Abstract
BACKGROUND
Renin-angiotensin-aldosterone system (RAAS) inhibitors are associated with hyperkalemia, but there is little evidence demonstrating patients who receive potassium monitoring have a lower rate of hyperkalemia.
OBJECTIVE
To evaluate the association between potassium monitoring and serious hyperkalemia-associated adverse outcomes among patients with diabetes newly initiating RAAS inhibitor therapy.
DESIGN
Retrospective observational study.
PARTICIPANTS
Patients with diabetes without end-stage renal disease initiating RAAS inhibitor therapy between 2001 and 2006 at three integrated health care systems.
MEASUREMENTS
Potassium monitoring and first hyperkalemia-associated adverse event during the initial year of therapy. Hyperkalemia-associated adverse events included hospitalizations, emergency department visits or deaths within 24 h of hyperkalemia diagnosis and/or diagnostic potassium ≥6 mmol/l. Incidence rates were calculated in person-years (p-y). We used inverse probability propensity score weighting to adjust for differences between patients with and without monitoring; Poisson regression was used to obtain adjusted relative risks.
RESULTS
A total of 19,391 of 27,355 patients (71%) received potassium monitoring. Serious hyperkalemia-associated events occurred at an incidence rate of 10.2 per 1,000 p-y. Compared to patients without monitoring, adjusted relative risk of hyperkalemia-associated adverse events among all patients with monitoring was 0.50 (0.37, 0.66); in the subset of patients who also had chronic kidney disease (n = 2,176), adjusted relative risk was 0.29 (0.18, 0.46).
CONCLUSIONS
Patients prescribed RAAS inhibitors who have both diabetes and chronic kidney disease and receive potassium monitoring are less likely to experience a serious hyperkalemia-associated adverse event compared to similar patients who did not receive potassium monitoring. This evidence supports existing consensus-based guidelines.
KEY WORDS: hyperkalemia, hyperpotassemia, angiotensin-converting enzyme inhibitor, ACEi, angiotensin receptor blocker, ARB, spironolactone, RAAS inhibitor
BACKGROUND AND OBJECTIVES
Renin-angiotensin-aldosterone system (RAAS) inhibitors, including angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB) and aldosterone receptor antagonists such as spironolactone, are used to treat hypertension and heart failure, to decrease cardiovascular morbidity and death after myocardial infarction, and to slow renal disease progression in patients with type 2 diabetes and hypertension.1–15 Although the mechanisms of action differ, these drugs are all associated with hyperkalemia.16–21 Patients with diabetes and renal insufficiency are considered to be at greatest risk of hyperkalemia.17,20–25
Widespread agreement exists that serum potassium monitoring is a component of good clinical care for patients prescribed RAAS inhibitors. However, available monitoring guidelines are based on opinion, provide varying suggested monitoring frequencies and timing, and are not tailored to patient-specific risks.13,15–18,24,26–30 Importantly, there is a paucity of evidence demonstrating that patients who receive potassium monitoring have a lower risk of hyperkalemia than patients who are not monitored.
The aim of this study was to evaluate the association between receipt of serum potassium monitoring and occurrence of serious hyperkalemia-associated adverse outcomes among ambulatory patients with diabetes newly initiating ACEi, ARB and/or spironolactone therapy. We hypothesized that there would be a decreased risk of hyperkalemia-associated adverse outcomes in patients with diabetes who were new users of ACEi, ARB and/or spironolactone who received serum potassium monitoring compared to similar patients who did not receive monitoring. We also hypothesized that this association would be stronger in subsets of patients with selected risk factors such as heart failure or chronic kidney disease.
METHODS
Study Design, Setting, and Participants
This retrospective observational study was conducted at three integrated health care delivery systems, including Kaiser Permanente Colorado (Denver, CO), Kaiser Permanente Northwest (Portland, OR) and Kaiser Permanente Georgia (Atlanta, GA), with a 2007 combined membership of over 1,200,000 individuals. The Institutional Review Boards of each participating site approved this study and waived the requirement for informed consent.
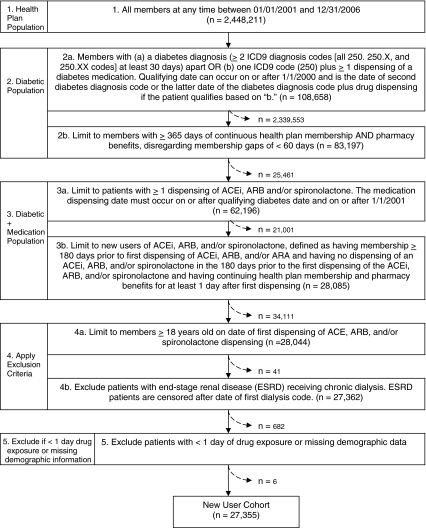
The study population included individuals with at least 12 months of continuous health plan membership and pharmacy benefits between January 1, 2001 and December 31, 2006 (Fig. 1). The study cohort was limited to patients 18 years old and older who had diabetes prior to initiating an ACEi, ARB or spironolactone, defined as at least two International Classification of Diseases, Ninth Revision, Clinical Modification (ICD9-CM) diagnosis codes (250.##) at least 30 days apart, or one ICD9-CM code plus at least one dispensing of a medication for diabetes. We excluded patients with pre-existing end-stage renal disease (ESRD) receiving chronic hemodialysis because their potassium status is subject to frequent fluctuation and different monitoring standards.
Figure 1.
Selection of study patients.
