Abstract
Diabetic ketoacidosis (DKA) has been the hallmark of a life-threatening medical emergency for poorly controlled or newly diagnosed type 1 diabetics. In the last two decades, this traditional association has been challenged with increasing reports of type 2 diabetics presenting with DKA. We report the case of a 75-year-old woman with known type 2 diabetes who presented in DKA and was found to have pancreatic adenocarcinoma. A link between diabetes mellitus and pancreatic cancer has been investigated, but the literature remains inconclusive as to whether diabetes mellitus (DM) is a cause or result of pancreatic cancer or simply the confluence of two common entities. Previous case reports of pancreatic tumors presenting with DKA all represented neuroendocrine tumors. Adenocarcinoma of the pancreas should be considered in the list of precipitants for DKA in type 2 DM.
KEY WORDS: diabetic ketoacidosis, type 2 diabetics, pancreatic adenocarcinoma
INTRODUCTION
Diabetic ketoacidosis (DKA) was classically considered to occur only in persons with type 1 diabetes mellitus. Hyperglycemia in type 2 diabetes was thought to lead only to hyperosmolar hyperglycemic state (HHS) without ketosis. However, a retrospective review found that among adult patients presenting with DKA, 47% had known type 1 diabetes, 26% had known type 2 diabetes and 27% had newly diagnosed diabetes1. Of those with newly diagnosed diabetes, one quarter did not require insulin 12 months later. The occurrence of DKA in type 2 diabetics is commonly associated with conditions of extreme stress but there is no correlation between a particular precipitant and the development of DKA or HHS.
The leading causes for the development DKA are inadequate insulin treatment or non-adherence to therapy, followed by new onset diabetes. Acute illnesses and some drugs are also significant causes. In one study1, 24% of patients presenting with DKA had no clear identifiable cause, and “stress” was considered the precipitant.
This case reports an older woman with known type 2 diabetes who presented in DKA with a history of several days of confusion and vomiting. CT imaging and biopsy showed a large pancreatic adenocarcinoma. To our knowledge, this is the first case of pancreatic adenocarcinoma presenting with DKA and it raises the question of a relationship between DKA, type 2 diabetes and pancreatic cancer.
Case Description
A 75-year-old woman with a 15-year history of type 2 diabetes mellitus, hypertension, hypercholesterolemia, and gastroesophageal reflux disease was admitted with confusion and vomiting. Three days prior to admission, her family noted that she was intermittently confused, had occasional vomiting episodes, and had refused to eat. There was no history of polyuria, polydipsia or diarrhea. For two months prior to admission, she reported a 10–15 pound weight loss, suprapubic discomfort, anorexia and malaise. An upper endoscopy showed changes consistent with Barrett’s esophagus. Colonoscopy and abdominal CT were scheduled but not completed prior to admission.
Her diabetes had been well controlled (HbA1c 6–7%) while taking oral hypoglycemic agents (metformin, glipizide and pioglitazone) for several years. One year prior to admission, her primary care physician noted that her blood sugar was elevated despite being adherent to her medications. Insulin (Levemir) was added 6 months later, but adequate glucose control was not achieved. She did not have a history of gallstones or pancreatitis. She was a non-smoker, non-drinker, and she lived alone. She had no family history of diabetes mellitus.
On presentation to the emergency room, she was lethargic. Blood pressure was 167/98 mmHg, heart rate 97 beats per minute, respiratory rate 18 breaths per minute and oxygen saturation 97% on room air. She had dry mucous membranes and decreased skin turgor. Breath sounds were present and equal bilaterally, without crackles or wheezes. Heart sounds were normal with a systolic ejection murmur at the left sternal border. Her abdomen was soft and non-distended, with mild suprapubic tenderness, no masses, and normal bowel sounds. On neurological examination, the patient was disoriented to place and time. She was able to move all four extremities. There were no focal neurologic signs.
