Abstract
Histone deacetylase inhibitor n-butyrate induced proliferative unresponsiveness in antigen-stimulated murine CD4+ T cells. T cells anergized by n-butyrate demonstrated reduced interleukin-2 (IL-2) secretion and decreased activating protein 1 (AP-1) activity upon restimulation. Mechanistic studies determined that the cyclin-dependent kinase (cdk) inhibitor p21Cip1 was up-regulated in the anergic CD4+ T cells. p21Cip1 is known to inhibit the cell cycle through its interaction with cdk, proliferating cell nuclear antigen (PCNA) or c-Jun N-terminal kinase (JNK). p21Cip1 did not preferentially associate with PCNA or cdk in anergic T helper type 1 (Th1) cells. Instead, among the three interaction partners, p21Cip1 was found to interact with phospho-JNK and phospho-c-jun selectively in the anergic CD4+ T cells. The activity of c-jun and downstream transcription factor AP-1 were suppressed in the anergic Th1 cells. In contrast, p21Cip1 and the two phospho-proteins were never detected concurrently in the control CD4+ T cells. The n-butyrate-induced p21Cip1-mediated inhibition of JNK and c-jun represents a novel potential mechanism by which proliferative unresponsiveness was maintained in CD4+ T cells.
Keywords: anergy, signalling/signal transduction, T cells, tolerance
Introduction
The induction of T-cell anergy results in the inability to respond to antigen-stimulated proliferative signals. Regardless of the method used to induce T-cell anergy the resulting proliferative unresponsiveness is associated with G1 cell cycle blockade.1–4 Examining the connection between G1 blockade and anergy induction led to the finding that the histone deacetylase (HDAC) inhibitor and G1 blocker n-butyrate could itself induce proliferative unresponsiveness in CD4+ T cells.5–7 The n-butyrate-induced anergy process required new protein synthesis, and was only induced in antigen-activated CD4+ T cells, not resting CD4+ T cells.8,9 Unlike these earlier studies, the antigen alone control group included in the current study helped to prove that the observed phenomenon was anergy and not the unresponsiveness due to the recent antigen stimulation.
As an HDAC inhibitor, n-butyrate alters the expression of a number of genes and their resulting proteins. Among these proteins, the one best known to inhibit proliferation is the cyclin-dependent kinase (cdk) inhibitor p21Cip1.10 p21Cip1 was up-regulated in T helper type 1 (Th1) cells anergized by exposure to n-butyrate.8 Recent studies in this model showed that p21Cip1-deficient CD4+ T cells were less sensitive than p21Cip1 wild-type CD4+ T cells to n-butyrate-induced anergy.11 p21Cip1 was not needed for the initial cell cycle blockade involved in anergy induction by HDAC inhibitors, but was required to maintain proliferative unresponsiveness when the anergic CD4+ T cells were restimulated with antigen. The mechanism by which p21Cip1 inhibited proliferation in the anergic CD4+ T cells was not defined, nor was it clear how p21Cip1, which is up-regulated under stimulatory as well as tolerogenic conditions in CD4+ T cells, albeit with different kinetics, inhibits proliferation in the latter but not the former.
p21Cip1 can inhibit cellular proliferation through at least three different mechanisms. As a cdk inhibitor, p21Cip1 selectively inhibits the enzymatic activity that is required for retinoblastoma protein phosphorylation and S phase entry. In accordance with this activity, overexpression of p21Cip1 has been shown to suppress cdk activity and cause G1 cell cycle arrest.12 p21Cip1 is also a potent inhibitor of the proliferating-cell nuclear antigen (PCNA), which is the processivity factor that functions as the sliding clamp on the DNA polymerase delta, the principal replicative DNA polymerase. In resting T cells PCNA is low, whereas upon stimulation, PCNA expression increases 1000-fold during mid-G113 Inhibition of PCNA by p21Cip1 has been reported to inhibit the cell cycle in both G1 and G2 phases in Jurkat T cells.14
The third mechanism by which p21Cip1 can block the cell cycle is through the inhibition of c-Jun N-terminal kniase (JNK). p21Cip1 has been shown to interact with JNK in vitro and to inhibit JNK activity in several cell types, including fibroblasts and T cells.15–17 JNK is a member of the mitogen-activated protein kinase (MAPK) signalling pathway that is activated by antigen stimulation in T cells. Triggering of the MAPK pathway in T cells normally leads to the activation of transcription factors such as activation protein 1 (AP-1), and to an associated increase in interleukin-2 (IL-2) transcription. However, in anergic T cells, defective IL-2 production has been linked to defects of JNK function, AP-1 activity and AP-1-dependent transactivation of IL-2 promoter,18–20 although the mechanisms for the defects observed are still unclear.
Here, we examined whether the proliferative unresponsiveness observed in CD4+ T cells rendered anergic by exposure to n-butyrate was mediated by p21Cip1 through the inhibition of members of the MAPK pathway, JNK in particular, or through the inhibition of its classical interaction partners PCNA and cdk. Also, the expression kinetics and protein associations of p21Cip1 in activated and anergic CD4+ T cells were compared to address the question why p21Cip1 interferes with cell division in the latter, but not the former.
Materials and methods
Animals and reagents
Male C57BL/10 mice at 6–8 weeks of age were purchased from Harlan Sprague Dawley (Indianapolis, IN). Protocols for the use of mice were approved by the University of Arkansas for Medical Sciences Animal Care and Use Committee. Keyhole limpet haemocyanin (KLH) (Imject) was purchased from Pierce (Rockford, IL). The antibodies specific for p21Cip1 [clone SMX30, mouse immunoglobulin G1 (IgG1)], mouse IgG1 (clone A85-1, rat IgG1), CD3 (clone 145-2C11, hamster IgG1), CD28 (clone 37.51, hamster IgG2), p27Kip1 (clone G173-524, mouse IgG1) and the horseradish peroxidase (HRP) -labelled goat anti-mouse IgG antibody were purchased from BD Biosciences (San Jose, CA). The anti-cdk2 antibody (rabbit IgG), anti-cdk6 antibody (rabbit IgG), anti-actin antibody (clone C-2, mouse IgG1), anti-cyclin D2 antibody (clone34B1-3, rat IgG2a), anti-cyclin D3 antibody (clone 18B6-10, rat IgG2a), anti-cyclin E antibody (rabbit IgG), HRP-labelled goat anti-rat IgG antibody, anti-PCNA antibody (clone PC-10, mouse IgG2a) and anti-U1 SnRNP antibody (goat IgG) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The HRP-labelled goat anti-rabbit IgG was purchased from BioRad (Hercules, CA). The anti-cdk4 monoclonal antibody (clone C-22, mouse IgG1), anti-p-JNK antibody (rabbit IgG), anti-p-c-jun antibody (rabbit IgG), anti-JNK (clone G9, mouse IgG1) were purchased from Cell Signaling Technology (Beverly, MA). Sodium butyrate (n-butyrate) and anti-p38 (clone P38-YNP, mouse IgG2b) was purchased from Sigma (St Louis, MO). Goat anti-rabbit IgG Fc antibody was purchased from Jackson ImmunoResearch (West Grove, PA). The JNK inhibitor SP600125 was purchased from Calbiochem (San Diego, CA).
Th1 clones
The KLH-specific Th1 cells (clone D9) were developed as described previously21 using C57BL/10 mice and KLH as the antigen. The Th1 clones were passaged every 6–10 days using 25 μg/ml KLH, irradiated syngeneic splenic antigen-presenting cells (APC) and 20% IL-2-containing concanavalin A (Con A) -stimulated conditioned medium (CM).
