Abstract
Conditional ablation of Indian hedgehog (Ihh) in the murine uterus results in mice that are sterile because of defects in embryo implantation. We performed microarray analysis on these mice at the time point at which the Ihh target genes are induced by the administration of exogenous hormone to mimic Day 3.5 of pregnancy. This analysis identified 863 genes altered by the conditional ablation of Ihh. Of these, genes that regulated the cell cycle were overrepresented. In addition, genes involved in epidermal growth factor (EGF) and estrogen (E2) signaling were found to be deregulated upon Ihh ablation. Furthermore, upon conditional ablation of Ihh, 15-mo-old mice exhibited hallmarks of estrogenized uteri, such as cystically dilated glands and hyalinized stroma. Thus, Ihh regulates embryo implantation by having an impact on the cell cycle, EGF signaling, and E2 signaling.
Keywords: estrogen, implantation, Indian Hedgehog, mouse, uterus
Gene expression profiling reveals the critical role of Indian hedgehog in cell cycle progression, EGF signaling, and the regulation of estrogen signaling in the uterus during embryo implantation.
INTRODUCTION
A critical event in the establishment of a successful pregnancy is embryo implantation in which the blastocyst attaches to and invades through the luminal epithelium of the uterus and into the stroma [1]. The ovarian steroid hormones, estrogen (E2) and progesterone (P4), acting through their cognate receptors, the estrogen receptor (ESR1) and the progesterone receptor (PGR), are necessary for these events in early pregnancy [2]. On Day 0.5 (d0.5; 0.5 = vaginal plug) of pregnancy in mice, a preovulatory surge of E2 stimulates uterine epithelial cell proliferation. Upon formation of the corpus luteum, there is an increase in P4 levels, resulting in uterine stromal cell proliferation on d2.5. On d3.5, there is an acute spike of E2, which in combination with the luteal P4, maintains uterine stromal cell proliferation, which renders the uterus receptive to the implanting embryo. Implantation can only occur if the uterus is receptive to the incoming blastocyst and there is a defined “window of receptivity” during which E2 is the primary determinant [3]. Embryo implantation in the mouse occurs on d4.5. Female Pgr knockout (PgrKO) mice are sterile due to a failure in embryo implantation, demonstrating the critical role of P4 signaling in this process [4]. To mechanistically understand how P4 exerts its effect during embryo implantation, downstream target genes of uterine PGR need to be identified.
Indian hedgehog (Ihh), which is a member of the Hedgehog family of ligands, was identified as a P4-regulated gene in the uterus [5, 6]. Hedgehog signaling has been shown to be important for the development of multiple tissues, including (but not limited to) the limbs, cerebellum, bone, cartilage, gonads, and heart [7]. Deregulation of hedgehog signaling has also been implicated in cancers, such as basal cell carcinoma, medulloblastoma, pancreatic cancer, prostate cancer, and lung cancer [8]. Ihh is expressed in the mouse uterine luminal epithelium in the preimplantation period, with its highest expression on d2.5, whereas its downstream target genes, patched 1 (Ptch1) and chicken ovalbumin upstream promoter transcription factor II (COUP-TFII, official symbol Nr2f2), are expressed in the uterine stroma, with their highest expression on d3.5 during the “window of receptivity” [5, 6, 9]. In the human endometrium, IHH expression significantly decreases during the transition from the early to the mid secretory phase, which is associated with a downregulation of cellular division [10].
Ihh has been shown to be critical for uterine function because conditional ablation of Ihh (PRcre/+Ihhf/f, official allele symbols Pgrtm2(cre)Lyd/+Ihhtm1Blan) in the mouse uterus results in infertility due to a failure of embryo implantation [11]. Rather than successful apposition and attachment of the blastocyst to the uterine luminal epithelium, embryos in the Pgrtm2(cre)Lyd/+ Ihhtm1Blan uterus were found to be floating in the uterine lumen. This was partly due to increased expression of mucin 1 (Muc1) in the Pgrtm2(cre)Lyd/+ Ihhtm1Blan uterus, the loss of which is necessary for successful embryo implantation [12]. In addition to the attachment defect, the Pgrtm2(cre)Lyd/+ Ihhtm1Blan uterus failed to undergo the decidualization reaction due to deficient preparation in the preimplantation period, as seen by decreased stromal cell proliferation and decreased vascularization of the stromal compartment [11]. Examination of genes known to be involved in embryo implantation, such as homeobox A10 (Hoxa10) and leukemia inhibitory factor (Lif), revealed them to be unaltered by Ihh ablation. Interestingly, whereas the epidermal growth factor (EGF) ligands heparin-binding epidermal growth factor (Hbegf) and amphiregulin (Areg) were not altered in the Pgrtm2(cre)Lyd/+ Ihhtm1Blan uterus, one of their receptors, epidermal growth factor receptor (Egfr), was significantly reduced in the Pgrtm2(cre)Lyd/+ Ihhtm1Blan subepithelial stroma. These data demonstrate a critical role for Ihh in the preimplantation period and suggest that Ihh regulates multiple pathways during this critical time point.
Further demonstration of the critical role of Ihh in the preimplantation period was the female infertility defect displayed by female mice with Nr2f2 ablation (either as heterozygotes or conditional uterine ablation) [13, 14]. These mice phenocopied the Pgrtm2(cre)Lyd/+ Ihhtm1Blan mice in that they exhibited defective embryo implantation with failed embryo attachment and a reduced decidual response. Again, this defect was due to improper preparation of the uterus, as there were increased Muc-1 levels, decreased stromal cell proliferation, and incomplete vascularization of the stroma. In addition, ablation of Nr2f2 increased ESR1 levels in the luminal epithelium, resulting in increased ESR1 activity in the uterus, suggesting that Hedgehog signaling may be a critical regulator of E2 signaling in the uterus.
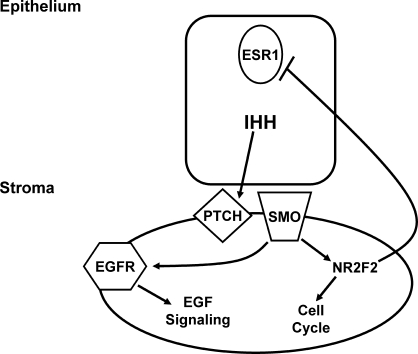
Here, we have performed microarray analysis on the Pgrtm2(cre)Lyd/+ Ihhtm1Blan uteri to identify the pathways regulated by Ihh during embryo implantation [11, 15]. From this analysis, we have identified multiple pathways as being Ihh regulated in the uterus during the preimplantation period. Among these, we observed a decrease in genes necessary for cell cycle progression and a deregulation of the EGF signaling pathway. Furthermore, we observed increased E2 signaling in the Pgrtm2(cre)Lyd/+ Ihhtm1Blan uterus, which resulted in a pathological phenotype. Thus, we have demonstrated a critical role for Ihh in the preparation of the uterus for the incoming embryo in the preimplantation period as a mediator of cell cycle progression as well as EGF and E2 signaling.