RAAS Inhibitor Dispensing
The study cohort was limited to new users of ACEi, ARB and/or spironolactone, defined as having no dispensing of an ACEi, ARB and/or spironolactone within the 180 days prior to the first dispensing during the study period. The duration of RAAS therapy was determined by the number and timing of prescriptions. New users were defined as having continuing RAAS inhibitor therapy if any gap between the end of one dispensings days’ supply and the date of the next dispensing was less than 30 days. The study follow-up period continued for each member of the study cohort until drug discontinuation (or maximum of 365 days) or censoring event (see below). For each dispensing, information obtained included patient identifier, NDC, dispensing date, strength and days’ supply. We also obtained this information for medications considered to be potential confounders. To ensure evaluation of hyperkalemia-associated adverse outcomes resulting in drug discontinuation, we ascertained outcomes for 30 days after the end date of the last dispensed days’ supply. Patients who switched drugs within a class were followed in their original patient-drug therapy group (i.e., initial therapy). Patients who switched drug classes or had a drug added from a new class were considered in the new patient-drug group beginning with the date the new drug was prescribed or added (i.e., consecutive therapy).
Assessment of Potassium Monitoring and Hyperkalemia Outcomes
Serum potassium monitoring was defined as electronic documentation of a potassium test using each site’s laboratory test code(s) (e.g., CPT codes and site-specific codes) and laboratory test result values within the timeframe of RAAS inhibitor therapy. The date of the laboratory test code(s) was considered the monitoring date(s). The location of the laboratory test (e.g., ambulatory, inpatient) was determined.
Hyperkalemia risk assessment is complicated by lack of consistent definitions of hyperkalemia across published studies.4,7,8,19,31 Therefore, we used a consistent, stringent definition of a serious hyperkalemia-associated adverse outcome associated with ACEi, ARB and/or spironolactone therapy to assess the primary outcome. This definition included an elevated serum potassium concentration of ≥6 mmol/l and/or a coded diagnosis of hyperkalemia (ICD9 code 276.7) that occurred in conjunction with an emergency department (ED) visit, an inpatient hospitalization (IP) or death attributed to hyperkalemia (coded ICD10 code E87.5). A hyperkalemia-associated outcome could not be identified on the basis of an elevated potassium concentration alone; all hyperkalemia cases required at least an IP or ED visit in conjunction with the elevated potassium and/or coded diagnosis. If one patient had more than one serious hyperkalemia-associated adverse event, only the first adverse event was included. To ensure complete capture of deaths, we determined mortality from multiple sources including disenrollment records and hospital discharge status (at all participating sites), and death certificates (at one site).
Potential Risk Factors and Confounders
Because diagnostic coding practices for chronic kidney disease (CKD) underestimate patients with estimated glomerular filtration rates (eGFR) ≤60 ml/min/1.73 m2,32–35 we determined moderate to severe (stages 3 and 4)36 CKD from coded diagnoses (ICD9 codes 585, 585.3, 585.4, 585.9, 586 or 593.9) or from: at least two eGFR ≤59 ml/min/1.73 m2, estimated from the Modification of Diet in Renal Disease (MDRD) equation and measured more than 90 days apart; at least two creatinine clearance rates ≤59 ml/min estimated from the Cockcroft-Gault equation and measured more than 90 days apart or ≥2 serum creatinine concentrations >1.5 mg/dl and measured more than 90 days apart.36–38 We conducted sub-analyses in which we stratified patients by the presence or absence of CKD.
Other variables measured as potential confounders included age, gender, the presence of chronic diseases (using the chronic disease score method of Clark et al.39), the presence of heart failure (ICD9 codes 428.#, 402.01, 402.11, 402.91), diagnosis of hypertension, provider identifier and dispensings of concomitant medications that can affect potassium (triamterene, amiloride, digoxin, potassium supplements, nonsteroidal anti-inflammatory agents, trimethoprim, beta-blockers, thiazide diuretics and loop diuretics). Further, we assessed whether potassium monitoring had occurred within the 30 days before initiating RAAS inhibitor therapy and whether a diagnosis of hyperkalemia had occurred within the 180 days prior to starting RAAS inhibitor therapy.
Data Sources, Data Management and Statistical Analysis
All study data were derived from existing automated databases. Using methods we have successfully applied previously,40–42 the programmer at the lead site developed and tested SAS programs, workplans and data dictionaries to develop the study dataset. All data checks and analyses were performed with SAS version 9.1 (SAS Institute Inc., Cary, NC).
We compared characteristics of patients with and without potassium monitoring using chi-square, Fisher's exact or Wilcoxon rank sum tests. Crude incidence rates of hyperkalemia-associated adverse outcomes were calculated using person time (as person-years, p-y) for each patient measured as time from drug initiation to first adverse outcome, drug discontinuation, health plan disenrollment, ESRD diagnosis, kidney transplant, death or end of study follow-up (365 days), whichever occurred sooner. Unadjusted relative risks (RR) of outcomes between monitored and not monitored patients were calculated assuming a Poisson distribution for the total number of outcomes for each treatment group and accounting for person time for the study cohort overall and stratified by age, gender, RAAS inhibitor drug group, CKD and heart failure.
Because patients with and without potassium monitoring are likely to differ in characteristics that can be related to potassium monitoring and hyperkalemia outcomes, we used propensity scores to estimate potassium monitoring (i.e., exposure) effects.43 The propensity scores helped to ensure that any difference in hyperkalemia outcomes between patients with and without monitoring were not attributable to the difference in the measured confounders. We generated a propensity score for each patient in a logistic regression model and then used the inverse probability propensity scores as weights to determine the adjusted RR for outcome with the same assumptions as those in calculations of the unadjusted RR.44–47 Variables included in the propensity scores were study site, RAAS inhibitor drug group, gender, age, kidney transplant, IP and/or ED within 6 months prior to study entry, heart failure diagnosis, CKD, hypertension diagnosis, diagnosis of hyperkalemia within 180 days prior to starting ACEi/ARB/spironolactone, whether or not a potassium concentration was measured within 30 days prior to initiating ACEi/ARB/spironolactone, chronic disease score, provider and concomitant therapy with digoxin, a diuretic, a beta-blocker or a potassium supplement. In calculations of adjusted RR for outcome, the propensity score functioned as a summary of potential confounders, along with potassium monitoring as the exposure to estimate the association of hyperkalemia-associated adverse outcome and receipt of monitoring.
