Abstract
Lesch–Nyhan disease is a neurogenetic disorder caused by deficiency of the enzyme hypoxanthine–guanine phosphoribosyltransferase. The classic form of the disease is described by a characteristic syndrome that includes overproduction of uric acid, severe generalized dystonia, cognitive disability and self-injurious behaviour. In addition to the classic disease, variant forms of the disease occur wherein some clinical features are absent or unusually mild. The current studies provide the results of a prospective and multi-centre international study focusing on neurological manifestations of the largest cohort of Lesch–Nyhan disease variants evaluated to date, with 46 patients from 3 to 65 years of age coming from 34 families. All had evidence for overproduction of uric acid. Motor abnormalities were evident in 42 (91%), ranging from subtle clumsiness to severely disabling generalized dystonia. Cognitive function was affected in 31 (67%) but it was never severe. Though none exhibited self-injurious behaviours, many exhibited behaviours that were maladaptive. Only three patients had no evidence of neurological dysfunction. Our results were compared with a comprehensive review of 78 prior reports describing a total of 127 Lesch–Nyhan disease variants. Together these results define the spectrum of clinical features associated with hypoxanthine–guanine phosphoribosyltransferase deficiency. At one end of the spectrum are patients with classic Lesch–Nyhan disease and the full clinical phenotype. At the other end of the spectrum are patients with overproduction of uric acid but no apparent neurological or behavioural deficits. Inbetween are patients with varying degrees of motor, cognitive, or behavioural abnormalities. Recognition of this spectrum is valuable for understanding the pathogenesis and diagnosis of all forms of hypoxanthine–guanine phosphoribosyltransferase deficiency.
Keywords: neurogenetics, genotype–phenotype correlation, metabolic disease, uric acid, dystonia, behaviour, Kelly–Seegmiller syndrome
Introduction
Complete deficiency of the purine recycling enzyme, hypoxanthine–guanine phosphoribosyltransferase (HPRT), causes Lesch-Nyhan disease (LND). Affected individuals typically suffer from overproduction of uric acid that may lead to hyperuricaemia, nephrolithiasis, gout or subcutaneous deposits of tophi (Lesch and Nyhan, 1964; Jinnah and Friedmann, 2001). Neurologically, all patients have severe motor disability that is dominated by dystonia, with occasional choreoathetosis or spasticity (Jinnah et al., 2006). Most also exhibit recurrent self-injurious behaviour, often with other difficult behaviours such as impulsivity, striking or spitting at others, or use of socially unacceptable language (Nyhan, 1976; Anderson and Ernst, 1994; Schretlen et al., 2005). Finally, most have intellectual disability (Anderson et al., 1992; Matthews et al., 1995; Schretlen et al., 2001).
Although complete HPRT deficiency typically results in the stereotypical LND syndrome, partial deficiency more often causes a phenotype in which some features are attenuated or absent (Kelley et al., 1969; Emmerson and Thompson, 1973; Jinnah and Friedmann, 2001; Puig et al., 2001). Collectively, these patients are labelled LND variants. All of these patients produce excess uric acid, but broad variations in the neurological and behavioural features have been described. Because all patients overproduce uric acid, efforts to classify the spectrum of disease have focussed on differences in neurological and behavioural manifestations. Initial attempts classified patients in two groups, including those with the complete phenotype, and those with only minor neurobehavioural manifestations (Kelley et al., 1969). However, increasing recognition of cases with intermediate severity led to later classification systems that involved three or four groups, based on specific neurobehavioural features. The earliest of these focussed on differences in intellectual function (Page et al., 1981; Hersh et al., 1986; Page and Nyhan, 1989). This focus proved difficult because of challenges inherent in comprehensive cognitive assessments, together with wide variations in normal individuals. More recent proposals have focussed on variations in the motor disorder (Sege-Peterson et al., 1992; Jinnah et al., 2000; Puig et al., 2001).
Most proposed nosological classification systems were derived from relatively small numbers of patients evaluated at individual centres, each with different assessment protocols and varying expertise with the many manifestations. These studies have led to different opinions on the relative importance of individual clinical features for classification. The goal of the current studies was to delineate more comprehensively the spectrum of neurological abnormalities in an international multi-centre study of a large group of LND variants.
Materials and methods
Patients
All 46 LND variants were recruited after referral to centres with expertise in the evaluation and management of LND and related conditions. The diagnosis of a variant form of LND required evidence for an HPRT gene mutation or reduced HPRT enzyme activity in a male patient without the self-injurious behaviour typical of classic cases. Self-injurious behaviour was defined as any self-directed behaviour leading to tissue injury. Neurological and/or behavioural difficulties were not required in the LND variants.
Since the main focus was the LND variants, classic cases of LND were excluded. The diagnosis of classic LND was made in accordance with prior studies (Jinnah et al., 2006) and included expression of the full phenotype with evidence for overproduction of uric acid, severe motor disability, cognitive dysfunction and self-injurious behaviour. The diagnosis of classic LND was supported by documentation of an HPRT gene mutation predicting null activity or reduced HPRT enzyme activity in fibroblasts or blood cells. Although classic LND cases were not evaluated for this study, data from our previously published cases are presented for comparisons (Jinnah et al., 2006).
Evaluation
The first author directly evaluated 37 patients. Nine others were evaluated by clinicians who worked with the first author on other cases, and videotapes were prepared for review. The evaluation included a detailed history with attention to early development and behaviour. It also included a complete neurological examination with specific attention to the motor features as previously described (Jinnah et al., 2006). Because dystonia was the most common and severe problem, the Burke–Fahn–Marsden (BFM) rating scale was used to estimate overall severity of motor dysfunction (Burke et al., 1985). Results from neuropsychological or diagnostic testing were summarized when available from the clinical records or prior studies (Schretlen et al., 2001).
Literature review
The Medline database through July 2009 was searched for reports with the keywords ‘Lesch–Nyhan’, ‘hypoxanthine–guanine phosphoribosyltransferase’, or ‘Kelley–Seegmiller’. Other reports were found through the bibliographies of these articles.
Among 78 articles describing 127 LND variants retrieved, four were from the French literature, two were from the German literature, one was from the Spanish literature and the rest were in English. To avoid redundancy, multiple reports for the same case were combined, and any cases that were re-evaluated and presented as part of the prospective evaluation were excluded from the summary of prior reports, leaving 109 unique cases in 60 reports.
Prospective evaluation
Demographics
All 46 LND variants were male, ranging from 3 to 65 years of age (Table 1). Most were taking allopurinol to reduce uric acid, and three were taking antihypertensives. Other medications occasionally used to reduce excess muscle tone included baclofen, benzodiazepines and trihexyphenidyl.
Table 1.
Case demographics
| Case- family | Other identifiers | HPRT mutation | Conse- quence | Residual functiona | Presenting problem | Presenting age (years) | Age last seen (years) | Non-neurologic problems | Medications | Prior reports |
|---|---|---|---|---|---|---|---|---|---|---|
| 1–1 | None | 239-240delGA insTT | D80F | ND (EL) | Motor delay and posturing | 0.5 | 3.4 | Multiple scars on hands | Allopurinol, diazepam | None |
| 2–2 | PB | IVS4-1G>A | Splice error | ND (EL) | Nephrolithiasis | 0.25 | 7 | Allopurinol | None | |
| 3–3 | JLY | IVS1+1G>A | Splice error | 0.1% (EL) | Hypotonia | 0.5 | 7 | Nephrolithiasis, hydronephrosis | Allopurinol, baclofen | (Marcus et al., 1993) |
| 4–4 | Zarazoga I | G397A | V133M | 9.4% (EL), 54% (LE) | Renal failure | Infancy | 8 | Renal insufficiency | Allopurinol | (Jinnah et al., 2000 Torres et al., 2000; Puig et al., 2001) |
| 5–5 | NF | T203C | L68P | ND (EL) | Motor delay | 0.5 | 9 | Nephrocalcinosis | Allopurinol | None |
| 6–6 | DoA | G143A | R48H | ND (EL) | Motor delay | 2 | 9 | Nephrolithiasis | Allopurinol | None |
| 7–7 | JW | C193T | L65P | 10% (EL) | Motor delay | 6 | 10 | Hypertension | Allopurinol, cetirizine | (Srivastava et al., 2002) |
| 8–8 | P4 | None | mRNA decrease | 6% (EL), 98% (LE) | Hyperuricaemia | 10 | 10 | NA | (Garcia et al., 2008) | |
| 9–5 | DiA | T203C presumed | L68P | 7% (EL) | Motor delay | 2 | 11 | NA | Allopurinol | None |
| 10–8 | P3 | None | mRNA decrease | 6% (EL), 98% (LE) | Hyperuricaemia | 12 | 12 | NA | (Garcia et al., 2008) | |
| 11–9 | JF | T548C | I183T | <1% (EL) 8% (LF) | Motor delay | Infancy | 14 | NA | Allopurinol | (Hersh et al., 1986; Sege-Peterson et al., 1992; Jinnah et al., 2000;) |
| 12–8 | P2 | None | mRNA decrease | 6% (EL), 98% (LE) | Hyperuricaemia | 15 | 16 | NA | NA | (Garcia et al., 2008) |
| 13–10 | IRdL | IVS4-2A>G | Splice error | ND (EL), 0.1% (LE) | Motor delay | 16 | 16 | NA | Allopurinol | (Torres et al., 2010) |
| 14–11 | RB | G601A | D201N | 60% (LF) | Crystalluria | 0.5 | 17 | Nephrolithiasis | Allopurinol, metoprolol, amlodipine | (Sege-Peterson et al., 1992; Jinnah et al., 2000;) |
| 15–9 | BF | T548C | I183T | <1% (EL) 8% (LF) | Crystalluria | 1 | 17 | NA | Allopurinol | (Hersh et al., 1986; Sege-Peterson et al., 1992; Jinnah et al., 2000) |
| 16–12 | Madrid I | G212T | G71V | ND (EL), 0.2% (LE) | Motor delay | 1 | 17 | Nephrolithiasis, hypertension, macrocytic anaemia | Allopurinol, baclofen, nifedipine | (Bouwens-Rombouts et al., 1993; Torres et al., 2000) |
| 17–13 | BT | G599C | R200T | 0-26% (EL)b | Hypotonia | 2 | 17 | Nephrolithiasis, macrocytic anaemia | Allopurinol | (Hidalgo-Laos et al., 1997; Jinnah et al., 2000) |
| 18–14 | TH | G143A | R48H | ND (EL), 39% (LF) | ADHD | 6 | 17 | Macrocytosis | Allopurinol | None |
| 19–15 | None | G500C | R167T | 8.7% (LL) | Nephrolithiasis, gout | 10 | 17 | Nephrolithiasis, cystitis, gout | Allopurinol | None |
