Abstract
The hierarchical competing systems model (HCSM) provides a framework for understanding the emergence and early development of executive function – the cognitive processes underlying the conscious control of behavior – in the context of search for hidden objects. According to this model, behavior is determined by the joint influence of a developmentally invariant habit system and a conscious representational system that becomes increasingly influential as children develop. This article describes a computational formalization of the HCSM, reviews behavioral and computational research consistent with the model, and suggests directions for future research on the development of executive function.
Introduction
The term executive function refers to the cognitive processes involved in goal-directed problem solving – processes such as working memory, inhibitory control, and error correction. Research on executive function indicates that it emerges in infancy and continues to develop well into adolescence (see Diamond, 2002, and Zelazo, Carlson & Kesek, 2008, for reviews). Much of the recent research on the development of executive function, however, has focused on the marked changes occurring between about 3 and 5 years of age (e.g. Carlson, 2005; Kirkham, Cruess & Diamond, 2003; Espy, 1997; Munakata & Yerys, 2001; Zelazo, Müller, Frye & Marcovitch, 2003) – changes that are correlated with key aspects of self- and social-understanding (i.e. theory of mind) in both typically and atypically developing children (Perner & Lang, 1999). These changes have been observed, for example, on the Dimensional Change Card Sort (Zelazo et al., 2003), the ‘Less is More’ task (Carlson, Davis & Leach, 2005), and the Day-Night Stroop Task (Gerstadt, Hong & Diamond, 1994); on all of these tasks, 3-year-olds perform poorly, whereas 5-year-olds perform well. Experimental investigations using these tasks have supported the formulation of detailed theories of the mechanisms underlying executive function and its development in this age range, and these theories have informed our understanding of executive function in general.
In contrast to the burgeoning literature on executive function in preschool age children, however, relatively little is known about the origins of executive function in infancy and the way in which executive function develops during the first few years of life. Research on the emergence of executive function in infancy has the potential to reveal the cognitive architecture that provides the foundation for executive function and its development in later years. This article summarizes research on the early development of executive function – measured at the end of the first year of life in the context of search for hidden objects – and then presents a formal theory of the processes underlying executive function: the Hierarchical Competing Systems Model (HCSM). The HCSM characterizes the starting-state of executive function development, and highlights the inter-relations among associative learning, conscious reflection, and language acquisition. Although these domains have been studied independently, there have been few attempts to provide an integrated account that focuses on their combined influence and explains how they may develop to allow for the conscious cognitive control of behavior.
Search for hidden objects
Piaget (1954) observed that infants who display secondary circular reactions (i.e. substage III, 4 to 8 months of age) search manually for hidden objects. Their search ability is constrained, however, to situations where the object is visible when the reach begins. For example, Piaget reported that infants will search if the object is only partially hidden, and Bower and Wishart (1972) found that infants who were unable to locate a toy under a cup were indeed able to reach for an attractive object after the lights were turned out (see also Clifton, Muir, Ashmead & Clarkson, 1993; Hood & Willats, 1986). Typically, these tasks involve one hiding location, as the phenomenon of interest is whether infants will reach for the attractive object.
A classic paradigm for research on executive function in infants and toddlers is the search task with multiple locations (see, e.g. Diamond, 1985; Marcovitch & Zelazo, 1999). In a typical search task, children watch as an object is conspicuously hidden at one of several locations, a delay is imposed, and then children must search for the object. Although the task is relatively simple (allowing it to be used when infants are old enough to reach; i.e. older than about 6 months of age), it contains all the elements of a typical measure of executive function with children. To solve the task, children must represent the object's current location, keep this information in mind, and then use it to guide their search. If they err, they must detect their errors and correct them. These elements also correspond to the latent variables associated with executive functions in adults reported by Miyake, Friedman, Emerson, Witzki, Howerter and Wager (2000): inhibiting, shifting, and updating. Children must inhibit their learned response to search at Location A, children must shift from the task of searching at A to searching at B, and update their working memory of the hiding event from Location A to Location B.
The use of search tasks to study cognition in young children has a long history, and was pioneered by Walter Hunter. In a seminal article, Hunter (1917) described his experiments on the ‘delayed reaction’ in his daughter, Thayer, when she was between 13 and 16 months old (prior to vocal language). In these tasks, an attractive object was conspicuously hidden in one of three boxes. After a delay during which Thayer was distracted, she was oriented towards the middle box and then allowed to search for the object. Hunter found that by 16 months of age, Thayer was typically successful when the delay was less than 24 seconds, but increasingly error prone as the delays increased. Integrating these findings with his earlier work with non-human animals (Hunter, 1913), Hunter revealed that Thayer's performance was superior to ‘Rat No. 9’ on a similar task, but inferior to ‘Bob’ the raccoon and ‘Blackie’ the dog. Hunter concluded that vocal language was not necessary for success, and he speculated that the acquisition of vocal language would render the task trivial, even over long delays.
The delayed reaction task (also called ‘delayed response’ task) has been used extensively as an assessment of frontal lobe function in non-human primates, and lesion studies indicate clearly that successful performance on the task depends on the integrity of prefrontal cortex – especially dorsolateral prefrontal cortex (see Fuster, 1980; Goldman-Rakic, 1987; Jacobsen, 1936). For this reason, it has also been used to examine prefrontally mediated memory (especially simple working memory) in infancy, childhood, and across the lifespan. For example, Diamond and Doar (1989) administered a delayed response task longitudinally from 6 to 12 months of age. The infants received 16 trials in which they searched for an object that was hidden in one of two identical hiding wells. The results confirmed that infants can tolerate longer delays as they get older (roughly an additional 2.1 seconds per month), which is consistent with the idea that performance on the delayed response task in infancy reflects the growth of prefrontal cortex and the development of working memory.
More direct evidence of the role of prefrontally mediated working memory in infant search comes from work by Baird, Kagan, Gaudette, Walz, Hershlag and Boas (2002), who used near infrared spectroscopy (NIRS) to compare changes in cerebral blood flow between infants (5 to 12 months) who reliably searched for hidden objects and infants who did not. Infants who searched showed an increase in blood flow (total haemoglobin) in prefrontal cortex, whereas those who did not search showed a decrease from baseline.
Studies using the delayed response task have generally confirmed that it is a useful measure for both short- and long-term memory not only in infancy, but also in early childhood and indeed across the lifespan. Schutte and Spencer (2002, Experiments 1 and 2), for example, employed a variant of the delayed response task in a continuous search space with 3-year-olds. In this task, children searched for a spaceship by moving a handheld rocket to where a spaceship had previously appeared. One advantage of using a continuous search space (i.e. no distinct hiding locations) is that it provides a more sensitive measure of the magnitude of errors: the distance between the actual location and the children's responses. In Schutte and Spencer's work, children were biased to search towards the center of the targets, and this bias increased with the length of the delay. The authors concluded that the bias arises from both the blending in long-term memory of all target locations and children's natural tendency to search towards the middle of a search space.
Lyons-Warren, Lillie and Hershey (2004) also employed a delayed response task in a continuous search space to assess both short- and long-term memory from late childhood across the lifespan (7–80 years). While engaged in an object recognition task, participants used a mouse to move a cursor to the location where they had previously seen a stimulus appear. On trials with short delays (thought to reflect short-term memory), performance improved with age throughout childhood and adolescence, but deteriorated with age throughout adulthood. In contrast, on trials with long delays (thought to reflect long-term memory), performance improved steadily with age through adolescence, but changed very little across the remainder of the lifespan.
Although many varieties of delayed response task have been employed in work with infants and children, by far the most common is a specific version pioneered by Piaget (1954): the A-not-B paradigm. In a typical A-not-B task, children observe an object hidden at one of two or more locations (Location A), and search for the object after a delay. After successfully retrieving the object a number of times, children then observe the object conspicuously hidden at a second location (Location B). The A-not-B error arises when participants search incorrectly (and perseveratively) at Location A instead of Location B. Perseverative search on the B trial (i.e. the A-not-B error) may arise due to a failure to represent the object's current location or to difficulties using a correct representation of the object's location to constrain their search.
The vast majority of research using the A-not-B task has been conducted with infants between 8 and 12 months of age, and it is well established that A-not-B errors are common between 8 and 10 months but that older infants often search successfully on the B trials. Marcovitch and Zelazo (1999) conducted a meta-analysis of research on this task that revealed the following: (a) performance improved with age (e.g. Sophian & Wellman, 1983), (b) errors were more likely to occur after longer delays (e.g. Gratch, Appel, Evans, LeCompte & Wright, 1974), (c) errors decreased as the distance between the hiding locations increased (e.g. Horobin & Acredolo, 1986), (d) increasing the number of hiding locations decreased perseverative behavior, but increased the likelihood of searching between the A and B locations (e.g. Diamond, Cruttenden & Neiderman, 1994), and (e) errors increased proportionally to the number of A trials.
The conclusion that the number of A trials affected performance was particularly interesting for a number of reasons. First, a previous meta-analysis of the A-not-B error (Wellman, Cross & Bartsch, 1986) had failed to reveal an effect of A trial experience. Second, only one study (Landers, 1971) had ever revealed a significant relation between the number of A trials and the A-not-B error, while several studies did not report a significant relation despite trends in the appropriate direction (Butterworth, 1977; Evans, 1973; Sophian & Wellman, 1983). Third, and finally, despite the uncertainty concerning the effect of A trial experience, and the assumption by some authors that no effect existed (e.g. Harris, 1989; Hofstadter & Reznick, 1996), several theoretical accounts predicted such an effect (e.g. Diamond et al., 1994; Munakata, 1997; Thelen & Smith, 1994). As will be discussed in a later section, the role of the number of A trials has since been tested directly (Marcovitch & Zelazo, 2006; Marcovitch, Zelazo & Schmuckler, 2002; Smith, Thelen, Titzer & McLin, 1999).
Piaget (1954) originally attributed the A-not-B error to an incomplete understanding of object permanence. On this view, infants egocentrically assume that the reappearance of the hidden object depends on their reaches, and so they continue to reach to Location A in an effort to reproduce the object. Although some authors have continued to attribute the error at least in part to an incomplete understanding of object permanence (e.g. Harris, 1989), Piaget's interpretation has fallen out of favor with most contemporary researchers for at least two reasons:
Studies that used looking time, as opposed to searching accuracy, as a dependent measure have revealed that children exhibit a sensitivity to object permanence as early as 3.5 months of age (Baillargeon & DeVos, 1991). Indeed, Ahmed and Ruffman (1998, Experiment 1) provided a striking example of 8- to 12-month-old infants who committed the A-not-B error when they searched manually, but were surprised (indicated by longer looking times) when the experimenter retrieved the object from the seemingly impossible Location A. From this point of view, the A-not-B error does not reflect a deficit in object permanence understanding, but rather an inability to control motor behavior effectively. This is consistent with an executive function explanation of the A-not-B task, according to which infants have difficulty using their knowledge to guide their search (e.g. because of difficulty inhibiting a previously correct response).
