Abstract
RNA interference (RNAi) mediates sequence-specific gene silencing, which can be harnessed to silencing disease-causing genes for therapy. Particularly suitable diseases are those caused by dominant, gain-of-function type of gene mutations. In these diseases, the mutant gene generates a mutant protein or RNA product, which possesses toxic properties that harm cells. By silencing the mutant gene, the toxicity can be lessened because the amount of the toxic product is lowered in cells. In this report, we tested RNAi therapy in a mouse model for amyotrophic lateral sclerosis (ALS), which causes motor neuron degeneration, paralysis, and death. We used a transgenic model that overexpresses mutant Cu, Zn superoxide dismutase (SOD1G93A), which causes ALS by a gained toxic property. We delivered RNAi using recombinant adenovirus (RAd) and adeno-associated virus serotype 2 (AAV2). We compared the efficiency of RNAi delivery between injecting the viral vectors into muscle and into nerve, and found that nerve injetion is more efficient in delivering RNAi to motor neurons. Based on this data, we conducted therapeutic trials in the mouse model and found that nerve injection of RAd, but not AAV2, at the disease onset had a modest therapeutic efficacy. These results highlight the potential and the challenges in delivering RNAi therapy by gene therepy. Antioxid. Redox Signal. 11, 1523–1534.
Introduction
Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disease that causes motor neuron degeneration, paralysis, and death. Approximately 10% of ALS cases are familial and 90% are sporadic. Gene mutations are known to underlie familial ALS. The genes where mutations cause familial ALS include Cu, Zn superoxide dismutase (SOD1), Alsin, senataxin, dynactin, VAMP-associated protein B (VAPB), and TAR DNA binding protein 43 KD (TDP-43) (25, 37, 47). Mutations in all these genes except Alsin are dominantly inherited. In contrast to the familial cases, no obvious cause is known for sporadic ALS. However, recent studies have discovered TDP-43 as a prominent component in the ubiquitin-positive intracellular inclusions in sporadic ALS (2, 36), thus suggesting that TDP-43 is involved in the pathogenesis of sporadic ALS.
Mutations in the SOD1 gene were the first discovered genetic cause for ALS and they cause ∼20% of familial ALS cases (37). In the past 16 years following this discovery, much progress has been made in our understanding of the mechanism whereby the mutant SOD1 causes this disease (5). Among the most important findings is the proof that mutant SOD1 causes motor neuron degeneration by a gain of a toxic property rather than a loss of the enzymatic function of SOD1. First, there is no correlation between the retention of the enzyme activity and the disease-causing propensity in the SOD1 mutants. While some mutations retain normal levels of superoxide dismutation activity, others lose almost all the enzyme activity (8). In addition, the presence of mutant enzyme does not affect the activity and stability of the normal enzyme despite the formation of mutant-wild type heterodimer (7). Second, transgenic mice expressing the mutant SOD1 develop motor neuron degeneration and ALS without lowering the level of superoxide dismutase activity (19, 57). Third, neither overexpression of the wild-type SOD1 nor deletion of the SOD1 gene leads to ALS in mice (19, 41, 57), indicating that alteration in normal SOD1 activity is not a direct cause of this disease. Fourth, overexpression of wild-type SOD1 does not alleviate, but instead, accelerates the disease; and knockout of the endogenous SOD1 does not significantly alter the course of the disease (10, 14, 23), indicating that the level of the superoxide dismutase activity is not related to the pathogenesis of ALS.
Since a toxic property in the mutant SOD1 causes motor neuron degeneration, we can predict that the higher the mutant protein expression, the stronger the toxicity, and consequently, the more severe the disease. Indeed, in different transgenic lines that express mutant SOD1, the higher the expression levels, the more severe the disease, as manifested by earlier disease onset and more rapid disease progression (12, 55, 57). With this knowledge, we can conclude that lowering the mutant SOD1 expression will be therapeutic and RNA interference (RNAi) may be harnessed for silencing the mutant SOD1 expression (16).
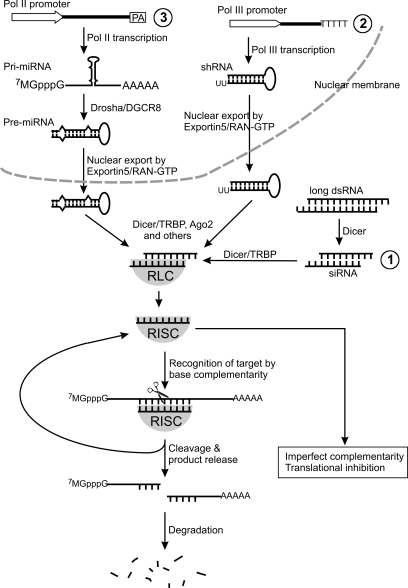
RNAi is a widely conserved eukaryotic function (35). Triggered in cells by double-stranded RNA (dsRNA), RNAi destroys the target RNA that shares sequence homology with the dsRNA. The main steps of the RNAi mechanism can be simplified as the following steps (Fig. 1): First, Dicer, an enzyme of the RNase III family, initiates ATP-dependent fragmentation of long dsRNA into 21–25 nucleotide double-stranded fragments, termed small interfering RNAs (siRNAs). Second, the siRNA duplexes bind with proteins Dicer and TRBP (or R2D2 for invertebrates), which facilitate the formation of a siRNA/multi-protein complex called RISC loading complex (RLC). Third, the siRNA duplex in RLC unwinds, which involves the protein Ago2 to cleave the passenger strand) to form an active RNA-induced silencing complex (RISC) that contains a single-stranded RNA (called the guide strand). Fourth, the RISC recognizes the target RNA by Watson–Crick base pairing with the guide strand and cleaves the target RNA. Finally, the RISC releases its cleaved product and goes on to catalyze a new cycle of target recognition and cleavage (39, 50).
