Abstract
Background
Low-carbohydrate (LC) and high-fat, low-carbohydrate (HFLC) dietary preparations may enhance 18F-FDG-PET-based imaging of small, inflamed structures near the heart by suppressing myocardial FDG signal. We compared myocardial 18F-FDG uptake in patients randomized to LC, HFLC, and unrestricted (UR) preparations prior to 18F-FDG-PET.
Methods and Results
We randomized 63 outpatients referred for oncologic 18F-FDG-PET to LC, HFLC, or UR dietary preparations (1:1:1 allocation) starting the evening before PET. After eating dinner according to instructions, UR and LC patients fasted until FDG injection (mean time 745 minutes for UR, 899 minutes for LC), and HFLC patients drank a fatty drink 60-70 minutes prior to FDG injection. Attenuation-corrected PET imaging was performed 60 minutes after FDG administration. Maximal myocardial standard uptake values (MyoSUVmax) were systematically measured in axial view and compared between the three groups. Using UR patients as reference, mean MyoSUVmax was lower in LC patients (3.3 ± 2.7 vs 6.2 ± 5.2, P = .03) but not in HFLC patients (5.5 ± 4.2, P = .63). Ratios of MyoSUVmax to liver SUVmax, calculated to control for background uptake, were not significantly different amongst the groups (1.9 ± 2.1 LC, 2.6 ± 2.3 HFLC, 3.6 ± 3.5 UR).
Conclusion
In this small randomized controlled trial using UR diet as reference, LC dietary preparation followed by extended fasting resulted in significant myocardial uptake suppression.
Keywords: Carbohydrates, diet, fatty acids, 18F-FDG, myocardium, PET
Introduction
A major obstacle in using 18F-FDG to image structures adjacent to the heart rests with the myocardium, which can significantly interfere with visualization by exhibiting high FDG uptake.1,2 Control of this unwanted uptake can potentially improve the use of 18F-FDG-PET for identifying thoracic diseases such as cardiac sarcoidosis and malignancies neighboring or infiltrating the cardiac chambers. In addition, reducing artifact from high myocardial FDG signal has become important as investigators have garnered increasing interest in noninvasive detection of the inflamed, rupture-prone3-6 sub-centimeter coronary artery plaque with 18F-FDG, based on the radionuclide’s prior success with large artery inflammation.7-10
Dietary modification appears to affect myocardial 18F-FDG metabolism. Lum et al showed that carbohydrate restriction prior to PET reduced artifact from myocardial 18F-FDG uptake.11,12 Recently, Williams and Kolodny published an observational, historical-control study in which patients who consumed a very high-fat, low-carbohydrate diet before FDG injection exhibited markedly reduced maximal myocardial 18F-FDG uptake.13 The same group has subsequently reported preliminary success in using this technique to facilitate 18F-FDG-PET imaging of coronary artery inflammation.14 Although encouraging, these findings were not from prospective, controlled studies. To systematically address the impact of diet on myocardial glucose metabolism, we conducted a prospective comparison of myocardial FDG uptake in patients randomized to unrestricted (UR), low-carbohydrate (LC), high-fat and low-carbohydrate (HFLC) dietary preparations.
Materials and Methods
Patient Population and Dietary Assignment
Using computerized randomization, we assigned 63 outpatients without exclusion criteria (acute inflammatory gallbladder or pancreatic disease, serum triglycerides >600 mg/dL, or solid food dysphagia) referred to Cedars-Sinai Medical Center for whole-body 18F-FDG-PET to one of 3 pre-scan dietary plans: UR, LC, or HFLC. Reason for referral was cancer staging in 58 patients and lung mass evaluation in 5. The study was approved by the Cedars-Sinai Medical Center institutional review board.