| 20–16 | VC | G143A | R48H | ND (EL) | Motor delay | <1 | 18 | NA | Allopurinol | (Larovere et al., 2007) |
| 21–17 | None | A584C | Y195S | ND (EL) | Uric acid crystal in finger | 14 | 18 | NA | NA | (Larovere et al., 2004, 2007) |
| 22–18 | MM | IVS1+1 G>T | Splice error | 5% (EL), 1% (LF) | Motor delay | 1 | 20 | Multiple scars on chin | Allopurinol, trihexyphenidyl, balcofen, diazepam | (Jinnah et al., 2000) |
| 23–14 | GH | G143A | R48H | 15% (LF) | Clumsiness | 2.5 | 20 | Macrocytosis | Allopurinol | None |
| 24–19 | P1 | None | Decreased mRNA | 5% (EL), 64% (LE) | Hyperuricaemia | 14 | 20 | Hypothyroidism | Allopurinol | (Garcia et al., 2008) |
| 25–20 | Santona (family S2) | T125C | I42T | ND (EL), 0.6% (LE) | Motor delay, dystonic postures | 14 | 21 | NA | Allopurinol | (Puig et al., 2001) |
| 26–21 | None | A602G | Splice errorc | NA | Gout | 2 | 23 | Gout, migraines, macrocytic anaemia | Allopurinol, baclofen | None |
| 27–22 | None | A584C | Y195S | ND (EL) | Motor delay | 2 | 24 | Tophi, renal insufficiency, nephrolithiasis, dysphornismd | Allopurinol, enalapril | (Larovere et al., 2004 ,2007) |
| 28–23 | Madrid II (family G) | G143A | R48H | 0.3% (EL), 9.2% (LE) | Dystonic gait | 13 | 24 | Allopurinol | (Andres et al., 1987; Puig et al., 2001) | |
| 29–5 | HA | T203C | L68P | AFM | Motor delay | 2.3 | 27 | Nephrolithiasis | Allopurinol | None |
| 30–24 | DD | G143A | R48H | 20% (LF) | NA | 29 | 29 | NA | Allopurinol | (Sege-Peterson et al., 1992; Jinnah et al., 2000) |
| 31–25 | Tsou | G152A | R51Q | NA | Gout | 13 | 30 | Tophi | Allopurinol | (Chang et al., 1999) |
| 32–26 | DM; GM 1622 | E2-3 duplication | Partial reversion | ND (EL), 1.6% (LF) | Motor delay | 0.5 | 31 | Multiple scars on all limbs | Allopurinol | (Gottlieb et al., 1982; Yang et al., 1984,1988; Sege-Peterson et al., 1992; Adler and Wrabetz, 1996; Jinnah et al., 2000) |
| 33–27 | Sardinia | C463T | P155S | ND (EL or LE), 2-4% (LF) | Motor delay | 1–2 | 32 | Gout, nephrolithiasis, G6PD deficiency | Allopurinol | (Cossu et al., 2002, 2006) |
| 34–28 | LW | G148C | A50P | 2.5 (LF) | Motor delay | 0.6 | 34 | Nephrolithiasis | Allopurinol, diazepam | (Sege-Peterson et al., 1992) |
| 35–22 | None | A584C | Y195S | ND (EL) | Gout | 19 | 35 | NA | (Larovere et al., 2004, 2007) | |
| 36–16 | None | G143A presumed | R48H | AFM | Motor delay | 1.5 | 37 | Gout, recurrent tophi, nephrolithiasis | Allopurinol | (Larovere et al., 2007) |
| 37–29 | Salamanca | T128G, G130A | M34R, D44N | 7.8% (LF) | Motor delay | 1.5 | 42 | NA | Allopurinol | (Page et al., 1987; Sege-Peterson et al., 1992; Jinnah et al., 2000; Torres et al., 2000; Puig et al., 2001) |
| 38–30 | LP | T596G | F199C | 8% (EL) | Motor delay | 2.3 | 43 | Nephrolithiasis, gout | Allopurinol | (Ea et al., 2009) |
| 39–29 | Salamanca | T128G, G130A | M34R, D44N | 7.8% (LF) | Motor delay | 6 | 45 | NA | Allopurinol | (Page et al., 1987; Sege-Peterson et al., 1992; Jinnah et al., 2000 Torres et al., 2000; Puig et al., 2001, 2008) |
| 40–25 | Tsou | G152A | R51Q | NA | Gout | 30 | 45 | Tophi | Allopurinol | (Chang et al., 1999) |
| 41–31 | Arlington | A239T | D80V | ND (EL) | Motor delay | 5 | 46 | Hypertension, migraines | Allopurinol | (Davidson et al., 1989) |
| 42–32 | Chia-yi | T93G | D31E | 5% (EL) | Gout | 27 | 53 | Nephrocalcinosis | Allopurinol | (Wu et al., 2007) |
| 43–16 | G143A | R48H | AFM | Tophus on knee | 28 | 56 | Tophi, nephrolithiasis, renal insufficiency, diabetes mellitus | Allopurinol, glibencamide | (Larovere et al., 2007) | |
| 44–33 | Moosejaw | C582G | D194E | 10.8% (EL) | NA | 58 | NA | Allopurinol | (Jinnah et al., 2000; Lightfoot et al., 1994; Snyder et al., 1984) | |
| 45–34 | Marseille | T407C | I136T | 1.4% (EL), 5.4% (L) | Gout | 40 | 60 | Severe gouty arthritis, urate nephropathy, scoliosis | Allopurinol | (Dussol et al., 2004) |
| 46–33 | Moosejaw | C582G | D194E | 8.4% (EL) | Haematuria | 22 | 65 | Gout, tophi, macrocytic anaemia, renal insufficiency | Allopurinol | (Snyder et al., 1984; Lightfoot et al., 1994; Jinnah et al., 2000) |
Some information was not available (NA) because knowledgeable informants or records could not be located. For nosological classification, a BFM score of 6 or more was used to define patients as having HPRT-related neurological dysfunction (HND), while those with scores of 5 or below were considered to have HPRT-related hyperuricaemia (HRH).
aResults are shown as percent of normal control from different assays used in different clinical laboratories as noted: AFM = testing conducted in affected family member only; EL = erythrocyte lysates, FL = fibroblast lysates, LE = live erythrocytes, LF = live fibroblasts, LL = lymphocyte lysates. If normal controls were presented as a range, the percent of control was based on the lower limit of normal.
bThe test result varied according to phosphoribosyl pyrophosphate substrate applied.
cGenomic DNA revealed the base substitution A602G predicting D201G, but mRNA showed exclusion of exon 8, suggesting a coding region error leading to a splicing defect.
dDysmorphic features included coarse facial features and hair, very short thumbs and great toes, and clubbed first and second fingers.
Presentation
Information concerning presenting signs was available for 44 cases (96%). The most frequent presenting problems were neurological, in 26 cases (Table 1). Delayed acquisition of motor or speech skills in early childhood was common. Another 18 patients came to medical attention as a result of overproduction of uric acid. Among these were eight with gout, six with problems involving the kidneys or urogenital tract, four with asymptomatic hyperuricaemia and one with a tophus.
Motor function
Motor abnormalities occurred in 42 patients (91%). Functional severity varied from incapacitating to barely detectable with specific tasks. The most seriously affected cases exhibited a motor syndrome indistinguishable from classic LND, with profoundly disabling and generalized dystonia. Among the LND variants with prominent dystonia, two had chorea and ballism and two had dystonic myoclonus. Moderately affected patients were less disabled but exhibited dystonia with repetitive abnormal posturing of the limbs and overflow posturing. Mildly affected cases had dystonic overflow only when performing specific tasks, or exercise-induced dystonia. The least severely affected cases had subtle motor signs that were clearly abnormal but not readily classified as dystonic. Examples included slight slowing or clumsiness of fine dexterous movements of the fingers and hands, or an awkward or stiff-appearing gait. Overall, obvious dystonic movements were evident in 27 (58.7%), probable dystonia limited to overflow posturing was seen in five (10.9%), and possible dystonia defined only by slow or awkward movements without overt twisting or posturing occurred in seven (15.2%).
Although slight slowing of movements was common, significant bradykinesia was evident only for four. Other parkinsonian features included resting tremor in two, rigidity without cogwheeling in two, rigidity with cogwheeling in one, hypomimia in two, and hypophonia in one. Mild postural or kinetic tremors occurred in two. Three had tic-like movements.
Pyramidal signs also were common. Hyperreflexia occurred in 23 (50%), being limited to the legs in 12 and the arms in one. Clonus occurred in 14, limited to the ankles. Only three had a rate-dependent increase in limb tone with a catch indicative of spasticity. The frequency of the extensor plantar reflex was not summarized because it could not be discriminated reliably from the dystonic toe response (Nausieda et al., 1980; Ashour et al., 2005).
Some patients also exhibited irregular timing and coordination of movements suggestive of cerebellar ataxia, for example overshooting a target on finger-to-nose pointing or irregular hand tapping. However, all of these patients also had severe generalized dystonia, and their irregular movements seemed more related to dystonic dysfunction than true cerebellar ataxia. More definitive features indicative of cerebellar involvement were absent, including isolated ataxia, ocular hypermetria or nystagmus, or scanning speech.
Speech
Dysarthria occurred in 35 cases (76%), developing in all during early childhood. The most severely affected had obvious dystonic dysarthria with slow and laboured articulation accompanied by overactivity of orolingual and jaw muscles, with overflow to other craniofacial muscles. In these patients, speech often was limited to single words or short phrases, and was difficult to follow. Moderately affected patients exhibited speech that was slow and laboured, often with overflow muscle activation typical of dystonia. A task-specific jaw dystonia occurred with speaking in three, and six had stuttering or hesitant speech. Less severely affected patients had speech that was difficult to characterize, being only slightly indistinct or slow. The least severely affected patients had histories of transient speech impediment during childhood or transient decompensation as adults during periods of stress or fatigue.
One patient had a high-pitched nasal voice suggestive of spastic dysarthria. Eleven had normal speech. Vocal cord involvement suggestive of spasmodic dysphonia was absent.
Gait
A gait disorder occurred in 33 cases (72%), emerging typically during childhood. Those most severely affected could not stand or walk, with the dominating problem being hypotonia with superimposed dystonic posturing of the legs and trunk with attempts to stand or walk. In these cases, muscle bulk was significantly reduced distally in the legs due to disuse. Moderately affected patients could stand with support, but independent ambulation was difficult due to leg or trunk posturing. Less severely affected cases exhibited a very laboured and stiff-appearing gait, with overflow activation of truncal or limb muscles. In the least severely affected cases, the gait had a stiff or heavy appearance without other obvious signs of dystonia. Thirteen had normal gaits.
Cognition
Cognitive skills varied from moderately impaired to above normal. Some type of learning impediment was evident in 31 cases (67%). This impediment was expressed as a need for special education, or being recognized as a slow learner in school. Attention-deficit hyperactivity disorder (ADHD) was diagnosed in seven. Several others were described by parents as having problems with attention but were not formally diagnosed.
Formal neuropsychological testing was available for 21 patients (Table 2). Most fell in the mildly impaired to low-average range. Only seven had IQ scores of 90 or above, and two of these were diagnosed with ADHD. No case had severe cognitive disability. The most seriously affected case was also unusual with dysmorphic features suggestive of a superimposed congenital defect (Table 1).
Table 2.
