Abstract
Biomarkers are urgently needed for the diagnosis and monitoring of disease progression in Parkinson’s disease. Both DJ-1 and α-synuclein, two proteins critically involved in Parkinson’s disease pathogenesis, have been tested as disease biomarkers in several recent studies with inconsistent results. These have been largely due to variation in the protein species detected by different antibodies, limited numbers of patients in some studies, or inadequate control of several important variables. In this study, the nature of DJ-1 and α-synuclein in human cerebrospinal fluid was studied by a combination of western blotting, gel filtration and mass spectrometry. Sensitive and quantitative Luminex assays detecting most, if not all, species of DJ-1 and α-synuclein in human cerebrospinal fluid were established. Cerebrospinal fluid concentrations of DJ-1 and α-synuclein from 117 patients with Parkinson’s disease, 132 healthy individuals and 50 patients with Alzheimer’s disease were analysed using newly developed, highly sensitive Luminex technology while controlling for several major confounders. A total of 299 individuals and 389 samples were analysed. The results showed that cerebrospinal fluid DJ-1 and α-synuclein levels were dependent on age and influenced by the extent of blood contamination in cerebrospinal fluid. Both DJ-1 and α-synuclein levels were decreased in Parkinson’s patients versus controls or Alzheimer’s patients when blood contamination was controlled for. In the population aged ≥65 years, when cut-off values of 40 and 0.5 ng/ml were chosen for DJ-1 and α-synuclein, respectively, the sensitivity and specificity for patients with Parkinson’s disease versus controls were 90 and 70% for DJ-1, and 92 and 58% for α-synuclein. A combination of the two markers did not enhance the test performance. There was no association between DJ-1 or α-synuclein and the severity of Parkinson’s disease. Taken together, this represents the largest scale study for DJ-1 or α-synuclein in human cerebrospinal fluid so far, while using newly established sensitive Luminex assays, with controls for multiple variables. We have demonstrated that total DJ-1 and α-synuclein in human cerebrospinal fluid are helpful diagnostic markers for Parkinson’s disease, if variables such as blood contamination and age are taken into consideration.
Keywords: cerebrospinal fluid, Parkinson’s disease, biomarker, DJ-1, α-synuclein
Introduction
The diagnosis of Parkinson’s disease, a progressive movement disorder, can be difficult, particularly in early stages, because its symptoms overlap with other diseases e.g. essential tremor, multiple system atrophy and progressive supranuclear palsy (Galvin et al., 2001; Burn and Lees, 2002). Additionally, other than limited clinical and neuroimaging evaluation, which is typically expensive, there is no established objective and/or efficient method to follow Parkinson’s disease progression or evaluate the efficacy of experimental therapies.
Gene mutations of α-synuclein lead to familial Parkinson’s disease and its protein product is intimately associated with the pathogenesis of Parkinson’s disease (familial or sporadic) (Lucking and Brice, 2000; Braak et al., 2003). DJ-1, another gene product that has been linked to both familial and sporadic Parkinson’s disease, appears to be related to oxidative stress, a critical process related to Parkinson’s disease (Choi et al., 2006). Hence, DJ-1 and α-synuclein have been considered leading candidates as possible Parkinson’s disease biomarkers in human cerebrospinal fluid (CSF) as well as in plasma or serum. A previous study using western blotting suggested that CSF DJ-1 levels in Parkinson’s disease were higher than those in non-Parkinson’s disease controls (Waragai et al., 2006) whereas results for serum DJ-1 levels in Parkinson’s disease were inconsistent, with experiments showing no change or increased levels (Waragai et al., 2007; Maita et al., 2008). Similarly, several studies measuring CSF α-synuclein levels in Parkinson’s disease have yielded conflicting results. Both Mollenhauer et al. (2008) and Tokuda et al. (2006) have reported that α-synuclein was decreased in Parkinson’s disease, compared with the control group. However, Öhrfelt et al. (2009) showed that there was no significant difference between the levels of α-synuclein in Parkinson’s disease and controls. The disparity among these studies probably resulted from several factors, including variation in antibodies that might detect different species of DJ-1 or α-synuclein, limited numbers of patients in some investigations [e.g. only 15 cases of Parkinson’s disease in one report (Öhrfelt et al., 2009)], a less than rigorous quantitative method, or inadequate control for important confounding factors, particularly blood contamination in CSF.
To address problems of study design and issues such as blood contamination, as well as to clarify the utility of DJ-1 and α-synuclein in CSF as biomarkers of Parkinson’s disease diagnosis and/or severity, we established a new robust technology for DJ-1 and α-synuclein quantitation. The method is a bead-based flow cytometric assay, commonly known as Luminex, which is usually with much higher sensitivity, throughput and efficiency (Vignali 2000; Dupont et al., 2005) when compared to enzyme-linked immunosorbent assay (ELISA) or western blotting. Furthermore, several factors (i.e. blood contamination, rostrocaudal gradient within CSF, gender- or age-dependence) were evaluated for their effects on CSF DJ-1 and α-synuclein levels. Finally, diagnostic values for DJ-1 and α-synuclein were determined, alone and in combination, in a large cohort of patients with Parkinson’s disease at various stages, as compared to patients with Alzheimer’s disease and healthy controls.
Materials and methods
Participants
The Human Subject Institutional Review Boards of Baylor College of Medicine, Oregon Health and Science University, the University of California at San Diego, VA Puget Sound Health Care System at Seattle and the University of Washington approved this study. All individuals provided informed consent and underwent evaluation that consisted of medical history, physical and neurological examinations, laboratory tests and neuropsychological assessment. Laboratory evaluation included complete blood count; serum electrolytes, blood urea nitrogen, creatinine, glucose, vitamin B12 and thyroid stimulating hormone; all results were within normal limits. The inclusion and exclusion criteria for normal controls and patients with Alzheimer’s disease or Parkinson’s disease are provided in the online Supplementary methods. Demographic information is listed in Table 1 for all subjects/patients.
Table 1.
Summary of demographics and CSF DJ-1 and α-synuclein values of donors
| Control |
Parkinson’s disease stage (Hoehn and Yahr's Scale) |
Alzheimer’s disease | |||||
|---|---|---|---|---|---|---|---|
| I | II | III | IV | Total | |||
| Number of cases | 132 | 21 | 38 | 51 | 7 | 117 | 50 |
| Sex (F/M) | 61/71 | 7/14 | 7/31 | 14/37 | 2/5 | 30/87 | 19/31 |
| Age (years) | |||||||
| Mean±SD | 58.8 ± 17.8 | 60.0 ± 9.1 | 59.2 ± 11.1 | 67.0 ± 9.1 | 72.7 ± 9.8 | 63.7 ± 10.6 | 68.1 ± 9.5 |
| Range | 21–90 | 42–75 | 37–83 | 46–84 | 58–83 | 37–84 | 51–86 |
| Duration of disease (years) | |||||||
| Mean ± SD | – | 3.2 ± 2.5 | 8.7 ± 6.4 | 8.9 ± 6.8 | 15.4 ± 3.4 | 8.1 ± 6.5 | – |
| Range | 0–8 | 1–27 | 1–42 | 12–20 | 0–42 | ||
| Cases of drug treatmenta | – | 10:5:1:5 | 24:7:1:6 | 43:2:1:5 | 7:0:0:0 | 84:14:3:16 | – |
| CSF total protein (mg/dl) | |||||||
| Mean ± SD | 41.7 ± 11.7 | 42.1 ± 15.3 | 49.0 ± 30.7 | 44.3 ± 15.1 | 50.6 ± 17.6 | 45.8 ± 21.4 | 52.1 ± 29.0 |
| Range | 22–86 | 25–80 | 17–175 | 22–102 | 31–75 | 17–175 | 18–189 |
| CSF haemoglobin (ng/ml) | |||||||
| Mean ± SD | 219 ± 784 | 275 ± 1180 | 1231 ± 2460 | 680 ± 1551 | 1441 ± 2122 | 842 ± 1905 | 631 ± 1457 |
| Range | 0–6493 | 0–5425 | 0–9271 | 0–5275 | 0–4956 | 0–9271 | 0–6723 |
| CSF DJ-1 (ng/ml; all cases) | |||||||
| Mean ± SD | 36.7 ± 15.3 | 27.6 ± 7.1 | 30.6 ± 9.3 | 30.7 ± 9.1 | 32.5 ± 8.0 | 30.4 ± 8.6 | 43.1 ± 15.4 |
| Range | 11.1–97.6 | 8.9–38.4 | 14.5–55.5 | 13–50.7 | 21.8–42.5 | 8.9–55.4 | 14.2–80.4 |
| CSF DJ-1 (ng/ml; cases with haemoglobin <200 ng/ml) | |||||||
| Mean ± SD | 38.2 ± 15.2 | 27.1 ± 6.8 | 28.7 ± 7.2 | 28.5 ± 8.0 | 29.9 ± 11.1 | 28.4 ± 7.4 | 41.1 ± 15.5 |
| Range | 11.1–97.6 | 8.9–35.1 | 14.5–47.0 | 13–49.8 | 21.8–42.5 | 8.9–49.8 | 14.2–72.2 |
| CSF α-synuclein (ng/ml; all cases) | |||||||
| Mean ± SD | 0.47 ± 0.31 | 0.43 ± 0.40 | 0.56 ± 0.47 | 0.48 ± 0.31 | 0.47 ± 0.24 | 0.50 ± 0.38 | 0.67 ± 0.46 |
| Range | 0.14–4.32 | 0.2–2.13 | 0.16–2.45 | 0.14–1.9 | 0.21–0.90 | 0.14–2.45 | 0.27–3.16 |
| CSF α-synuclein (ng/ml; cases with haemoglobin <200 ng/ml) | |||||||
| Mean ± SD | 0.47 ± 0.16 | 0.34 ± 0.09 | 0.38 ± 0.11 | 0.37 ± 0.09 | 0.31 ± 0.12 | 0.36 ± 0.10 | 0.55 ± 0.15 |
| Range | 0.22–1.09 | 0.20–0.50 | 0.16–0.79 | 0.14–0.58 | 0.21–0.45 | 0.14–0.79 | 0.27–0.9 |
a Number of patients with Parkinson’s disease who were treated with carbidopa/levodopa alone or together with other anti-parkinsonism drugs versus those treated with dopamine agonists but not levodopa versus those treated with other anti-Parkinson’s disease medications (e.g. monoamine oxidase B inhibitors and amantadine) only versus those not treated with any anti-parkinsonism drugs (no Parkinson’s disease med) when the CSF samples were obtained.