MATERIALS AND METHODS
Animals and Hormone Treatments
Mice were maintained in the designated animal care facility at Baylor College of Medicine according to the institutional guidelines for the care and use of laboratory animals. Mice were treated with an abbreviated protocol used to elicit an artificial decidual response, as previously described [16]. Briefly, 6-wk-old female wild-type, Pgrtm2(cre)Lyd/+ , Pgr+/+Ihhtm1Blan, and Pgrtm2(cre)Lyd/+ Ihhtm1Blan mice were ovariectomized and rested for 2 wk before treatment with three s.c. daily injections of 100 ng of E2 per mouse. After 2 days of rest, mice were then treated with daily injections of 1 mg of P4 and 6.7 ng of E2 per mouse s.c. for 2 days. Mice were killed 6 h after the last E2 plus P4 injection by cervical dislocation while under anesthetic (Avertin [2,2-tribromoethyl alcohol]; Sigma-Aldrich, St. Louis, MO). After tissue dissection, uterine tissues were placed in 4% paraformaldehyde or flash frozen and stored at −80°C. Serum testosterone levels were measured in 15-mo-old mice by radioimmunoassay by the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core. Serum estradiol levels were measured in 3-mo-old mice using the Estradiol Kit (no. 33540) for the Beckman Coulter Access 2 System (Beckman Coulter Inc., Fullerton, CA).
RNA Isolation and Microarray Hybridization
Total RNA was extracted from uterine tissues using the Qiagen RNAeasy total RNA isolation kit (Qiagen, Valencia, CA). The RNA was pooled from the uteri of three mice per genotype. All RNA samples were analyzed with a Bioanalyzer 2100 (Agilent Technologies, Wilmington, DE) before microarray hybridization. Microarray analysis was performed by the Baylor College of Medicine Microarray Core Facility using Affymetrix murine genome 430 2.0 mouse oligonucleotide arrays (Affymetrix, Santa Clara, CA), as previously described [17]. All experiments were performed in triplicate with independent pools of RNA.
Data Analysis
Microarray data analysis was performed as previously described [17, 18]. DNA chip analyzer dChip was used to adjust arrays to a common baseline using invariant set normalization [19]. To estimate expression, the PM-only model developed by Li and Wong [20] and Li and Hung Wong [21] was used. We selected differentially expressed genes in the Pgr+/+Ihhtm1Blan and Pgrtm2(cre)Lyd/+ Ihhtm1Blan mice using a two-sample comparison according to the following criteria: lower boundary of 90% confidence interval of fold-change greater than 1.2 and an absolute value of difference between group means greater than 50. Differentially expressed genes were classified according to Gene Ontology function using Affymetrix annotation (NetAffx; http://affymetrix.com/index.affx), and pathway analysis was performed using DAVID Analysis [22, 23] and Ingenuity Systems Software (Ingenuity Systems Inc., Redwood City, CA).
Real-Time RT-PCR Analysis
Total RNA was extracted from uterine tissues (n = 7 per genotype) using the Trizol reagent according to manufacturer's instructions (Invitrogen, Carlsbad, CA). Total RNA (1 μg) was reverse transcribed into cDNA with M-MLV (Invitrogen) in a 20-μl volume. Expression levels of mRNA were measured by real-time RT-PCR TaqMan analysis using the ABI Prism 7700 Sequence Detector System according to the manufacturer's instructions (PE Applied Biosystems, Foster City, CA). Real-time probes and primers were purchased from Applied Biosystems; for a complete list, see Supplemental Table S1 (all supplemental files for this article are available online at www.biolreprod.org). All real-time RT-PCR was performed using independent RNA sets. All mRNA quantities were normalized against 18S RNA using ABI rRNA control reagents (Applied Biosystems). Statistical analyses used one-way ANOVA followed by Tukey posthoc multiple range test with the Instat package from GraphPad (San Diego, CA).
Immunohistochemistry
Uteri were fixed overnight in 4% paraformaldehyde (vol/vol), followed by thorough washing in 70% ethanol, and tissues were processed, embedded in paraffin, and sectioned. Uterine sections from paraffin-embedded tissue were cut at 5 μm and mounted on silane-coated slides, deparaffinized, and rehydrated in a graded alcohol series. Sections were preincubated with 10% normal goat serum in PBS (pH 7.5) and then incubated with anti-CCND1 (1:500; NeoMarkers, Fremont, CA), anti-MCM3 (1:500; Santa Cruz Biotechnology, Santa Cruz, CA), or anti-ERα (ESR1; 1:200; DAKO, Carpinteria, CA) in 10% normal serum in PBS (pH 7.5). On the following day, sections were washed in PBS and incubated with biotinylated secondary antibody (5 μl/ml; Vector Laboratories, Burlingame, CA) for 1 h at room temperature. Immunoreactivity was detected using the DAB Substrate kit (Vector Laboratories); immunoreactivity was visualized as intense brown staining. Masson trichrome staining was performed by the Baylor College of Medicine Center for Comparative Medicine Comparative Pathology Laboratory using standard protocols.