A study investigator gave detailed instructions by phone and in print to each patient. UR patients were instructed to eat a typical meal without restrictions in the evening prior to PET, then fast for at least 6 hours prior to 18F-FDG injection (patients could thus eat breakfast, as long as the meal was 6 hours or more beforehand). LC patients were instructed to eat a dinner containing <5 g of carbohydrates in the evening prior to PET, after which they should fast until 18F-FDG injection. HFLC patients were instructed to eat a dinner containing >35 g of fat and <5 g of carbohydrates in the evening prior to PET, then fast until 60-70 minutes prior to 18F-FDG administration, at which time they would drink a 250 mL liquid mixture of 45 mL of RESOURCE Benecalorie® (a low-residue, no-carbohydrate calorie supplement containing 33 g of fat, 91% of which is unsaturated; Novartis Medical, Basel, Switzerland), sucralose-based Crystal Light® flavoring (Kraft Foods, Northfield, IL), and water. Benecalorie was chosen because it was a fatty-acid supplement that did not require large-volume preparation, could be consumed quickly, and was easy to implement. All patients were instructed to avoid exercise and caffeine for at least 24 hours prior to and to record foods consumed within 18 hours before 18F-FDG injection. Written instructions for LC and HFLC patients also listed appropriate and inappropriate food items.
18F-FDG-PET Imaging Protocol
Dose of intravenous 18F-FDG was calculated based on body mass, using a reference of 370 MBq for 65 kg and not exceeding 555 MBq. Insulin was not used to control blood glucose prior to FDG injection. If the requested PET scan included diagnostic computed tomography (CT), thin oral barium contrast without any flavoring agent was given. After administration of 18F-FDG, patients rested in a quiet room for 60 minutes. CT and PET images were then acquired in a Gemini PET/CT scanner (Philips Healthcare, Bothell, Washington). While the patient breathed normally, CT imaging from the eyes to the mid thighs were performed with the 16-slice multi-detector scanner using the following parameters: 0.5 seconds per rotation, 100 mA tube current, 120 kVp tube voltage, 5 mm slice thickness, and 4.25 mm slice interval. PET images were acquired using 4 mm slice thickness and 5 minutes per bed position for the area of interest and 3 minutes per bed position for all other areas (9 total bed positions for a typical scan). Acquired images were iteratively reconstructed with CT-based attenuation correction.
PET Image Analysis
All reconstructed, attenuation-corrected PET images were sent to a Sun Microsystems workstation (Santa Clara, CA) and evaluated using the Philips Scintigraph software (Bothell, Washington). An experienced, blinded reader assessed each study to determine image quality and generate clinical interpretations. A separate analysis was done by a study investigator (V.C.) to specifically quantify myocardial 18F-FDG uptake. For scans in which myocardial uptake appeared uniform, maximal standard uptake value (SUVmax) was obtained by drawing a region of interest around the entire visible left ventricle in axial view, at the level of the lateral papillary muscle. If the papillary muscle was not visible, the reader visually selected the axial slice containing the largest cardiac footprint. If myocardial uptake appeared heterogeneous, the region of interest was drawn in the axial view containing the highest visual uptake. In addition, SUVmax were obtained from representative areas in the right lung apex and the liver dome that did not exhibit abnormal activity—these areas served as controls.
Statistical Methods
Based on work from Lum et al,11,12 we estimated a reduction of at least 3.0 in mean myocardial SUVmax with either LC or HFLC diet, compared to UR. Using a two-sided α of 0.05 and β of 0.20, 21 patients per group would provide 82% power to detect this estimated difference.
All categorical variables were described as frequencies and percents. Group comparisons of categorical variables were made using Pearson’s chi-squared test or Fisher Exact test, where appropriate. Continuous variables were described as mean ± standard deviation. Comparisons of continuous variables between any 2 groups were made with the Student’s t-test and amongst all 3 groups were made using analysis of variance (ANOVA), with Tukey and Dunnett post-hoc testing whenever there were statistically significant differences. For all analyses, differences with a P-value <.05 were considered significant. All statistical analyses were performed using Analyze-It software, version 2.10 (Leeds, UK).