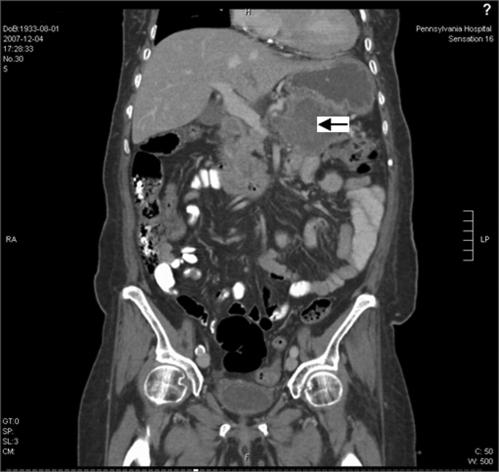
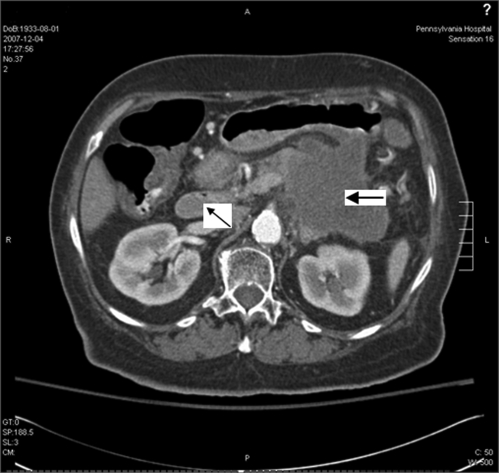
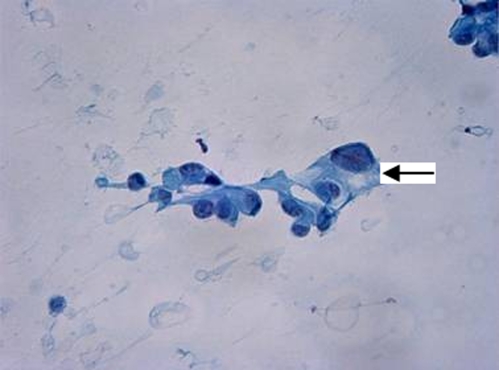
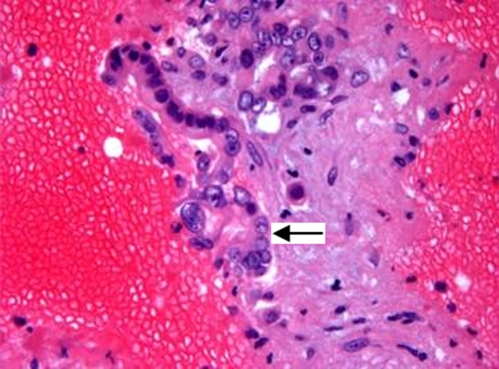
Admission laboratory data showed blood glucose of 450 mg/dl, urinary ketones of 150 mg/dL, and small acetone level in blood. Urinalysis and microscopic examination was negative for nitrite or leukocyte esterase with 0–2 white blood cells and moderate squamous epithelial cells. There was also glucosuria (1000 mg) and mild proteinuria (25 mg/dL). Arterial blood pH revealed metabolic acidosis (pH 7.28) with an anion gap of 25. There was a mild leukocytosis of 11.9 × 103 /uL (normal 4.0–11.0 × 103 /uL), elevated alkaline phosphatase of 360 IU/L (25–150 IU/L), gamma-glutamyl transpeptidase of 346 IU/L (5–80 IU/L) and total bilirubin of 2.3 mg/dL (0.1–1.2 mg/dL) with indirect bilirubin of 2.2 mg/dL. Her serum alanine transaminase, aspartate transaminase, albumin, amylase, lipase and coagulation profile were within normal limits. HbA1c was 9.5% (normal <7%). CT of the head was negative for acute pathology. TSH, RPR, vitamin B12 and folic acid were all within the normal limits. Serum acetaminophen level was normal. Additional toxicology screening was not done. She was treated appropriately for diabetic ketoacidosis with normalization of glucose, ketones, and anion gap within 6 hours. Subsequently, her mental status improved, and she was awake, alert and oriented. Abdominal ultrasound showed a 7 × 7 × 5 cm indeterminate heterogeneous avascular solid lesion in the pancreas. CT of the abdomen/pelvis confirmed a pancreatic tail mass of 9 × 7 cm, with atrophy of the body and head of the pancreas, multiple mesenteric nodular masses, and pelvic ascites. There was a hypodense lesion in the liver, and nodules at the left lung base and left adrenal gland shown in Figures 1 and 2. CT guided biopsy of the pancreatic mass revealed malignant cells consistent with pancreatic adenocarcinoma (Figs. 3 and 4).
Figure 1.