Neurological features
| Case- family | Speech | Gait | Extrapyramidal features | BFM | Other motor features | Cognition | Behaviour | Evolution |
|---|---|---|---|---|---|---|---|---|
| 1–1 | Moderate dystonic dysarthria | Limited by dystonic posturing but stands with support | Moderate generalized dystonia affecting face, trunk, limbs | 48 | Brisk leg reflexes, ankle clonus | NA | Aggressive | Hypotonia and posturing at 6 months; stable by 2 years |
| 2–2 | Severe dystonic dysarthria | Severe posturing prevents standing or walking | Severe generalized dystonia affecting face, neck, trunk, limbs | 66 | Brisk leg reflexes, ankle clonus | NA | Aggressive, coprolalia, ‘likes to flirt with danger’ | Motor and speech delay noted by 1 year; involuntary movements by 2 years; stable thereafter |
| 3–3 | Severe dystonic dysarthria | Severe posturing prevents standing or walking | Severe generalized dystonia affecting face, neck, trunk, limbs | 82.5 | Mild leg spasticity | Poor attention | Oppositional behaviour | NA |
| 4–4 | Normal | Normal | Mild overflow posturing of hands | 2 | Poor attention, IQ = 89 | Normal | Floppy head noted at 6 months; normal thereafter | |
| 5–5 | Severe dystonic dysarthria | Severe posturing prevents standing or walking | Severe generalized dystonia affecting face, neck, trunk, limbs; rare myoclonic jerks | 47 | Brisk arm and leg reflexes | Special education | Inappropriate affection, even with strangers | Motor and speech delay noted by 1 year; stable thereafter |
| 6–6 | Moderate dystonic dysarthria, hesitant, telegraphic | Normal but awkward running and hopping | Mild generalized dystonia | 19 | Brisk leg reflexes | Special education, IQ = 79 | Impulsive, oppositional | Motor and speech delay noted by 2 years; stable thereafter |
| 7–7 | Transient childhood speech disorder | Normal | Mild hand clumsiness with overflow posturing | 8 | ADHD, IQ = 108 | Onychophagia, impulsive, Asperger syndrome | Persistent stable clumsiness noted by 1 year; transient speech disorder | |
| 8–8 | Normal | Normal | Chronic tic disorder | 0 | Normal, IQ = 146 | Normal | None | |
| 9–5 | Moderate dystonic dysarthria | Independent but very slow and laboured with very stiff appearance | Moderate generalized dystonia with mild rigidity and myoclonic jerks | 34 | Brisk leg reflexes, ankle clonus | Special education | Normal | Motor and speech delay noted by 2–4 years; stable thereafter |
| 10–8 | Normal | Normal | Slightly slow/clumsy hand movements with exercise-induced dystonia | 7a | ADHD, IQ = 117 | Normal | None | |
| 11–9 | Normal | Independent but heavy appearance, awkward | Slow hand movements, overflow hand posturing | 2 | Brisk leg reflexes, ankle clonus, | IQ = 82 | Normal | Transient hypotonia in infancy |
| 12–8 | Slight dystonic dysarthria | Slightly slowed, reduced arm swing | Slow hand movements | 1 | None | Normal, IQ = 134 | obsessive-compulsive disorder | None |
| 13–10 | Moderate dystonic dysarthria | Leg spasms prevent standing or walking | Severe generalized dystonia | NA | Brisk leg reflexes, ankle clonus | Special education | Normal | Drinks 3l/day of water |
| 14–11 | Slightly indistinct | Independent but heavy appearance, cannot edge walk | Slow/clumsy limb movements, rare facial twitches | 3 | Ankle clonus | IQ = 56 | Normal | None |
| 15–9 | Tongue–jaw synkinesis and transient childhood speech disorder | Normal | Slow hand movements, tic-like shoulder shrug and head roll | 0.5 | Brisk leg reflexes, ankle clonus | IQ = 66 | Normal | Transient childhood speech impediment |
| 16–12 | Moderate dystonic dysarthria | Truncal hypotonia with dystonic leg posturing prevents standing or walking | Moderate generalized dystonia affecting face, neck, trunk, limbs; bradykinesia and rigidity without cogwheeling | 22.5 | Two seizures during childhood | IQ = 86 | Normal | Motor and speech delay noted by 1 year; involuntary movements 2–4 years; progressive gait disability from 8 years |
| 17–13 | History of stress-induced dysarthria | Normal | None | 0 | Brisk arm and leg reflexes, ankle clonus | Poor school performance, IQ = 86 | Severe onychophagia sometimes to bleeding | Motor delay and sialorrhea noted by 2 years; resolved thereafter |
| 18–14 | Normal | Normal | None | 0 | None | ADHD, IQ = 89 | Onychophagia, impulsive, bad behaviors (lying, stealing, etc.) | None |
| 19–15 | Mild dystonic dysarthria | Normal but cannot edge walk | Slightly slowed/clumsy hand movements | 7.5 | Brisk leg reflexes, ankle clonus | Normal | NA | NA |
| 20–16 | Slightly slow and indistinct | Slightly slowed | Hypomimia, slow hand movements with overflow posturing | 6 | Brisk leg reflexes, ankle clonus | Significantly impaired | Normal | Motor and speech delay noted by 2 years; stable thereafter |
| 21–17 | Hypophonic | Normal | Slightly slowed hand movements | 2 | None | Repeated 1st grade | Aggressive, labile mood, ‘rebellious’ | NA |
| 22–18 | Severe dystonic dysarthria | Severe posturing prevents standing or walking | Severe generalized dystonia affecting face, neck, trunk, limbs | 60 | Brisk arm and leg reflexes, reduced distal muscle bulk | IQ = 67 | None | Motor and speech delay noted by 1 year; progressive gait disability with falls from 6 to 12 years |
| 23–14 | Sightly slowed and indistinct, intermittent jaw dystonia, childhood stuttering | Normal except overflow hand posturing | Slow/clumsy hand movements | 7 | None | IQ = 83 | None | Clumsiness progressively apparent from 3 to 8 years; stable thereafter |
| 24–19 | Normal | Reduced arm swing | Slow arm and hand movements | NA | None | IQ = 97 | Normal | None |
| 25–20 | Severe dystonic dysarthria | Extreme leg spasms prevent standing or walking | Severe generalized dystonia | 12.5 | Brisk arm and leg reflexes, ankle clonus | ADHD, IQ = 86 | Anxiety disorder | None |
| 26–21 | Moderate dystonic dysarthria, childhood stuttering | Independent but very slow and laboured with very stiff appearance | Moderate generalized dystonia affecting face, neck, trunk, limbs; bradykinesia | 39 | None | ADHD, special education, IQ = 78 | Incarcerated for inappropriate behaviour | Motor and speech delay noted by 2 years; stable clumsiness until 9 years when stuttering speech began; progressive gait disability with falls at 18 years |
| 27–22 | Slightly indistinct | Normal but can’t edge-walking | Slow/clumsy hand movements | 6 | Brisk arm and leg reflexes, ankle clonus | Marked cognitive impairment | Aggressive | Motor and speech delay noted by 2 years; stable thereafter |
| 28–23 | Slight dystonic dysarthria | Mildly dystonic | Hypomimia, mild generalized dystonia, affecting face, trunk, limbs | NA | Brisk leg reflexes | Special education | Normal | None |
| 29–5 | Moderate dystonic dysarthria with jaw dystonia | Independent but hyperlordotic with very slow and laboured stiff appearance | Moderate generalized dystonia affecting face, trunk, limbs with bradykinesia, rigidity | 37 | Brisk leg reflexes, ankle clonus | Special education | Normal | Motor and speech delay noted at 2–4 years; stable thereafter |
| 30–24 | High-pitched nasal voice | Moderately slow with stiff appearance | Mild generalized dystonia affecting face and limbs | 16 | Brisk arm and leg reflexes, ankle clonus | IQ = 96 | Onychophagia when stressed | NA |
| 31–25 | Normal | Normal | Postural and kinetic tremor | NF | None | NA | Normal | None |
| 32–26 | Severe dystonic dysarthria | Severe posturing prevents standing or walking | Severe generalized dystonia affecting face, neck, trunk, limbs; occasional chorea and ballismic limb flailing | 61 | Reduced distal muscle bulk | IQ = 87, 77 | None | Motor and speech delay noted at 1 year; involuntary movements progressively apparent from 2 to 4 years; stable thereafter |
| 33–27 | Moderate dystonic dysarthria | Independent but very slow and stiff appearance with rare skip-like postural adjustments | Moderate generalized dystonia affecting face, trunk, limbs; bradykinesia | 16 | None | ADHD, IQ = 74 | Normal | Motor delay by 1 year, progressive clumsiness from 2–4 years, progressive gait disability beyond 20 years |
| 34–28 | Severe dystonic dysarthria | Truncal hypotonia with dystonic leg posturing prevents standing or walking | Resting hypotonia, severe generalized dystonia affecting face, neck, trunk, limbs; occasional choreiform and ballismic limb flailing | 68 | Brisk arm reflexes | IQ = 49 | Sits on arms to avoid hitting bystanders, story fabrication | Motor and speech delay with involuntary movements noted by 1 year; involuntary movements increasingly apparent through 30 years |
| 35–22 | Normal | Normal | Minor overflow posturing of one hand | 1 | None | Mild executive syndrome | Normal | None |
| 36–16 | Moderate dystonic dysarthria | Slightly slowed and heavy appearance | Slow/clumsy limb movements, stereotypical action-induced elevation of one shoulder | 11 | Brisk arm and leg reflexes, neuropathy | Poor school performance | Onychophagia | Motor and speech delay noted at 2–4 years; stable until >20 years when additional disability due to joint deformity began |
| 37–29 | Mild dystonic dysarthria with slight stuttering | Independent but hyperlordotic and stiff appearance | Slow/clumsy hand movements with overflow posturing | 14.5 | None | ADHD, could not finish public school, IQ = 68 | Onychophagia | Motor and speech delay noted at 2–4 years; stable clumsiness thereafter |
| 38–30 | Slightly slowed and indistinct | Slowed and stiff appearance; desires support at all times | Mild generalized dystonia with slow/clumsy hand movements | 22.5 | Brisk arm and leg reflexes | Frontal syndrome | Normal | Motor and speech delay by 2 years, sudden gait decline at 30 years after dropping daughterb |
| 39–29 | Mild dystonic dysarthria with severe jaw dystonia and stuttering | Independent but hyperlordotic and stiff appearance | Blepharospasm | 5 | None | IQ = 68 | Normal | Motor and speech delay noted at 2–4 years; stable clumsiness thereafter |
| 40–25 | Normal | Impaired by joint deformities from tophaceous gout | None | 0 | None | Normal | Progressive disability due to joint deformity | |
| 41–31 | Slightly slowed and indistinct | Extreme leg spasms prevent standing or walkingb | Painful transient leg spasms, postural and kinetic tremor | 6 | None | Slow learner | Impulsive | Leg braces from 5 to 6 years, mild dystonic posturing until sudden decline at 40 years due to painful leg spasms |
| 42–32 | Normal | Normal | None | 0 | None | NA | Normal | None |
| 43–16 | Moderate dystonic dysarthria | Slightly slowed, cannot toe or heel walk due to joint deformity from tophaceous gout | NF | Brisk leg reflexes, peripheral neuropathy | NA | Emotional lability | Progressive hand disability due to joint deformity | |
| 44–33 | Slightly indistinct | Normal | Slight cogwheeling and slowing of hand movements | 5 | Brisk arm and leg reflexes, mild spasticity | Slow learner, IQ = 78 | Normal | None |
| 45–34 | Normal | Impaired by joint deformity from tophaceous gout | None | 4 | Normal, no testing | Normal | Progressive disability due to joint deformity beyond age 40 years | |
| 46–33 | Slightly indistinct with intermittent stuttering | Normal | Slight slowing of hand movements | 5 | Brisk arm and leg reflexes, mild spasticity | Slow learner, IQ = 93 | Normal | Stable stuttering since early childhood |
Some information was not available (NA). BFM = Burke–Fahn–Marsden dystonia rating scale; NF = not feasible because of severe tophaceous gout with joint deformity precluding meaningful motor exam or other confounding problems. The extensor plantar reflex was not included among the pyramidal signs because it could not be unequivocally distinguished from the dystonic toe response.
aBFM score taken at baseline, not after exercise.
bFunctional decline suspected to be psychogenic. One patient developed sudden deterioration of gait with examination features suggestive of fear of falling after he dropped his infant daughter. The other suddenly developed incapacitating painful leg spasms that were inducible following a minor knee injury. In both, there was no evidence for parallel deterioration of speech or hand skills.
Behaviour
Overt self-injurious behaviour was absent because it was an exclusion criterion. However, some LND variants exhibited potentially related behaviours. For example, severe onychophagia was evident in six cases. Obsessive-compulsive disorder and an anxiety disorder were diagnosed in one patient each. Other psychosocial problems included five with impulsivity (including two severe enough to quality for impulse control disorder), four with clinically apparent problems with aggression, three others considered oppositional by parents, one who was incarcerated for inappropriate social behaviour, and one with Asperger syndrome.