Collection of CSF and quality control
All CSF samples were obtained by lumbar puncture in the morning. Up to 25 ml CSF was taken from each subject, with every 5 ml pooled into one fraction. These were aliquoted (0.5 ml) into polypropylene cryotubes, flash frozen and stored at −80°C. To study any potential variations arising from a rostro-caudal gradient, the fractions of the control group (45 individuals) in <10th ml, 10th–15th ml and 15th–20th ml were assessed. To identify and characterize the proteins of interest in human CSF and to develop assays, reference CSF was obtained from the clinical laboratory at Harborview Medical Centre (Seattle, WA). See the Supplementary methods for details.
Quantitative western blotting
Proteins in 500 µl pooled reference CSF sample was precipitated with 20% trichloroacetic acid, washed with six volumes of cold acetone, dissolved with a urea/loading buffer (6 M urea, 2% sodium dodecyl sulphate, 10% glycerol, 62.5 mM Tris, pH 6.8) and loaded onto 8–16% sodium dodecyl sulphate polyacrylamide gels for electrophoresis. Recombinant human DJ-1 (Covance, Berkeley, CA, USA) and α-synuclein (rPeptide, Athens, GA, USA) proteins were used as standards, respectively. Primary antibodies used were mouse monoclonal anti-α-synuclein (BD Biosciences, San Diego, CA, USA; 1:1000) and rabbit polyclonal anti-PARK7 (DJ-1) (Novus Biologicals, Littleton, CO, USA; 1:2000). Blots were scanned and band intensities were quantified by densitometric analysis using a VersoDoc 3000 imaging system (Bio-Rad, Hercules, CA, USA) and the Quantity One software (version 4.6.3, Bio-Rad).
In-gel digestion and mass spectrometry
Proteins from pooled CSF samples were precipitated with 20% trichloroacetic acid, and ∼300 μg of proteins for each lane were separated on an 8–16% tris–glycine gel, and stained using Coomassie blue G-250. Lanes of protein matching the corresponding western blot were excised from the gel and destained. In-gel trypsin digestion was then carried out for 15 h using a standard protocol (Hong et al., 2009).
To validate the presence of DJ-1 and α-synuclein in human CSF, both proteins were identified by a 4800 matrix-assisted laser desorption ionization-time of flight-time of flight-tandem mass spectrometer (Applied Biosystems, Foster City, USA). Details related to mass spectrometry-based protein identification can be found in the Supplementary methods.
Size-exclusion chromatography
Five millilitres of CSF sample was loaded onto a HiPrep 16/60 Sephacryl S-100 high resolution column (Amersham Biosciences, Piscataway, NJ, USA) and eluted with 160 mM ammonium acetate (pH 7.4) at a flow rate of 0.25 ml/min using a BioLogic Duo-Flow fast protein liquid chromatography system (Bio-Rad). Absorbance of the eluted material was monitored at 280 nm. Detailed method can be found in the Supplementary methods.
Development of bead-based Luminex assay
Covalent coupling of capturing antibodies to LiquiChip activated beads
LiquiChip Activated Beads (Qiagen, Valencia, CA, USA) were chemically coupled with a rabbit anti-α-synuclein antibody [(Lindersson et al., 2004; Fjorback et al., 2007: used in the final CSF assay), a mouse anti-α-synuclein antibody (LB509) (Covance), a mouse anti-α-synuclein antibody (211) (Invitrogen, Carlsbad, CA, USA) and a rabbit anti-DJ-1 antibody (NB100-483, Novus; used in the final CSF assay) or a mouse anti-DJ-1 antibody (SIG-39830, Covance) according to the manufacturer’s protocol with minor modifications. Briefly, 10–20 µg of antibodies in 50 mM 2-(N-morpholino)ethanesulfonic acid (pH 6.5) was added to 100 µl of LiquiChip Activated Beads, and then agitated at 900 rpm on a plate shaker overnight at 4°C and for another 2 h at room temperature in the dark. Coupled beads were collected, resuspended in 1% bovine serum albumin/phosphate buffered saline and stored at 4°C before use. The concentration of beads in the suspension was determined by using the LiquiChip Reader. In-process control of antibody-coupling homogeneities was done by determining (i) the coefficient of variation for counting of 100 beads from the same region; and (ii) the signal-to-noise ratio.
Bead-based Luminex analyses
For the functional assay, a sandwich-type of assay was performed. All incubations were performed in 96 well MultiScreen Filter plates (Millipore, Billerica, MA, USA) in the dark at ambient temperature. The plate was pre-wetted with a washing buffer (0.1% Tween-20 and 0.1% triton X-100 in phosphate buffered saline, pH 7.4) before use and washed two to four times between each step. The capturing antibody-coupled beads were resuspended and mixed thoroughly by vortexing and brief sonication and were then added (∼3000 beads per well) to the pre-wetted plate. For α-synuclein detection, 25 µl CSF was treated with 25 µl 2× radioimmunoprecipitation assay buffer for 0.5 h, and diluted with 50 µl assay diluent (0.1% bovine serum albumin/phosphate buffered saline); for DJ-1 detection, 50 µl CSF was diluted with 50 µl assay diluent directly. CSF samples were then loaded (100 µl/well) and incubated for 3 h at 600 rpm on a plate shaker. After the sample solution was removed, the detecting antibodies in assay diluent [1 ng/μl; biotinylated anti-human α-synuclein antibody (R&D systems, Minneapolis, MN, USA) or biotinylated anti-human DJ-1 antibody (R&D systems)] were added at 100 μl/well and incubated for 3 h on a plate shaker (600 rpm). After the detecting antibody solution was removed, diluted streptavidin-R-PE (1 ng/μl; Qiagen) in assay diluent was added (100 μl/well) and incubated for 0.5 h on a plate shaker (600 rpm). The plate was then washed and 100 µl of washing buffer was added to each well. After a brief incubation on a plate shaker, the plate was read on a LiquiChip Luminex 200™ Workstation (Qiagen). For each set of microspheres, 100 beads were analysed and median values were reported. A series of recombinant, full-length human α-synuclein (rPeptide) or DJ-1 (Covance) standards diluted in assay diluent solution were run in parallel. 0.1% bovine serum albumin/phosphate buffered saline was used as a blank, and as internal standard, aliquots of two previously prepared CSF specimens with known target-protein concentrations were included in each plate. As controls, aliquots of frozen adult human and mouse brain tissues were homogenized and processed as described (Liu et al., 2008).
Details on curve fitting and value reading can be found in the Supplementary methods.