Role of the Funding Source
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant no. R21DK075076. NIDDK did not have any role in the study design, conduct or reporting.
RESULTS
We identified 27,355 individuals with diabetes who were new users of ACEi, ARB or spironolactone (Fig. 1), including 2,176 who also had stage 3 or 4 CKD (Table 1). Most (n = 25,240, 92.3%) of these individuals were initially dispensed an ACEi (Table 1). Seventy-one percent (n = 19,391) had at least one serum potassium concentration measured during their ACEi, ARB or spironolactone therapy (Table 1). Individuals with and without monitoring were different in measured demographic and clinical characteristics (Table 1). For individuals with potassium monitoring, the median (5th, 95th percentile) time between RAAS inhibitor initiation and first monitoring was 15 (0, 252) days.
Table 1.
Characteristics of Patients in the Study Cohort a
| Characteristic | All patients (N = 27,355) | Patients with ≥1 screening potassium monitoring (N = 19,391; 71%) | Patients without screening potassium monitoring (N = 7,964; 29%) | P Value b |
|---|---|---|---|---|
| Mean age in years (SD) | 59.0 (13.3) | 60.4 (13.0) | 55.5 (13.2) | <0.001 |
| Gender: number female (%) | 13,258 (48.5) | 9,544 (49.2) | 3,714 (46.6) | <0.001 |
| Number of patients in each initial patient-drug group (%) c | <0.001 | |||
| ACEi | 25,240 (92.3) | 17,825 (91.9) | 7,415 (93.1) | |
| ARB | 1,538 (5.6) | 1,099 (5.7) | 439 (5.5) | |
| Spironolactone | 456 (1.7) | 361 (1.9) | 95 (1.2) | |
| ACEi + spironolactone | 95 (0.3) | 85 (0.4) | 10 (0.1) | |
| ACEi + ARB | 16 (0.1) | 13 (<0.1) | 3 (<0.1) | |
| ARB + spironolactone | 10 (<0.1) | 8 (<0.1) | 2 (<0.1) | |
| Duration of initial drug therapy (days) | <0.001 | |||
| Mean (SD) | 209 (143) | 238 (139) | 138 (128) | |
| Median (5th, 95th percentile) | 194 (30, 365) | 321 (30, 365) | 70 (30, 365) | |
| Duration of consecutive drug therapy (days) | <0.001 | |||
| Mean (SD) | 225 (143) | 259 (134) | 143 (130) | |
| Median (5th, 95th percentile) | 264 (30, 365) | 365 (30, 365) | 79 (30, 365) | |
| Number of chronic diseases within 6 months prior to study entry | <0.001 | |||
| Mean (SD) | 6.3 (2.3) | 6.5 (2.3) | 5.9 (2.1) | |
| Median (5th, 95th percentile) | 6 (3, 10) | 6 (3, 11) | 6 (3, 9) | |
| Hospitalization(s) and/or emergency department visits, any within 6 months prior to study entry (%) | 6,067 (22.2) | 4,549 (23.5) | 1,518 (19.1) | <0.001 |
| Outpatient visits within 6 months prior to study entry | <0.001 | |||
| Mean (SD) | 4.6 (4.7) | 4.8 (4.9) | 4.2 (4.2) | |
| Median (5th, 95th percentile) | 3 (0, 14) | 3 (0, 14) | 3 (0, 13) | |
| Heart failure diagnosis (%) d | 2,009 (7.3) | 1,727 (8.9) | 282 (3.5) | <0.001 |
| Hypertension diagnosis (%) d | 18,709 (68.4) | 14,135 (72.9) | 4,574 (57.4) | <0.001 |
| Chronic kidney disease (CKD, %) d, e | 2,176 (8.0) | 1,937 (10.0) | 239 (3.0) | <0.001 |
| CKD diagnosis code (%) | 1,812 (6.6) | 1,601 (8.3) | 211 (2.7) | <0.001 |
| CKD by creatinine clearance or glomerular filtration rate estimate (%) | 364 (1.3) | 336 (1.7) | 28 (0.4) | |
| End-stage renal disease diagnosis during study period (%) | 163 (0.6) | 132 (0.7) | 31 (0.4) | 0.004 |
| Kidney transplant (%) d | ||||
| Prior to study period | 56 (0.2) | 54 (0.3) | 2 (0.1) | <0.001 |
| During study period | 3 (<0.1) | 3 (<0.1) | 0 | |
| Deaths during study period (%) | 259 (1.0) | 207 (1.1) | 52(0.7) | <0.001 |
| Concomitant therapy f | ||||
| Digoxin | 973 (3.6) | 847 (4.4) | 126 (1.6) | <0.001 |
| Diuretic | 8,788 (32.1) | 7,200 (37.1) | 1,588 (20.0) | <0.001 |
| Potassium supplement | 3,072 (11.2) | 2,695 (13.9) | 377 (4.7) | <0.001 |
| Digoxin and potassium supplement | 411 (1.5) | 377 (1.9) | 34 (0.4) | <0.001 |
| Digoxin and diuretic | 688 (2.5) | 619 (3.2) | 69 (0.9) | <0.001 |
| Diuretic and potassium supplement | 2,821 (10.3) | 2,481 (12.8) | 340 (4.3) | <0.001 |
| Nonsteroidal anti-inflammatory agents | 5,323 (19.5) | 4,076 (21.0) | 1,247 (15.7) | <0.001 |
| Trimethoprim | 694 (2.5) | 572 (2.9) | 122 (1.5) | <0.001 |
| Beta-blocker | 8,223 (30.1) | 6,668 (34.4) | 1,555 (19.5) | <0.001 |
| Patients with potassium level measured within 30 days prior to initiating ACEi/ARB/spironolactone therapy | 244 (0.9) | 170 (0.9) | 74 (0.9) | 0.68 |
| Patients with potassium level ≥6.0 mmol/l within 180 days prior to study period | 23 (0.1) | 20 (0.1) | 3 (<0.1) | 0.09 |
| Patients with a diagnosis of hyperkalemia within 180 days prior to starting ACEi/ARB/spironolactone therapy | 140 (0.5) | 110 (0.6) | 30 (0.4) | 0.05 |
aPatients with diabetes who were new users of a RAAS inhibitor [i.e., angiotensin-converting enzyme inhibitor (ACEi), angiotensin receptor blocker (ARB) or spironolactone], with one or more dispensings during the study timeframe
bChi square, Fisher’s exact or Wilcoxon rank sum tests
