Abstract
T-cell exhaustion is characterized by the stepwise and progressive loss of T-cell functions and can culminate in the physical deletion of the responding cells. Exhaustion is well-defined during chronic lymphocytic choriomeningitis virus infection and commonly develops under conditions of antigen-persistence, which occur following many chronic infections that are of significant public health concern including hepatitis B virus, hepatitis C virus and human immunodeficiency virus infections, as well as during tumour outgrowth. Exhaustion is not a uniformly disabled setting as a gradation of phenotypic and functional defects can manifest, and these cells are distinct from prototypic effector, memory and also anergic T cells. We are gaining insights into the extrinsic and intrinsic factors that determine the severity of exhaustion. These include the duration and magnitude of antigenic activation, availability of CD4 T-cell help, the levels of stimulatory and suppressive cytokines, as well as the expression of activatory and inhibitory receptors. More information is now becoming available regarding the molecular mechanisms that attenuate the responsiveness of exhausted T cells. As the parameters that dictate exhaustion are more thoroughly defined, this is fostering the development of methods that prevent and rejuvenate functionally inferior responses. In this article we discuss our current understanding of the properties of exhausted T cells and the mechanisms that promote and maintain this state.
Keywords: chronic infections, cytokines, effector functions, inhibitory receptors, T cell exhaustion
Qualitative differences in T-cell responses
T-cell exhaustion was first described as the clonal deletion of virus-specific CD8 T cells that occurs during high-grade chronic infections.1 Technological advancements, especially the production of major histocompatibility complex multimers which can identify antigen-specific T cells without relying on functional readouts, as well as the development of enhanced methods to assess the phenotypic and functional portfolios of single cells have improved our understanding of the complexities of the exhausted state.2 It is now clear that T cells are not necessarily physically deleted under conditions of antigen persistence but can instead become functionally inept and incapable of elaborating the usual array of effector activities typically associated with robust, protective, effector and memory T-cell populations. Exhaustion is not limited to CD8 T-cell responses as CD4 T cells have also been shown to develop functional unresponsiveness following several infections.3–11 During the last decade the characteristics of exhausted cells have become better defined and we now appreciate that exhausted T cells are quite distinct from prototypic effector and memory T cells (Fig. 1).
Figure 1.
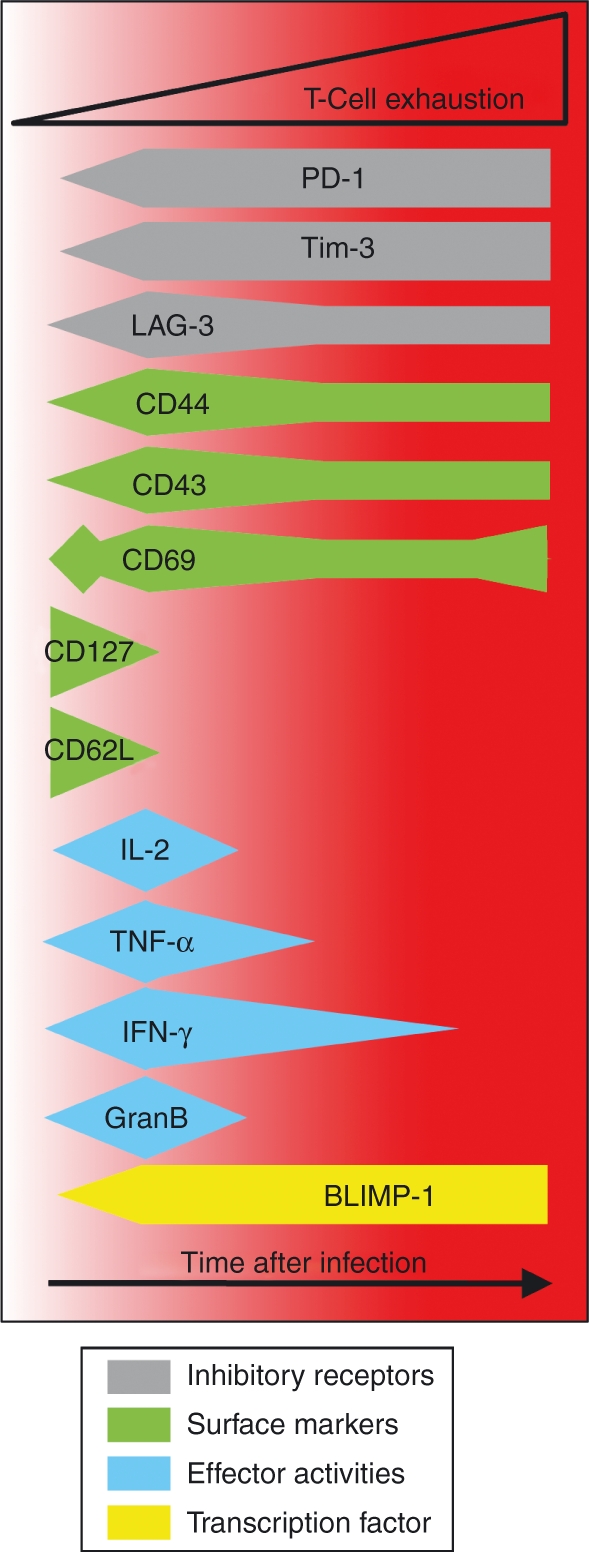
As exhausted T cells emerge sequential phenotypic and functional changes occur. Exhausted T cells express arrays of inhibitory molecules and distinctive patterns of cytokine receptors, transcription factors and effector molecules, which distinguish these cells from conventional effector, memory and anergic T cells. The changes depicted represent only a small subset of the overall alterations that manifest as the exhausted state develops. GranB, granzyme B; IFN-γ, interferon-γ; IL-2, interleukin-2; TNF-α, tumour necrosis factor-α.