Inducing tolerance in Th1 cells
The Th1 cells were incubated in primary cultures at 5 × 105 cells/ml along with 5 × 106/ml irradiated syngeneic spleen cells as APCs, with KLH (50 μg/ml) in 10% CM. The next day n-butyrate (Sigma) was added to the cultures at 1·1 mm. Control primary cultures either received antigen and APCs in CM without n-butyrate or received n-butyrate alone. After incubation in primary cultures for 2–6 days, the viable cells were washed and reincubated in the secondary cultures at 5 × 105/ml along with 5 × 106/ml irradiated syngeneic spleen cells as APCs and increasing concentrations of KLH. After 24 hr, the Th1 cells were pulsed with [3H]thymidine for 12 hr to assess their proliferative capacity. In some experiments, Th1 cells were instead stimulated with streptavidin-coated magnetic beads (Dynal, Great Neck, NY) that had been previously incubated (1 hr at 4°) with biotinylated anti-CD3 and anti-CD28 antibody to asses the proliferative capacity of the Th1 cells.
Lysis
Th1 cells were harvested at different time-points either during the course of primary cultures or in the secondary cultures. The cells were passed over Ficoll–Hypaque to remove the irradiated APCs, counted and disrupted with modified lysis buffer containing 10 mm KCl, 10 mm HEPES, 1% Nonidet P-40, 1 mm NaVO4, aprotinin (10 mg/ml), leupeptin (10 mg/ml), and 0·5 mm phenylmethylsulphonyl fluoride. In some cases, the cells were lysed with hypotonic buffer (20 mm HEPES; pH 7·5, 5 mm NaF, 0·1 nm ethylenediaminetetraacetic acid, 10 μm Na2MoO4 and protease inhibitors) and the nuclei were pelleted with centrifugation at 14 000 g for 10 min. Following the removal of the cytoplasmic fraction, nuclear proteins were then extracted from the isolated nuclei in modified lysis buffer by sonification followed by agitation on a horizontal rotator on ice for 20 min.
Western blotting
Equivalent amounts of protein (50–100 μg) from Th1 cell lysates were separated on 12% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) Ready Gels (BioRad). The proteins were electrotransferred onto nitrocellulose (Amersham Life Sciences, Buckinghamshire, UK) and subsequently immunoblotted with different primary antibodies (1–3 μg/ml) and appropriate secondary antibodies: HRP-conjugated goat anti-mouse IgG (1 : 1000), HRP-conjugated goat anti-rabbit IgG (1 : 1000) or HRP-conjugated goat anti-rat IgG (1 : 500). Immunodetection was performed by Super Signal West Pico Chemiluminescent Substrate (Pierce). To test for appropriate protein loading, some blots were stripped with the Western blot recycling kit (BioRad) and reprobed with the anti-actin antibody. To test for appropriate cytoplasmic/nuclear fractionation, some blots were stripped and reprobed with the anti-U1 SnRNP 70 antibody.
Immunoprecipitation
Streptavidin-coated magnetic beads (Dynal) (30 μl) were incubated (30 min at 4°) with the appropriate biotinylated secondary antibody (either goat anti-rabbit IgG Fc Ab or rat anti-mouse IgG1 mAb) followed by incubation (30 min at 4°) with the appropriate primary antibody directed against the target protein. The Th1 cell lysates (100–200 μg/sample) were then incubated with the beads overnight at 4°. The magnetic beads with the immunoprecipitated protein were washed three times in lysis buffer, boiled with loading buffer for 5 min, resolved on 12% SDS–PAGE and immunoblotted with antibodies specific for p21Cip1 and the immunoprecipitated proteins. The p-c-jun immunoprecipitates could not be immunoblotted for p-c-jun to confirm the success of immunoprecipitation due to the immunoglobulin heavy chain interference and unavailability of a monoclonal anti-p-c-jun antibody.
Cytokine production
Following anergy induction in the primary cultures, anergic and control Th1 cells were harvested, washed, counted and restimulated with streptavidin-coated magnetic beads (Dynal) that had been previously incubated for (1 hr at 4°) with biotinylated anti-CD3 and anti-CD28 antibody at 1 : 1, 1 : 2 or 1 : 4 bead to cell ratio in the presence of anti-IL-2 receptor-α antibody to prevent the attachment of secreted IL-2 to the cells. After 24 hr, cell culture supernatants were collected and analysed for the cytokine content by flow cytometry using a Mouse Th1/Th2 Cytokine Cytometric Bead Array (CBA) kit (BD, San Diego, CA) according to manufacturer’s protocol on FACSCalibur.
Transcription factor enzyme-linked immunsorbent assay
Following primary cultures, control or anergic Th1 cells were isolated and restimulated using anti-CD3 and anti-CD28 antibody-coated magnetic beads at 1 : 4 bead to cell ratio for 0–24 hr. Nuclear lysates were then prepared using Nuclear Extract kit (Active Motif, Carlsbad, CA). Previously untreated resting Th1 cells were also included as a measure of the baseline level of transcription factor activity. c-Fos and c-jun activity was measured using TransAM Transcription Factor Activity Assay kits (Active Motif) according to the manufacturer’s protocol. Briefly, duplicate wells of 96-well plates to which the consensus-binding site oligo has been immobilized were incubated with 20 μg lysate/sample. The wells were then washed and the transcription factor of interest that was bound specifically to the coated oligonucleotide was detected by primary antibody specific for an epitope on the bound and active form of the transcription factor. Subsequent incubation with secondary antibody and developing solution provided a colorimetric readout that was acquired at 450 nm.
Data analysis
Data are presented as mean ± standard deviation (SD). The statistical analysis of the data was performed using that paired Student’s t-test. A P-value ≤ 0·01 was considered significant.
Results
n-Butyrate induced unresponsiveness in Th1 cells
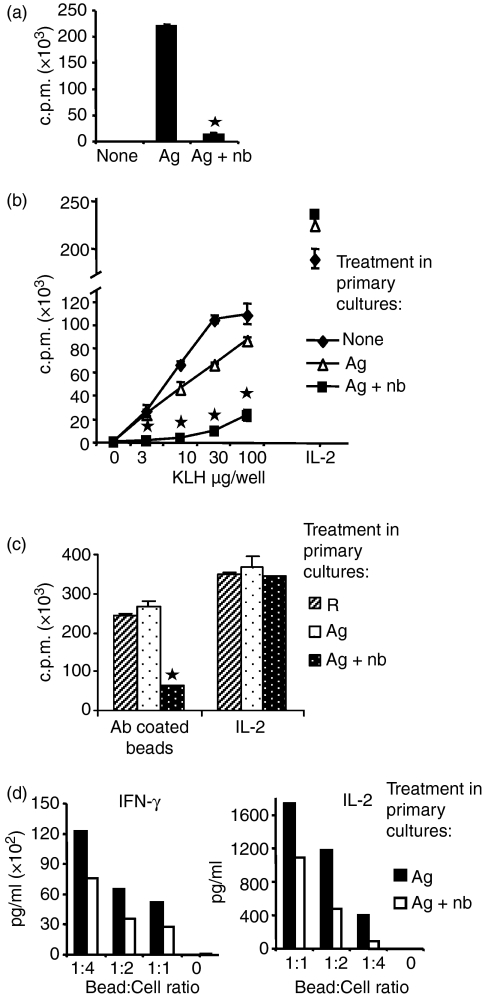
n-Butyrate effectively blocked the proliferation of antigen-stimulated cells in primary cultures (Fig. 1a). In accordance with earlier studies,5,8 Th1 cells that were antigen-stimulated in the presence of n-butyrate in primary cultures were largely unresponsive when restimulated with antigen in the absence of n-butyrate in secondary cultures (Fig. 1b). In contrast, the Th1 cells that were stimulated with antigen in the absence of n-butyrate in the primary cultures proliferated in the secondary cultures as well as previously untreated Th1 cells. Although unresponsive to antigen stimulation, anergic Th1 cells proliferated in response to exogenous IL-2, indicating no loss in cell viability.