cFor analysis, the ACEi, ARB and ACEi + ARB groups were considered together, and the spironolactone, ACEi + spironolactone and ARB + spironolactone patient-drug groups were considered together
dDiagnosis codes were determined from up to 180 days prior to the study period through the end of the study period
eMany of the 1,812 patients in the “diagnosis code” grouping also had creatinine clearance or GFR estimates during the study period that reflected CKD stage 3 or 4. The 364 patients in the “creatinine clearance or GFR estimate” grouping had CKD stage 3 or 4 based on at least two creatinine clearance or GFR estimates ≥90 days apart during their study period, but did not have any diagnosis code for CKD during the study timeframe
fNot mutually exclusive, that is, a patient prescribed digoxin could have also been prescribed a diuretic, etc. Diuretic concomitant therapy does not include patients receiving spironolactone
A serious hyperkalemia-associated adverse outcome occurred in 184 (0.7%) individuals, with an overall population incidence rate of 10.2/1,000 p-y (Table 2). The incidence rate of hyperkalemia-associated outcomes did not differ between individuals prescribed an ACEi or ARB (p = 0.36), and therefore the ACEi and ARB groups were considered together for other analyses. However, the incidence rate of hyperkalemia among patients prescribed spironolactone was 96.0/1,000 p-y, whereas among patients prescribed an ACEi or ARB, the incidence rate was 8.8/1,000 p-y. Individuals who received potassium monitoring were 50 percent less likely to experience a hyperkalemia-associated adverse outcome [adjusted RR 0.50 (95% CI 0.37, 0.66)] than individuals who did not receive monitoring (Table 2). When stratified by those prescribed spironolactone and those prescribed an ACEi or ARB, potassium monitoring remained protective against hyperkalemia in both groups (Table 2).
Table 2.
Relative Risks of Serious Hyperkalemia-Associated Adverse Outcomes among Patients with Diabetes Who Are New Users of ACEi, ARB and/or Spironolactone Therapy
| Serious Hyperkalemia outcome (serum potassium ≥6.0 mmol/l or ICD9 code 276.7 plus an emergency department visit or hospitalization) | All patients | Patients with ≥1 screening potassium monitoring | Patients without screening potassium monitoring | Unadjusted relative risk (95% CI) a | Adjusted relative risk (95% CI) b | |||
|---|---|---|---|---|---|---|---|---|
| Number of outcomes | Incidence rate (per 1,000 person-years) | Number of outcomes | Incidence rate (per 1,000 person-years) | Number of outcomes | Incidence rate (per 1,000 person-years) | |||
| Entire study cohort | ||||||||
| All patients (n = 27,361) | 184 | 10.2 | 154 | 10.7 | 30 | 8.3 | 1.29 (0.87, 1.90) | 0.50 (0.37, 0.66) |
| Drug subcohorts | ||||||||
| ACEi or ARB alone or in combination (n = 26,794) | 155 | 8.8 | 131 | 9.3 | 24 | 6.7 | 1.38 (0.90, 2.14) | 0.46 (0.34, 0.63) |
| Spironolactone alone or in combination (n = 561) | 29 | 96.0 | 23 | 83.5 | 6 | 220.2 | 0.38 (0.15, 0.93) | 0.26 (0.11, 0.60) |
| Subcohorts of patients without chronic kidney diseasec or heart failure diagnosis | ||||||||
| No chronic kidney disease and no heart failure, ACEi or ARB alone or in combination (n = 23,539) | 39 | 2.5 | 32 | 2.7 | 7 | 2.1 | 1.28 (0.57, 2.91) | 1.08 (0.48, 2.40) |
| No chronic kidney disease and no heart failure, spironolactone (n = 266) | 7 | 55.8 | 4 | 37.1 | 3 | 170.6 | 0.22 (0.05, 0.97) | 0.16 (0.04, 0.67) |
| Subcohorts of patients with chronic kidney diseasec | ||||||||
| Chronic kidney disease stage 3 or 4; ACEi or ARB alone or in combination (n = 1,482) | 44 | 41.3 | 34 | 34.6 | 10 | 121.7 | 0.28 (0.14, 0.57) | 0.19 (0.11, 0.36) |
| Chronic kidney disease stage 3 or 4, spironolactone (n = 42) | 4 | 179.0 | 4 | 187.5 | 0 | 0 | -- | -- |
| Subcohorts of patients with heart failure diagnosis | ||||||||
| Heart failure, ACEi or ARB alone or in combination (n = 1,232) | 31 | 36.3 | 27 | 35.7 | 4 | 41.1 | 0.87 (0.30, 2.48) | 1.04 (0.32, 3.40) |
| Heart failure, spironolactone (n = 142) | 6 | 67.8 | 5 | 60.6 | 1 | 170.2 | 0.35 (0.04, 3.05) | 0.40 (0.06, 2.82) |
| Subcohorts of patients with chronic kidney diseasec and heart failure diagnosis | ||||||||
| Chronic Kidney disease stage 3 or 4 and heart failure, ACEi or ARB alone or in combination (n = 541) | 41 | 115.1 | 38 | 112.0 | 3 | 176.7 | 0.63 (0.20, 2.05) | 0.46 (0.16, 1.36) |
| Chronic kidney disease stage 3 or 4 and heart failure, spironolactone (n = 112) | 12 | 180.0 | 10 | 156.5 | 2 | 722.1 | 0.22 (0.05, 0.99) | 0.26 (0.01, 4.79) |
aComparing patients with monitoring to patients without monitoring based on Poisson regression accounting for person time
bComparing patients with monitoring to patients without monitoring based on Poisson regression accounting for person time and weighting with the inverse of propensity scores. For variables included in the propensity score models, please see the Methods section
