Abstract
Neurodegeneration with brain iron accumulation encompasses a heterogeneous group of rare neurodegenerative disorders that are characterized by iron accumulation in the brain. Severe generalized dystonia is frequently a prominent symptom and can be very disabling, causing gait impairment, difficulty with speech and swallowing, pain and respiratory distress. Several case reports and one case series have been published concerning therapeutic outcome of pallidal deep brain stimulation in dystonia caused by neurodegeneration with brain iron degeneration, reporting mostly favourable outcomes. However, with case studies, there may be a reporting bias towards favourable outcome. Thus, we undertook this multi-centre retrospective study to gather worldwide experiences with bilateral pallidal deep brain stimulation in patients with neurodegeneration with brain iron accumulation. A total of 16 centres contributed 23 patients with confirmed neurodegeneration with brain iron accumulation and bilateral pallidal deep brain stimulation. Patient details including gender, age at onset, age at operation, genetic status, magnetic resonance imaging status, history and clinical findings were requested. Data on severity of dystonia (Burke Fahn Marsden Dystonia Rating Scale—Motor Scale, Barry Albright Dystonia Scale), disability (Burke Fahn Marsden Dystonia Rating Scale—Disability Scale), quality of life (subjective global rating from 1 to 10 obtained retrospectively from patient and caregiver) as well as data on supportive therapy, concurrent pharmacotherapy, stimulation settings, adverse events and side effects were collected. Data were collected once preoperatively and at 2–6 and 9–15 months postoperatively. The primary outcome measure was change in severity of dystonia. The mean improvement in severity of dystonia was 28.5% at 2–6 months and 25.7% at 9–15 months. At 9–15 months postoperatively, 66.7% of patients showed an improvement of 20% or more in severity of dystonia, and 31.3% showed an improvement of 20% or more in disability. Global quality of life ratings showed a median improvement of 83.3% at 9–15 months. Severity of dystonia preoperatively and disease duration predicted improvement in severity of dystonia at 2–6 months; this failed to reach significance at 9–15 months. The study confirms that dystonia in neurodegeneration with brain iron accumulation improves with bilateral pallidal deep brain stimulation, although this improvement is not as great as the benefit reported in patients with primary generalized dystonias or some other secondary dystonias. The patients with more severe dystonia seem to benefit more. A well-controlled, multi-centre prospective study is necessary to enable evidence-based therapeutic decisions and better predict therapeutic outcomes.
Keywords: Neurodegeneration with brain iron accumulation, dystonia, deep brain stimulation, globus pallidus
Introduction
Neurodegeneration with brain iron accumulation (NBIA) encompasses a heterogeneous group of rare progressive disorders which are characterized by iron accumulation in the brain (Hayflick et al., 2003). Diagnosis is usually made according to the following criteria: (i) progressive disorder; with (ii) at least one of the following symptoms: dystonia, rigidity, tremor, bradykinesia or choreoathetosis; and (iii) an abnormal MRI with hypointensity of the pallidum on the T2-weighted images (Swaiman, 2001).
Several genetic subtypes of NBIA are currently emerging. The most common subtype, pantothenate kinase-associated neurodegeneration, is caused by mutations in the PANK2 gene (Zhou et al., 2001) and accounts for ∼50% of cases of NBIA (Hayflick et al., 2003). Further genetic defects associated with NBIA are mutations in the PLA2G6 gene (Morgan et al., 2006), and disorders of iron metabolism, such as aceruloplasminaemia (Harris, 1995) and neuroferritinopathy (Curtis et al., 2001). Patients exhibiting symptoms typical of NBIA without any of these known mutations are classified as ‘idiopathic NBIA’ and their disease is probably caused by, as yet unknown, other genes (Gregory et al., 2009).
The clinical presentation, the age of onset and the progression rate of NBIA are highly variable, even amongst siblings with identical underlying mutations, and there is a considerable degree of phenotypic heterogeneity even in cases with PANK2 mutation-positive status (Thomas et al., 2004). Severe generalized dystonia is frequently a prominent clinical symptom of NBIA, which can be very disabling and cause gait impairment, difficulty with speech and swallowing, pain, respiratory distress and, in some cases, death due to dystonic storm, respiratory failure or infection. Due to small patient numbers, pharmacotherapy of dystonia in NBIA is not evidence-based, and thus guided by therapeutic experiences in other forms of dystonia. Patients are often treated with anticholinergics, such as trihexiphenidyl or gamma-aminobutyric acid agonists such as benzodiazepines or baclofen, orally or intrathecally (Albright et al., 1996). There are some reports of botulinum toxin being effective (Dressler et al., 2001) for particularly bothersome focal symptoms, and many patients receive regular botulinum toxin injections. Levodopa has been reported to have a positive effect on some of the disabling movements seen in NBIA patients without PANK2 mutations (Gregory and Hayflick 2005; Clement et al., 2007). However, in most cases with severe dystonia, pharmacological therapy, including botulinum toxin injections, is unsatisfactory. The limited benefits of medical treatments have prompted early attempts with neurosurgical therapy.
Several cases have been published in which there has been improvement in dystonia associated with NBIA after bilateral thalamotomy (Tsukamoto et al., 1992) as well as unilateral (Justesen et al., 1999) or bilateral pallidotomy (Kyriagis et al., 2004; Balas et al., 2006). However, since stereotactic lesioning is an irreversible procedure, its use in children and adolescents raises several concerns (especially for bilateral procedures, which have higher risk of severe side effects). With the advent of deep brain stimulation (DBS) as an effective and reversible therapeutic measure to treat dystonia, the number of stereotactic lesioning procedures has dropped considerably. The DBS target of choice in dystonia is presently the globus pallidus internus (GPi). Safety and efficacy of bilateral pallidal DBS (hereafter referred to as GPi-DBS) in primary dystonia has been documented in two prospective double-blind trials with either primary generalized or primary segmental dystonia (Vidailhet et al., 2005; Kupsch et al., 2006). For secondary dystonias, data are more limited and therapeutic outcomes are mixed. Good results have so far been obtained in patients with tardive dystonias (Trottenberg et al., 2005; Sako et al., 2008). In other forms of secondary dystonias, such as dystonia-plus syndromes, post-traumatic, post-anoxic, post-encephalitic or cerebral palsy associated dystonia, small cohorts and single cases have been reported and the results are variable (Loher et al., 2000; Zorzi et al., 2005; Vidailhet et al., 2009).
To date, several case reports (Umemura et al., 2004; Sharma et al., 2005; Krause et al., 2006; Shields et al., 2007; Mikati et al., 2009) and one series of six patients (Castelnau et al., 2005) have been published concerning therapeutic outcome of bilateral GPi-DBS in NBIA-dystonia. Four of the five cases report a favourable outcome. However, with case studies, there may be a reporting bias towards favourable outcome. Furthermore, factors predicting a favourable outcome of DBS cannot be estimated from small series.
Thus, we initiated a multi-centre retrospective study with the objective of gathering worldwide experiences with bilateral GPi-DBS in patients with NBIA. Our goal was to provide a wide and unselected coverage of NBIA patients operated on so far by contacting movement disorders centres, surgical centres and patient support groups worldwide. We hypothesized that GPi-DBS in patients with NBIA reduces dystonia as assessed by the Burke Fahn Marsden Dystonia Rating Scale (BFMDRS), but is overall less effective than reported in single cases and small series published thus far.