Haemoglobin test
The haemoglobin levels in CSF samples were measured using a Human haemoglobin ELISA Quantitation Kit from Bethyl Lab Inc (Montgomery, TX, USA) according to the manufacturer’s instructions. This was done to establish an index of the degree of red blood cell contamination of CSF.
Statistical analysis
All analyses were performed with the Statistical Package for the Social Sciences for Windows (version 12.0.1, SPSS Inc., Chicago, IL, USA). To compare demographic, clinical and CSF baseline data between groups, a non-parametric Kruskal–Wallis ANOVA was used, followed by a Mann–Whitney U-test for continuous variables. Additionally, Cox proportional hazards models were employed to separately estimate the effects of different baseline risk factors on the relative possibility of changing of analyte (DJ-1 or α-synuclein) levels. The analyses were done with and without adjustment for potential confounding of the baseline variables—i.e. CSF fraction, age, gender, or haemoglobin concentration. A receiver operating characteristic (ROC) curve was used to calculate the relationship between sensitivity and specificity for the disease group versus healthy controls, and hence evaluate the diagnostic performance of the analytes. The ‘optimum’ cut-off value from the ROC curve is the point at which the sum of sensitivity and specificity is maximal.
Results
Characterization of DJ-1 and α-synuclein in human CSF
By western blotting under denatured conditions, both DJ-1 and α-synuclein were observed in the pooled reference human CSF (Supplementary Figs S1A and S3A), consistent with previous studies (Borghi et al., 2000; Waragai et al., 2006). The presence of both DJ-1 and α-synuclein in human CSF was also confirmed by in-gel digestion and mass spectrometry (Supplementary Fig. S1B and Table S1). Mass spectrometry data for α-synuclein were similar to a previous study (Mollenhauer et al., 2008) and thus not shown. To our knowledge, this is the first time that DJ-1 is confirmed in human CSF by mass spectrometry. The nature of CSF DJ-1 and α-synuclein under native conditions was further studied by size exclusion chromatography (Supplementary Figs S1C, S2 and S3B). It appeared that almost all DJ-1 signals, as assessed by western blotting, came from the size exclusion chromatography fractions corresponding to molecular weights of 25–30 kDa, while most α-synuclein species present in native CSF corresponded to molecular weights of 45 kDa with some signals detected beyond 45 kDa. It is unclear, however, whether these high molecular α-synuclein species were oligomers or monomers conjugated with other macromolecules. The concentrations of DJ-1 and α-synuclein in CSF were estimated to be 90 ± 21 and 1.36 ± 0.35 ng/ml, respectively, using serially diluted recombinant human DJ-1 or α-synuclein proteins as standards (Supplementary Figs S1A and S3A). These results were largely confirmed by Luminex assays (Supplementary Figs S1D and S3C), to be discussed later.
Establishment of the Luminex assays
To quantify DJ-1 and α-synuclein levels in human CSF reliably, a specific and sensitive novel Luminex method was developed. Luminex assays mainly differ from conventional ELISA in that they use polystyrene microspheres in suspension (instead of a microplate well) to immobilize a capturing antibody.
The sensitivity of the established DJ-1 Luminex ranged as low as 0.05 ng/ml of human DJ-1, which is 20-fold higher than a previously reported ELISA (Maita et al., 2008). In addition, the CSF DJ-1 signal-to-background ratio was 66, an over 7-fold improvement over the prior ELISA (Maita et al., 2008). Finally, the Luminex assay demonstrated low day-to-day as well as plate-to-plate signal variability (<10% with >60 plates analysed), with high signal reproducibility and linear performance in the low pg range (Supplementary Fig. S4A). The assay specificity was confirmed by human CSF with DJ-1 depleted using a mouse anti-DJ-1 antibody (Covance) that was not used in the Luminex assay and using wild-type versus DJ-1 knock-out mouse specimens (Supplementary Fig. S4B). Under the optimized conditions (Supplementary Fig. S4C), DJ-1 concentration of the reference CSF was around 78 ng/ml, comparable to the values determined by immunoblotting (Supplementary Fig. S1A). Furthermore, as mentioned, DJ-1 concentrations in each size exclusion chromatography fraction were also measured by the Luminex assay, producing results similar to those obtained by western blotting (Supplementary Fig. S1C and D). The assay’s accuracy was also measured by spiking human recombinant DJ-1 proteins in CSF and the recovery was about 81% (Supplementary Fig. S4D).
For the established α-synuclein Luminex assay, the sensitivity ranged as low as 0.009 ng/ml of human α-synuclein, which is 11-fold higher than a previously reported ELISA (Mollenhauer et al., 2008). In addition, the CSF α-synuclein signal-to-background ratio was 70, an over 19-fold increase compared to the prior ELISA (Mollenhauer et al., 2008). The α-synuclein Luminex assay also demonstrated low day-to-day as well as plate-to-plate signal variability (<5% with >80 plates analysed), with high signal reproducibility and linear performance in the low pg range (Supplementary Fig. S5A). The specificity of the signal was confirmed by using wild-type versus α-synuclein knock-out mouse specimens (Supplementary Fig. S5B). Under the optimized conditions (Supplementary Fig. S5C), α-synuclein concentration of the reference CSF was around 1.3 ng/ml, which was in excellent agreement with the immunoblotting results of 1.36 ± 0.35 ng/ml (Supplementary Fig. S3A). Furthermore, when the α-synuclein signal was determined in each size exclusion chromatography fraction with the Luminex assay, the pattern of α-synuclein levels was consistent with that found by western blotting (Supplementary Fig. S3B and C). The assay accuracy was also measured by spiking human recombinant α-synuclein proteins in CSF and the recovery was about 93% (Supplementary Fig. S5D).
For both DJ-1 and α-synuclein, no apparent alterations were seen when the reference CSF samples were frozen and thawed twice or stored at −80°C for up to 3 months (Supplementary Figs S4C and S5C).
Effects of blood contamination, rostrocaudal gradient, age and gender on CSF DJ-1 and α-synuclein levels
Blood contamination
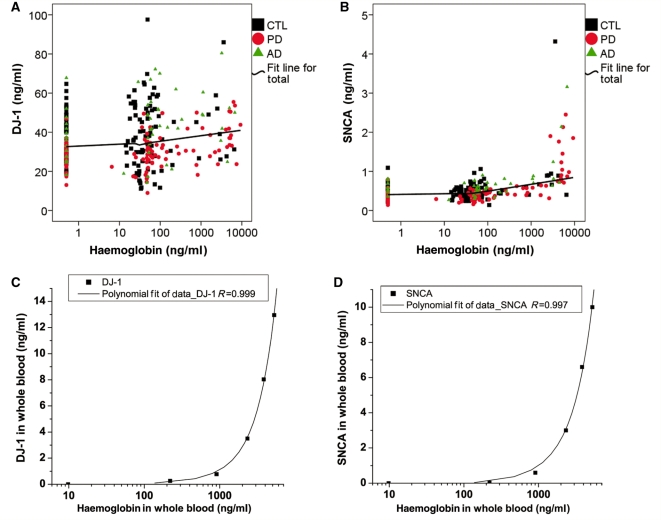
Because the levels of DJ-1 and α-synuclein stored in human blood cells are much higher than in CSF (Supplementary Fig. S6), it is expected that a minimal contamination of blood in CSF could have a significant impact on CSF levels of either DJ-1 or α-synuclein. To control for this variable, haemoglobin level was evaluated in all CSF samples. As seen in Fig. 1A and B, the range of haemoglobin levels in this cohort (controls and diseased subjects) varied between 0 and ∼9000 ng/ml, with a majority of samples (81.9%) having <200 ng/ml. Furthermore, both DJ-1 and α-synuclein levels increased substantially when haemoglobin level was >200 ng/ml. More importantly, when the haemoglobin level of CSF was <200 ng/ml, the enhancement of DJ-1 and α-synuclein by spiking lysed whole blood into CSF was around 0.2 and 0.01 ng/ml, respectively, which constituted no more than 1% of DJ-1 or α-synuclein values measured in the reference CSF sample. This result was further confirmed by spiking variable amounts of whole blood into assay diluent (Fig 1C and D). Hence, in addition to analysing all the samples, to minimize the influence of blood contamination in the results, additional analysis was also performed in the cases with haemoglobin levels <200 ng/ml.
Figure 1.
