Abstract
Background
Opioids have been linked to increased risk of fractures, but little is known about how opioid dose affects fracture risk.
Objective
To assess whether risk of fracture increases with opioid dose among older patients initiating sustained use of opioids for chronic non-cancer pain.
Design
A cohort study that uses Cox proportional hazards models to compare fracture risk among current opioid users vs. persons no longer using opioids.
Participants
Members of an integrated health care plan (N = 2,341) age 60 years and older who received 3+ opioid prescriptions within a 90-day period for chronic, non-cancer pain between 2000 and 2005.
Measurements
Time-varying measures of opioid use and average daily dose in morphine equivalents were calculated from automated data. Fractures were identified from automated data and then validated through medical record review.
Results
Compared with persons not currently using opioids, opioid use was associated with a trend towards increased fracture risk (1.28 (95% CI (0.99, 1.64 )). Higher dose opioid use (≥50 mg/day) was associated with a 9.95% annual fracture rate and a twofold increase in fracture risk (2.00 (95% CI (1.24, 3.24)). Of the fractures in the study cohort, 34% were of the hip or pelvis, and 37% were associated with inpatient care.
Conclusions
Higher doses (≥50 mg/day) of opioids for chronic non-cancer pain were associated with a 2.00 increase in risk of fracture confirmed by medical record review. Clinicians should consider fracture risk when prescribing higher-dose opioid therapy for older adults.
KEY WORDS: fracture, opioids, elderly, survival, chronic pain
BACKGROUND
Long-term use of opioid medications for chronic non-cancer pain has come under growing scrutiny in the wake of large increases in prescribing rates over the last two decades1,2. Changes in opioid prescribing practices have occurred in the absence of definitive evidence of the efficacy and safety of long-term opioid therapy for chronic non-cancer pain3–6. Between 1998 and 2006, more than three% of persons 70 years and older in the U.S. were estimated to be regular users of prescription opioid medications compared to less than 1% of people under 407. These high rates of opioid use in older adults7–9 underscore the importance of quantifying the risks of prescription opioid use for chronic non-cancer pain among older persons.
Among older people, bone fractures are common adverse events10 that may be influenced by opioid use. Opioid-related side effects, such as dizziness and sedation, may lead to falls and, sometimes, fractures11–13. Several studies have reported an association between opioids and risk of fracture11,12,14–19. A recent meta-analysis20, based on six studies, concluded that opioids are associated with a moderate, but clinically relevant (38%) increased fracture risk, though it noted the need for large studies that minimize selection bias and more accurately measure fracture risk20. In particular, research that evaluates whether fracture risk is associated with high-dose opioid use is lacking20.
The purpose of the current study was to examine the association between opioid use and fracture risk in older people, focusing on whether there is a relationship between opioid dose and risk of fracture.
RESEARCH DESIGN AND METHODS
This paper reports findings of a cohort study from CONSORT—CONsortium to Study Opioid Risks and Trends—on fracture risks among older patients prescribed opioids for chronic non-cancer pain21. The health plan studied, Group Health Cooperative (GHC), provides comprehensive care on a pre-paid basis to about 500,000 persons in Washington State who are demographically representative of the general population22. The study was reviewed and approved by the GHC Institutional Review Board.
Participants
Chronic pain was defined by repeated opioid analgesic prescriptions for a pain problem. Inclusion criteria were (i) adults aged 60 or over initiating an episode of opioid use (no prescriptions filled for opioids in the previous 6 months) between 2000 and 2005, (ii) three or more opioid prescriptions in the first 90 days of the episode, and (iii) a diagnosis of a non-cancer pain problem from the prescribing physician in the two weeks prior to the initial opioid prescription. Pain diagnoses included back or neck pain, osteoarthritis, headache, extremity pain, abdominal pain/hernia, menstrual/menopausal pain, and temporomandibular disorder pain (TMD). Persons entered the cohort on the 90th day of their opioid use episode, once their eligibility was established.
Exclusion criteria were (i) cancer (except non-melanoma skin cancer) diagnosed in 2006 or earlier according to a registry covering northwest Washington State maintained as part of the Surveillance, Epidemiology and End Results Program, (ii) two or more cancer diagnoses identified from automated data between the episode start date and the censor date, (iii) fewer than 270 days of enrollment in GHC in the year prior to cohort entry.