Figure 1.
n-Butyrate induced anergy in T helper type 1 (Th1) cells. Th1 cells were stimulated with keyhole limpet haemocyanin (KLH) in the presence or absence of n-butyrate in primary cultures. (a) Following 24 hr in the primary cultures, proliferation was measured as a function of [3H]thymidine incorporation. (b) Following 6 days in primary cultures, Th1 cells were isolated, and along with previously untreated Th1 cells, were stimulated in equivalent numbers for 24 hr with KLH or with interleukin-2 (IL-2) -containing conditioned medium. Proliferation results are presented as mean ± standard error. This experiment was repeated nine times with similar results. Asterisk indicates statistical difference between anergic and control Th1 cells. (c) Following 6 days in primary cultures, Th1 cells were isolated and were stimulated in equivalent numbers for 24 hr with anti-CD3 and anti-CD28 antibody-coated beads at a 1 : 4 bead to cell ratio or with IL-2-containing conditioned medium. (d) Following 6 days in primary cultures, Th1 cells stimulated with antigen ±n-butyrate were restimulated with anti-CD3 and anti-CD28 antibody-coated beads at a 1 : 1, 1 : 2 or 1 : 4 bead to cell ratio for 24 hr. Culture supernatants were collected and IL-2 and interferon-γ levels were measured.
In later experiments, antigen restimulation was preferred when possible because it was more physiological. However, when measuring parameters that involve short-term stimulation and preparation of cell lysates antibody-coated beads were used for restimulation of CD4+ T cells eliminating the requirement of separating the APCs. Irradiated splenocytes that were used as a source of APCs in our experiments could be treated with Ficoll–Hypaque and separated from the CD4+ T cells only after 1 day in cultures. In preparation for later experiments, Fig. 1(c) was included, showing that anergy could be demonstrated using beads instead of antigen to stimulate secondary cultures.
In addition to proliferative unresponsiveness, Th1 cells stimulated with antigen in the presence of n-butyrate demonstrated a 37–77% decrease in IL-2 and a 26–55% decrease in interferon-γ secretion when stimulated in secondary culture with three different stimulation indices (Fig. 1d). Hence, n-butyrate-induced anergy was demonstrated by a loss of both antigen-induced proliferation and cytokine production.
p21Cip1 induced by n-butyrate was maintained in anergic Th1 cells; other cell cycle proteins were not altered
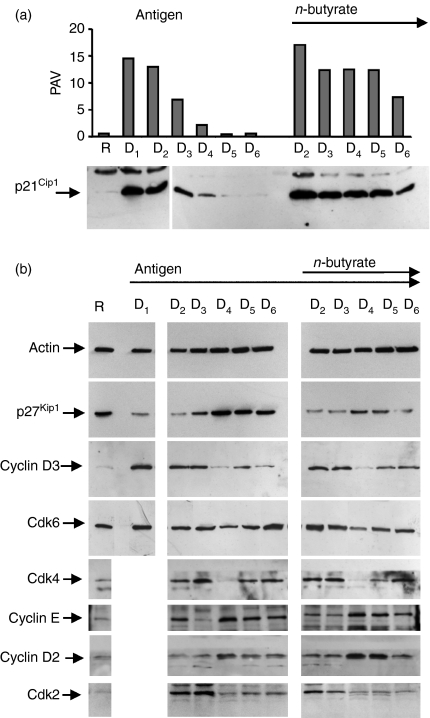
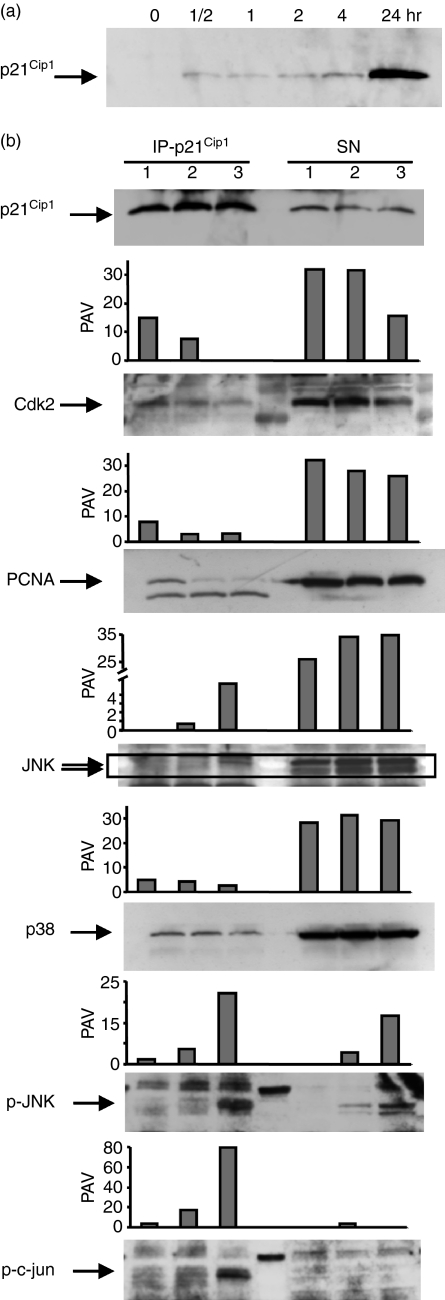
It has been reported previously that n-butyrate increased p21Cip1expression in antigen-stimulated Th1 cells.8 However, p21Cip1 is also induced in antigen-stimulated Th1 cells in the absence of n-butyrate. Consequently, the kinetics of p21Cip1 up-regulation was studied in antigen-stimulated Th1 cells in the presence and absence of n-butyrate during the 6-day primary cultures to compare the two groups for any possible difference in p21Cip1 expression. When antigen was added in the initiation of the primary culture (day 0), p21Cip1 was up-regulated in control Th1 cells by day 1, remained high on day 2, but decreased significantly by day 3 and was back to resting levels by day 5 (Fig. 2a). In contrast, when antigen was added on day 0 and n-butyrate was added on day 1, the p21Cip1 levels remained elevated in anergic Th1 cells during the entire 6-day primary culture.
Figure 2.
p21Cip1 induced by n-butyrate was maintained in anergic T helper type 1 (Th1) cells. The Th1 cells were stimulated with keyhole limpet haemocyanin (day 0). n-Butyrate was added to some of the Th1 cells on day 1 (D1). Total cell lysates were prepared daily during the 6-day primary culture and Western blot analysis of p21Cip1 (a) and other cell-cycle proteins (b) was performed. Because n-butyrate was added to the cultures at the end of day 1, there is a common day 1 (D1) lane for the two groups. As there was insufficient material, the D1 level of some proteins could not be included. Densitometric analysis of the p21Cip1 levels was performed and the are results presented as per cent adjusted volume (PAV). Day 6 levels of p21Cip1 were studied in four separate experiments with consistent results.
p27Kip1 is another cdk inhibitor thought to play a role in T-cell anergy. As expected, p27Kip1 was high in resting Th1 cells. Its level decreased with the antigen stimulation and was later restored to resting levels in control Th1 cells by day 5 of the primary cultures. In contrast, p27Kip1 levels failed to be completely restored in Th1 cells incubated with antigen and n-butyrate in 6-day primary cultures (Fig. 2b). Hence, because p21Cip1 rather than p27Kip1 was high in the anergic Th1 cells at the end of the 6-day primary cultures, subsequent experiments were focused on the role of p21Cip1 in maintaining proliferative unresponsiveness.