RESULTS
Identification of Indian Hedgehog-Regulated Genes During Embryo Implantation
Previously, we generated mice in which Ihh was conditionally ablated in the murine uterus using the Pgrcre mouse model (Pgrtm2(cre)Lyd/+ Ihhtm1Blan) [11, 15]. These mice were found to be infertile, demonstrating an inability of the uterus to undergo embryo attachment and decidualization. To identify the pathways that Ihh regulates during these processes, we performed high-density DNA microarray analysis on the uteri from these mice. Ihh expression is highest on d2.5 of pregnancy, whereas the expression of its downstream target genes Ptch1 and Nr2f2 is highest on d3.5 of pregnancy, which is just prior to embryo implantation [5, 6]. For this reason, our microarray analysis was conducted at this time point. However, to avoid variability due to the presence of a dormant blastocyst [24] or variations in staging of pregnancy due to differing times of coitus, we simulated d3.5 of pregnancy by administering ovariectomized mice with exogenous steroid hormones using a modification of the protocol used to induce a decidual response (see Materials and Methods) [16]. We had previously determined that 30 h after the first E2 plus P4 injection used in the artificially induced decidual response gave full activation of the Ihh signaling pathway (Fig. 1) [9]. Total RNA extracts were subjected to microarray analysis using the Affymetrix mouse genome 430 2.0 arrays. This analysis revealed 429 and 434 transcripts whose abundance was significantly increased or decreased, respectively, in the Pgrtm2(cre)Lyd/+ Ihhtm1Blan uterus compared with Pgr+/+Ihhtm1Blan controls. A complete list of the genes whose transcripts increase or decrease in abundance can be found in Supplemental Tables S2 and S3, respectively. To determine which pathways are regulated by Ihh at this time point, we performed pathway analysis using DAVID Analysis and Ingenuity Systems Software [22, 23]. A complete list of the significantly regulated pathways can be found in Supplemental Table S4. The altered pathways included (but were not limited to) those involved in the cell cycle, WNT signaling, MAPK signaling, TGFB signaling, JNK signaling and EGF signaling (Supplemental Table S4). These pathways have all been implicated in embryo implantation, suggesting that Ihh is a critical mediator of multiple aspects of making the uterus receptive to an incoming embryo [1, 2]. To begin to dissect out these pathways, we first examined those shown to be previously involved in the phenotypes of the Pgrtm2(cre)Lyd/+ Ihhtm1Blan uterus [11].
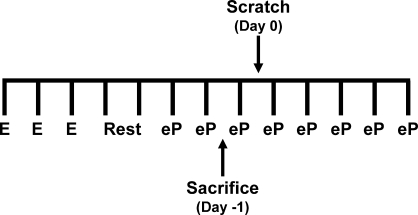
FIG. 1.
Schematic of microarray analysis experimental design. Microarray analysis was performed using an abbreviated artificial decidual response protocol in which the uteri were harvested 30 h after the first E2 plus P4 injection. Each vertical bar marks 1 day. E, estrogen injection (100 ng); Rest, no injection; eP, estrogen plus progesterone injection (6.7 ng and 1 mg, respectively).
Cell Cycle Genes Are Deregulated by Ablation of Ihh
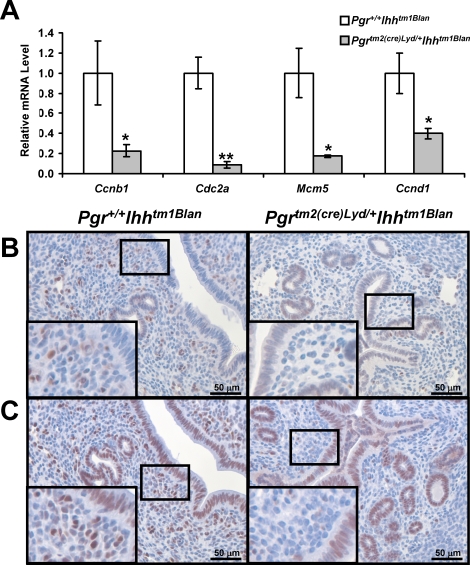
Analysis of the differentially regulated genes demonstrated that 23 genes that regulate cell cycle progression were significantly decreased in the Pgrtm2(cre)Lyd/+ Ihhtm1Blan uterus. Among these altered genes were the components of the mitosis-promoting factor, cyclin B1 (Ccnb1); cell division cycle 2 homolog A (Cdc2a/CDK1); the minichromosome maintenance (MCM) family of proteins that are involved in DNA replication; and cyclin D1 (CCND1), a protein necessary for uterine cell proliferation [25–27]. Both Ccnb1 and Cdc2a were reduced in the Pgrtm2(cre)Lyd/+ Ihhtm1Blan uterus (Fig. 2A). The mRNA expression level of Mcm5, a member of the MCM family of proteins, was also significantly reduced, as was that of Ccnd1 (Fig. 2A). These changes in gene expression of the cell cycle genes were not affected by the presence of Cre in the Pgrtm2(cre)Lyd/+ Ihhtm1Blan mice, because Pgrtm2(cre)Lyd/+ mice displayed the same expression of Ccnb1, Cdc2a, Ccnd1, and Mcm5 as wild-type mice (Supplemental Fig. S1A). CCND1 protein was primarily located in the uterine stroma, with some staining in the glandular epithelium of the Pgr+/+ Ihhtm1Blan uterus (Fig. 2B). Although CCND1 levels were comparable in the epithelial cells of the Pgrtm2(cre)Lyd/+ Ihhtm1Blan uterus, they were reduced in the uterine stroma when compared to Pgr+/+ Ihhtm1Blan uteri (Fig. 2B). In addition, another member of the MCM family of proteins, MCM3, was reduced in the Pgrtm2(cre)Lyd/+ Ihhtm1Blan stroma compared with Pgr+/+ Ihhtm1Blan mice (Fig. 2C). MCM3 exhibited robust expression in the luminal and glandular epithelium of both genotypes. This deregulation of genes involved in cell cycle progression may explain the stromal cell proliferation defect observed in the Pgrtm2(cre)Lyd/+ Ihhtm1Blan uterus [11].
FIG. 2.
Cell cycle genes are deregulated by Ihh ablation. A) Real-time RT-PCR analysis of cell cycle genes (Ccnb1, Cdc2a, Mcm5, Ccnd1). The results represent the mean ± SEM. *P < 0.05; **P < 0.01. B) Immunohistochemical analysis of CCND1 in Pgr+/+ Ihhtm1Blan (left) and Pgrtm2(cre)Lyd/+ Ihhtm1Blan (right) mice. Positive staining is seen by a brown signal. Nuclei were counterstained with hematoxylin. C) Immunohistochemical analysis of MCM3 in Pgr+/+ Ihhtm1Blan (left) and Pgrtm2(cre)Lyd/+ Ihhtm1Blan (right) mice. Positive staining is seen by a brown signal. Nuclei were counterstained with hematoxylin. Original magnification of insets ×40.