Results
As shown in Table 1, randomization resulted in 3 similar groups. Neither of the patients with diabetes (one in UR and one in LC) used insulin. Frequencies of beta-blocker use, which may affect myocardial glucose metabolism, were similar. Food diary review indicated full adherence to diet assignment for all UR and LC patients. Two UR patients ate breakfast on the day of PET (fasting times were 360 and 390 minutes). The remaining 19 UR patients and all 21 LC patients fasted after dinner until 18F-FDG injection. All UR patients reported eating >5 g of carbohydrates and <35 g of fat in the meal prior to 18F-FDG injection. All LC and all HFLC patients reported restricting carbohydrate intake to <5 g in the dinner prior to 18F-FDG injection. For those assigned to HFLC diet, estimated fat consumption during dinner prior to PET was <35 g in 8 patients (38%), and all drank the Benecalorie-based mixture. Mean fasting time before 18F-FDG injection was significantly shorter for HFLC patients (61 minutes vs 745 minutes for UR and 899 minutes for LC, P < .001).
Table 1.
Demographics of study population (n = 63)*
| Unrestricted (UR) | Low-carbohydrate (LC) | High-fat and low-carbohydrate (HFLC) | P-value | |
|---|---|---|---|---|
| Age (years) | 56 ± 16 | 61 ± 15 | 62 ± 14 | NS |
| BMI (kg/m2) | 26 ± 5 | 24 ± 4 | 25 ± 5 | NS |
| Female | 13 (62) | 14 (67) | 14 (67) | NS |
| Diabetes | 1 (5) | 1 (5) | 2 (10) | NS |
| Dyslipidemia | 4 (19) | 5 (24) | 6 (29) | NS |
| Prior MI | 0 (0) | 0 (0) | 0 (0) | NS |
| Prior CABG | 0 (0) | 0 (0) | 0 (0) | NS |
| Prior PCI | 0 (0) | 0 (0) | 2 (10) | NS |
| Beta-blocker use | 4 (19) | 4 (19) | 5 (24) | NS |
| Cancer staging as indication for PET | 20 (95) | 20 (95) | 18 (86) | NS |
| Fasting time before 18F-FDG (min) | 745 ± 159 | 899 ± 149 | 61 ± 21 | <0.001† |
| 18F-FDG dose (MBq) | 466 ± 56 | 429 ± 70 | 426 ± 78 | NS |
BMI, Body mass index; MI, myocardial infarction; CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention; PET, positron-emission tomography; FDG, fluorodeoxyglucose; NS, not significant.
*Where appropriate, results are shown as mean ± standard deviation or number and percentage (in parenthesis).
†Significant for comparison amongst all 3 groups, for LC vs UR, and LC vs HFLC.
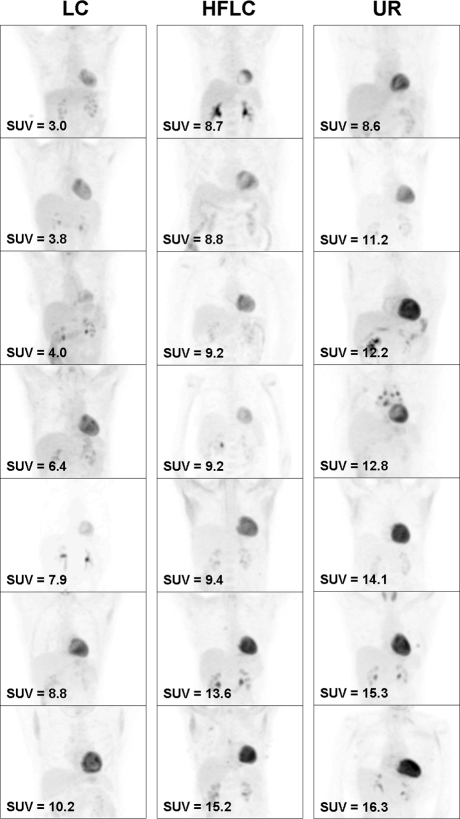
Ranges of myocardial SUVmax were 1.2 to 16.3 for UR, 1.0 to 10.2 for LC, and 1.4 to 15.2 for HFLC diets (Figure 1). Compared to UR patients, mean myocardial SUVmax was significantly reduced in LC patients (3.3 ± 2.7 vs 6.2 ± 5.2, 95% confidence interval 0.32 to 5.48, P = .03) but not in HFLC patients (5.5 ± 4.2, 95% confidence interval −2.25 to 3.65, P = .63). Seventeen (81%) LC, 12 (57%) HFLC, and 13 (62%) UR patients met the suggested criterion of successful suppression: myocardial SUVmax ≤ 5.0.14 When myocardial SUVmax was divided by liver lobe SUVmax to account for background uptake, LC patients had the lowest mean