Exhausted T-cell responses have been documented following numerous infections, including lymphocytic choriomeningitis virus (LCMV), polyoma virus, adenovirus, Friend leukaemia virus, mouse hepatitis virus, human immunodeficiency virus (HIV), hepatitis B virus (HBV) and hepatitis C virus (HCV), and have also been observed in patients with malignancies.1,6,10,12–29 In each of these cases initial T-cell responses are elicited, but a spectrum of phenotypic and functional defects arises as the responding cells lose their functional capabilities in a progressive and stepwise manner. Interleukin-2 (IL-2) production is one of the first effector activities to be extinguished, followed by tumour necrosis factor-α (TNF-α)production, whereas the ability to produce interferon-γ (IFN-γ) is more resistant to inactivation (Fig. 2) 6,7,15 Although cytotoxic functions by exhausted T cells can be difficult to measure in vitro, sensitive in vivo assays can detect some level of killing activity.6,30 The biological significance of this is uncertain because these cells do not contain the infection. Counterintuitively, despite their functional ineptness, exhausted T cells display high levels of CD43 (1B11), CD69 and inhibitory receptors but low levels of CD62L and CD127, which is an expression profile typically associated with effector T cells (Fig. 1).6,7,15,26,31–34 Unlike prototypic memory T cells that are maintained at remarkably stable levels in the absence of antigen, severely exhausted T cells can succumb to deletion. This physical loss of the cells is probably the result of several factors including shifts in the expression of pro- and anti-apoptotic factors as well as an inability to respond to IL-7 and IL-15, which normally regulate T-cell homeostasis.22,31,32,35
Figure 2.
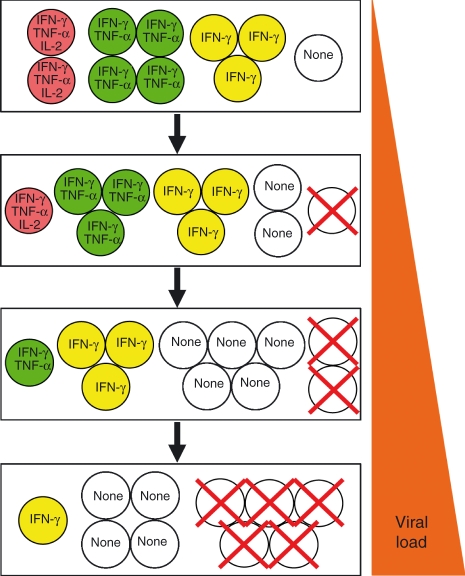
T-cell exhaustion develops in a stepwise and progressive manner and can culminate in the deletion of the virus-specific T cells. The overall ensemble of T cells elicited during the initial stages of infection is comprised of a series of subsets with different functional attributes. If the infection is not brought under control then composition of this population changes as the more polyfunctional cells are lost. Sustained high viral loads result in further reductions in the functional potential of the population and severely exhausted T cells are not maintained. IFN-γ, interferon-γ; IL-2, interleukin-2; TNF-α, tumour necrosis factor-α.
Exhausted T cells most commonly emerge during high-grade chronic infections, and the levels and duration of antigenic stimulation are critical determinants of this process.6,36–38 Infections elicit multi-epitope-specific T-cell responses, but not all specificities are equally prone to exhaustion.6,15,26,34 This differential silencing of responses, even within the same host, may be consequential because the specificities of T cells that are most effective at eradicating the pathogen may become functionally inactivated more rapidly and quickly deleted. Decreasing antigen availability, as occurs during the gradual resolution of infections and following intervention strategies that promote viral control, generally help the exhausted T-cell population regain polyfunctional attributes and more closely match typical memory T cells (Fig. 3).4,6,31,39–43 As with the development of the effector-function-negative state, the resurrection is also unequal and severely exhausted T cells may be refractory to reactivation.6,22,44
Figure 3.
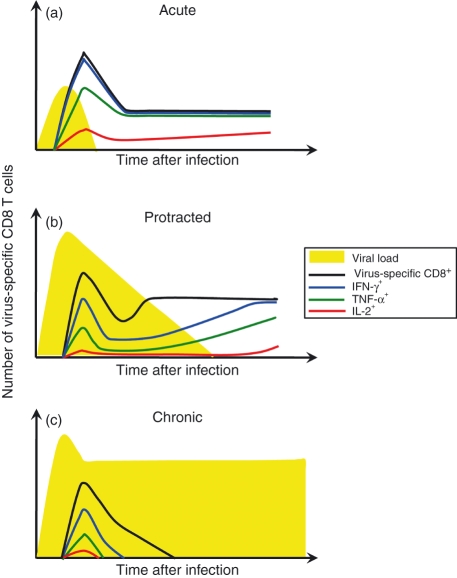
Divergent patterns of CD8 T-cell responses develop following acute, protracted, and chronic infections. (a) Acute viral infections are rapidly contained and establish stably maintained memory T-cell pools. These memory T cells are capable of elaborating many effector functions including interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α) production and a subset produce interleukin-2 (IL-2). (b) During more protracted infections the initial burst size and the functional quality of the response are often reduced, and further development of an exhausted phenotype occurs as viral loads remain high. Gradual resolution of the infection may result in a progressive resurgence of the response. (c) Exhaustion develops more rapidly and the responding T cells do not recover function if viral loads are not contained and a high-grade chronic infection ensues. (Based on Fuller et al., 6)
In addition to antigen availability other factors, including cytokine levels and the presence of CD4 T cells dictate the development of exhaustion, as discussed further in this article. In many ways this loss of effectiveness is paradoxical because the principle function of the immune response is to protect the host. So why inactivate the response? Perhaps the cardinal rule is to ‘do no harm’ and the stepwise mechanism of dampening T-cell responses that are overwhelmed by a chronic infection provides the flexibility to allow a level of infection control while attenuating immunopathology.