The A-not-B error can be elicited in older children who clearly do not have difficulty with the object permanence concept (Marcovitch & Zelazo, 2006; Sophian & Wellman, 1983; Spencer, Smith & Thelen, 2001; Zelazo, Reznick & Spinazzola, 1998). In these studies, the standard task is usually modified. For example, Sophian and Wellman (1983) elicited perseverative search in 2-year-old children by surreptitiously hiding the object on B trials while stating verbally the correct location (e.g. ‘The soap will be in the bird's box’, p. 382). Presumably, observing the conspicuous hiding of the object is necessary for 2-year-olds to use the current information to override the influence of previous experience on the A trials. In another variant of the A-not-B task, Zelazo et al. (1998) elicited perseveration when the crucial choice of where to search was embedded within a sequence of steps. To retrieve a hidden candy, children were required to execute a four-step retrieval process: (a) remove a foam barrier, (b) pull a tray, (c) choose a stimulus, and (d) pull the stimulus to reveal the candy. Perseveration in this version of the task may reflect the fact that children have particular difficulty analyzing practiced routines into separate steps and modifying an intermediate step (e.g. Karmiloff-Smith, 1992). There is some indication, however, that children beyond 12 months of age may continue to perseverate even in the standard version of the A-not-B task, at least under some circumstances. Espy, Kaufmann, McDiarmid and Glisky (1999) found that with a 10-second delay between hiding and finding, even preschoolers made perseverative errors, although the number of perseverative errors decreased between the ages of 23 and 66 months. Overall, then, it is now clear that the A-not-B error is not limited to infancy, but rather that it can continue to be elicited later in childhood. This finding suggests that perseverative influences on search persist beyond the developmental period during which it is plausible to invoke conceptual explanations of the A-not-B phenomenon.
Rather than postulate conceptual difficulties, most contemporary explanations of the A-not-B error have focused on cognitive mechanisms such as memory, inhibitory control, and some combination of the two. First, some authors have suggested that infants have difficulty keeping the object's new location in mind – essentially a problem of simple working memory (e.g. Cummings & Bjork, 1983; Fox, Kagan & Weiskopf, 1979; Harris, 1973; Munakata, McClelland, Johnson & Siegler, 1997; Schacter, Moscovitch, Tulving, McLachlan & Freedman, 1986). According to one version of this account (Cummings & Bjork, 1983), infants encode the general vicinity of the hiding location but lack the sophistication to encode the specific location; hence, they are likely to confuse the A and B locations. Support for the role of memory difficulties in bringing about the A-not-B error comes from research demonstrating that the likelihood of perseveration is affected by the duration of the delay between hiding and finding (Diamond, 1985; Harris, 1973).
Second, an alternative to these memory accounts has been to hypothesize that infants have difficulty inhibiting a prepotent tendency to reach to Location A despite keeping the object's correct location in mind (e.g. Baillargeon, Graber, DeVos & Black, 1990; Bjorklund & Harnishfeger, 1990; Dempster, 1992). This hypothesis is consistent with research revealing that infants sometimes perseverate even when the object is visible at Location B (and hence no memory in required; Butterworth, 1977; Harris, 1974) or even while looking at the correct location (Diamond, 1985; Piaget, 1954).
Finally, Diamond (1985; Diamond et al., 1994) has proposed that correct responding on an A-not-B task requires both the memory of the correct location and inhibition of the previously correct response (i.e. memory + inhibition hypothesis; Diamond et al., 1994). Diamond et al. provided compelling evidence for this hypothesis using a 7-location A-not-B task. According to the logic of the memory account put forth by Cummings and Bjork (1983), on B trials, infants should search in the vicinity of Location B but not necessarily precisely at Location B. Thus, if A-not-B errors on a standard 2-location task resulted only from failures in memory, then errors in the 7-location task should cluster around Location B. Similarly, if A-not-B errors resulted only from failures in inhibition, then errors in the 7-location task should cluster around Location A. However, if failures in both memory and inhibition account for A-not-B errors, then infants should search between Locations A and B (i.e. errors should tend to be clustered between these locations). Indeed, infants' search was best characterized by the memory + inhibition account (but see Marcovitch & Zelazo, 1999, for a computational account that does not require inhibition). Although this approach has been influential and instrumental in stimulating research on the topic, the desire for increased theoretical precision regarding the mechanisms underlying search has motivated some authors to develop computational models of A-not-B performance, and it is to these models that we now turn.
Computational theories of A-not-B search
In recent years, there have been several attempts to provide formal models of the processes underlying A-not-B search. The value of computational modeling for understanding cognitive development is now well established (e.g. Elman, 1990; Marcovitch & Zelazo, 2000; Morton & Munakata, 2002; Shultz, 2003; Simon & Halford, 1995; Thelen, Schöner, Scheier & Smith, 2001). Among other things, computational modeling requires identifying and formalizing the mechanisms of developmental change, and simulations based on computational models often generate novel hypotheses – including hypotheses that are unlikely to have been obvious in the absence of a formal model. As such, computational models may be used to guide a program of experimental behavioral research.
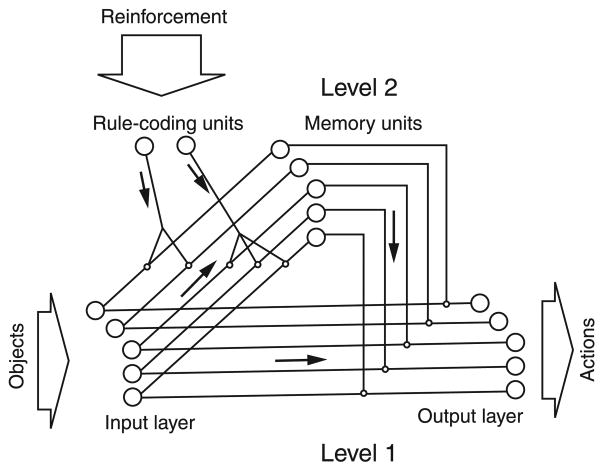
One of the first computational models of the A-not-B search was the neural network model of prefrontal cortex put forth by Dehaene and Changeux (1989). This model was designed to account for performance on delayed response tasks, including the A-not-B task. Input units encode features of an object and its hiding location while output units orient the organism towards one of the hiding locations. It is interesting to note that orientation towards a particular location can be based on a feature of the object (e.g. orient towards the blue one) or a feature of the hiding location (e.g. orient towards the one on the left). Behavior is positively reinforced (i.e. strengthening the connectivity between units) when orientation is towards the correct object and punished (i.e. weakening the connectivity between units) when orientation is towards the wrong object.
In Level 1 of the model, input units are connected directly to output units (see Figure 1). Simulations with Level 1 produce correct orientation on A trials. On B trials, however, the model initially orients towards Location A (i.e. commits the A-not-B error), followed by a period of random responding, and then eventually begins to orient consistently towards Location B. After a period of correct B trials, the Level 1 model perseverates again when the object is switched back to Location A. In other words, the Level 1 model can never succeed at both locations at the same time. Thus, the Level 1 model adequately simulates the behavior of 7.5- to 9-month-old infants who search successfully at the first location and then perseverate to the recently correct location whenever the object is moved (e.g. Diamond & Doar, 1989; Hofstadter & Reznick, 1996).
Figure 1.
Dehaene and Changeux's (1989) neural network used to simulate search errors in infants. See text for details. (Figure reproduced from Dehaene, S., & Changeux, J.-P. (1989). A simple model of prefrontal cortex function in delayed response tasks. Journal of Cognitive Neuroscience, 1, 244–261.)
Level 2 of the model includes two additional layers of units organized hierarchically (see Figure 1). The first layer of memory units stores information about the object features, but only if the appropriate rule coding units in the second layer are active. Appropriate rules are reinforced across trials. For example, in an A-not-B task, the location of the object is more important than the color of the object. Across trials, the ‘location rule’ will be activated and input from the location input units becomes associated with the memory units. This additional input allows active memory units to maintain their activation across a delay, and hence to influence the orientation of the network. Simulations using both Levels 1 and 2 together demonstrate performance equivalent to 12-month-old infants on the A-not-B task (i.e. correct performance regardless of whether the location was switched from the previous trial). Based on these simulations, Dehaene and Changeux (1989) emphasize the role that prefrontal cortex plays in allowing infants to override Level 1 tendencies to search at Location A.
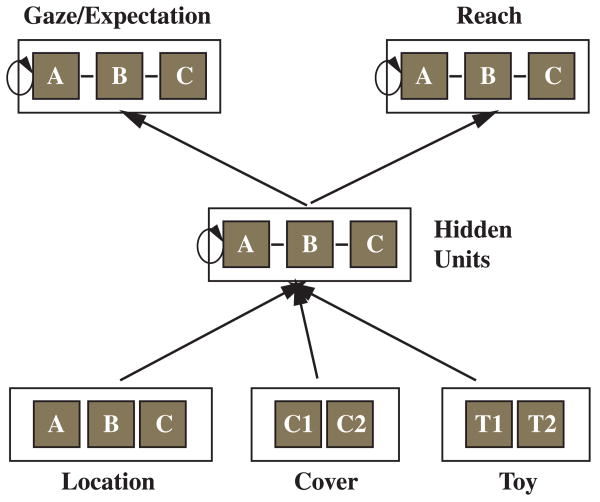
Munakata (1998) proposed a different model of the role of prefrontal cortex in search. She suggested that the development of prefrontal cortex affects active, but not latent, memory traces. Latent memory traces evolve from processing a stimulus so that the organism may react differently to the stimulus in the future. In contrast, active memory traces involve the active maintenance of a representation that remains accessible over a delay during which the stimulus is absent. Munakata (1998) implemented a parallel distributed processing (PDP) model to demonstrate how the A-not-B error can be attributed to weak active traces for Location B failing to override latent traces for Location A.
According to Munakata (1998), latent traces in PDP networks are instantiated as changes to the connection weights between units whereas active traces arise from the sustained activation of units. Her model consists of input units that independently code for the hiding location, the type of cover, and the type of object; hidden units that embody a representation of the hiding locations; and output units that reveal the location at which the network will gaze and the location at which the network will reach (see Figure 2). Connections to the reaching units are updated only once every trial during the A-not-B task (at the point where reaching would occur), but connections to the gaze units are updated throughout the trial. Finally, the hidden and output units have recurrent connections back to each unit, and these allow for sustained activation. Munakata manipulated the weights of the recurrent connections to simulate networks with different representational capabilities (related to the ‘age’ of the network and the growth of active memory).
Figure 2.
Munakata's (1998) neural network used to simulate search errors in infants. See text for details. (Figure reproduced from Munakata, Y. (1998). Infant perseveration and implications for object permanence theories: a PDP model of the AB task. Developmental Science, 2, 161–184.)