If eligible subjects disenrolled from GHC after study entry they were censored on their disenrollment date; otherwise, subjects were censored on December 31, 2006 (the end of the study period). A subject’s follow-up period is the 90th day of the episode until the censor date.
Measurements
Classification of Opioids
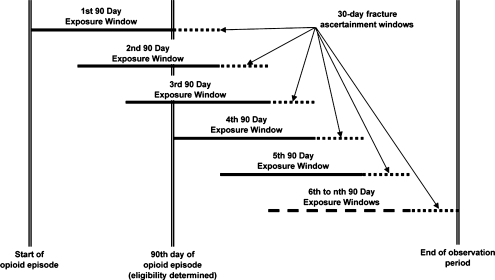
Medication data were obtained from GHC automated pharmacy files where enrollees report obtaining over 90% of their prescription medications22. Morphine equivalent dose (MED) was calculated for each opioid prescription, defined by the quantity of pills dispensed multiplied by strength (in milligrams (mg)) and by a conversion factor21. To achieve stable estimates of opioid dose, which varies over follow-up, dose was measured within 90-day time periods, starting with the initial 90-day period when the patient became eligible for the study. These 90-day opioid exposure periods were estimated with 30 days increments, so they overlap from one 90-day period to the next (see Fig. 1). Total morphine equivalent dose was summed for each 90-day exposure window and divided by 90 to estimate average daily MED. Based on distributional considerations, the average daily opioid dose was categorized into none, 1 to 19 mg, 20 to 49 mg, and ≥50 mg.
Figure 1.
Determination of exposure windows and ascertainment of fracture.
Definition of Fractures
Potential fractures were first identified using ICD-9 codes in automated encounter data that indicate fracture (800xx–804xx; 807xx–809xx; 810xx–829xx). All potential fracture diagnoses between 2000 and 2006 were identified. Vertebral fractures were excluded because it was often impossible to distinguish incident from existing fractures. Next, one of the authors (KS) examined patient medical records to confirm that an incident fracture occurred on the event date. This record review was carried out without knowledge of opioid exposure status. The first fracture that met full case criteria (i.e. after medical record review) was analyzed for each case; his or her records were censored at this event. Consequences of the confirmed fractures were assessed using automated data, including hospitalization within 2 days, nursing facility admission within 1 month, and death within 2 months.
Covariate Data Collection
Covariates were obtained from automated health care data, including age and sex, tobacco use, use of hormone replacement therapy (HRT) or bisphosphonates, and a diagnosis of depression, substance abuse, or dementia in the two years prior to entry into the cohort. The type of pain diagnosis at the index visit was identified. Because chronic disease comorbidity (and associated frailty) is a potential risk factor for fractures, chronic disease comorbidity adjustors were calculated at the time of the index visit: RxRISK risk23 and the Romano version of the Charlson Score24. Use of sedative-hypnotics (sedative hypnotics, barbiturates and muscle relaxants) and antidepressants was assessed for each 90-day exposure window. Because prior fractures are a risk factor for future fractures25, we identified fractures occurring in the 270 days prior to entry into the risk set.
Design and Analyses
We used a Cox proportional hazards model to estimate the association between current opioid use and average daily dose of opioids and the risk of fracture26. Opioid use (or dose) was included as a time varying covariate estimated for each 90-day exposure window, and updated at 30-day intervals (see Fig. 1).The reference group was those not currently using opioids, defined as not receiving opioids from GHC pharmacies during the relevant 90-day exposure window. While opioid use was one of the study’s inclusion criteria, participants could discontinue opioid use during the follow-up period. Fracture status (present / absent) was ascertained for each participant during the 30-day interval immediately following each 90-day exposure window during the follow-up period. This means that ascertained fractures could occur no more than 30 days after the time window in which opioid use and dose were measured. Dose response was examined by entering continuous dose into the model. Hazard ratios were adjusted for age (60–64; 65–74; 75–84; 85+), sex, tobacco use, depression diagnosis, substance abuse diagnosis, dementia diagnosis, index pain diagnosis (except for TMD due to sparse data), chronic disease comorbidity adjustors, sedative-hypnotic use, antidepressant use, HRT/ bisphosphonate use, and prior fractures.