CD4 T cells help sustain CD8 T-cell responses
CD4 T cells have multiple effects on the overall immune response following infection and are often required for optimal CD8 T-cell responses. CD4 T cells deliver these helper functions in several ways. They activate professional antigen-presenting cells such as dendritic cells via CD40 and CD40 ligand interactions, secrete chemokines and cytokines, which guide naive T cells to the sites of priming in secondary lymphoid organs and activated T cells to the location of the infection, and also produce supportive cytokines including IL-2 and IL-21, which can act directly on the responding CD8 T cells.45–52 As a consequence, any abnormalities in, or loss of, CD4 T cells during the course of an infection, which can occur as these cells succumb to exhaustion during HBV, HCV, or LCMV infections; or undergo deletion during HIV-1 infection, probably feed back on CD8 T cells, rendering them less effective.3–11 The absence of CD4 T cells during acute infections can result in the generation of defective memory CD8 T cells, which may exhibit altered homeostatic turnover, a reduced functional repertoire including the loss of IL-2 production, and the inability to mount vigorous secondary responses.53 Under conditions of antigen-persistence, which occurs during chronic infections, the requirements for CD4 T cells are even more stringent because CD8 T cells generally succumb to severe exhaustion and may be deleted without T-cell help.26
Several recent studies have demonstrated a pivotal role for IL-21, probably produced by CD4 T cells, in sustaining CD8 T-cell responses to chronic infections.49–51 The absence of IL-21 or its receptor does not have a significant impact on primary CD8 T-cell responses induced following infection with LCMV clone 13 or Docile strains; however, the virus-specific cells subsequently lose their ability to produce IFN-γ, TNF-α and IL-2, and also display other hallmarks of the exhausted state including high levels of CD43 (1B11) and programmed death-1 (PD-1) expression. Significantly, IL-21 or IL-21 receptor-deficient mice are unable to control the infection, and these outcomes closely parallel those observed in CD4-deficient mice, implicating IL-21 as a vital helper factor.49–51 During HIV-1 infection the levels of IL-21 in the circulation correlate with CD4 T-cell counts, and individuals with higher levels of IL-21 have increased frequencies of HIV-specific CD8 T cells.54 In CD4-deficient mice infected with LCMV clone 13, the administration of IL-21 has been shown to reduce viral loads, enhance the responsiveness of the virus-specific CD8 T cells and suppress the usual deletion of epitope-specific populations which quickly succumb to exhaustion.51 A downside to the IL-21 therapy is that the treated mice became moribund, most likely as a result of immunopathology caused by the enhanced immune response.
Interleukin-21 has pleiotropic effects so it is likely that it impacts multiple aspects of the immune response in persistently infected hosts. Nevertheless, simultaneous comparisons of the activities and fates of CD8 T cells which express the IL-21 receptor, and can perceive IL-21-dependent signals, with those cells which cannot, show that IL-21 acts directly on the CD8 T cells to sustain their presence and activity during chronic LCMV infections.49–51 The related and genetically linked common γ-chain cytokine IL-2 has also been shown to augment CD8 T-cell responses, and CD8 T cells which cannot perceive IL-2 because of IL-2 receptor-α chain deficiency are also rapidly lost during chronic LCMV infections.47,55–57 IL-21 appears to have far less of an impact on the induction and maintenance of anti-viral CD8 T cells following acute infections.49,50 Given the differential requirements for IL-21 during acute and chronic infections, and that this cytokine has been proposed to limit the rapid generation of terminally differentiated effector-like T cells and attenuate T-cell senescence, it is tempting to speculate that these parameters are of particular importance for sustaining T-cell activities during chronic infections.58,59
Suppressive cytokines: IL-10 and transforming growth factor-β
The composition of the cytokine milieu can have both a positive and a negative influence on the development of adaptive immune responses. Several chronic infections including Epstein–Barr virus (EBV), HBV, HCV, HIV and LCMV are associated with the production of increased levels of the immunosuppressive cytokine, IL-10.60–67 In addition to the production of endogenous IL-10 by the infected host’s own cells, EBV, cytomegalovirus, as well as the Orf Parapoxvirus, encode IL-10 homologues, which can also negatively regulate the immune response.68 The analyses of polymorphisms within the IL-10 promoter provide further evidence of the influence of this cytokine on the outcomes of infection. Promoter sequences associated with low IL-10 production are more prevalent in individuals with asymptomatic HBV infection, and those associated with high IL-10 production are a risk factor for the development of chronic HCV infection.69–71 Interleukin-10 has multiple effects and has been shown to reduce pro-inflammatory cytokine production, impede the functions of antigen-presenting cells, dampen T-cell responses and also affect B cells.68,72 This cytokine is produced by CD4 T cells, including regulatory T cells, as well as by many other cell types such as dendritic cells, macrophages, B cells and CD8 T cells.68,72
The relationship between IL-10 and T-cell exhaustion has been well studied using the LCMV system. Comparative analyses of mice undergoing acute (LCMV-Armstrong) and chronic (LCMV clone 13) infections revealed that both IL-10 protein and messenger RNA levels were higher in the chronically infected cohort.63–65 Because chronic LCMV infection is associated with T-cell exhaustion and elevated levels of IL-10 are observed under these conditions, the next steps were taken to test whether reducing the availability of IL-10 would enhance responses and promote viral control. The administration of antibodies that block the IL-10 receptor immediately following infection as well as a therapeutic regimen given after the infection had taken hold resulted in lower viral loads, decreased expression of PD-1, and improved the functionality of the virus-specific T cells.63,64 Significantly, these changes occurred without any signs of overt immunopathology. Additional studies in which neutralizing anti-IL-10 antibodies were administered also showed comparable initial effects; however, the infection was not cleared and exhaustion was observed at later time-points highlighting the central importance of antigen persistence in sustaining exhaustion.65
The utility of IL-10 blockade approaches for rejuvenating exhausted responses and accelerating viral clearance has been further shown following therapeutic vaccination, again in the setting of chronic LCMV infection. Therapeutic DNA vaccination alone was ineffective; however, when given together with anti-IL-10 receptor antibodies, virus-specific CD4 and CD8 T-cell responses were enhanced and viral loads were better contained.73 Collectively, the findings from the studies outlined above demonstrate that the levels of a single cytokine, in this case IL-10, can have a profound influence on the outcome of infection and the quality of the cellular immune response. However, it is not yet defined whether exhaustion is the result of the direct actions of IL-10 on T cells. Moreover, although results indicate that IL-10 and PD-1 promote and maintain exhaustion independently, less is known regarding how the roles of IL-10 integrate with additional parameters, including the levels of other cytokines, that determine the fate of the response.74
The immunosuppressive cytokine transforming growth factor-β (TGF-β) has been implicated in regulating the size of the pathogen-specific T-cell responses and the propensity of these cells to undergo apoptosis.75,76 This is especially relevant during chronic infections as blunted initial responses are often observed and severely exhausted cells succumb to deletion. During acute infections, TGF-β restricts the size of both the effector and memory CD8 T-cell pool, most likely by inducing the expression of the pro-apoptotic protein Bim, as well as by down-regulating the expression of the anti-apoptotic gene Bcl-2.75 The significance of the TGF-β pathway on the development of exhaustion has been further dissected following LCMV clone 13 infection. Interestingly, elevated levels of TGF-β are observed under these conditions and the TGF-β pathway appears to regulate the size of the response but not directly influence the functional capacity of the cells.76 Nevertheless, circumventing this attenuation of the response was beneficial because increasing the numbers of virus-specific T cells brought about viral clearance.