EGF Signaling Is Altered by Ihh Ablation
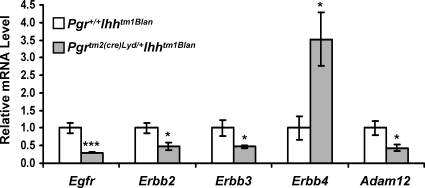
Previously, we demonstrated that the EGF ligands, amphiregulin (Areg) and heparin-binding epidermal growth factor (Hbegf), were unaltered by Ihh ablation, but that the epidermal growth factor receptor (EGFR/ERBB1) was lost in the subepithelial stroma [11]. In addition, we now show that Ihh ablation alters the expression of the other members of the ERBB receptor family, with V-ERB-B2 avian erythroblastic leukemia viral oncogene homolog 2 (Erbb2/Her2) and V-ERB-B2 avian erythroblastic leukemia viral oncogene homolog 3 (Erbb3/Her3) being significantly reduced and V-ERB-B2 avian erythroblastic leukemia viral oncogene homolog 4 (Erbb4/Her4) significantly increased (Fig. 3). In addition, a disintegrin and metalloproteinase domain 12 (Adam12) expression was significantly reduced. ADAM12 has been shown to be involved in the processing of HBEGF to generate its active form [28]. The expression of Egfr, Erbb2, Erbb3, Erbb4, and Adam12 was unaffected in the Pgrtm2(cre)Lyd/+ uteri compared with wild-type controls (Supplemental Fig. S1B). Therefore, Ihh regulates the EGF signaling pathway not by regulating the expression of the ligands but by regulating the expression of the receptors that bind the ligands and the enzymes responsible for processing the ligands.
FIG. 3.
EGF signaling is altered by ablation of Ihh. Real-time RT-PCR analysis of the EGF signaling genes Egfr, Erbb2, Erbb3, Erbb4, and Adam12. The results represent the mean ± SEM. *P < 0.05; ***P < 0.001.
Ihh Acts to Repress E2 Signaling in the Uterus
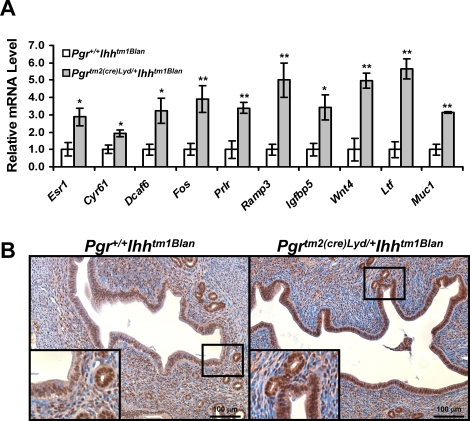
From the microarray analysis, we observed an increase in Esr1 expression which was validated by real-time RT-PCR analysis (Fig. 4A). Therefore, we wanted to determine whether there was increased ESR1 activity in the Pgrtm2(cre)Lyd/+ Ihhtm1Blan uterus. Therefore, we compared the list of differentially regulated genes in the Pgrtm2(cre)Lyd/+ Ihhtm1Blan uterus to the genes identified as being E2 regulated in wild-type mice by Hewitt et al. [29]. This analysis revealed that 20 (2.3%) of the 863 genes altered in the Pgrtm2(cre)Lyd/+ Ihhtm1Blan array are in common with genes identified as being altered in the uteri of wild-type mice treated with E2 (Supplemental Table S5). A selection of these E2-regulated genes was validated by real-time RT-PCR analysis (Fig. 4A). In this independent sample set, we again observed increased expression of the E2 target gene Muc1, confirming our previous finding. Analysis of these genes in the Pgrtm2(cre)Lyd/+ mouse compared with controls revealed no alteration in the mRNA expression level for the majority of genes; however, the expression ofDcaf6 was significantly increased, albeit not to the magnitude of the Pgrtm2(cre)Lyd/+ Ihhtm1Blan mice (1.4-fold in the Pgrtm2(cre)Lyd/+uteri vs. 3.2-fold in the Pgrtm2(cre)Lyd/+ Ihhtm1Blan uteri), and the expression of Prlr was significantly reduced, which is opposite to that of the Pgrtm2(cre)Lyd/+ Ihhtm1Blan mice (Supplemental Fig. S1C). Because ESR1 is located in both the epithelium and stroma of the uterus, we wanted to determine whether there was a compartment-specific effect of Ihh ablation on E2 signaling. ESR1 protein remained unchanged in the uterine stroma or glandular epithelium, but it was markedly increased in the uterine luminal epithelium of the Pgrtm2(cre)Lyd/+ Ihhtm1Blan uterus compared with controls (Fig. 4B).
FIG. 4.
Estrogen signaling is increased by Ihh ablation. A) Identification of E2- and Ihh-regulated genes during embryo implantation. Genes differentially regulated by Ihh were compared to genes regulated by E2 in wild-type mice identified by Hewitt et al. [29]. B) Real-time RT-PCR analysis of E2-regulated genes. The results represent the mean ± SEM. *P < 0.05; **P < 0.01. B) Immunohistochemical analysis of ESR1 in Pgr+/+ Ihhtm1Blan (left) and Pgrtm2(cre)Lyd/+ Ihhtm1Blan (right) mice. Positive staining is seen by a brown signal. Nuclei were counterstained with hematoxylin. Original magnification of insets ×40.
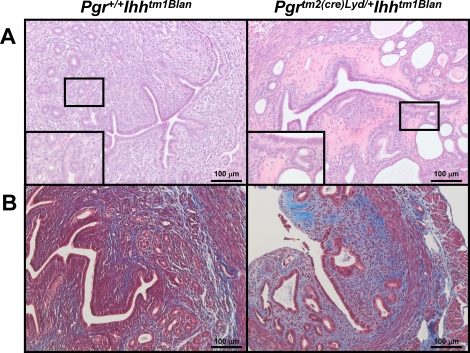
Because unopposed E2 signaling is a hallmark of uterine diseases, we wanted to determine whether the increased E2 signaling observed in the Pgrtm2(cre)Lyd/+ Ihhtm1Blan mice resulted in a pathological condition. Therefore, we examined the histology of untreated, intact Pgr+/+Ihhtm1Blan and Pgrtm2(cre)Lyd/+ Ihhtm1Blan mice at 15 mo of age and observed that 50% of the Pgrtm2(cre)Lyd/+ Ihhtm1Blan mice (n = 12) displayed an abnormal appearance. Microscopically, the Pgrtm2(cre)Lyd/+ Ihhtm1Blan uteri had increased numbers of cystically dilated endometrial glands compared with the control Pgr+/+ Ihhtm1Blan mice (Fig. 5A). These dilated endometrial glands were typically lined by flattened epithelial cells and contained cellular debris within the glandular lumens. In addition, the endometrial stroma from the Pgrtm2(cre)Lyd/+ Ihhtm1Blan uteri contained foci of homogeneous eosinophilic staining, which is indicative of stromal hyalinization. Staining for Masson trichrome indicated increased collagen deposition in the endometrial stroma of the Pgrtm2(cre)Lyd/+ Ihhtm1Blan uteri (Fig. 5B). These results are consistent with previous findings on chronic treatment of E2 in mice [30]. This phenotype was not due to altered ovarian function as the mice exhibited normal serum testosterone (63.93 ± 6.37 ng/dl [Pgr+/+ Ihhtm1Blan] and 60.01 ± 3.86 ng/dl [Pgrtm2(cre)Lyd/+ Ihhtm1Blan]) and estradiol (2.22 ± 0.55 ng/dl [Pgr+/+Ihhtm1Blan] and 1.91 ± 0.48 ng/dl [Pgrtm2(cre)Lyd/+ Ihhtm1Blan]) levels. Thus, Ihh acts as a critical mediator of E2 signaling such that its loss results not only in altered uterine receptivity but also a pathological condition.