CT scan of abdomen and pelvis in coronal section showing a large pancreatic tail mass (arrow) with multiple mesenteric and peritoneal nodular masses and a small amount of pelvic ascites.
Figure 2.
CT scan of abdomen showing a 9 × 7 cm pancreatic mass (large arrow) with age-appropriate atrophy of the head and neck of the pancreas (small arrow).
Figure 3.
Smear from fine-needle aspiration (FNA) biopsy of the pancreas showing pleomorphic malignant cells (arrow) with eccentric nuclei, prominent nucleoli, and transparent cytoplasm, consistent with adenocarcinoma. (Papanicolaou-stain, x400).
Figure 4.
Cell block preparation from the FNA biopsy showing malignant cells forming glands (arrow) (H&E stain, x400).
The patient declined further treatment and was discharged home with pain medication, insulin (Levemir) 30 units daily and home hospice service. She died three months later in inpatient hospice.
DISCUSSION
DKA is less common in type 2 diabetics compared to type 1 diabetics because these patients are thought to be insulin resistant rather than insulin deficient. When it occurs, common precipitating factors are discontinuation of medication, infection, myocardial infarction, inadequate insulin therapy, or newly diagnosed diabetes. Our patient had pancreatic cancer, profound weight loss and poor nutrition. Although these factors clearly constitute acute stress and may have predisposed and contributed towards her decompensation, none of them individually are usually considered precipitants of DKA. We will review what is known about type 2 diabetics and DKA, and discuss the relationship between DKA, diabetes, and pancreatic cancer.
Pathophysiology and Mechanism of DKA in Type2 Diabetics
An underlying deficiency of insulin, with elevation in counter-regulatory hormones, is found in DKA. There are three proposed mechanisms of DKA in type 2 diabetics: 1) Insulinopenia, 2) Elevation of counter regulatory stress hormones, and 3) Increase in free fatty acids. Some authors/studies believe that only the first one of these, insulinopenia, is significant in type 2 diabetics.2
In the largest study of DKA in type 2 diabetics, Linfoot2 found, using c-peptide concentration as a marker for insulin, a significant decrease in plasma c-peptide concentration at the time of DKA presentation in type 1 diabetics and ketosis-prone type 2 diabetics. The stressors precipitating DKA have been postulated to cause a relative rather than a definitive deficiency of insulin. Possible causes for this relative insulin deficiency include impairment of insulin secretion due to chronic exposure of insulin secreting or islet cells to high levels of glucose of free fatty acids.3 Additional causes are hypokalemia, which can impair insulin secretion, and prolonged fasting, which increases the rate of ketosis, and may decrease insulin secretion. Intensified diabetic management in type 2 diabetics with DKA results in significant improvement in beta-cell function and insulin sensitivity which later leads to insulin discontinuation.1,4
Although elevation of counter-regulatory stress hormones and free fatty acids are postulated mechanisms, Linfoot2 found that the plasma concentrations of the stress hormones and free fatty acids were not significantly different in ketosis prone, and non ketosis prone, type 2 diabetics. Thus he believed the predominant mechanism for ketosis in these patients is the deficiency or decrease in insulin secretion. Unfortunately c-peptide was not measured in our patient, and therefore we can only hypothesize about the possible role of insulinopenia in the development of her DKA. However, it is hard to ignore the possible role of counter regulatory hormones in our patient with a poor oral intake and the stress of a large tumor burden. Simple starvation ketosis could have been a contributing factor to ketoacidosis. However when ketosis is associated with starvation, it is usually mild, without hyperglycemia, and with a plasma bicarbonate level in the minimally low or normal range, thus making starvation ketosis a less likely precipitating factor in our patient.