Materials and methods
A total of 16 international centres were involved in this retrospective study. The centres are listed in Appendix A.
Recruitment
DBS and movement disorders centres around the world were contacted and asked to contribute patients with NBIA who were treated with GPi-DBS. We received a contact list of all centres worldwide known to implant DBS devices by the only manufacturer at the time (Medtronic Inc.). All of these were contacted a minimum of two times, either via email or phone. A similar recruitment approach has been used previously in retrospective multi-centre studies (Voon et al., 2008). In parallel, contacts with patients and families were established through patient organizations, most notably ‘Hoffnungsbaum e. V.’, the German NBIA Association, as well as the NBIA Disorders Association, which is the patient organization in the United States. Additionally, two of the patients were extracted from previous publications, either entirely (Umemura et al., 2004) or partially (Krause et al., 2006). Centres were asked to contribute every patient regardless of outcome.
Inclusion criteria
Inclusion criteria were: (i) confirmed NBIA (as assessed by a specialized centre); (ii) presence of moderate to severe dystonia; and (iii) implantation of bilateral GPi-DBS to treat dystonia. Diagnostic criteria for NBIA used here were: (i) progressive disorder; with (ii) at least one of the following features: dystonia, rigidity, tremor, bradykinesia, choreoathetosis; and (iii) the presence of abnormal MRI with hyperintensity of the pallidum on T1 images and hypointensity on T2 images (Swaiman, 2001).
Protocol
All centres able to contribute data or cases were sent a standardized data sheet. First, patient details including gender, age at onset, age at diagnosis, age at operation, genetic status, MRI status, history and clinical findings were requested. Secondly, data on severity of dystonia were collected, including (if available) the BFMDRS motor (BFMDRS-M) and disability (BFMDRS-D) scores (Burke et al., 1985) and the Barry Albright Dystonia Scale (Barry et al., 1999). Thirdly, data on quality of life were requested in the form of a retrospective subjective global rating (from 1 to 10; 0 equalling no quality of life, 10 equivalent to maximal quality of life) by patient and caregiver. Finally, data on supportive therapy, concurrent pharmacotherapy, stimulation settings, adverse events and side effects were also requested. Measures of severity of dystonia, quality of life, stimulation settings and concurrent pharmacotherapy were collected preoperatively, at 2–6 months postoperatively, as well as at 9–15 months postoperatively. A summary of all data collected during the study can be found in Table 1.
Table 1.
Parameters collected during the study
| Study protocol |
|---|
| Preoperative assessment |
| Patient history (age at operation, age at onset, disease duration, date of birth, gender) |
| Neurological examination |
| Brain MRI status |
| Genetic testing (if available) |
| Burke Fahn Marsden Dystonia Rating Scale (severity of dystonia and disability) |
| Barry Albright Dystonia Scale (severity of dystonia) |
| Subjective quality of life rating (patient and caregiver) |
| Medication, supportive therapy |
| Assessment at 2–6 and 9–15 months postoperatively |
| Burke Fahn Marsden Dystonia Rating Scale (severity of dystonia and disability) |
| Barry Albright Dystonia Scale (severity of dystonia) |
| Subjective quality of life rating (patient and caregiver) |
| Stimulation settings, target point |
| Medication, supportive therapy |
| Adverse events, side effects |
Outcome measures
Change in severity of dystonia as measured by the BFMDRS-M (Burke et al., 1985) was chosen as the primary outcome measure. The clinically relevant difference for BFMDRS-M was set at 20% or more, in analogy to previous studies of secondary dystonia (Vidailhet et al., 2009). To confirm the findings of the BFMDRS-M in this heterogeneous cohort, we employed a second dystonia scale, the Barry Albright Dystonia Scale (Barry et al., 1999), which is particularly suited for paediatric dystonia. Secondary outcome measures were (i) disability (as assessed using the BFMDRS-D); and (ii) quality of life as assessed using a global rating of quality of life by caregiver. An improvement of 20% or more in the BFMDRS-D was considered clinically relevant, as was a 20% improvement in global quality of life ratings.
Data analysis
One patient was excluded from the analysis because the stimulation electrodes were not located in the GPi. Normality of data distribution was tested using a Kolmogorov–Smirnov Test. All data were normally distributed. Percentages of patients who attained the clinically relevant difference were calculated. Then, t-tests for dependent samples were performed comparing values obtained for preoperative severity of dystonia, disability and quality of life to values obtained at 2–6 months as well as at 9–15 months postoperatively. To account for cases in which data were missing, analysis was performed for each variable (e.g. BFMDRS-M) using only those cases for which values were available at all three time points. Thus, different cases may be included for different parameters. For the secondary outcome measures, we performed a Bonferroni correction to account for multiple t-tests. We also performed a linear regression to determine if we could identify, preoperatively, factors that were predictive of improvement in severity of dystonia and other measures of therapeutic outcome. Because several linear regressions were calculated, a Bonferroni correction was applied to adjust the level of significance accordingly. Data analysis was performed using Statistical Package for the Social Sciences 17.0.
The study was conducted with approval of the local Ethics Committee of the University Hospital of Cologne, and was carried out according to the Guidelines of the Declaration of Helsinki. Informed written consent was obtained either from patients or, in the case of patients who were either unable to give their written consent or were minors, from their legal representatives. All data were then entered into a database in an anonymous format according to the data protection laws in Germany.
Results
In total, 23 patients were included in the data analysis. Four of these have already been published elsewhere (Kurlemann et al., 1991; Umemura et al., 2004; Krause et al., 2006; Shields et al., 2007). Patient details can be found in Table 2; preoperative mean descriptive group statistics are given in Table 3.
Table 2.