Multiple scars under the chin from ‘repeated falls’ were seen in one case, and two others had multiple scars on their limbs from ‘accidents’ due to ‘bad wheelchair driving’. These patients and their caregivers did not view the scars as evidence of unwanted self-injurious behaviour. However, the stereotypical location in the case with chin scars and the unusually large numbers of limb scars in the other two are not typical of patients with other similar motor handicaps, leading the examiners to question whether they qualified as self-injurious behaviour.
Development and progression
Among the cases where sufficient information regarding early development could be obtained, the most common pattern involved a delay in motor or speech development in early childhood. The most severely affected cases were identified during their first year of life with hypotonia or delayed acquisition of motor milestones. Involuntary movements evolved between 6 months and 4 years. Less severely affected cases were not identified until 2–6 years of age when persistent clumsiness or overflow posturing became increasingly apparent with the increasingly complex motor skills expected during development. The least severely affected cases had transient motor or speech impediments during early childhood.
In most cases, motor disability worsened only during early childhood and remained static thereafter. Adult-onset disability did not occur, and there was no evidence of dementia with ageing. The lack of progression was supported by the lack of any significant correlation of either BFM dystonia score or IQ with age. However, worsening motor function with advancing age was evident for 10 cases. In three of these, progressive gait or hand disability was attributable to obvious joint destruction from tophaceous gout. In two others a psychogenic process was suspected due to sudden onset after a traumatic event (Table 2). The remaining five had worsening motor disability suggestive of evolution of their neurological disorder. For example, four with signs of mild motor delay during the first year of life eventually became ambulatory, albeit with slightly clumsy gaits. Walking became progressively awkward later in childhood or adolescence, with development of falls severe enough to require wheelchairs by early adulthood.
Previously reported cases
Presentation
Among 109 LND variants published, the age at presentation was noted for 78. The average age was 12.4 years, with a range of <1 month to 55 years. Initial presenting problems were reported for 97 patients (Table 3). Among these, 67 (69%) presented with issues related to the overproduction of uric acid. There were 38 cases with urological presentations including renal colic, renal failure, nephrolithiasis, haematuria, or crystalluria. Gout was the presenting problem for 26, and asymptomatic hyperuricaemia was the initial clue for three. Only 19 (20%) presented with neurological abnormalities. Most common were signs of delayed motor development during early childhood.
Table 3.
Presenting features in previously reported cases
| Presenting feature | Number (n = 97) | Percent of total |
|---|---|---|
| Neurological | 19 | 19.6 |
| Motor delay | 12 | 12.4 |
| Seizures | 3 | 3.1 |
| Speech impediment | 2 | 2.1 |
| Encephalopathy | 1 | 1.0 |
| Toe walking | 1 | 1.0 |
| Urological | 38 | 39.2 |
| Colic | 17 | 17.5 |
| Renal failure | 13 | 13.4 |
| Crystalluria | 9 | 9.3 |
| Haematuria | 8 | 8.2 |
| Nephrolithiasis | 7 | 7.2 |
| Dysuria | 2 | 2.1 |
| Other urate-related | 29 | 29.9 |
| Gout | 26 | 26.8 |
| Hyperuricemia | 3 | 3.1 |
| Miscellaneous | 11 | 11.3 |
| Affected relative | 7 | 7.2 |
| Failure to thrive | 2 | 2.1 |
| Screening program | 1 | 1.0 |
| Fevers | 1 | 1.0 |
Presenting features in 97 of the 109 unique cases where information concerning presentation was available. The subgroups may sum to more than the total since some cases presented with more than one problem.
Motor abnormalities
At any time during the illness, the most commonly reported motor problem was dysarthria, in 19 cases (Table 4). Extrapyramidal signs included dystonia or choreoathetosis in nine each, and athetosis in four. Pyramidal signs included hyperreflexia in 14, spasticity in 11 and clonus in two.
Table 4.
Neurological features in previously reported cases
| Feature | Number (n = 47) | Percent of total |
|---|---|---|
| Extrapyramidal | 18 | 38.3 |
| Choreoathetosis | 9 | 19.1 |
| Dystonia | 9 | 19.1 |
| Athetosis | 4 | 8.5 |
| Pyramidal | 19 | 40.4 |
| Hyperreflexia | 14 | 29.8 |
| Spasticity | 11 | 23.4 |
| Clonus | 2 | 4.3 |
| Other | 28 | 59.6 |
| Dysarthria | 19 | 40.4 |
| Seizures | 7 | 14.9 |
| Ataxia | 2 | 4.3 |
| Tremor | 2 | 4.3 |
Neurological features in 47 of the 109 unique cases were information was presented. The subgroups may sum to more than the total since some cases presented with more than one problem. The table is based on originally reported terminology with no effort to re-interpret accuracy when the term conflicted with actual clinical descriptions.
Less frequent problems included seizures in seven, and postural or kinetic tremors in two. One case was reported as having ataxia, and two others were reported as suffering from a spinocerebellar syndrome. Another 11 were described as being clumsy or poorly coordinated, or as having ‘minor’ neurological problems. Notably absent were bradykinesia, resting tremor or cogwheel rigidity, tics or myoclonus.
Cognitive abnormalities
Among 44 cases where cognition was addressed, formal neuropsychological testing yielded IQ scores below 90 for eight. In 16 others, cognitive impairments were suspected on the basis of poor school performance or other clinical benchmarks. IQ scores of 90 or greater were documented for only four cases. Another 15 cases were considered to be cognitively normal on the basis of clinical impressions.
Behavioural abnormalities
Overt self-injurious behaviour was absent among the cases reviewed because it was an exclusion criterion for defining a variant form of LND. However, several potentially related problems were reported. Habitual fingernail biting was noted for five cases. Impulsivity was a problem for five cases, including one in whom the impulses were destructive. One patient was diagnosed with hyperactivity, one with obsessive-compulsive disorder, and another was institutionalized in a psychiatric ward for unspecified reasons.
Discussion
The LND variants are defined by HPRT deficiency without self-injurious behaviour, a hallmark feature of classic LND. The current study provides the largest and most comprehensive summary of the neurological features of these variants to date. The relatively large number of patients permits the delineation of a characteristic phenotype with a graded spectrum of severity, rather than a variable assortment of unrelated abnormalities (Fig. 1). The spectrum of variation is evident for each of the major clinical features. Below we summarize this phenotypic spectrum, its relevance for nosological classification of HPRT deficiency, and its biological basis. Finally, we review the implications of phenotypic variation for diagnostic assessment and treatment.
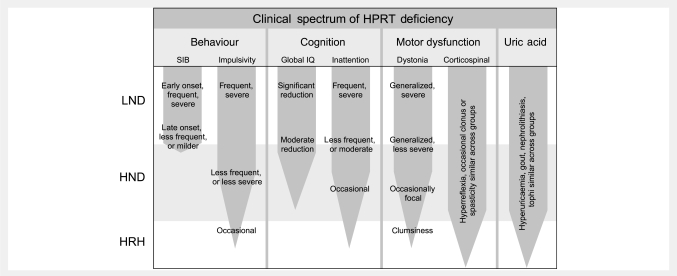
Figure 1.
Schematic representation of the spectrum of clinical features in LND and its variants. Patients are divided into subgroups with the most severe being LND, the intermediate form being HPRT-related neurological dysfunction (HND), and the least severely affected being HPRT-related hyperuricaemia (HRH). The frequency or severity of each problem is depicted by the thickness of the tapering bar, with description of the spectrum of the problem across the groups.
The spectrum of motor abnormalities
The results of our evaluations are compatible with the literature, with small differences attributable to methodology. A shared conclusion is that motor abnormalities are common in the LND variants, with a spectrum that ranges from subtle clumsiness to severe disability. While prior reports suggest the majority of LND variants present with problems related to uric acid, our results suggest the majority present with neurological problems. Our studies also suggest a higher frequency of motor abnormalities in comparison to prior reports, with a high proportion of patients having some form of dystonia. The most likely explanation for these differences is that the current study involved a more methodical evaluation, which had higher sensitivity for revealing neurobehavioural problems in comparison with the majority of prior studies conducted by investigators specializing in genetics or metabolic disease.
The LND variants provide an unusual window on the spectrum of dystonia. Dystonia is obvious when it is fully developed with twisting movements and odd postures, but its mildest expressions often are harder to recognize. By extrapolating from what is more clearly dystonia in more seriously affected classic LND cases, it seems likely that more mildly affected cases exemplify more subtle forms of dystonia. For example, the hyperlordotic postures seen in some variants may reflect milder expressions of the more severe truncal dystonia and opisthotonic postures common in classic LND (Jinnah et al., 2006). Stuttering and hesitant speech may constitute an action dystonia, as previously suggested (Kiziltan and Akalin, 1996; Puig et al., 2008). Clumsy hand movements or gaits with a ‘stiff’ or ‘heavy’ appearance may reflect the mildest expressions of dystonia. If this interpretation is correct, the implication is that the frequency of dystonia is much higher than current appreciated in the LND variants and perhaps other disorders too.
The spectrum of cognitive abnormalities
Our studies and others in the literature also call attention to cognitive dysfunction in the LND variants (Schretlen et al., 2001). Our studies suggest that cognitive dysfunction is more frequent than previously appreciated. The difference again probably involves methods of assessment. Cognitive assessments frequently under-estimate disability when they rely only on clinical impressions without formal neuropsychological testing. Several of our patients thought to be cognitively normal based on global clinical impressions were found to have significant cognitive disability after formal neuropsychological testing. Others with broadly normal IQs showed more selective deficits in specific cognitive domains. However, cognitive dysfunction usually is not severe. Most patients had IQ scores in the borderline to low-average range (70–89).
The spectrum of behavioural abnormalities
Patients with classic LND display a characteristic behavioural phenotype that includes self-injurious behaviours, impulsive acts of aggression such as striking out or spitting, and use of foul or sexually charged language (Nyhan, 1976; Anderson and Ernst, 1994; Schretlen et al., 2005). Self-injurious behaviour was an exclusionary criterion for defining a variant form of LND, so it was absent from the current series. However, our studies are consistent with the literature in implying that behaviour in the LND variants may not always be normal. Five LND variants exhibited behaviours potentially related to self-injury, such as habitual fingernail biting. It is tempting to speculate that this onychophagia is a forme fruste of more serious finger biting, which is the commonest expression of self-injury in classic LND (Anderson and Ernst, 1994; Schretlen et al., 2005).
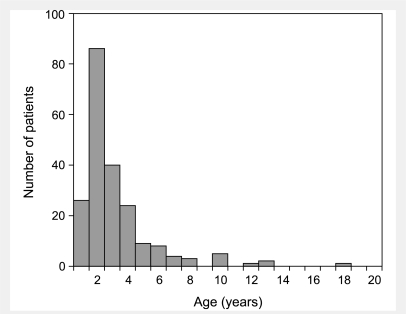
Other socially difficult behaviours, such as impulsivity, severe enough to warrant medical attention also were common in the LND variants. Similar problems have been described before, such as one case who was noted to act on impulses to jump from a moving vehicle or insert a nail into an electric outlet (Geerdink et al., 1973). Another LND variant was noted to have precipitously pulled out a large patch of hair from his head for no apparent reason, and to have exhibited antisocial behaviour that led to incarceration (Nyhan, 1978). These cases may not reach strict definitions of self-injurious behaviour that involve tissue injury, but it is important to acknowledge that the criterion for tissue injury for definition of self-injury is somewhat arbitrary. Distinctions between the variant and LND cases are further blurred by classic cases of LND with very mild or late-onset of self-injury. One of our classic LND patients had very mild self-injury limited to a hypertrophic abrasion on one thumb due to repetitive hand-to-mouth behaviour, but without overt bleeding from biting (Jinnah et al., 2006). A review of the literature discloses that self-injury typically arises before 4 years of age, but may be delayed until late teenage years, when it often is infrequent or mild (Fig. 2). These observations suggest a spectrum of maladaptive behaviour across classic and variant LND rather than an all-or-none phenomenon, a suggestion supported by a study with standardized behavioural rating scales showing the LND variants score between those of normal and classic LND in nearly every problem behaviour category (Schretlen et al., 2005).