Besides simulating the A-not-B error in a canonical task, Munakata's (1998) model impressively accounts for a wide range of empirical findings, such as eliciting the error when A experience is observational (e.g. when infants simply watch an object being hidden during the A trials but do not have an opportunity to retrieve the object at Location A; Smith et al., 1999). The model also accounts for improved performance: (1) in looking procedures, (2) with age, (3) across shorter delays, (4) with multiple locations (more than 2), (5) with distinctive covers, and (6) when the object is hidden only on the B trials. However, one limitation of Munakata's model is in explaining perseverative behavior throughout the lifespan within the context of a single model. As simulations of older children (and a fortiori adults) must begin with stronger recurrent weights than the simulations of successful infants, it is difficult to conceive of how this framework could account for A-not-B type errors later in life. In later work, Morton and Munakata (2002) presented a different neural network model of perseveration of preschool children, but the nature of the preschoolers' task was different enough that it is difficult to compare and integrate the two models (although they are both based on the same fundamental principles of active versus latent traces of memory).
Another contemporary computational theory that addresses the A-not-B error is Dynamic Systems Theory (DST; Smith et al., 1999; Thelen & Smith, 1994; Thelen et al., 2001), which postulates that search behavior can be predicted by the internal dynamic relations between a host of psychological systems, including the perception of the experimental context, the encoding of the hiding event, and the memory resulting from previous searches. According to DST, search behavior is influenced by the coupling of looking and reaching. On the first A trial, infants' attention is recruited by the hiding event, and this increases the probability that the infant will be looking at, and therefore reaching to, Location A. However, this bias is ephemeral, and may not be strong enough to initiate reaching behavior, which explains why many infants do not search on the first A trial (see Smith et al., 1999, for details). On subsequent A trials, the probability of searching at Location A increases as the magnitude of the bias increases. Furthermore, once reaching to Location A has occurred, the memory of these reaches at Location A reinforces the looking/reaching systems' proclivity towards Location A. The A-not-B error results from the combined influence of two factors: (a) a strong bias to search towards Location A that was established on the A trials, and (b) the likelihood that infants' attention towards Location B at the time of hiding will shift away during the delay period. If the locations are visually similar to one another, infants may shift their attention to the wrong location, which in turn will elicit inappropriate searching behavior. Eight- to 12-month-old infants are poorly skilled at reaching (which is coupled to looking), so they are easily attracted to locations that look the same as the target location. In other words, for these infants, the visually similar target is insufficient to override the experience at Location A. Indeed, when Location B is visually distinct from Location A, the probability of making the A-not-B error is reduced, although it is not eliminated (A. Bremner & Bryant, 2001; Smith et al., 1999).
Thelen et al. (2001) provide a comprehensive description of the computational details behind the DST. In this account, reaching behavior reflects the state of activation of a motor planning field. On any given trial, the activation of this field is a function of the dynamic looking pattern on that trial and the specific events of looking and reaching that occurred on previous trials. More specifically, the model includes a function S(x, t) for the motor planning field that relies on three sources of input: (a) task input, (b) specific input, and (c) memory input. The task input is constant and captures the activation caused by the persistent perceptual elements of the task (e.g. the potential hiding locations). In contrast, the specific input is short lived, varies from trial to trial, and captures the activation caused by the cueing event (e.g. hiding an object, waving a lid). The final input, memory input, has a recursive relation to the motor planning field. Specifically, memory is derived directly from the cumulative activation of all previous trials (and, in a broader sense, the entire history of the organism, although this is not an explicit input in the Thelen et al. model). With appropriate parameterization, the model was very effective in simulating the canonical A-not-B error, including the effects of age, delay, and the role of distinctive covers. Recently, the theory has been extended to account for a variety of other phenomena, including the increased tendency to search away from Location A and towards Location B (i.e. spatial drift) across delays (Schutte & Spencer, 2002) and the increased precision of spatial working memory across development (Spencer & Schutte, 2004; see also Schutte, Spencer & Schöner, 2003; Spencer et al., 2001).
To summarize, there are now several computational models of A-not-B search, and these models have been able to account for an impressive number of empirical findings. Besides generating novel empirical predictions that can be tested in children, the computational nature of these models has allowed precise quantification of the psychological processes involved, and it has increased our understanding of how these processes may change over time. This kind of formalization is exactly what is necessary for a unified developmental theory. From our perspective, one limitation of most of these models is that it remains unclear how they may account for further improvements in executive function beyond infancy – including those occurring during childhood and into adolescence (e.g. Diamond, 2002; Zelazo & Müller, 2002). One notable exception is DST, which relies on the relative strengths of competing responses to determine behavior. In that spirit, but in an effort to address the development of consciousness, the hierarchical competing systems model (HCSM), described below, is intended to serve as a foundation for a more comprehensive model of the development of executive function.
Overview of the hierarchical competing systems model
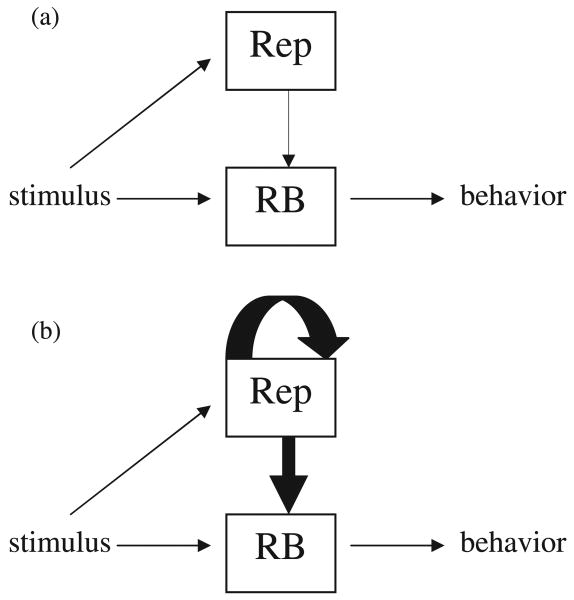
The hierarchical competing systems model (HCSM) is a refinement and updated formalization of ideas introduced in a series of articles by Marcovitch and Zelazo (1999, 2006; Marcovitch et al., 2002). The HCSM postulates that goal-directed behavior is influenced by two hierarchically arranged systems, a habit system that is dependent on previous experience, and a representational system that captures the influence of conscious reflection on behavior and that develops over the course of childhood (Zelazo, 2004). The two systems compete to guide behavior such that the representational system can influence, and potentially override, the habit system. As can be seen in Figure 3a, activity is elicited simultaneously in both systems, and in the absence of conscious reflection, behavior is determined jointly by the two systems but primarily by the response-based system. In Figure 3b, conscious reflection is captured by the ability to reflect on the contents of the representational system, as indicated by the reciprocal arrow. The influence of the representational system on behavior is magnified, and may even override the influence of previous behavior (Marcovitch & Zelazo, 2006).
Figure 3.
Conceptualization of the HCSM. The representational system exerts an influence on the habit system which produces behavior. In (a), the absence of reflection produces a relatively weak influence on behavior, while in (b), the presence of reflection magnifies the influence of the representational system. (Figure reproduced from Marcovitch, S., & Zelazo, P.D. (2006). Non-monotonic influence of number of A trials on 2-years-olds' perseverative search: a test of the hierarchical competing systems model. Journal of Cognition and Development, 7, 477–501.)
The emergence and early development of top-down, cognitive control is modeled in the context of the A-not-B task with infants and young children. In essence, the HCSM postulates that the A-not-B error can only result if: (a) there is a strong enough habit to reach at Location A, and (b) there is no conscious reflection at the moment of search that will guide behavior to the correct location. Although it is focused on executive function in infants and young children, the HCSM is intended to serve as a foundation for an account of executive function across the lifespan, emphasizing the role of reflection in processes such as shifting, updating, and inhibition (Miyake et al., 2000). Note that the HCSM is similar, but distinct from other views that behavior arises from the product of multiple psychological systems. For example, Norman and Shallice (1986) postulated a Supervisory Attentional System (SAS) that exerts control over the activation and inhibition values of various schemas. This HCSM differs from this model in its emphasis on the role of reflection in bringing about the selection of the relevant schema.
For the purpose of the HCSM, reflection on a mental state is tantamount to that mental state being the contents of consciousness (cf. Perner & Dienes, 2003; Rosenthal, 1997). This is a recursive process, in that the reflected state can then also become the contents of consciousness at a higher level of consciousness (Zelazo, 2004; Zelazo & Zelazo, 1998). It is assumed that reflection in this sense is necessary for the deliberate selection among possible response options. Thus, although behavior is heavily influenced by the amount and quality of previous experience, a deliberate and conscious decision to perform an action can override the influence of habit and allow one to exert top-down control over one's behavior (see also Botvinick, Braver, Barch, Carter & Cohen, 2001, and Zelazo et al., 2008, for models of executive function that suggest that conflict monitoring triggers top-down control processes).
According to the HCSM, the amount of experience a participant has reaching at the A location (i.e. the number of A trials) will influence both the habit strength and the cumulative probability of reflection. Thus, on the one hand, more experience at Location A will produce a stronger habit to search at the Location A (cf. Hull, Felsinger, Gladstone & Yamaguchi, 1947). On the other hand, more experience with the task in general (including searches at Location A) will provide more opportunities for the participant to reflect upon their representation of the hidden objects – something that likely interacts with a growing appreciation of the task constraints and affordances, including the possible relevance of Location B. If this reflection occurs prior to the object being hidden at Location B, then the participant will search correctly on the B trial, no matter how strong the habit to search at A.1
Spatial cues play an important role in search behavior, and infants are more likely to search correctly when the locations are visually distinct from one another (e.g. Noland, 2007; Smith et al., 1999). According to the HCSM, these cues would serve to increase the influence of the representational system, in part by providing additional cues to be labeled. This is generally consistent with A. Bremner and Bryant's (2001; see also J.G. Bremner, 1978; Butterworth, Jarrett & Hicks, 1982) view that strong spatial cues encourage updates of the object's locations. Note that as long as participants reach to Location A on A trials, the HCSM does not predict that the spatial cues (i.e. distinctive covers) would affect the habit system. This prediction is consistent with DST (Thelen et al., 2001),2 but counter to Munakata's (1998) assertion that distinctive covers reduce associations to Location A.
In many respects, the HCSM is compatible with other computational models of A-not-B search, particularly in emphasizing the importance of previous experience at Location A. However, the HCSM differs from these models in two ways:
The HCSM postulates that because of conscious reflection, increases in the number of A trials will eventually improve search performance on the B trials. Other models, in contrast, propose that increases in the number of A trials will never decrease the probability of perseveration – indeed, they should increase it.
Whereas other models suggest that improved performance results from simple rule learning (Dehaene & Changeux, 1989), or increases in active memory (Munakata, 1998), the HCSM postulates a conscious representational system that has the potential to develop through a series of degrees of reflection, or levels of consciousness (Zelazo, 2004). According to the model, consciousness can operate at multiple discrete levels, and these levels have a hierarchical structure – they vary from a first-order level of consciousness to higher-order reflective levels. Higher levels of consciousness are brought about through an iterative process of reflection, or the recursive reprocessing of the contents of consciousness. Each degree of reprocessing results in a higher level of consciousness, and this in turn allows for a stimulus to be considered relative to a larger interpretive context, with consequence for behavioral control.