The effects of blood contamination on DJ-1 or α-synuclein levels in CSF. CSF DJ-1 (A) and α-synuclein (SNCA) (B) levels were measured in individual control (CTL) and disease (Alzheimer’s disease, AD; Parkinson’s disease, PD) samples by Luminex, while the haemoglobin levels were measured using an ELISA kit. The haemoglobin variable was log transformed (log10) for the correlation study due to its positively skewed distribution. A fit line is shown for all the samples tested. A positive correlation between haemoglobin and DJ-1 (C) or α-synuclein (D) levels was also observed in whole blood serially diluted in the Luminex assay diluent (0.1% bovine serum albumin/phosphate buffered saline).
Rostrocaudal gradient of DJ-1 and α-synuclein
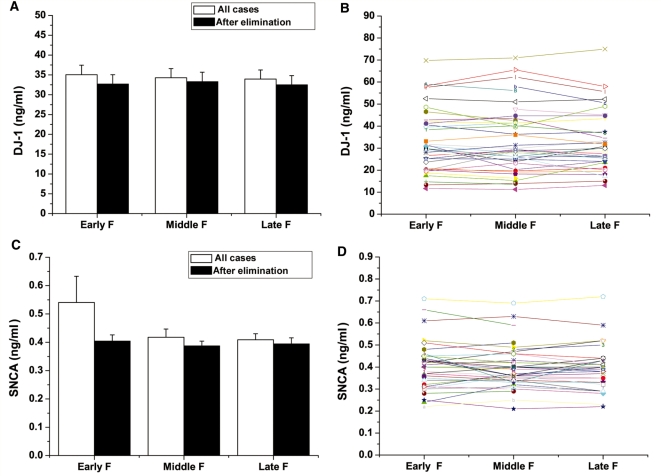
To assess whether the rostrocaudal gradient contributed to variation of DJ-1 and α-synuclein levels in CSF, the analyte levels in three different fractions (i.e. <10th ml, 10–15th ml and 15–20th ml) of control individuals were compared. Although there was a trend for α-synuclein to increase in lower fractions (i.e. the fractions similar to those obtained clinically for diagnostic purpose), no statistically significant difference was observed among sequential fractions, with or without eliminating the samples with high haemoglobin levels (Fig. 2A and C). Furthermore, as shown in Fig. 2B and D, no significant variation was observed in different fractions when an individual subject was analysed.
Figure 2.
Rostrocaudal gradient of CSF and DJ-1 or α-synuclein levels in CSF. The early CSF fractions (<10th ml, Early F), middle fractions (10–15th ml, Middle F) and late fractions (15–20th ml, Late F) in the control group were assessed. Samples included young (mean 29.1, range 21–40, n = 15), middle age (mean 59.8, range 55–64, n = 15) and old (mean 78.8, range 75–83, n = 15) controls. No significant difference was observed in different fractions when the average DJ-1 (A) or α-synuclein (SNCA) (C) levels were compared, with or without eliminating the samples with >200 ng/ml haemoglobin levels. The levels of DJ-1 (B) or α-synuclein (D) in different CSF fractions in individual samples were also compared after cases with high haemoglobin levels were omitted. Each symbol represented one participant and the three fractions from the same participant were linked. No significant difference among fractions was observed in individual samples.
Gender and age dependence
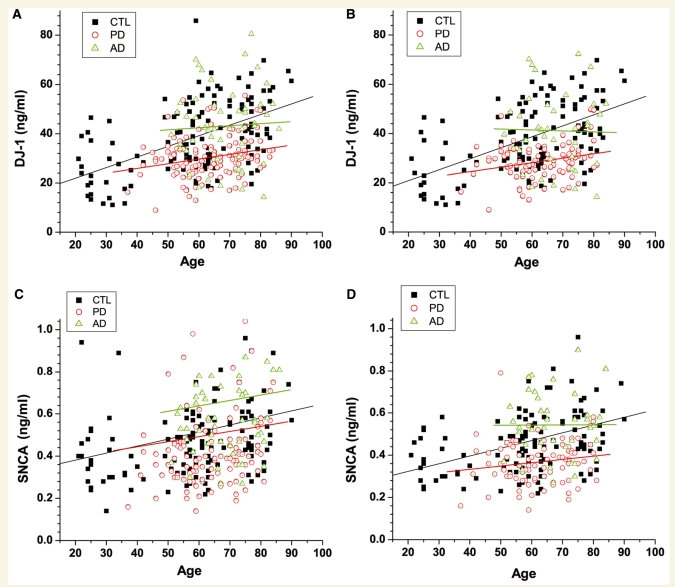
Given that no apparent rostrocaudal gradient was observed for either DJ-1 or α-synuclein, the age and gender dependence were analysed with fractions below the 10th ml, which is typically obtained clinically. The results demonstrated that both DJ-1 and α-synuclein levels in the CSF were significantly increased as a function of age, especially in normal controls (Fig. 3). The age-dependence became weaker in patients with Parkinson’s disease. More specifically, in the control group, after eliminating the samples with high haemoglobin levels, linear regression analysis revealed R = 0.51 (P < 0.0001) and R = 0.40 (P < 0.0001) for DJ-1 and α-synuclein, respectively. In contrast, R = 0.26 (P = 0.02) and R = 0.16 (P = 0.14) were obtained for DJ-1 and α-synuclein, respectively, in patients with Parkinson’s disease. Similarly, the age-dependence in patients with Alzheimer’s disease was R = –0.02 (P = 0.89) and R = 0.00 (P = 0.98) for DJ-1 and α-synuclein, respectively. This observation will become exceedingly significant when DJ-1 and α-synuclein are used as diagnostic markers in later sections (Fig. 5 and Table 2).
Figure 3.
Age dependence of DJ-1 or α-synuclein levels in CSF. CSF DJ-1 (A, B) and α-synuclein (SNCA) (C, D) levels were measured in individual control (CTL) and disease (Alzheimer’s disease, AD; Parkinson’s disease, PD) samples by Luminex. Data shown are before (A, C) or after (B, D) elimination of cases with high haemoglobin levels. The correlation coefficient (R) and P-value of linear regression for each group were as the following: DJ-1 in CTL, R = 0.50 (P < 0.0001) and R = 0.51 (P < 0.0001), for before and after elimination of contaminated cases, respectively; DJ-1 in Parkinson’s disease, R = 0.23 (P = 0.01) and R = 0.26 (P = 0.02), for before and after elimination of contaminated cases, respectively; DJ-1 in Alzheimer’s disease, R = 0.05 (P = 0.72) and R =−0.02 (P = 0.89), for before and after elimination of contaminated cases, respectively. α-synuclein in the control, R = 0.16 (P = 0.07) and R = 0.40 (P < 0.0001), for before and after elimination of contaminated cases, respectively; α-synuclein in Parkinson’s disease, R = 0.07 (P = 0.47) and R = 0.16 (P = 0.14), for before and after elimination of contaminated cases, respectively; α-synuclein in Alzheimer’s disease, R = 0.05 (P = 0.71) and R = 0.00 (P = 0.98) for before and after elimination of contaminated cases, respectively.
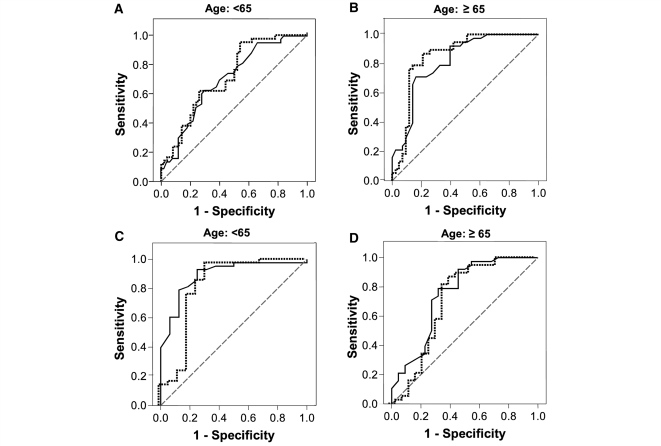
Figure 5.
ROC curves to evaluate the CSF DJ-1 and/or α-synuclein as Parkinson’s disease biomarkers. (A, B) Combined ROC curves for the DJ-1 (dotted line) and α-synuclein (solid line) of control versus Parkinson’s disease with donor’s age <65 (A) or ≥65 years (B). (C, D) Combined ROC curves for the DJ-1 (dotted line) and α-synuclein (solid line) of Parkinson’s disease versus Alzheimer’s disease with donor’s age <65 (C) or ≥65 years (D).
Table 2.