Whether TGF-β and IL-10 levels are universal determinants of exhaustion during all types of persistent infections remains undefined. It is plausible that other cytokines, such as IL-27 or IL-35, also influence the quality of anti-viral T-cell responses. Interleukin-27 regulates the immune response during certain parasitic infections and autoimmune diseases by dampening T-cell activities, whereas IL-35 secretion by regulatory T cells is associated with immunosuppression, making either of these cytokines excellent candidates for promoting T-cell exhaustion.77,78
Inhibitory receptors
As T-cell responses are elicited following infection, dramatic changes occur in gene expression, including the up-regulation of inhibitory receptors.33,38 During acute infections these receptors function to limit the severity of the response but are then down-regulated as the pathogen is cleared and the memory pool forms. This pattern diverges during chronic infections and the establishment of the exhausted state is associated with the constitutive expression of constellations of inhibitory receptors, which collectively operate to negatively regulate the functional and proliferative potential of the responding cells. The identification of the importance of inhibitory receptors in the dysregulation of cellular immune responses in chronically infected hosts has revealed new potential therapeutic targets for restoring immune functions and decreasing viral loads.
The significance of the CD28 family member PD-1 (CD279) in exhaustion was first discovered following micro-array analysis of the gene expression profiles of virus-specific CD8 T cells during chronic LCMV infection.79 PD-1 plays a role in establishing peripheral tolerance and inhibits the proliferation and function of T cells. In the LCMV system, PD-1 is markedly up-regulated on exhausted T cells but only transiently expressed on effector T cells during acute infections, and is not present on functionally competent memory T cells. Blocking anti-PD-L1 antibody treatment during chronic LCMV infections promotes the proliferation of virus-specific T cells, improves their functionality, and reduces viral loads, even in cohorts of CD4-depleted mice, which develop severe T-cell exhaustion.79
During HIV-1 infection PD-1 is expressed by both virus-specific CD8 and CD4 T cells.80–82 Notably, the levels and frequency of PD-1 expression positively correlate with viral loads and inversely correlate with CD4 T-cell counts. The expression is also dynamic and declines following the initiation of anti-viral therapy. In long-term non-progressors the levels of PD-1 are low on HIV-specific T cells and these populations are more polyfunctional. In vitro studies show that blocking the PD-1 pathway allows exhaustion to be overcome by enhancing the proliferation of CD8 and CD4 T cells and promoting cytokine production.80–82 Significantly, blocking PD-1 antibody treatments during the early or later phases of simian immunodeficiency virus (SIV) infection showed remarkably promising results. The frequencies and functional quality of SIV-specific CD8 T cells detectable in the blood and gut increased, viral loads dropped, and the survival rate of the infected macaques improved.43 The dynamics and significance of PD-1 expression has also been examined following HBV and HCV infections.3,22,24,83,84 PD-1 levels are up-regulated on virus-specific CD8 T cells during the acute phase of the infection, but tend to be lower if the infection is resolved; however, higher PD-1 expression is associated with an exhausted phenotype in individuals with chronic infections.3,22,24,83,84 PD-1 expression is markedly up-regulated on HCV-specific CD8 T cells in the liver; however, the function of these cells is not enhanced by PD-1 blockade.84 This illustrates that multiple factors must regulate the maintenance of exhaustion and also suggests that the severity of exhaustion is impacted by localized levels of viral antigen and the compartmentalization of the T cells.
Although PD-1 is the best characterized inhibitory receptor associated with exhaustion, several other receptors have also been shown to impair T-cell responses during chronic infections. Cytotoxic T-lymphocyte antigen 4 (CTLA-4), which like PD-1 is a CD28 family member, has been shown to impact the functional quality of the T-cell response during HIV-1 and HCV infections of humans.85–87 Treatment of SIV-infected macaques with blocking CTLA-4 antibodies reveals some of the potential complications of ‘inhibiting inhibitors’ to promote immune responses and viral control. Viral loads were higher in infected macaques administered CTLA-4 blocking antibodies from the onset of infection, and treatment at later time-points reduced the effectiveness of anti-retroviral chemotherapy.86 These changes may be attributable to increased T-cell activation caused by releasing the cells from the attenuating effects of CTLA-4 and consequently providing a greater pool of infectable and infected targets.