cChronic kidney disease was determined from any of the following: an ICD9 code indicating chronic kidney disease, at least two eGFR ≤59 ml/min/1.73 m2, estimated from the Modification of Diet in Renal Disease (MDRD) equation and measured at least 91 days apart, at least two creatinine clearance rates ≤59 ml/min estimated from the Cockcroft-Gault equation and measured at least 91 days apart, or at least two serum creatinine concentrations >1.5 mg/dl
Across the entire study cohort, hyperkalemia outcomes occurred far more often among patients who also had CKD (overall incidence rate 66.8/1000 p-y compared to 5.0/1,000 p-y among those without CKD). Among all patients with CKD, the adjusted RR of a hyperkalemia-associated adverse outcome for those who were monitored compared to those who were not was 0.29 (0.18, 0.46), while among all patients without CKD, the adjusted RR of a hyperkalemia-associated adverse outcome for those who were monitored compared to those who were not was 0.99 (0.58, 1.71; data not shown in table).
When stratified into ACEi, ARB alone/in combination and spironolactone subcohorts, monitoring was protective among patients with CKD (Table 2). However, for patients with heart failure, monitoring was not protective (Table 2). Among patients without either CKD or heart failure dispensed an ACEi or ARB alone or in combination, monitoring was of questionable value (Table 2).
Potassium monitoring was protective against hyperkalemia-associated outcomes across all age groups except patients less than 50 years of age [<50 years: adjusted RR 4.07 (0.72, 23.19); 50–59 years: adjusted RR 0.48 (0.25, 0.93); 60–69 years: adjusted RR 0.56 (0.29, 1.09); ≥70 years: adjusted RR 0.49 (0.30, 0.82; data not shown).
DISCUSSION
Our results demonstrate that patients with diabetes who received serum potassium monitoring during their first year of therapy with an ACEi, ARB or spironolactone were less likely to experience a hyperkalemia-associated adverse event than patients who did not receive monitoring. This protective effect of monitoring was primarily evident in patients who also had CKD stage 3 or 4. To our knowledge, this study is the first to provide evidence that supports the consensus-based guidelines followed by clinicians who routinely monitor serum potassium in an effort to avoid hyperkalemia-associated adverse events among patients prescribed an ACEi, ARB or spironolactone.
Patients with diabetes are at risk of developing hyperkalemia because of impaired kidney potassium excretion related to hyporeninemic hypoaldosteronism,19,48–50 impaired renal tubular function,17 insulin deficiency that limits the ability of the body to shift potassium into cells17 and concomitant medications (e.g., potassium-sparing diuretics).51–53 In patients who also have CKD, the risk of hyperkalemia is likely further increased because of decreased urinary potassium excretion associated with impaired GFR and declines in aldosterone levels.54
The existence of a relationship among hyperkalemia, RAAS inhibitor therapy and CKD is well established.19,31,54,55 De Denus and colleagues suggested that, among patients with heart failure receiving an ACEi, each mg/dl increase in serum creatinine nearly doubled the risk of hyperkalemia in patients with CKD.31 Further, Maddirala et al. documented that, among patients receiving an ACEi, the incidence of hyperkalemia was nearly 3- and 13-fold higher among patients with stage 3 and 4 CKD, respectively, than among patients with stage 1 or 2 CKD.54 While our study was not designed to determine factors associated with the development of hyperkalemia, our results are consistent with the findings of these previous studies, and our results underscore the importance of evaluating renal function and serum potassium in patients in whom RAAS inhibitor therapy is initiated.31,54 In our study, 29 percent of patients with diabetes initiating RAAS inhibitor therapy did not have serum potassium monitoring. Our finding is consistent with the 28 to 39 percent of patients without monitoring documented in previous investigations.40–42,56
Because of the perceived hyperkalemia risk, the National Committee on Quality Assurance (NCQA) includes monitoring creatinine and potassium for patients prescribed ACEi, ARB and diuretics as a Health Plan Employer Data and Information Set (HEDIS®) measure for performance quality.57 While our study addressed only the subpopulation of patients with diabetes, our results call into question whether routine potassium monitoring is either beneficial or cost effective in the broad population included in the HEDIS® measure. Targeting monitoring toward those with selected comorbidities may be more efficient.