Summary of ROC results
|
All cases (n = 91 control; 80 Parkinson’s disease; 38 Alzheimer’s disease) |
Cases with age <65 (n = 48 control; 42 Parkinson’s disease; 16 Alzheimer’s disease) |
Cases with age ≥65 (n = 43 control; 38 Parkinson’s disease; 22 Alzheimer’s disease) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC | P-value | Cut-off value (ng/ml) | Sens (%) | Spec (%) | AUC | P-value | Cut-off value (ng/ml) | Sens (%) | Spec (%) | AUC | P-value | Cut-offvalue (ng/ml) | Sens (%) | Spec (%) | |
| Parkinson’s disease versus control | |||||||||||||||
| DJ-1 | 0.77 | <0.001 | 40 | 94 | 50 | 0.72 | <0.001 | 40 | 98 | 34 | 0.85 | <0.001 | 40 | 90 | 70 |
| α-synuclein | 0.73 | <0.001 | 0.50 | 93 | 39 | 0.62 | 0.003 | 0.50 | 93 | 22 | 0.82 | <0.001 | 0.50 | 92 | 58 |
| Both | 0.77 | <0.001 | 0.26 | 95 | 42 | 0.72 | <0.001 | 0.26 | 98 | 30 | 0.87 | <0.001 | 0.26 | 92 | 56 |
| Parkinson’s disease versus Alzheimer’s disease | |||||||||||||||
| DJ-1 | 0.75 | <0.001 | 40 | 94 | 53 | 0.81 | <0.001 | 40 | 98 | 50 | 0.69 | 0.017 | 40 | 90 | 55 |
| α-synuclein | 0.83 | <0.001 | 0.50 | 93 | 63 | 0.89 | <0.001 | 0.50 | 93 | 75 | 0.75 | 0.001 | 0.50 | 92 | 55 |
| Both | 0.83 | <0.001 | 0.40 | 95 | 50 | 0.89 | <0.001 | 0.40 | 98 | 44 | 0.75 | 0.001 | 0.40 | 92 | 55 |
AUC = area under curve; ROC = receiver operating characteristics; Sens = sensitivity; Spec = specificity. These analyses excluded subjects with CSF haemoglobin >200 ng/ml or age <50. For each of the pair wise comparisons of disease categories, ROC analysis was performed for DJ-1 and then α-synuclein. After a logistic regression analysis, a prediction score was obtained based on a linear combination of DJ-1 and log of α-synuclein. This prediction score was then used to run the third ROC analysis.
No association of DJ-1 or α-synuclein levels with gender was found, regardless of the levels of haemoglobin levels in this cohort (data not shown).
CSF DJ-1 and α-synuclein in controls and patients with Parkinson’s disease and Alzheimer’s disease
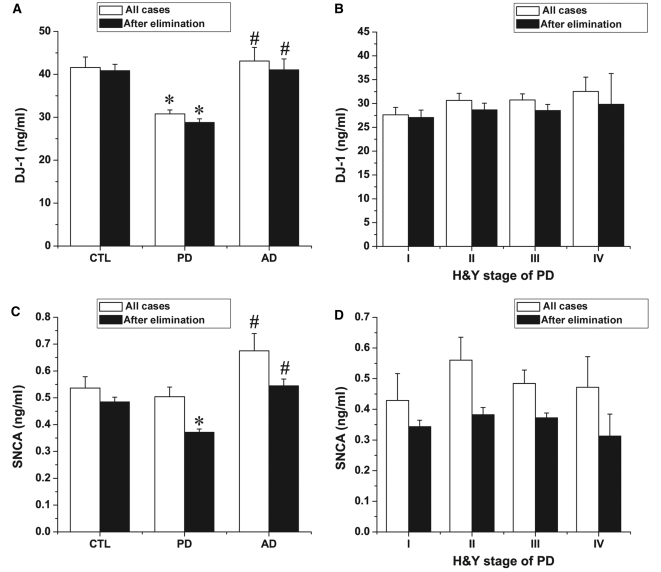
To compare the DJ-1 levels in controls and patients with Parkinson’s disease and Alzheimer’s disease, only aliquots from fractions below the 10th ml were considered. In addition, since DJ-1 levels in human CSF were dependent on age, only control cases matched with the mean as well as the range of Parkinson’s disease and Alzheimer’s disease groups were used, i.e. the subjects younger than 50 were excluded from this analysis (a total of nine cases, constituting about 7.7% of the cohort, were excluded from the Parkinson’s disease group). Another reason to eliminate Parkinson’s disease cases with younger ages relates to a potential contribution of disease causing gene mutations in these cases. Finally, there was no Alzheimer’s disease case in the age group of <50 in this study. The age range of cases included in the analysis were as follows (Supplementary Fig. S7): (i) controls, mean 66.4 years, range 50–84, n = 98; (ii) Parkinson’s disease, mean 65.3, range 50–84, n = 108; and (iii) Alzheimer’s disease, mean 68.1, range 51–86, n = 50. As shown in Fig. 4A and Table 1, when all samples were analysed (regardless of haemoglobin levels), DJ-1 levels in patients with Parkinson’s disease were significantly lower than those in controls (P = 5.53E-10) and patients with Alzheimer’s disease (P = 1.87E-9). There was no significant difference between controls and patients with Alzheimer’s disease (P = 0.56). After deleting the subjects with high haemoglobin levels (>200 ng/ml) (the mean and range of age of this subset of subjects were: control, mean 65.9, range 50–83, n = 91; Parkinson’s disease, mean 64.5, range 50–81, n = 80; Alzheimer’s disease, mean 67.4, range 51–84, n = 38), the overall conclusion did not change, although variations among the subjects became much smaller (Parkinson’s disease versus control: P = 9.86E-11; Parkinson’s disease versus Alzheimer’s disease: P = 4.15E-8; Alzheimer’s disease versus control: P = 0.94).
Figure 4.
Cross-sectional examination of CSF DJ-1 and α-synuclein by Luminex. (A, C) Quantitative Luminex analysis of CSF DJ-1 or α-synuclein (SNCA) levels in patients with Parkinson’s disease, Alzheimer’s disease and healthy controls (CTL) before and after elimination of contaminated cases (200 ng/ml haemoglobin was used as a cut-off). Only age matched samples were included. *P < 0.05 versus control group; #P < 0.05 versus Parkinson’s disease group. (B, D) Statistical analysis of CSF DJ-1 and α-synuclein levels in Hoehn and Yahr's stages of Parkinson’s disease. Data shown are mean ± SEM; cases studies in each group can be found in Table 1.
Identical analyses were also performed for α-synuclein (Fig. 4C). Before eliminating cases with high haemoglobin levels, although there was a trend for the α-synuclein level to decrease in Parkinson’s disease, no significant difference was observed between Parkinson’s disease and control groups (P = 0.56). However, a statistical significance between control and Parkinson’s disease (P = 3.04E–7) was found after deleting the samples with high haemoglobin concentrations (>200 ng/ml). As for Alzheimer’s disease, α-synuclein levels tended to increase in patients, but no statistical significance was found with or without eliminating the samples with high haemoglobin levels (P = 0.07 and P = 0.06, respectively). Remarkably, a significant difference was achieved between Parkinson’s disease and Alzheimer’s disease, regardless of whether haemoglobin level was factored in or not (P = 0.01 and P = 3.85E–11, respectively).
To determine if CSF DJ-1 and/or α-synuclein levels were altered during the course of disease progression, the CSF DJ-1 and α-synuclein were analysed according to the clinical stage of Parkinson’s disease based on Hoehn and Yahr's; scale (H-Y scores). The average levels of DJ-1 in the CSF are shown in Fig. 4B and Table 1. Statistical analysis using ANOVA with post hoc Bonferroni’s test revealed no correlation between the CSF DJ-1 level and the different stages of Parkinson’s disease either before (P = 0.47) or after (P = 0.86) eliminating cases with high haemoglobin levels. Similarly, there was no correlation between α-synuclein concentration and H-Y scores, with or without omitting the samples with high haemoglobin levels (P = 0.62 and P = 0.48, respectively) (Fig. 4D). Additionally, the values of CSF DJ-1 and α-synuclein were not correlated with disease duration of Parkinson’s disease (Supplementary Fig. S8), with or without omitting cases with high haemoglobin levels. This is not entirely surprising, given that H-Y stages, on average, are typically correlated with disease duration. Finally, there was no significant difference in DJ-1 or α-synuclein levels between different stages of Parkinson’s disease, when DJ-1 and α-synuclein levels were normalized to CSF total protein concentrations (data not shown).