The importance of counterbalancing potentially detrimental responses with the ability to elicit and sustain sufficiently protective T-cell responses is further exemplified by studies of T-cell immunoglobulin and mucin domain-containing protein-3 (TIM-3), because this receptor functions to attenuate autoimmune responses and has also been shown to influence the exhausted state. During HIV-1, HCV and LCMV infections TIM-3 is expressed by virus-specific T cells, and the frequencies and levels of expression parallel the exhausted state of the cells and the severity of infection.8,88,89 As with PD-1, blockade of TIM-3 improves the responsiveness and proliferation of the exhausted cells in vitro, but interestingly, TIM-3 is not necessarily co-expressed with PD-1 and can therefore identify a distinct subset of exhausted but PD-1-negative T cells.8,88
The hierarchies of inhibitory receptor expression by exhausted cells have been documented during chronic LCMV infection, and these populations of CD8 T cells can be segregated into a series of discrete subsets that express different numbers and combinations of inhibitory receptors.33,38 More severely exhausted cells express a greater number of inhibitory receptors. The roles of each individual receptor in promoting and sustaining exhaustion are less clear. The inhibitory molecule lymphocyte-activated gene-3 (LAG-3) is widely expressed on exhausted LCMV-specific CD8 T cells.38,89 Nevertheless, LAG-3 blockade alone is less effective at reversing exhaustion and lowering viral levels than a combined PD-L1 and LAG-3 blocking approach; moreover, the establishment of exhaustion occurs similarly in wild-type and LAG-3-deficient mice.38,89 Therefore, deciphering how specific inhibitory receptor signals integrate to promote and maintain exhaustion will be important next steps.
Molecular control of exhaustion
Exhausted T cells can be distinguished from typical effector and memory cells in many ways (Fig. 1).33 As the exhausted state develops in a stepwise, progressive, and often predictable manner, it is plausible that distinct, but overlapping, mechanisms independently regulate successive waves of changes in cellular activities such as susceptibility to apoptosis, proliferative capacity, ability to elaborate select effector functions, and receptor and adhesion molecule expression. This modular regulatory process is supported by studies of exhausted LCMV and HIV-specific T cells showing that defective nuclear translocation of nuclear factor of activated T cell 2 (NFAT-2) ablates IFN-γ secretion while degranulation and killing functions remain intact.30
Cbl-b is a RING-type E3 ubiquitin ligase that down-modulates TCR signalling and is strongly induced in anergic T cells.90 Deletion of Cbl-b results in independence from co-stimulation, hyperproliferation and enhanced IL-2 production. During acute LCMV infection Cbl-b deficiency prevents TCR down-regulation and increases the levels of IFN-γ production but does not impact cytotoxicity.91 During chronic LCMV infections the loss of Cbl-b restores IFN-γ production and confers resistance to deletion.92 This enhancement of the response increases morbidity and mortality, exemplifying the importance of exhaustion in preventing immunopathology. Gene related to anergy in lymphocytes (GRAIL) is also an E3 ubiquitin ligase that is associated with anergy; however, this molecule is not markedly up-regulated in exhausted LCMV-specific CD8 T cells.33,93 This further supports the concept that a unique ensemble of molecular regulators governs the exhausted setting and distinguishes these cells from conventional anergic, effector and memory T cells.
The transcriptional repressor BLIMP-1 (Pdrm1) regulates the terminal differentiation of CD8 T cells and is highly expressed during chronic infections.94 In the LCMV system, BLIMP-1 deficiency in T cells prevents the development of some of the characteristic signatures of exhaustion as the cells express CD127, retain the ability to produce IFN-γ and IL-2, and express fewer inhibitory receptors despite a failure to contain the infection.94 Intriguingly, haplo-insufficiency of BLIMP-1 enhances immune-mediated viral control. The levels rather than the presence or absence of BLIMP-1 critically influences the outcome of the infection and quality of the T-cell response so it will be important to fully understand how BLIMP-1 expression is regulated. One regulator of BLIMP-1 is IL-21, as IL-21 signalling can induce BLIMP-1 in pre-activated CD8 T cells; however, additional factors must regulate BLIMP-1 expression because this molecule is required for exhaustion, which is augmented by the absence of IL-21.49–51,95
Future prospects
In many ways the factors that promote and maintain exhaustion are still being defined. As more regulatory parameters are uncovered, it will be necessary to dissect how these individual mechanisms network together to fine-tune the phenotypic and functional set-points of the response. Gene-expression analyses suggest that metabolic defects manifest in exhausted T cells, and several recent reports have implicated changes in cellular metabolism and the mammalian target of rapamycin pathway in the generation of effector and memory T-cell responses.33,96–98 It will be of interest to determine whether and how these pathways influence the quality of T-cell responses during chronic infections. Importantly, several approaches have now been shown to improve responses and in certain cases lower viral loads. Nevertheless, whether all subsets of exhausted cells are susceptible to these regenerative methods, and whether these cells are fully reactivated and truly comparable in function and proliferative potential to bona fide memory cells, requires further evaluation. The key will be to devise tailored approaches, possibly using a combination of strategies that promote viral clearance while avoiding immunopathology and unintended consequences such as enhanced infection, autoimmunity or a deterioration of responses.
Acknowledgments
We wish to thank Laurie Harrington and all of the members of the Zajac laboratory for their advice and critical reading of this manuscript. This work was supported in part by grants R01 AI049360, R01 AI067933, and U01 AI082966 (to A.J.Z.) and T32 AI007051 (to J.S.Y.) from the National Institutes of Health. As a result of the space constraints, we apologize that we were unable to cite all our colleagues that have made an impact in defining our current knowledge of T-cell exhaustion.
Disclosure
The authors have no conflicts of interests to disclose.