Patient characteristics
| Patient number | Age at onset of symptoms (years) | Age at time of diagnosis (years) | Age at operation (years) | Duration of disease (years) | Gender | Genetics | MRI |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 5 | 6 | 5 | Female | PKAN | Eye of the tiger |
| 2 | 1 | 6 | 9 | 8 | Male | PKAN | Eye of the tiger |
| 3 | 2 | 11 | 16 | 14 | Male | PKAN | Eye of the tiger |
| 4 | 2 | 5 | 12 | 10 | Female | Not tested | Eye of the tiger |
| 5 | 2 | 5 | 6 | 4 | Female | PKAN | Eye of the tiger |
| 6 | 2 | 5 | 9 | 7 | Female | Not tested | Eye of the tiger |
| 7 | 3 | 5 | 12 | 9 | Male | Not tested | Eye of the tiger |
| 8 | 4 | 8 | 14 | 10 | Male | PKAN | Eye of the tiger |
| 9a | 6 | 10 | 13 | 7 | Male | PKAN | Eye of the tiger |
| 10b | 8 | 10 | 17 | 9 | Male | PKAN | Eye of the tiger |
| 11c | 8 | 36 | 36 | 28 | Male | Not tested | Eye of the tiger |
| 12 | 9 | 11 | 13 | 4 | Male | PKAN | Eye of the tiger |
| 13 | 9 | 11 | 16 | 7 | Female | PKAN | Eye of the tiger |
| 14 | 9 | 13 | 17 | 8 | Male | PKAN | Eye of the tiger |
| 15 | 10 | 13 | 17 | 7 | Male | Not tested | Eye of the tiger |
| 16d | 11 | 13 | 29 | 18 | Female | Non-PKAN | Eye of the tiger |
| 17 | 12 | 16 | 32 | 20 | Female | PKAN | Eye of the tiger |
| 18 | 12 | 12 | 15 | 3 | Male | PKAN | Eye of the tiger |
| 19 | 12 | 13 | 24 | 12 | Female | PKAN | Eye of the tiger |
| 20 | 14 | 16 | 20 | 6 | Female | Not tested | Eye of the tiger |
| 21 | 14 | 33 | 36 | 22 | Male | PKAN | Eye of the tiger |
| 22 | 14 | 19 | 27 | 13 | Male | Not tested | Eye of the tiger |
| 23 | 15 | 15 | 19 | 4 | Male | Not tested | Eye of the tiger |
| Mean ± SD | 7.8 ± 4.8 | 12.7 ± 8.0 | 18.0 ± 8.8 | 10.2 ± 6.4 | 10 females (43.5 %) 13 males (56.5 %) | 60.9% PKAN 4.3 % non-PKAN 34.8 % not tested |
Table 3.
Mean characteristics of group preoperatively
| n | Mean ± SD | Range | |
|---|---|---|---|
| Age at onset (years) | 23 | 7.8 ± 4.8 | 1.0–15.0 |
| Age at diagnosis (years) | 23 | 12.7 ± 8.0 | 5.0–36.0 |
| Age at operation (years) | 23 | 18.0 ± 8.8 | 6.0–36.0 |
| Disease duration (years) | 23 | 10.2 ± 6.4 | 3.0–28.0 |
| BFMDRS-M (out of 120) | 21 | 71.2 ± 26.0 | 21.0–112.0 |
| BFMDRS-D (out of 30) | 22 | 21.0 ± 5.8 | 9.0–30.0 |
| Barry Albright Dystonia Scale (out of 32) | 21 | 21.0 ± 6.3 | 6.0–30.0 |
| Global quality of life—patient (out of 10) | 16 | 3.7 ± 2.8 | 0–9.0 |
| Global quality of life—caregiver (out of 10) | 21 | 3.0 ± 2.5 | 0–9.0 |
| Care and Comfort Hypertonicity Questionnaire (out of 189) | 17 | 104.1 ± 41.8 | 31.0–177.0 |
Primary outcome measure
Severity of dystonia
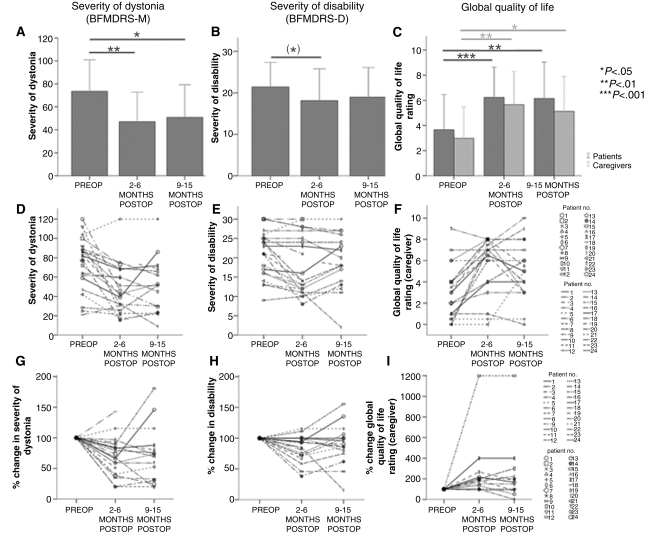
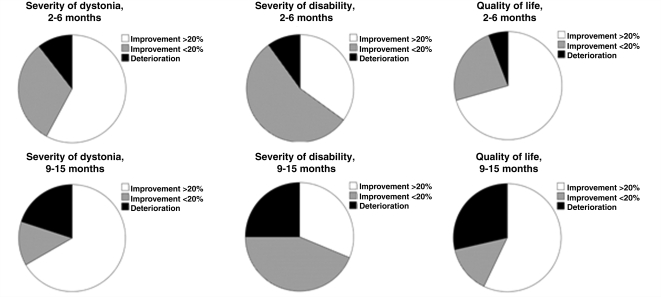
BFMDRS-M at all three time points were available for 14 patients. The severity of dystonia, as assessed by the BFMDRS-M, improved significantly after GPi-DBS both at 2–6 months (T = 3.6, P < 0.01) and at 9–15 months postoperatively (T = 2.7, P < 0.05) compared to before surgery (Fig. 1A). The mean improvement in severity of dystonia was 28.5% at 2–6 months and 25.7% at 9-15 months. Of 19 patients for whom a BFMDRS-M was available at 2–6 months, 11 (57.9%) showed an improvement of 20% or more. At 9–15 months, 10 of 15 patients (66.7%) showed an improvement of 20% or more on the BFMDRS-M (see also pie chart in Fig. 2A; for analysis based on absolute values, see Supplementary Fig. S1). A line diagram of individual patients’ outcomes is shown in Fig. 1D and G: of 11 patients who had a clinically relevant improvement at 2–6 months, 8 (72.7%) maintained an improvement of 20% or more, one declined, and no information was available on the remaining two. Of the eight patients who did not improve at 2–6 months, two (25%) improved more than 20% at 9–15 months, two (25%) remained the same and one (12.5%) worsened. No information was available on the other three patients at 9–15 months.
Figure 1.
Bar charts (A–C) and line plots (D–I) of outcomes for BFMDRS-M (A, D and G), BFMDRS-D (B, E and H) and global quality of life ratings (C, F and I). Means ± 2 SEM are shown in the bar charts. The line plots show individual values per patient plotted for each time point, the middle panel showing original data, the lower panel depicting percentage change at 2–6 and 9–15 months with preoperative values set to 100% to make improvements and deteriorations easier to distinguish. Significance levels are given on the right. Parentheses signify that the result is not significant any longer after Bonferroni correction.
Figure 2.
Pie charts of relative improvements in (A) severity of dystonia (BFMDRS-M), (B) severity of disability (BFMDRS-D), and (C) global quality of life ratings rated by caregivers. The upper panel shows change at 2–6 months, the lower panel shows change at 9–15 months.
The results obtained from the Barry Albright Dystonia Scale showed comparable results (n = 14) with a significant improvement in severity of dystonia both at 2–6 months (T = 4.3, P < 0.001) as well as at 9–15 months (T = 3.2, P < 0.01).
Secondary outcome measures
Two secondary outcome measures were investigated, namely the BFMDRS disability scale and the global quality of life ratings by the caregivers. Thus, using an initial significance level of P = 0.05, after Bonferroni correction (two measures, two paired t-tests each), the adjusted significance level applied was P = 0.01.