value (1.9 ± 2.1), but this was not statistically significant compared to other preparations. No significant difference in mean myocardial SUVmax was found between HFLC patients who consumed <35 or ≥35 g fat for dinner (6.0 ± 4.3 vs 5.2 ± 4.3, P = .66). Mean SUVmax of the right lung apex and liver lobe were similar for all groups. Comparisons of mean SUVmax measured from all regions of interest are detailed in Table 2. Figure 2 shows images from the 7 cases with highest myocardial SUVmax from each diet group.
Figure 1.
Distribution of maximal myocardial 18F-FDG uptake by dietary assignment (LC, Low-carbohydrate; HFLC, high-fat and low-carbohydrate; UR, unrestricted), as measured by standard uptake value (SUVmax). LC patients had significantly lower mean SUVmax than UR patients (P = .03)
Table 2.
Maximal standard uptake value (SUVmax) measures of 18F-FDG uptake by dietary assignment*
| Low-carbohydrate (LC) | High-fat and low-carbohydrate (HFLC) | Unrestricted (UR) | P-value | |
|---|---|---|---|---|
| Right lung apex | 0.6 ± 0.7 | 0.6 ± 0.2 | 0.6 ± 0.3 | NS |
| Liver lobe | 2.1 ± 0.7 | 2.3 ± 0.6 | 2.0 ± 0.5 | NS |
| Myocardium | 3.3 ± 2.7 | 5.5 ± 4.2 | 6.2 ± 5.2 | 0.03† |
| Number with SUVmax ≤ 5.0 | 17 (81%) | 12 (57%) | 13 (62%) | NS |
| Myocardial SUVmax/liver SUVmax | 1.9 ± 2.1 | 2.6 ± 2.3 | 3.6 ± 3.5 | NS |
| Number with ratio ≤1.0 | 13 (62%) | 8 (38%) | 9 (43%) | NS |
*Where appropriate, results are shown as mean ± standard deviation or number and percentage.
†Significant for LC vs UR.
Figure 2.
Representative images from 7 patients with the highest maximal myocardial standard uptake value (SUVmax) from each diet plan (LC, Low-carbohydrate; HFLC, high-fat and low-carbohydrate; UR, unrestricted). In all cases, the myocardium exhibits greater uptake than the liver and mediastinum. Only LC dietary preparation resulted in patients with SUVmax < 5.0 in this group (the top 3 examples in the leftmost column). The visual impression that not all myocardial uptake appeared to increase in correspondence to increasing SUVmax (e.g., the LC patient with SUVmax of 7.9) is because image contrast has been individually adjusted to show neighboring structures
Discussion
LC and HFLC dietary preparations aim to diminish myocardial 18F-FDG uptake by providing the myocardium increased access to fatty acids to reduce glucose-based metabolism.15-19 Our randomized trial found that, compared to UR diet, a simple LC diet followed by extended fasting effectively reduced myocardial 18F-FDG uptake, while the addition of fatty-acid loading did not achieve a significant reduction. These results suggest that LC may be preferable to HFLC dietary preparation for myocardial 18F-FDG suppression. Besides the lack of significant myocardial 18F-FDG signal suppression in this trial, fatty acid loading may be more difficult for patients to perform and may not be well-received by particularly diet-conscious patients.