RESULTS
Table 1 presents the characteristics of the sample (N = 2,341), of whom about two-thirds were female. Back pain, extremity pain and osteoarthritis were the most common pain conditions. The mean daily dose of opioids prescribed was 12.8 mg (morphine equivalents). Among 42% of the cohort, hydrocodone was the most common opioid prescribed. Use of antidepressant and sedative medications during the follow-up period was common, with about three-fifths of subjects using each type at some time during the follow-up period.
Table 1.
Characteristics of Sample of Older Adults with Sustained Opioid Use for Chronic Non-cancer Pain (N = 2,341)
| Characteristic | Quantity |
|---|---|
| Female (%) | 65.6 |
| Age, mean (s.d.) | 72.9 (8.8) |
| Tobacco use (%) | 16.5 |
| Depression diagnosis (%) | 22.4 |
| Substance abuse diagnosis (%) | 3.8 |
| Dementia diagnosis (%) | 4.8 |
| Prior fracture (%)a | 2.6 |
| HRT/bisphosphonate use (%) | 33.7 |
| Comorbidity | |
| Rxrisk, mean (s.d.) | 4272 (2455) |
| Charlson Index , mean (s.d.) | 1.32 (2.0) |
| Pain diagnosis at the index visit (% of cohort) | |
| Back pain | 41.6 |
| Neck pain | 4.8 |
| Osteoarthritis | 24.9 |
| Headache | 2.4 |
| Extremity pain | 33.6 |
| Abdominal pain/hernia | 5.3 |
| Menstrual/Menopausal pain | 0.6 |
| Temporomandibular disorder pain | 0.2 |
| Person months of follow-up, mean (s.d.) | 32.7 (21.3) |
| Daily dose of opioids (milligrams of morphine equivalent) | |
| Mean daily dose (s.d.) | 12.8 mg (17.0) |
| Median daily dose (s.d.) | 7.6 mg (0.4) |
| Sedative hypnotic use during follow-up (%) | 59.5 |
| Antidepressant use during follow-up (%) | 56.9 |
| Most common opioids prescribedb during follow-up (% of cohort) | |
| Hydrocodone | 41.7 |
| Oxycodone | 23.6 |
| Codeine combination | 14.4 |
| Long-acting morphine | 8.3 |
aFracture during 9 months prior to entry into risk set
bTop 4 shown. Based on number of days opioids were prescribed during follow-up
Subjects were followed for a mean of 32.7 months (range 1 to 83 months). About one-quarter of subjects not experiencing a fracture were censored prior to the end of the study period (12/31/06)—about half due to death and half due to disenrollment from the health plan. About three-quarters of subjects were not using opioids in at least one 90-day window (but could have resumed opioid use subsequently). We compared persons who stopped using opioids to those who sustained opioid use over their entire follow-up period on the 19 covariates included in the multivariate analysis. There were no significant differences between persons who stopped opioid use and those who continued on 13 of the 19 variables examined. Persons who stopped were significantly less likely to take sedative and antidepressant medications, less likely to have received an index pain diagnosis of back pain or headache and had less comorbidity as reflected by lower RxRISK and Romano scores.
Tables 2 and 3 show data pertaining to fractures. Among 477 subjects receiving an ICD-9 diagnosis of fracture during the study period, 320 (67.1%) were found to have valid incident non-vertebral fractures upon medical record review. This resulted in an overall annual fracture rate of 5.0%. Most non-qualifying fractures were due to rule-out diagnoses unconfirmed by radiographic studies and for follow-up visits for fractures that had occurred prior to entry into the risk set. About one-third of validated fractures were of the hip or pelvis. Fractures often had serious health consequences with about 40% resulting in hospitalization, institutionalization or death (Table 3).
Table 2.
Validation of Fractures Identified Through Automated Data (N = 477)
| Characteristic | Quantity |
|---|---|
| Validated through medical record review, N (%) | 320 (67.1) |
| Reasons for non-validation, N (%) | |
| Non-incident fracture | 60 (12.5) |
| Fracture ruled out by radiograph | 56 (11.7) |
| Compression fracture | 19 (4.0) |
| No information in record | 12 (2.5) |
| Hip replacement | 10 (2.1) |
Table 3.