Our study has several strengths. The study population included a large number of new users of RAAS inhibitors. We applied a rigorous, standardized definition of hyperkalemia outcome. In addition, we used propensity scores to balance anticipated differences in measured confounders between patients with and without monitoring. The importance of adjusting for confounding was highlighted by the change from the unadjusted RR [1.29, 95% CI (0.87, 1.90)] to the adjusted RR [0. 50, 95% CI (0.37, 0.66)]; if differences in confounders between groups had not been considered, results could have been erroneously interpreted. Finally, to ensure correct classification of patients with CKD, we evaluated not only diagnosis codes, but also laboratory values to identify patients with estimated glomerular filtration rates and creatinine clearance values consistent with stage 3 and 4 CKD. This resulted in classifying an additional 364 (17%) patients as having CKD.
As with all observational studies, this study is subject to misclassification biases. Misclassification of outcomes could have occurred if hyperkalemia was not coded as a diagnosis. Additionally, we likely did not identify hyperkalemia-associated deaths in the community because hyperkalemia-associated arrhythmias resulting in death are unlikely to be listed on the death certificate. Misclassification of risk factors could have occurred if we did not identify all patients with CKD or heart failure or had incomplete disease severity information.33 Finally, while propensity scores assist in reducing bias associated with measured confounders, their use cannot reduce bias associated with unmeasured confounding.
Study data did not permit evaluation of whether the prescribing physician ordered a laboratory test that the patient did not complete. Thus, we could not differentiate between patient and physician factors that contributed to lack of monitoring. We also did not have blood glucose information available for study patients and therefore could not include severe hyperglycemia (i.e., contributing to intravascular potassium shifts) in the potential confounders.
Because this study was conducted within integrated healthcare systems, the results may not be generalizable to other settings. Yet, patients with diabetes or those prescribed RAAS inhibitors are unlikely to have differing characteristics simply because of the delivery system in which they enroll. Further, because we compared risks between monitored and non-monitored groups, these results should be applicable to patients in other health care settings.
The results of this work provide guidance in developing a framework for evidence-based potassium-monitoring recommendations for patients with diabetes prescribed RAAS inhibitors. Because randomized trials of monitoring practices are unlikely to be undertaken, such guidelines will of necessity be based on analysis of existing data. It will be important in future studies to determine more precisely the patient groups that benefit most from monitoring, such as whether monitoring is beneficial for patients without diabetes who have CKD. It will also be important to study whether interventions intended to improve potassium monitoring are effective, and to evaluate the cost effectiveness of both monitoring and of interventions.
We conclude that patients newly prescribed ACEi, ARB and spironolactone who have both diabetes and CKD and receive serum potassium monitoring are much less likely to experience a serious hyperkalemia-associated adverse event than similar patients without monitoring. This study offers tangible evidence to support published guidelines that recommend monitoring serum potassium among patients with these clinical characteristics who are started on RAAS inhibitors.
Acknowledgements
“Diabetes and Drug-Associated Hyperkalemia: Effect of Laboratory Monitoring” was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) under the following grant number: R21DK075076. NIDDK did not participate in any of the following: design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript.
We thank Xiuhai Yang, MS, Hans Petersen, MS, and Junling Ren, MEd, for programming efforts, and Leslie A. Wright, MA, and Jill Mesa for project management.
Conflicts of Interest Disclosure Marsha Raebel reports being a co-investigator in a research study funded by Sanofi-Aventis. This study does not involve the evaluation of any commercial product. Dr. Raebel also owns ≤400 shares of stock in each of Merck, Genentech, Teva, Biogen and Pfizer. Douglas Roblin reports receiving research grants within the last 3 years from GlaxoSmithKline and Gilead. Craig Cheetham reports being a co-investigator on a research study funded by Merck, owns stock in Allergan Pharmaceuticals and has a family member employed by Allergan Pharmaceuticals. Christopher Blanchette was previously employed by GlaxoSmithKline, during which time he owned stock in that company. He has received research grants from GlaxoSmithKline, AstraZeneca, Wyeth, Amgen and Premier, Inc., and serves as a consultant for NovoNordisk, GlaxoSmithKline, AstraZeneca, Sepracor and Viostat. David Smith has received research grants from Genzyme, Sanofi-Aventis, Amgen and Abbott. These research grants do not involve the evaluation of any commercial product. Colleen Ross, Stanley Xu and Gwyn Saylor report no potential conflicts of interest.