Our findings differ from observations in two recent publications from the Beth Israel Deaconess Medical Center. These studies indicated improved myocardial 18F-FDG uptake suppression with a HFLC diet when compared to standard “fasting” preparations.13,14 A large portion of the difference can be attributed to the mean myocardial SUVmax of 3.3 in our LC patients, which was markedly lower than the 8.8 reported by Williams and Kolodny, who studied a similar population under a similar fasting protocol.13 In addition, myocardial 18F-FDG uptake in our HFLC patients (mean SUVmax of 5.5) was higher than the 3.9 reported by these authors.13 While presence of type II error was possible (our study design had a power of 80%), significant variability in the control of myocardial 18F-FDG uptake with fatty-acid loading remains the likely explanation. In the follow-up study from the Beth Israel Deaconess investigators to prospectively apply their fatty-acid loading protocol for coronary arterial imaging (Wykrzykowska et al), the outcome was not as impressive as initially reported14: mean myocardial SUVmax was 7.7 and 15 of 31 (48%) patients had recorded myocardial SUVmax > 5.0. Myocardial glucose metabolic activity in these patients was likely elevated in part due to high coronary artery disease burden—26 of 32 patients had abnormal results on invasive coronary angiography and 6 had prior myocardial infarctions.
There are at least two potential explanations for the myocardial activity suppression we observed in LC patients. First, in addition to carbohydrate restriction, prolonged fasting may have helped our patients maximize free fatty acid generation from triglyceride cleavage.16,20 Mean duration of fasting in our LC patients was 899 minutes; all except 1 fasted for at least 720 minutes. In the protocol described by Williams and Kolodny, some patients received instructions to fast for at least 240 minutes prior to 18F-FDG administration,13 and this may have been insufficient to induce optimal circulating fatty acid levels. Second, patients in our trial received more thorough dietary counseling than in real-life settings, contributing to very high rates of full diet assignment adherence and likely helping to optimize results in our LC patients.
Several potential reasons for why our HFLC preparation did not result in significant myocardial 18F-FDG uptake suppression should be mentioned. The time we allotted between fatty drink consumption and 18F-FDG injection (mean of 61 minutes) may have been too brief to capture peak circulating triglyceride levels.21 The sucralose-based sweetener we used to flavor the fatty drink could have adversely affected myocardial fatty acid metabolism. In addition, we could not verify whether fatty acid consumption based on our HFLC protocol met or exceeded the quantity necessary for optimal loading. Overloading the myocardium with fatty acids may adversely increase myocardial oxygen consumption, potentially prompting an unintended secondary increase in glucose metabolism, as shown in multiple prior experiments.22-25
Predictable control of myocardial 18F-FDG uptake is important when using PET to detect lesions close to the heart; these include noncardiac thoracic pathology and diseases of specific cardiac structures. In particular, prominent myocardial FDG signal can greatly hinder investigational techniques to visualize coronary vasculature and coronary arterial plaque inflammation. For this application, adequate suppression depends on both the myocardium and multiple characteristics of the target coronary artery, such as its location, motion, distance to the myocardium, and intensity of 18F-FDG uptake. Although a SUVmax threshold of >5.0 has been suggested to indicate insufficient suppression,14 true criteria for adequate suppression likely vary by individual and have yet to be systematically defined. In the absence of this definition, the amount of myocardial signal variability we observed (e.g., 4 LC patients (19%) and 9 HFLC patients (43%) had myocardial SUVmax > 5.0) denotes the need to improve the precision of any diet-based approach for controlling unwanted myocardial 18F-FDG uptake.