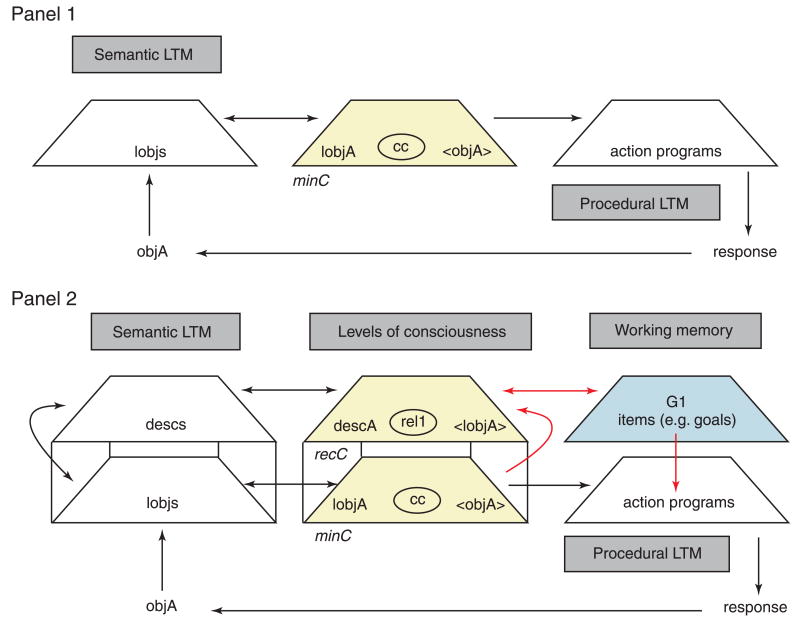
Figure 4 contrasts two cases in which action is based on different levels of consciousness. In Panel 1, action occurs in the absence of any reflection at all – it occurs on the basis of what is referred to as minimal consciousness (minC). An object in the environment (objA) triggers a salient, low-resolution ‘description’ from semantic long-term memory. In the context of the A-not-B task, objA is not the hidden object per se, but rather the location of the object in space (i.e. one of the hiding locations). This description (or IobjA, for ‘intentional object’) then becomes an intentional object of minC, by way of which it automatically triggers the most strongly associated action program in procedural long-term memory or elicits a stored stimulus–reward association. A particular hiding location, for example, may have been associated with interesting activity (e.g. a hiding event) or a reward (e.g. retrieving an object), and so, when seen, may elicit reaching toward that location.
Figure 4.
The implications of reflection (levels of consciousness) for search. Panel 1: Automatic action on the basis of unreflective consciousness. An object in the environment (objA) triggers an intentional representation of that object (IobjA) in semantic long-term memory (LTM); this IobjA, which is causally connected (cc) to a bracketed objA, becomes the content of consciousness (referred to at this level as minimal consciousness or minC). Panel 2: Action on the basis of one degree of reflection. Following minC processing of the objA, the contents of minC are then fed back into minC via a re-entrant feedback process, producing a new, more reflective level of consciousness referred to as recursive consciousness or recC. The contents of recC can be related (rel) in consciousness to a corresponding description (descA) or label, which can then be decoupled from the experience labeled and deposited into working memory (WM) where it can serve as a goal (G1) to trigger an action program in a top-down fashion from procedural LTM. (Figure reproduced from Zelazo, P.D. (2004). The development of conscious control in childhood. Trends in Cognitive Sciences, 8, 12–17.)
In Panel 2, action is based on one degree of reflection, resulting in a higher level of consciousness called recursive consciousness (recC). Now when objA triggers IobjA and becomes the content of minC, instead of triggering an associated action program directly, IobjA is fed back into minC (at a subsequent moment) where it can be related to a label (descA) from semantic long-term memory. This descA can then be decoupled from the minC experience that was labeled, and it can be deposited into long-term memory (where it provides a potentially enduring trace of the experience) and into working memory where it can serve as a goal (G1) that triggers an action program even in the absence of objA, and even if IobjA would otherwise trigger a different action program. For example, in the A-not-B task, the toddler may respond on the basis of a representation (in working memory) of the object at its current B location and avoid responding on the basis of an acquired tendency to reach to Location A. The toddler responds mediately to the decoupled label in working memory rather than immediately to a superficial gloss of the situation. In what follows, we state explicitly the roles of habit and reflection in search behavior, and attempt to show how these systems change with age and with various environmental influences.
Specific computational formalization of the HCSM
Habit system
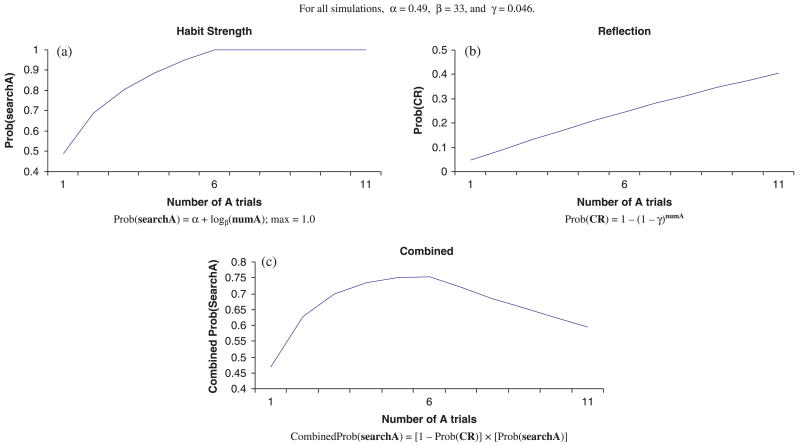
The influence of the habit system on search is directly proportional to the strength of the habit. As suggested by Marcovitch and Zelazo (1999) and others (e.g. Anderson, 1993; Hull, 1943), habit strength should be positively related to the amount of direct experience. It also seems reasonable that this function should be negatively accelerating and reach a maximal habit strength (i.e. the function should be asymptotic); an increase in habit strength may be expected between 1 and 6 A trials, but not necessarily between 101 and 106 A trials, for example. Thus, the model postulates that the probability of searching incorrectly on the first B trial due to habit is described by the following asymptotic function:
| (1) |
where Prob(searchA) is the probability of searching at A based on habit (max is 1.0), numA is the number of A trials, α is the parameter that accounts for the baseline probability of searching at Location A after 1 trial, and β is the parameter that captures the rate of increase in habit strength. Specifically, lower values for β will cause habit strength to reach an asymptote more quickly.
As will be discussed in the simulation section, plausible values for the parameters for 9-month-olds are α = .49 and β = 33. Figure 5a displays the probability of incorrectly searching at A as a function of the number of A trials using these parameter values. Note that the probability of searching incorrectly due to habit increases with number of A trials, but reaches its maximal influence after about 6 A trials (i.e. the influence of habit does not change between 6 and 11 A trials).
Figure 5.
The probability of: (a) incorrectly searching at A based on the effects of Habit Strength, (b) correctly searching at B based on the effects of Conscious Reflection, and (c) incorrectly searching at A based on the combined effects of Habit Strength and Conscious Reflection (see text for details).
Conscious representational system
One assumption of the HCSM is that a representation of the hidden object is formed after observing the hiding event (see Dehaene & Changeux, 1989; Diamond et al., 1994; Mareschal, Plunkett & Harris, 1995; Munakata, 1998; Schacter & Moscovitch, 1984; Thelen & Smith, 1994, for similar ideas). This representation will influence behavior, even in the absence of reflection on the representation. A number of research studies provide compelling evidence that search errors do not always occur exactly at Location A; rather, search errors occur between the A and B locations, as if the representation of the hidden object partially ‘pulls’ the reach towards the correct location (Diamond et al., 1994; Marcovitch & Zelazo, 2006; Schutte & Spencer, 2002; Spencer et al., 2001). The HCSM goes one step further – if appropriate reflection on the representation occurs prior to reaching (e.g. consciously noticing the current location of the object) then the representational system will influence behavior absolutely, leading to correct performance.
On any given trial, there is a baseline probability that the participant may reflect on the representation of the hidden object. This probability depends on the developmental level of the child (e.g. it will be higher for older children compared to younger children, and higher for typically developing children compared to delayed children) and task difficulty (i.e. it will be higher for simple tasks than for complex tasks). The model postulates that even if the probability of reflecting on a given trial is small (as you might expect from an infant or a toddler), this probability cumulates across trials and increases with task experience. Thus, the model works under the assumption that once reflection occurs on any trial during the task, it will re-occur on subsequent trials: children will have adopted a higher level of consciousness in this context. The probability that the participant will reflect at some time during the task is described by:
| (2) |
where Prob(CR) is the cumulative probability that reflection will have occurred, numA is the number of A trials, and γ is a parameter that represents the probability of reflection on any given trial. For γ = .046 (i.e. a low 4.6% chance of reflection on any given trial), the cumulative probability of correctly reflecting on the representation as a function of the number of A trials can be seen in Figure 5b. The cumulative probability of reflection, which guarantees correct search, increases with exposure to the task.
Combination of both systems
Combining Equations 1 and 2, incorrect search at A on the first B trial will occur: (a) if the participant has not reflected on the representation of the object, and (b) the habit system influences search at Location A. This is expressed by:
| (3) |
The probability of incorrect search as a function of the number of A trials is displayed in Figure 5c. Thus, with the above-mentioned parameter values, the HCSM predicts a non-monotonic, U-shaped relation between the number of A trials and the probability of incorrectly searching at A on the B trial. This feature of the model is unique; no other extant model of memory postulates a non-monotonic relation.
The psychological role of the parameters
Both α and β are parameters that influence habit strength. For the purpose of the present model, habit is defined as an implicit influence on behavior mediated by motor movements. Following the logic of DST (e.g. Clearfield, Diedrich, Smith & Thelen, 2006; Gershkoff-Stowe & Thelen, 2004; Thelen et al., 2001), as infants and young children develop, movements become more efficient (e.g. less jerky), which produces stronger motor memory traces. All things being equal, α (the probability of searching at A after a single A trial) should increase with age, while β should decrease with age (which results in a more efficient and faster increase of habit strength). In addition, the values of α and β should be affected by the complexity of the reaching movement. Simple reaches (e.g. direct unimpeded reaches towards the target) are more stable than complex reaches (e.g. reaching around an obstacle) and, according to the logic of Clearfield et al. (2006), will lead to stronger motor memory traces at any age.