Disability
BFMDRS-D at all three time points were available for 15 patients. For these 15, BFMDRS-D was not significantly improved at either 2–6 months postoperatively (T = 2.5), or at 9–15 months postoperatively (T = 1.6, Fig. 1B). Out of 20 patients for whom a BFMDRS-D was available at 2–6 months, 7 (35.0%) showed an improvement of more than 20%. Out of the 16 patients for whom a BFMDRS-D was available at 9–15 months, 5 (31.3%) showed an improvement of 20% or more (for a pie chart of these results, see Fig. 2B). However, the mean improvement for the entire group at 2–6 months was only 15.8% and decreased further at 9–15 months to 9.7%. A line diagram of individual patients’ outcomes is shown in Figs 1E and H.
Quality of life
The subjective global ratings obtained retrospectively from both patients and caregivers were used. Caregiver ratings were available for all three time points in 17 patients, whereas all patient ratings were only available for 14 patients. Since some of the patients probably had cognitive deficits and assessment of quality of life in children using abstract numbers is of limited validity, we decided to use only caregiver ratings as a secondary outcome measure. There was a significant improvement in quality of life as rated by the caregiver both at 2–6 months (T = 4.3, P < 0.001), and at 9–15 months (T = 3.1, P < 0.01). These results are shown alongside the patient ratings in Fig. 1C. There was an 80.4% median improvement in quality of life at three months and an 83.3% median improvement at 9–15 months. At 2–6 months, 14 (70.0%) out of 20 caregivers rated an improvement in quality of life of 20% or more. At 9–15 months, 11 (64.7%) out of 17 caregivers rated an improvement in quality of life for the patient of 20% or more. A pie chart of these results is shown in Fig. 2C. A line diagram of individual patients’ outcomes is shown in Fig. 1F and I. There was no significant difference in quality of life ratings between 2–6 months and 9–15 months.
Prediction of therapeutic outcome
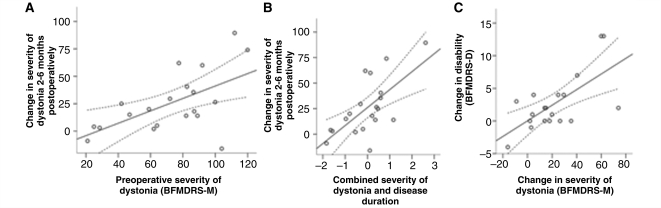
Given the wide range of outcomes in severity of dystonia, we atempted to identify predictors of outcome. We found that the preoperative severity of dystonia, as assessed by the BFMDRS-M, predicts improvement in dystonia at 2–6 months and at 9–15 months post-surgery (linear regression, corr. r2= 0.31, F = 9.4, P < 0.01, Fig. 3A; linear regression, corr. r2= 0.29, F = 7.3, P < 0.05, respectively). Thus, patients with more severe dystonia improved more. However, the finding at 9–15 months was not significant at the Bonferroni-corrected significance level of P = 0.01. When the absolute BFMDRS-M are replaced by percentage improvements, the correlation is no longer significant, although there is a trend for significance (P = 0.051 and P = 0.087, respectively). The improvement in severity of dystonia at 2–6 months is better predicted by duration of disease and severity of dystonia preoperatively (linear regression, corr. r2= 0.41, F = 14.1, P < 0.001, Fig. 3B). Thus, patients with more severe dystonia and longer (and thus possibly slower) disease duration improved significantly more. However, both parameters co-vary to a certain extent (longer disease duration frequently means more dystonia).
Figure 3.
Scatterplots of (A) severity of dystonia preoperatively against change in severity of dystonia at 2–6 months (linear regression, corr. r2= 0.31, F = 9.4, P < 0.01); (B) combined score for disease duration and severity of dystonia preoperatively (obtained using a principal component analysis) against change in dystonia at 2–6 months (linear regression, corr. r2= 0.41, F = 14.1, P < 0.001); and (C) change in severity of dystonia at 2–6 months versus change in disability at 2–6 months (linear regression, corr. r2= 0.40, F = 13.2, P < 0.005).
Improvement in disability at three months could be predicted by the improvement in severity of dystonia (linear regression, corr. r2= 0.40, F = 13.2, P < 0.005, Fig. 3C). Likewise, improvement in disability at 9–15 months could be predicted by the improvement in severity of dystonia at 9–15 months (linear regression, corr. r2= 0.50, F = 15.0, P < 0.005). However, factors available preoperatively such as extent of disability, severity of dystonia or other factors did not predict postoperative improvement in disability.
Genetic status: subanalysis inpatients with pantothenate kinase-associated neurodegeneration
Genetic testing was performed in 15 patients. Fourteen of these had a PANK2 gene mutation. In nine PANK2 mutation-positive patients in whom BFMDRS-M were available both at 2–6 and at 9–15 months, there was a significant improvement at 2–6 months (T = 2.9, P < 0.05), but not at 9–15 months (T = 1.3). Improvement in BFMDRS-M at 2–6 months was 27.2%, matching the outcome in the entire cohort.
Implantation and target point
The surgical target in all patients included in this study was the bilateral GPi. Out of 22 patients in whom information was available on anaesthesia, 3 were implanted under local anaesthesia, while the remaining patients were implanted under general anaesthesia. Information about the target coordinates was available in 12 of 23 patients. However, different localization techniques and coordinate systems were used across centres, and the information available regarding target localization techniques was insufficient to calculate a standard mean target coordinate. For those patients in whom information on target was available, target coordinates are listed in Supplementary Table S1.
Stimulation parameters
Mean and standard deviation of pulse frequency, pulse width and voltage after 2–6 and 9–15 months of continuous DBS are listed in Table 4. Due to small numbers of patients and different stimulation settings used in different countries, we found no pattern of stimulation parameters that proved particularly efficient in the treatment of NBIA-dystonia. However, stimulus duration tended to be longer at 9–15 months (P = 0.069). Patients with longer stimulus duration (over 240 µs) did not have a significantly better outcome than the rest of the group.
Table 4.
Mean stimulation parameters at 2–6 and 9–15 months postoperatively
| 2–6 months after DBS (n = 19); mean (range) |
9–15 months after DBS (n = 13); mean (range) |
|||
|---|---|---|---|---|
| Parameter | Left | Right | Left | Right |
| Pulse frequency (Hz) | 133.7 (60–215) | 133.7 (60–215) | 128.5 (60–185) | 128.5 (60–185) |
| Pulse width (µs) | 194.2 (60–450) | 197.4 (60–450) | 244.6 (60–450) | 244.6 (60–450) |
| Pulse amplitude (V) | 2.83 (1.0–5.0) | 2.78 (1.0–5.0) | 2.73 (1.3–4.6) | 2.76 (1.3–4.6) |
Data are presented as (mean ± SD).
Accompanying pharmacotherapy
Data on anti-dystonic pharmacotherapy were available for 22 patients at 2–6 months and for 18 patients at 9–15 months after surgery. All patients took at least one antidystonic medication; the maximum number of drugs was three. After 2–6 months of continuous neurostimulation, medication was reduced in nine patients (41%), while in 9 other patients (41%), pharmacological treatment remained unchanged. In four of the patients (18%), the dose of pharmacotherapy was increased. After 9–15 months of continuous GPi-DBS, nine patients (50%) had reduced anti-dystonic medication, whereas five patients (28%) were on the same medication and four patients (22%) were taking increased medication compared with their preoperative medication regimen.