The kinetics of other cell cycle proteins was also studied to assess their possible involvement in n-butyrate-induced T-cell anergy. No significant differences between the antigen-stimulated control and anergic Th1 cells were observed in the expression of cdk2, cdk4, cdk6, cyclin D2, cyclin D3 and cyclin E (Fig. 2b). In summary, the kinetics studies on cell cycle proteins revealed that the most detectable difference between anergic and control Th1 cells was the high level of p21Cip1 maintained throughout the primary cultures in the anergic Th1 cells.
p21Cip1 was found in both cytoplasm and nucleus of anergic Th1 cells
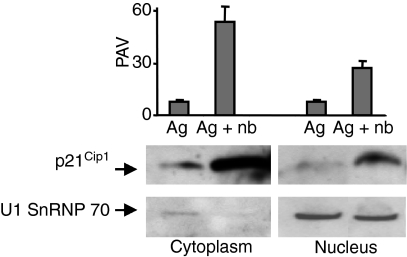
Localization of proteins such as p21Cip1 in the cell can have important functional consequences.22 Among the interaction partners of p21Cip1, active cyclin-cdk complexes and PCNA are localized in the nucleus, whereas most MAPK are localized to the cytoplasm. Phosphorylated JNK (p-JNK) can be found in the nucleus as well as in the cytoplasm. Following a 6-day primary culture, anergic Th1 cells contained p21Cip1 in both cytoplasmic and nuclear fractions, although there was more p21Cip1 in the cytoplasmic fraction than the nuclear fraction (Fig. 3). In contrast, control Th1 cells contained little p21Cip1 in either fraction at the end of the 6-day primary culture. The presence of U1, a small nuclear ribonuclear protein of molecular weight 70 000 (SnRNP 70) in the nuclear fractions from both anergic and control Th1 cells confirmed efficient nuclear fractionation.
Figure 3.
p21Cip1 was detected in both cytoplasmic and nuclear fractions of anergic T helper type 1 (Th1) cells. The Th1 cells were incubated in primary cultures with antigen ± n-butyrate. Following 6 days in primary cultures, viable cells were isolated and cell lysates were prepared. Western blot analysis of p21Cip1 and U1 SnRNP 70 was performed. This experiment has been repeated once with similar results.
p21Cip1 interaction with cdk was not altered in anergic Th1 cells
The mechanistic significance of the p21Cip1 detected in the anergic Th1 cells was examined in the next series of experiments. As p21Cip1 was found in both cytoplasm and nucleus of anergic Th1 cells, all three interaction partners of p21Cip1 known to mediate cell cycle inhibition, namely cdk, PCNA and JNK, were examined for their association with p21Cip1 in these cells.
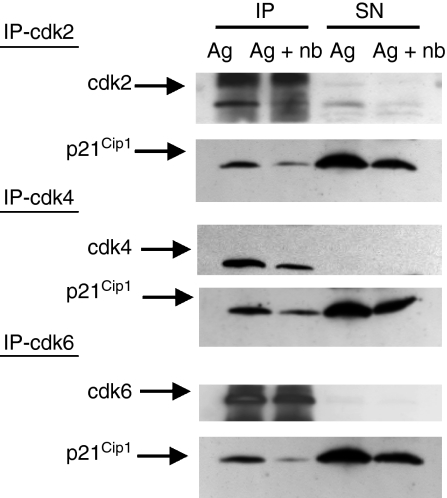
p21Cip1 was first examined for its ability to bind to cdk. Cdk2, cdk4 and cdk6 were examined for coprecipitation with p21Cip1 in anergic and control Th1 cells following antigen restimulation. The restimulation period was extended to 36 hr to allow enough time for the control Th1 cells to up-regulate p21Cip1. The upper blots demonstrated that the cdk were immunoprecipitated efficiently such that very little of the relevant cdk remained in the supernatant (Fig. 4). It was noted that anergic Th1 cells contained little cdk2, probably because of the requirement for IL-2 in cdk2 up-regulation. As expected, p21Cip1 was found associated with cdk2, cdk4 and cdk6 in control Th1 cells 36 hr after antigen stimulation. However, p21Cip1 in the anergic Th1 cells did not demonstrate an increased association with cdk compared with the control Th1 cells. Proliferative unresponsiveness in the anergic Th1 cells therefore could not be attributed to preferential p21Cip1 interaction with cdk.
Figure 4.
p21Cip1 did not preferentially interact with cyclin-dependent kinases (cdk) in anergic T helper type 1 (Th1) cells. The Th1 cells were incubated in primary cultures with antigen ± n-butyrate. Following 6 days in primary cultures, viable Th1 cells were isolated and then restimulated with antigen for 36 hr. Following restimulation, whole cell lysates were prepared, and cdk2, cdk4 and cdk6 were immunoprecipitated from the lysates. The immunoprecipitated proteins (IP) and the proteins remaining in the lysate supernatant (SN) were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis and then immunoblotted with antibodies specific for p21Cip1 and the respective cdk.
Considering the possibility that p21Cip1 interaction with cdk could have taken place in the anergic Th1 cells earlier in the secondary cultures before p21Cip1 was up-regulated in the control cells, the p21Cip1–cdk interactions were examined in lysates obtained from anergic Th1 cells restimulated for only 2 hr. Control lysates were obtained from Th1 cells that were restimulated for 24 hr to up-regulate sufficient p21Cip1 levels for detection (Fig. 5a). p21Cip1 was immunoprecipitated from the lysates and examined for binding partners. All experimental groups, including 24-hr-stimulated control Th1 cells, anergic Th1 cells before restimulation and anergic Th1cells following 2 hr of restimulation, contained p21Cip1 that was immunoprecipitated successfully from all three lysates (Fig. 5b). Examination of cdk2 interaction revealed that most of this protein was not associated with p21Cip1 and the amount of cdk2 that was associated with p21Cip1 was not higher in the anergic Th1 cells than in control Th1 cells. This result confirmed the earlier finding that in the anergic cells p21Cip1 did not appear to be acting through cdk inhibition.
Figure 5.
p21Cip1 preferentially interacted with phosphorylated c-Jun N-terminla kinase (p-JNK) and p-c-jun in anergic T helper type 1 (Th1) cells. (a) The Th1 cells were stimulated with anti-CD3 and anti-CD28 antibody-coated beads for 0–24 hr, lysed at different time-points, separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE)and immunoblotted with antibody specific for p21Cip1. (b) The Th1 cells were stimulated with antigen in the presence or absence of n-butyrate in primary cultures. Viable control or anergic Th1 cells were then isolated. Control Th1 cells were stimulated for 24 hr with anti-CD3 and anti-CD28 antibody-coated beads (lane 1). febs_Half of the anergic Th1 cells were lysed at the end of primary cultures (lane 2) and the other half were restimulated with anti-CD3 and anti-CD28 antibody-coated beads for 2 hr before lysis (lane 3). Cell lysates were prepared from the three different groups and p21Cip1 was immunoprecipitated from the lysates. The immunoprecipitated p21cip1 as well as the remaining lysate supernatant (SN) were separated on SDS–PAGE and then immunoblotted with antibodies specific for p21Cip1, cdk2, PCNA, JNK, p38, p-JNK and p-c-jun. This experiment was repeated once with similar results.
p21Cip1 did not interact with PCNA preferentially in the anergic Th1 cells
To determine whether p21Cip1 inhibited proliferation in the secondary cultures through interaction with and inhibition of PCNA, p21Cip1 coprecipitation with PCNA was also examined. Most of the PCNA did not associate with p21Cip1 in either control Th1 cells or anergic Th1 cells, regardless of restimulation (Fig. 5b). In addition, the amount of PCNA that was associated with p21Cip1 was not higher in the anergic Th1 cells than the control cells. This result suggested that in the anergic Th1 cells p21Cip1 was not acting through preferential PCNA binding and inhibition.
p21Cip1 interacted with p-JNK and p-c-jun in the restimulated anergic Th1 cells
As a third possible mechanism, p21Cip1 interactions with members of the MAPK pathway were studied. Under the same experimental conditions in which p21Cip1–cdk2 and p21Cip1–PCNA interactions were studied, p21Cip1–JNK coprecipitation was examined. The majority of JNK protein was not associated with p21Cip1 in any of the groups. However, a small amount of JNK coprecipitated with p21Cip1 in 2-hr restimulated anergic Th1 cells (Fig. 5b).