The parameter γ is the probability of reflection on a particular trial, and it is postulated to increase with age as a function of the development of consciousness and to vary as a function of other influences on representational strength, such as divided attention (e.g. Zelazo, 2004). Indeed, at any given age, representational strength can be increased by directing attention to the appropriate stimulus via labeling. Labeling prompts reflection on the object labeled (Jacques & Zelazo, 2005) and it may facilitate the influence of the conscious representational system in at least two ways. First, when the contents of subjective experience are labeled, the label can then be decoupled from the immediate situation and be maintained in working memory (as shown in Figure 4, Panel 2). This provides the child with a potentially enduring trace of his or her experience, and allows him or her to act in the absence of, or even in spite of, direct environmental stimulation (e.g. noticing the old Location A). Second, labeling transforms what was subjective into an object of conscious consideration; it makes one's representations an object of reflection. Without language (or a comparable symbol system), children would be limited to unreflective consciousness of immediate interoor exteroceptor stimulation (i.e. responding immediately to perceptual stimuli).
From this perspective, age-related changes in reflection can be explained by changes in self-initiated labeling strategies. For example, 12-month-old infants respond to labeled objects which may indicate early emergence of reflection – the ability to match a perceptual experience that resides in minimal consciousness with a label (i.e. recursive consciousness; Zelazo, 2004). According to the HCSM, infants at this age (and not 9-month-olds) typically pass the standard A-not-B task because of recursive consciousness of the appropriate representation. Note that recursive consciousness per se does not have to be explicit. It is only when recursive consciousness itself becomes the contents of consciousness that the process is explicit, which is when children are aware of the labels and can use them effectively. Increases in task experience (and with specific stimuli) will afford more opportunity to engage in recursive consciousness. By 2 years of age, many children will readily label familiar objects (i.e. evidence of another level of reflection), although sometimes only when prompted. Thus according to the HCSM, children demonstrate powerful reflective tendencies when labeling occurs (either spontaneously or after a prompt), and would be able to override relatively simple prepotent responses (e.g. searching at a previous location). Further age-related increases in reflection come about with more advanced labeling strategies (e.g. generating mnemonics) and these can be used to override more powerful prepotent tendencies (e.g. solving multiplication problems incorrectly).
Hypotheses generated from the model
The HCSM makes a number of predictions, six of which are presented here. Where possible, we summarize behavioral findings relevant to these predictions.
H1: Under certain conditions, the relation between the number of A trials and performance on the B trials is non-monotonic. That is to say, children will be more likely to search perseveratively (i.e. incorrectly searching at A on B trials) after a moderate number of A trials than after relatively few or relatively many trials.
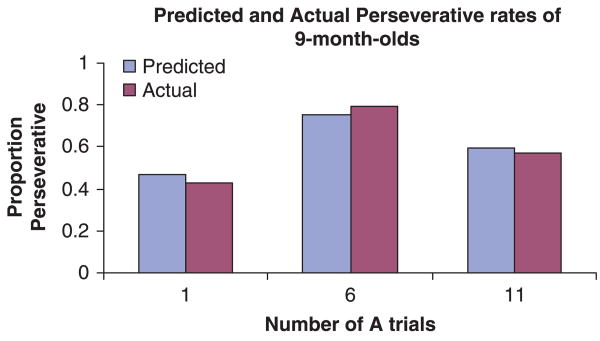
H1 has been confirmed in a standard A-not-B task conducted with 9-month-old infants (Marcovitch et al., 2002). In this study, infants searched for an object either 1, 6, or 11 times at Location A. Then, when the object was conspicuously hidden at Location B, infants were more likely to search incorrectly after 6 A trials (79%) than after 1 A trial (43%) or 11 A trials (57%). Figure 6 displays the remarkably close fit between the actual data and the proposed computational model, when the parameters are set to α = .49, β = 33, and γ = .046 (i.e. the same parameter values as listed above). The decrease in performance between 1 and 6 A trials is caused primarily by increases in habit strength, whereas the improved performance after 11 A trials indicates the influence of reflection.
Figure 6.
The predictions from the quantification of the Hierarchical Competing Systems Model (α = .49. β = 33, and γ = .046) and actual performance of 9-month-olds (Marcovitch et al., 2002).
An alternative mechanism that may account for the U-shaped pattern of results is habituation. From this perspective, infants habituate after 11 A trials and begin to respond randomly, which appears to be an improvement compared to the systematic perseveration after 6 A trials. Although this cannot be ruled out completely, Marcovitch et al. (2002) argued that if infants habituate after many trials, then they should respond randomly on the final A trials prior to the B trial. Furthermore, across all B trials there should be random, not systematic, performance. Results indicated that infants rarely erred on the final A trials, and B trial performance was systematic (i.e. once a correct response was produced, all subsequent responses were correct).
Habituation may also play a role in the sense that after 11 trials, infants are habituated to events at Location A and thus show increased attention to novel events, such as hiding at Location B. This view would be consistent with DST, as infants are likely to search where they are attending. As argued elsewhere (Marcovitch & Zelazo, 2006), we contend that this view is not incompatible with the HCSM; rather, the automaticity of responses after many A trials may be what frees up cognitive resources to allow for the opportunity to reflect.
The U-shaped effect was also elicited with 2-year-old children in an age-appropriate modification of the A-not-B task (Marcovitch & Zelazo, 2006, Experiment 1). In this study, 2-year-olds were tested on the multistep, multilocation search task. To find the reward, children engaged in a multistep retrieval procedure: (a) they removed a foam barrier, (b) they pulled out a tray which revealed 5 symbols, (c) they pulled on the symbol that was attached by a string to a plastic bag that contained the reward. Children were more likely to make a perseverative error (i.e. incorrectly search at Location A on the first B trial) after searching correctly on 6 A trials (63%), as opposed to 1 A trial (29%) or 11 A trials (39%).
Further evidence for a U-shaped effect with 2-year-olds was demonstrated in an A-not-B variant where the search space was homogeneous (i.e. continuous) rather than heterogeneous (i.e. discrete). In this study (Marcovitch & Zelazo, 2006, Experiment 2), which used a task developed by Spencer et al. (2001), objects were hidden in a sandbox and the amount of time needed to find the object was taken as an index of perseverative behavior (i.e. if children were incorrectly searching at Location A, it would take them more time to find the object at the correct Location B). Once again, a U-shaped relation was apparent: children took the longest time to find the object after 11 A trials (mean = 20.8 s), as opposed to after 3 A trials (mean = 8.6 s), 7 A trials (mean = 10.7 s), or 15 A trials (mean = 9.2 s). Thus, the U-shaped effect postulated by the HCSM has now been replicated across three different tasks and two different age groups. Because it is not obvious how other models could be modified to account for them, these findings provide important support for the HCSM relative to other accounts of perseveration.
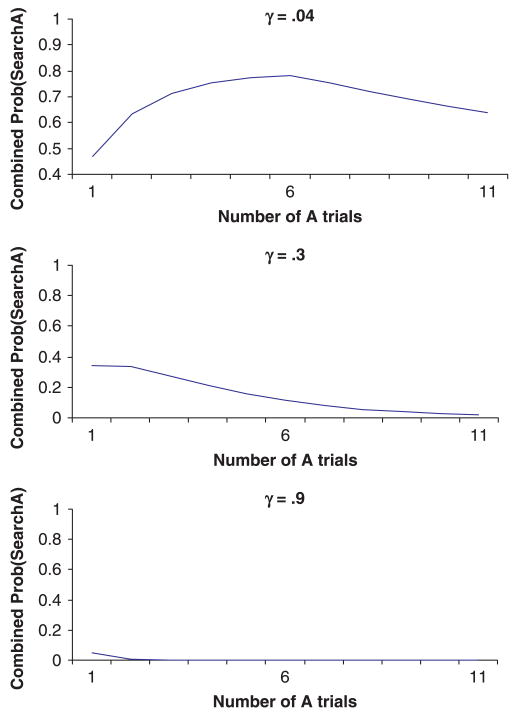
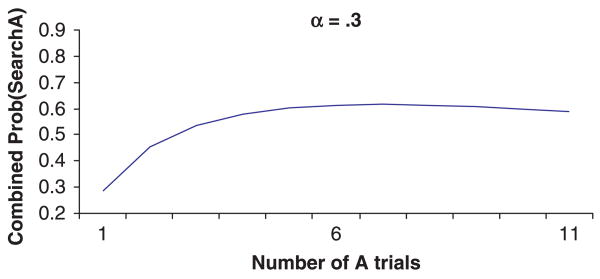
H2: On a given task and all things being equal, as infants and children get older the relation between perseverative behavior and the number of A trials will shift from being U-shaped to being monotonically decreasing to eventually disappear due to a ceiling effect.
H2 was derived from simulations where α and β were kept constant, and γ (postulated to increase with age) was manipulated. As shown in Figure 7, as γ increases, the relation shifts from U-shaped (γ = .04), to monotonically decreasing (γ = .3), to near perfect performance (γ = .9). This prediction is consistent with Marcovitch and Zelazo's (2001) suggestion that even after hundreds of A trials in a simple A-not-B task, an attentive adult will search correctly on the B trial, easily overriding the habit system (although a distracted adult may indeed perseverate; see H4 below). Thus, age-related improvements in representation will not only result in better performance but will also change the nature of the relation between the number of A trials and perseverative behavior.
Figure 7.
Results from simulations where γ varies from .04 to .3 to .9.
This prediction remains to be tested empirically, but it is consistent with the results of research assessing adults' performance on the Wisconsin Card Sorting Test (WCST; Grant & Berg, 1948; Grant & Cost, 1954). In the WCST, participants are given test cards that vary on three dimensions (shape, color, and number), and must discover the rule for matching them to target cards that also vary on these dimensions. After sorting correctly by this rule on a certain number of consecutive trials (i.e. the number of reinforcing trials), the rule changes, and participants must infer the new rule. This task is obviously more complex than the A-not-B task, but it does require keeping a new rule in mind and using it to guide responding despite interference from a tendency to persist in initial fashion. Indeed, patients with lesions to dorsolateral prefrontal cortex often perseverate on this task (e.g. Milner, 1964), and the analogy to infants' performance on the A-not-B task has frequently been noted. Consistent with H2, Grant and colleagues varied the number of reinforcing trials, and found that adults were significantly less likely to perseverate, and more likely to switch, as a direct function of the number of reinforcing trials (see Figure 8). Rather than a clear U-shaped effect, as might be expected with younger participants, the effect of number of reinforcing trials was monotonically decreasing.
Figure 8.