Adverse events
Information on adverse events and side effects was available for 22 out of 23 patients. Adverse event information was reviewed by two neurologists (A.P. and L.T.) and classified according to the following criteria: (i) adverse events that occurred within 30 days after the surgical intervention and could be related to surgery were regarded as surgical; (ii) adverse events that were reversible by modifying the stimulation parameters were regarded as stimulation-related; (iii) adverse events that related to the technical integrity of the implanted stimulation system were classified as device-related; and (iv) all other adverse events which could either arise from the disease or its progression or other comorbidities were classified as patient-related. A summary of adverse events is given in Table 5.
Table 5.
Serious adverse events and stimulation-related adverse events reported by patients and centres
| Event | SAE | AE |
|---|---|---|
| Surgical adverse events | ||
| Wound healing disorder | x | |
| Fracture of femur due to dystonic exacerbation one day after implantation | x | |
| Patient-related adverse events | ||
| Pneumonia two years after implantation resulting in deatha | x | |
| Dystonic storm (onset prior to DBS) resulting in death | x | |
| Subluxation of hip with necrosis of head of femur and chondrolysis necessitating hospitalization | x | |
| Viral infection with hospitalization | x | |
| Fall with consecutive worsening of symptoms and hospitalization | x | |
| Stimulation-related adverse events | ||
| Visual disturbance (reversible) | x | |
| Mild hyperkinesia (reversible) | x | |
| Paraesthesias (reversible) | x | |
| Worsening of gait and balance (reversible) | x | |
| Worsening of gait freezing (reversible) | x | |
| Blepharospasm under monopolar stimulation | x | |
| Device-related adverse events | ||
| Paraesthesias in the area of the generator implant | x | |
| Dislocation of pulse generator and cables necessitating surgical revisiona | x |
a: These events occurred outside the formal period of observation (preoperative to 15 months postoperatively).
Surgical adverse events
Patient 2 had a wound-healing problem in the abdomen at the site of pulse generator implantation. The day after DBS surgery, Patient 10 sustained a dystonic storm leading to a spontaneous open fracture of the left femoral bone. The initial worsening of symptoms resolved after several days of stimulation and he subsequently experienced marked benefit in severity of dystonia.
Stimulation-related adverse events
Patient 1 suffered from blepharospasm under monopolar stimulation, which resolved under bipolar stimulation. Patient 9 displayed mild worsening of gait and balance after initial programming which resolved after adjustment of stimulation parameters. Patient 10 complained about phosphenes and paraesthesia due to neurostimulation, which subsided with prolonged stimulation and gradual adjustment of the stimulation parameters. Patient 11 reported hyperkinetic movements of neck and trunk when voltage of stimulation was increased too quickly. Patient 22 suffered from worsening of pre-existing gait freezing under high amplitude stimulation of more caudal electrode contacts.
Device-related adverse events
Patient 20 experienced paraesthesias in the area around the generator implant which were found to be due to leakage of electricity. Patient 3 suffered from a dislocation of the pulse generator and cables that required surgical correction 22 months after the initial implantation, at a time point that was outside of the observation period of this study. Out of 19 patients in whom this information was available, one patient needed a change of battery due to exhaustion during the 15 months observation period.
Patient-related adverse events
Two years after DBS implantation (and thus outside of the formal observation period), Patient 4 developed aspiration pneumonia with lethal outcome. Patient 8 was scheduled for elective DBS but suffered a dystonic crisis with respiratory insufficiency. Regrettably, emergency GPi-DBS in combination with intrathecal baclofen did not improve his clinical situation and he died of a dystonic crisis six weeks after surgery. Patient 1 suffered a severe systemic viral infection seven months after electrode implantation with hospitalization and subsequent decline of motor control and speech. Patient 3 suffered a subluxation of the left hip. An osteotomy procedure was conducted nine months after DBS surgery with subsequent complications (necrosis of the head of the femur and chondrolysis), which considerably limited his mobility and quality of life. Patient 13 suffered a serious fall six months after GPi-DBS followed by a continuous deterioration of his condition.
Discussion
These results indicate that bilateral GPi-DBS is effective in improving the severity of dystonia in both the short (2–6 months) and long-term (9–15 months). The mean improvement in severity of dystonia at 9–15 months was 25.7%. Compared with preoperative status for the whole group, quality of life also showed an improvement, whereas disability did not improve significantly. At 9–15 months postoperatively, 66.7% of patients showed an improvement in severity of dystonia of 20% or more, and 31.3% showed an improvement in disability of 20% or more. Global quality of life ratings showed a median improvement of 83.3% at 9–15 months, 64.7% of patients improved by 20% or more. Severity of dystonia preoperatively and disease duration predicted improvement in severity of dystonia at 2–6 months. Improvement in disability at 2–6 months, as well as at 9–15 months, correlated with improvement in severity of dystonia. However, none of the preoperative scores were successful in predicting improvement in disability and quality of life. Pharmacotherapy was reduced in 50% of patients 9–15 months after GPi-DBS.
Although these findings are positive and encouraging, our study has several limitations. Due to the retrospective and multi-centre nature of this study, some data were missing. By working in parallel through movement disorders centres, surgical centres and patient support groups we tried to identify as many patients with NBIA implanted with DBS as possible. However, patients who were not organized within the support groups, or underwent surgery at centres not able or willing to participate, may have escaped our survey. A further problem was that due to the wide variability of the NBIA phenotype, clinical scales do not always entirely suit all individuals. In addition, retrospectively obtained caregiver quality of life ratings are subject to bias, as all subjects received GPi-DBS. Furthermore, retrospective assessment of quality of life will exaggerate the effect of therapy due to recall bias. Despite these limitations, we believe that our study shows a realistic picture of the outcome of GPi-DBS in dystonia in NBIA patients. In particular, these results are important because all cases, including those with non-favourable outcomes, were included. This study had no formal monitoring, but was carried out through careful retrospective evaluation of patient records. Thus, under-reporting of adverse events is possible.
In our patient sample, we were not able to assess the efficiency of GPi-DBS in different genetic subtypes of NBIA because all patients in whom genetic testing had been performed had pantothenate kinase-associated neurodegeneration except for one, in whom no mutation was identified. Thus, a statistical comparison between pantothenate kinase-associated and non-pantothenate kinase-associated neurodegeneration was not possible. However, we predict that severity of dystonia, as well as presence or absence of other symptoms, are more powerful predictors of therapeutic outcome than genetic status.
There was a discrepancy in the current survey between the motor outcome of GPi-DBS and the comparatively small effect on disability as assessed using the BFMDRS disability scale. There are several reasons why this might have been the case. The current study was not blinded, causing a potential observer bias by the clinical rater assessing the dystonia. Furthermore, the BFMDRS disability scale was developed for use in patients with primary torsion dystonia and may not be well suited for some of the patients in this cohort, who can be paediatric and severely disabled. For example, the BFMDRS only distinguishes between ‘walking with help’ and ‘wheelchair-bound’. The ability to control the wheelchair independently, or the autonomous transfer in and out of it, is not taken into account. Specific scales for patients with NBIA are unavailable. A scale used in paediatric settings is the Care and Comfort Hypertonicity Questionnaire (Nemer McCoy et al., 2006), which was developed for use in children with cerebral palsy. Since DBS in paediatric patients is still relatively uncommon, patients are frequently attended by adult neurologists, who more frequently use the BFMDRS and are often unfamiliar with paediatric scales. Thus, the BFMDRS as the most frequently employed scale is valuable because it allows comparison with other studies, although it might not adequately reflect changes at all levels of severity.