References
- 1.Brenner BM, Cooper ME, Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 2.Lindholm LH, Ibsen H, Dahlof B, et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomized trial against atenolol. Lancet. 2002;359:1004–1010. doi: 10.1016/S0140-6736(02)08090-X. [DOI] [PubMed] [Google Scholar]
- 3.HOPE (Heart Outcomes Prevention Evaluation) Study Investigators Effects of an angiotensin converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 4.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 5.Chobanian AV, Bakris GI, Black HR, Cushman WC, Green LA, Izzo JL. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. JAMA. 2003;39(suppl 1):S94–S98. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 6.Cohn JN, Tognoni G. for the Valsartan heart failure trial investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 7.McMurray JJV, Ostergren J, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767–771. doi: 10.1016/S0140-6736(03)14283-3. [DOI] [PubMed] [Google Scholar]
- 8.Pfeffer MA, McMurray JJV, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–1906. doi: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- 9.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 10.Pfeffer MA. ACE inhibition in acute myocardial infarction. N Engl J Med. 1995;332:118–120. doi: 10.1056/NEJM199501123320210. [DOI] [PubMed] [Google Scholar]
- 11.Pfeffer MA, Braunwald E, Moye LA. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the Survival and Ventricular Enlargement trial. N Engl J Med. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 12.Gruppo Italiano per lo Studio della Sopravvivenza nell'infart Miocardico GISSI-3: effects of lisinopril and transfermal glyceryl trinitrate singly and together on 6-week mortality and ventricular function after myocardial infarction. Lancet. 1994;343:1115–1122. [PubMed] [Google Scholar]
- 13.Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. A report of the American college of cardiology/American heart association task force on practice guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2001;38:2101–2113. doi: 10.1016/S0735-1097(01)01683-7. [DOI] [PubMed] [Google Scholar]
- 14.Wolf G, Ritz E. Combination therapy with ACE inhibitors and angiotensin II receptor blockers to halt progression of chronic renal disease: Pathophysiology and indications. Kidney Int. 2005;67:799–812. doi: 10.1111/j.1523-1755.2005.00145.x. [DOI] [PubMed] [Google Scholar]
- 15.The RALES Investigators Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (the randomized aldactone evaluation study [RALES]) Am J Cardiol. 1996;78:902–907. doi: 10.1016/S0002-9149(96)00465-1. [DOI] [PubMed] [Google Scholar]
- 16.Palmer BF. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what to do if the serum creatinine and/or serum potassium concentration rises. Nephrol Dial Transplant. 2003;18:1973–1975. doi: 10.1093/ndt/gfg282. [DOI] [PubMed] [Google Scholar]
- 17.Palmer BF. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med. 2004;351:585–592. doi: 10.1056/NEJMra035279. [DOI] [PubMed] [Google Scholar]
- 18.Juurlink DN, Mamdani MM, Lee DS, et al. Rates of hyperkalemia after publication of the randomized aldactone evaluation study. N Engl J Med. 2004;351:543–551. doi: 10.1056/NEJMoa040135. [DOI] [PubMed] [Google Scholar]
- 19.Ahuja TS, Freeman D, Jr, Mahnken JD, Agraharkar M, Siddiqui M, Memon A. Predictors of the development of hyperkalemia in patients using angiotensin-converting enzyme inhibitors. Am J Nephrol. 2000;20:268–272. doi: 10.1159/000013599. [DOI] [PubMed] [Google Scholar]
- 20.Desai AS, Swedberg K, McMurray JJV, et al. Incidence and predictors of hyperkalemia in patients with heart failure: An analysis of the CHARM program. J Am Coll Cardiol. 2007;50:1959–1966. doi: 10.1016/j.jacc.2007.07.067. [DOI] [PubMed] [Google Scholar]
- 21.Ramadan FH, Masoodi N, El-Solh AA. Clinical factors associate with hyperkalemia in patients with congestive heart failure. J Clin Pharm Therapeutics. 2005;30:233–239. doi: 10.1111/j.1365-2710.2005.00638.x. [DOI] [PubMed] [Google Scholar]
- 22.Bakris G, Williams M, Dworkin L. Preserving renal function in adults wit hypertension and diabetes: a consensus approach. Am J Kidney Dis. 2000;36:646–661. doi: 10.1053/ajkd.2000.16225. [DOI] [PubMed] [Google Scholar]
- 23.Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685–693. doi: 10.1001/archinte.160.5.685. [DOI] [PubMed] [Google Scholar]
- 24.Schepkens H, Vanholder R, Billiouw JM, Lameire N. Life-threatening hyperkalemia during combined therapy with angiotensin-converting enzyme inhibitors and spironolactone: an analysis of 25 cases. Am J Med. 2001;110:438–441. doi: 10.1016/S0002-9343(01)00642-8. [DOI] [PubMed] [Google Scholar]
- 25.Schaefer TJ, Wolford RW. Disorders of potassium. Emerg Med Clin N Am. 2005;23:723–747. doi: 10.1016/j.emc.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 26.Berry C, McMurray J. Life-threatening hyperkalemia during combined therapy with angiotensin-converting enzyme inhibitors and spironolactone. Am J Med. 2001;111:587. doi: 10.1016/s0002-9343(01)00927-5. [DOI] [PubMed] [Google Scholar]
- 27.Knight EL, Avorn J. Quality indicators for appropriate medication use in vulnerable elders. Ann Intern Med. 2001;135:703–710. doi: 10.7326/0003-4819-135-8_part_2-200110161-00009. [DOI] [PubMed] [Google Scholar]
- 28.Lloyd SJ, Mauro VF. Spironolactone in the treatment of congestive heart failure. Ann Pharmacother. 2000;34:1336–1340. doi: 10.1345/aph.10104. [DOI] [PubMed] [Google Scholar]
- 29.Shah KB, Rao K, Sawyer R, Gottlieb SS. The adequacy of laboratory monitoring in patients treated with spironolactone for congestive heart failure. J Am Coll Cardiol. 2005;46:845–849. doi: 10.1016/j.jacc.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 31.Denus S, Tardif J-C, White M, et al. Quantification of the risk and predictors of hyperkalemia in patients with left ventricular dysfunction: A retrospective analysis of the Studies of Left Ventricular Dysfunction (SOLVD) trials. Am Heart J. 2006;152:705–712. doi: 10.1016/j.ahj.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 32.Stevens LA, Fares G, Fleming J, et al. Low rates of testing and diagnostic codes usage in a commercial clinical laboratory: evidnece for lack of physician awareness of chronic kidney disease. J Am Soc Nephrol. 2005;16:2439–2448. doi: 10.1681/ASN.2005020192. [DOI] [PubMed] [Google Scholar]
- 33.Duru OK, Vargas RB, Kermah D, Nissenson AR, Norris KC. High prevalence of stage 3 chronic kidney disease in older adults despite normal serum creatinine. J Gen Intern Med. 2009;24:86–92. doi: 10.1007/s11606-008-0850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller WG. Reporting estimated GFR from serum creatinine: Recommendations from the laboratory working group of the National Kidney Diabetes Education Program. Oral presentation from the 2006 annual meeting of the American Association of Clinical Chemistry. 2006. Available from: http://www.aacc.org/events/expert_access/2006/kidneydisease/Pages/default.aspx Accessed Dec. 2009.