Figure 2.
Histogram showing age at onset of self-injury in previously reported cases of classic LND. Among 349 classic LND cases described in 133 previous reports, the age at onset of self-injury was available for 212 cases. Cases were binned in yearly increments, with a mean age of 3.1 ± 2.5 and a median age of 2 years.
The spectrum of uric acid abnormalities
There also are significant variations in the severity of problems due to uric acid, though these were not systematically evaluated in our patients. Efforts to address uric acid problems are challenging because its overproduction is treated with allopurinol as soon as it is recognized. As a result, uric acid complications reflect primarily the efficacy of treatment rather than variations in disease severity.
Prior studies have indicated that overproduction of uric acid does not differ between classic and variant cases (Mateos and Puig, 1994; Jinnah and Friedmann, 2001; Puig et al., 2001). However, these studies were limited by relatively small numbers. When considering all available uric acid measures in untreated patients reported in the literature, a significant difference between the patient subgroups becomes evident (Table 5). Further studies are needed that control for age, renal function, and other variables known to affect uric acid measures independent of HPRT deficiency.
Table 5.
Serum and urine uric acid in classic and variant LND
| Patient group | Serum uric acid (mg/dl) | Urine uric acid (mg/kg per 24 h) | Urine uric acid/ creatinine ratio |
|---|---|---|---|
| Classic LND | 11.7 ± 4.8 | 42.6 ± 17.3 | 3.2 ± 1.1 |
| HPRT-related neurological dysfunction | 13.0 ± 3.8 | 33.6 ± 13.1 | 1.6 ± 0.8 |
| HPRT-related hyperuricaemia | 12.4 ± 5.7 | 23.9 ± 9.8 | 1.0 ± 0.5 |
Uric acid measures for 349 classic and 125 variant cases of LND reported in the prior literature. To avoid skewing the results by over-representation of individual samples, multiple values or ranges of values reported for any one case were averaged to give a single value. When multiple values were reported over several years for one case, only the first value was used, since serum uric acid varies according to age and the values could not be averaged. We excluded values from patients who were receiving drugs known to alter uric acid. Statistical comparisons were conducted via the Kruskal–Wallis test for non-parametric data, which revealed significant group differences for 24 h urinary uric acid (P = 0.003) and uric acid/creatinine ratios (P < 0.001) but not for serum uric acid (P = 0.13).
Despite the spectrum of problems related to uric acid, it seems unlikely that they are causally related to the neurological or behavioural problems in LND. Treatment of LND patients from birth with allopurinol does not influence the development of neurobehavioural problems, and there are other clinical disorders with excessive production of uric acid but without the neurobehavioural problems of LND (Jinnah and Friedmann, 2001).
Patterns of disease and nosology
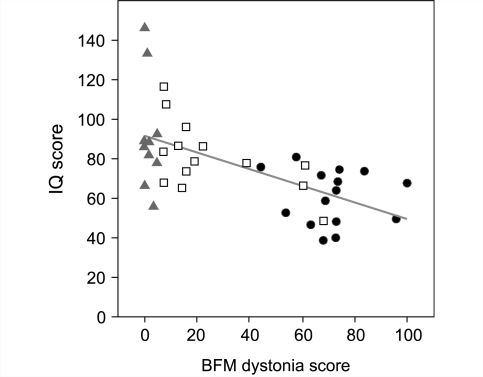
The relatively large number of LND variants evaluated here combined with our prior study of classic LND (Jinnah et al., 2006) facilitates the identification of patterns of disease rather than a random assortment of phenotypic abnormalities (Fig. 1). For example, self-injurious behaviour in classic LND typically is associated with the most severe motor dysfunction and the most prominent cognitive disability. Patients with little or no motor abnormalities appear to have less cognitive impairment, with a significant correlation of BFM scores with IQ (Fig. 3). Although exceptions exist, these observations suggest the overall clinical phenotype occurs as a continuously graded spectrum of severity.
Figure 3.
Correlations between dystonia and cognition. Dystonia was rated with the BFM dystonia rating scale, with mild motor deficits scored as mild expressions of dystonia. Scores for patients with LND come from our previous study (Jinnah et al., 2006) while those for variants come from Tables 1 and 2. Cognition was assessed with IQ, which was taken from the results of clinical diagnostic testing or previous publications. Patient subgroups are LND (circles), HPRT-related neurological dysfunction (squares), and HPRT-related hyperuricaemia (triangles). Patients with LND and HPRT-related neurological dysfunction were distinguished by the presence of self-injurious behaviour. The HPRT-related hyperuricaemia group was defined as clinically insignificant motor dysfunction with a BFM score of 5 or less. There was a significant negative correlation between BFM and IQ scores (Spearman rho = −0.64, P < 0.001). This correlation remained after controlling for age (Spearman rho = −0.63, P < 0.001). There was no significant correlation between BFM and age (Spearman rho = 0.20, P = 0.21).
Despite this spectrum, there is both heuristic and practical value for defining subgroups for clinical studies, treatment of specific features, and counselling. The spectrum of disease most commonly has been divided into three groups (Sege-Peterson et al., 1992; Jinnah and Friedmann, 2001). The most severe phenotype is designated as classic LND, which encompasses overproduction of uric acid with all the neurological manifestations including self-injurious behaviours. An intermediate group includes uric acid overproduction with varying degrees of motor disability, but self-injury is absent. These patients have been designated HPRT-related neurological dysfunction. The least severely affected group has been designated HPRT-related hyperuricaemia, which includes patients with uric acid overproduction, but clinically insignificant neurological or behavioural deficits. Essentially, the occurrence of self-injurious behaviour distinguishes classic patients from variants, and clinically apparent motor disability distinguishes the variants into those with and without neurological impairment.
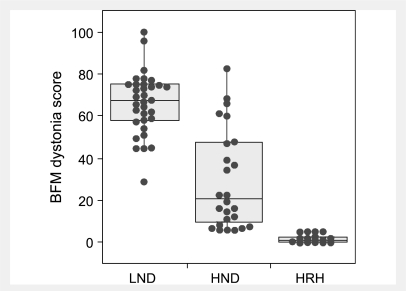
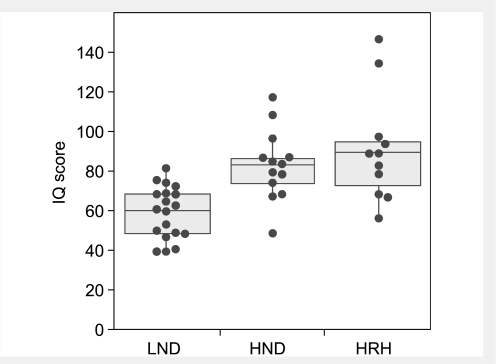
When dystonia is rated with the BFM scale and stratified according to these subgroups, there is considerable overlap but good correspondence between severity and clinical subgroup (Fig. 4). This result is expected because motor dysfunction falls on a continuous spectrum and is a criterion for distinguishing the LND variants. The BFM also discriminates LND from variants, even though it is not a criterion for separating these groups. Using IQ as an estimate of cognition, there again is significant overlap but a good correspondence between median scores and clinical subgroup (Fig. 5), even though IQ is not a criterion for discriminating groups. Thus the clinically defined groups appear to be internally consistent in distinguishing grades of severity.
Figure 4.
Severity of dystonia according to patient subgroup. Dystonia was rated with the BFM dystonia rating scale, with mild motor deficits scored as mild expressions of dystonia. Patient subgroups are LND, HPRT-related neurological dysfunction (HND), and HPRT-related hyperuricaemia (HRH). Patients with LND and HPRT-related neurological dysfunction were distinguished by the presence of self-injurious behaviour. The HPRT-related hyperuricaemia group was defined as clinically insignificant motor dysfunction with a BFM score of 5 or less. Scores for patients with LND come from our previous study (Jinnah et al., 2006) while those for variants come from Tables 1 and 2. Individual scores are overlaid with a box-whisker plot, where the middle horizontal line in each box shows the median. The upper and lower limits of the box define the upper and lower quartiles. Whiskers span the entire data range excepting outliers, defined as values that fell outside the upper or lower quartile plus 1.5 times the inter-quartile distance. The groups were compared statistically using the Kruskal–Wallis H-statistic, which revealed overall significance at P < 0.0001. Post hoc Wilcoxin signed ranked tests revealed significant differences (P < 0.001) between each of the groups.
Figure 5.
IQ scores according to patient subgroup. The IQ was not methodically assessed with a standardized instrument due to differences in age and language, but instead was taken from the results of clinical diagnostic testing or previous publications. Patient subgroups include LND, HPRT-related neurological dysfunction (HND), and HPRT-related hyperuricaemia (HRH). Patients with LND and HPRT-related neurological dysfunction were distinguished by the presence of self-injurious behaviour. The HPRT-related hyperuricaemia group was defined as clinically insignificant motor dysfunction with a BFM score of 5 or less. Scores for patients with LND come from our previous study (Jinnah et al., 2006) while those for variants come from Tables 1 and 2. Individual scores are overlaid with a box-whisker plot, where the middle horizontal line in each box shows the median. The upper and lower limits of the box define the upper and lower quartiles. Whiskers span the entire data range excepting outliers, defined as values that fell outside the upper or lower quartile plus 1.5 times the inter-quartile distance. The groups were compared statistically using the Kruskal–Wallis H-statistic, which revealed overall significance at P < 0.0001. Post hoc Wilcoxin signed ranked tests revealed significant differences (P < 0.001) between the LND and each of the other two groups. The difference between HPRT-related neurological dysfunction and HPRT-related hyperuricaemia groups was not significant (P = 0.56).
Some caveats regarding nosology
Despite the internal consistency of the proposed nosological classification, some caveats must be noted. First, BFM scores are not normally distributed, but instead suggest a bimodal population (Figs 3 and 4). This finding might support a two-group classification system as previously suggested (Kelley et al., 1969). However, the lack of a normal distribution could reflect an artefact of insufficient numbers of patients with intermediate severity, non-linearity of the BFM scale, more frequent progression of patients with intermediate scores, or recurrent mutations that result in non-random clinical outcomes. For example, the C151T hotspot that encodes a null enzyme generates an overrepresentation of classic patients (Jinnah et al., 2000, 2006), and the recurrent G143A mutation that encodes a partially dysfunctional enzyme creates an overrepresentation of very mildly affected patients (Tables 1 and 2).
The second caveat is that despite the overall correlation between BFM scores and IQ in our patients, significant discrepancies exist, suggesting the lack of exact correspondence between motor and cognitive function (Fig. 3). It is important to acknowledge that cognitive function was not methodically assessed with the same instruments across all patients due to differences in age and language, and IQ scores may not capture significant deficits in specific cognitive domains. Further studies of cognitive domains most affected, such as attention, may provide more useful measures than overall IQ for defining clinically relevant subgroups. However, since motor and cognitive dysfunction may be dissociable, it seems reasonable to consider significant cognitive dysfunction as a criterion for reclassifying a patient with HPRT-related hyperuricaemia to HPRT-related neurological dysfunction, regardless of any motor disability.