Likewise, there was a discrepancy between the small effect on disability and the rather impressive improvement in quality of life. Of course, retrospective quality of life ratings may have overestimated the effect of therapy due to a recall bias. However, quality of life in dystonia also reflects numerous factors beyond disability, such as pain due to dystonia, stigma, fatigue due to medication and several other factors (Mueller et al., 2008), all of which are not addressed in the BFMDRS-D. Although not quantified in the current survey, pain was reported to be reduced in many of the patients in the cohort, as was social stigma due to very visible dystonia. Thus, it is conceivable that quality of life improved considerably despite relatively small changes in disability.
The study by Castelnau and colleagues (2005) reported a 74.6% improvement in severity of dystonia, compared with 25.7% in the current study, as well as a 53% improvement in disability compared with 15.8% improvement in the current study. The patient samples did not differ significantly in age at onset, disease duration, age at surgery, severity of dystonia or severity of disability. The cases reported by Castelnau et al. (2005) were stimulated with very long impulse durations which differ from those used in the current study. Furthermore, Castelnau and colleagues (2005) have considerable experience with DBS in secondary childhood dystonia, and thus, may have selected suitable patients more carefully than other centres. Also, postoperative programming may have been carried out based on more expertise. The strength of the current study is that patients were included regardless of outcome, thus giving a realistic picture of the outcomes of GPi-DBS in NBIA. In particular, no lethal outcomes were reported in the Castelnau et al. (2005) study. In our sample, one patient died during the period of observation due to dystonic storm with onset before the operation. A second patient died of pneumonia two years after surgery. No systematic quantification of any accompanying symptoms, such as those due to pyramidal tract degeneration, was done in either the Castelnau et al. (2005) study or our study. Thus, the difference in outcome may be related to differences in neurological signs and symptoms, other than dystonia itself, between the two population samples. This may be due to differences in (not explicitly stated) selection criteria between different centres.
We found no significant difference in severity of dystonia, disability and quality of life between 2–6 months and 9–15 months after surgery. Castelneau and colleagues (2005) also report a sustained response over a maximum of 42 months. Although there was no significant difference between 2–6 months and 9–15 months outcomes, the data in Fig. 1 suggest a relative outcome decline at least in some patients. We think this decline is due to disease progression. In cases where MRI imaging has been repeatedly performed, there is an increase in the area of T2 hypointensity in the putamen and globus pallidus which is paralleled by clinical deterioration (Hayflick et al., 2006). Of course, there is always the possibility of a placebo effect or an observer bias component, which cannot be ruled out in an open trial. However, the placebo effect of GPi-DBS in dystonia seems to be relatively small (Kupsch et al., 2006). Recurrence of dystonia during temporary reductions in amplitude, or while turning off the stimulation to change settings, suggest a maintained stimulation effect throughout. Neither this study nor the Castelneau et al. (2005) study had a control group of patients with NBIA who did not undergo GPi-DBS. However, a randomization of NBIA patients deemed suitable for DBS into a GPi-DBS group and a non-GPi-DBS group is difficult to justify ethically. An open control group of patients who choose not to undergo GPi-DBS may serve as a viable compromise control group for further studies.
Since this was a retrospective study, factors other than GPi-DBS to treat dystonia, e.g. pharmacotherapy, were reported but not controlled. However, out of 22 patients for whom information on pharmacotherapy was available, 18 (82%) either had reduced medication, or their pharmacotherapy was unaltered at three months. Thus, the antidystonic effect can be safely attributed to GPi-DBS and is unlikely to be due to changes in pharmacotherapy. Although the reduction in medication is mostly dictated by the treating physician, it may be relevant because antidystonic drugs frequently cause side effects such as drowsiness. A reduction in medication will reduce side effects, thereby possibly improving quality of life.
We found in patients with NBIA that more severe dystonia preoperatively predicts greater improvement postoperatively. In contrast, in primary generalized dystonia subjects, Vasques and colleagues (2009) found that higher preoperative BFMDRS scores were associated with less improvement in primary generalized dystonia postoperatively. In addition, longer disease duration and the presence of fixed, skeletal deformities in those with primary generalized dystonia has been shown to be associated with less favourable outcome after treatment with GPi-DBS (Isaias et al., 2008; Vasques et al., 2009). These differences in our findings may be related to the course of dystonia in patients with NBIA as compared to patients with primary generalized dystonia. In NBIA, dystonia can develop relatively rapidly, resulting in severe generalized dystonia without fixed skeletal deformities. Therefore, the issue of disease duration and the presence of fixed skeletal deformities may not be relevant to the NBIA patient. Hence, the significant improvement observed in severely affected NBIA patients encourages the consideration of GPi-DBS as a viable treatment in those with severe dystonia and underlying NBIA since skeletal deformities are less likely in these patients.
There were no systematic differences in terms of stimulation settings between patients with good and bad outcome. Given the sample size, the heterogeneity of the clinical picture and the number of other factors likely contributing to outcome, this is not surprising. Other studies of dystonia with larger, more homogeneous patient samples have also not found any particular settings which are more effective than others (Vasques et al., 2009).
In summary, we present evidence that secondary dystonia in NBIA improves with bilateral GPi-DBS. However, this improvement does not seem to be equal to the benefit reported in patients with primary generalized or tardive dystonias (Trottenberg et al., 2005; Vidailhet et al., 2005; Kupsch et al., 2006). NBIA patients with more severe dystonia seem to derive greater benefit from GPi-DBS. We recommend operating on patients as soon as dystonia becomes disabling and before any possible secondary skeletal deformities arise. A multi-centre, well-controlled prospective study is necessary to get large numbers of cases of this heterogeneous condition and thus be able to better predict the outcome from surgery. For this purpose, we are maintaining a prospective database of patients with NBIA undergoing DBS. We encourage the DBS community to contribute patients to this database with the aim of collecting systematic evidence of treatment effects in this rare condition.
Funding
Hoffnungsbaum e. V. (to A.P., C.W., L.T.); the Klüh-Foundation (to L.T.); the Manfred-and-Ursula-Müller-Foundation (to L.T.); the German Bundesministerium für Bildung und Forschung (BMBF, German Ministry for Education and Research: to L.T.); the Research Program MŠM 0021620849 of the Czech Ministry of Education (to R.J.) and by grants IGAMZ 1A/8629-5 and GAČR 309/09/1145 (to R.J.); Brain Research Trust UK (to S.T.); and Action Medical Research (to S.T.).