FIG. 5.
Examination of 15-mo-old mice. A) Hematoxylin-eosin staining of 15-mo-old Pgr+/+ Ihhtm1Blan (left) and Pgrtm2(cre)Lyd/+ Ihhtm1Blan (right) mice. B) Masson trichrome staining of 15-mo-old Pgr+/+ Ihhtm1Blan (left) and Pgrtm2(cre)Lyd/+ Ihhtm1Blan (right) mice. Blue signal indicates collagen. Red signal indicates muscle. Black signal indicates nuclei. Original magnification of insets ×40.
DISCUSSION
Ihh has been shown to be critical for normal adult uterine function as conditional ablation of Ihh in the uterus renders mice infertile due to defective embryo attachment and decidualization [11]. The highest expression of Ihh is on d2.5 of pregnancy in the uterine epithelium, whereas that of its target genes Ptch1 and Nr2f2 is highest on d3.5 in the uterine stroma [5, 6]. Previously, we determined that these gene expression changes could be mimicked by the administration of exogenous hormones such that the highest expression of the Ihh target genes correlated with 30 h after the first E2 plus P4 injection in the artificially induced decidual reaction [9, 16]. We performed microarray analysis at this time point to determine which pathways are regulated by Ihh at the time of embryo implantation and identified 863 Ihh-regulated genes. In the analysis, we used whole uterus to extract the RNA, which may have limited the number of genes identified because Ihh signaling is focally activated in the subepithelial stroma, which makes up a relatively small proportion of the total cell population. Nonetheless, we observed an alteration in multiple pathways, including cell cycle progression, EGF signaling, and E2 signaling (Fig. 6). Although the Pgrtm2(cre)Lyd/+ Ihhtm1Blan uterus exhibited decreased vascularization, we did not observe a significant alteration in genes involved in this process, possibly because of the relative abundance of the vasculature in comparison with the epithelial or stroma cells in the uterus. For the majority of genes examined, these changes in gene expression were not due to the presence of Cre, because wild-type and Pgrtm2(cre)Lyd/+ mice exhibited similar gene expression patterns (Supplemental Fig. S1). However, for Dcaf6 and Prlr the expression levels differed in the Pgrtm2(cre)Lyd/+ uteri compared with controls, although not in the same magnitude or direction. Despite the loss of one Pgr allele, the Pgrtm2(cre)Lyd/+ mouse is phenotypically normal, and no changes in P4-regulated gene expression have been observed. In addition, neither Dcaf6 nor Prlr has been shown to be P4 regulated in the uterus by microarray analysis [17]. Therefore, these changes in gene expression in the Pgrtm2(cre)Lyd/+ mouse model may be due to those particular genes having increased sensitivity to Cre expression [31].
FIG. 6.
Model of Ihh signaling in the uterus during preimplantation. Ihh acts as a mediator of epithelial-stromal communication by mediating multiple signaling pathways in the preimplantation period, such as cell cycle progression, EGF signaling, and E2 signaling.
Stromal cell proliferation is a P4-driven process critical for embryo implantation and subsequent decidualization, and Ihh has been shown to be critical for this proliferation, as the Pgrtm2(cre)Lyd/+ Ihhtm1Blan uterus exhibited decreased stromal cell proliferation [11, 32]. In the human endometrium, expression of IHH is correlated with cell division as its expression decreases from the early to mid secretory phase, when cellular division is also decreased [10]. Conversely, the Hedgehog signaling axis has been shown to be overexpressed in endometrial cancer and to act as a critical mediator of growth in endometrial carcinoma cells [33]. CCNB1 and CDC2A together form the mitosis-promoting factor, which phosphorylates numerous proteins necessary for entry into mitosis [25]. PTCH1 has been shown to physically interact with CCNB1, resulting in the inhibition of its nuclear localization, thereby inhibiting entry into mitosis [34, 35]. In addition, we now show that Ihh regulates the expression of these two critical proteins, demonstrating an additional mechanism by which Hedgehog signaling regulates entry into mitosis (Fig. 2A). Ccnd1 has been shown to be critical for cell proliferation in the uterus as a target of P4 inhibition of E2-induced proliferation [27, 36, 37]. Here, we show that Ihh regulates Ccnd1 expression in the uterus, corroborating previous findings in Drosophila and mouse cerebellar granule neuron precursors (Fig. 2A) [38, 39]. Interestingly, this loss of expression appears confined to the uterine stroma, where the proliferation defect was observed in the Pgrtm2(cre)Lyd/+ Ihhtm1Blan mice (Fig. 2B) [11]. The MCM family of proteins is characterized by their binding to chromatin to regulate DNA replication. One of these proteins is MCM5 (also known as CDC46), which has been shown to be critical for replication in both yeast [40] and humans [26]. Here, we show that Ihh regulates the expression of Mcm5 (Fig. 2A). Another member of this family, MCM3, was also reduced upon Ihh ablation, and this reduction appears confined to the uterine stroma at this time point (Fig. 2C). Previously, the localization of MCM3 has been shown to be regulated by steroid hormones in the uterus, with E2 plus P4 treatment resulting in strong stromal staining and weak epithelial staining [41]. Many of the cell cycle genes regulated by Ihh, such as Ccnd1, Mcm5, and Mcm3, have also been implicated in E2-induced luminal epithelial proliferation at the time of implantation (Supplemental Table S6) [41]. However, even though we observed increased E2 sensitivity in the epithelium of the Pgrtm2(cre)Lyd/+ Ihhtm1Blan uteri, there was no difference in epithelial proliferation when compared to controls. Decreased proliferation was only observed in the endometrial stroma. Thus, the changes in proliferation that were observed were due to the direct action of Ihh ablation and not because of the heightened E2 sensitivity of the epithelium.