References
- 1.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–61. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 2.Welsh RM. Assessing CD8 T cell number and dysfunction in the presence of antigen. J Exp Med. 2001;193:F19–22. doi: 10.1084/jem.193.5.f19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boni C, Fisicaro P, Valdatta C, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215–25. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks DG, McGavern DB, Oldstone MB. Reprogramming of antiviral T cells prevents inactivation and restores T cell activity during persistent viral infection. J Clin Invest. 2006;116:1675–85. doi: 10.1172/JCI26856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks DG, Teyton L, Oldstone MB, McGavern DB. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J Virol. 2005;79:10514–27. doi: 10.1128/JVI.79.16.10514-10527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuller MJ, Khanolkar A, Tebo AE, Zajac AJ. Maintenance, loss, and resurgence of T cell responses during acute, protracted, and chronic viral infections. J Immunol. 2004;172:4204–14. doi: 10.4049/jimmunol.172.7.4204. [DOI] [PubMed] [Google Scholar]
- 7.Fuller MJ, Zajac AJ. Ablation of CD8 and CD4 T cell responses by high viral loads. J Immunol. 2003;170:477–86. doi: 10.4049/jimmunol.170.1.477. [DOI] [PubMed] [Google Scholar]
- 8.Golden-Mason L, Palmer BE, Kassam N, et al. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol. 2009;83:9122–30. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iyasere C, Tilton JC, Johnson AJ, et al. Diminished proliferation of human immunodeficiency virus-specific CD4+ T cells is associated with diminished interleukin-2 (IL-2) production and is recovered by exogenous IL-2. J Virol. 2003;77:10900–9. doi: 10.1128/JVI.77.20.10900-10909.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oxenius A, Zinkernagel RM, Hengartner H. Comparison of activation versus induction of unresponsiveness of virus-specific CD4+ and CD8+ T cells upon acute versus persistent viral infection. Immunity. 1998;9:449–57. doi: 10.1016/s1074-7613(00)80628-7. [DOI] [PubMed] [Google Scholar]
- 11.Sester U, Presser D, Dirks J, Gartner BC, Kohler H, Sester M. PD-1 expression and IL-2 loss of cytomegalovirus- specific T cells correlates with viremia and reversible functional anergy. Am J Transplant. 2008;8:1486–97. doi: 10.1111/j.1600-6143.2008.02279.x. [DOI] [PubMed] [Google Scholar]
- 12.Gallimore A, Glithero A, Godkin A, Tissot AC, Pluckthun A, Elliott T, Hengartner H, Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I–peptide complexes. J Exp Med. 1998;187:1383–93. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goepfert PA, Bansal A, Edwards BH, Ritter GD, Jr, Tellez I, McPherson SA, Sabbaj S, Mulligan MJ. A significant number of human immunodeficiency virus epitope-specific cytotoxic T lymphocytes detected by tetramer binding do not produce gamma interferon. J Virol. 2000;74:10249–55. doi: 10.1128/jvi.74.21.10249-10255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gruener NH, Lechner F, Jung MC, et al. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J Virol. 2001;75:5550–8. doi: 10.1128/JVI.75.12.5550-5558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–27. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krebs P, Scandella E, Odermatt B, Ludewig B. Rapid functional exhaustion and deletion of CTL following immunization with recombinant adenovirus. J Immunol. 2005;174:4559–66. doi: 10.4049/jimmunol.174.8.4559. [DOI] [PubMed] [Google Scholar]
- 17.Ladell K, Hellerstein MK, Cesar D, Busch R, Boban D, McCune JM. Central memory CD8+ T cells appear to have a shorter lifespan and reduced abundance as a function of HIV disease progression. J Immunol. 2008;180:7907–18. doi: 10.4049/jimmunol.180.12.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee PP, Yee C, Savage PA, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–85. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 19.Migueles SA, Weeks KA, Nou E, et al. Defective human immunodeficiency virus-specific CD8+ T-cell polyfunctionality, proliferation, and cytotoxicity are not restored by antiretroviral therapy. J Virol. 2009;83:11876–89. doi: 10.1128/JVI.01153-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moser JM, Altman JD, Lukacher AE. Antiviral CD8+ T cell responses in neonatal mice: susceptibility to polyoma virus-induced tumors is associated with lack of cytotoxic function by viral antigen-specific T cells. J Exp Med. 2001;193:595–606. doi: 10.1084/jem.193.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mumprecht S, Schurch C, Schwaller J, Solenthaler M, Ochsenbein AF. Programmed death 1 signaling on chronic myeloid leukemia-specific T cells results in T-cell exhaustion and disease progression. Blood. 2009;114:1528–36. doi: 10.1182/blood-2008-09-179697. [DOI] [PubMed] [Google Scholar]
- 22.Radziewicz H, Ibegbu CC, Fernandez ML, et al. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007;81:2545–53. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reignat S, Webster GJ, Brown D, et al. Escaping high viral load exhaustion: CD8 cells with altered tetramer binding in chronic hepatitis B virus infection. J Exp Med. 2002;195:1089–101. doi: 10.1084/jem.20011723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urbani S, Boni C, Missale G, Elia G, Cavallo C, Massari M, Raimondo G, Ferrari C. Virus-specific CD8+ lymphocytes share the same effector-memory phenotype but exhibit functional differences in acute hepatitis B and C. J Virol. 2002;76:12423–34. doi: 10.1128/JVI.76.24.12423-12434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–13. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zelinskyy G, Robertson SJ, Schimmer S, Messer RJ, Hasenkrug KJ, Dittmer U. CD8+ T-cell dysfunction due to cytolytic granule deficiency in persistent Friend retrovirus infection. J Virol. 2005;79:10619–26. doi: 10.1128/JVI.79.16.10619-10626.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dittmer U, He H, Messer RJ, et al. Functional impairment of CD8+ T cells by regulatory T cells during persistent retroviral infection. Immunity. 2004;20:293–303. doi: 10.1016/s1074-7613(04)00054-8. [DOI] [PubMed] [Google Scholar]
- 29.Zhang D, Shankar P, Xu Z, et al. Most antiviral CD8 T cells during chronic viral infection do not express high levels of perforin and are not directly cytotoxic. Blood. 2003;101:226–35. doi: 10.1182/blood-2002-03-0791. [DOI] [PubMed] [Google Scholar]