FIG. 1.
RNAi therapeutic strategies: (1) siRNA may be delivered directly to the CNS to silence disease genes; (2) Pol III constructs synthesizing shRNA; or (3) Pol II constructs synthesizing miRNA can be placed in viral vectors and delivered into the CNS cells for long-term silencing of disease genes. See text for description of the different strategies and the RNAi mechanism.
In differentiated mammalian cells, long dsRNA activates RNA-dependent protein kinase PKR and type I interferon response, which leads to a nonspecific global translation depression and apoptosis (18). However, this nonspecific reaction can be circumvented by introduction of synthetic siRNA (11, 17), which can go into the RNAi pathway similar to the siRNAs produced from long dsRNA (Fig. 1, #1). Alternatively, RNAi may be triggered by a short hairpin RNA (shRNA) synthesized by a Pol III promoter (Fig. 1, #2) or by a microRNA (miRNA) that can be synthesized by either a Pol II or Pol III promoter (Fig. 1, #3) (9, 44). The shRNA is a single-stranded RNA folded into a simple hairpin, composed of a perfectly base-paired stem of 21 to 23 nt and a loop. It is synthesized by a Pol III promoter (e.g., U6) as in the nucleus (44). In contrast, the miRNA is synthesized as a long transcript, called primary miRNA (pri-miRNA) (27). The pri-miRNA need to be processed by RNase III enzyme Drosha and its partner DGCR8 (or Pasha in invertebrates) to form precursor miRNA (pre-miRNA), which is ∼70 nt long and folds into a hairpin structure composed of an imperfectly base-paired stem and a loop. The pre-miRNA then shares the same downstream processing pathway with the shRNA. Both are exported by exportin 5/RAN-GTP from the nucleus to the cytoplasm, where they are further processed to form a single-stranded miRNA or siRNA. This processing step is tightly coupled with loading the guide strand of miRNA or siRNA into the RISC, which is capable of either cleaving the target RNA if the target perfectly complements the miRNA in sequence, or mediating translational gene silencing if the miRNA mismatches the target RNA at multiple base-pairs (Fig. 1).
Because RISC recognizes its target by Watson–Crick base pairing with the guide strand of the siRNA, destruction of the target RNA can be specific (43). This property of RNAi can be harnessed to target specific mRNA species for destruction, and therefore, to silence the expression of the toxic protein encoded by the mRNA for therapy. Thus far, RNAi therapy for CNS diseases has been delivered by direct administration of synthetic siRNA or genes that encode shRNA or miRNA using viral vectors. Both delivery methods have shown therapeutic efficacy in animal models for neurodegenerative diseases, and thus, are potential therapeutic strategies for humans (15, 20, 45, 54, 58).
The viral delivery method (gene therapy) delivers transgenes that are composed of a promoter and a desired transgene cassette to the CNS cells. In recent years, viral vector technology for gene delivery into the brain has improved substantially (33, 42, 52). A sustained expression extending beyond 12 months has been achieved using recombinant adenovirus (RAd)-, lentivirus-, adeno-associated virus (AAV)-, and herpes simplex-1 virus-derived vectors in animal studies (32). Viral vector systems, including both adenovirus and AAV, have been developed to the point where clinical gene therapy for brain diseases is now possible for both acute and chronic central nervous system pathologies. Taking advantage of these viral vectors, several groups have tested viral delivery of RNAi therapy for neurological diseases. These tests have shown efficacy in models for polyglutamine diseases, Alzheimer disease, and ALS (13).
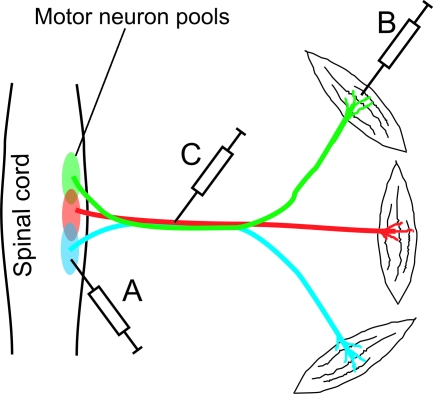
In these studies, two transgenes were incorporated into AAV or lentiviral vectors. One transgene expressed a marker gene (e.g., EGFP) driven by a Pol II promoter (e.g., CMV) and the other expressed a shRNA driven by a Pol III promoter (e.g., U6). The marker gene provided an indicator as to efficiency with which the transgene is delivered. The shRNA silences the expression of the mutated disease gene or a gene that is in the disease pathway. In the cases of polyglutamine diseases and Alzheimer disease, the viral vectors were directly injected into the relevant disease areas in the brain. However, in treating ALS, the limited spread of virus presents a particular challenge for administering the virus to wide groups of motor neurons distributed along the spinal cord and in the motor cortex. Injection into a single or a few spots can only cover a small fraction of motor neurons that are degenerating (Fig. 2A). Indeed, injection of a lentivirus delivering RNAi against human SOD1 into the spinal cord of an ALS mouse model led to a local functional improvement but no extension of survival (40).