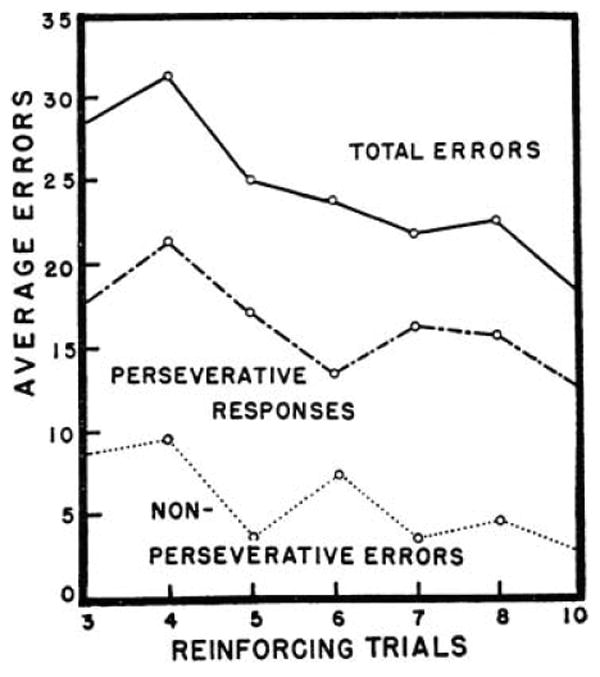
The effect of number of reinforcing trials (trials to criterion) on adults' performance (errors) on the Wisconsin Card Sorting Test. (Figure reproduced from Grant, D.A., & Berg, E.A. (1948). A behavioral analysis of degree of reinforcement and ease of shifting to new responses in a Weigl-type-card-sorting problem. Journal of Experimental Psychology, 38, 404–411.)
This monotonically decreasing function is also consistent with a model of conscious and unconscious cognition put forth by Cleeremans (2006). He contends that experience strengthens the quality of a representation such that implicit cognition corresponds to low quality representations, explicit cognition corresponds to moderate quality representations, and automaticity corresponds to high quality representations. From this perspective, experience with a representation will make it more explicit which in turn renders it more likely to govern behavior.
H3: At any given age, the likelihood of successful search can be increased by providing cues, such as labels, that call attention to an object or otherwise cause a participant to reflect on it. All things being equal, cues should also change the effect of number of A trials on the probability of perseveration: with additional cues, the effect should shift from being U-shaped to being monotonically decreasing to eventually disappearing due to a ceiling effect.
H3 arises from the proposal that γ can be increased via cues, such as labels, that encourage reflection (Homer & Nelson, 2005; Jacques & Zelazo, 2005; Zelazo, 2004). Support for this claim was provided by Homer and Nelson (2005) who assessed the role of labeling on a variant of DeLoache's (e.g. 1991) scale model search task. In the standard version of this task, children observe a toy being hidden in a three-dimensional scale model of a room and are then required to use this information to find an analogous toy that had been hidden in the corresponding place in the room itself. Typically, 2.5-year-old children search perseveratively on this task (O'Sullivan, Mitchell & Daehler, 2001). However, Homer and Nelson reported that young children's performance improved when they labeled the hiding location, suggesting that labeling is an effective metacognitive cue that encourages reflection – at least in children at this age.
The specific role of labeling in effecting reflection can be seen in work by Jacques, Zelazo, Lourenco and Sutherland (2006), who found that labeling improved cognitive flexibility on the Flexible Item Selection Task. On each trial of this task, children are shown sets of three items designed so one pair matches on one dimension, and a different pair matches on a different dimension (e.g. a small yellow teapot, a large yellow teapot, and a large yellow shoe). Children are first asked to select one pair (i.e. Selection 1), and then asked to select a different pair (i.e. Selection 2). To respond correctly, children must represent the pivot item (i.e. the large yellow teapot) according to both dimensions. Four-year-olds generally perform well on Selection 1 but poorly on Selection 2, indicating inflexibility. However, asking children to label their perspective on Selection 1 (e.g. ‘Why do those two pictures go together?’) increased the likelihood that they would adopt a different perspective on Selection 2. This is consistent with the suggestion that labeling one's perspective on a situation encourages reflection on that perspective – it helps make it an object of explicit consideration, thereby allowing one to detach oneself from that perspective and select an alternative perspective on the same situation.
H4: At any given age, the likelihood of successful search will be affected by manipulations that decrease the likelihood of reflection. All things being equal, these manipulations should also change the effect of number of A trials on the probability of perseveration: the relation between number of A trials and the probability of perseveration should become more like that characteristic of younger participants (see H2).
Manipulations that impair processing of the task context and decrease attention to a hidden object will decrease γ. One example is divided attention, which can be achieved experimentally by employing a dual-task paradigm (e.g. Pashler, 1992). Under dual-task constraints, participants will perform more poorly, but more important, they will show patterns of responding that are characteristic of younger children (H2). Specifically, dual-task constraints should change the effect of number of A trials on the probability of perseveration. For example, in adults, the effect should shift from no effect to monotonically decreasing or to U-shaped. This specific prediction is unique to the HCSM and remains to be tested.
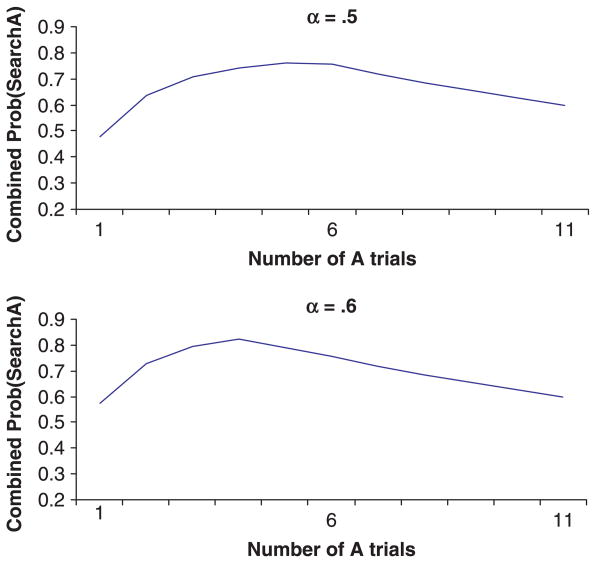
H5: Infants and children with better motor coordination (and hence better motor memory – resulting in greater habit strength) will display greater levels of perseveration, and require fewer trials at A to achieve these levels, than their peers with poorer motor coordination.
H5 is based on simulations where γ and β were kept constant, and α (postulated to increase with improved motor coordination) is manipulated (see Figure 9). Note that identical predictions are generated if α is kept constant and β is free to vary. When α = .5, the U-shaped pattern observed with 9-month-olds is apparent, with maximal perseveration after 5 or 6 A trials. As α increases (i.e. α = .6), the point of maximum perseveration decreases (after 4 A trials), which is consistent with the notion that in a highly coordinated individual, fewer trials are needed to maximize habit strength.
Figure 9.
Results from simulations where α varies from .5 to .6.
H6: Children with motor delays will not exhibit the U-shaped relation. After relatively few A trials, they will make fewer errors than their typically developing peers. In addition, the relation between number of A trials and perseveration will be asymptotic.
Simulations where α is decreased significantly (i.e. α = .3, see Figure 10) reveal lower levels of perseveration and the absence of a U-shaped relation. Rivière and Lecuyer (2003) have provided evidence for improved performance on search tasks for children with motor delays. In their study, 29-month-old children with spinal muscular atrophy searched more correctly than healthy controls of the same age on a modified invisible displacement task. This is consistent with the idea that perseverative behavior is manifested by motor memory towards Location A, and this can only be achieved through consistent, stable reaches (Clearfield et al., 2006). Children who cannot coordinate a stable reach, whether because of age or physical disability, will not form as strong a habit toward Location A and will therefore be more likely to succeed on the A-not-B task.
Figure 10.
Results from a simulation where α = .3.
This set of hypotheses is unique, and no extant theory of perseverative search or executive function makes this same set of predictions. Although other models posit competing processes akin to the habit system and representational system (e.g. Dehaene & Changeux, 1989; Diamond et al., 1994; Mareschal, Plunkett & Harris, 1999; Munakata, 1998; Schacter & Moscovitch, 1984; Thelen et al., 2001), the HCSM is the only contemporary theory of perseverative search to emphasize the role of reflection. Besides generating novel hypotheses, the HCSM's focus on reflection allows it to serve as the foundation for a model of the development of executive function across the lifespan (see, e.g. Zelazo, Craik & Booth, 2004; Zelazo et al., 2003).
Model extensions and future directions
In one sense, the proposed quantification of the HCSM has been successful, as it has generated testable hypotheses that may serve to increase our understanding of search behavior and the development of executive function from infancy throughout the preschool years and beyond. However, the model is still incomplete. The following are five extensions of the model that might usefully be implemented:
Further investigation is needed to explore how the parameters help us understand the psychological organization of the cognitive systems involved in search. For example, is one parameter (i.e. γ) sufficient to account for the variety of ways that representational abilities can change? Do α and β map on to distinct psychological processes (and if not should one of the parameters be eliminated)?
-
The model currently does not address non-perseverative incorrect responding (i.e. errors that occur at a location other than Location A). A complete computational model must account for the established tendency to search between the perseverative and correct location (e.g. Diamond et al., 1994; Marcovitch & Zelazo, 1999, 2006; Schutte & Spencer, 2002; Spencer et al., 2001), as well as the observed relation between the number of A trials and searching exactly at Location A (Marcovitch & Zelazo, 2006).
One way that the current model can be applied to paradigms that yield search between the A and B locations is to adopt a more liberal definition of perseveration. Specifically, drawing upon previous work by Diamond et al. (2004), Spencer et al. (2001), and Marcovitch and Zelazo (2006), perseverative search can encompass all behavior that is biased, even partially, by experience at Location A. Thus, the HCSM's prediction of perseverative responding can include all reaches at Location A, or between the A and B locations. A future challenge, of course, is to use the HCSM to quantify the degree of perseveration (i.e. how much the reach is biased in the A direction). We have proposed previously that search between locations can be modeled by summing the effects of a Gaussian distribution with an exponential distribution (see Marcovitch & Zelazo, 1999, for details), and perhaps this can be integrated into the model in the future.
The proposed model accounts for the role of habits created by motor movements. However, habits can be purely representational (e.g. observing the object being hidden multiple times at a particular location without being allowed to reach may create an expectation to see the object at that location). One possibility is that representational habits exhibit the same influence as motor habits (cf. Kirkham et al., 2003; Munakata, 1998; Zelazo et al., 1998). Indeed, a number of researchers have postulated mechanisms that map observed action onto motor representations of that action (e.g. Flanagan & Johansson, 2003; Hommel, Müsseler, Aschersleben & Prinz, 2001), and this mapping may be mediated by mirror neurons (Rizzolatti, Fadiga, Gallese & Fogassi, 1996). Alternatively, representational habits may be dependent on the representational system rather than the habit system.
The computational model computes the probability of searching perseveratively as its output. The model should be extended to account for other dependent measures, which may be more sensitive to the degree of perseveration (e.g. response time and the error run, or the number of B trials on which children err prior to searching correctly).
The computational model deals with discrete responses (i.e. heterogeneous search spaces). The model needs to be extended to deal with homogeneous search spaces (e.g. sandbox task; Marcovitch & Zelazo, 2006; Spencer et al., 2001).