Relationship between DM and Pancreatic Cancer
DKA as a presentation of pancreatic tumors has only been documented with glucagon-secreting pancreatic islet cell neoplasm and somatostatinomas, which are rare.5,6 Pancreatic adenocarcinoma is not known to be a presentation, complication or cause of DKA. However, the relationship between diabetes and pancreatic cancer is controversial. While some investigators assume that long standing type 2 diabetes predisposes to pancreatic cancer7,8, a recent study showed that in many pancreatic cancer patients, glucose intolerance and new onset diabetes occurs at the initial presentation.9,10
The evidence for diabetes as a cause of pancreatic cancer is based on limited animal studies. It has been postulated that prolonged exposure to hyperinsulinemia stimulates the growth of pancreatic cancers via the insulin receptors found on the pancreatic cells.11–13
In humans, the link between diabetes and pancreatic cancer is based primarily on a temporal relationship. A meta-analysis9 showed that individuals diagnosed with diabetes had a 50% greater risk of pancreatic malignancy in the 4 years following diagnosis compared with individuals who had diabetes for more than 5 years. When looking at individuals with pancreatic cancer, diabetes is diagnosed within the previous 5 years up to 40% of the time. These are intriguing, but inconclusive linkages, and do not apply to our patient who had diabetes for 15 years prior to presentation with pancreatic cancer. Thus despite the numerous studies performed to elucidate the relationship between these two diseases, there is no conclusive evidence regarding cause and effect.14
Additional efforts to examine the relationship between diabetes and pancreatic cancer have looked at glucose metabolism and insulin response. Peripheral insulin resistance and concurrent impairment of beta cell response to glucose are suggested as primary mechanisms for diabetes in pancreatic cancer.15,16 Insulin-receptor binding and cellular glucose transportation were impaired in patients with pancreatic cancer.
Reduced glycogen synthesis and storage in skeletal muscle, contributing to hyperglycemia has also been observed.17 This hypothesis was further supported by a clinical study18 which showed that the baseline C-peptide level in patients with pancreatic adenocarcinoma was higher than normal. After tumor resection, there was improvement of insulin sensitivity and diabetes status.17
The simple loss of normal functioning pancreatic mass has always been a consideration in the genesis of diabetes. Pancreatogenic diabetes has been described in patients with pancreatic resection secondary to chronic pancreatitis or acute fulminating hemorrhagic pancreatitis, with nearly 100% of patient developing diabetes after 80–95% pancreatectomy.19,20 It has been argued that cancer, local tumor invasion, beta cell destruction, duct obstruction and fibrotic pancreatitis can contribute to the development of glucose intolerance and diabetes. However, because islet cells surrounding tumor have the appearance of normal pancreatic islet cells, because only 15–20% of the beta cell mass is required to maintain a non-diabetic state, and because a small pancreatic adenocarcinoma can also cause hyperglycemia in cancer patients,17 these hypotheses regarding the cancer-diabetes link remain unproven.
DKA can result from a significant loss of beta cell and pancreatic polypeptide (PP)-secreting cells. PP cells are primarily located in the ventral pancreatic head and uncinate process. These cells inhibit exocrine pancreatic secretion and gallbladder contraction and act as hormonal mediators of glucose metabolism. Deficiency of PP cells is associated with hepatic resistance to insulin and inappropriate hepatic glucose production, contributing to persistent hyperglycemia.19 Our patient had a large mass in the tail of the pancreas and atrophy of the head and body, which could have caused a decrease in pancreatic polypeptide cell secretion and a decrease in beta cell mass with consequent decreased insulin secretion.
Two other postulated mechanisms, islet cell dysfunction21,22 and diabetes as a paraneoplastic phenomenon23–26, have been offered to explain the temporal link between diabetes and pancreatic cancer. Despite the preservation of endocrine pancreatic function after a large loss of pancreatic islets, there is minimal increase in C-peptide concentration after intravenous glucagon stimulation in pancreatic adenocarcinoma patients.22 This suggests that insulin release in response to glucose stimuli is reduced in these patients. Additional evidence for dysfunctional islet cells includes a delay in proinsulin maturation27 and increased amylin (islet amyloid polypeptide) secretion in pancreatic cancer patients.28
The suggestion that diabetes could be a paraneoplastic phenomenon comes from animal models in which pancreatic cancer cells secreted non-immunological mediators that interfered with glucose metabolism, amylin secretion, and peripheral action of insulin.24,25
In our patient, her longstanding diabetes prior to the development of DKA with recently poorly controlled diabetes mellitus may be consistent with the undiagnosed pancreatic cancer. Despite the large tumor mass upon presentation, the change in her diabetic pattern and pancreatic failure are likely secondary to intrinsic mechanisms, rather than the local effect of the tumor. Transient insulinopenia, peripheral insulin resistance and beta cell dysfunction secondary to the presence of pancreatic cancer may have contributed to the persistent hyperglycemic state. The associated stress of poor nutrition, profound weight loss and large tumor burden may have predisposed her metabolic decompensation and led to DKA.