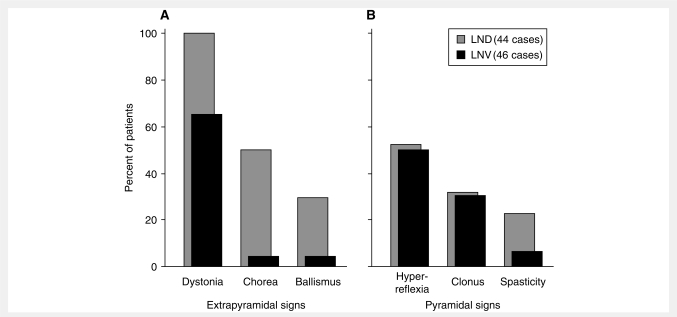
The third caveat is that corticospinal motor signs do no seem to show graded severity across the patient groups, since they are equally frequent and severe in both classic and variant LND (Fig. 6). This finding may suggest the pathogenesis of corticospinal problems is unrelated to the pathogenesis of the extrapyramidal features. However, corticospinal signs are minor compared to extrapyramidal signs, and they may not warrant consideration in patient classification. There also are rare reports of patients with clinical features not found in our patients. Two cases from the literature were reported to have a spinocerebellar syndrome (Kelley et al., 1969) and another was reported to have ataxia with dystonia (Adler and Wrabetz, 1996). However, the first two cases were re-evaluated by others who described an extrapyramidal syndrome rather than ataxia (Nyhan, 1978). The other case was re-evaluated here (case DM). Though he had dysmetric limb movements, they were considered secondary to his severe generalized dystonia rather than true cerebellar ataxia. This view is supported by the lack of other features supportive of cerebellar dysfunction in any of our variant or classic LND cases (Jinnah et al., 2001, 2006). Thus there seems little evidence for true cerebellar ataxia in LND or its variants, and this feature seems sufficiently infrequent to be considered atypical. Although our studies focus on the use of clinical features alone for nosological classification, additional molecular and biochemical measures of the disease may be helpful for validating or refining the classification further.
Figure 6.
Motor features in classic versus variant LND. The percent of patients in the current series of LND variants (LNV; n = 46, black bars) is compared with the percent of patients with classic LND (n = 44, grey bars) from our prior studies (Jinnah et al., 2006). Panel A depicts extrapyramidal features while Panel B depicts pyramidal features.
Pathogenesis of phenotypic variation
LND and its variants are caused by different mutations in the HPRT gene (Table 6). Prior genotype–phenotype comparisons have suggested that the location and type of gene mutation are less relevant for predicting the clinical phenotype than the effect of the mutation on residual enzyme function (Jinnah et al., 2000, 2004). Mutations resulting in little or no residual enzyme function typically cause classic LND, while mutations permitting residual activity more often lead to less severely affected LND variants. The mutations in the current series support this concept, with the majority resulting in single amino acid substitutions compatible with residual enzyme function (Table 1). There also were five patients with splicing mutations, which are known to be ‘leaky’ and permit variable residual activity (Hunter et al., 1996; O'N;eill et al., 1998; Mak et al., 2000; Gaigl et al., 2001). Four others had low but detectable mRNA levels leading to low enzyme activity. One patient had a duplication involving exons 2–3, previously shown to undergo partial reversion leaving a small amount of residual enzyme activity (Yang et al., 1988).
Table 6.
Mutations of the HPRT gene in classic and variant LND
| Mutation class | LND (n = 280) | LNV (n = 101) | NA (n = 9) | Total (n = 390) |
|---|---|---|---|---|
| Single base substitution | ||||
| Missensea | 86 | 76 | 4 | 167 |
| Nonsenseb | 29 | 1 | 1 | 31 |
| Splice sitec | 44 | 11 | 0 | 55 |
| Deletions | ||||
| Coding sequences | 80 | 2 | 4 | 86 |
| Splice site | 5 | 0 | 0 | 5 |
| Insertions | ||||
| Coding sequences | 22 | 1 | 0 | 23 |
| Splice site | 1 | 0 | 0 | 1 |
| Miscellaneous | ||||
| Duplications | 3 | 3 | 0 | 6 |
| Substitutions | 4 | 1 | 0 | 5 |
| Regulatory elementsd | 0 | 3 | 0 | 3 |
| Female casese | 6 | 1 | 0 | 7 |
| Double mutants | 0 | 2 | 0 | 2 |
The genetic mutations in the HPRT gene for both classic cases (LND) and the less seriously affected variants (LNV). The clinical subtype for some patients could not be determined because of insufficient information in some of the reports (NA). A complete list of individual mutations and associated publications can be found at http://www.lesch-nyhan.org.
aSingle base change leading to single amino acid subsitution.
bSingle base change leading to premature termination of protein translation.
cSingle base change leading to intron/exon splicing defect.
dUnidentified promoter or enhancer non-coding sequence change resulting in reduced mRNA.
eAll females have had an identifiable mutation on one allele combined with non-random X-inactivation.
The concept that the severest phenotype occurs when HPRT activity is absent whereas the milder variants have residual enzyme function is supported by most studies in which enzyme activity was measured using assay conditions that mimic the natural state in cultured fibroblasts, lymphocytes, or intact erythrocytes (Page et al., 1981; Fairbanks et al., 1987; Page and Nyhan, 1989; Puig et al., 2001). For most cases, there is a good correlation between clinical severity and residual enzyme activity. Rare case reports where enzyme activity lacks correlation with phenotypic severity sometimes are presented as evidence against this idea (Rijksen et al., 1981; Cossu et al., 2002). These apparent exceptions most often occur when the enzyme is measured via assays that do not replicate natural conditions (McDonald and Kelley, 1971; Dancis et al., 1973; Holland et al., 1976; Bakay et al., 1979; Cameron et al., 1984; Hersh et al., 1986; Fairbanks et al., 1987; Zoref-Shani et al., 2000; Jinnah et al., 2004). All of our LND variants who had enzyme activity measured in live cells displayed measurable residual activity, and discrepancies between assays from live cells versus lysates were evident for many cases that had both assays (Table 1). These observations highlight some problems associated with the lack of standardized biochemical testing. Though assays based on live cells are more accurate than those based on cell lysates, most diagnostic centres use lysate-based assays because they are technically simpler and less expensive.
The current studies also are consistent with prior studies of classic LND indicating that pathogenesis involves dysfunction of basal ganglia circuits (Visser et al., 2000). These circuits have been divided into several parallel but segregated pathways serving motor function, cognition, oculomotor control, and behavioural or ‘limbic’ functions. The most prominent motor abnormality in LND and its variants is dystonia, which may be attributed to dysfunction of motor circuits involving the putamen and motor cortex (Jinnah et al., 2006). Cognitive disability with prominent defects in attention may be attributed to dysfunction of circuits involving the caudate and frontal cortices (Schretlen et al., 2001). The characteristic ocular motor apraxia with saccadic distractability in LND and its variants resembles the oculomotor defects of Huntington’s disease, which have been linked with the caudate and frontal cortex eye fields (Jinnah et al., 2001). Finally, self-injurious and other difficult behaviours can be attributed to pathways through the ventral striatum and mediobasal frontal cortex (Visser et al., 2000).
Dysfunction of basal ganglia circuits in LND and its variants appears to be related to selective vulnerability of dopaminergic neurons. Although these constitute only small population of neurons, they have a profound modulatory influence on corticostriatal physiology. Post-mortem neurochemical studies have revealed 60–90% loss of dopamine in the basal ganglia (Lloyd et al., 1981; Saito et al., 1999), and PET studies have shown similar reductions in fluorodopa uptake or dopamine transporters in the basal ganglia (Ernst et al., 1996; Wong et al., 1996). A selective loss of dopamine also is seen in the basal ganglia in HPRT knockout mice (Jinnah et al., 1992, 1994, 1999), and in cultured HPRT-deficient dopaminergic neurons (Bitler and Howard, 1986; Yeh et al., 1998; Lewers et al., 2008). Histopathological studies of autopsied brain tissue or the HPRT knockout mice do not show a loss of midbrain dopamine neurons, suggesting the loss of dopamine reflects a metabolic rather than a degenerative process (Del Bigio and Halliday, 2007; Egami et al., 2007; Ceballos-Picot et al., 2009). In this regard, LND resembles DOPA-responsive dystonia more than it resembles Parkinson’s disease.
Although the most prominent clinical features in LND and its variants may be attributed to dysfunction of the basal ganglia, other regions may not be spared entirely. For example, hyperreflexia and clonus provide evidence for dysfunction of corticospinal motor pathways. Cognitive limitations also may reflect involvement of the cerebral cortex. Contrary to a recent claim (Del Bigio and Halliday, 2007), there seems little evidence for clinically significant cerebellar ataxia.
Diagnosis
The diagnosis of classic LND is relatively straightforward when all the telltale clinical features, including self-injury, are apparent. Diagnosis is more challenging in variants with attenuated syndromes. The differential diagnosis of early-onset dystonia or clumsiness, with or without cognitive impairment, is broad. Ancillary neurological testing with neuroimaging or EEG has limited value. Instead, overproduction of uric acid, frequently evident as an elevated serum uric acid, is one of the most useful clues (Jinnah and Friedmann, 2001). Hyperuricaemia is uncommon below 40 years of age and should prompt further evaluation, especially if it is combined with evidence for motor or cognitive impairment. Patients for whom diagnoses are delayed ultimately develop one of the consequences of hyperuricaemia, such as nephrolithiasis or gout, both of which are uncommon before 40 years of age (Cameron et al., 1993). The development of either problem in a young person with motor or cognitive abnormalities also should lead to further testing.
Hyperuricaemia provides a useful early clue, but it is not adequate for definitive diagnosis. Serum uric acid is highly dependent on many factors including hydration, diet, medications and renal efficiency. A few LND variants have persistently normal serum uric acid, and many have elevations that are sufficiently small to escape notice. Molecular testing for a mutation in the HPRT gene provides a reliable means of diagnosis (Jinnah et al., 2000). The gene test also facilitates carrier diagnosis and prenatal testing. The main shortcoming of molecular testing is that mutations must be identified by sequencing the gene because they are heterogeneous. Since this process is time-consuming and expensive, it is offered by only a handful of centres worldwide (http://www.lesch-nyhan.org). Another shortcoming is that mutation screening will miss the unusual cases of HPRT deficiency that are due to non-coding reductions in HPRT mRNA expression (Dawson et al., 2005; Garcia et al., 2008). Finally, unique mutations have little prognostic value.
Another option for diagnostic testing is biochemical measurement of HPRT enzyme activity (Jinnah et al., 2004). Since assays of live cells provide enzyme measures that correlate with disease severity, they may have predictive value for prognosis. However, they are technically demanding and offered by only a few centres worldwide. Nevertheless, further studies addressing the most appropriate biochemical assays could be valuable for counselling of cases diagnosed early.
Summary
These studies provide the largest summary to date for the spectrum of neurological manifestations that can occur in association with HPRT deficiency. The results are valuable for raising awareness of atypical presentations of this rare disease, where diagnosis is challenging. The LND variants may lack clinically apparent cognitive dysfunction or overtly abnormal behaviour characteristic of the classic phenotype. Motor function may range from normal to severe disability, with the most common problem being varying manifestations of dystonia. These studies also are valuable for pointing to clues for the diagnosis, as well as diagnostic methods and their limitations. Early diagnosis is important because some of the manifestations such as those related to uric acid are treatable, and because early recognition facilitates carrier identification for family counselling.
Funding
Association Lesch-Nyhan Action; Centro de Investigaciones Biomedicas en Red para el Estudio de las Enfermedades Raras (CIBERER); Fondo de Investigaciones Sanitarias (FIS 06/0019 and FIS 08/0009); Lesch-Nyhan Syndrome Children’s Research Foundation; the National Institutes of Health (HD53312 and DK82840).
Acknowledgements
The authors gratefully acknowledge the patients and their families for participating in these studies. They thank Patrick O’Neill for verifying the mutations in some patients. They also thank their many colleagues who referred cases.