Previously, we demonstrated that Ihh regulated the expression of EGFR in the subepithelial stroma [11]. Here, we show that Ihh also regulates the expression of the other members of the ERBB family of receptors using real-time RT-PCR analysis, with Erbb2 and Erbb3 being downregulated and Erbb4 upregulated in the Pgrtm2(cre)Lyd/+ Ihhtm1Blan uterus (Fig. 3). These genes were not identified as being Ihh regulated by microarray analysis, but this may be due to the false-negative rate of the analysis. Previous work on the role of EGF signaling in the uterus during embryo implantation examined the EGF ligands Areg and Hbgef, neither of which was altered in the Pgrtm2(cre)Lyd/+ Ihhtm1Blan uterus. Both Hbegf−/− and Pgrtm2(cre)Lyd/+ Hbegf/f mice exhibit embryo implantation defects, suggesting an important role for EGF signaling during early pregnancy, whereas Areg−/− mice fail to exhibit a uterine phenotype, possibly because of compensation by the other EGF ligands [42, 43]. Adam12, a known processor of EGF ligands, was downregulated by ablation of Ihh (Fig. 2). Recently, ADAM12 has been shown to be a critical regulator of stromal cell decidualization, which is necessary for successful embryo implantation [44]. ADAM12 has been shown to process not only HBEGF but also Delta-like 1, a ligand for the Notch receptors [28, 45]. In addition, ADAM12 has been shown to act in focal adhesion formation through the β1 and β3 integrins (ITGB1 and ITGB3) and in transforming growth factor-β (TGFB) signaling through interactions with the TGFB type II receptor [46, 47]. All of these pathways have been shown to be active during embryo implantation, and thus Ihh through ADAM12 may regulate these pathways during embryo implantation [48–50]. Thus, in the uterus, Ihh may be a major mediator of not only EGF but also other signaling pathways. Interestingly, its regulation of EGF signaling occurs not at the level of ligand expression but at the receptor level and also in the processing of the ligands.
Estrogen signaling is critical to uterine function during early pregnancy because it defines the window of receptivity [3]. High or sustained levels of E2 make the uterus refractory toward embryo implantation. In the Pgrtm2(cre)Lyd/+ Ihhtm1Blan uterus, we observed increased levels of ESR1 at both the mRNA and protein levels with the protein markedly increased in the uterine luminal epithelium, which was accompanied by increased expression of E2-regulated genes (Fig. 4). To identify these E2-regulated genes, our gene list was compared to that from Hewitt et al. [29], in which microarrays were performed on ovariectomized wild-type mice treated with E2. We identified 19 genes induced by E2 in the Hewitt et al. data set that were upregulated upon ablation of Ihh (Supplemental Table S5). These results agree with previous findings in the Pgrtm2(cre)Lyd/+ Nr2f2tm1Vco mice, in which the E2-regulated genes lactoferrin, component C3, and chloride channel-activated 3 were upregulated [14]. Thus, the repressive actions of Ihh on the luminal epithelium may occur through Nr2f2 in the uterine stroma. In addition, the E2 target gene Muc1 was upregulated in both the Pgrtm2(cre)Lyd/+ Ihhtm1Blan and Pgrtm2(cre)Lyd/+ Nr2f2tm1Vco uterus [11, 14]. Muc1 is a glycoprotein that lines the uterine epithelium and whose loss is necessary for embryo attachment and subsequent implantation to occur [12]. It was previously shown to be E2 regulated, although it failed to appear in the Hewitt et al. data set [12, 29]. Therefore, there may be additional E2-regulated genes that were altered by Ihh ablation that were not identified in this analysis. Thus, the Ihh signaling axis regulates embryo implantation through a downregulation of E2 signaling.
Furthermore, at 15 mo of age, Pgrtm2(cre)Lyd/+ Ihhtm1Blan uteri exhibit characteristics of an estrogenized uterus, such as cystic glands and hyalinized stroma (Fig. 5). This phenotype may be attributed to altered ovarian function as serum estradiol and testosterone levels decrease with age because of atresia of the ovarian follicles, and a defect in this decrease could result in increased circulating estradiol levels [51]. However, we detected serum testosterone and estradiol levels that were comparable between Pgr+/+ Ihhtm1Blan and Pgrtm2(cre)Lyd/+ Ihhtm1Blan mice, indicating that ovarian function was not affected by Ihh ablation. In addition, in our previous analysis of the Pgrtm2(cre)Lyd/+ Ihhtm1Blan mice, we did not observe an ovarian phenotype, likely because of the nonoverlapping expression patterns of Ihh and Pgrtm2(cre)Lyd/+ in the ovary [11, 15, 52]. An indirect hypothalmo-pituitary-ovarian action may also be a potential reason for this phenomenon; however, previously we observed normal serum progesterone levels, and here we demonstrate normal serum testosterone and estradiol levels, indicating that this axis is intact [11]. Thus, the observed phenotype is likely due to the increased E2 signaling resulting from Ihh ablation, suggesting that Ihh may also play a role in endometrial dysfunction as aberrant E2 signaling is a hallmark of uterine diseases such as endometriosis and endometrial cancer [53–55]. Recently, IHH was identified as a gene significantly downregulated in patients with endometriosis [56]. Thus, the Ihh signaling axis may be an important therapeutic target for these diseases as a potential means to target increased E2 signaling.
In conclusion, conditional ablation of Ihh in the murine uterus renders female mice infertile [11]. Microarray analysis conducted at the point at which the Ihh target genes have their highest expression identified 863 Ihh-regulated genes. Of these genes, those that regulate the cell cycle, EGF signaling, and E2 signaling were identified and validated. Thus, Ihh affects embryo implantation through its regulation of stromal cell proliferation and inhibition of epithelial E2 signaling, events that are necessary for successful embryo implantation. In addition, the increased E2 signaling observed in the Pgrtm2(cre)Lyd/+ Ihhtm1Blan uterus and the downregulation of IHH observed in endometriosis suggest that Hedgehog signaling may play a role in the development of endometrial diseases, such as endometriosis and endometrial cancer. Further examination of the role of Hedgehog signaling in the uterus may open avenues for new treatments of these diseases.
Acknowledgments
We thank Jinghua Li, Jie Yang, and Bryan Ngo for technical assistance, and Janet DeMayo, MS, for manuscript preparation. We would also like to thank Lata Murthy, Peter Cook, and Dr. Dolores J. Lamb for assistance with the estradiol assay.