As a control, another MAPK that is reported to interact with p21Cip1in vitro,15 namely p38, was examined for its interaction with p21Cip1 in the anergic Th1 cells. Little p38 could be detected in the p21Cip1 immunoprecipitates except a small band that was present equally in all groups (Fig. 5b). Most of the p38 in all the lysates was not associated with p21Cip1. This result suggested that the low level p21Cip1–JNK interaction observed in the anergic restimulated Th1 cells was specific for JNK and did not encompass another MAPK p38.
Unlike JNK and p38, which are present in relatively unchanged levels throughout T-cell activation, phosphorylated versions of MAPK such as p-JNK and p-c-jun are only found in T cells for the initial few hours following stimulation. The interaction of p21Cip1 with JNK in the anergic Th1 cells was detected early in restimulation and was not present in the absence of restimulation, so the possibility that p21Cip1 preferentially associated with p-JNK was explored. Among the three experimental groups, only the 2-hr restimulated anergic Th1 cells contained p-JNK as expected (Fig. 5b). Interestingly, more than half of the p-JNK in the anergic restimulated Th1 cells was found to be associated with p21Cip1. The interaction between p21Cip1 and p-c-jun was also examined. Similar to p-JNK, only the 2-hr restimulated anergic Th1 cells contained p-c-jun. Almost all of the p-c-jun in the anergic group appeared to be associated with p21Cip1. Hence, unlike cdk and PCNA, certain members of the MAPK pathway, especially in their phosphorylated forms, appeared to bind p21Cip1 in anergic Th1 cells.
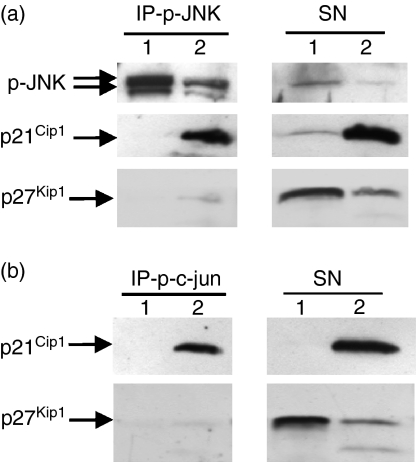
p21Cip1 interaction with p-JNK and p-c-jun in the anergic Th1 cells was confirmed in reciprocal immunoprecipitations
To further examine the interaction of p21Cip1 with p-JNK and p-c-jun in anergic Th1 cells, reciprocal immunoprecipitation experiments were performed. The anergic and control Th1 cells were restimulated for 2 hr to up-regulate p-JNK and p-c-jun levels. Control cells that were stimulated for 2 hr did not contain p21Cip1 yet, whereas, the anergic cells that were restimulated for 2 hr contained high levels of p21Cip1 (Fig. 6a). A significant proportion of the p21Cip1 in the restimulated anergic Th1 cells was found to be associated with p-JNK. Similarly, reciprocal p-c-jun immunoprecipitation was performed. Again, a significant amount of the p21Cip1 in the restimulated anergic Th1 cells was found to be associated with p-c-jun (Fig. 6b).
Figure 6.
p21Cip1 interacted with phosphorylated c-Jun N-terminal kinase (p-JNK) and p-c-jun in anergic T helper type 1 (Th1) cells in reciprocal immunoprecipitations. Th1 cells from primary cultures stimulated with antigen in the absence (lane 1) or presence (lane 2) of n-butyrate were restimulated with anti-CD3 and anti-CD28 antibody-coated beads for 2 hr before lysis. p-JNK (a) and p-c-jun (b) were immunoprecipitated from lysates, separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis and then immunoblotted with antibodies specific for p21Cip1, p27Kip1and p-JNK.
p21Cip1and p27Kip1 share similar N-terminal domains but show no resemblance in their C termini.23 That is why p27Kip1 does not possess PCNA binding activity; because p21Cip1 interacts with JNK through its N-terminal,15 such interaction could be shared with p27Kip1. However, in contrast to p21Cip1, p27Kip1 did not coprecipitate with p-JNK or p-c-jun in the anergic Th1 cells (Fig. 6a,b). This finding underlined the selectivity of the p21Cip1–p-JNK and p21Cip1–p-c-jun interactions.
c-fos and c-jun transcription factor binding is inhibited in the anergic Th1 cells
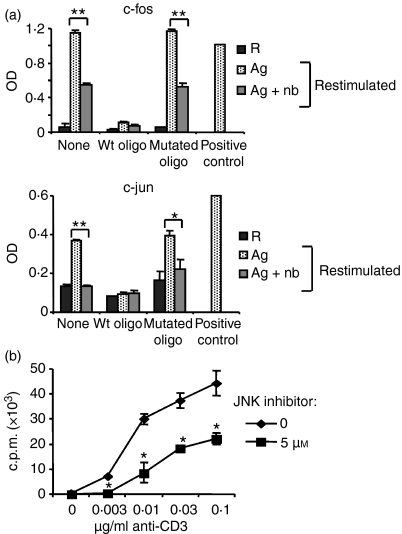
If the interaction of p21Cip1 with p-JNK and p-c-jun had functional consequences, then it should be possible to detect changes in the activity of the downstream transcription factors such as AP-1. Initial experiments were conducted to determine maximum activity kinetics of c-fos and c-jun, two components of AP-1, using an electrophoretic mobility shift assay alternative enzyme-linked immunosorbent assay (ELISA) -based method. Maximum activity of c-fos occurred 2 hr following Th1 cell stimulation, whereas maximum activity of c-jun was observed 1 hr after Th1 cell stimulation (data not shown). Nuclear cell lysates from anergic and control Th1 cells restimulated for the appropriate time periods were then prepared and the transcription factor activities in the two groups were compared. Unstimulated resting Th1 cells yielded low activity for both c-fos and c-jun (Fig. 7a). As expected, restimulated control Th1 cells showed increased activity levels compared with resting Th1 cells. Interestingly, a significant decrease was detected in the activity of both AP-1 components in the anergic Th1 cells compared with the control Th1 cells upon restimulation. The binding levels detected for both transcription factors decreased down to baseline levels upon addition of the wild-type oligonucleotides, but were not altered by the addition of mutated oligonucleotides, confirming the specificity of the assay.
Figure 7.
c-Fos and c-jun activity was inhibited in the anergic T helper type 1 (Th1) cells. (a) Th1 cells were stimulated with antigen ± n-butyrate in primary cultures. The Th1 cells were then restimulated using anti-CD3 and anti-CD28 antibody-coated beads for 2 hr for c-fos assay and 1 hr for c-jun assay. Nuclear lysates were prepared from restimulated Th1 cells as well as from unstimulated resting Th1 cells (group R). c-fos and c-jun activity was measured using TransAM Transcription Factor Activity Assay kits. This experiment was performed twice with consistent results. Statistical differences between control and anergic Th1 cells are indicated as **P< 0·01 or *P < 0·05. (b) Th1 cells were stimulated with immobilized anti-CD3 and anti-CD28 antibodies in the presence or absence of 5 μm SP600125 for 24 hr. Proliferation was measured by [3H]thymidine incorporation. This experiment has been repeated twice with similar results. Asterisk indicates statistical difference differences between Th1 cells incubated in the presence or absence of SP600125.