Conclusion
Among extant theories of search behavior in infancy and toddlerhood, the HCSM is unique in addressing directly the role of conscious control, and it makes several unique predictions as a result. In this article, we proposed a quantification of the HCSM that relies on the parameterization of two simple mathematical formulae. This quantification helps clarify what is assumed by the model, as well as what is postulated to develop. Computational simulations based on this model were used to derive predictions and suggest how the model may account for extant data. Further research is needed to test predictions from the model, as well as to generalize the model to account for a wider range of results and measures, but it is hoped that the model may serve as the foundation of a more comprehensive model of the development of executive function during childhood – one that addresses further age-related increases in the conscious control of behavior, such as those seen between the ages of 3 and 5 years.
Acknowledgments
Preparation of this article was supported by National Institute of Child Health and Human Development Grant 1R0HD054609-01A1 to SM and by grants from NSERC of Canada and the Canada Research Chairs program to PDZ.
Footnotes
For commentaries on this article see Cooper (2008), Diamond (2008) and Smith (2008).
Note that the model currently operates under the assumption that search behavior will be correct on all A trials, which is often, but not always the case. This assumption is advantageous as it allows for the experience on each A trial to contribute to both the habit and representational systems. Strictly speaking, however, incorrect A trials should disrupt (and perhaps weaken) the habit while still providing opportunity for reflection. Although this issue needs to be addressed to explain the wide range of possible behaviors, it does not impact on any of the major arguments presented in this article. Rather, the empirical investigations inspired by the model should be sensitive to differences in A trial performance. We thank an anonymous reviewer for pointing this out.
DST does contend that distinctive covers may lead to a weaker habit, but only if this input encourages incorrect reaches to B during the A trials.
References
- Ahmed A, Ruffman T. Why do infants make A not B errors in a search task, yet show memory for the location of hidden objects in a nonsearch task? Developmental Psychology. 1998;34:441–453. doi: 10.1037//0012-1649.34.3.441. [DOI] [PubMed] [Google Scholar]
- Anderson JR. Problem solving and learning. American Psychologist. 1993;38:35–44. [Google Scholar]
- Baillargeon R, DeVos J. Object permanence in young infants: further evidence. Child Development. 1991;62:1227–1246. [PubMed] [Google Scholar]
- Baillargeon R, Graber M, DeVos J, Black J. Why do young infants fail to search for hidden objects? Cognition. 1990;36:255–284. doi: 10.1016/0010-0277(90)90059-s. [DOI] [PubMed] [Google Scholar]
- Baird AA, Kagan J, Gaudette T, Walz K, Hershlag N, Boas D. Frontal lobe activation during object permanence: evidence from near infrared spectroscopy. NeuroImage. 2002;16:1120–1126. doi: 10.1006/nimg.2002.1170. [DOI] [PubMed] [Google Scholar]
- Bjorklund DF, Harnishfeger KK. The resources construct in cognitive development: diverse sources of evidence and a theory of inefficient inhibition. Developmental Review. 1990;10:48–71. [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bower TGR, Wishart J. The effects of motor skill on object permanence. Cognition. 1972;1:165–172. [Google Scholar]
- Bremner A, Bryant P. The effect of spatial cues on infants' responses in the AB task, with and without a hidden object. Developmental Science. 2001;4:408–415. [Google Scholar]
- Bremner JG. Spatial errors made by infants: inadequate spatial cues or evidence of egocentrism? British Journal of Psychology. 1978;69:77–84. doi: 10.1111/j.2044-8295.1978.tb01634.x. [DOI] [PubMed] [Google Scholar]
- Butterworth GE. Object disappearance and error in Piaget's stage IV task. Journal of Experimental Child Psychology. 1977;23:391–401. [Google Scholar]
- Butterworth GE, Jarrett N, Hicks L. Spatiotemporal identity in infancy: perceptual competence or conceptual deficit? Developmental Psychology. 1982;18:435–449. [Google Scholar]
- Carlson SM. Developmentally sensitive measures of executive function in preschool children. Developmental Neuropsychology. 2005;28:595–616. doi: 10.1207/s15326942dn2802_3. [DOI] [PubMed] [Google Scholar]
- Carlson SM, Davis AC, Leach JG. Less is more: executive function and symbolic representation in young children. Psychological Science. 2005;16:609–616. doi: 10.1111/j.1467-9280.2005.01583.x. [DOI] [PubMed] [Google Scholar]
- Clearfield MW, Diedrich FJ, Smith LB, Thelen E. Young infants reach correctly in A-not-B tasks: on the development of stability and perseveration. Infant Behavior and Development. 2006;29:435–444. doi: 10.1016/j.infbeh.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Cleeremans A. Conscious and unconscious cognition: a graded, dynamic, perspective. In: Jing Q, Rosenzweig MR, d'Ydewalle G, Zhang H, Chen HC, Zhang K, editors. Progress in psychological science around the world Vol I Neural, cognitive and developmental issues. Hove: Psychology Press; 2006. pp. 401–418. [Google Scholar]
- Clifton RK, Muir DW, Ashmead DH, Clarkson MG. Is visually guided reaching in early infancy a myth? Child Development. 1993;64:1099–1110. [PubMed] [Google Scholar]
- Cummings EM, Bjork EL. Search behavior on multi-choice hiding tasks: evidence for an objective conception of space in infancy. International Journal of Behavioral Development. 1983;6:71–87. [Google Scholar]
- Dehaene S, Changeux JP. A simple model of prefrontal cortex function in delayed response tasks. Journal of Cognitive Neuroscience. 1989;1:244–261. doi: 10.1162/jocn.1989.1.3.244. [DOI] [PubMed] [Google Scholar]
- Deloache JS. Symbolic functioning in very young children: understanding of pictures and models. Child Development. 1991;62:736–752. [PubMed] [Google Scholar]
- Dempster F. The rise and fall of the inhibitory mechanism: toward a unified theory of cognitive development and aging. Developmental Review. 1992;12:45–75. [Google Scholar]
- Diamond A. Development of the ability to use recall to guide action, as indicated by infants' performance on AB. Child Development. 1985;56:868–883. [PubMed] [Google Scholar]
- Diamond A. Normal development of prefrontal cortex from birth to young adulthood: cognitive functions, anatomy, and biochemistry. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. London: Oxford University Press; 2002. pp. 466–503. [Google Scholar]
- Diamond A, Cruttenden L, Neiderman D. AB with multiple wells: 1. Why are multiple wells sometimes easier than two wells? 2. Memory or memory + inhibition? Developmental Psychology. 1994;30:192–205. [Google Scholar]
- Diamond A, Doar B. The performance of human infants on a measure of frontal cortex function, the delayed response task. Developmental Psychobiology. 1989;22:271–294. doi: 10.1002/dev.420220307. [DOI] [PubMed] [Google Scholar]
- Elman JL. Finding structure in time. Cognitive Science. 1990;14:179–211. [Google Scholar]
- Espy KA. The shape school: assessing executive function in preschool children. Developmental Neuropsychology. 1997;13:495–499. doi: 10.1207/s15326942dn2601_3. [DOI] [PubMed] [Google Scholar]
- Espy K, Kaufmann PM, McDiarmid MD, Glisky ML. Executive functioning in preschool children: performance on A-not-B and other delayed response format tasks. Brain and Cognition. 1999;41:178–199. doi: 10.1006/brcg.1999.1117. [DOI] [PubMed] [Google Scholar]
- Evans WF. The stage IV error in Piaget's theory of object concept development: an investigation of the role of activity. University of Houston; Houston, TX: 1973. Unpublished dissertation proposal. [Google Scholar]
- Flanagan JR, Johansson RS. Action plans used in action observation. Nature. 2003;424:769–771. doi: 10.1038/nature01861. [DOI] [PubMed] [Google Scholar]
- Fox N, Kagan J, Weiskopf S. The growth of memory during infancy. Genetic Psychology Monographs. 1979;99:91–130. [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex. New York: Raven; 1980. [Google Scholar]
- Gershkoff-Stowe L, Thelen E. U-shaped changes in behavior: a dynamic systems perspective. Journal of Cognition and Development. 2004;5:11–36. [Google Scholar]
- Gerstadt CL, Hong YJ, Diamond A. The relationship between cognition and action: performance of children years old on a Stroop-like day-night test. Cognition. 1994;53:129–153. doi: 10.1016/0010-0277(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In: Plum F, Mountcastle V, editors. Handbook of Physiology. Vol. 5. Washington, DC: The American Physiological Society; 1987. pp. 373–517. [Google Scholar]
- Grant DA, Berg EA. A behavioral analysis of degree of reinforcement and ease of shifting to new responses in a Weigl-type card-sorting problem. Journal of Experimental Psychology. 1948;38:404–411. doi: 10.1037/h0059831. [DOI] [PubMed] [Google Scholar]
- Grant DA, Cost JR. Continuities and discontinuities in conceptual behavior in a card sorting problem. Journal of General Psychology. 1954;50:237–244. [Google Scholar]
- Gratch G, Appel KJ, Evans WF, LeCompte GK, Wright NA. Piaget's stage IV object concept error: evidence of forgetting or object conception. Child Development. 1974;45:71–77. [PubMed] [Google Scholar]
- Harris PL. Perseverative errors in search by young infants. Child Development. 1973;44:28–33. [PubMed] [Google Scholar]
- Harris PL. Perseverative search at a visibly empty place by young infants. Journal of Experimental Child Psychology. 1974;18:535–542. [Google Scholar]
- Harris PL. Object permanence in infancy. In: Slater A, Bremner G, editors. Infant development. Hillsdale, NJ: Erlbaum; 1989. pp. 103–121. [Google Scholar]
- Hofstadter M, Reznick JS. Response modality affects human infant delayed-response performance. Child Development. 1996;67:646–658. [PubMed] [Google Scholar]
- Homer BD, Nelson K. Seeing objects as symbols and symbols as objects: language and the development of dual representation. In: Homer BD, Tamis-LeMonda CS, editors. The development of social cognition and communication. Mahwah, NJ: Lawrence Erlbaum Associates; 2005. pp. 29–52. [Google Scholar]
- Hommel B, Müsseler J, Aschersleben G, Prinz W. The Theory for Event Coding (TEC): a framework for perception and action planning. Behavioral and Brain Sciences. 2001;24:849–878. doi: 10.1017/s0140525x01000103. [DOI] [PubMed] [Google Scholar]
- Hood B, Willats P. Reaching in the dark to an object's remembered position: evidence for object permanence in 5-month-old infants. British Journal of Developmental Psychology. 1986;4:57–65. [Google Scholar]
