-glucosamine: incorporation and ultrastructural analysis. Biol Reprod 1977; 16: 564–570. [DOI] [PubMed] [Google Scholar]
- Toleman C, Paterson AJ, Whisenhunt TR, Kudlow JE.Characterization of the histone acetyltransferase (HAT) domain of a bifunctional protein with activable O-GlcNAcase and HAT activities. J Biol Chem 2004; 279: 53665–53673. [DOI] [PubMed] [Google Scholar]
- Whisenhunt TR, Yang X, Bowe DB, Paterson AJ, Van Tine BA, Kudlow JE.Disrupting the enzyme complex regulating O-GlcNAcylation blocks signaling and development. Glycobiology 2006; 16: 551–563. [DOI] [PubMed] [Google Scholar]
- Kreppel LK, Blomberg MA, Hart GW.Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J Biol Chem 1997; 272: 9308–9315. [DOI] [PubMed] [Google Scholar]
- Crane RK, Sols A.The non-competitive inhibition of brain hexokinase by glucose-6-phosphate and related compounds. J Biol Chem 1954; 210: 597–606. [PubMed] [Google Scholar]
- D'Alessandris C, Andreozzi F, Federici M, Cardellini M, Brunetti A, Ranalli M, Del Guerra S, Lauro D, Del Prato S, Marchetti P, Lauro R, Sesti G.Increased O-glycosylation of insulin signaling proteins results in their impaired activation and enhanced susceptibility to apoptosis in pancreatic beta-cells. FASEB J 2004; 18: 959–961. [DOI] [PubMed] [Google Scholar]
- Jansen S, Cashman K, Thompson JG, Pantaleon M, Kaye PL.Glucose deprivation, oxidative stress and peroxisome proliferator-activated receptor-alpha (PPARA) cause peroxisome proliferation in preimplantation mouse embryos. Reproduction 2009; 138: 493–505. [DOI] [PubMed] [Google Scholar]
- Wu G, Haynes TE, Li H, Yan W, Meininger CJ.Glutamine metabolism to glucosamine is necessary for glutamine inhibition of endothelial nitric oxide synthesis. Biochem J 2001; 353: 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horal M, Zhang Z, Stanton R, Virkamaki A, Loeken MR.Activation of the hexosamine pathway causes oxidative stress and abnormal embryo gene expression: involvement in diabetic teratogenesis. Birth Defects Res A Clin Mol Teratol 2004; 70: 519–527. [DOI] [PubMed] [Google Scholar]
- Riley JK, Carayannopoulos MO, Wyman AH, Chi M, Moley KH.Phosphatidylinositol 3-kinase activity is critical for glucose metabolism and embryo survival in murine blastocysts. J Biol Chem 2006; 281: 6010–6019. [DOI] [PubMed] [Google Scholar]
- Li Y, Chandrakanthan V, Day ML, O'Neill C.Direct evidence for the action of phosphatidylinositol (3,4,5)-trisphosphate-mediated signal transduction in the 2-cell mouse embryo. Biol Reprod 2007; 77: 813–821. [DOI] [PubMed] [Google Scholar]
- Pantaleon M, Kaye PL.IGF-I and insulin regulate glucose transport in mouse blastocysts via IGF-I receptor. Mol Reprod Dev 1996; 44: 71–76. [DOI] [PubMed] [Google Scholar]
- Vosseller K, Wells L, Lane MD, Hart GW.Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc Natl Acad Sci U S A 2002; 99: 5313–5318. [DOI] [PMC free article] [PubMed] [Google Scholar]