Conflict of interest
Lars Timmermann has received honoraria from Medtronic for lecturing and consulting services. Amande Pauls reports no conflict of interest. Karolin Wieland reports no conflict of interest. Robert Jech reports no conflict of interest. Gerhard Kurlemann reports no conflict of interest. Nutan Sharma has been on the speaker's bureau for Allergan until October 1st 2009. Steven Gill reports no conflict of interest. Charles Haenggeli reports no conflict of interest. Susan Hayflick reports no conflict of interest. Penny Hogarth reports no conflict of interest. Nico Leenders reports no conflict of interest. Patricia Limousin has occasionally received honoraria from Medtronic unrelated to this work. Carl J. Malanga has no conflicts of interest to report. Elena Moro has occasionally received honoraria from Medtronic for lecturing and consulting services. Jill Ostrem receives research grant support from St Jude Medical. Fredy Revilla performs DBS Programming Training for Medtronic. Patrick Santens has received speaker's salaries from Medtronic in the past. Alfons Schnitzler reports no conflict of interest. Stephen Tisch reports no conflict of interest. Francesc Valldeoriola has received honoraria from Medtronic for organising teaching courses and giving lectures. Jan Vesper has received honoraria from Medtronic for consulting services. Jens Volkmann has received speaking and advisory honoraria from Medtronic Inc. Dirk Woitalla reports no conflict of interest. Selcuk Peker reports no conflict of interest.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Acknowledgements
We would like to thank Angelika Klucken from Hoffnungsbaum e. V. and Patty Wood from the NBIA Disorders Association for their outstanding support, and all the patients and families who participated in the study. We also thank Eva Luise Köhler, wife of the President of the Federal Republic of Germany and patron of ACHSE e. V. (the German Alliance for Rare Diseases) for her explicit endorsement of this project.
Glossary
Abbreviations
- BFMDRS
Burke Fahn Marsden Dystonia Rating Scale
- GPi-DBS
bilateral pallidal deep brain stimulation
- NBIA
neurodegeneration with brain iron accumulation
Appendix A—Participating centres
Department of Neurology, Charles University in Prague, First Faculty of Medicine, Prague, Czech Republic; Department of Paediatric Neurology, University Children's Hospital, Muenster, Germany; Department of Neurology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA; Department of Neurology, Neurosciences, Frenchay Hospital, Bristol, UK; Hôpital des Enfants, Geneve, Switzerland; Department of Neurology, University Medical Centre Groningen, Groningen, Netherlands; Unit of Functional Neurosurgery and Department of Neurology, Sobell Department of Motor Neuroscience and Movement Disorders, Institute of Neurology, University College London, London, UK; Department of Neurology, Division of Child Neurology, University of North Carolina at Chapel Hill, NC, USA; Department of Neurology, Univ. of California at San Francisco, San Francisco, CA, USA; Department of Neurology, Movement Disorders Centre, University of Cincinnati, Cincinnati, OH, USA; Department of Neurology, Gent University Hospital, Ghent, Belgium; Hospital Clinic, Institut de Neurociencies, Universitat de Barcelona, Barcelona, Spain; Department of Neurology and Department of Neurosurgery, Heinrich Heine University, Düsseldorf, Germany; Department of Neurology, St. Josef Hospital, Ruhr University, Bochum, Germany; Department of Neurosurgery, Hospital for Neurological Sciences, Acibadem University School of Medicine, Istanbul, Turkey.
References
- Albright AL, Barry MJ, Fasick P, Barron W, Shultz B. Continuous intrathecal baclofen infusion for symptomatic generalized dystonia. Neurosurgery. 1996;38:934–8. doi: 10.1097/00006123-199605000-00015. discussion 938–9. [DOI] [PubMed] [Google Scholar]
- Balas I, Kovacs N, Hollody K. Staged bilateral stereotactic pallidothalamotomy for life-threatening dystonia in a child with Hallervorden-Spatz disease. Mov Disord. 2006;21:82–5. doi: 10.1002/mds.20655. [DOI] [PubMed] [Google Scholar]
- Barry MJ, VanSwearingen JM, Albright AL. Reliability and responsiveness of the Barry-Albright Dystonia Scale. Dev Med Child Neurol. 1999;41:404–11. doi: 10.1017/s0012162299000870. [DOI] [PubMed] [Google Scholar]
- Burke RE, Fahn S, Marsden CD, Bressman SB, Moskowitz C, Friedman J. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology. 1985;35:73–7. doi: 10.1212/wnl.35.1.73. [DOI] [PubMed] [Google Scholar]
- Castelnau P, Cif L, Valente EM, Vayssiere N, Hemm S, Gannau A, et al. Pallidal stimulation improves pantothenate kinase-associated neurodegeneration. Ann Neurol. 2005;57:738–41. doi: 10.1002/ana.20457. [DOI] [PubMed] [Google Scholar]
- Clement F, Devos D, Moreau C, Coubes P, Destee A, Defebvre L. Neurodegeneration with brain iron accumulation: clinical, radiographic and genetic heterogeneity and corresponding therapeutic options. Acta Neurol Belg. 2007;107:26–31. [PubMed] [Google Scholar]
- Curtis AR, Fey C, Morris CM, Bindoff LA, Ince PG, Chinnery PF, et al. Mutation in the gene encoding ferritin light polypeptide causes dominant adult-onset basal ganglia disease. Nat Genet. 2001;28:350–4. doi: 10.1038/ng571. [DOI] [PubMed] [Google Scholar]
- Dressler D, Wittstock M, Benecke R. Botulinum toxin for treatment of jaw opening dystonia in Hallervorden-Spatz syndrome. Eur Neurol. 2001;45:287–8. doi: 10.1159/000052146. [DOI] [PubMed] [Google Scholar]
- Gregory A, Hayflick SJ. Neurodegeneration with brain iron accumulation. Folia Neuropathol. 2005;43:286–96. [PMC free article] [PubMed] [Google Scholar]
- Gregory A, Polster BJ, Hayflick SJ. Clinical and genetic delineation of neurodegeneration with brain iron accumulation. J Med Genet. 2009;46:73–80. doi: 10.1136/jmg.2008.061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris ED. The iron-copper connection: the link to ceruloplasmin grows stronger. Nutr Rev. 1995;53:170–3. doi: 10.1111/j.1753-4887.1995.tb01545.x. [DOI] [PubMed] [Google Scholar]
- Hayflick SJ, Westaway SK, Levinson B, Zhou B, Johnson MA, Ching KH, et al. Genetic, clinical, and radiographic delineation of Hallervorden-Spatz syndrome. N Engl J Med. 2003;348:33–40. doi: 10.1056/NEJMoa020817. [DOI] [PubMed] [Google Scholar]