- 30.Agnellini P, Wolint P, Rehr M, Cahenzli J, Karrer U, Oxenius A. Impaired NFAT nuclear translocation results in split exhaustion of virus-specific CD8+ T cell functions during chronic viral infection. Proc Natl Acad Sci U S A. 2007;104:4565–70. doi: 10.1073/pnas.0610335104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuller MJ, Hildeman DA, Sabbaj S, Gaddis DE, Tebo AE, Shang L, Goepfert PA, Zajac AJ. Cutting edge: emergence of CD127high functionally competent memory T cells is compromised by high viral loads and inadequate T cell help. J Immunol. 2005;174:5926–30. doi: 10.4049/jimmunol.174.10.5926. [DOI] [PubMed] [Google Scholar]
- 32.Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci U S A. 2004;101:16004–9. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wherry EJ, Ha SJ, Kaech SM, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–84. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Zhou S, Ou R, Huang L, Price GE, Moskophidis D. Differential tissue-specific regulation of antiviral CD8+ T-cell immune responses during chronic viral infection. J Virol. 2004;78:3578–600. doi: 10.1128/JVI.78.7.3578-3600.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grayson JM, Weant AE, Holbrook BC, Hildeman D. Role of Bim in regulating CD8+ T-cell responses during chronic viral infection. J Virol. 2006;80:8627–38. doi: 10.1128/JVI.00855-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mueller SN, Ahmed R. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2009;106:8623–8. doi: 10.1073/pnas.0809818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Q, Skinner PJ, Ha SJ, et al. Visualizing antigen-specific and infected cells in situ predicts outcomes in early viral infection. Science. 2009;323:1726–9. doi: 10.1126/science.1168676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blackburn SD, Shin H, Haining WN, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lechner F, Wong DK, Dunbar PR, et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sabbaj S, Heath SL, Bansal A, Vohra S, Kilby JM, Zajac AJ, Goepfert PA. Functionally competent antigen-specific CD127hi memory CD8+ T cells are preserved only in HIV-infected individuals receiving early treatment. J Infect Dis. 2007;195:108–17. doi: 10.1086/509510. [DOI] [PubMed] [Google Scholar]
- 41.Caetano J, Martinho A, Paiva A, Pais B, Valente C, Luxo C. Differences in hepatitis C virus (HCV)-specific CD8 T-cell phenotype during pegylated alpha interferon and ribavirin treatment are related to response to antiviral therapy in patients chronically infected with HCV. J Virol. 2008;82:7567–77. doi: 10.1128/JVI.02175-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenberg ES, Altfeld M, Poon SH, et al. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407:523–6. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 43.Velu V, Titanji K, Zhu B, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–10. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc Natl Acad Sci U S A. 2008;105:15016–21. doi: 10.1073/pnas.0801497105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell–dendritic cell interaction. Nature. 2006;440:890–5. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 46.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–3. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bachmann MF, Wolint P, Walton S, Schwarz K, Oxenius A. Differential role of IL-2R signaling for CD8+ T cell responses in acute and chronic viral infections. Eur J Immunol. 2007;37:1502–12. doi: 10.1002/eji.200637023. [DOI] [PubMed] [Google Scholar]
- 48.Williams MA, Holmes BJ, Sun JC, Bevan MJ. Developing and maintaining protective CD8+ memory T cells. Immunol Rev. 2006;211:146–53. doi: 10.1111/j.0105-2896.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- 49.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–72. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frohlich A, Kisielow J, Schmitz I, et al. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–80. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 51.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–6. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8+ T lymphocyte mobilization to virus-infected tissue requires CD4+ T-cell help. Nature. 2009;462:510–3. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bevan MJ. Helping the CD8+ T-cell response. Nat Rev Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 54.Iannello A, Boulassel MR, Samarani S, Debbeche O, Tremblay C, Toma E, Routy JP, Ahmad A. Dynamics and consequences of IL-21 production in HIV-infected individuals: a longitudinal and cross-sectional study. J Immunol. 2009;184:114–26. doi: 10.4049/jimmunol.0901967. [DOI] [PubMed] [Google Scholar]
- 55.Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9:540–7. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 56.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–6. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 57.Molloy MJ, Zhang W, Usherwood EJ. Cutting edge: IL-2 immune complexes as a therapy for persistent virus infection. J Immunol. 2009;182:4512–5. doi: 10.4049/jimmunol.0804175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hinrichs CS, Spolski R, Paulos CM, et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–33. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen H, Weng NP. IL-21 preferentially enhances IL-15-mediated homeostatic proliferation of human CD28+ CD8 memory T cells throughout the adult age span. J Leukoc Biol. 2009;87:43–9. doi: 10.1189/jlb.0209086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clerici M, Wynn TA, Berzofsky JA, Blatt SP, Hendrix CW, Sher A, Coffman RL, Shearer GM. Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with the human immunodeficiency virus. J Clin Invest. 1994;93:768–75. doi: 10.1172/JCI117031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hyodo N, Nakamura I, Imawari M. Hepatitis B core antigen stimulates interleukin-10 secretion by both T cells and monocytes from peripheral blood of patients with chronic hepatitis B virus infection. Clin Exp Immunol. 2004;135:462–6. doi: 10.1111/j.1365-2249.2003.02376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ohga S, Nomura A, Takada H, et al. Dominant expression of interleukin-10 and transforming growth factor-beta genes in activated T-cells of chronic active Epstein–Barr virus infection. J Med Virol. 2004;74:449–58. doi: 10.1002/jmv.20197. [DOI] [PubMed] [Google Scholar]