- 35.Winkelmayer WC, Schneeweiss S, Mogun H, Patrick AR, Avorn J, Solomon DH. Identification of Individuals with CKD from Medicare Claims Data: a validation study. Am J Kidney Dis. 2005;46:225–232. doi: 10.1053/j.ajkd.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 36.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. doi: 10.1016/S0272-6386(02)70081-4. [DOI] [PubMed] [Google Scholar]
- 37.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 38.Cockcroft Dw, Gault MH. Prediction o f creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 39.Clark DO, Korff M, Saunders K, Baluch WM, Simon GE. A chronic disease score with empirically derived weights. Med Care. 1995;33:783–795. doi: 10.1097/00005650-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Raebel MA, McClure DL, Simon SR, et al. Laboratory monitoring of angiotensin converting enzyme inhibitors and angiotensin receptor blockers in ambulatory patients. Pharmacoepidemiol Drug Saf. 2007;16:55–64. doi: 10.1002/pds.1217. [DOI] [PubMed] [Google Scholar]
- 41.Raebel MA, McClure DL, Andrade SE, et al. Laboratory evaluation of potassium and creatinine among ambulatory patients prescribed spironolactone: are we monitoring for Hyperkalemia? Ann Pharmacother. 2007;41:193–200. doi: 10.1345/aph.1H520. [DOI] [PubMed] [Google Scholar]
- 42.Raebel MA, Lyons EE, Andrade SE, et al. Laboratory monitoring of high risk drugs at initiation of therapy in ambulatory care. J Gen Intern Med. 2005;20:1120–1126. doi: 10.1111/j.1525-1497.2005.0257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. doi: 10.1093/biomet/70.1.41. [DOI] [Google Scholar]
- 44.Robins JM, Herman MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiol. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 45.Rosenbaum PR. Model-Based Direct Adjustment. J Am Stat Assoc. 1987;82:387–394. doi: 10.2307/2289440. [DOI] [Google Scholar]
- 46.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Sturmer T. Variable selection for propensity score model. Am J Epidemiol. 2006;163:1149–1156. doi: 10.1093/aje/kwj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu S, Ross C, Raebel MA, Shetterly S, Blanchette C, Smith D. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value in Health. doi:10.1111/j.1524-4733.2009.00671.x. [DOI] [PMC free article] [PubMed]
- 48.Arruda J, Batlle D, Sehy J, Roseman M, Baronowski R, Kurtzman NA. Hyperkalemia and renal insufficiency: role of selective aldosterone deficiency and tubular unresponsiveness to aldosterone. Am J Nephrol. 1981;1:160–167. doi: 10.1159/000166533. [DOI] [PubMed] [Google Scholar]
- 49.DeFronzo RA. Hyperkalemia and hyporeninemic hypoaldosteronism. Kidney Int. 1980;17:118–134. doi: 10.1038/ki.1980.14. [DOI] [PubMed] [Google Scholar]
- 50.Schaefer TJ, Wolford RW. Disorders of potassium. Emerg Med Clin N Am. 2005;23:723–747. doi: 10.1016/j.emc.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 51.Chiu TF, Bullard MJ, Chen JC, Liaw SJ, Ng CJ. Rapid life-threatening hyperkalemia after addition of amiloride HCl/hydrochlorothiazide to angiotensin-converting enzyme inhibitor therapy. Ann Emerg Med. 1997;30:612–615. doi: 10.1016/S0196-0644(97)70078-7. [DOI] [PubMed] [Google Scholar]
- 52.Kurata C, Uehara A, Sugi T, Yamazaki K. Syncope caused by nonsteroidal anti-inflammatory drugs and angiotensin-converting enzyme inhibitors. Jpn Circ J. 1999;63:1002–1003. doi: 10.1253/jcj.63.1002. [DOI] [PubMed] [Google Scholar]
- 53.Juurlink DN, Mamdani M, Kopp A, Laupacis A, Redelmeier DA. Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA. 2003;289:1652–1658. doi: 10.1001/jama.289.13.1652. [DOI] [PubMed] [Google Scholar]
- 54.Maddirala S, Khan A, Vincent A, Lau K. Effect of angiotensin converting enzyme inhibitors and angiotensin receptor blockers on serum potassium levels and renal function in ambulatory outpatients: risk factors analysis. Am J Med Sci. 2008;336:330–335. doi: 10.1097/MAJ.0b013e3181836ac7. [DOI] [PubMed] [Google Scholar]
- 55.Reardon LC, Macpherson DS. Hyperkalemia in outpatients using angiotensin-converting enzyme inhibitors. Arch Intern Med. 1998;158:26–32. doi: 10.1001/archinte.158.1.26. [DOI] [PubMed] [Google Scholar]
- 56.Shah KB, Rao K, Sawyer R, Gottlieb SS. The adequacy of laboratory monitoring in patients treated with spironolactone for congestive heart failure. J Am Coll Cardiol. 2005;46:845–849. doi: 10.1016/j.jacc.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 57.NCQA. Annual monitoring for patients on persistent medications: new measure for HEDIS 2006. NCQA . 2006. Available from: http://www.ncqa.org/Portals/0/HEDISQM/Archives/2006/MeasuresList.pdf Accessed December 2009.