The relationship between CSF DJ-1 or α-synuclein levels and Alzheimer’s disease severity, approximated by mini-mental state exam (MMSE) scores, was also examined. Among the Alzheimer’s disease cases, eight cases had an MMSE score <10, 19 cases with an MMSE score of 10–19, and 22 cases with an MMSE score of 20-26. No correlation (by linear regression) between the CSF DJ-1 or α-synuclein level and the MMSE score was found before (P = 0.41 for DJ-1, P = 0.53 for α-synuclein) or after (P = 0.81 for DJ-1, P = 0.60 for α-synuclein) cases with high haemoglobin levels were eliminated.
Evaluation of CSF DJ-1 and/or α-synuclein as Parkinson’s disease diagnostic biomarkers
To evaluate the utility of CSF DJ-1 and α-synuclein levels in discriminating cases of Parkinson’s disease from normal or Alzheimer’s disease cases, ROC curve analysis was performed. As shown in Table 2 and Fig. 5, when the cut-off values of 40 ng/ml and 0.5 ng/ml were chosen for DJ-1 and α-synuclein, respectively, the sensitivity and specificity for distinguishing Parkinson’s disease from control were 94 and 50% for DJ-1, and 93 and 39% for α-synuclein. The combination of DJ-1 and α-synuclein in CSF did not enhance the performance of discrimination significantly.
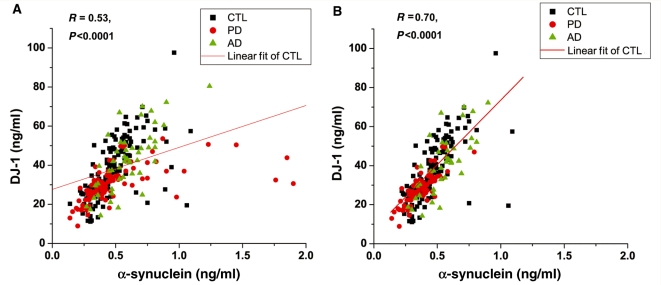
As discussed in early sections, there was a significant age-dependence of both DJ-1 and α-synuclein levels in CSF. Therefore, the diagnostic performance of DJ-1 and α-synuclein, using the same cut-off values discussed above, was further analysed based on age, i.e. those <65 and ≥65 years old. As shown in Table 2, the sensitivity and specificity of DJ-1 to segregate Parkinson’s disease from controls were 98 and 34% versus 90 and 70% for the younger and older group, respectively. Similarly the sensitivity and specificity of α-synuclein to distinguish Parkinson’s disease from controls were 93 and 22% versus 92 and 58% for the younger and older group, respectively. Again, a combination of the two markers did not enhance the performance for either marker. In fact, this outcome was expected based on a strong relationship between DJ-1 and α-synuclein values measured in each individual subject (Fig. 6).
Figure 6.
Relationship between DJ-1 and α-synuclein in CSF. Data shown are before (A) or after (B) eliminating cases containing high haemoglobin levels (200 ng/ml was used as a cut-off). The correlation coefficient and P-value of linear regression for controls are shown in each panel. CTL = control; PD = Parkinson's disease; AD = Alzheimer's disease.
For differential diagnosis between Parkinson’s disease and Alzheimer’s disease, when the cut-off values of 40 ng/ml for DJ-1 and 0.5 ng/ml for α-synuclein were chosen, based on ROC analysis, the sensitivity and specificity were 94 and 53% for DJ-1 and 93 and 63% for α-synuclein. Sensitivity and specificity varied with age, with a slightly better diagnostic discrimination in those <65 versus ≥65 years old (Table 2 and Fig. 5).
Discussion
Several major advances have been made in this investigation: (i) confirmation as well as characterization of the nature of DJ-1 and α-synuclein in human CSF by a number of methods, including mass spectrometry analysis, with the results correlating with those determined by newly established Luminex assays; (ii) determination of several factors critical to DJ-1 and α-synuclein levels in human CSF, i.e. the influence of blood contamination, rostrocaudal gradient, gender and age, as well as the effects of freeze/thaw cycles and storage time of the samples; and (iii) evaluation of the utility of DJ-1 and α-synuclein in CSF, alone and in combination, as biomarkers of Parkinson’s disease diagnosis and severity in a large cohort of patients with Parkinson’s disease at various stages, along with healthy as well as diseased controls.
Luminex assays established in this study detected most, if not all, species of DJ-1 and α-synuclein in human CSF based on its measurement of all species resolved by size exclusion chromatography. It is unclear, however, whether high molecular species of α-synuclein (as determined by size exclusion chromatography) were oligomers, monomers/oligomers conjugated with other macromolecules, or even just conformational changes of monomeric α-synuclein that gave rise to an appearance of high molecular weight species when assessed by size exclusion chromatography. Compared to controls, both DJ-1 and α-synuclein were significantly decreased in patients with Parkinson’s disease when age and blood contamination were taken into consideration. DJ-1 and α-synuclein discriminated patients with Parkinson’s patients from controls, as determined by ROC (Fig. 5 and Table 2), with a sensitivity and specificity of 90 and 70% for DJ-1 and 92 and 58% for α-synuclein, respectively, in this large cohort. However, no association between CSF DJ-1 or α-synuclein levels and disease severity was observed for patients with Parkinson’s disease.
Notably, in comparison to non-Parkinson’s disease and patients with advanced Parkinson’s disease, CSF DJ-1 level were shown to be higher in patients with early Parkinson’s disease in a previous study using western blotting (Waragai et al., 2006). The discrepancy in DJ-1 results between this study and those reported by western blotting can be attributed to several major differences between the two investigations. First, compared to western blotting, Luminex assays are more robustly quantitative with a much greater dynamic range, have a standard curve that can assess DJ-1 levels precisely, whether at low- or high-end, and have much reduced variability. Additionally, it was clear that DJ-1 levels in CSF were dependant strongly on the magnitude of blood contamination in CSF; thus it is unclear whether a significant variation seen in Waragai et al.’s study (2006) were due to the western blot assay itself or the extent of blood contamination in each sample (a variable not mentioned in the report). Finally, the current investigation is advantageous because a much larger cohort (132 normal controls versus 117 patients with Parkinson’s disease) was studied, thus allowing adequate power to resolve several factors, age-dependence in particular, which significantly influence DJ-1 levels in CSF.
The data accumulated thus far on CSF α-synuclein have been inconsistent, showing evidence for a decrease or no change of α-synuclein in patients with Parkinson’s disease compared to controls (Tokuda et al., 2006; Mollenhauer et al., 2008; Öhrfelt et al., 2009). The results on α-synuclein obtained in this investigation were largely consistent with those reported by Mollenhauer et al. (2008), i.e. a decreased α-synuclein level in Parkinson’s disease versus controls, with the exception that the value of total α-synuclein in uncontaminated CSF was lower in the current investigation (about 50% of what was reported previously). The different antibodies used in the two studies might have contributed, at least in part, to the disparity in the α-synuclein levels. Additionally, a much longer incubation period, a procedure known to increase not only the analyte signal but also non-specific background, was used when α-synuclein was captured by the antibody used in the prior ELISA assay (Mollenhauer et al., 2008). More importantly, however, it is the observation that no statistical difference was observed between patients with Parkinson’s disease and controls if the samples with high haemoglobin levels were included in the calculation. To state it differently, our results would be consistent with Öhrfelt et al.’s (2009), had we not controlled for blood contamination in α-synuclein assays.
While evaluating DJ-1 and α-synuclein in CSF is likely to help with Parkinson’s disease diagnosis, others have measured DJ-1 and α-synuclein in plasma/serum, a sample source that is much easier to access. Unfortunately, the results obtained from plasma/serum are highly variable, with experiments showing no change or increased DJ-1 levels in Parkinson’s disease (Waragai et al., 2007; Maita et al., 2008). As for α-synuclein, Lee et al. (2006) reported increased plasma α-synuclein levels in Parkinson’s disease and multiple system atrophy compared to aged-matched healthy controls using a commercially available ELISA kit, while Li et al. (2007) reported decreased plasma α-synuclein levels in Parkinson’s disease compared to normal controls measured by western blotting. In addition, El-Agnaf et al. (2006) have shown a significant increase in α-synuclein oligomers in plasma in patients with Parkinson’s disease compared with controls. Thus, the discrepancy among different investigations could be due to the variations in oligomers and/or other cross-reactive molecules detected by the ELISA technique (Li et al., 2007) or the limitation of the ELISA kit. For example, the α-synuclein values reported by Lee et al. (2006) were below the detection limit of the kit used (Fjorback et al., 2007). Finally, inadequate control of several important variables, such as the extent of haemolysis, whether in vivo or ex vivo (red blood cells are the major source of α-synuclein and DJ-1 in whole blood; unpublished data), and residual platelets (Li et al., 2002; Michell et al., 2005) in plasma, might have also contributed to the variable results. For future reference, however, measuring DJ-1/α-synuclein in CSF and plasma or serum simultaneously (with control for major variables) could obtain indices by which intrathecal production of DJ-1 and/or α-synuclein can be calculated.
