Abstract
The lectin pathway of complement is activated upon binding of mannan-binding lectin (MBL) or ficolins (FCNs) to their targets. Upon recognition of targets, the MBL-and FCN-associated serine proteases (MASPs) are activated, allowing them to generate the C3 convertase C4b2a. Recent findings indicate that the MASPs also activate components of the coagulation system. We have previously shown that MASP-1 has thrombin-like activity whereby it cleaves and activates fibrinogen and factor XIII. MASP-2 has factor Xa-like activity and activates prothrombin through cleavage to form thrombin. We now report that purified L-FCN-MASPs complexes, bound from serum to N-acetylcysteine-Sepharose, or MBL-MASPs complexes, bound to mannan-agarose, generate clots when incubated with calcified plasma or purified fibrinogen and factor XIII. Plasmin digestion of the clot and analysis using anti-D-dimer antibodies revealed that the clot was made up of fibrin and was similar to that generated by thrombin in normal human plasma. Fibrinopeptides A and B (FPA and FPB, respectively) were released after fibrinogen cleavage by L-FCN-MASPs complexes captured on N-acetylcysteine-Sepharose. Studies of inhibition of fibrinopeptide release indicated that the dominant pathway for clotting catalysed by the MASPs is via MASP-2 and prothrombin activation, as hirudin, a thrombin inhibitor that does not inhibit MASP-1 and MASP-2, substantially inhibits fibrinopeptide release. In the light of their potent chemoattractant effects on neutrophil and fibroblast recruitment, the MASP-mediated release of FPA and FPB may play a role in early immune activation. Additionally, MASP-catalysed deposition and polymerization of fibrin on the surface of micro-organisms may be protective by limiting the dissemination of infection.
Keywords: coagulation, lectin pathway, mannan-binding lectin, mannan-binding lectin-associated serine proteases
Introduction
Proteases are involved in a variety of physiological process and often act in a sequential manner generally known as a proteolytic cascade, for example, in complement activation and the coagulation pathway. The coagulation cascade results in the generation of a blood clot after injury to the vasculature, whereas the complement cascade leads to the opsonization or killing of invading micro-organisms. The coagulation and complement cascades are intended to act locally, that is, coagulation is activated at a site of injury and complement at a site of infection. The complement cascade is activated through three different pathways, the classical, the alternative and the lectin pathways. The three pathways converge in their terminal steps, resulting in the opsonization and lysis of invading micro-organisms.
The lectin complement pathway is activated upon target recognition by pattern recognition receptors such as mannan-binding lectin (MBL), H-ficolin, L-ficolin and M-ficolin (H-FCN, L-FCN and M-FCN, respectively)1, each of which associates with the MBL-associated serine proteases (MASPs). Three MASPs, termed MASP-1, MASP-2 and MASP-3, are known.2 Additionally, MASP-2 exists in an alternatively spliced form, known as Map19, that lacks the serine protease domain. Each MBL or FCN may associate with a homodimer of one of these proteases, so that, in the blood, there are varying amounts of complexes with composition MBL-(MASP-1)2, MBL-(MASP-2)2, MBL-(MASP-3)2 and MBL-(MAp19)2, and finally MBL with no proteases attached. The FCNs are likely to form a similar range of complexes.3,4
Upon binding of MBL/MASPs or FCN/MASPs complexes to targets, autoactivation of MASP-1 and MASP-2 occurs, while MASP-3 does not appear to autoactivate. MASP activation allows the generation of the complement system C3-activating protease (C3 convertase) C4b2a, with subsequent activation of C3 and the later complement proteins C5 to C9. This in turn leads to the generation of two pro-inflammatory anaphylotoxins, C3a and C5a, and the membrane attack complex.5,6
Among the three MASPs, a natural substrate for MASP-3 has not yet been identified. However, activated MASP-2 is known to cleave and activate complement components C2 and C4, forming C4b2a. Recently it was demonstrated that activated MASP-2 also cleaves prothrombin into thrombin,7 leading to the formation of a fibrin clot. MASP-1 has been shown to cleave C2, but not C4. In principle, generation of C4b2a requires the simultaneous cleavage of C4 and C2. It has been proposed that MASP-1 augments complement activation by cleaving C2 after it has bound to the C4b fragment but cannot initiate complement fixation in the absence of MASP-2.8 Using synthetic substrates, we earlier reported that human MASP-1 has a specificity profile similar to that of thrombin and cleaves the thrombin substrate Val-Pro-Arg-aminomethylcoumarin (VPR-AMC).9,10 We have also found that isolated MASP-1 cleaves two protein substrates of thrombin, namely factor XIII (plasma transglutaminase) and fibrinogen,11 although the catalytic activity is several hundred-fold less than that of thrombin.12 Thus MASP-1 is capable of catalysing the formation of cross-linked fibrin.12
We now report that the activation of the MASPs by binding of either MBL-MASPs or FCN-MASPs to their respective ligands leads to the generation of a fibrin clot that is indistinguishable from the clot generated through the conventional clotting cascade. It is proposed that the fibrin clot-forming ability of MASPs may represent an ancient mechanism to limit the spread of infectious agents.
Materials and methods
Materials
Human citrated plasma (pooled) was from HD Supplies (Aylesbury, UK) and human plasmin was obtained from KABI (Copenhagen, Denmark). Human factor XIII and prothrombin were purchased from Haematologic Technologies Inc. (Essex Junction, Vermont, USA) while mouse monoclonal antibody to fibrin D-dimer (DD1; Cat No. ab10050) was from Abcam (Cambridge, UK). Human fibrinogen was purchased from Calbiochem (San Diego, CA). As with most commercial fibrinogen preparations, the fibrinogen contains factor XIII and fibronectin as minor contaminants. Human thrombin, hirudin, ethylenediaminetetraacetic acid (EDTA), soybean trypsin inhibitor (SBTI), human antithrombin III (ATIII) and heparin were purchased from Sigma-Aldrich (Poole, UK). C1inhibitor was isolated from human plasma as described by Sim and Reboul.13 Pooled human plasma was obtained from H.D. Supplies (Berks, UK) All the other reagents were of analytical grade.
Polyclonal rabbit anti-L-FCN antiserum was prepared using native L-FCN, purified as described by Krarup et al.14 as immunogen. Rabbit polyclonal antiserum was raised against the N-terminal 15 residues of the MASP-2 A chain (corresponding to TPLGPKWPEPVFGRL) conjugated to keyhole limpet haemocyanin, and rabbit anti-MBL antibodies were prepared using the recombinant lectin domains of MBL as immunogen, as described in Arnold et al.15 Rabbit polyclonal antiserum raised against the last 19 residues of the C-terminus (B chain) of human MASP-1 was a kind gift from Dr S. Thiel (University of Aarhus, Aarhus, Denmark). N-acetylcysteine Sepharose beads were prepared as described by Krarup et al.14
Purification of L-FCN-MASPs and MBL-MASPs
For the isolation of complexes between L-FCN and MASPs or between MBL and MASPs (L-FCN-MASPs or MBL-MASPs), serum was prepared by calcifying human plasma to 20 mM CaCl2 (final concentration).
L-FCN-MASPs were purified by minor modifications of the protocol developed by Krarup et al.14 The 4–8% polyethylene glycol (PEG) 3350 precipitate of human serum (1 l) was dissolved in 200 ml of 20 mm HEPES containing 500 mm NaCl, 1·5 mm NaN3, 5 mm CaCl2 and 0·01% (v/v) polyoxyethylene 10 tridecyl ether (Emulfogen; Sigma-Aldrich), pH 7·5 (loading buffer), and loaded onto an 8-ml column of N-acetylcysteine-Sepharose. After washing with loading buffer, the bound L-FCN-MASPs were eluted with 10 mm Tris–HCl, 20 mm NaCl, 1·5 mm NaN3, 2 mm EDTA and 0·01% (v/v) Emulfogen, pH 7·5.
Eluted fractions were adjusted to 10 mm CaCl2 and analysed for L-FCN by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis under reducing conditions. The association of MASPs with L-FCN was checked by assaying the fractions for L-FCN-associated MASP-1/MASP-2 activity. This was done similarly to the quantification of MBL-associated MASP-1 activity as follows : aliquots (100 μl) of fractions were incubated in microtitre plates coated with N-acetylated bovine serum albumin (BSA) to allow L-FCN binding to the N-acetylated BSA.3,16 Unbound material was removed by washing, and MASP-1/MASP-2 activity bound to the plate via L-FCN was quantified by hydrolysis of methylsulphonyl D-Phe-Gly-Arg-7-amino-4-methylcoumarin (FGR-AMC), which is cleaved by both MASP-1 and MASP-2.9
Pooled fractions (8 ml) were diluted to 50 ml with low salt buffer (20 mm HEPES containing 20 mm NaCl, 1·5 mm NaN3, 5 mm CaCl2 and 0·01% Emulfogen, pH 7·5) and passed onto a 1-ml Mono Q column (GE Healthcare, Little Chalfont, UK) attached to an AKTA FPLC (GE Healthcare). The bound material was eluted using a 20-ml linear NaCl gradient from 20 to 500 mm in 20 mm HEPES buffer, pH 7·5, containing 10 mm CaCl2, and the fractions containing L-FCN were identified using an N-acetyl BSA-based L-FCN ELISA.17 Briefly, BSA was coated onto microtitre plates and then acetylation was performed using acetic anhydride. After washing and further blocking, the fractions were incubated in coated wells overnight and subsequently followed by detection with polyclonal rabbit anti-L-FCN antibodies and alkaline phosphatase-labelled mouse anti-rabbit immunoglobulin G (IgG) antibodies.