The results presented suggested that p21Cip1 interacted with members of the MAPK pathway, specifically p-JNK and p-c-jun, resulting in an inhibition in proliferation and IL-2 secretion in anergic Th1 cells. To demonstrate that inhibition of JNK function is sufficient to inhibit Th1 cell proliferation in this model, the specific JNK inhibitor SP60012524 was used. At a non-toxic concentration of inhibitor (5 μm), SP600125 significantly suppressed the proliferation of anti-CD3 and anti-CD28 antibody-stimulated Th1 cells (Fig. 7b). Antibody stimulation was used instead of antigen stimulation to demonstrate the direct effect of the inhibitor on Th1 cells and to discount the indirect effects on APCs. Inhibition of JNK activity by SP600125 was sufficient to suppress the proliferation of the KLH-specific Th1 cells, indicating that Th1 cells used in this model are no different from primary CD4+ T cells in that the inhibition of JNK alone is sufficient to block proliferation.
In conclusion, p21Cip1-mediated suppression of JNK activity in anergic Th1 cells is a novel potential mechanism that could account for the proliferative unresponsiveness found in these cells.
Discussion
This manuscript examined the role of p21Cip1 in maintaining the proliferative unresponsiveness found in Th1 cells anergized by exposure to antigen and n-butyrate. The results presented in this work suggest that p21Cip1 functions in these Th1 cells primarily through the inhibition of members of the MAPK family rather than inhibition of its classical interaction partners, namely cdk. p21Cip1 has long been described as a negative regulator of the cdk-mediated G1 to S phase transition.25 However, based on the association pattern of p21Cip1 and cdk in anergic compared to control Th1 cells, the p21Cip1 inhibition of cdk activity does not appear to be the primary mechanism for cell cycle inhibition. Instead, the results suggest that p21Cip1 specifically interacts with p-JNK and p-c-jun in antigen-restimulated anergic Th1 cells.
The role of p21Cip1 in the normal cell cycle has been at variance in different studies. Eventually, a dual role has been suggested for p21Cip1 in which low levels of p21Cip1 facilitated the cell cycle by promoting cdk–cyclin complex assembly whereas high levels inhibited cdk activity.25–27 The role of p21Cip1 in normal T-cell activation is not clear. T cells from the p21Cip1-deficient mice exhibited enhanced homeostatic proliferation and increased the frequency of cycling T cells.28 Another study using p21Cip1-deficient mice reported that p21Cip1 did not affect primary proliferation of naïve T cells, but was required for the regulation of activated/memory T-cell proliferation.29 In the present study, control Th1 cells stimulated for 36 hr with antigen contained appreciable amounts of p21Cip1, much of which associated with cdk2, cdk4 and cdk6. It would therefore seem likely that at least some of the regulatory effect of p21Cip1 in stimulated control Th1 cells in our system involves interaction with cdk. The amount and timing of p21Cip1 induced in activated T cells may be sufficient to promote cdk–cyclin assembly but not enough to block cdk activity. Alternatively, p21Cip1 may be up-regulated in activated T cells as a fail-safe mechanism in case some kind of cellular stress necessitates regulation of DNA replication or repair. In either case the p21Cip1 in activated T cells would be subjected to proteasome-mediated degradation and/or diluted in actively dividing T cells. In contrast, in anergic Th1 cells, p21Cip1 persists and is available to bind to inhibit the MAPK important in early T-cell activation.
In addition to partnering with cdk, p21Cip1 can form a binary complex with PCNA.30 PCNA is induced in activated T cells and when T-cell proliferation ceases, synthesis and accumulation of PCNA also stops.13 In case of genetic damage, p53-dependent up-regulation of p21Cip1 leads to cdk-independent inhibition of PCNA-dependent DNA replication allowing time for DNA repair.30,31 p21Cip1 interaction with PCNA results in the inhibition of PCNA and thereby causes G1 and G2 block in T cells.14,32 There was some association of p21Cip1 with PCNA in stimulated control Th1 cells, but the functional significance of this low-level interaction was not determined. The interaction between p21Cip1 and PCNA was not increased in anergic Th1 cells, which suggests that PCNA inhibition by p21Cip1 is probably not the cause of proliferative unresponsiveness in these Th1 cells.
p21Cip1 in anergic Th1 cells instead appears to work via the inhibition of MAPK, specifically p-JNK and p-c-jun. In T cells, productive antigen stimulation triggers the activation of MAPK including extracellular signal-regulated kinase, p38 and JNK.33 The JNK is activated through the dual phosphorylation of its Thr and Tyr residues by mitogen-activated kinase kinase 4 (MKK4) and MKK7. Activated JNK in turn phosphorylates c-jun in its N terminus, activating the c-jun-containing AP-1 complexes.34 Activation of AP-1 transcription factor eventually results in increased IL-2 transcription. Others have shown defective expression and function of the AP-1 transcription factor as well as reduced JNK activity in anergic T cells.18–20,35 In accordance with these earlier studies, c-fos and c-jun activity was decreased in Th1 cells anergized by exposure to n-butyrate. Although an ELISA-based method was used, the readout reflects the activity rather than the binding of the transcription factors because the primary antibody provided with this kit is specific for an epitope on the bound and active form of the transcription factor. p21Cip1 has been shown to interact with JNK and inhibit its activity.15,16 p21Cip1-deficient fibroblasts had higher basal levels of JNK1 than controls, an effect that was reversed if the cells were transfected with p21Cip1.16 In T cells JNK activation following release from G1 arrest correlated with dissociation from p21Cip1.17 In T cells, JNK not only promotes IL-2 gene transcription through the activation of c-jun and AP-1,36 but also directly promotes IL-2 messenger RNA stability.37 Consequently, the finding that p21Cip1 interacts with p-JNK and p-c-jun in Th1 cells anergized by exposure to n-butyrate could explain the lack of IL-2 production and related proliferation in these Th1 cells. p21Cip1 is not known to inhibit the janus kinase and signal transducer and activator of transcription (JAK-STAT) pathway, so IL-2 signalling probably takes place normally in anergic Th1 cells. Consequently, a mechanism by which p21Cip1 binds to and inhibits AP-1 components should not block the ability of anergic Th1 cells to proliferate in response to exogenous IL-2 in secondary n-butyrate-free cultures. In contrast to anergic Th1 cells, there was no p21Cip1 in control Th1 cells before restimulation. p21Cip1 gradually accumulated in the control Th1 cells, demonstrating very low levels at the early time periods at which p-JNK or p-c-jun were up-regulated in response to antigen restimulation. Therefore, in the control Th1 cells, early activation events were completed before p21Cip1 reached detectable levels, possibly explaining why p21Cip1 did not block initial cell division in control Th1 cells unlike the anergic Th1 cells.
In the immunoprecipitation experiments, most of the JNK in the cell lysates did not associate with p21Cip1 except for a small amount in the anergic Th1 cells restimulated for 2 hr. Normally, only a small portion of JNK present in the cell becomes phosphorylated upon T-cell receptor stimulation. As the JNK antibody used in this study recognizes p-JNK as well as unphosphorylated JNK, the thin band of JNK that was associated with p21Cip1 in the restimulated anergic group could represent the phosphorylated form of JNK. p21Cip1 interaction with p-JNK and p-c-jun was demonstrated in this study. It is not clear why p21Cip1 would bind preferentially to the phosphorylated forms of these proteins, but phosphorylation-dependent confirmation changes may be in effect regulating this interaction. This interaction was confirmed in reciprocal immunoprecipitations.