FIG. 2.
A schematic illustration of different methods of administering viral vectors for delivering RNAi to spinal motor neurons: (A) direct spinal cord injection, (B) muscle injection, and (C) nerve injection. Different colors mark the different motor neuron pools that innervate different muscles. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Because of the limitation in injecting the viral vector directly into the CNS, other groups have injected the virus into muscles (Fig. 2B). These experiments have demonstrated that some virus such as adenovirus, AAV2, and Rabies glycoprotein-pseudotyped lentivirus can be taken up by the nerve terminals and retrogradely transported to the spinal cord motor neurons (3, 26, 34). However, because each muscle only receives a small number of axonal projections, injection of many muscles were required to cover multiple motor neuron pools. Because of the large tissue mass of the muscles, these injections also require relatively large doses of the virus. Perhaps to reduce the required dose, Ralph and colleagues injected a lentivirus delivering RNAi against mutant SOD1 into the muscles in 7-day-old mice, where muscle mass is small and immune response underdeveloped. They observed retrograde transport of the virus and the transgene expression. This treatment resulted in a large extension (∼70%) in lifespan of the mutant SOD1 mouse model (38). This is an encouraging result for RNAi therapy but the treatment may not be practical in humans. Administration of lentivirus to adult humans in muscles throughout the whole body will require even larger doses of virus, which will not only increase the cost, but also put the patient at risk due to untoward side effects, including immune responses, transduction of irrelevant cells (e.g., germline cells), and the potential to cause insertion mutagenesis and neoplasia formation (28). In addition, it was unclear from this experiment whether silencing of mutant SOD1 at the disease onset will be effective, because the therapy was administrated long before the disease onset in the mouse model. In order to improve the delivery of viral vectors to motor neurons, we administered viral vectors by nerve injection.
Materials and Methods
Construction and production of RAd and AAV2 vectors
To generate the RAd/U6-shR-SOD1/CMV-EGFP vector, the CMV promoter and enhanced green fluorescent fragment (CMV-EGFP) were cut from pcDNA-EGFP and inserted into XhoI and EcoRV sites of pΔE1sp1A (Microbix Biosystems, Inc., Toronto, Ontario, Canada). This generates the plasmid pΔE1sp1A/CMV-EGFP. The U6-shRNA expression cassette was then cut from pBSENU6-shRNA (63) and inserted into XhoI and PmeI sites of pΔE1sp1A/CMV-EGFP. The RAd/CMV-EGFP/U6-blank was generated using similar method except without the shRNA. The RAd that was used for nerve injection was produced according to our previously published protocol (46). Briefly, vectors were scaled up by infecting human embryonic kidney HEK 293 cells with a multiplicity of infection of three plaque-forming units (pfu)/cell of vector seed stock. The cells were harvested 48 h later, lysed with 5% deoxycholate and deoxyribonulease I, and the RAd were purified by ultracentrifugation over two cesium chloride step gradients. Vectors were titered in triplicate by end-point dilution cytopathic effect assay and screened for the presence of replication-competent adenovirus (RCA) and for lipopolysaccharide (LPS) contamination (Cambrex, East Rutherford, NJ). Vector preparations were free from RCA and LPS contamination.
To generate the AAV2/U6–shR–SOD1/CMV–EGFP, the U6-shRNA expression cassette was cut from pBSENU6-shRNA (63) and inserted into XhoI site of pcDNA-EGFP to generate the U6–shRNA/CMV–EGFP plasmid. The CMV–EGFP/U6–shRNA was then cut out and inserted into the NotI site of the pAAV2-MCS (Stratagene, San Francisco, CA). Small scale production of AAV2/U6–shRNA/CMV–EGFP was conducted based on a previously published protocol (61). For large productions, the U6–shRNA/CMV–EGFP cassette was inserted into the NotI site in the pFBGR plasmid and the AAV2 vector was produced by the Gene Transfer Vector Core at University of Iowa based on a previously published protocol (51).
Animals
The high expression line of transgenic mice expressing human mutant SOD1G93A (19) was purchased from The Jackson Lab (Bar Harbor, Maine). The mice have been bred to FVB background for >10 generations and identified using PCR as described previously (21). Animals were housed in a standard environment with 12/12 light cycle. All animal procedures were approved by the institutional animal care and use committee (IACUC). For monitoring disease progression, body weight or rotorod test was conducted as described previously (6, 59). The early stage of the disease is defined by a drop of the mouse body weight of 10% or more from the peak body weight. The end stage of the disease is defined by a complete paralysis of any two limbs. In a vast majority of the mice, the hinder limbs are paralyzed before the fore limbs. Therefore, by this assay a delay in hinder limb paralysis will result in a delay in the onset of the end stage.