MBL-MASP complexes were isolated from normal human serum by the method of Tan et al.,18 with modifications. The serum (1 l) was subjected to 7% PEG (3350) precipitation and the precipitate was dissolved in 20 mm HEPES buffer containing 1 m NaCl and 10 mm CaCl2, pH 7·8. Mannan-agarose beads (5 ml; Sigma-Aldrich) washed in this buffer were incubated with dissolved PEG precipitate for 4 hr at 4°. After incubation the resin was washed with the buffer and bound MBL-MASPs were eluted with 20 mm HEPES, 1 m NaCl and 0·01% (v/v) Emulfogen, pH 7·8, containing 10 mm EDTA. Fractions containing MBL-MASPs were pooled, dialysed in the same buffer in the absence of EDTA, recalcified by the addition of CaCl2 to a final concentration of 10 mm and subjected to ion exchange chromatography using a Mono Q column, as described above for L-FCN-MASPs. MBL-MASP-containing fractions were selected by SDS-PAGE, and analysed by western blot using anti-MBL, MASP-1 and MASP-2 antibodies as well as N-terminal sequencing (results not shown).
Fibrinogen cleavage by purified L-FCN-MASPs or MBL-MASPs
Fibrinogen was incubated with various concentrations of purified L-FCN-MASPs (in 20 mm HEPES, 145 mm NaCl and 5mm CaCl2, pH 7·5) or MBL-MASPs for 14 hr at 37° and samples were subjected to SDS-PAGE on 4–12% NuPage Novex Bis Tris gels (Invitrogen, Paisley, UK) under reducing and non-reducing conditions to monitor cleavage.
Activation of factor XIII by purified L-FCN-MASPs or MBL-MASPs
Factor XIII was incubated with various concentrations of purified L-FCN-MASPs or MBL-MASPs at 37° for different time intervals and then subjected to SDS-PAGE (10% gel) under reducing conditions to monitor cleavage.
Fibrinopeptide release by L-FCN-MASPs captured from normal human serum on an affinity resin
Fresh N-acetylcysteine-Sepharose beads (100 μl, packed volume) were washed thoroughly with buffer A (20 mm HEPES, 145 mm NaCl and 10 mm CaCl2, pH 7·4) and incubated with normal human serum (10 ml) for 3 hr on a rotary mixer at 4° to trap L-FCN-MASPs. Cysteine-Sepharose and underivatized Sepharose were used as controls. After incubation, beads were washed three times with buffer A containing 0·05% Tween20 and incubated overnight with fibrinogen (500 ug; 200 μl of 2·5 mg/ml fibrinogen in buffer A) at 37° on a rotary mixer. After incubation, beads, together with any clots formed, were mixed well and centrifuged and the supernatant was subjected to fibrinopeptide separation. Fibrinopeptides were separated from the proteins (fibrin/fibrinogen) by filtering the supernatant through a 10-kDa cut-off centrifugal filtration unit (Vivaspin; Sartorius, Epsom, UK) The flow-through was treated as a fibrinopeptide preparation and subjected to high-performance liquid chromatography (HPLC) analysis as described previously.12 FPA and FPB were identified initially as peaks with the same retention time as standard FPA and FPB, and then further analysed by mass spectrometry.
Mass/charge ratio determination by mass spectrometry
Standard FPA and FPB (Bachem, St Helens, UK) and fractions eluted by HPLC (section Fibrinopeptide release by L-FCN-MASPs captured from normal human serum on an affinity resin) were subjected to mass spectrometry (Ettan MALDI-ToF Pro mass spectrometer; GE Healthcare) to determine the m/z ratio. Fractions were collected based on the peaks in the HPLC profile. Samples collected in microcentrifuge tubes were dried in a Speedvac (Savant, Holbrook, NY) and taken up in 10 μl of 0·1% trifluoroacetic acid. From this solution, 1 μl was taken and mixed with 1 μl of a saturated α-cyano matrix solution and 0·5 μl was loaded onto the MALDI target.
Effect of inhibitors on fibrinopeptide release
After capturing the L-FCN-MASPs from normal human serum using N-acetylcysteine-Sepharose, as in section Fibrinopeptide release by L-FCN-MASPs captured from normal human serum on an affinity resin, the resin was washed thoroughly with buffer A (20 mm HEPES, 145 mm NaCl and 10 mm CaCl2, pH 7·4) and incubated with various inhibitors (final concentrations as shown in Table 1) in buffer A, for 30 min, with shaking at 37°. The final volume after the addition of inhibitor was kept constant. After incubation, the resin was separated from the inhibitor by centrifugation and washing in buffer A, and fibrinogen was added along with the same inhibitor at the same concentration as before, and incubated overnight at 37° with shaking. Fibrinopeptides were separated and analysed as in section Fibrinopeptide release by L-FCN-MASPs captured from normal human serum on an affinity resin.
Table 1.
Inhibitor profile of fibrinopeptide release by L-ficolin mannan-binding lectin-associated serine proteases (MASPs) bound to CysNAc-Sepharose from normal human serum
| Inhibitor (concentration) | % inhibition of fibrinopeptide release |
|---|---|
| C1 inhibitor (4 μg/ml) | 90 |
| Hirudin (1·75 μg/ml) | 80 |
| EDTA (10 mm)1 | 90 |
| Soybean trypsin inhibitor (10 μg/ml) | 0 |
| Pefabloc SC (1 mm) | 95 |
| Anti-thrombin III (2 μg/ml) | 60 |
| Anti-thrombin + heparin (2 μg/ml + 55 μg/ml) | 90 |
| α2-macroglobulin (25 ng/ml) | 55 |
Ethylenediaminetetraacetic acid (EDTA) was added to serum to a final concentration of 10 mm and a washing buffer containing 10 mm EDTA was used. For the other inhibitors.
Fibrinopeptide release by purified L-FCN-MASPs in the presence and absence of prothrombin
To study the role of prothrombin in fibrinogen cleavage, soluble purified L-FCN-MASPs (8 μg) was incubated with fibrinogen (500 μg; 200 μl of 2·5 mg/ml of fibrinogen in buffer A) overnight at 37° with or without prothrombin (10 μg) in buffer A. Vials were centrifuged and the supernatant subjected to fibrinopeptide separation and analysis by HPLC as in section Fibrinopeptide release by L-FCN-MASPs captured from normal human serum on an affinity resin. In another experiment, prothrombin (10 μg) was incubated with purified L-FCN-MASPs (8 μg) with N-acetylcysteine Sepharose resin (100 μl) for 4 hr at 4°. Further, beads were either washed three times with 0·01% Tween 20 in buffer A or incubated directly with fibrinogen (500 μg; 200 μl of 2·5 mg/ml of fibrinogen in buffer A) overnight at 37°. After incubation, vials were centrifuged and the supernatant was subjected to fibrinopeptide analysis as in section Fibrinopeptide release by L-FCN-MASPs captured from normal human serum on an affinity resin.
Generation of a clot from plasma by L-FCN -MASPs or MBL-MASPs
Human blood was collected in heparin (Sigma-Aldrich; final concentration 1 unit/ml). After centrifugation to remove cells, plasma was calcified (20 mm CaCl2, final concentration). Fresh mannan-agarose or N-acetylcysteine-Sepharose resin (100 μl) was washed with buffer A and incubated with 1 ml of normal human serum for 2 hr at 4° to capture MBL-MASPs or L-FCN-MASPs, respectively. The beads were then washed with buffer A and incubated at 37°C for 1 hr with 1 ml of calcified plasma diluted 1 : 1 (v/v) with 0·1 m Na bicarbonate (pH 8·5). After incubation, the clot and trapped beads were washed with buffer A and the clot subjected to plasmin digestion.
Clot generation by L-FCN-MASPs or MBL-MASPs with pure fibrinogen
L-FCN-MASPs or MBL-MASPs were captured directly from serum on N-acetylcysteine-Sepharose or mannan-agarose as described in section Generation of a clot from plasma by L-FCN -MASPs or MBL-MASPs and incubated with 200 μl of fibrinogen (3 mg/ml) in the presence of factor XIII (0·5 mg/ml) or iodoacetamide (IAM) (1 mm) in buffer A for 2 hr at 37°. Similarly, after capturing the L-FCN-MASPs or MBL-MASPs on N-acetylcysteine-Sepharose or mannan-agarose, resin was incubated with soybean trypsin inhibitor (2 ml of 50 μg/ml of soybean trypsin inhibitor to inhibit plasmin) for 30 min at 37°, washed and then incubated with fibrinogen in the presence of factor XIII (0·5 mg/ml) with shaking. After 2 hr at 37°, tubes were shaken and photographed.