- Horobin KM, Acredolo LP. The role of attentiveness, mobility history, and separation of hiding sites on stage IV search behavior. Journal of Experimental Child Psychology. 1986;41:114–127. [Google Scholar]
- Hull CL. Principles of behavior. New York: Appleton-Century; 1943. [Google Scholar]
- Hull CL, Felsinger J, Gladstone I, Yamaguchi H. A proposed quantification of habit strength. Psychological Review. 1947;54:237–254. doi: 10.1037/h0062631. [DOI] [PubMed] [Google Scholar]
- Hunter WS. The delayed reaction in animals and children. Behavior Monographs. 1913;2:1–86. [Google Scholar]
- Hunter WS. The delayed reaction in a child. Psychological Review. 1917;24:74–87. [Google Scholar]
- Jacobsen CF. Studies of cerebral function in primates: I. The functions of the frontal association areas in monkeys. Comparative Psychology Monographs. 1936;13:1–68. [Google Scholar]
- Jacques S, Zelazo PD. On the possible socio-communicative roots of cognitive flexibility. In: Homer B, Tamis-LeMonda C, editors. The development of social understanding and communication. Mahwah, NJ: Lawrence Erlbaum Associates; 2005. pp. 53–81. [Google Scholar]
- Jacques S, Zelazo PD, Lourenco SF, Sutherland AE. The roles of labeling and abstraction in the development of cognitive flexibility. 2006 Manuscript under review. [Google Scholar]
- Karmiloff-Smith A. Beyond modularity. Cambridge, MA: MIT Press; 1992. [Google Scholar]
- Kirkham NZ, Cruess LM, Diamond A. Helping children apply their knowledge to their behavior on a dimension-switching task. Developmental Science. 2003;6:449–467. [Google Scholar]
- Landers WF. Effects of differential experience on infants' performance in a Piagetian Stage IV object-concept task. Developmental Psychology. 1971;5:48–54. [Google Scholar]
- Luce RD. Where is mathematical modeling in psychology headed? Theory and Psychology. 1999;9:723–737. [Google Scholar]
- Lyons-Warren A, Lillie R, Hershey T. Short- and long-term spatial delayed response performance across the lifespan. Developmental Neuropsychology. 2004;26:661–678. doi: 10.1207/s15326942dn2603_1. [DOI] [PubMed] [Google Scholar]
- Marcovitch S, Zelazo PD. The A-not-B error: results from a logistic meta-analysis. Child Development. 1999;70:1297–1313. [Google Scholar]
- Marcovitch S, Zelazo PD. Proceedings of the Twenty-second Annual Conference of the Cognitive Science Society. Mahwah, NJ: Lawrence Erlbaum Associates; 2000. A generative connectionist model of the development of rule use in children; pp. 334–339. [Google Scholar]
- Marcovitch S, Zelazo PD. On the need for conscious control and conceptual understanding. Behavioral and Brain Sciences. 2001;24:48–49. Commentary on Thelen et al. [Google Scholar]
- Marcovitch S, Zelazo PD. Non-monotonic influence of number of A trials on 2-years-olds' perseverative search: a test of the hierarchical competing systems model. Journal of Cognition and Development. 2006;7:477–501. [Google Scholar]
- Marcovitch S, Zelazo PD, Schmuckler MA. The effect of number of A trials on performance on the A-not-B task. Infancy. 2002;3:519–529. [Google Scholar]
- Mareschal D, Plunkett K, Harris P. Developing object permanence: a connectionist model. In: Moore JD, Lehman JF, editors. Proceedings of the Seventeenth Annual Conference of the Cognitive Science Society. Mahwah, NJ: Lawrence Erlbaum Associates; 1995. pp. 170–175. [Google Scholar]
- Mareschal D, Plunkett K, Harris P. A computational and neuropsychological account of object-oriented behaviours in infancy. Developmental Science. 1999;2:306–317. [Google Scholar]
- Milner B. Some effects of frontal lobectomy in man. In: Warren J, Ackert K, editors. The frontal granular cortex and behavior. New York: McGraw-Hill; 1964. pp. 313–334. [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex ‘frontal lobe’ tasks: a latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Morton JB, Munakata Y. Active vs. latent representations: a neural network model of perseveration, dissociation, and decalage. Developmental Psychobiology. 2002;40:255–265. doi: 10.1002/dev.10033. [DOI] [PubMed] [Google Scholar]
- Munakata Y. Perseverative reaching in infancy: the roles of hidden toys and motor history in the AB task. Infant Behavior and Development. 1997;20:405–416. [Google Scholar]
- Munakata Y. Infant perseveration and implications for object permanence theories: a PDP model of the AB task. Developmental Science. 1998;2:161–184. [Google Scholar]
- Munakata Y, McClelland JL, Johnson MH, Siegler R. Rethinking infant knowledge: toward an adaptive process account of successes and failures in object permanence tasks. Psychological Review. 1997;104:686–713. doi: 10.1037/0033-295x.104.4.686. [DOI] [PubMed] [Google Scholar]
- Munakata Y, Yerys BE. All together now: when dissociations between knowledge and action disappear. Psychological Science. 2001;12:335–337. doi: 10.1111/1467-9280.00361. [DOI] [PubMed] [Google Scholar]
- Noland JS. It is not just about location: infants perseverate to container shape during object search. Infancy. 2007;11:295–303. doi: 10.1111/j.1532-7078.2007.tb00228.x. [DOI] [PubMed] [Google Scholar]
- Norman D, Shallice T. Attention to action: willed and automatic control of behavior. In: Davidson R, Schwartz G, Shapiro D, editors. Consciousness and self-regulation: Advances in research and theory. Vol. 4. New York: Plenum; 1986. pp. 1–18. [Google Scholar]
- O'Sullivan LP, Mitchell LL, Daehler MW. Representation and perseveration: influences on young children's representational insight. Journal of Cognition and Development. 2001;2:339–365. [Google Scholar]
- Pashler H. Attentional limitations in doing two tasks at the same time. Current Directions in Psychological Science. 1992;1:44–48. [Google Scholar]
- Perner J, Dienes Z. Developmental aspects of consciousness: how much of a theory of mind do you need to be consciously aware? Consciousness and Cognition. 2003;12:63–82. doi: 10.1016/s1053-8100(02)00010-7. [DOI] [PubMed] [Google Scholar]
- Perner J, Lang B. Development of theory of mind and cognitive control. Trends in Cognitive Sciences. 1999;3:337–344. doi: 10.1016/s1364-6613(99)01362-5. [DOI] [PubMed] [Google Scholar]
- Piaget J. The construction of reality in the child. New York: Basic Books; 1954. [Google Scholar]
- Rivière J, Lecuyer R. The C-not-B error: a comparative study. Cognitive Development. 2003;18:285–297. [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Cognitive Brain Research. 1996;3:131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Rosenthal DM. A theory of consciousness. In: Block N, Flanagan O, Güzeldere G, editors. The nature of consciousness: Philosophical debates. Cambridge, MA: MIT Press; 1997. pp. 729–753. [Google Scholar]
- Schacter DL, Moscovitch M. Infants, amnesics, and dissociable memory systems. In: Moscovitch M, editor. Infant memory. New York: Plenum; 1984. pp. 173–216. [Google Scholar]
- Schacter DL, Moscovitch M, Tulving E, McLachlan DR, Freedman M. Mnemonic precedence in amnesic patients: an analogue of the AB error in infants? Child Development. 1986;57:816–823. [PubMed] [Google Scholar]
- Schutte AR, Spencer JP. Generalizing the dynamic field theory of the A-not-B error beyond infancy: three-year-olds' delay- and experience-dependent location memory biases. Child Development. 2002;73:377–404. doi: 10.1111/1467-8624.00413. [DOI] [PubMed] [Google Scholar]
- Schutte AR, Spencer JP, Schöner G. Testing the dynamic field theory: working memory for locations becomes more spatially precise over development. Child Development. 2003;74:1393–1417. doi: 10.1111/1467-8624.00614. [DOI] [PubMed] [Google Scholar]
- Shultz TR. Computational developmental Psychology. Cambridge, MA: MIT Press; 2003. [Google Scholar]
- Simon TJ, Halford GS, editors. Developing cognitive competence: New approaches to process modeling. Hillsdale, NJ: Lawrence Erlbaum Associates; 1995. [Google Scholar]
- Smith LB, Thelen E, Titzer R, McLin D. Knowing in the context of acting: the task dynamics of the A-not-B error. Psychological Review. 1999;106:235–260. doi: 10.1037/0033-295x.106.2.235. [DOI] [PubMed] [Google Scholar]
- Sophian C, Wellman HM. Selective information use and perseveration in the search behavior of infants and young children. Journal of Experimental Child Psychology. 1983;35:369–390. doi: 10.1016/0022-0965(83)90015-2. [DOI] [PubMed] [Google Scholar]
- Spencer JP, Schutte AR. Unifying representations and responses: perseverative biases arise from a single behavioral system. Psychological Science. 2004;15:187–193. doi: 10.1111/j.0956-7976.2004.01503007.x. [DOI] [PubMed] [Google Scholar]
- Spencer JP, Smith LB, Thelen E. Tests of a dynamic systems account of the A-not-B error: the influence of prior experience on the spatial memory abilities of 2-year-olds. Child Development. 2001;72:1327–1346. doi: 10.1111/1467-8624.00351. [DOI] [PubMed] [Google Scholar]
- Thelen E, Schöner G, Scheier C, Smith LB. A dynamic field theory of infant perseverative errors. Behavioral and Brain Sciences. 2001;24:1–18. doi: 10.1017/s0140525x01003910. [DOI] [PubMed] [Google Scholar]
- Thelen E, Smith LB. A dynamical systems approach to the development of cognition and action. Cambridge, MA: Bradford Books, MIT Press; 1994. [Google Scholar]
- Wellman HM, Cross D, Bartsch K. Infant search and object permanence: a meta-analysis of the A-not-B error. Monographs of the Society for Research in Child Development. 1986;51(3) Serial No. 214. [PubMed] [Google Scholar]
- Zelazo PD. The development of conscious control in childhood. Trends in Cognitive Sciences. 2004;8:12–17. doi: 10.1016/j.tics.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Carlson SM, Kesek A. Development of executive function in childhood. In: Nelson CA, Luciana M, editors. Handbook of developmental cognitive neuro-science. 2nd. Cambridge, MA: MIT Press; 2008. pp. 553–574. [Google Scholar]
- Zelazo PD, Craik FIM, Booth L. Executive function across the life span. Acta Psychologica. 2004;115:167–184. doi: 10.1016/j.actpsy.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Müller U. Executive functions in typical and atypical development. In: Goswami U, editor. Handbook of childhood cognitive development. Oxford: Blackwell; 2002. pp. 445–469. [Google Scholar]
- Zelazo PD, Müller U, Frye D, Marcovitch S. The development of executive function in early childhood. Monographs of the Society for Research in Child Development. 2003;68(3) doi: 10.1111/j.0037-976x.2003.00260.x. Serial No. 274. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Reznick JS, Spinazzola J. Representational flexibility and response control in a multistep multilocation search task. Developmental Psychology. 1998;34:203–214. doi: 10.1037//0012-1649.34.2.203. [DOI] [PubMed] [Google Scholar]
- Zelazo PR, Zelazo PD. The emergence of consciousness. Advances in Neurology. 1998;77:149–165. [PubMed] [Google Scholar]