Injection of viral vectors and horseradish peroxidase conjugated cholera toxin subunit B (HRP-CTB)
Mice were anesthetized with avertin and were placed on a flat surface. The nerves were exposed at the level of thigh. Approximately 7 μl of RAd or AAV2 (various amounts, see Results), or 3 μl of HRP-CTB (1 mg/ml, List Biological Laboratories Inc., Campbell, CA) were injected into the nerves using a 5-μl Hamilton syringe with a 33-gauge needle. The skin was then sutured closed, and the mice were allowed to recover on a warm pad. At various times after the injection of the viral vectors or 3 days after the injection of HRP-CTB, mice were anesthetized with avertin and transcardiacly perfused with PBS and then with perfusion buffer (2% paraformaldehyde and 0.1% glutaraldehyde in PBS). The spinal cord and the nerves were removed and postfixed with the perfusion buffer at 4°C.
Histology
Spinal cords were sectioned longitudinally (40 μm thick) using a vibratome. EGFP signal was observed directly using a fluorescent microscope. To detect SOD1, the sections were incubated in blocking solution (5% donkey serum, 0.5% Triton X-100, and 2% fat-free dry milk in PBS) in room temperature for 30 min and then in the blocking solution containing primary antibody sheep anti-SOD1 (Biodesign, Saco, ME, at 1:300) at 4°C overnight. After washing, the sections were incubated with the secondary antibody (rhodamine-conjugated donkey anti-sheep IgG, Jackson ImmunoResearch, West Grove, PA, 1:100) for 3 h at room temperature. The SOD1 antibody recognizes both human and mouse SOD1. However, the human SOD1 is expressed in the transgenic mice at 17 times of the mouse endogenous levels (24). Therefore, the staining intensity in these mice almost entirely represents human SOD1. To detect the peroxidase reactivity, the sections were developed using a diaminobenzidine (DAB) kit (Vector Laboratories, Inc., Burlingame, CA).
Statistical analysis
Two-way ANOVA was used to compare the weight changes between the RAd-RNAi injected and the RAd-blank injected animals. Wilcoxon Rank Sum test was used to compare the survival between these two groups of animals.
Results
Efficient delivery of RAd and AAV2 to motor neurons by nerve injection
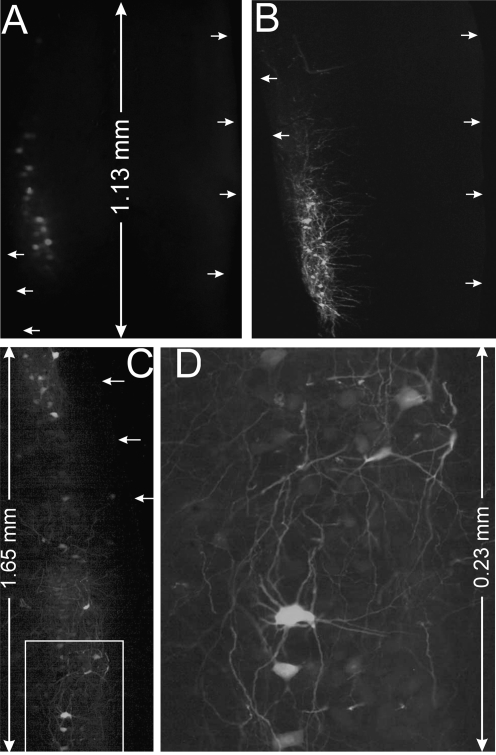
We reasoned that injection of viral vectors directly into the large nerve trunks such as the sciatic nerve can expose the virus to a large number of axons, and therefore, has the potential to deliver the transgene to the largest population of motor neurons through a single injection (Fig. 2C). To determine whether this is true, we prepared a batch of RAd carrying an EGFP gene (CMV-EGFP) and injected it into gastrocnemius muscle and sciatic nerve in equal doses (1.25 × 109 particles). We observed expression of EGFP in relatively few spinal cord motor neurons after muscle injection (Fig. 3A). In contrast, in the sciatic nerve injected animal, we observed a large number of EGFP expressing motor neurons (Fig. 3B). In some injections, motor neurons expressed EGFP in a long segment of the lateral motor column, which covered an area as long as ∼3.6 mm (Fig. 3C and D). This observation confirms our hypothesis that nerve injection is more efficient in delivering viral vectors to motor neurons than the muscle injection.
FIG. 3.
Comparison of motor neuron expression of EGFP between injection of RAd into gastrocnemius muscle and into sciatic nerve. Fewer motor neurons express express EGFP in mouse spinal cord 7 days after injection of RAd (1.25 × 109 particles) into gastrocnemius muscle (A) compared with the injection of the sciatic nerve (B). Notice the levels of EGFP in individual motor neurons are also lower in the muscle-injected spinal cord, compared with the nerve-injected spinal cord. By the same exposure, in the nerve-injected spinal cord the motor neurons show bright fluorescence and visible neurites, whereas in the muscle-injected spinal cord the motor neurons are dimly labeled and the neurites are not visible. (C) In another experiment, wide motor neuron expression of EGFP is observed 5 days after injection of 108 PFU into sciatic nerve. (D) High magnification view of labeled motor neurons in (C). The images are horizontal longitudinal sections of the spinal cord. In (C) and (D), only the injected sides are shown. Arrows point to the boards of lateral spinal cord.