Generation of the fibrin clot by thrombin in the presence and absence of factor XIII
Fibrinogen (3 mg/ml) was prepared in buffer B (10 mm HEPES, 145 mm NaCl and 5 mm CaCl2, pH 7·4). Thrombin and factor XIII were diluted in the same buffer. Clots were generated by combining the following reagents: 66·6 μl of fibrinogen (3 mg/ml), 10 μl of factor XIII (0·5 mg/ml), 5 μl of thrombin (20 units/ml) and 18·4 μl of buffer B to make a total volume of 100 μl; or 66·6 μl of fibrinogen (3 mg/ml), 4 μl of iodoacetamide (25 mm), 5 μl of thrombin (20 units/ml) and 24·6 μl of buffer B. After incubation for 2 hr at 37°, clots were washed three times with buffer B and stored at −20°.
Characterization of the clot
Digestion of clot by plasmin
Clots generated by the different methods were subjected to plasmin digestion according to Terasawa et al.19 with minor modifications. Washed clots (30 mg wet weight) were incubated with 200 μl of plasmin (0·12 CU/ml) at 37° for 18 hr with intermittent shaking. After incubation, digested clots were shaken well and centrifuged and the supernatant fractions were collected.
Detection of D-dimer or fibrin degradation products
Plasmin digest products were analysed by SDS-PAGE (8% acrylamide) under reducing and non-reducing conditions followed by Coomassie blue staining of the proteins. Alternatively, proteins in the gel were electroblotted onto a polyvinylidene difluoride membrane (GE Healthcare) and probed with a monoclonal antibody specific for the D-dimer. Antibody binding was detected using horseradish peroxidase-conjugated secondary antibodies (Sigma-Aldrich) in conjunction with an enhanced chemiluminescence (ECL) reagent (GE Healthcare).
Results
Purification of FCN-MASPs
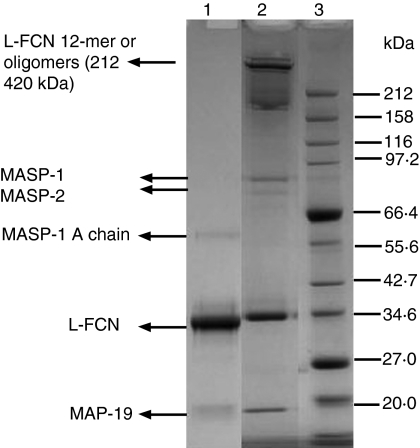
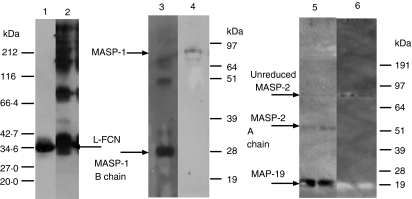
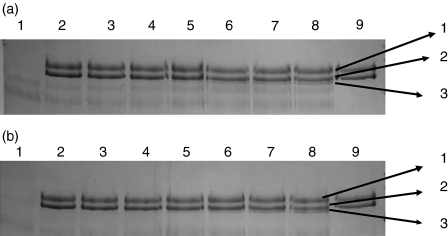
To determine the clot-forming activities of MASPs, L-FCN-MASP complexes were purified from human serum. Figure 1 shows the SDS-PAGE pattern of purified L-FCN-MASP complexes under reducing and non-reducing conditions. Without reduction, higher molecular weight oligomers of L-FCN are visible (lane 2), which disappear after reduction (lane 1). In Fig. 1 (lane 2), the two bands at about 77 and 87 kDa are likely to be MASP-2 and MASP-1 (expected molecular weights 74 kDa and 88 kDa, respectively). These are not visible on reduction (lane 1), indicating that the MASPs are fully activated. The band at about 60 kDa is likely to be the MASP-1 A chain (expected molecular weights of A chains are 60 kDa for MASP1 and 48kDa for MASP2). MAp19 is also visible in lane 2. Figure 2 depicts a western blot analysis of L-FCN-MASPs complexes. Bands are seen when blots were probed with anti-L-FCN, anti-MASP-1 and anti-MASP-2 antibodies, indicating that the purified mixture of complexes does indeed contain L-FCN, MASP-1, MASP-2 and MAp19. The MASP-1 B chain is not clearly visible in Fig. 1, but is clearly seen on the blot in Fig. 2 (lane 3) at the expected molecular weight of 28 kDa. The two chains of activated MASP-2 are not clearly visible in Fig. 1 (lane 1), but the A chain of MASP-2 is visible on the blot in Fig. 2 (lane 5) near the expected molecular. weight of 48 kDa, as is the unreduced MASP-2 (lane 6, about 74 kDa). The blot of MASP-1 (Fig. 2) shows unreduced activated MASP-1 at about 88 kDa (lane 4) and the 28-kDa B chain in lane 3. Two minor bands in lane 3 at about 50 and 70 kDa may be traces of minor degradation products. None of the antibodies used detect MASP-3, but it may be present.
Figure 1.
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) pattern of L-FCN-MASPs. Purified L-FCN-MASPs were separated under reducing (lane 1) and non-reducing (lane 2) conditions. Markers: lane 3. Gels were stained with Coomassie brilliant blue.
Figure 2.
Western blots of the L-FCN preparation. Western blots were probed with anti-L-FCN (lanes 1 and 2), anti-MASP-1 (lanes 3 and 4) and anti-MASP-2 (lanes 5 and 6) under reducing and non-reducing conditions, respectively. As can be observed, L-FCN ran as a single polypeptide band of 35 kDa under reducing conditions while under non-reducing conditions it formed a ladder of bands. This running pattern is characteristic of a homo-oligomeric protein such as FCN. When a similar blot was probed with anti-MASP-1 (B-chain specific) antibodies only the MASP-1 B chain was detectable under reducing conditions, while the A and B chain complex could be detected under non-reducing conditions. On the blot probed with anti-MASP-2 (A-chain specific) antibodies both the MASP-2 A chain and MAp19 could be detected under reducing conditions while only the A and B chain complex could be detected in the non-reducing conditions.
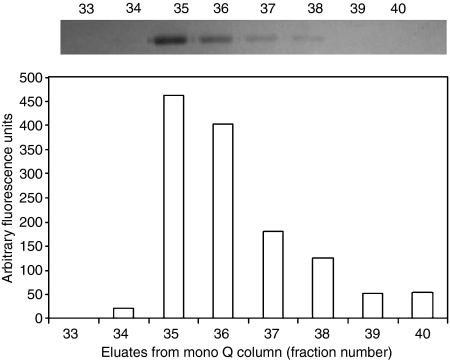
Figure 3 depicts the cleavage of a synthetic peptide substrate, FGR-AMC, by L-FCN-associated MASP-1/MASP-2 in mono Q eluates bound to N-acetylated BSA-coated plates. As the concentration of L-FCN in the eluted fraction increases, the cleavage of substrate FGR-AMC also increases. This clearly indicates that eluted fractions contain L-FCN-MASP complexes. Similar analyses were performed for MBL-MASP complexes during purification (data not shown) and the results confirmed isolation of active MBL-MASP complex.
Figure 3.
Cleavage of a synthetic peptide FGR-AMC by L-FCN-MASPs in mono Q eluates. The top panel shows the amount of 35-kDa L-FCN present in fractions eluted from a mono Q column, as indicated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE). The chart shows the corresponding proteolytic activity of the L-FCN-associated MASP, following capture of the L-ficolin on an N-acetyl bovine serum albumin (BSA) surface. MASP activity in the individual fractions was quantified by measuring the fluorescence increase after cleavage of the peptide derivative FGR-AMC (Presanis et al., 2004). The results indicate that the L-FCN detected by SDS-PAGE was indeed complexed with active MASPs.
Fibrinogen cleavage by purified L-FCN-MASP or MBL-MASP complexes
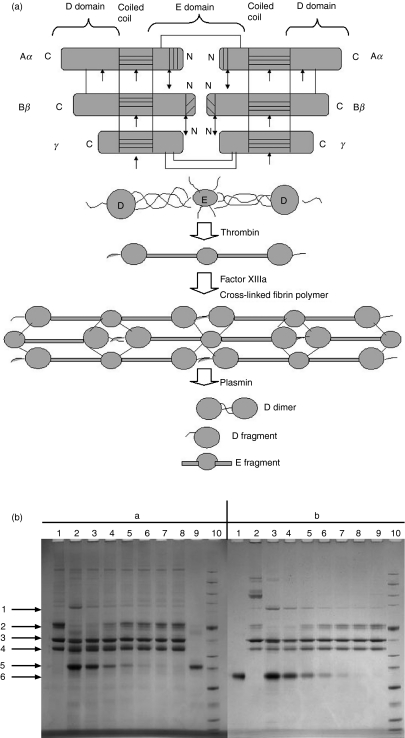
Fibrinogen has a molecular weight of 340 kDa and is composed of three pairs of disulphide-bound polypeptide chains named Aα, Bβ and γ (Fig. 4a). The polypeptides are oriented so that all six N-terminal ends meet to form the central E domain. Two regions of coiled coil alpha helices stretch out on either side of the E domain, each consisting of one Aα, one Bβ and one γ polypeptide. Each coiled coil region ends in a globular D domain consisting of the C-terminal ends of Bβ and γ as well as part of Aα. Both the E and D domains contain important binding sites for the conversion of fibrinogen to fibrin, for fibrin assembly and cross-linking and for platelet aggregation. Proteolysis by thrombin results in release of FPA (Aα 1–16) followed by FPB (Bβ 1–14) and the fibrin monomers that result polymerize in a half-overlap fashion to form insoluble fibrin fibrils. The chains of fibrin are referred to as α, β and γ, because of the removal of FPA and FPB. The polymerized fibrin is subsequently stabilized by the transglutaminase, activated factor XIII, which forms amide linkages between the γ and α chains of the fibrin molecules. As with fibrinogen, the activation of factor XIII is thrombin-mediated and factor XIII is a heterotetrameric protein complex composed of two A chains and two B chains. Thrombin cleaves the A chain, enabling the A and B chains to dissociate. This leaves the functional transglutaminase (the cleaved A chain) free to cross-link fibrin molecules and other suitable substrates by forming bonds between lysine and glutamine side chains on polypeptides.20
Figure 4.