CONCLUSION
DKA is no longer exclusively associated with type 1 diabetics. While the majority of patients presenting with DKA are type 1 diabetics, a minority will be patients with type 2 diabetes. In many of these patients, an obvious precipitant will be found. Considering pancreatic cancer in both newly diagnosed and known type 2 diabetics presenting with DKA without obvious precipitant(s) may be appropriate. This case also raises the possibility of pancreatic cancer related pancreatic failure, paraneoplastic syndrome, and cachexia with starvation ketosis, as contributory factors in the development of DKA in this setting.
Summary
Patients with type 2 diabetes can develop DKA.
Pancreatic cancer should be considered as a possible precipitant of DKA if common factors are not identified.
The stressors precipitating DKA in type 2 diabetics cause transient insulinopenia rather definitive insulin deficiency.
Evidence is inconclusive regarding the cause and effect between diabetes mellitus and pancreatic cancer.
Acknowledgments
We thank the patient’s family for their consent to allow us to use this case for teaching. We also thank Dennis Policastro, MD and Mindi Roeser, MD for advice and assistance, Michiko Riko Nagamine, MD for providing the pathology slides and Steve Bieko, MD for the CT images.
There was no funding associated with the manuscript preparation.
Conflict of Interest None disclosed.
References
- 1.Westphal SA. The occurrence of diabetic ketoacidosis in non-insulin-dependent diabetes and newly diagnosed diabetic adults. Am J Med. 1996;101(1):19–24. doi: 10.1016/S0002-9343(96)00076-9. [DOI] [PubMed] [Google Scholar]
- 2.Linfoot P, Bergstrom C, Ipp E. Pathophysiology of ketoacidosis in Type 2 diabetes mellitus. Diabet Med. 2005;22(10):1414–1419. doi: 10.1111/j.1464-5491.2005.01660.x. [DOI] [PubMed] [Google Scholar]
- 3.Poitout Vincent. Glucolipotoxicity of the pancreatic cell: myth or reality? Biochem Soc Trans. 2008;36:901–904. doi: 10.1042/BST0360901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umpierrez GE, Smiley D, Kitabchi AE. Narrative review: ketosis-prone type 2 diabetes mellitus. Ann Intern Med. 2006;144(5):350–357. doi: 10.7326/0003-4819-144-5-200603070-00011. [DOI] [PubMed] [Google Scholar]
- 5.Fenkci SM, Fidan Yaylali G, Sermez Y, Akdam H, Sabir N, Kiraç S. Malign cystic glucagonoma presented with diabetic ketoacidosis: case report with an update. Endocr Relat Cancer. 2005;12(2):449–454. doi: 10.1677/erc.1.00957. [DOI] [PubMed] [Google Scholar]
- 6.Anthony LB, Sharp SC, May ME. Case report: diabetic ketoacidosis in a patient with glucagonoma. Am J Med Sci. 1995;309(6):326–327. doi: 10.1097/00000441-199506000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA. 1995;273(20):1605–1609. doi: 10.1001/jama.273.20.1605. [DOI] [PubMed] [Google Scholar]
- 8.Yalniz M, Pour PM. Diabetes mellitus: a risk factor for pancreatic cancer? Langenbeck’s Arch Surg. 2005;390(1):66–72. doi: 10.1007/s00423-004-0469-8. [DOI] [PubMed] [Google Scholar]
- 9.Huxley R, Ansary-Moghaddam A, Berrington de González A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92(11):2076–2083. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chari ST, Leibson CL, Rabe KG, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology. 2008;134(1):95–101. doi: 10.1053/j.gastro.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher WE, Boros LG, Schirmer WJ. Insulin promotes pancreatic cancer: evidence for endocrine influence on exocrine pancreatic tumors. J Surg Res. 1996;63(1):310–313. doi: 10.1006/jsre.1996.0266. [DOI] [PubMed] [Google Scholar]
- 12.Fisher WE, Boros LG, Schirmer WJ. Reversal of enhanced pancreatic cancer growth in diabetes by insulin. Surgery. 1995;118(2):453–457. doi: 10.1016/S0039-6060(05)80358-7. [DOI] [PubMed] [Google Scholar]