Footnotes
Supported by National Institutes of Health (NIH) grant R01HD042311 to F.J.D., NIH grant R01HD057873 to J.-W.J., NIH grant R01CA77530 and the Susan G. Komen Award BCTR0503763 to J.P.L, National Institute of Arthritis and Musculoskeletal and Skin Diseases NIH grant R01AR50560 to B.L., Specialized Programs of Research Excellence (SPORE) in Uterine Cancer NIH grant P50CA098258 to R.R.B., Reproductive Biology Training grant 5T32HD07165 and a scholarship from Baylor Research Advocates for Student Scientists to H.L.F., and the Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH through cooperative agreements U54HD0077495 to F.J.D. and U54HD28934 to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
REFERENCES
- Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, Yoshinaga K.Embryo implantation. Dev Biol 2000; 223: 217–237. [DOI] [PubMed] [Google Scholar]
- Wang H, Dey SK.Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet 2006; 7: 185–199. [DOI] [PubMed] [Google Scholar]
- Ma WG, Song H, Das SK, Paria BC, Dey SK.Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci U S A 2003; 100: 2963–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O'Malley BW.Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 1995; 9: 2266–2278. [DOI] [PubMed] [Google Scholar]
- Takamoto N, Zhao B, Tsai SY, DeMayo FJ.Identification of Indian hedgehog as a progesterone-responsive gene in the murine uterus. Mol Endocrinol 2002; 16: 2338–2348. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Zhao X, Das SK, Hogan BL, Dey SK.Indian hedgehog as a progesterone-responsive factor mediating epithelial-mesenchymal interactions in the mouse uterus. Dev Biol 2002; 245: 280–290. [DOI] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP.Hedgehog signaling in animal development: paradigms and principles. Genes Dev 2001; 15: 3059–3087. [DOI] [PubMed] [Google Scholar]
- Wang Y, McMahon AP, Allen BL.Shifting paradigms in Hedgehog signaling. Curr Opin Cell Biol 2007; 19: 159–165. [DOI] [PubMed] [Google Scholar]
- Lee K, Jeong J, Tsai MJ, Tsai S, Lydon JP, DeMayo FJ.Molecular mechanisms involved in progesterone receptor regulation of uterine function. J Steroid Biochem Mol Biol 2006; 102: 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, Nayak NR, Giudice LC.Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology 2006; 147: 1097–1121. [DOI] [PubMed] [Google Scholar]
- Lee K, Jeong J, Kwak I, Yu CT, Lanske B, Soegiarto DW, Toftgard R, Tsai MJ, Tsai S, Lydon JP, DeMayo FJ.Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet 2006; 38: 1204–1209. [DOI] [PubMed] [Google Scholar]
- Surveyor GA, Gendler SJ, Pemberton L, Das SK, Chakraborty I, Julian J, Pimental RA, Wegner CC, Dey SK, Carson DD.Expression and steroid hormonal control of Muc-1 in the mouse uterus. Endocrinology 1995; 136: 3639–3647. [DOI] [PubMed] [Google Scholar]
- Takamoto N, Kurihara I, Lee K, Demayo FJ, Tsai MJ, Tsai SY.Haploinsufficiency of chicken ovalbumin upstream promoter transcription factor II in female reproduction. Mol Endocrinol 2005; 19: 2299–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara I, Lee DK, Petit FG, Jeong J, Lee K, Lydon JP, DeMayo FJ, Tsai MJ, Tsai SY.COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet 2007; 3: e102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ, Lydon JP.Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 2005; 41: 58–66. [DOI] [PubMed] [Google Scholar]
- Finn CA, Martin L.Endocrine control of the timing of endometrial sensitivity to a decidual stimulus. Biol Reprod 1972; 7: 82–86. [DOI] [PubMed] [Google Scholar]
- Jeong JW, Lee KY, Kwak I, White LD, Hilsenbeck SG, Lydon JP, DeMayo FJ.Identification of murine uterine genes regulated in a ligand-dependent manner by the progesterone receptor. Endocrinology 2005; 146: 3490–3505. [DOI] [PubMed] [Google Scholar]
- Jeong JW, Lee KY, Han SJ, Aronow BJ, Lydon JP, O'Malley BW, DeMayo FJ.The p160 steroid receptor coactivator 2, SRC-2, regulates murine endometrial function and regulates progesterone-independent and -dependent gene expression. Endocrinology 2007; 148: 4238–4250. [DOI] [PubMed] [Google Scholar]
- Schadt EE, Li C, Ellis B, Wong WH.Feature extraction and normalization algorithms for high-density oligonucleotide gene expression array data. J Cell Biochem Suppl 2001 37(suppl): 120–125. [DOI] [PubMed] [Google Scholar]
- Li C, Wong WH.Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci U S A 2001; 98: 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Hung Wong W.Model-based analysis of oligonucleotide arrays: model validation, design issues and standard error application. Genome Biol 2001; 2: RESEARCH0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA.DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol 2003; 4: P3 [PubMed] [Google Scholar]
- Huang daW, Sherman BT, Lempicki RA.Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009; 4: 44–57. [DOI] [PubMed] [Google Scholar]
- Hamatani T, Daikoku T, Wang H, Matsumoto H, Carter MG, Ko MS, Dey SK.Global gene expression analysis identifies molecular pathways distinguishing blastocyst dormancy and activation. Proc Natl Acad Sci U S A 2004; 101: 10326–10331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassermann F, Peschel C, Duyster J.Mitotic entry: a matter of oscillating destruction. Cell Cycle 2005; 4: 1515–1517. [DOI] [PubMed] [Google Scholar]
- Krude T, Musahl C, Laskey RA, Knippers R.Human replication proteins hCdc21, hCdc46 and P1Mcm3 bind chromatin uniformly before S-phase and are displaced locally during DNA replication. J Cell Sci 1996; 109(pt 2):309–318. [DOI] [PubMed] [Google Scholar]
- Chen B, Pan H, Zhu L, Deng Y, Pollard JW.Progesterone inhibits the estrogen-induced phosphoinositide 3-kinase→AKT→GSK-3beta→cyclin D1→pRB pathway to block uterine epithelial cell proliferation. Mol Endocrinol 2005; 19: 1978–1990. [DOI] [PubMed] [Google Scholar]