(a) Fibrin polymerization and lysis. The top panel shows polypeptide organization of fibrinogen. The area with horizontal lines is the coiled coil region. The area with vertical lines is fibrinopeptide A. The area with diagonal lines is fibrinopeptide B. Double arrows indicate the thrombin cleavage site. Single arrows indicate plasmin cleavage sites. The middle panel shows the domain organization of fibrinogen. The bottom panel shows cross-linking of the fibrin monomer. The E domain binds to the holes on up to four D domains, forming a long fibrous latticework. The clot is then stabilized through cross-linking. The clot can be degraded, yielding different degradation products if it has been cross-linked. The D fragment is released when the clot is not cross-linked by factor XIIIIa. (b) Fibrinogen cleavage by purified lectin-MASPs. Fibrinogen was incubated with various concentrations of purified lectin-MASPs and subjected to reducing sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE). Arrows indicate (1) the γ-dimer, (2) the α-chain, (3) the β-chain, (4) the γ-chain, (5) L-FCN and (6) mannan-binding lectin (MBL). Panels (a) and (b) show the results obtained with L-FCN-MASPs and MBL-MASPs, respectively. (a) Lane 1, fibrinogen (5 μg) incubated without L-FCN-MASPs at 37° for 14 hr; lanes 2–8, with 3·5, 1·5, 0·75, 0·4, 0·2, 0·1,0 05 and 0·025 μg of L-FCN-MASPs; lane 9, with 1·25 μg of L-FCN-MASPs; lane 10, marker. (b) Lane 1, MBL-MASPs (1 ug); lane 2, fibrinogen (1 ug); lanes 3–9, fibrinogen (1 ug) incubated with 3·0, 1·5, 0·75, 0·375, 0·18, 0·09 and 0·046 ug of MBL-MASPs; lane 10, marker. The results show that both L-FCN and MBL exhibit a dose-dependent cross-linking of the fibrinogen γ-chains, resulting in the generation of the γ-dimer as well as degradation of the α-chain. Additionally, a small change in the size of the β-chain could be observed, indicating the release of fibrinopeptide B.
Proteolysis of fibrinogen by plasmin initially liberates C-terminal residues from the Aα chain to produce fragment X (intact D-E-D, which is still clottable). Fragment X is further degraded to non-clottable fragments Y (D-E) and D. Fragment Y can be digested into its constituent D and E fragments. Digestion of non-cross-linked fibrin with plasmin is very similar to the digestion of fibrinogen, which results in the production of fragments D and E. Degradation of cross-linked fibrin by plasmin results in fragment DD (the D-dimer consisting of the D domains of two fibrin molecules cross-linked via the gamma chains), fragment E (the central E domain) and DDE, in which fragment E is non-covalently associated with DD (Fig. 4a).21
Fibrinogen was incubated with various concentrations of purified L-FCN-MASPs or MBL-MASPs and subjected to SDS-PAGE under reducing conditions (Fig. 4b). In both cases, cross-linking was evident from the formation of the gamma dimer when compared with fibrinogen incubated without L-FCN-MASPs or MBL-MASPs. As the concentration of L-FCN-MASPs or MBL-MASPs increased, the α-chain completely disappeared with the appearance of the gamma dimer, which is in accordance with the fibrinogen cleavage pattern obtained using pure recombinant MASP-1.12 Additionally, a small shift in the β-chain was observed at the high L-FCN/MBL-MASP concentrations, indicating that the complexes also mediate the release of FPB.
Factor XIII activation by purified L-FCN-MASPs or MBL-MASPs
Figure 5 shows factor XIII activation by purified MASP complexes incubated for different time intervals. Generation of factor XIIIa (doublet A) can be seen in lanes 6–8.
Figure 5.
Factor XIII cleavage by purified lectin mannan-binding lectin-associated serine proteases (MASPs). Factor XIII was incubated with L-FCN-MASPs and mannan-binding lectin (MBL) MASPs for different times at 37° and subjected to reducing sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE). Panels (a) and (b) show the results obtained with L-FCN-MASPs and MBL-MASPs, respectively. Arrows indicate (1) the B chain, (2) the A chain, (3) the cleaved A chain. (a) Lane 1, L-FCN-MASPs; lanes 2–8, factor XIII + L-FCN-MASPs incubated for 0 min, 30 min, 1 hr, 2 hr, 4 hr, 6 hr and 8 hr, respectively; lane 9, factor XIII alone incubated for 8 hr. (b) Factor XIII (1 μg) incubated with 2·5 μg of purified MBL-MASPs for different time intervals at 37° and subjected to SDS-PAGE under reducing conditions. Lane 1, MBL-MASPs; lanes 2–8, factor XIII + MBL-MASPs incubated for 0 min, 30 min, 1 hr, 2 hr, 4 hr, 6 hr and 8 hr; lane 9, factor XIII incubated for 8 hr. Arrows indicate (1) the B chain, (2) the A chain and (3) the cleaved A chain.
Fibrinopeptide release, and the effects of inhibitors
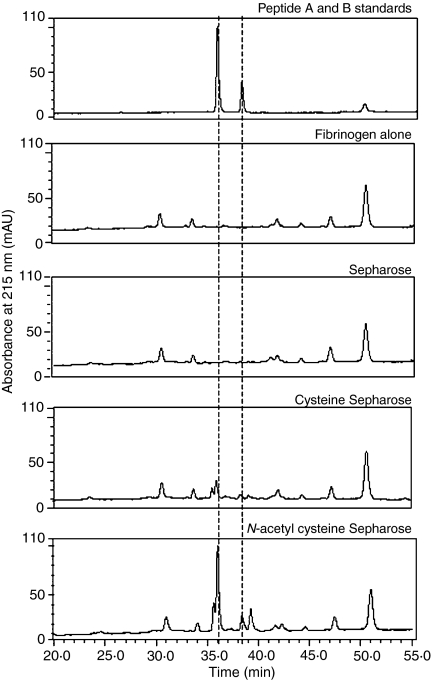
FPA and FPB release from fibrinogen is essential for the initiation of clot generation. Figure 6 shows fibrinopeptide release from fibrinogen by the proteins captured from serum when incubated with N-acetylcysteine-Sepharose, cysteine-Sepharose or underivatized Sepharose. It can be observed that peptides with identical retention times to FPA and FPB could only be detected when fibrinogen was incubated with serum proteins captured on N-acetylcysteine-Sepharose (panel 5, Fig 6). To further characterize these peptides, the peaks were subjected to MALDI-ToF spectrometry. Here the mass of peptide A was found to be 1536·267 (expected value 1536·56) and that of peptide B was 1552·302 (expected value 1552·58), confirming their identity. When incubated with cysteine-Sepharose (panel 4, Fig 6), underivatized Sepharose (panel 3, Fig. 6) or N-acetyl Sepharose that had not been preincubated with serum, no FPB could be detected and only a diminished peak at the elution point of FPA was observed when incubated with serum captured with cysteine Sepharose (panel 4, Fig. 6).
Figure 6.
Fibrinopeptide release by ficolin MASPs trapped on various matrices. Fibrinopeptides were released following cleavage of fibrinogen by L-FCN-MASPs as described in the Materials and methods.