Glossary
Abbreviations
- ADHD
attention-deficit hyperactivity disorder
- BFM
Burke–Fahn–Marsden scale
- HPRT
hypoxanthine–guanine phosphoribosyltransferase
- LND
Lesch–Nyhan disease
References
- Adler CH, Wrabetz L. Lesch-Nyhan variant: dystonia, ataxia, near-normal intelligence, and no self-mutilation. Mov Disord. 1996;11:583–4. doi: 10.1002/mds.870110519. [DOI] [PubMed] [Google Scholar]
- Anderson LT, Ernst M. Self-injury in Lesch-Nyhan disease. J Autism Dev Disord. 1994;24:67–81. doi: 10.1007/BF02172213. [DOI] [PubMed] [Google Scholar]
- Anderson LT, Ernst M, Davis SV. Cognitive abilities of patients with Lesch-Nyhan disease. J Autism Dev Disord. 1992;22:189–203. doi: 10.1007/BF01058150. [DOI] [PubMed] [Google Scholar]
- Andres A, Praga M, Ruilope LM, Martinez JM, Millet VG, Bellow I, et al. Partial deficit of hypoxanthine guanine phosphoribosyl transferase presenting as acute renal failure. Nephron. 1987;46:179–81. doi: 10.1159/000184337. [DOI] [PubMed] [Google Scholar]
- Ashour R, Tintner R, Jankovic J. Striatal deformities of the hand and foot in Parkinson's; disease. Lancet Neurol. 2005;4:423–31. doi: 10.1016/S1474-4422(05)70119-8. [DOI] [PubMed] [Google Scholar]
- Bakay B, Nissinen E, Sweetman L, Francke U, Nyhan WL. Utilization of purines by an HPRT variant in an intelligent, nonmutilative patient with features of the Lesch-Nyhan syndrome. Pediatr Res. 1979;13:1365–70. doi: 10.1203/00006450-197912000-00013. [DOI] [PubMed] [Google Scholar]
- Bitler CM, Howard BD. Dopamine metabolism in hypoxanthine-guanine phosphoribosyltransferase-deficient variants of PC12 cells. J Neurochem. 1986;47:107–12. doi: 10.1111/j.1471-4159.1986.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Bouwens-Rombouts AG, van den Boogaard MJ, Puig JG, Mateos FA, Hennekam RC, Tilanus MG. Identification of two new nucleotide mutations (HPRTUtrecht and HPRTMadrid) in exon 3 of the human hypoxanthine-guanine phosphoribosyltransferase (HPRT) gene. Hum Genet. 1993;91:451–4. doi: 10.1007/BF00217770. [DOI] [PubMed] [Google Scholar]
- Burke RE, Fahn S, Marsden CD, Bressman SB, Moskowitz C, Friedman J. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology. 1985;35:73–7. doi: 10.1212/wnl.35.1.73. [DOI] [PubMed] [Google Scholar]
- Cameron JS, Moro F, Simmonds HA. Gout, uric acid and purine metabolism in paediatric nephrology. Pediatr Nephrol. 1993;7:105–18. doi: 10.1007/BF00861588. [DOI] [PubMed] [Google Scholar]
- Cameron JS, Simmonds HA, Webster DR, Wass V, Sahota A. Problems of diagnosis in an adolescent with hypoxanthine-guanine phosphoribosyltransferase deficiency and acute renal failure. Adv Exp Med Biol. 1984;165A:7–12. doi: 10.1007/978-1-4684-4553-4_2. [DOI] [PubMed] [Google Scholar]
- Ceballos-Picot I, Mockel L, Potier MC, Dauphinot L, Shirley TL, Torero-Ibad R, et al. Hypoxanthine-guanine phosphoribosyl transferase regulates early developmental programming of dopamine neurons: implications for Lesch-Nyhan disease pathogenesis. Hum Mol Genet. 2009;18:2317–27. doi: 10.1093/hmg/ddp164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SJ, Chang JG, CHen CJ, Wang JC, Ou TT, Chang KL, et al. Identification of a new single nucleotide substitution on the hypoxanthine-guanine phosphoribosyltransferase gene (HPRTTsou) from a Taiwanese aboriginal family with severe gout. J Rheumatol. 1999;26:1802–7. [PubMed] [Google Scholar]
- Cossu A, Micheli V, Jacomelli G, Carcassi A. Kelley-Seegmiller syndrome in a patient with complete hypoxanthine-guanine phosphoribosyltransferase deficiency. Clin Exp Rhematol. 2002;19:851–3. [PubMed] [Google Scholar]
- Cossu A, Orru S, Jacomelli G, Carcassi C, Contu L, Sestini S, et al. HPRT-Sardinia: a new point mutation causing HPRT deficiency without Lesch-Nyhan disease. Biochim Biophys Acta. 2006;1762:29–33. doi: 10.1016/j.bbadis.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Dancis J, Yip LC, Cox RP, Piomelli S, Balis ME. Disparate enzyme activity in erythocytes and leukocytes: a variant of hypoxanthine phosphoribosyl-transferase deficiency with an unstable enzyme. J Clin Invest. 1973;52:2068–74. doi: 10.1172/JCI107391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson BL, Tarle SA, Palella TD, Kelley WN. Molecular basis of hypoxanthine-guanine phosphoribosyltransferase deficiency in ten subjects determined by direct sequencing of amplified transcripts. J Clin Invest. 1989;84:342–6. doi: 10.1172/JCI114160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson PA, Gordon RB, Keough DT, Emmerson BT. Normal HPRT coding region in a male with gout due to HPRT deficiency. Mol Genet Metab. 2005;85:78–80. doi: 10.1016/j.ymgme.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Del Bigio MR, Halliday WC. Multifocal atrophy of cerebellar internal granular neurons in Lesch-Nyhan disease: case reports and review. J Neuropathol Exp Neurol. 2007;66 doi: 10.1097/nen.0b013e3180515319. [DOI] [PubMed] [Google Scholar]
- Dussol B, Ceballos-Picot I, Aral B, Castera V, Philip N, Berland Y. Kelley-Seegmiller syndrome due to a new variant of the hypoxanthine-guanine phosphoribosyltransferase (I136T) encoding gene (HPRT Marseille) J Inherit Metab Dis. 2004;27:543–5. doi: 10.1023/b:boli.0000037399.72152.a9. [DOI] [PubMed] [Google Scholar]
- Ea HK, Bardin T, Jinnah HA, Aral B, Liote F, Ceballos-Picot I. Severe gouty arthritis and mild neurological symptoms due to a new variant of the hypoxanthine-guanine phosphoriboysltransferase (F199C) Arthritis Rheum. 2009;60:2201–4. doi: 10.1002/art.24617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egami K, Yitta S, Kasim S, Lewers JC, Roberts RC, Lehar M, et al. Basal ganglia dopamine loss due to defect in purine recycling. Neurobiol Dis. 2007;26:396–407. doi: 10.1016/j.nbd.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson BT, Thompson L. The spectrum of hypoxanthine-guanine phosphoribosyltranferase deficiency. Quart J Med. 1973;166:423–40. [PubMed] [Google Scholar]
- Ernst M, Zametkin AJ, Matochik JA, Pascualvaca D, Jons PH, Hardy K, et al. Presynaptic dopaminergic deficits in Lesch-Nyhan disease. N Engl J Med. 1996;334:1568–72. doi: 10.1056/NEJM199606133342403. [DOI] [PubMed] [Google Scholar]
- Fairbanks LD, Simmonds HA, Webster DR. Use of intact erythrocytes in the diagnosis of inherited purine and pyrimidine disorders. J Inherit Metab Dis. 1987;10:174–86. doi: 10.1007/BF01800045. [DOI] [PubMed] [Google Scholar]
- Gaigl Z, Shin YS, Gathof BS. Novel splice mutation in a patient with partial hypoxanthine-guanine phosphoribosyltransferase (HPRT) deficiency. J Inherit Metab Dis. 2001;24(Supp 1):142. [Google Scholar]
- Garcia MG, Torres RJ, Prior C, Puig JG. Normal HPRT coding region in complete and partial HPRT deficiency. Mol Genet Metab. 2008;94:167–72. doi: 10.1016/j.ymgme.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Geerdink RA, De Vries WHM, Willemse J, Oei TL, De Bruyn CHMM. An atypical case of hypoxanthine-guanine phosphoribosyltransferase deficiency (Lesch-Nyhan syndrome) I. clinical studies. Clin Genet. 1973;4:348–52. doi: 10.1111/j.1399-0004.1973.tb01930.x. [DOI] [PubMed] [Google Scholar]
- Gottlieb RP, Koppel MM, Nyhan WL, Bakay B, Nissinen E, Borden M, et al. Hyperuricaemia and choreoathetosis in a child without mental retardation or self-mutilation – a new HPRT variant. J Inherit Metab Dis. 1982;5:183–6. doi: 10.1007/BF02179135. [DOI] [PubMed] [Google Scholar]
- Hersh JH, Page T, Hand ME, Seegmiller JE, Nyhan WL, Weisskopf B. Clinical correlations in partial hypoxanthine guanine phosphoribosyltransferase deficiency. Pediatr Neurol. 1986;2:302–4. doi: 10.1016/0887-8994(86)90025-1. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Laos RI, Kedar A, Williams CA, Neiberger RE. A new point mutation in a hypoxanthine phosphoribosyltransferase-deficient patient. Pediatr Nephrol. 1997;11:645–8. doi: 10.1007/s004670050357. [DOI] [PubMed] [Google Scholar]
- Holland MJ, DiLorenzo AM, Dancis J, Balis ME, Yu TF, Cox RP. Hypoxanthine phosphoribosyltransferase activity in intact fibroblasts from patients with X-linked hyperuricemia. J Clin Invest. 1976;57:1600–5. doi: 10.1172/JCI108430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter TC, Melancon SB, Dallaire L, Taft S, Skopek TR, Albertini RJ, et al. Germinal HPRT splice donor site mutation results in multiple RNA splicing products in T-lymphocyte cultures. Somat. Cell Mol Genet. 1996;22:145–50. doi: 10.1007/BF02369904. [DOI] [PubMed] [Google Scholar]
- Jinnah HA, DeGregorio L, Harris JC, Nyhan WL, O'N;eill JP. The spectrum of inherited mutations causing HPRT deficiency: 75 new cases and a review of 196 previously reported cases. Mutat Res. 2000;463:309–26. doi: 10.1016/s1383-5742(00)00052-1. [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Friedmann T. Lesch-Nyhan disease and its variants. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill; 2001. pp. 2537–70. [Google Scholar]
- Jinnah HA, Harris JC, Nyhan WL, O'N;eill JP. The spectrum of mutations causing HPRT deficiency: an update. Nucleosides, Nucleotides, Nucleic Acids. 2004;23:1153–60. doi: 10.1081/NCN-200027400. [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Jones MD, Wojcik BE, Rothstein JD, Hess EJ, Friedmann T, et al. Influence of age and strain on striatal dopamine loss in a genetic mouse model of Lesch-Nyhan disease. J Neurochem. 1999;72:225–9. doi: 10.1046/j.1471-4159.1999.0720225.x. [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Langlais PJ, Friedmann T. Functional analysis of brain dopamine systems in a genetic mouse model of Lesch-Nyhan syndrome. J Pharmacol Exp Ther. 1992;263:596–607. [PubMed] [Google Scholar]