Characteristics of Fractures Validated by Medical Record Review (N = 320)
| Characteristic | Quantity |
|---|---|
| Annual fracture rate (%) | 5.0 |
| Fracture Site, N (%) | |
| Hip | 71 (22.2) |
| Pelvis | 39 (12.2) |
| Lower extremity | 74 (23.1) |
| Upper extremity | 81 (25.3) |
| Rib-sternum | 49 (15.3) |
| Skull/facial bone | 6 (1.9) |
| Consequences, N (%)a | |
| Hospitalized within 2 days | 117 (36.6) |
| Nursing home or skilled nursing home admission within 1 month | 76 (23.8) |
| Died within 2 months | 6 (1.9) |
| Any of Above | 127 (39.7) |
aTo be classified as a consequence of fracture, the hospitalization, institutionalization or death needed to be associated with a fracture diagnosis
Table 4 shows the unadjusted annual fracture rates by dosage level and hazard ratios for the relationship between current prescribed dosage level and fracture adjusted for potential confounders. The annual rate of fracture was 6.1% among all current opioid users, and 3.8% among persons not currently using opioids, an elevated fracture risk of 28% (hazard ratio, HR 1.28 (95% CI (0.99, 1.64 ) p = 0.06). Among persons taking opioids at doses of ≥50 mg/day, the fracture rate was 9.9%, a twofold increase in fracture risk compared to persons not currently taking opioids (HR 2.00 (95% C.I. (1.24, 3.24) p = 0.005). Hazard ratios for the intermediate dosages indicated an increased fracture risk relative to persons not currently taking opioids, but differences at intermediate dosage levels were not statistically significant. The overall test for dose response was of borderline significance (p = 0.06). Tests for interaction between opioids and sedatives and opioids and antidepressants were not significant.
Table 4.
Hazard Ratios for the Relationship Between Opioid Dosage Level and Fracture
| Opioid dosage level (mg/day) | Number of fractures | Person years | Fracture rate (per person year) | Hazard ratio (95% C.I.)a |
|---|---|---|---|---|
| 0 | 114 | 3,012 | 0.03784 | 1.00 |
| 1–<20 | 152 | 2,684 | 0.05663 | 1.20 (0.92, 1.56) |
| 20–<50 | 33 | 472 | 0.06993 | 1.34 (0.89, 2.01) |
| 50+ | 21 | 211 | 0.09953 | 2.00 (1.24, 3.24) |
| Any Use | 206 | 3,367 | 0.06118 | 1.28 (0.99, 1.64) |
aAdjusted for smoking, depression, substance abuse, dementia, comorbidity, pain site, age, gender, prior fracture, antidepressant use, sedative use, HRT/bisphosphonate use
DISCUSSION
In this population-based study, current use of opioids for chronic non-cancer pain among persons 60 or older was associated with a borderline significant 28% increase in fracture risk. Compared with persons not currently taking opioids, persons taking average daily doses of ≥50 mg/day were at twice the fracture risk (HR 2.00).
Although our finding of a 28% increased risk of fracture associated with opioid use did not reach statistical significance (p = 0.06) the point estimate is consistent with findings from several other studies12,20. A recent meta analysis based on six studies concluded that opioids were associated with a 1.38 (1.15, 1.66)) increased risk of fracture20. The authors pointed out the need for additional large prospective studies that minimize selection bias and provide more accurate measures of fracture risk.
The current study was designed to address several limitations of prior research. In contrast to some prior studies that compared opioid users to non-users (e.g. persons who may never have used opioids)12,16, the comparison (non-exposed) group in this study was persons who initiated and then discontinued use of prescribed opioids for chronic non-cancer pain. This comparison group was selected to control for selection bias, because patients receiving prescribed opioids for chronic non-cancer pain are likely to be different from those not prescribed opioids for chronic non-cancer pain. Other study features designed to improve evaluation of opioid risks included time-varying measures of opioid use based on comprehensive, high-quality automated data sources22, validation of outcome (fractures) through medical record review, and control for potential confounders, including time-varying measures of other medications that may be linked to fracture risk12,27,28.