Table 1 shows the effect of various inhibitors on fibrinopeptide release by L-FCN-MASPs bound to N-acetylcysteine-Sepharose. Inhibition (80%) by hirudin indicates the participation of thrombin in the observed cleavage of fibrinogen by FCN-MASPs. Hirudin does not inhibit MASP-1 or MASP-2 but inhibits thrombin.9,22 Anti-thrombin III + heparin inhibits MASP-1 and MASP-2 and also thrombin,23 so the 90% inhibition by anti-thrombin III + heparin does not distinguish between participation of MASPs and that of thrombin. However, inhibition by the C1 inhibitor indicates that MASPs are involved in the cleavage, because the C1 inhibitor inhibits both MASP-1 and MASP-2.9,24 A possible interaction between the C1 inhibitor and thrombin has previously been demonstrated, but this does not result in thrombin inhibition.25 Calcium ions are essential for binding of MASPs to L-FCN.26 Consistent with this requirement, incubation with EDTA (before final addition of fibrinogen) caused complete inhibition. Soybean trypsin inhibitor exhibited no inhibition of fibrinopeptide release. Lanchantin et al.27 reported that this inhibitor forms a one-to-one complex with bovine or human thrombin, thereby blocking its specific capacity to activate prothrombin. Nanninga and Guest28 reported plasmin to be inhibited by the soybean trypsin inhibitor. The C4 activation by the MBL-MASP complex has been reported to be inhibited by the soybean trypsin inhibitor.29 The fibrinopeptide released in the presence of the soybean trypsin inhibitor thus must be attributable to the activity of MASP-1 in the MBL-MASP complex.
Pefabloc [4-(2-aminoethyl)-benzenesulphonyl fluoride (AEBSF)] and α2-macroglobulin are broad inhibitors of various proteases but have been shown to inhibit both MASPs and thrombin, and both also showed an ability to inhibit the release of the fibrinopeptide.9,30
Inhibition by hirudin of fibrinopeptide release from L-FCN-MASPs captured from serum on N-acetylcysteine-Sepharose beads indicates the involvement of thrombin in the process. The thrombin must be formed by prothrombin activation by MASP-2, as inhibition by the C1 inhibitor indicates that preformed thrombin is not involved. Prothrombin could be present as a contaminant in L-FCN-MASP complex preparation bound to N-acetylcysteine-Sepharose. To investigate this possibility, we monitored the release of fibrinopeptides when fibrinogen was incubated with purified L-FCN-MASP complexes in the absence or presence of exogenously added prothrombin.
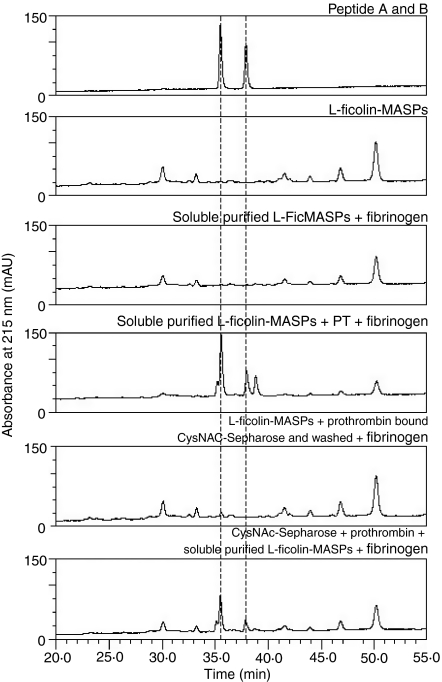
Although chromatographically purified, soluble L-FCN-MASPs were able to cleave fibrinogen, as determined by SDS-PAGE (Fig. 4), the amount of fibrinopeptides released was undetectable by the HPLC method of estimation (panel 3, Fig. 7). Fibrinopeptide release was observed when prothrombin was added to soluble purified L-FCN-MASPs incubated with fibrinogen (panel 4, Fig. 7). Fibrinopeptide release was not observed when purified L-FCN-MASP was incubated with N-acetylcysteine-Sepharose in the presence of prothrombin, washed and then incubated with fibrinogen (panel 5, Fig. 7).
Figure 7.
Effect of prothrombin addition to purified L-FCN-MASPs in fibrinopeptide release. Fibrinopeptides were released following cleavage of fibrinogen by L-FCN-MASP in the presence and absence of prothrombin, as described in the Materials and methods.
In comparison with the soluble state where fibrinogen, prothrombin and L-FCN-MASPs were incubated in solution, less fibrinopeptide release was observed when L-FCN-MASPs, prothrombin and fibrinogen were incubated with N-acetylcysteine-Sepharose (panel 6, Fig. 7). A possible reason may be better interaction between MASPs and high-molecular-weight fibrinogen in solution than in the solid phase. Panel 5 in Fig. 7 also confirms that neither prothrombin nor activated thrombin binds non-specifically to N-acetylcysteine-Sepharose, as washing after incubation of purified L-FCN-MASPs and prothrombin with N-acetylcysteine-Sepharose did not result in fibrinopeptide release. To eliminate the possibility of non-specific binding of prothrombin or thrombin from serum, fibrinogen was incubated with or without purified L-FCN-MASP complexes and prothrombin and fibrinopeptide release was only observed in the presence of N-acetyl-Sepharose-bound L-ficolin-MASP complexes and prothrombin (not shown). This showed that the main pathway for fibrinogen activation is via MASP2 and prothrombin.
Generation of a clot from purified fibrinogen by MBL-MASPs or L-FCN-MASPs
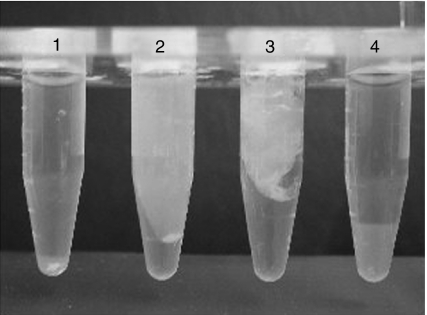
MBL-MASPs or L-FCN-MASPs were captured directly from serum on N-acetylcysteine-Sepharose or mannan-agarose and then incubated with purified fibrinogen. Figure 8 shows the generation of a clot from fibrinogen, in the presence or absence of added factor XIII, by the MASP complexes bound to affinity matrices. Factor XIII utilizes a cysteine residue to catalyse the cross-linking of α and γ chains in fibrin. When factor XIII was not added, iodoacetamide was added to inactivate any contaminating factor XIII from serum. In the absence of added factor XIII, the amount of clot formed was decreased. The soybean trypsin inhibitor did not appear to inhibit clot formation.
Figure 8.

Generation of a clot from fibrinogen in the presence or absence of factor XIII by MASPs captured on CysNAc-Sepharose or mannan-binding lectin (MBL) MASPs captured on mannan-agarose. 1, CysNAc-Sepharose + fibrinogen + iodoacetamide; 2, CysNAc-Sepharose + fibrinogen + factor XIII; 3, CysNAc-Sepharose + soybean trypsin inhibitor (SBTI) + fibrinogen + factor XIII; 4, mannan-agarose + fibrinogen + iodoacetamide; 5, mannan-agarose + fibrinogen + factor XIII; 6, mannan-agarose + SBTI + fibrinogen + factor XIII.
Generation of a clot by MBL-MASPs or L-FCN-MASPs from calcified plasma
MBL-MASPs or L-FCN-MASPs were captured from serum on mannan-agarose or N-acetylcysteine-Sepharose, respectively, and then incubated with calcified diluted heparinized plasma at pH 8·5. Figure 9 shows that MBL-MASPs or L-FCN-MASPs, captured from serum on mannan-agarose and N-acetylcysteine-Sepharose, were capable of catalysing clot formation. In contrast, no clots were detected when underivatized Sepharose was used, or mannan-agarose beads were incubated with normal human serum in the presence of EDTA. This result confirms that MBL-MASP or L-FCN-MASP complexes are capable of mediating clot formation in heparinized plasma.
Figure 9.
Clot generation by mannan-binding lectin (MBL)/MASPs bound to their respective matrices after incubation with diluted plasma. Lane 1, underivatized Sepharose; lane 2, CysNAc-Sepharose; lane 3, mannan-agarose; lane 4, mannan-agarose incubated with diluted plasma in the presence of 20 mm ethylenediaminetetraacetic acid (EDTA).
The composition of the clot generated by the present method was analysed further. For generation of these clots, blood was collected in 1 unit of heparin per ml of blood. ATIII in the presence of heparin at concentrations higher than this inhibits both MASP-1 and MASP-2, as well as thrombin.9 Blood was not collected in sodium citrate for this experiment because recalcification leads to the formation of a fibrin clot and depletes all the native fibrinogen in the plasma. In the absence of fibrinogen, a thick clot did not form when the lectin pathway was activated. However, clot formation in citrated serum was also achieved after it was spiked with pure fibrinogen.
To prevent inhibition of MASPs by serum C1 inhibitor, the pH was raised to 8·5, as serine protease inhibitor (serpin)-protease complexes are unstable at higher pH because of hydrolysis of the serpin-protease linkage.31 We observed previously that recombinant MASP-1 (rMASP-1) was not inhibited by a twofold molar excess of the C1 inhibitor at pH 8·5, while complete inhibition of rMASP-1 by the C1 inhibitor was seen at pH 7·4.9 When serum was used at pH 7·4, there was a >80% reduction in the amount of clot formed. However, a normal volume of clot was formed at pH 7·4 when purified fibrinogen was used (not shown).