- Hayflick SJ, Hartman M, Coryell J, Gitschier J, Rowley H. Brain MRI in neurodegeneration with brain iron accumulation with and without PANK2 mutations. AJNR Am J Neuroradiol. 2006;27:1230–3. [PMC free article] [PubMed] [Google Scholar]
- Isaias IU, Alterman RL, Tagliati M. Outcome predictors of pallidal stimulation in patients with primary dystonia: the role of disease duration. Brain. 2008;131:1895–902. doi: 10.1093/brain/awn120. [DOI] [PubMed] [Google Scholar]
- Justesen CR, Penn RD, Kroin JS, Egel RT. Stereotactic pallidotomy in a child with Hallervorden-Spatz disease. Case report. J Neurosurg. 1999;90:551–4. doi: 10.3171/jns.1999.90.3.0551. [DOI] [PubMed] [Google Scholar]
- Krause M, Fogel W, Tronnier V, Pohle S, Hortnagel K, Thyen U, et al. Long-term benefit to pallidal deep brain stimulation in a case of dystonia secondary to pantothenate kinase-associated neurodegeneration. Mov Disord. 2006;21:2255–7. doi: 10.1002/mds.21166. [DOI] [PubMed] [Google Scholar]
- Kupsch A, Benecke R, Muller J, Trottenberg T, Schneider GH, Poewe W, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med. 2006;355:1978–90. doi: 10.1056/NEJMoa063618. [DOI] [PubMed] [Google Scholar]
- Kurlemann G, Bongartz G, Kuchelmeister K, Palm DG. [Hallervorden-Spatz syndrome. Indicative findings in cranial computerized and magnetic resonance tomography for intra vitam diagnosis]. Monatsschr Kinderheilkd. 1991;139:626–8. [PubMed] [Google Scholar]
- Kyriagis M, Grattan-Smith P, Scheinberg A, Teo C, Nakaji N, Waugh M. Status dystonicus and Hallervorden-Spatz disease: treatment with intrathecal baclofen and pallidotomy. J Paediatr Child Health. 2004;40:322–5. doi: 10.1111/j.1440-1754.2004.00374.x. [DOI] [PubMed] [Google Scholar]
- Loher TJ, Hasdemir MG, Burgunder JM, Krauss JK. Long-term follow-up study of chronic globus pallidus internus stimulation for posttraumatic hemidystonia. J Neurosurg. 2000;92:457–60. doi: 10.3171/jns.2000.92.3.0457. [DOI] [PubMed] [Google Scholar]
- Mikati MA, Yehya A, Darwish H, Karam P, Comair Y. Deep brain stimulation as a mode of treatment of early onset pantothenate kinase-associated neurodegeneration. Eur J Paediatr Neurol. 2009;13:61–4. doi: 10.1016/j.ejpn.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Morgan NV, Westaway SK, Morton JE, Gregory A, Gissen P, Sonek S, et al. PLA2G6, encoding a phospholipase A2, is mutated in neurodegenerative disorders with high brain iron. Nat Genet. 2006;38:752–4. doi: 10.1038/ng1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller J, Skogseid IM, Benecke R, Kupsch A, Trottenberg T, Poewe W, et al. Pallidal deep brain stimulation improves quality of life in segmental and generalized dystonia: results from a prospective, randomized sham-controlled trial. Mov Disord. 2008;23:131–4. doi: 10.1002/mds.21783. [DOI] [PubMed] [Google Scholar]
- Nemer McCoy R, Blasco PA, Russman BS, O'Malley JP. Validation of a care and comfort hypertonicity questionnaire. Dev Med Child Neurol. 2006;48:181–7. doi: 10.1017/S0012162206000405. [DOI] [PubMed] [Google Scholar]
- Sako W, Goto S, Shimazu H, Murase N, Matsuzaki K, Tamura T, et al. Bilateral deep brain stimulation of the globus pallidus internus in tardive dystonia. Mov Disord. 2008;23:1929–31. doi: 10.1002/mds.22100. [DOI] [PubMed] [Google Scholar]
- Sharma MC, Aggarwal N, Bihari M, Goyal V, Gaikwed S, Vaishya S, et al. Hallervorden spatz disease: MR and pathological findings of a rare case. Neurol India. 2005;53:102–4. [PubMed] [Google Scholar]
- Shields DC, Sharma N, Gale JT, Eskandar EN. Pallidal stimulation for dystonia in pantothenate kinase-associated neurodegeneration. Pediatr Neurol. 2007;37:442–5. doi: 10.1016/j.pediatrneurol.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Swaiman KF. Hallervorden-Spatz syndrome. Pediatr Neurol. 2001;25:102–8. doi: 10.1016/s0887-8994(01)00253-3. [DOI] [PubMed] [Google Scholar]
- Thomas M, Hayflick SJ, Jankovic J. Clinical heterogeneity of neurodegeneration with brain iron accumulation (Hallervorden-Spatz syndrome) and pantothenate kinase-associated neurodegeneration. Mov Disord. 2004;19:36–42. doi: 10.1002/mds.10650. [DOI] [PubMed] [Google Scholar]
- Trottenberg T, Volkmann J, Deuschl G, Kuhn AA, Schneider GH, Muller J, et al. Treatment of severe tardive dystonia with pallidal deep brain stimulation. Neurology. 2005;64:344–6. doi: 10.1212/01.WNL.0000149762.80932.55. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H, Inui K, Taniike M, Nishimoto J, Midorikawa M, Yoshimine T, et al. A case of Hallervorden-Spatz disease: progressive and intractable dystonia controlled by bilateral thalamotomy. Brain Dev. 1992;14:269–72. doi: 10.1016/s0387-7604(12)80246-4. [DOI] [PubMed] [Google Scholar]
- Umemura A, Jaggi JL, Dolinskas CA, Stern MB, Baltuch GH. Pallidal deep brain stimulation for longstanding severe generalized dystonia in Hallervorden-Spatz syndrome. Case report. J Neurosurg. 2004;100:706–9. doi: 10.3171/jns.2004.100.4.0706. [DOI] [PubMed] [Google Scholar]
- Vasques X, Cif L, Gonzalez V, Nicholson C, Coubes P. Factors predicting improvement in primary generalized dystonia treated by pallidal deep brain stimulation. Mov Disord. 2009;24:846–53. doi: 10.1002/mds.22433. [DOI] [PubMed] [Google Scholar]
- Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Benabid AL, Cornu P, et al. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med. 2005;352:459–67. doi: 10.1056/NEJMoa042187. [DOI] [PubMed] [Google Scholar]
- Vidailhet M, Yelnik J, Lagrange C, Fraix V, Grabli D, Thobois S, et al. Bilateral pallidal deep brain stimulation for the treatment of patients with dystonia-choreoathetosis cerebral palsy: a prospective pilot study. Lancet Neurol. 2009;8:709–17. doi: 10.1016/S1474-4422(09)70151-6. [DOI] [PubMed] [Google Scholar]
- Voon V, Krack P, Lang AE, Lozano AM, Dujardin K, Schupbach M, et al. A multicentre study on suicide outcomes following subthalamic stimulation for Parkinson's disease. Brain. 2008;131:2720–8. doi: 10.1093/brain/awn214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Westaway SK, Levinson B, Johnson MA, Gitschier J, Hayflick SJ. A novel pantothenate kinase gene (PANK2) is defective in Hallervorden-Spatz syndrome. Nat Genet. 2001;28:345–9. doi: 10.1038/ng572. [DOI] [PubMed] [Google Scholar]
- Zorzi G, Marras C, Nardocci N, Franzini A, Chiapparini L, Maccagnano E, et al. Stimulation of the globus pallidus internus for childhood-onset dystonia. Mov Disord. 2005;20:1194–200. doi: 10.1002/mds.20510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.