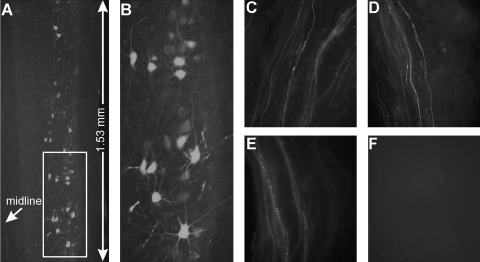
To determine whether the nerve injection of AAV is also more efficient than muscle injection, we tested AAV2, which was shown previously to be retrogradely transported and to mediate transgene expression in motor neurons (26). We injected AAV2 (5 × 108) into the gastrocnemius muscle or the sciatic nerve. At 2–3 weeks after the muscle injection, we could not find EGFP expressing neurons in the spinal cord (data not shown). This lack of EGFP-expressing neurons after muscle injection was most likely caused by the low viral particle number that we injected, as a minimum of 109 viral particles is required to see the transduced motor neurons in the spinal cord (26). However, we were able to readily find many EGFP-expressing motor neurons in the spinal cord after the nerve injection (Fig. 4). Based on the successful labeling of motor neurons through nerve injection but not the muscle injection, together with the data from the RAd (Fig. 3), we conclude that nerve injection is superior to muscle injection in delivering RAd and AAV2 to spinal motor neurons.
FIG. 4.
EGFP expression in spinal motor neurons by retrograde transport following sciatic nerve injection of AAV2. (A) Three weeks after injection of AAV2/CMV-EGFP. (B) A large magnification view of the boxed area in (A). Notice that besides the brightly labeled neurons, there are also many weakly labeled motor neurons in the ventral horn area. Similar strongly and weakly labeled EGFP-positive axons can be observed in the sciatic nerve: (C) a segment above the injection site, (D) a segment at the injection site, (E) a segment below the injection site. (F) A sciatic nerve segment taken from the uninjected sciatic nerve from the same animal.
RNAi delivered by RAd through nerve injection can mediate silencing of SOD1 in mouse spinal cords
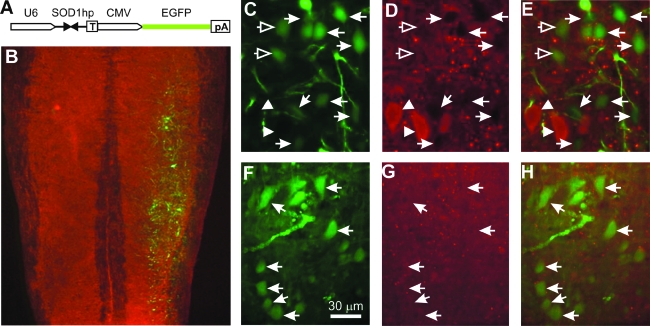
To test whether nerve injection of RAd can be used to administer RNAi therapy for ALS, we inserted a transgene that drives the expression of an shRNA against human SOD1 by the U6 promoter (U6–shR–SOD1a) (60) into the RAd vector that also expresses EGFP (Fig. 5A). Therefore, this RAd/U6–shR–SOD1/CMV–EGFP vector expresses both shR-SOD1 for silencing mutant SOD1 and EGFP for monitoring motor neuron transduction. As a control, we also constructed a RAd/U6–blank/CMV–EGFP vector, which expresses EGFP only. We tested these vectors by infecting cultured HEK293 cells transfected with a myc-tagged human SOD1. By Western blot analysis, we found that the vector knocked down both the transfected and the endogenous SOD1, as reported previously (60) (data not shown). To test the silencing activity of the vector in vivo, we injected these vectors into sciatic nerves of mutant SOD1G93A transgenic mice. Seven days after vector delivery, we dissected the lower lumbar spinal cord on the injected side and extracted proteins. By Western blot analysis, we did not detect knockdown of SOD1 (data not shown). This was not surprising since the shRNA was only expressed in motor neurons. To specifically examine motor neurons, we stained for SOD1 signal in the spinal cord sections using immunofluorescence. We observed robust expression of EGFP in motor neurons in the injected side of the spinal cord (Fig. 5B). Furthermore, in mice injected with RAd/U6–shR–SOD1/CMV–EGFP, EGFP-expressing motor neurons are correlated with lowered SOD1 staining (Fig. 5C–E, arrows), in contrast to the motor neurons without EGFP expression, which displayed strong SOD1 staining (Fig. 5C–E, arrowheads). A minority of the motor neurons expressed EGFP but did not show strongly decreased SOD1 staining (Fig. 5C–E, open arrows). This could be caused by a low U6 promoter activity in some cells because the shRNA and EGFP are driven by independent promoters.