Our study has several limitations. The entire study population underwent 18F-FDG-PET for suspected or confirmed oncologic disease, not coronary or cardiac disease. Hence, these patients may pose some difference in myocardial glucose metabolism compared to patients with coronary artery disease, particularly those with active myocardial ischemia. To date, FDG-based imaging for coronary artery disease is experimental and not used clinically; we chose not to enroll patients referred for 18F-FDG-based viability assessment for the concern that the clinically important test accuracy characteristics may be altered by changes in dietary preparation. We did not collect lipid profile and glucose laboratory data to confirm patient history. We did not ascertain serum triglyceride changes to verify peak effect of the high-fat drink. Adherence to dietary instructions was based on self-report. Our technique of measuring myocardial SUVmax was biased towards reporting the “worst-case” scenario and was more prone to variability and noise than whole-heart sampling. We chose this design to be conservative about quantifying myocardial uptake suppression. Additional interventions that may impact myocardial glucose metabolism, such as peri-imaging beta-adrenergic receptor blockade, were not evaluated.
Conclusion
In this randomized controlled trial, a low-carbohydrate diet with extended fasting resulted in suppression of myocardial 18F-FDG uptake during PET. Low-carbohydrate diet followed by extended fasting prior to 18F-FDG injection should be considered a useful protocol for the purpose of developing 18F-FDG-PET to investigate coronary arterial inflammation.
Acknowledgment
The authors thank the Society of Nuclear Medicine and Covidien for funding this work.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Funding: This work was supported by the Society of Nuclear Medicine Covidien Seed Grant, through the Education and Research Foundation for the Society of Nuclear Medicine.
References
- 1.Engel H, Steinhart H, Buck A, Berthold T, Huch Boni RA, von Schulthess GK. Whole body PET: Physiological and artifactual fluorodeoxyglucose accumulations. J Nucl Med. 1996;37:441–446. [PubMed] [Google Scholar]
- 2.Shreve P, Anzai Y, Wahl RL. Pitfalls in oncologic diagnosis with FDG PET imaging: Physiologic and benign variants. Radiographics. 1999;19:61–77. doi: 10.1148/radiographics.19.1.g99ja0761. [DOI] [PubMed] [Google Scholar]
- 3.Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986;6:131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- 4.van der Wal AC, Das PK, Bentz van de Berg D, van der Loos CM, Becker AE. Atherosclerotic lesions in humans: In situ immunophenotypic analysis suggesting an immune mediated response. Lab Invest. 1989;61:166–170. [PubMed] [Google Scholar]
- 5.Ogawa M, Ishino S, Mukai T, Asano D, Teramoto N, Watabe H, et al. (18)F-FDG accumulation in atherosclerotic plaques: Immunohistochemical and PET imaging study. J Nucl Med. 2004;45:1245–1250. [PubMed] [Google Scholar]
- 6.Davies JR, Rudd JH, Weissberg PL, Narula J. Radionuclide imaging for the detection of inflammation in vulnerable plaques. J Am Coll Cardiol. 2006;47:C57–C68. doi: 10.1016/j.jacc.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 7.Yun M, Yeh D, Araujo LI, Jang S, Newberg A, Alavi A. F-18 FDG uptake in the large arteries: A new observation. Clin Nucl Med. 2001;26:314–319. doi: 10.1097/00003072-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Rudd JH, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105:2708–2711. doi: 10.1161/01.CIR.0000020548.60110.76. [DOI] [PubMed] [Google Scholar]
- 9.Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48:1818–1824. doi: 10.1016/j.jacc.2006.05.076. [DOI] [PubMed] [Google Scholar]