- Asakura M, Kitakaze M, Takashima S, Liao Y, Ishikura F, Yoshinaka T, Ohmoto H, Node K, Yoshino K, Ishiguro H, Asanuma H, Sanada S, et al. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat Med 2002; 8: 35–40. [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Deroo BJ, Hansen K, Collins J, Grissom S, Afshari CA, Korach KS.Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen. Mol Endocrinol 2003; 17: 2070–2083. [DOI] [PubMed] [Google Scholar]
- Gunin AG, Mashin IN, Zakharov DA.Proliferation, mitosis orientation and morphogenetic changes in the uterus of mice following chronic treatment with both estrogen and glucocorticoid hormones. J Endocrinol 2001; 169: 23–31. [DOI] [PubMed] [Google Scholar]
- Schmidt-Supprian M, Rajewsky K.Vagaries of conditional gene targeting. Nat Immunol 2007; 8: 665–668. [DOI] [PubMed] [Google Scholar]
- Franco HL, Jeong JW, Tsai SY, Lydon JP, DeMayo FJ.In vivo analysis of progesterone receptor action in the uterus during embryo implantation. Semin Cell Dev Biol 2008; 19: 178–186. [DOI] [PubMed] [Google Scholar]
- Feng YZ, Shiozawa T, Miyamoto T, Kashima H, Kurai M, Suzuki A, Ying-Song J, Konishi I.Overexpression of hedgehog signaling molecules and its involvement in the proliferation of endometrial carcinoma cells. Clin Cancer Res 2007; 13: 1389–1398. [DOI] [PubMed] [Google Scholar]
- Adolphe C, Hetherington R, Ellis T, Wainwright B.Patched1 functions as a gatekeeper by promoting cell cycle progression. Cancer Res 2006; 66: 2081–2088. [DOI] [PubMed] [Google Scholar]
- Barnes EA, Kong M, Ollendorff V, Donoghue DJ.Patched1 interacts with cyclin B1 to regulate cell cycle progression. EMBO J 2001; 20: 2214–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Feng H, Bi C, Zhu L, Pollard JW, Chen B.GSK-3beta mediates in the progesterone inhibition of estrogen induced cyclin D2 nuclear localization and cell proliferation in cyclin D1-/- mouse uterine epithelium. FEBS Lett 2007; 581: 3069–3075. [DOI] [PubMed] [Google Scholar]
- Zhu L, Pollard JW.Estradiol-17beta regulates mouse uterine epithelial cell proliferation through insulin-like growth factor 1 signaling. Proc Natl Acad Sci U S A 2007; 104: 15847–15851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman-Scheel M, Weng L, Xin S, Du W.Hedgehog regulates cell growth and proliferation by inducing Cyclin D and Cyclin E. Nature 2002; 417: 299–304. [DOI] [PubMed] [Google Scholar]
- Kenney AM, Rowitch DH.Sonic hedgehog promotes G(1) cyclin expression and sustained cell cycle progression in mammalian neuronal precursors. Mol Cell Biol 2000; 20: 9055–9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hennessy KM, Botstein D, Tye BK.CDC46/MCM5, a yeast protein whose subcellular localization is cell cycle-regulated, is involved in DNA replication at autonomously replicating sequences. Proc Natl Acad Sci U S A 1992; 89: 10459–10463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Deng Y, Pollard JW.Progesterone blocks estrogen-induced DNA synthesis through the inhibition of replication licensing. Proc Natl Acad Sci U S A 2006; 103: 14021–14026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetteke NC, Qiu TH, Fenton SE, Troyer KL, Riedel RF, Chang A, Lee DC.Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development 1999; 126: 2739–2750. [DOI] [PubMed] [Google Scholar]
- Xie H, Wang H, Tranguch S, Iwamoto R, Mekada E, Demayo FJ, Lydon JP, Das SK, Dey SK.Maternal heparin-binding-EGF deficiency limits pregnancy success in mice. Proc Natl Acad Sci U S A 2007; 104: 18315–18320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Guo W, Chen Q, Fan X, Zhang Y, Duan E.Adam12 plays a role during uterine decidualization in mice. Cell Tissue Res 2009; 338: 413–421. [DOI] [PubMed] [Google Scholar]
- Dyczynska E, Sun D, Yi H, Sehara-Fujisawa A, Blobel CP, Zolkiewska A.Proteolytic processing of delta-like 1 by ADAM proteases. J Biol Chem 2007; 282: 436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atfi A, Dumont E, Colland F, Bonnier D, L'Helgoualc'h A, Prunier C, Ferrand N, Clement B, Wewer UM, Theret N.The disintegrin and metalloproteinase ADAM12 contributes to TGF-beta signaling through interaction with the type II receptor. J Cell Biol 2007; 178: 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thodeti CK, Frohlich C, Nielsen CK, Takada Y, Fassler R, Albrechtsen R, Wewer UM.ADAM12-mediated focal adhesion formation is differently regulated by beta1 and beta3 integrins. FEBS Lett 2005; 579: 5589–5595. [DOI] [PubMed] [Google Scholar]
- Afshar Y, Stanculescu A, Miele L, Fazleabas AT.The role of chorionic gonadotropin and Notch1 in implantation. J Assist Reprod Genet 2007; 24: 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaghay M, Lessey BA.Uterine receptivity: alterations associated with benign gynecological disease. Semin Reprod Med 2007; 25: 461–475. [DOI] [PubMed] [Google Scholar]
- Jones RL, Stoikos C, Findlay JK, Salamonsen LA.TGF-beta superfamily expression and actions in the endometrium and placenta. Reproduction 2006; 132: 217–232. [DOI] [PubMed] [Google Scholar]
- Shifren JL, Schiff I.The aging ovary. J Womens Health Gend Based Med 2000; 9(suppl 1):S3–S7. [DOI] [PubMed] [Google Scholar]
- Russell MC, Cowan RG, Harman RM, Walker AL, Quirk SM.The hedgehog signaling pathway in the mouse ovary. Biol Reprod 2007; 77: 226–236. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Cheng YH, Yin P, Imir G, Utsunomiya H, Attar E, Innes J, Julie Kim J.Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol 2006; 248: 94–103. [DOI] [PubMed] [Google Scholar]
- Ito K, Utsunomiya H, Yaegashi N, Sasano H.Biological roles of estrogen and progesterone in human endometrial carcinoma–new developments in potential endocrine therapy for endometrial cancer. Endocr J 2007; 54: 667–679. [DOI] [PubMed] [Google Scholar]
- Rose PG.Endometrial carcinoma. N Engl J Med 1996; 335: 640–649. [DOI] [PubMed] [Google Scholar]
- Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC.Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 2007; 148: 3814–3826. [DOI] [PubMed] [Google Scholar]