Characterization of the fibrinogen clots
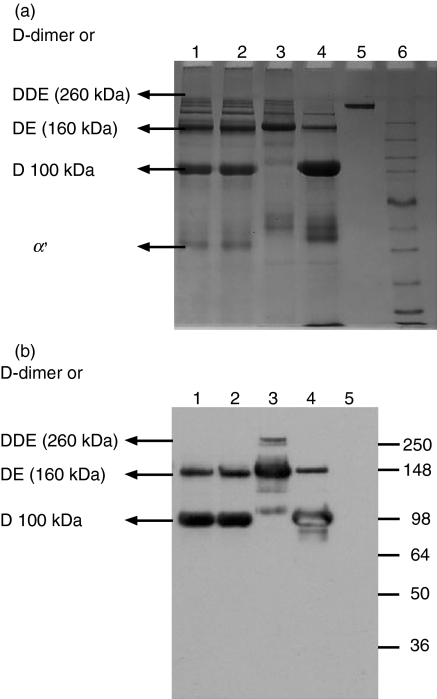
To analyse the composition of the clot, clots were generated by the following methods: (i) L-FCN-MASPs captured from serum and incubated with diluted plasma at pH 8·5; (ii) MBL-MASPs captured from serum and incubated with diluted plasma at pH 8·5; (iii) polymerization of purified fibrinogen by incubation in the presence of thrombin and factor XIII at pH 7·5; and (iv) as (iii), but without the addition of factor XIII and in the presence of 1 mm iodoacetamide at pH 7·5. Figure 10a shows the SDS-PAGE pattern under non-reducing conditions of plasmin digests of clots generated by the above-mentioned methods. From Fig. 10 it is evident that DDE of molecular weight 260 kDa and DE of molecular weight 160 kDa appear in the plasmin digest of the clot generated by incubating purified fibrinogen, thrombin and factor XIII (lane 3). A similar pattern was obtained for the clots generated by L-FCN-MASPs and MBL-MASPs when incubated with calcified diluted plasma (lanes 1 and 2). Additionally, a D-monomer of molecular weight 100 kDa can be seen in the plasmin digest of the clot generated by MBL-MASPs or L-FCN-MASPs as well as for the plasmin digest of the clot generated by incubating purified fibrinogen, thrombin and iodoacetamide (lane 4). In general, a decreased amount of highly cross-linked clots appeared with L-FCN-MASPs and MBL-MASPs compared with thrombin-mediated clots. The above results strongly suggest that the composition of the clot generated by lectin MBL-MASPs or FCN-MASPs is similar to that of a conventional fibrin clot, i.e. thrombin-mediated, although there is less cross-linking. This was confirmed by subjecting plasmin digests separated by SDS-PAGE to western blotting using monoclonal antibody specific for the D-dimer (Fig. 10b). Under non-reducing conditions, the D and DE fragments were visible and these were generated as a result of plasmin digestion of the clot generated by L-FCN- or MBL-MASPs (lanes 1 and 2). In the plasmin digest of clots generated by L-FCN-MASPs or MBL-MASPs, the intensities of D and DE bands matched both the plasmin digests of the clot generated by thrombin in the absence of factor XIII (in the presence of iodoacetamide). The poor detection or non-detection of high-molecular-weight DDE and D-dimer bands by this approach in western blots (Fig. 10b, lanes 1 and 2) may be attributable to both the inefficiency of transfer of very high-molecular-weight proteins by the semi-dry blot method and the lower concentration of high-molecular-weight fragments in lanes 1 and 2. It is also possible that the antibody may have been incapable of detecting highly cross-linked fibrin forms. The pattern obtained matches very well with the clot generated using purified thrombin/fibrinogen, thus confirming that the clot generated by L-FCN-MASPs or MBL-MASPs is made up of fibrin.
Figure 10.
(a) Comparison of plasmin digests of generated clots. Lane 1, plasmin digest of a clot generated from plasma by CysNAc-Sepharose; lane 2, plasmin digest of a clot generated from plasma by mannan-Sepharose; lane 3, plasmin digest of a clot generated by thrombin and fibrinogen in the presence of factor XIII; 4, plasmin digest of a clot generated by thrombin and fibrinogen in the absence of factor XIII; 5, fibrinogen; 6, marker. (b) Western blots of plasmin digests of the generated clots. Lane 1, plasmin digest of a clot generated from plasma by CysNAc-Sepharose; lane 2, plasmin digest of a clot generated from plasma by mannan-Sepharose; lane 3, plasmin digest of a clot generated by thrombin and fibrinogen in the presence of factor XIII; lane 4, plasmin digest of a clot generated by thrombin and fibrinogen in the absence of factor XIII; lane 5, fibrinogen; lane 6, marker.
Discussion
Our earlier studies have shown that native and recombinant MASP-1 show thrombin-like activity, as indicated by its enzymatic activity towards fibrinogen and factor XIII.11,12 Additionally, purified L-FCN-MASP and MBL-MASP complexes containing MASP-1 (as determined by western blot) were also capable of cleaving fibrinogen in a fashion similar to recombinant MASP-1.12 Furthermore, these complexes are also capable of cleaving FGR-AMC, which is a synthetic substrate for MASP-1 and MASP-2.9 MASP-2 does not cleave fibrinogen or factor XIII directly but is capable of mediating fibrinogen and factor XIII turnover through the activation of prothrombin.7 The MBL-MASP and FCN-MASP complexes were also capable of generating insoluble clots (Fig. 9).
Clot generation by MASP complexes was observed and confirmed by two different methods: (i) L-FCN-MASPs captured on N-acetylcysteine-Sepharose from normal human serum incubated with fibrinogen in the presence or absence of added factor XIII (Fig. 8); and (ii) L-FCN-MASPs captured on N-acetylcysteine-Sepharose from normal human serum incubated with human plasma diluted 1 : 1 with 0·1m NaHCO3, pH 8·5 (Fig. 9). The composition of the MASP-generated clots was compared with that of thrombin-mediated clots and we found that they were similar but the latter were more extensively cross-linked (Fig. 10a,b).
FPA and FPB release was confirmed by HPLC and mass spectrometry. The inhibitory profile (Table 1) confirmed the involvement of MASPs as well as thrombin in the generation of FPA and FPB. The inhibition of fibrinopeptide release by hirudin (close to 90%) indicates that the L-FCN-MASPs captured from serum were able to generate thrombin from prothrombin. This indicates that the MASP-2 + prothrombin pathway is quantitatively much more important in forming fibrinopeptides than MASP-1-mediated fibrinogen/factor XIII activation.7 Prothrombin might be associated with the L-FCN-MASP complex in a non-specific manner. When chromotographically purified L-FCN-MASP complexes were captured on N-acetylcysteine beads, prothrombin was added and the beads were then washed, prothrombin does not appear to remain attached to the complexes. This is inferred because fibrinopeptides could not be detected. We speculate that another plasma/serum protein might be required to make prothrombin associate with the L-FCN-MASP complex bound to the beads.
The finding that fibrinogen cleavage and subsequent clot formation can be accomplished by activated FCN-MASP and MBL-MASP complexes strongly supports the idea that activation of the MASPs leads to the formation of a fibrin clot. Activation of complement proteins by coagulation proteases has been previously reported (but generally not in much detail) but not the converse.32,33 Localized coagulation may help by restricting the spread of infection.34–37
In insects, haemolymph coagulation is important for sealing wounds, trapping microbes and blocking their entry.38 The Drosophila soft clot is composed of several proteins including haemolectin. Haemolectin is a multidomain C-type lectin which, among others, contains complement control protein domains.39 Confinement of an infection to a local site in the body might be an important function of neutrophil extracellular traps (NETs).40,41 Trapping mechanisms found in mammals also include the salivary scavenger receptor gp340 and lung surfactant proteins SP-A and SP-D. The oligomeric gp340 receptor aggregates streptococci and other bacteria at the mucosal surface and might generate an extracellular network that traps both pathogen and innate defence molecules in close proximity.42 Surfactant proteins A and D bind to pneumonia-causing bacteria, causing their aggregation.43,44 SP-D is involved in bacterial clearance during the early stages of infection.45
There is also evidence supporting the idea that coagulation may function protectively during infection where activation of the coagulation system at the bacterial interface contributes to the pathophysiology of bacterial infectious diseases.46
Fibrin performs a critical protective function during infection by the obligate intracellular protozoan parasite Toxoplasma gondii. 47 Here fibrin suppresses an otherwise lethal haemorrhagic pathology that accompanies the robust immune response prompted by toxoplasmosi.47 Mullarky et al.48 applied a new tool to study the role of coagulation during bacterial infection. They used fibrinogen (k/o)-deficient mice and demonstrated that mice lacking fibrin(ogen) succumb to doses of Listeria monocytogenes that genetically matched control mice survive, thereby establishing that fibrin(ogen) performs important host protective functions during listeriosis. They also found that mice treated with warfarin, a well-characterized pharmaceutical anticoagulant, also succumb to doses of L. monocytogenes that control mice survive. As warfarin suppresses fibrin formation without affecting circulating levels of fibrinogen, these data suggest that fibrin, as opposed to fibrinogen, is the important determinant of survival in this model. They also found that fibrin(ogen) plays an important role in restraining the growth of L. monocytogenes within infected hepatic tissue. Fibrinogen was also shown to aid clearance of Staphylococcus aureus from the peritoneal cavity. Mullarky et al.48 ascribe the fibrin(ogen)-mediated control of bacterial growth within hepatic tissue to the physical attributes of fibrin, which is well known to trap circulating blood cells during the formation of a blood clot.