The principal contribution of this study is estimation of time-varying average consumed daily dose based on timing of refills, number of pills and strength of pills in morphine equivalents derived from comprehensive, high quality automated data sources. Few studies have assessed the association of opioid dose with fracture risk among community practice patients. For example, the meta-analysis mentioned above lacked information about the association of fracture risk with opioid dose. In a study among employees included in a Medicare database, researchers found a dose response relationship between propoxyphene use of ≥260 mg /day (60 mg morphine equivalents) and hip fracture risk16. A case-control study that assessed the association of fracture risk with the cumulative dose of specific opioids, rather than average daily dose, generally did not detect a dose-response effect11. Other researchers, in the absence of high-quality computerized prescription data, have adopted proxy measures of dose including strength of the pill14 and daily versus less frequent use of opioids over two weeks12. Based on a measure of average daily dose derived from high quality automated sources, we conclude that there is a clinically and statistically significant increase in fracture risk at doses of ≥50 mg /day MED.
What is less clear is the exact relationship between fracture risk and dose level. The formal test of dose response was borderline significant (p = 0.06) and associations between the lower dose subgroups and fractures were not statistically significant. However, failure to reach statistical significance does not necessarily mean that low opioid doses are as safe as non-use. For example, the confidence interval indicates that fracture risk among persons using 20–50 mg of opioids per day could range from 10% lower to 100% greater than the fracture risk observed among persons not using opioids. Confidence intervals for both of the lower dosage categories reflect considerable uncertainty about the association of opioid use with fracture risk at these dosage levels, suggesting the need for analyses in larger cohorts than we were able to assemble in this study.
Several mechanisms have been suggested to explain the putative impact of opioids on fracture risk. Researchers have hypothesized that side effects of opioid use, such as dizziness, sedation, lightheadedness and nausea, increase the risk of falls, and consequently, fractures11,12. Others, reporting an association between opioid use and reductions in bone mineral density, propose that opioids might interfere with bone formation through suppression of endogenous sex hormone production29. Ensrud et al. suggested that opioids might directly affect neuromuscular function but acknowledged that such impairments could be caused by other characteristics associated with the opioid users in their study—e.g. a higher prevalence of comorbid medical conditions12. Whatever the cause, our finding of a twofold increase in fracture risk associated with higher opioid doses has potential public health implications. A recent study estimated that 3% of older Americans used opioids regularly7. Fractures in older adults are often sentinel health events resulting in long-term reductions in mobility and capacity for independent living30. Indeed, more than one-third of fractures in this study resulted in hospitalization or other institutional care. One in ten study subjects taking 50 mg/day or more experienced a fracture in a year. Our results suggest that clinicians should consider fracture risk when deciding whether to initiate opioid therapy—particularly at higher dosage levels—in older adults.
Physicians face a dilemma when choosing medications for treating chronic pain among the elderly. The American Geriatrics Society recently recommended opioids as preferable to NSAIDs in the treatment of moderate-severe persistent pain in older adults31. While there is no ideal pain medication for this sub-population, an appropriate clinical response to pain and suffering is needed32. Decisions about medication selection and dose should consider each patient’s risk profile and preferences, along with available non-pharmacologic options for managing chronic pain. The results of the current study underscore the need for future research that evaluates how to reduce risks of potential adverse effects of sustained use of opioid medications, particularly at higher dosage levels.
This study has several significant limitations that need to be considered in interpreting results. As in any observational study, it is possible that residual confounding remains although we controlled for many potential confounding variables. Because of our reliance on automated data, we were not able to control for some potential confounders such as bone mineral density, gait, activities of daily living, frailty, family history of fracture, and pain severity. Measures of use and average daily dose were based on timing of refills determined by automated data, but we cannot definitely establish how patients used the medications. We were unable to examine whether the increased risk associated with higher dose opioids is due to how quickly opioid dosage levels were increased. Finally, our study may be subject to bias since only fractures brought to medical attention were included.