Unlike p21Cip1, p27Kip1 did not seem to associate with the MAPK in the anergic Th1 cells. p27Kip1 has been suggested to be a mediator of T-cell tolerance in a study of human alloantigen-specific T-cell tolerance in which over-expression of p27Kip1 in primary cultures was shown to result in unresponsiveness in T-cell clones upon rechallenge in secondary cultures.3 In addition, p27Kip1 was recently shown to be required for transplantation tolerance induced in vivo by costimulation blockade.38 Yet in one study, the role of p27Kip1 in T-cell anergy was questioned by investigators who showed that anti-TCR antibody could induce tolerance in p27Kip1-deficient CD4+ T cells in vitro.39 In our model, anergy induced by exposure to HDAC inhibitors, known to be potent stimulators of p21Cip1, seems to primarily rely on this CDK inhibitor rather than p27Kip1. The levels of p27Kip1 were not higher in the anergic Th1 cells than control Th1 cells at the end of 6-day primary cultures. p27Kip1 down-regulated rather than up-regulated in T cells treated with antigen and n-butyrate appeared to contradict reports in the literature describing an increase in p27Kip1 following exposure to n-butyrate. The apparent discrepancy may be because the n-butyrate is added 16–18 hr after the initial setting up of the anergy experiments as this delay in n-butyrate addition increased the yields in the anergy group significantly. The regulation of p27Kip1 by n-butyrate occurs post-translationally via the suppression of Skp1–Cul1–F-box-protein (SCF) (skp2) ubiquitin ligase that targets p27Kip1 for destruction.40 In the anergy group, p27Kip1 might have been already ubiquitinylated or degraded before the addition of n-butyrate.
HDAC inhibitors are undergoing clinical trials as antitumour agents. Recent studies highlighted their anti-inflammatory effects through the modulation of dendritic cell function41 and regulatory T-cell numbers and function.42 This study focused on the anergic effects of the HDAC inhibitor n-butyrate on KLH-specific CD4+ T cells. The results presented describe a mechanism by which p21Cip1 could maintain proliferative unresponsiveness in anergic CD4+ T cells by interfering with the signalling pathways downstream of the T-cell receptor, particularly through the inhibition of MAPK and prevention of IL-2 synthesis. Aside from T-cell anergy induced by HDAC inhibitors, other anergy-inducing methods such as exposure to anti-CD3 antibody have been shown to up-regulate p21Cip1.43 The in vivo significance of p21Cip1 was underlined in studies showing that a peptidyl mimic of p21Cip1 inhibited T-cell proliferation and abrogated autoimmune disease development,44 while a p21Cip1 deficiency promoted autoimmune disease and enhanced the expansion of activated/memory T-cells.29,45 By describing an interaction between p21Cip1 and MAPK in anergic CD4+ T cells the results provide a mechanism by which p21Cip1 could maintain proliferative unresponsiveness and demonstrate cross-talk between two pathways that regulate the cell cycle in T cells; signalling cascades downstream of T-cell receptor ligation and basic cell cycle machinery composed of cdk inhibitors.
Acknowledgments
We would like to thank Annick DeLoose for her excellent technical assistance. This work was supported by the National Science Foundation, Arkansas Biosciences Institute and UAMS Graduate Student Research Funds.
Glossary
Abbreviations:
- AP-1
activating protein-1
- APC
antigen-presenting cells
- cdk
cyclin-dependent kinase
- CM
interleukin-2-containing concanavalin A-stimulated conditioned medium
- ELISA
enzyme-linked immunosorbent assay
- HDAC
histone deacetylase
- HRP
horseradish peroxidase
- IgG
immunoglobulin G
- IL-2
interleukin-2
- JNK
c-Jun N-terminal kinase
- KLH
keyhole limpet haemocyanin
- MAPK
mitogen-activated protein kinase
- PCNA
proliferating-cell nuclear antigen
- SDS–PAGE
sodium dodecyl sulphate–polyacrylamide gel electrophoresis
- Th1
T helper type 1
Disclosures
The authors have no conflict of interests.
References
- 1.Gilbert KM, Ernst DN, Hobbs MV, Weigle WO. Effects of tolerance induction on early cell cycle progression by Th1 clones. Cell Immunol. 1992;141:362–72. doi: 10.1016/0008-8749(92)90155-i. [DOI] [PubMed] [Google Scholar]
- 2.Powell JD, Lerner CG, Schwartz RH. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J Immunol. 1999;162:2775–84. [PubMed] [Google Scholar]
- 3.Boussiotis VA, Freeman GJ, Taylor PA, Berezovskaya A, Grass I, Blazar BR, Nadler LM. p27kip1 functions as an anergy factor inhibiting interleukin 2 transcription and clonal expansion of alloreactive human and mouse helper T lymphocytes. Nat Med. 2000;6:290–6. doi: 10.1038/73144. [DOI] [PubMed] [Google Scholar]
- 4.Greenwald RJ, Boussiotis VA, Lorsbach RB, Abbas AK, Sharpe AH. CTLA-4 regulates induction of anergy in vivo. Immunity. 2001;14:145–55. doi: 10.1016/s1074-7613(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert KM, Weigle WO. Th1 cell anergy and blockade in G1a phase of the cell cycle. J Immunol. 1993;151:1245–54. [PubMed] [Google Scholar]
- 6.Edens RE, Dagtas S, Gilbert KM. Histone deacetylase inhibitors induce antigen specific anergy in lymphocytes: a comparative study. Int Immunopharmacol. 2006;6:1673–81. doi: 10.1016/j.intimp.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert KM, Boger S, Fifer EK. Butyric acid derivate induces allospecific T cell anergy and prevents graft-versus-host disease. Immunopharmcol Immunotoxicol. 2002;25:13–27. doi: 10.1081/iph-120018280. [DOI] [PubMed] [Google Scholar]
- 8.Jackson SK, DeLoose A, Gilbert KM. Induction of anergy in Th1 cells is associated with increased levels of cyclin-dependent kinase inhibitors p21cip1 and p27kip1. J Immunol. 2001;166:952–8. doi: 10.4049/jimmunol.166.2.952. [DOI] [PubMed] [Google Scholar]
- 9.Jackson SK, DeLoose A, Gilbert KM. The ability of antigen, but not IL-2, to promote n-butyrate-induced Th1 cell anergy is associated with increased expression and altered association patterns of cyclin dependent kinase inhibitors. Immunology. 2002;106:486–95. doi: 10.1046/j.1365-2567.2002.01457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richon VM, Sandhoff TW, Rifkind RA, Marks PA. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc Natl Acad Sci USA. 2000;97:10014–9. doi: 10.1073/pnas.180316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilbert KM, Boger S, Price P, Fifer EK. T cell tolerance induced by histone deacetylase inhibitor is mediated by p21cip1. Immunopharmcol Immunotoxicol. 2005;27:545–64. doi: 10.1080/08923970500416749. [DOI] [PubMed] [Google Scholar]
- 12.Harper JW, Elledge SJ, Keyomarsi K. Inhibition of cyclin-dependent kinase by p21. Mol Biol Cell. 1995;6:387–400. doi: 10.1091/mbc.6.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore KS, Sullivan K, Tan EM, Prystowsky MB. Proliferating cell nuclear antigen/cyclin is an interleukin 2-responsive gene. J Biol Chem. 1987;262:8447–50. [PubMed] [Google Scholar]