- 63.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–9. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, von HerrathMG. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med. 2006;203:2461–72. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maris CH, Chappell CP, Jacob J. Interleukin-10 plays an early role in generating virus-specific T cell anergy. BMC Immunol. 2007;8:8. doi: 10.1186/1471-2172-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaplan DE, Ikeda F, Li Y, et al. Peripheral virus-specific T-cell interleukin-10 responses develop early in acute hepatitis C infection and become dominant in chronic hepatitis. J Hepatol. 2008;48:903–13. doi: 10.1016/j.jhep.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brockman MA, Kwon DS, Tighe DP, et al. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood. 2009;114:346–56. doi: 10.1182/blood-2008-12-191296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–79. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 69.Miyazoe S, Hamasaki K, Nakata K, et al. Influence of interleukin-10 gene promoter polymorphisms on disease progression in patients chronically infected with hepatitis B virus. Am J Gastroenterol. 2002;97:2086–92. doi: 10.1111/j.1572-0241.2002.05926.x. [DOI] [PubMed] [Google Scholar]
- 70.Knapp S, Hennig BJ, Frodsham AJ, et al. Interleukin-10 promoter polymorphisms and the outcome of hepatitis C virus infection. Immunogenetics. 2003;55:362–9. doi: 10.1007/s00251-003-0594-5. [DOI] [PubMed] [Google Scholar]
- 71.Paladino N, Fainboim H, Theiler G, Schroder T, Munoz AE, Flores AC, Galdame O, Fainboim L. Gender susceptibility to chronic hepatitis C virus infection associated with interleukin 10 promoter polymorphism. J Virol. 2006;80:9144–50. doi: 10.1128/JVI.00339-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 73.Brooks DG, Lee AM, Elsaesser H, McGavern DB, Oldstone MB. IL-10 blockade facilitates DNA vaccine-induced T cell responses and enhances clearance of persistent virus infection. J Exp Med. 2008;205:533–41. doi: 10.1084/jem.20071948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brooks DG, Ha SJ, Elsaesser H, Sharpe AH, Freeman GJ, Oldstone MB. IL-10 and PD-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proc Natl Acad Sci U S A. 2008;105:20428–33. doi: 10.1073/pnas.0811139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–71. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 76.Tinoco R, Alcalde V, Yang Y, Sauer K, Zuniga EI. Cell-intrinsic transforming growth factor-beta signaling mediates virus-specific CD8+ T cell deletion and viral persistence in vivo. Immunity. 2009;31:145–57. doi: 10.1016/j.immuni.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Collison LW, Vignali DA. Interleukin-35: odd one out or part of the family? Immunol Rev. 2008;226:248–62. doi: 10.1111/j.1600-065X.2008.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stumhofer JS, Hunter CA. Advances in understanding the anti-inflammatory properties of IL-27. Immunol Lett. 2008;117:123–30. doi: 10.1016/j.imlet.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 80.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–4. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 81.Petrovas C, Casazza JP, Brenchley JM, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–92. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 83.Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249–58. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nakamoto N, Kaplan DE, Coleclough J, et al. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology. 2008;134:1927–37. doi: 10.1053/j.gastro.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaufmann DE, Kavanagh DG, Pereyra F, et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol. 2007;8:1246–54. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 86.Cecchinato V, Tryniszewska E, Ma ZM, et al. Immune activation driven by CTLA-4 blockade augments viral replication at mucosal sites in simian immunodeficiency virus infection. J Immunol. 2008;180:5439–47. doi: 10.4049/jimmunol.180.8.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakamoto N, Cho H, Shaked A, et al. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 2009;5:e1000313. doi: 10.1371/journal.ppat.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jones RB, Ndhlovu LC, Barbour JD, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–79. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Richter K, Agnellini P, Oxenius A. On the role of the inhibitory receptor LAG-3 in acute and chronic LCMV infection. Int Immunol. 2010;22:13–23. doi: 10.1093/intimm/dxp107. [DOI] [PubMed] [Google Scholar]
- 90.Zheng Y, Zha Y, Gajewski TF. Molecular regulation of T-cell anergy. EMBO Rep. 2008;9:50–5. doi: 10.1038/sj.embor.7401138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shamim M, Nanjappa SG, Singh A, et al. Cbl-b regulates antigen-induced TCR down-regulation and IFN-gamma production by effector CD8 T cells without affecting functional avidity. J Immunol. 2007;179:7233–43. doi: 10.4049/jimmunol.179.11.7233. [DOI] [PubMed] [Google Scholar]
- 92.Ou R, Zhang M, Huang L, Moskophidis D. Control of virus-specific CD8+ T-cell exhaustion and immune-mediated pathology by E3 ubiquitin ligase Cbl-b during chronic viral infection. J Virol. 2008;82:3353–68. doi: 10.1128/JVI.01350-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Anandasabapathy N, Ford GS, Bloom D, et al. GRAIL: an E3 ubiquitin ligase that inhibits cytokine gene transcription is expressed in anergic CD4+ T cells. Immunity. 2003;18:535–47. doi: 10.1016/s1074-7613(03)00084-0. [DOI] [PubMed] [Google Scholar]
- 94.Shin H, Blackburn SD, Intlekofer AM, Kao C, Angelosanto JM, Reiner SL, Wherry EJ. A role for the transcriptional repressor Blimp-1 in CD8+ T cell exhaustion during chronic viral infection. Immunity. 2009;31:309–20. doi: 10.1016/j.immuni.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kwon H, Theirry-Mieg D, Thierry-Mieg J, et al. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity. 2009;31:941–52. doi: 10.1016/j.immuni.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–12. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Delgoffe GM, Kole TP, Zheng Y, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–44. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–7. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]