In conclusion, this study found that persons age 60 years and older who used opioids at dosages of ≥50 mg/day were at double the fracture risk compared to older persons who had discontinued opioid use. Clinicians should consider this risk when prescribing higher opioid dosage levels to older people. More research is needed to evaluate the association of opioid dose with fracture risk among older adults, and to determine whether enhanced monitoring, modifying opioid doses, and/or non-pharmacologic options for managing chronic pain might reduce fracture risk among older adults while affording pain control acceptable to patients.
Acknowledgments
This research was supported by a grant to Dr. Michael Von Korff from the National Institute of Drug Abuse [DA022557].
Conflict of Interest Statement Ms. Saunders owns stock in for-profit companies. Dr. Von Korff owns stock in for-profit companies and has received a grant from Johnson & Johnson. In the past three years Dr. Sullivan has received grants from Wyeth, Eli Lilly and Company, Aetna, and Ortho McNeil and has consulted for Eli Lilly and Company. Otherwise, the authors report no conflicts of interest.
References
- 1.Zerzan JT, Morden NE, Soumerai S, et al. Trends and geographic variation of opiate medication use in state Medicaid fee-for-service programs, 1996 to 2002. Med Care. 2006;44:1005–1010. doi: 10.1097/01.mlr.0000228025.04535.25. [DOI] [PubMed] [Google Scholar]
- 2.Caudill-Slosberg M, Schwartz L, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs. 2000. Pain. 2004;109:514–519. doi: 10.1016/j.pain.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349(20):1943–1953. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- 4.Martell BA, O’Connor PG, Kerns RD, et al. Systematic review: opioid treatment for chronic back pain: prevalence, efficacy, and association with addiction. Ann Intern Med. 2007;146(2):116–127. doi: 10.7326/0003-4819-146-2-200701160-00006. [DOI] [PubMed] [Google Scholar]
- 5.Ballantyne JC, Shin NS. Efficacy of opioids for chronic pain: a review of the evidence. Clin J Pain. 2008;24(6):469–478. doi: 10.1097/AJP.0b013e31816b2f26. [DOI] [PubMed] [Google Scholar]
- 6.Noble M, Tregear SJ, Treadwell JR, Schoelles K. Long-term opioid therapy for chronic noncancer pain:a systematic review and meta-analysis of efficacy and safety. J Pain Symptom Manage. 2008;35:214–228. doi: 10.1016/j.jpainsymman.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Kelly J, Cook S, Kaufman D, Anderson T, Rosenberg L, Mitchell A. Prevalence and characteristics of opioid use in the US adult population. Pain. 2008;138:507–513. doi: 10.1016/j.pain.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Paulose-Ram R, Hirsch R, Dillon C, Losonczy K, Cooper M, Ostchega Y. Prescription and non-prescription analgesic use among the US adult population: results from the third National Health and Nutrition Examination Survey (NHANES III) Pharmacoepidemiol Drug Saf. 2003;12:315–326. doi: 10.1002/pds.755. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan MD, Edlund MJ, Zhang L, Unutzer J, Wells KB. Association between mental health disorders, problem drug use, and regular prescription opioid use. Arch Intern Med. 2006;166:2087–2093. doi: 10.1001/archinte.166.19.2087. [DOI] [PubMed] [Google Scholar]
- 10.Braithwaite RS, Col N, Wong J. Estimating hip fracture morbidity, mortality and costs. JAGS. 2003;51:364–370. doi: 10.1046/j.1532-5415.2003.51110.x. [DOI] [PubMed] [Google Scholar]
- 11.Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with the use of morphine and opiates. J Intern Med. 2006;260:76–87. doi: 10.1111/j.1365-2796.2006.01667.x. [DOI] [PubMed] [Google Scholar]
- 12.Ensrud K, Blackwell T, Mangione C, et al. Central nervous system active medications and risk for fractures in older women. Arch Intern Med. 2003;163:949–957. doi: 10.1001/archinte.163.8.949. [DOI] [PubMed] [Google Scholar]
- 13.Vestergaard P. Pain-relief medication and risk of fractures. Curr Drug Saf. 2008;3:199–203. doi: 10.2174/157488608785699504. [DOI] [PubMed] [Google Scholar]