FIG. 5.
RAd-delivered RNAi can knockdown mutant SOD1 level in motor neurons. (A) The shRNA- and GFP-expression cassette that is placed in RAd. U6 and CMV promoters direct synthesis of shRNA against SOD1 and EGFP, respectively. “T” indicates the Pol III transcription termination sequence. “pA” indicates the poly A signal. (B) Robust expression of EGFP in motor neurons 7 days after injection of the RAd/U6-hSOD1hp/CMV-EGFP into the right sciatic nerve. Red represents SOD1 staining. (C and D) The majority of the EGFP expressing neurons (arrows) have lowered SOD1 staining. Motor neurons that do not express EGFP (arrowheads) have high SOD1 staining. (F–H) A control mouse injected with RAd/U6-SCRAMBLEhp/CMV-EGFP shows EGFP expression in motor neurons (arrows) but no reduced levels of SOD1. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
In contrast to the RAd/U6–shR–SOD1/CMV–EGFP injected animals, we did not find that EGFP-expressing motor neurons in RAd/U6–blank/CMV–EGFP injected animals have lowered SOD1 expression (Fig. 5F–H, arrows). Thus, the lowered mutant SOD1 levels in RAd/U6–shR-SOD1/CMV–EGFP injected animals are caused by the specific silencing mediated by the shRNA against SOD1.
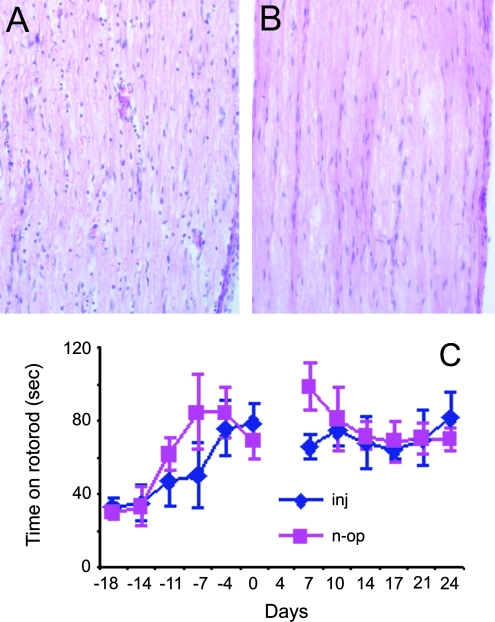
Nerve injection of RAd caused inflammation but did not affect the general motor function
To determine how much injury nerve injections cause to the nerve, we injected the right sciatic nerve of wild-type mice with RAd/CMV–EGFP and sacrificed the animals 1 week after the injection. Examination of the injected segment showed an increased cell (macrophage and lymphocyte) infiltration (Fig. 6A) compared with the uninjected side (Fig. 6B), indicating the presence of inflammation. This is not surprising since the first generation of RAd is known to cause inflammation. To determine whether nerve injection caused local motor defects, we carried out an experiment to assess the effect of RAd injection on motor function. We divided 8 SOD1G93A animals (68 days old) into two groups and trained them for a rotorod test twice weekly for two and half weeks. We then injected RAd into both sciatic nerves in four animals. We skipped one rotorod test following the operation to let the mice recover from the wound and then tested them for another 3 weeks. The four injected animals performed comparably with the four nonoperated animals (Fig. 6C). This result suggests that RAd injection did not accelerate motor neuron degeneration and will not interfere with our observation on the disease progression after the nerve injection. This was also consistent with our previous observation that axotomy (a more severe injury than viral injection) does not accelerate motor neuron degeneration (30) and the well-established observation that rodents tolerate the first generation RAd well.
FIG. 6.
The effect of RAd injection on the sciatic nerve. (A) Hematoxylin and Eosin (H&E) stained section from the injected segment of the sciatic nerve. (B) A section from the uninjected side. (C) Rotorod test of G93A animals before and after injection of RAd to both sciatic nerves. “inj”, injected group; “n-op”, nonoperated group. The date at which the injected group was operated on was day 0. One test was skipped after the injection to avoid possible aggravation of the operation wounds. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
RNAi delivered by RAd, but not by AAV2, through nerve injection at the disease onset extends the survival of SOD1G93A transgenic mice
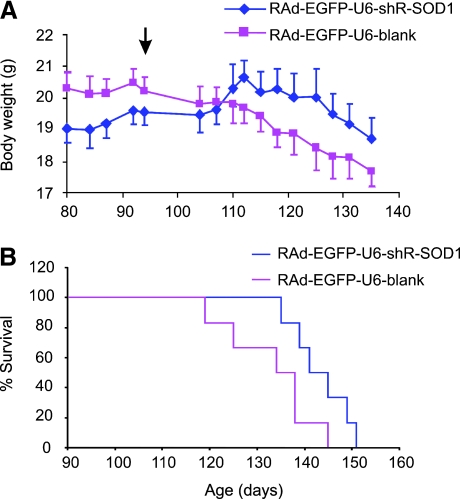
Based on the above observations, we conducted a therapeutic trial using RAd/U6–shR–SOD1/CMV–EGFP. We injected this vector into both sides of each animal at 94 days of age, which is an average age of disease onset for our mouse colony based on the peak weight measurement. The control group was the littermates injected with RAd/U6–blank/CMV–EGFP. We assessed the disease progression by the changes in the animals' body weight (6). On average, the control group continued to lose weight after the viral administration whereas the experimental group gained weight and this trend of change is statistically significant (Fig. 7A), suggesting that the disease progression has been slowed. Confirming the slowing of the disease progression, we observed an extension of survival in the experimental group (143 ± 6 days) compared with the control group (133 ± 10 days) (Fig. 7B). These data suggest that knockdown of mutant SOD1 expression in the motor neurons at the disease onset can slow the disease progression. This result confirms our other knockdown experiment where a chemically stabilized siRNA were infused into the spinal cord at the disease onset (54).