Activated monocytes possess an alternative fibrinolytic pathway that uses the cellular adherence receptor MAC1 (CD11b/CD18), which directly binds and internalizes fibrinogen resulting in lysosomal degradation.49 Furthermore, as a protein that is deposited in the form of a provisional fibrin matrix at virtually any site of overt tissue damage, fibrin could serve as a significant non-diffusible cue for regulating leucocyte targeting. Consistent with this general concept, multiple in vitro studies have shown leucocyte involvement, leading to changes in cell migration, phagocytosis, nuclear factor (NF)-κB-mediated transcription, production of chemokines and cytokines, degranulation and other processes.35,50–52 These findings have driven efforts to better define the molecular details of the interaction between fibrinogen and integrin αmβ2. One notable facet of the binding interaction is that both fibrin and immobilized fibrinogen are bound with high avidity by αmβ2, whereas soluble fibrinogen is a relatively poor ligand.53,54 This conformation-dependent binding implies that αmβ2 would generally not be occupied when circulating leucocytes passively encounter plasma fibrinogen. The integrin, however, would mediate avid cellular engagements of immobilized fibrin(ogen) at a site of tissue damage, locations where copious fibrin deposition would be universally observed.
The complement system has a more ancient origin in evolution than acquired immunity. The central component of the complement system, the C3 protein, on which the three activation pathways converge has been identified in jawless vertebrates, the lampreys and the hagfish as well as in deuterostome invertebrates, ascidians, amphioxus, and sea urchins (echinoderms). Sea squirts (ascidians) occupy a pivotal intermediary position between invertebrates and vertebrates. Two lectins corresponding to mammalian MBLs and FCNs, two MASPs, C3, C2 and a C3 receptor have been identified in ascidians.55 Therefore, the primitive complement system seems to have been established in the deuterosome lineage; the classical pathway of activation was then acquired in the jawed vertebrate lineage before acquired immunity arose.55,56 From an evolutionary point of view, the primitive lectin pathway in innate immunity appears to have developed into the more sophisticated, multifunctional complement system of the classical pathway through gene duplication, to serve as an effector of acquired immunity.
We propose that, when L-FCN-MASPs bind to pathogens, MASP-1 becomes activated and cleaves fibrinogen, thereby generating chemotactic fibrinopeptides that are capable of recruiting neutrophils to the site of infection.57 In parallel with this process, MASP-2 is activated and produces active thrombin from prothrombin.7 This latter process overwhelms the former. It is also proposed that the fibrin clot-forming ability of MASPs may be a remnant of an efficient ancient mechanism to confine infectious particles to a small area. During evolution, the clot-forming function has been substantially but not completely taken over by the more specific protease thrombin and MASPs have become an effector system for acquired immunity.
Acknowledgments
KH is supported by Marie Curie International Incoming Fellowship PROLECT 040215, and KCG was the recipient of a Commonwealth split-site doctoral fellowship. KG is supported by UGC INDIA. AK and RBS were supported by the MRC (UK).
Disclosures
The authors have no conflict of interest.
References
- 1.Fujita T. Evolution of the lectin-complement pathway and its role in innate immunity. Nat Rev Immunol. 2002;2:346–53. doi: 10.1038/nri800. [DOI] [PubMed] [Google Scholar]
- 2.Gal P, Dobo J, Zavodszky P, Sim RB. Early complement proteases: C1r, C1s and MASPs. A structural insight into activation and functions. Mol Immunol. 46:2745–52. doi: 10.1016/j.molimm.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 3.Mayilyan KR, Presanis JS, Arnold JN, Hajela K, Sim RB. Heterogeneity of MBL- MASP complexes. Mol Immunol. 2006;43:1286–92. doi: 10.1016/j.molimm.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Mayilyan KR, Presanis JS, Arnold JN, Sim RB. Discrete MBL-MASP complexes show wide inter-individual variability in concentration: data from UK vs Armenian populations. Int J Immunopathol Pharmacol. 2006;19:567–80. doi: 10.1177/039463200601900313. [DOI] [PubMed] [Google Scholar]
- 5.Bhakdi S, Tranum-Jensen J. Complement lysis: a hole is a hole. Immunol Today. 1991;12:318–21. doi: 10.1016/0167-5699(91)90007-G. [DOI] [PubMed] [Google Scholar]
- 6.Sim RB, Tsiftsoglou SA. Proteases of the complement system. Biochem Soc Trans. 2004;32:21–7. doi: 10.1042/bst0320021. [DOI] [PubMed] [Google Scholar]
- 7.Krarup A, Wallis R, Presanis JS, Gal P, Sim RB. Simultaneous activation of complement and coagulation by MBL-associated serine protease 2. PLoS One. 2007;2:e623. doi: 10.1371/journal.pone.0000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CB, Wallis R. Two mechanisms for mannose-binding protein modulation of the activity of its associated serine proteases. J Biol Chem. 2004;279:26058–65. doi: 10.1074/jbc.M401318200. [DOI] [PubMed] [Google Scholar]
- 9.Presanis JS, Hajela K, Ambrus G, Gal P, Sim RB. Differential substrate and inhibitor profiles for human MASP-1 and MASP-2. Mol Immunol. 2004;40:921–9. doi: 10.1016/j.molimm.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Morita T, Kato H, Iwanaga S, Takada K, Kimura T. New fluorogenic substrates for alpha-thrombin, factor Xa, kallikreins, and urokinase. J Biochem (Tokyo) 1977;82:1495–8. doi: 10.1093/oxfordjournals.jbchem.a131840. [DOI] [PubMed] [Google Scholar]
- 11.Hajela K, Kojima M, Ambrus G, et al. The biological functions of MBL-associated serine proteases (MASPs) Immunobiology. 2002;205:467–75. doi: 10.1078/0171-2985-00147. [DOI] [PubMed] [Google Scholar]
- 12.Krarup A, Gulla KC, Gal P, Hajela K, Sim RB. The action of MBL-associated serine protease 1 (MASP1) on factor XIII and fibrinogen. Biochim Biophys Acta. 2008;1784:1294–300. doi: 10.1016/j.bbapap.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 13.Sim RB, Reboul A. Preparation and properties of human C1 inhibitor. Methods Enzymol. 1981;80:43–54. doi: 10.1016/s0076-6879(81)80007-9. [DOI] [PubMed] [Google Scholar]
- 14.Krarup A, Thiel S, Hansen A, Fujita T, Jensenius JC. L-ficolin is a pattern recognition molecule specific for acetyl groups. J Biol Chem. 2004;279:47513–9. doi: 10.1074/jbc.M407161200. [DOI] [PubMed] [Google Scholar]
- 15.Arnold JN, Radcliffe CM, Wormald MR, et al. The glycosylation of human serum IgD and IgE and the accessibility of identified oligomannose structures for interaction with mannan-binding lectin. J Immunol. 2004;173:6831–40. doi: 10.4049/jimmunol.173.11.6831. [DOI] [PubMed] [Google Scholar]
- 16.Frederiksen PD, Thiel S, Larsen CB, Jensenius JC. M-ficolin, an innate immune defence molecule, binds patterns of acetyl groups and activates complement. Scand J Immunol. 2005;62:462–73. doi: 10.1111/j.1365-3083.2005.01685.x. [DOI] [PubMed] [Google Scholar]
- 17.Krarup A. The Lectin Pathway of Complement Activation. Oxford: University of Oxford; 2007. DPhil Thesis. [Google Scholar]
- 18.Tan SM, Chung MC, Kon OL, Thiel S, Lee SH, Lu J. Improvements on the purification of mannan-binindg lectin and demonstration of its Ca2+-independent assocation with a C1s-like serine proteases. Biochem J. 1996;319:329–32. doi: 10.1042/bj3190329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terasawa F, Kani S, Hongo M, Okumura N. In vitro fibrin clot formation and fibrinolysis using heterozygous plasma fibrinogen from gammaAsn319, Asp320 deletion dysfibrinogen, Otsu I. Thromb Res. 2006;118:651–61. doi: 10.1016/j.thromres.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Esposito C, Caputo I. Mammalian transglutaminases. Identification of substrates as a key to physiological function and physiopathological relevance. FEBS J. 2005;272:615–63. doi: 10.1111/j.1742-4658.2004.04476.x. [DOI] [PubMed] [Google Scholar]
- 21.Gaffney PJ. A review of structures found in vitro and in vivo. Ann N Y Acad Sci. 2001;936:594–10. [PubMed] [Google Scholar]
- 22.Stone SR, Maraganore JM. Hirudin and hirudin based peptides. Methods Enzymol. 1993;80:43–54. doi: 10.1016/0076-6879(93)23053-p. [DOI] [PubMed] [Google Scholar]