Two additional important observations need to be discussed further. First, the diagnostic performance of DJ-1 was not enhanced by α-synuclein, or vice versa. This result was expected because there was a remarkably close correlation between these two values in each individual, whether in controls or diseased individuals (Fig. 6). A decrease in CSF α-synuclein has been hypothesized to be secondary to sequestration of α-synuclein in brain tissue (Westerlund et al., 2008), resembling a scenario of Aβ in patients with Alzheimer’s disease (Hansson et al., 2006). Notably, a recent study showed that the expression of DJ-1 in the human brain was also significantly reduced in sporadic Parkinson’s disease (Nural et al., 2009). Thus, given the protective role of DJ-1 (e.g. anti-oxidative stress and anti-apoptosis) and its tendency to increase in healthy subjects, it can be speculated that patients with Parkinson’s disease might have suffered from a lack of adaptive response during the ageing process. Alternatively, a decrease in DJ-1 could be secondary to its sequestration with α-synuclein in brain tissue. The support for this argument can be found in the experiments demonstrating that DJ-1 and α-synuclein are associated with each other, either directly or indirectly (Meulener et al., 2005; Jin et al., 2007).
The other significant observation is that the sensitivity and specificity of DJ-1 and α-synuclein appear to be dependant on age, meaning that the specificity increased for both indices in the population of age ≥ 65 (Fig. 5 and Table 2). This occurred because levels of both DJ-1 and α-synuclein, while decreasing in diseased populations, tended to increase, age-dependently, in healthy controls. Previous studies reported conflicting results regarding age dependence of CSF α-synuclein, showing evidence for a decrease (Tokuda et al., 2006) or no change of α-synuclein with age (van Geel et al., 2008). Besides the issues discussed previously (low sensitivity of the earlier assays, blood contamination, limited number of cases, etc.), the study reporting a decrease of α-synuclein in CSF with age (Tokuda et al., 2006) concentrated CSF by vacuum centrifugation, which could be another source of variation of the results.
Though disappointing, it is important to emphasize that no apparent association was found between CSF DJ-1 or α-synuclein levels and the severity of Parkinson’s disease. While this observation has to be validated in samples collected longitudinally (as opposed to a cross-sectional study in the current investigation), it could mean that neither DJ-1 nor α-synuclein can be used as Parkinson’s disease progression markers. In other words, additional markers, particularly those reflecting Parkinson’s disease pathogenesis and/or early disease processes, are needed to enhance biochemical assessment of Parkinson’s disease.
It is unclear whether changes in DJ-1 and/or α-synuclein values correlate with development of cognitive impairment in Parkinson’s disease. In this regard, though still controversial, it has been suggested that tau increased while Aβ decreased in CSF of Parkinson’s disease patients with dementia compared with those without dementia (Mollenhauer et al., 2006), i.e. a pattern resembling changes in patients with Alzheimer’s disease. Thus, we are actively recruiting Parkinson’s disease patients with cognitive impairment, allowing us to test whether DJ-1 and/or α-synuclein levels, either alone or in combination with tau/Aβ, can predict the development of cognitive impairment, a major non-motor complication, particularly in the advanced stages of Parkinson’s disease.
CSF α-synuclein levels in patients with Alzheimer’s disease were examined in several previous studies, which also yielded conflicting results. Öhrfelt et al. (2009) reported that patients with Alzheimer’s disease had significantly lower α-synuclein levels than controls, and patients with MMSE scores below 20 had significantly lower α-synuclein than patients with MMSE scores of 20 or higher. Others observed no significant difference in CSF α-synuclein levels between patients with Alzheimer’s disease and healthy or neurological controls with non-neurodegenerative diagnoses (Mollenhauer et al., 2006; Spies et al., 2009); and no correlation between α-synuclein levels and MMSE scores (Spies et al., 2009). Control of important variables, such as blood contamination in CSF, was not mentioned in any of these reports. In the current investigation, no significant difference in α-synuclein levels was observed between patients with Alzheimer’s disease and controls, and no correlation was found between α-synuclein levels and Alzheimer’s disease severity (approximated by MMSE scores) with or without eliminating the samples with high haemoglobin levels. However, we only had 50 Alzheimer’s disease cases in this study, i.e. more cases might be needed to clarify the discrepancy.
One limitation of our investigation that has not been addressed is the potential confounding effect of pharmacotherapy, for example by l-dopa (Fahn et al., 2004). A previous study reported the expression of dopamine receptors in the choroid plexus (Amenta et al., 2000), which could alter CSF homeostasis and hence influence the levels of DJ-1 and α-synuclein in medicated patients with parkinsonism. In our cohort, 16 of the 117 Parkinson's; cases were de novo, i.e. without being treated with any anti-parkinsonism drugs when the CSF samples were obtained. Another three patients were treated with non-dopamine drugs only (Table 1). The DJ-1 and α-synuclein values in the de novo patients were not statistically different from patients treated with all anti-parkinsonism drugs or dopamine-specific drugs (l-dopa and dopamine agonists) only. Additionally, the results were not changed when these de novo patients were eliminated from the analysis (data not shown). Thus, these data, though preliminary, seemed to suggest that pharmacotherapy (particularly dopamine-specific drugs) has no apparent effect on CSF DJ-1 or α-synuclein levels. That said, a more definitive study will be needed, using a larger number of unmedicated subjects, or examining the effects of pharmacotherapy in animal models of Parkinson’s disease, to address this potential confounder. It would also be necessary to study the effects of drug dosages on CSF DJ-1 or α-synuclein levels, but it is rather difficult to obtain precise cumulative dosages of drug treatment in a retrospective investigation, because most patients had to alter not only the types of medication but also the dosage of a particular drug, e.g. levodopa, over the years. Thus, one solution to this challenge in future studies could be to closely follow a cohort of 25–30 de novo patients for 3–5 years with precise annual recording of all drugs taken/alterations as well as CSF taps. The samples collected longitudinally will provide an excellent opportunity for us to re-evaluate the role of DJ-1 and α-synuclein in Parkinson’s disease progression in addition to determination of the effects of dopamine drugs on CSF DJ-1 and α-synuclein levels.
Another limitation of this study is that we did not evaluate CSF DJ-1 or α-synuclein levels in other synucleinopathies or related disorders (e.g. multiple system atrophy or progressive supranuclear palsy), i.e. diseases that overlap with Parkinson’s disease clinically. In a preliminary analysis, Lee et al. (2006) reported that plasma α-synuclein levels were increased in multiple system atrophy. Further studies with a more robust quantitative method and adequate control for important confounding factors are necessary to validate this result and to determine whether DJ-1 as well as α-synuclein could differentiate Parkinson’s disease from those related disorders.
In summary, this study has demonstrated the usefulness of newly developed Luminex assays in assessing DJ-1 and α-synuclein in human CSF as helpful diagnostic markers, with relatively high sensitivity and moderate specificity. Furthermore, this investigation has addressed several major factors that could have contributed to variable results on both DJ-1 and α-synuclein reported previously. Despite these advances, our results underscore the need for additional novel markers for screening and monitoring of patients with Parkinson’s disease as well as evaluating the effectiveness of current and future therapeutic interventions.
Funding
National Institutes of Health grants (ES004696, NS057567, AG025327, AG033398, NS060252, NS062684, AG05136, AG08017, AG08671 and UL1 RR025014-01); Dana Foundation; The Michael J. Fox Foundation; The Friends of Alzheimer’s Research; The Alzheimer’s Association of Western and Central Washington; The Department of Veterans Affairs.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Acknowledgements
The authors deeply appreciate those who have donated their CSF for their studies.