FIG. 7.
RAd-delivered RNAi slows disease progression and extends survival of SOD1G93A mice. (A) Six pairs of female SOD1G93A littermates were divided into two groups. One was injected with RAd expressing shRNA against SOD1 and EGFP and the other was injected with RAd expressing EGFP alone. The animals were all injected at 94 days of age (arrow) and weighed before and after the injection. The average weight changes are plotted. Error bars represent standard error. We compared the weight and its changes over time (including times before and after viral injection) between the two groups using two-way ANOVA. There was no significant difference between the weights of the two groups (F = 1.6; p = 0.21). However, there was a significant difference in the weight changes over time during aging between the two groups (F = 1.8; p = 0.04). There was no significant interaction between the weight of the mice and the weight changes over time (F = 1.5; p = 0.09). (B) Animals injected with RAd expressing the shRNA lived significantly longer than those injected with the control RAd (Wilcoxon rank sum test, p = 0.022). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
These therapeutic effects observed using the RAd vector are encouraging and demonstrate that nerve injection of the retrogradely transportable viral vectors can be an efficient way of delivering RNAi therapy for ALS. However, two limitations prevented us from gaining an even more robust therapeutic efficacy. First, the RAd-delivered transgene expression was only transient. By a longitudinal examination of the expression levels of EGFP at various times after the RAd administration, we found that the EGPF level peaked at ∼1 week after the RAd injection and gradually declined to only a few scattered motor neurons persistently expressing EGFP in the following 2 weeks (data not shown). Second, only the sciatic nerve was injected in this experiment. Future efforts using the improved versions of RAd (e.g., the gutless RAd) and injection of other nerves that contain axons from important motor neuron pools (see below) may lead to persistent expression of the shRNA and an enhancement of the therapeutic efficacy.
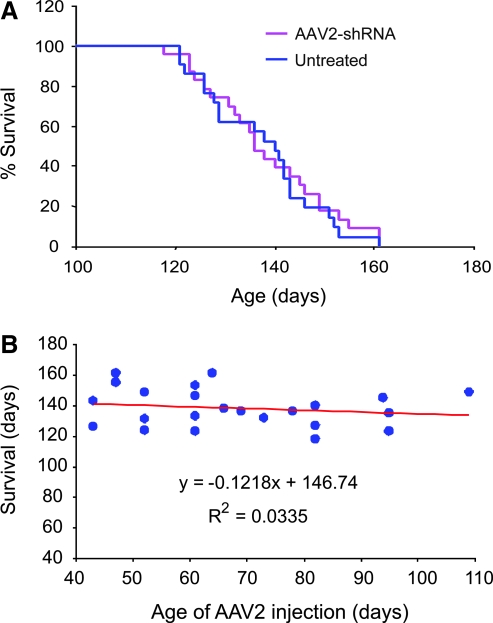
To determine whether using AAV2 to deliver RNAi can also yield therapeutic efficacy, we tested therapy using the same U6–shRNA and CMV–EGFP construct packaged into an AAV2 vector (AAV2/U6–shR-SOD1/CMV–EGFP). Similar to the RAd vector, we also tested this vector in cultured cells and determined it had silencing activity (data not shown). In our preliminary tests in vivo, we found that AAV2-mediated expression of EGFP persisted for at least 3 months after the initial vector injection and that freshly prepared viral particles worked much better than the frozen viral particles in transducing motor neurons by nerve injection (data not shown). We divided SOD1G93A mouse littermates at various ages into an experimental group and a control group and administered the AAV2/U6–shR-SOD1/CMV–EGFP to the experimental group. We found no significant difference between the treated and the control groups in survival (Fig. 8A). Because the animals in the experimental group were injected at different ages, we also analyzed whether the injection age affected the survival outcome. We found no significant relationship between the injection age and the survival outcome (Fig. 8B). Given the persistent transgene expression mediated by the AAV2 vector, the absence of therapeutic efficacy is surprising. It might be caused by fewer motor neurons being transduced because overall we observed that AAV2 transduced fewer motor neurons than the RAd. Another potential problem is an insufficient expression of the shRNA to knockdown the extremely high levels of expression of mutant SOD1 in this transgenic mouse model. Indeed, when we examined the SOD1G93A expression in four AAV2/U6–shR-SOD1/CMV–EGFP-injected mice using the same immunofluorescence technique as shown in Fig. 5, we could not find a significant difference between the EGFP-positive cells and the EGFP-negative cells in their SOD1 staining intensity (data not shown). Thus, further improvement of delivering RNAi using AAV may need to focus on enhancing transduction efficiency and the RNAi efficacy.