- 14.Lorens JB, Bennett MK, Pearsall DM, et al. Retroviral delivery of peptide modulators of cellular functions. Mol Ther. 2000;1:438–47. doi: 10.1006/mthe.2000.0063. [DOI] [PubMed] [Google Scholar]
- 15.Shim J, Lee H, Park J, Kim H, Choi EJ. A non-enzymatic p21 protein inhibitor of stress-activated protein kinases. Nature. 1996;381:804–6. doi: 10.1038/381804a0. [DOI] [PubMed] [Google Scholar]
- 16.Xue Y, Ramaswamy NT, Hong X, Pelling JC. Association of JNK1 with p21waf1 and p53: modulation of JNK1 activity. Mol Carcinog. 2003;36:38–44. doi: 10.1002/mc.10096. [DOI] [PubMed] [Google Scholar]
- 17.Patel R, Bartosch B, Blank JL. p21waf1 is dynamically associated with JNK in human T-lymphocytes during cell cycle progression. J Cell Sci. 1998;111:2247–55. doi: 10.1242/jcs.111.15.2247. [DOI] [PubMed] [Google Scholar]
- 18.Sunstedt A, Dohlsten M. In vivo anergized CD4+ T cells have defective expression and function of the activating protein-1 transcription factor. J Immunol. 1998;161:5930–6. [PubMed] [Google Scholar]
- 19.DeSilva DR, Feeser WS, Tancula EJ, Scherle PA. Anergic T cells are defective in both Jun NH2-terminal kinase and mitogen-activated kinase signaling pathways. J Exp Med. 1996;183:2017–23. doi: 10.1084/jem.183.5.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang SM, Beverly B, Tran AC, Brorson K, Schwartz RH, Lenardo MJ. Transactivation by AP-1 is a molecular target of T cell clonal anergy. Science. 1992;257:1134–8. doi: 10.1126/science.257.5073.1134. [DOI] [PubMed] [Google Scholar]
- 21.Gilbert KM, Hoang KD, Weigle WO. Th1 and Th2 clones differ in their response to a tolerogenic signal. J Immunol. 1990;144:2063–71. [PubMed] [Google Scholar]
- 22.Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol. 2001;3:245–52. doi: 10.1038/35060032. [DOI] [PubMed] [Google Scholar]
- 23.Luo Y, Hurwitz J, Massague J. Cell-cycle inhibition by independent CDK and PCNA binding domains in p21Cip1. Nature. 1995;375:159–61. doi: 10.1038/375159a0. [DOI] [PubMed] [Google Scholar]
- 24.Han Z, Boyle DL, Chang L, Bennett B, Karin M, Yang L, Manning AM, Firestein GS. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest. 2001;108:73–81. doi: 10.1172/JCI12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–16. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 26.LaBaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, Fattaey A, Harlow E. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11:847–62. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Hannon GJ, Beach D. p21-containing cyclin kinases exist in both active and inactive states. Genes Dev. 1994;8:1750–9. doi: 10.1101/gad.8.15.1750. [DOI] [PubMed] [Google Scholar]
- 28.Santiago-Raber ML, Lawson BR, Dummer W, Barnhouse M, Koundouris S, Wilson CB, Kono DH, Theofilopoulos AN. Role of cyclin kinase inhibitor p21 in systemic autoimmunity. J Immunol. 2001;167:4067–74. doi: 10.4049/jimmunol.167.7.4067. [DOI] [PubMed] [Google Scholar]
- 29.Arias CF, Ballesteros-Tato A, Garcia MI, Martin-Caballero J, Flores JM, Martinez A, Balomenos D. p21CIP1/WAF1 controls proliferation of activated/memory T cells and affects homeostasis and memory T cell responses. J Immunol. 2007;178:2296–306. doi: 10.4049/jimmunol.178.4.2296. [DOI] [PubMed] [Google Scholar]
- 30.Waga S, Li R, Stillman B. p53-induced p21 controls DNA replication. Leukemia. 1997;11(Suppl. 3):321–3. [PubMed] [Google Scholar]
- 31.Li R, Waga S, Hannon GJ, Beach D, Stillman B. Differential effects by the p21 CDK inhibitor on PCNA-dependent DNA replication and repair. Nature. 1994;371:534–7. doi: 10.1038/371534a0. [DOI] [PubMed] [Google Scholar]
- 32.Kurki P, Lotz M, Ogata K, Tan EM. Proliferating cell nuclear antigen (PCNA)/cyclin in activated human T lymphocytes. J Immunol. 1987;138:4114–20. [PubMed] [Google Scholar]
- 33.Rincon M. MAP-kinase signaling pathways in T cells. Curr Opin Immunol. 2001;13:339–45. doi: 10.1016/s0952-7915(00)00224-7. [DOI] [PubMed] [Google Scholar]
- 34.Derijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–37. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 35.Li W, Whaley CD, Mondino A, Mueller DL. Blocked signal transduction to the ERK and JNK protein kinases in anergic CD4+ T cells. Science. 1996;271:1272–5. doi: 10.1126/science.271.5253.1272. [DOI] [PubMed] [Google Scholar]
- 36.Matsuda S, Moriguchi T, Koyasu S, Nishida E. T lymphocyte activation signals for interleukin-2 production involve activation of MKK6-p38 and MKK7-SAPK/JNK signaling pathways sensitive to cyclosporin A. J Biol Chem. 1998;273:12378–82. doi: 10.1074/jbc.273.20.12378. [DOI] [PubMed] [Google Scholar]
- 37.Chen CY, Del Gatto-Konczak F, Wu Z, Karin M. Stabilization of interleukin-2 mRNA by the c-Jun NH2-terminal kinase pathway. Science. 1998;280:945–9. doi: 10.1126/science.280.5371.1945. [DOI] [PubMed] [Google Scholar]
- 38.Rowell EA, Wang L, Hancock WW, Wells AD. The cyclin-dependent kinase inhibitor p27Kip1 is required for transplantation tolerance induced by costimulatory blockade. J Immunol. 2006;177:5169–76. doi: 10.4049/jimmunol.177.8.5169. [DOI] [PubMed] [Google Scholar]
- 39.Powell JD, Bruniquel D, Schwartz RH. TCR engagement in the absence of cell cycle progression leads to T cell anergy independent of p27(Kip1) Eur J Immunol. 2001;31:3737–46. doi: 10.1002/1521-4141(200112)31:12<3737::aid-immu3737>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 40.Finzer P, Kuntzen C, Soto U, zur HH, Rosl F. Inhibitors of histone deacetylase arrest cell cycle and induce apoptosis in cervical carcinoma cells circumventing human papillomavirus oncogene expression. Oncogene. 2001;20:4768–76. doi: 10.1038/sj.onc.1204652. [DOI] [PubMed] [Google Scholar]
- 41.Reddy P, Sun Y, Toubai T, et al. Histone deacetylase inhibition modulates indoleamine 2,3-dioxygenase-dependent DC functions and regulates experimental graft-versus-host disease in mice. J Clin Invest. 2008;118:2562–73. doi: 10.1172/JCI34712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tao R, de Zoeten EF, Ozkaynak E, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 43.Chen D, Heath V, O’Garra A, Johnston J, McMahon M. Sustained activation of the raf-MEK-ERK pathway elicits cytokine unresponsiveness in T cells. J Immunol. 1999;163:5796–805. [PubMed] [Google Scholar]
- 44.Goulvestre C, Chereau C, Nicco C, Mouthon L, Weill B, Batteux F. A mimic of p21WAF1/CIP1 ameliorates murine lupus. J Immunol. 2005;175:6959–67. doi: 10.4049/jimmunol.175.10.6959. [DOI] [PubMed] [Google Scholar]
- 45.Balomenos D, Martin-Caballero J, Garcia MI, Prieto I, Flores JM, Serrano M, Martinez-A C. The cell cycle inhibitor p21 controls T cell proliferation and sex-linked lupus development. Nat Med. 2000;6:171–6. doi: 10.1038/72272. [DOI] [PubMed] [Google Scholar]