- 10.Rudd JH, Myers KS, Bansilal S, Machac J, Rafique A, Farkouh M, et al. 18Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: Implications for atherosclerosis therapy trials. J Am Coll Cardiol. 2007;50:892–896. doi: 10.1016/j.jacc.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 11.Lum DP, Wandell S, Ko J, Coe MN. Reduction of myocardial fluorodeoxyglucose uptake artifacts with a carbohydrate restricted diet. Clin Positron Imaging. 2000;3:155. doi: 10.1016/S1095-0397(00)00067-4. [DOI] [PubMed] [Google Scholar]
- 12.Lum DP, Wandell S, Ko J, Coel MN. Reduction of myocardial 2-deoxy-2-[18F]fluoro-D-glucose uptake artifacts in positron emission tomography using dietary carbohydrate restriction. Mol Imaging Biol. 2002;4:232–237. doi: 10.1016/S1095-0397(01)00062-0. [DOI] [PubMed] [Google Scholar]
- 13.Williams G, Kolodny GM. Suppression of myocardial 18F-FDG uptake by preparing patients with a high-fat, low-carbohydrate diet. Am J Roentgenol. 2008;190:W151–W156. doi: 10.2214/AJR.07.2409. [DOI] [PubMed] [Google Scholar]
- 14.Wykrzykowska J, Lehman S, Williams G, Parker JA, Palmer MR, Varkey S, et al. Imaging of inflamed and vulnerable plaque in coronary arteries with 18F-FDG PET/CT in patients with suppression of myocardial uptake using a low-carbohydrate, high-fat preparation. J Nucl Med. 2009;50:563–568. doi: 10.2967/jnumed.108.055616. [DOI] [PubMed] [Google Scholar]
- 15.Bing RJ, Siegel A, Ungar I, Gilbert M. Metabolism of the human heart II. Studies on fat, ketone and amino acid metabolism. Am J Med. 1954;16:504–515. doi: 10.1016/0002-9343(54)90365-4. [DOI] [PubMed] [Google Scholar]
- 16.Opie LH, Evans JR, Shipp JC. Effect of fasting on glucose and palmitate metabolism of perfused rat heart. Am J Physiol. 1963;205:1203–1208. doi: 10.1152/ajplegacy.1963.205.6.1203. [DOI] [PubMed] [Google Scholar]
- 17.Neely JR, Morgan HE. Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Annu Rev Physiol. 1974;36:413–459. doi: 10.1146/annurev.ph.36.030174.002213. [DOI] [PubMed] [Google Scholar]
- 18.Wisneski JA, Gert EW, Neese RA, Mayr M. Myocardial metabolism of free fatty acids. Studies with 14C-labeled substrates in humans. J Clin Invest. 1987;79:359–366. doi: 10.1172/JCI112820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frayn KN. The glucose-fatty acid cycle: A physiological perspective. Biochem Soc Trans. 2003;31:1115–1119. doi: 10.1042/BST0311115. [DOI] [PubMed] [Google Scholar]
- 20.Yamanouchi M, Yoshia K, Niwayama H, Nakagawa K, Aioi S, Shikama N, et al. Effect of the duration of fasting on myocardial fluorine-18-fluorodeoxyglucose positron emission tomography images in normal males. Jpn Circ J. 1996;60:319–327. doi: 10.1253/jcj.60.319. [DOI] [PubMed] [Google Scholar]
- 21.Thomas TR, Fischer BA, Kist WB, Horner KE, Cox RH. Effects of exercise and n-3 fatty acids on postprandial lipemia. J Appl Physiol. 2000;88:2199–2204. doi: 10.1152/jappl.2000.88.6.2199. [DOI] [PubMed] [Google Scholar]
- 22.Mjos OD. Effect of free fatty acids on myocardial function and oxygen consumption in intact dogs. J Clin Invest. 1971;50:1386–1389. doi: 10.1172/JCI106621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kjekshus JK, Mjos OD. Effect of free fatty acids on myocardial function and metabolism in the ischemic dog heart. J Clin Invest. 1972;51:1767–1776. doi: 10.1172/JCI106978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mjos OD, Kjekshus JK, Lekvan J. Importance of free fatty acids as a determinant of myocardial oxygen consumption and myocardial ischemic injury during norepinephrine infusion in dogs. J Clin Invest. 1974;53:1290–1299. doi: 10.1172/JCI107676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vik-Mo H, Mjos OD. Influence of free fatty acids on myocardial oxygen consumption and ischemic injury. Am J Cardiol. 1981;48:361–365. doi: 10.1016/0002-9149(81)90621-4. [DOI] [PubMed] [Google Scholar]