- 23.Ersdal-Badju E, Lu A, Zuo Y, Picard V, Bock SC. Identification of the antithrombin III heparin binding site. J.Biol Chem. 1997;272:19393–400. doi: 10.1074/jbc.272.31.19393. [DOI] [PubMed] [Google Scholar]
- 24.Davis AE, III, Mejia P, Lu F. Biological activities of C1 inhibitor. Mol Immunol. 2008;45:4057–63. doi: 10.1016/j.molimm.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cugno M, Bos I, Lubbers Y, Hack CE, Agostoni A. In vitro interaction of C1-inhibitor with thrombin. Blood Coagul Fibrinolysis. 2001;12:253–60. doi: 10.1097/00001721-200106000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Thiel S. Complement activating soluble pattern recognition molecules with collagen-like regions, mannan-binding lectin, ficolins and associated proteins. Mol Immunol. 2007;44:3875–88. doi: 10.1016/j.molimm.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Lanchantin GF, Friedmann JA, Hart DW. Interaction of soybean trypsin inhibitor with thrombin and its effect on prothrombin activation. J Biol Chem. 1969;244:865–75. [PubMed] [Google Scholar]
- 28.Nanninga L, Guest M. On the interaction of fibrinolysin (plasmin) with inhibitors antifibrinolysin and soybean trypsin inhibitor. Arch Biochem Biophys. 1964;108:542–51. doi: 10.1016/0003-9861(64)90440-0. [DOI] [PubMed] [Google Scholar]
- 29.Petersen SV, Thiel S, Jensen L, Vorup-Jensen T, Koch C, Jensenius JC. Control of the classical and the MBL pathway of complement activation. Mol Immunol. 2000;37:803–11. doi: 10.1016/s0161-5890(01)00004-9. [DOI] [PubMed] [Google Scholar]
- 30.Ambrus G, Gal P, Kojima M, et al. Natural substrates and inhibitors of mannan-binding lectin-associated serine protease-1 and -2: a study on recombinant catalytic fragments. J Immunol. 2003;170:1374–82. doi: 10.4049/jimmunol.170.3.1374. [DOI] [PubMed] [Google Scholar]
- 31.Calugaru SV, Swanson R, Olson ST. The pH dependence of serpin proteinase complex dissociation reveals a mechanism of complex stabilization involving inactive and active conformational states of the proteinase which are perturbable by calcium. J Biol Chem. 2001;276:32446–55. doi: 10.1074/jbc.M104731200. [DOI] [PubMed] [Google Scholar]
- 32.Huber-Lang M, Sarma JV, Zetoune FS, et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12:682–7. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- 33.Markiewski MM, Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD. Complement and coagulation: strangers or partners in crime? Trends Immunol. 2007;28:184–92. doi: 10.1016/j.it.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Dunn DL, Simmons RL. Fibrin in peritonitis. III. The mechanism of bacterial trapping by polymerizing fibrin. Surgery. 1982;92:513–9. [PubMed] [Google Scholar]
- 35.Echtenacher B, Weigl K, Lehn N, Mannel DN. Tumor necrosis factor-dependent adhesions as a major protective mechanism early in septic peritonitis in mice. Infect Immun. 2001;69:3550–5. doi: 10.1128/IAI.69.6.3550-3555.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rotstein OD. Role of fibrin deposition in the pathogenesis of intraabdominal infection. Eur J Clin Microbiol Infect Dis. 1992;11:1064–8. doi: 10.1007/BF01967800. [DOI] [PubMed] [Google Scholar]
- 37.Zinsser HH, Pryde AW. Experimental study of physical factors, including fibrin formation, influencing the spread of fluids and small particles within and from the peritoneal cavity of the dog. Ann Surg. 1952;136:818–27. doi: 10.1097/00000658-195211000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Theopold U, Li D, Fabbri M, Scherfer C, Schmidt O. The coagulation of insect hemolymph. Cell Mol Life Sci. 2002;59:363–72. doi: 10.1007/s00018-002-8428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lesch C, Goto A, Lindgren M, Bidla G, Dushay MS, Theopold U. A role for hemolectin in coagulation and immunity in Drosophila melanogaster developmental & comparative. Immunology. 2007;31:1255–63. doi: 10.1016/j.dci.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 40.Wartha F, Beiter K, Normark S, Henriques-Normark B. Neutrophil extracellular traps: casting the NET over pathogenesis. Curr Opin Microbiol. 2007;10:52–6. doi: 10.1016/j.mib.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol. 2007;5:577–82. doi: 10.1038/nrmicro1710. [DOI] [PubMed] [Google Scholar]
- 42.Loimaranta V, Jakubovics NS, Hytonen J, Finne J, Jenkinson HF, Stromberg N. Fluid- or surface-phase human salivary scavenger protein gp340 exposes different bacterial recognition properties. Infect Immun. 2005;73:2245–52. doi: 10.1128/IAI.73.4.2245-2252.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lim BL, Wang JY, Holmskov U, Hoppe HJ, Reid KB. Expression of the carbohydrate recognition domain of lung surfactant protein D and demonstration of its binding to lipopolysaccharides of gram-negative bacteria. Biochem Biophys Res Commun. 1994;202:1674–80. doi: 10.1006/bbrc.1994.2127. [DOI] [PubMed] [Google Scholar]
- 44.Hartshorn KL, Crouch E, White MR, Colamussi ML, Kakkanatt A, Tauber B, Shepherd V, Sastry KN. Pulmonary surfactant proteins A and D enhance neutrophil uptake of bacteria. Am J Physiol. 1998;274:L958–69. doi: 10.1152/ajplung.1998.274.6.L958. [DOI] [PubMed] [Google Scholar]
- 45.Jounblat R, Clark H, Eggleton P, Hawgood S, Andrew PW, Kadioglu A. The role of surfactant protein D in the colonisation of the respiratory tract and onset of bacteraemia during pneumococcal pneumonia. Respir Res. 2005;6:126. doi: 10.1186/1465-9921-6-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Persson K, Russell W, Morgelin M, Herwald H. The conversion of fibrinogen to fibrin at the surface of curliated Escherichia coli bacteria leads to the generation of proinflammatory fibrinopeptides. J Biol Chem. 2003;278:31884–90. doi: 10.1074/jbc.M302522200. [DOI] [PubMed] [Google Scholar]
- 47.Johnson LL, Berggren KN, Szaba FM, Chen W, Smiley ST. Fibrin-mediated protection against infection-stimulated immunopathology. J Exp Med. 2003;197:801–6. doi: 10.1084/jem.20021493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mullarky IK, Szaba FM, Berggren KN, Parent MA, Kummer LW, Chen W, Johnso LL. Smiley ST. Infection-stimulated fibrin deposition controls hemorrhage and limits hepatic bacterial growth during listeriosis. Infect Immun. 2005;73:3888–95. doi: 10.1128/IAI.73.7.3888-3895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simon DI, Ezratty AM, Francis SA, Rennke H, Loscalzo J. Fibrin(ogen) is internalized and degraded by activated human monocytoid cells via Mac-1 (CD11b/CD18): a nonplasmin fibrinolytic pathway. Blood. 1993;82:2414–22. [PubMed] [Google Scholar]
- 50.Dempfle CE. Coagulopathy of sepsis. Thromb Haemost. 2004;91:213–24. doi: 10.1160/TH03-03-0182. [DOI] [PubMed] [Google Scholar]
- 51.Dhainaut JF, Yan SB, Margolis BD, et al. Drotrecogin alfa (activated) (recombinant human activated protein C) reduces host coagulopathy response in patients with severe sepsis. Thromb Haemost. 2003;90:642–53. doi: 10.1160/TH02-11-0270. [DOI] [PubMed] [Google Scholar]
- 52.Doganay M. Listeriosis: clinical presentation. FEMS Immunol Med Microbiol. 2003;35:173–5. doi: 10.1016/S0928-8244(02)00467-4. [DOI] [PubMed] [Google Scholar]
- 53.Flick MJ, Du X, Witte DP, Jirouskova M, Soloviev DA, Busuttil SJ, Plow EF, Degen JL. Leukocyte engagement of fibrin(ogen) via the integrin receptor _M_2/Mac-1 is critical for host inflammatory response in vivo. J Clin Invest. 2004;113:1596–606. doi: 10.1172/JCI20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Foster TJ, Hook M. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 1998;6:484–8. doi: 10.1016/s0966-842x(98)01400-0. [DOI] [PubMed] [Google Scholar]
- 55.Fujita T, Matsushita M, Endo Y. The lectin-complement pathway – its role in innate immunity and evolution. Immunol Rev. 2004;198:185–202. doi: 10.1111/j.0105-2896.2004.0123.x. [DOI] [PubMed] [Google Scholar]
- 56.Dodds AW. Which came first, the lectin/classical pathway or the alternative pathway of complement? Immunobiology. 2002;205:340–54. doi: 10.1078/0171-2985-00137. [DOI] [PubMed] [Google Scholar]
- 57.Skogen WF, Senior RM, Griffin GL, Wilner GD. Fibrinogen-derived peptide B beta 1-42 is a multidomained neutrophil chemoattractant. Blood. 1988;71:1475–9. [PubMed] [Google Scholar]