- 14.Shorr RI, Griffin MR, Daugherty JR, Ray WA. Opioid analgesics and the risk of hip fracture in the elderly: codeine and propoxyphene. J Gerontol. 1992;47:M111–M115. doi: 10.1093/geronj/47.4.m111. [DOI] [PubMed] [Google Scholar]
- 15.Guo Z, Wills P, Viitanen M, Fastborn J, Winblad B. Cognitive impairment, drug use, and the risk of hip fracture in persons over 75 years old: a community-based prospective study. Am J Epidemiol. 1998;148:887–892. doi: 10.1093/oxfordjournals.aje.a009714. [DOI] [PubMed] [Google Scholar]
- 16.Kamal-Bahl S, Stuart B, Beers M. Propoxyphene use and risk for hip fractures in older adults. Am J Geriatr Pharmacother. 2006;4:219–226. doi: 10.1016/j.amjopharm.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 17.French D, Campbell R, Spehar A, Rubenstein LZ, Branch L, Cunningham F. National outpatient medication profiling: medications associated with outpatient fractures in community-dwelling elderly veterans. Br J Clin Pharmacol. 2007;63(2):238–244. doi: 10.1111/j.1365-2125.2006.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spector W, Shaffer T, Potter E, Correa-de-Araujo R, Rhona Limcangco M. Risk factors associated with the occurrence of fractures in U.S. nursing homes: resident and facility characteristics and prescription medications. JAGS. 2007;55:327–333. doi: 10.1111/j.1532-5415.2007.01081.x. [DOI] [PubMed] [Google Scholar]
- 19.Abrahamsen B, Brixen K. Mapping the prescriptiome to fractures in men—a national analysis of prescription history and fracture risk. Osteoporosis Int. 2008; Aug 9 [Epub ahead of print]. [DOI] [PubMed]
- 20.Takkouche B, Montes-Martinez A, Gill S, Etminan M. Psychotropic medications and the risk of fracture. Drug Saf. 2007;30(2):171–184. doi: 10.2165/00002018-200730020-00006. [DOI] [PubMed] [Google Scholar]
- 21.Korff M, Saunders K, Ray T, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24(6):521–527. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saunders K, Davis R, Stergachis A. Group Health Cooperative. In: Strom B, editor. Pharmacoepidemiology. West Sussex: John Wiley and Sons; 2005. pp. 223–239. [Google Scholar]
- 23.Fishman P, Goodman M, Hornbrook M, et al. Risk adjustment using automated pharmacy data: the Rx Risk Model. Med Care. 2003;41:84–99. doi: 10.1097/00005650-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Romano PS, Roos L, Jollis J. Further evidence concerning the use of a clinical comorbidity index with ICD-9-CM administrative data. J Clin Epidemiol. 1993;46:1085–1090. doi: 10.1016/0895-4356(93)90106-B. [DOI] [PubMed] [Google Scholar]
- 25.Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. N Engl J Med. 1995;332:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 26.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York: Springer-Verlag; 2000. [Google Scholar]
- 27.Cumming R. Epidemiology of medication-related falls and fractures in the elderly. Drugs Aging. 1998;12:43–53. doi: 10.2165/00002512-199812010-00005. [DOI] [PubMed] [Google Scholar]
- 28.Wagner AK, Zhang F, Soumerai S, et al. Benzodiazepine use and hip fractures in the elderly: who is at greatest risk? Arch Intern Med. 2004;164:1567–1572. doi: 10.1001/archinte.164.14.1567. [DOI] [PubMed] [Google Scholar]
- 29.Kinjo M, Setoguchi S, Schneeweiss S, Solomon D. Bone mineral density in subjects using central nervous system-active medications. Am J Med. 2005;118:1414.e7–1414.e12. doi: 10.1016/j.amjmed.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 30.Haentjens P, Lamraski G, Boonen S. Costs and consequences of hip fracture occurrence in old age: an economic perspective. Disabil Rehabil. 2005;27:1129–1141. doi: 10.1080/09638280500055529. [DOI] [PubMed] [Google Scholar]
- 31.AGS Panel on the Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc (in press).
- 32.Barkin R, Barkin S, Barkin D. Perception, assessment, treatment and management of pain in the elderly. Clin Geriatr Med. 2005;21:465–490. doi: 10.1016/j.cger.2005.02.006. [DOI] [PubMed] [Google Scholar]