- 13.Pour PM, Stepan K. of pancreatic carcinogenesis in the hamster model. VIII. Inhibitory effect of exogenous insulin. J Natl Cancer Inst. 1984;72(5):1205–1208. [PubMed] [Google Scholar]
- 14.Wang F, Herrington M, Larsson J, Permert J. The relationship between diabetes and pancreatic cancer. Mol Cancer. 2003;2:4. doi: 10.1186/1476-4598-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cersosimo E, Pisters PW, Pesola G, McDermott K, Bajorunas D, Brennan MF. Insulin secretion and action in patients with pancreatic cancer. Cancer. 1991;67(2):486–493. doi: 10.1002/1097-0142(19910115)67:2<486::AID-CNCR2820670228>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Basso D, Plebani M, Fogar P, et al. Beta-cell function in pancreatic adenocarcinoma. Pancreas. 1994;9(3):332–335. doi: 10.1097/00006676-199405000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Knezetic JA, Strömmer L, Permert J, Larsson J, Adrian TE. The intracellular mechanism of insulin resistance in pancreatic cancer patients. J Clin Endocrinol Metab. 2000;85(3):1232–1238. doi: 10.1210/jc.85.3.1232. [DOI] [PubMed] [Google Scholar]
- 18.Mustafa C, Ramiz C, Bayram F, et al. High prevalence of diabetes in patients with pancreatic cancer in central Anatolia, Turkey. Diabetes Res Clin Pract. 2002;58:97–100. doi: 10.1016/S0168-8227(02)00130-4. [DOI] [PubMed] [Google Scholar]
- 19.Slezak L, Andersen DK. Pancreatic resection: effects on glucose metabolism. World J Surg. 2001;25:452–460. doi: 10.1007/s002680020337. [DOI] [PubMed] [Google Scholar]
- 20.Bonner-Weir S, Trent DF, Weir GC. Partial pancreatectomy in the rat and subsequent defect in glucose-induced insulin release. J Clin Invest. 1983;71(6):1544–1553. doi: 10.1172/JCI110910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cersosimo E, Pisters PW, Pesola G, et al. Insulin secretion and action in patients with pancreatic cancer. Cancer. 1991;67:486–493. doi: 10.1002/1097-0142(19910115)67:2<486::AID-CNCR2820670228>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 22.Fox JN, Frier BM, Armitage M, Ashby JP. Abnormal insulin secretion in carcinoma of the pancreas: response to glucagon stimulation. Diabet Med. 1985;2:113–116. doi: 10.1111/j.1464-5491.1985.tb00612.x. [DOI] [PubMed] [Google Scholar]
- 23.Basso D, Greco E, Fogar P, et al. Pancreatic cancer-associated diabetes mellitus: an open field for proteomic applications. Clin Chim Acta. 2005;357:184–189. doi: 10.1016/j.cccn.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 24.Basso D, Valerio A, Brigato L, Panozzo MP, Miola M, Lucca T, Ujka F, Zaninotto M, Avogaro A, Plebani M. An unidentified pancreatic cancer cell product alters some intracellular pathways of glucose metabolism in isolated rat hepatocytes. Pancreas. 1997;15(2):132–138. doi: 10.1097/00006676-199708000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Basso D, Brigato L, Veronesi A, Panozzo MP, Amadori A, Plebani M. The pancreatic cancer cell line MIA PaCa2 produces one or more factors able to induce hyperglycemia in SCID mice. Anticancer Res. 1995;15(6B):2585–2588. [PubMed] [Google Scholar]
- 26.Permert J, Adrian TE, Jacobsson P, Jorfelt L, Fruin AB, Larsson J. Is profound peripheral insulin resistance in patients with pancreatic cancer caused by a tumor-associated factor? Am J Surg. 1993;165(1):61–66. doi: 10.1016/S0002-9610(05)80405-2. [DOI] [PubMed] [Google Scholar]
- 27.Nakamori S, Ishikawa O, Ohigashi H, et al. Increased blood proinsulin and decreased C-peptide levels in patients with pancreatic cancer. Hepatogastroenterology. 1999;46(25):16–24. [PubMed] [Google Scholar]
- 28.Ding X, Flatt PR, Permert J, Adrian TE. Pancreatic cancer cells selectively stimulate islet beta cells to secrete amylin. Gastroenterology. 1998;114(1):130–138. doi: 10.1016/S0016-5085(98)70641-9. [DOI] [PubMed] [Google Scholar]