- Jinnah HA, Lewis RF, Visser JE, Eddey GE, Barabas G, Harris JC. Ocular motor dysfunction in Lesch-Nyhan disease. Pediatr Neurol. 2001;24:200–4. doi: 10.1016/s0887-8994(00)00265-4. [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Visser JE, Harris JC, Verdu A, Larovere L, Ceballos-Picot I, et al. Delineation of the motor disorder of Lesch-Nyhan disease. Brain. 2006;129:1201–17. doi: 10.1093/brain/awl056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinnah HA, Wojcik BE, Hunt MA, Narang N, Lee KY, Goldstein M, et al. Dopamine deficiency in a genetic mouse model of Lesch-Nyhan disease. J Neurosci. 1994;14:1164–75. doi: 10.1523/JNEUROSCI.14-03-01164.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WN, Greene ML, Rosenbloom FM, Henderson JF, Seegmiller JE. Hypoxanthine-guanine phosphoribosyltransferase deficiency in gout. Ann Int Med. 1969;70:155–206. doi: 10.7326/0003-4819-70-1-155. [DOI] [PubMed] [Google Scholar]
- Kiziltan G, Akalin MA. Stuttering may be a type of action dystonia. Mov Disord. 1996;11:278–82. doi: 10.1002/mds.870110311. [DOI] [PubMed] [Google Scholar]
- Larovere LE, O'N;eill JP, Randall M, Fairbanks LD, Guelbert N, Czornyi L, et al. Hypoxanthine-guanine phosphoribosyltransferase deficiency: biochemical and molecular findings in six Argentine patients. Nucleosides Nucleotides Nucleic Acids. 2007;26:255–8. doi: 10.1080/15257770701257269. [DOI] [PubMed] [Google Scholar]
- Larovere LE, Romero N, Fairbanks LD, Conde C, Guelbert N, Rosa AL, et al. A novel missense mutation, c.584A>C (Y195S), in two unrelated Argentine patients with hypoxanthine-guanine phosphoribosyl-transferase deficiency, neurological variant. Mol Genet Med. 2004;81:352–4. doi: 10.1016/j.ymgme.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Lesch M, Nyhan WL. A familial disorder of uric acid metabolism and central nervous system function. Am J Med. 1964;36:561–70. doi: 10.1016/0002-9343(64)90104-4. [DOI] [PubMed] [Google Scholar]
- Lewers JC, Ceballos-Picot I, Shirley TL, Mockel L, Egami K, Jinnah HA. Consequences of impaired purine recycling in dopaminergic neurons. Neuroscience. 2008;152:761–72. doi: 10.1016/j.neuroscience.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightfoot T, Lewkonia RM, Snyder FF. Sequence, expression, and characterization of HPRTMoose Jaw: a point mutation resulting in cooperativity and decreased substrate affinities. Hum Mol Genet. 1994;3:1377–81. doi: 10.1093/hmg/3.8.1377. [DOI] [PubMed] [Google Scholar]
- Lloyd KG, Hornykiewicz O, Davidson L, Shannak K, Farley I, Goldstein M, et al. Biochemical evidence of dysfunction of brain neurotransmitters in the Lesch-Nyhan syndrome. N Engl J Med. 1981;305:1106–11. doi: 10.1056/NEJM198111053051902. [DOI] [PubMed] [Google Scholar]
- Mak BS, Shi CS, Tsai CR, Lee WJ, Lin HY. New mutations of the HPRT gene in Lesch-Nyhan syndrome. Pediatr Neurol. 2000;23:332–5. doi: 10.1016/s0887-8994(00)00199-5. [DOI] [PubMed] [Google Scholar]
- Marcus S, Christensen E, Malm G. Molecular analysis of the mutations in five unrelated patients with the Lesch Nyhan syndrome. Hum Mutat. 1993;2:473–7. doi: 10.1002/humu.1380020608. [DOI] [PubMed] [Google Scholar]
- Mateos EA, Puig JG. Purine metabolism in Lesch-Nyhan syndrome versus Kelley-Seegmiller syndrome. J Inherit Metab Dis. 1994;17:138–42. doi: 10.1007/BF00735419. [DOI] [PubMed] [Google Scholar]
- Matthews WS, Solan A, Barabas G. Cognitive functioning in Lesch-Nyhan syndrome. Dev. Med. Child Neurol. 1995;37:715–22. doi: 10.1111/j.1469-8749.1995.tb15017.x. [DOI] [PubMed] [Google Scholar]
- McDonald JA, Kelley WN. Lesch-Nyhan syndrome: altered kinetic properties of mutant enzyme. Science. 1971;171:689–90. doi: 10.1126/science.171.3972.689. [DOI] [PubMed] [Google Scholar]
- Nausieda PA, Weiner WJ, Klawans HL. Dystonic foot response of Parkinsonism. Arch Neurol. 1980;37:132–6. doi: 10.1001/archneur.1980.00500520030003. [DOI] [PubMed] [Google Scholar]
- Nyhan WL. Behavior in the Lesch-Nyhan syndrome. J Autism Child Schizophren. 1976;6:235–52. doi: 10.1007/BF01543464. [DOI] [PubMed] [Google Scholar]
- Nyhan WL. Ataxia and disorders of purine metabolism: defects in hypoxanthine guanine phosphoribosyl transferase and clinical ataxia. Adv Neurol. 1978;21:279–87. [PubMed] [Google Scholar]
- O'N;eill JP, Rogan PK, Cariello N, Nicklas JA. Mutations that alter RNA splicing of the human HPRT gene: a review of the spectrum. Mutat Res. 1998;411:179–214. doi: 10.1016/s1383-5742(98)00013-1. [DOI] [PubMed] [Google Scholar]
- Page T, Bakay B, Nissinen E, Nyhan WL. Hypoxanthine-guanine phosphoribosyltranferase variants: correlation of clinical phenotype with enzyme activity. J Inherit Metab Dis. 1981;4:203–6. doi: 10.1007/BF02263652. [DOI] [PubMed] [Google Scholar]
- Page T, Nyhan WL. The spectrum of HPRT deficiency: an update. Adv Exp Med Biol. 1989;253A:129–33. doi: 10.1007/978-1-4684-5673-8_20. [DOI] [PubMed] [Google Scholar]
- Page T, Nyhan WL, Morena de Vega V. Syndrome of mild mental retardation, spastic gait, and skeletal malformations in a family with partial deficiency of hypoxathine-guanine phosphoribosyltransferase. Pediatrics. 1987;79:713–7. [PubMed] [Google Scholar]
- Puig JG, Torres RJ, Mateos FA, Ramos TH, Arcas JM, Buno AS, et al. The spectrum of hypoxanthine-guanine phosphoribosyltransferase deficiency: Clinical experience based on 22 patients from 18 Spanish families. Medicine. 2001;80:102–12. doi: 10.1097/00005792-200103000-00003. [DOI] [PubMed] [Google Scholar]
- Puig JG, Torres RJ, Verdu A, Jinnah HA. An unusual cause of stuttering. In: Reich SG, editor. Movement disorders: 100 instructive cases. London: Informa Healthcare; 2008. pp. 349–51. [Google Scholar]
- Rijksen G, Staal GEJ, van der Vlist MJM, Beemer FA, Troost J, Gutensohn W, et al. Partial hypoxanthine-guanine phosphoribosyl transferase deficiency with full expression of the Lesch-Nyhan syndrome. Hum Genet. 1981;57:39–47. doi: 10.1007/BF00271165. [DOI] [PubMed] [Google Scholar]
- Saito Y, Ito M, Hanaoka S, Ohama E, Akaboshi S, Takashima S. Dopamine receptor upregulation in Lesch-Nyhan syndrome: a postmortem study. Neuropediatrics. 1999;30:66–71. doi: 10.1055/s-2007-973462. [DOI] [PubMed] [Google Scholar]
- Schretlen DS, Harris JC, Park KS, Jinnah HA, Ojeda del Pozo N. Neurocognitive functioning in Lesch-Nyhan disease and partial hypoxanthine-guanine phosphoribosyltransferase deficiency. J Int Neuropsychol Soc. 2001;7:805–12. doi: 10.1017/s135561770177703x. [DOI] [PubMed] [Google Scholar]
- Schretlen DS, Ward J, Meyer SM, Yun J, Puig JG, Nyhan WL, et al. Behavioral aspects of Lesch-Nyhan disease and it variants. Dev Med Child Neurol. 2005;47:673–7. doi: 10.1017/S0012162205001374. [DOI] [PubMed] [Google Scholar]
- Sege-Peterson K, Chambers J, Page T, Jones OW, Nyhan WL. Characterization of mutations in phenotypic variants of hypoxanthine phosphoribosyltransferase deficiency. Hum Mol Genet. 1992;1:427–32. doi: 10.1093/hmg/1.6.427. [DOI] [PubMed] [Google Scholar]
- Snyder FF, Chudley AE, MacLeod PM, Carter RJ, Fung E, Lowe JK. Partial deficiency of hypoxanthine-guanine phosphoribosyltransferase with reduced affinity for PP-ribose-P in four related males with gout. Hum Genet. 1984;67:18–22. doi: 10.1007/BF00270552. [DOI] [PubMed] [Google Scholar]
- Srivastava T, O'N;eill JP, Dasouki M, Simckes AM. Childhood hyperuricemia and acute renal failure resulting from a missense mutation in the HPRT gene. Am J Med Genet. 2002;108:219–22. doi: 10.1002/ajmg.10217. [DOI] [PubMed] [Google Scholar]
- Torres RJ, Garcia MG, Puig JG. Nucleosides, Nucleotides, Nucl Acids. 2010. Partial HPRT deficiency phenotype and incomplete splicing mutation. (in press) [DOI] [PubMed] [Google Scholar]
- Torres RJ, Mateos FA, Molano J, Gathoff BS, O'N;eill JP, Gundel RM, et al. Molecular basis of hypoxanthine-guanine phosphoribosyltransferase deficiency in thirteen Spanish families. Hum Mutat. 2000;15:383. doi: 10.1002/(SICI)1098-1004(200004)15:4<383::AID-HUMU17>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Visser JE, Baer PR, Jinnah HA. Lesch-Nyhan syndrome and the basal ganglia. Brain Res Rev. 2000;32:449–75. doi: 10.1016/s0165-0173(99)00094-6. [DOI] [PubMed] [Google Scholar]
- Wong DF, Harris JC, Naidu S, Yokoi F, Marenco S, Dannals RF, et al. Dopamine transporters are markedly reduced in Lesch-Nyhan disease in vivo. Proc Natl Acad Sci USA. 1996;93:5539–43. doi: 10.1073/pnas.93.11.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CH, Lai HM, Yang MC, Liaw CC, Chang SJ, Ko YC, et al. Identification of a new single-nucleotide mutation on the hypoxanthine-guanine phosphoribosyltransferase gene from 983 cases with gout in Taiwan. J Rheumatol. 2007;34:794–7. [PubMed] [Google Scholar]
- Yang TP, Patel PI, Chinault AC, Stout JT, Jackson LG, Hildebrand BM, et al. Molecular evidence for new mutation at the hprt locus in Lesch-Nyhan patients. Nature. 1984;310:412–4. doi: 10.1038/310412a0. [DOI] [PubMed] [Google Scholar]
- Yang TP, Stout JT, Konecki DS, Patel PI, Alford RL, Caskey CT. Spontaneous reversion of novel Lesch-Nyhan mutation by HPRT gene rearrangement. Somat Cell Mol Genet. 1988;14:293–303. doi: 10.1007/BF01534590. [DOI] [PubMed] [Google Scholar]
- Yeh J, Zheng S, Howard BD. Impaired differentiation of HPRT-deficient dopaminergic neurons: a possible mechanism underlying neuronal dysfunction in Lesch-Nyhan syndrome. J Neurosci Res. 1998;53:78–85. doi: 10.1002/(SICI)1097-4547(19980701)53:1<78::AID-JNR8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Zoref-Shani E, Feinstein S, Frishberg Y, Sperling O. Kelley-Seegmiller syndrome due to a unique variant of hypoxanthine-guanine phosphoribosyltransferase: reduced affinity for 5-phosphoribosy1-pyrophosphate manifested only at low, physiological substrate concentrations. Biochim Biophys Acta. 2000;1500:197–203. doi: 10.1016/s0925-4439(99)00103-9. [DOI] [PubMed] [Google Scholar]