Glossary
Abbreviations
- ELISA
enzyme-linked immunosorbent assay
- MMSE
mini-mental state exam
- ROC
receiver operating characteristic
References
- Amenta F, Barili P, Bronzetti E, Felici L, Mignini F, Ricci A. Localization of dopamine receptor subtypes in systemic arteries. Clin Exp Hypertens. 2000;22:277–88. doi: 10.1081/ceh-100100077. [DOI] [PubMed] [Google Scholar]
- Borghi R, Marchese R, Negro A, Marinelli L, Forloni G, Zaccheo D, et al. Full length alpha-synuclein is present in cerebrospinal fluid from Parkinson's; disease and normal subjects. Neurosci Lett. 2000;287:65–7. doi: 10.1016/s0304-3940(00)01153-8. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's; disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Burn DJ, Lees AJ. Progressive supranuclear palsy: where are we now? Lancet Neurol. 2002;1:359–69. doi: 10.1016/s1474-4422(02)00161-8. [DOI] [PubMed] [Google Scholar]
- Choi J, Sullards MC, Olzmann JA, Rees HD, Weintraub ST, Bostwick DE, et al. Oxidative damage of DJ-1 is linked to sporadic Parkinson and Alzheimer diseases. J Biol Chem. 2006;281:10816–24. doi: 10.1074/jbc.M509079200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont NC, Wang K, Wadhwa PD, Culhane JF, Nelson EL. Validation and comparison of luminex multiplex cytokine analysis kits with ELISA: determinations of a panel of nine cytokines in clinical sample culture supernatants. J Reprod Immunol. 2005;66:175–91. doi: 10.1016/j.jri.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Agnaf OM, Salem SA, Paleologou KE, Curran MD, Gibson MJ, Court JA, et al. Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson's; disease. Faseb J. 2006;20:419–25. doi: 10.1096/fj.03-1449com. [DOI] [PubMed] [Google Scholar]
- Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, et al. Levodopa and the progression of Parkinson's; disease. N Engl J Med. 2004;351:2498–508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- Fjorback AW, Varming K, Jensen PH. Determination of alpha-synuclein concentration in human plasma using ELISA. Scand J Clin Lab Invest. 2007;67:431–5. doi: 10.1080/00365510601161497. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Lee VM, Trojanowski JQ. Synucleinopathies: clinical and pathological implications. Arch Neurol. 2001;58:186–90. doi: 10.1001/archneur.58.2.186. [DOI] [PubMed] [Google Scholar]
- Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer's; disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5:228–34. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- Hong Z, Zhang QY, Liu J, Wang ZQ, Zhang Y, Xiao Q, et al. Phosphoproteome study reveals Hsp27 as a novel signaling molecule involved in GDNF-induced neurite outgrowth. J Proteome Res. 2009;8:2768–87. doi: 10.1021/pr801052v. [DOI] [PubMed] [Google Scholar]
- Jin J, Davis J, Zhu D, Kashima DT, Leroueil M, Pan C, et al. Identification of novel proteins affected by rotenone in mitochondria of dopaminergic cells. BMC Neurosci. 2007;8:67. doi: 10.1186/1471-2202-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PH, Lee G, Park HJ, Bang OY, Joo IS, Huh K. The plasma alpha-synuclein levels in patients with Parkinson's; disease and multiple system atrophy. J Neural Transm. 2006;113:1435–9. doi: 10.1007/s00702-005-0427-9. [DOI] [PubMed] [Google Scholar]
- Li QX, Campbell BC, McLean CA, Thyagarajan D, Gai WP, Kapsa RM, et al. Platelet al.ha- and gamma-synucleins in Parkinson's; disease and normal control subjects. J Alzheimers Dis. 2002;4:309–15. doi: 10.3233/jad-2002-4406. [DOI] [PubMed] [Google Scholar]
- Li QX, Mok SS, Laughton KM, McLean CA, Cappai R, Masters CL, et al. Plasma alpha-synuclein is decreased in subjects with Parkinson's; disease. Exp Neurol. 2007;204:583–8. doi: 10.1016/j.expneurol.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Lindersson E, Beedholm R, Hojrup P, Moos T, Gai W, Hendil KB, et al. Proteasomal inhibition by alpha-synuclein filaments and oligomers. J Biol Chem. 2004;279:12924–34. doi: 10.1074/jbc.M306390200. [DOI] [PubMed] [Google Scholar]
- Liu J, Hong Z, Ding J, Liu J, Zhang J, Chen S. Predominant release of lysosomal enzymes by newborn rat microglia after LPS treatment revealed by proteomic studies. J Proteome Res. 2008;7:2033–49. doi: 10.1021/pr7007779. [DOI] [PubMed] [Google Scholar]
- Lucking CB, Brice A. Alpha-synuclein and Parkinson's; disease. Cell Mol Life Sci. 2000;57:1894–908. doi: 10.1007/PL00000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maita C, Tsuji S, Yabe I, Hamada S, Ogata A, Maita H, et al. Secretion of DJ-1 into the serum of patients with Parkinson's; disease. Neurosci Lett. 2008;431:86–9. doi: 10.1016/j.neulet.2007.11.027. [DOI] [PubMed] [Google Scholar]
- Meulener MC, Graves CL, Sampathu DM, Armstrong-Gold CE, Bonini NM, Giasson BI. DJ-1 is present in a large molecular complex in human brain tissue and interacts with alpha-synuclein. J Neurochem. 2005;93:1524–32. doi: 10.1111/j.1471-4159.2005.03145.x. [DOI] [PubMed] [Google Scholar]
- Michell AW, Luheshi LM, Barker RA. Skin and platelet al.ha-synuclein as peripheral biomarkers of Parkinson's; disease. Neurosci Lett. 2005;381:294–8. doi: 10.1016/j.neulet.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Mollenhauer B, Cullen V, Kahn I, Krastins B, Outeiro TF, Pepivani I, et al. Direct quantification of CSF alpha-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp Neurol. 2008;213:315–25. doi: 10.1016/j.expneurol.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Mollenhauer B, Trenkwalder C, von Ahsen N, Bibl M, Steinacker P, Brechlin P, et al. Beta-amlyoid 1-42 and tau-protein in cerebrospinal fluid of patients with Parkinson's; disease dementia. Dement Geriatr Cogn Disord. 2006;22:200–8. doi: 10.1159/000094871. [DOI] [PubMed] [Google Scholar]
- Nural H, He P, Beach T, Sue L, Xia W, Shen Y. Dissembled DJ-1 high molecular weight complex in cortex mitochondria from Parkinson's; disease patients. Mol Neurodegener. 2009;4:23. doi: 10.1186/1750-1326-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhrfelt A, Grognet P, Andreasen N, Wallin A, Vanmechelen E, Blennow K, et al. Cerebrospinal fluid alpha-synuclein in neurodegenerative disorders-a marker of synapse loss? Neurosci Lett. 2009;450:332–5. doi: 10.1016/j.neulet.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Spies PE, Melis RJ, Sjogren MJ, Rikkert MG, Verbeek MM. Cerebrospinal fluid alpha-synuclein does not discriminate between dementia disorders. J Alzheimers Dis. 2009;16:363–9. doi: 10.3233/JAD-2009-0955. [DOI] [PubMed] [Google Scholar]
- Tokuda T, Salem SA, Allsop D, Mizuno T, Nakagawa M, Qureshi MM, et al. Decreased alpha-synuclein in cerebrospinal fluid of aged individuals and subjects with Parkinson's; disease. Biochem Biophys Res Commun. 2006;349:162–6. doi: 10.1016/j.bbrc.2006.08.024. [DOI] [PubMed] [Google Scholar]
- van Geel WJ, Abdo WF, Melis R, Williams S, Bloem BR, Verbeek MM. A more efficient enzyme-linked immunosorbent assay for measurement of alpha-synuclein in cerebrospinal fluid. J Neurosci Methods. 2008;168:182–5. doi: 10.1016/j.jneumeth.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Vignali DA. Multiplexed particle-based flow cytometric assays. J Immunol Methods. 2000;243:243–55. doi: 10.1016/s0022-1759(00)00238-6. [DOI] [PubMed] [Google Scholar]
- Waragai M, Nakai M, Wei J, Fujita M, Mizuno H, Ho G, et al. Plasma levels of DJ-1 as a possible marker for progression of sporadic Parkinson's; disease. Neurosci Lett. 2007;425:18–22. doi: 10.1016/j.neulet.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Waragai M, Wei J, Fujita M, Nakai M, Ho GJ, Masliah E, et al. Increased level of DJ-1 in the cerebrospinal fluids of sporadic Parkinson's; disease. Biochem Biophys Res Commun. 2006;345:967–72. doi: 10.1016/j.bbrc.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Westerlund M, Belin AC, Anvret A, Hakansson A, Nissbrandt H, Lind C, et al. Cerebellar alpha-synuclein levels are decreased in Parkinson's; disease and do not correlate with SNCA polymorphisms associated with disease in a Swedish material. Faseb J. 2008;22:3509–14. doi: 10.1096/fj.08-110148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.