FIG. 8.
AAV2-delivered RNAi has no effect on the disease progression in SOD1G93A mice. (A) Female SOD1G93A mice were divided into two groups: one injected with AAV2 expressing shRNA against SOD1 (n = 23) and EGFP, and the other was untreated (n = 21). The AAV2 was injected into the sciatic nerve in mice at ages from 43 days to 109 days. This group survived for 139 ± 12 days. The untreated group survived for 138 ± 12 days. (B) The correlation between the AAV2-injection age and survival was not statistically significant (p = 0.2), indicating the age of AAV2 administration did not affect survival. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
The general practicality of the nerve-injection method
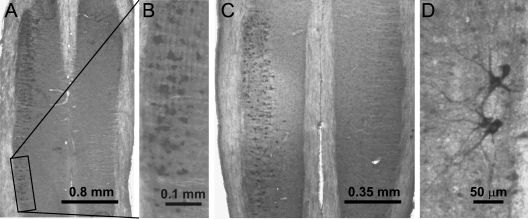
The above data establish that nerve injection is an effective method for delivering AAV and RAd to spinal cord motor neurons. Is the nerve injection practical in other nerves? Understanding this is important because knockdown of mutant SOD1 in several important motor neuron pools, including those that innervate the upper limbs, and probably most importantly, those that innervate the respiratory muscles, may further enhance the efficacy of RNAi therapy. To test this, we injected the retrograde tracer HRP-ChTxB (1) to various major nerve trunks before they branch into smaller branches. The advantages of this approach are that injection of the large nerves is relatively easy and can expose the injected agent to more motor axons so that the delivery efficiency can be enhanced. In addition to the sciatic nerve, we tested saphenous nerve, accessible from the ventral side of the upper thigh; the fore limb major nerves including musculospiral, median and ulnar nerves, easily accessible from the axilla; and the phrenic nerve, accessible from the C5 nerve roots. The phrenic nerve innervates the diaphragm and contains axons from the most important motor neuron pool that control breathing. Two to three days after the injection, we fixed the animal by perfusion and detected HRP labeling using DAB. We observed robust labeling of these motor neuron pools (Fig. 9A–D), suggesting that nerves are also accessible for injection of viral vectors.
FIG. 9.
Large nerve trunks are accessible for injections. (A) Motor neuron pool labeled by injection of HRP–ChTxB into the cervical VIII nerve. (B) Enlarged view of an area in (A). (C) Motor neuron pool labeled by injection into the saphenous nerve in lumbar spinal cord. (D) Motor neurons labeled by injection into the cervical V nerve.
Discussion
Our results demonstrate that administration of RAd and AAV2 by nerve injection efficiently delivers transgenes to spinal cord motor neurons. In addition, incorporation of RNAi-expression cassette in RAd can elicit gene silencing in these cells. When we administered RAd carrying the RNAi cassette against mutant SOD1 to the G93A transgenic mice at the disease onset, the disease progression was slowed and the survival extended. These results suggest that delivery of RNAi using gene therapy through nerve injection can be an effective therapeutic strategy.
Our study also indicates that further studies are necessary to enhance the RNAi therapeutic efficacy. The therapeutic efficacy that we have observed with RAd-RNAi vector is modest. This probably resulted from the transient expression mediated by this vector since we have observed that the expression of EGFP peaked at 1 week after the RAd administration, and thereafter, the expression declined and only few motor neurons maintained a sustained expression. Therefore, future experiments should focus on solving this problem by using the new generation of RAd vectors. The recently developed helper dependent gutless adenoviral vectors (HC-Ad), for example, have been demonstrated to have low toxicity and the capacity of maintaining a sustained high level expression of transgenes in the CNS, even in the presence of a systemic anti-adenoviral immune response as could be encountered in patients undergoing clinical trials (4, 29, 49, 62).
AAV has been increasingly used for gene deliveries in vivo because of its low toxicity and its capability to mediate long-term transgene expression. A previous study demonstrated that AAV2 can be retrogradely transported and mediate transgene expression in motor neurons after its injection into muscles (26). We compared nerve injection with muscle injection of AAV2 and found that nerve injection was more efficient in transduction motor neurons in the spinal cord. Although the number of motor neurons transduced by AAV2 and the intensity of EGFP expression appeared somewhat lower than in motor neurons transduced with RAd, AAV2-mediated transgene expression was sustained for a long period of time. Unfortunately, AAV2-RNAi vector administered by nerve injection did not result in an observable knockdown and therapeutic benefit. This could be due to the fewer transduced motor neurons and lower levels of shRNA expression compared with the RAd–RNAi vector. Thus, future studies needs to focus on further enhancing the efficiency of transducing motor neurons and silencing the target gene. This may be achieved by using AAV serotypes that have higher affinity to motor neurons and by optimizing the shRNA or miRNA sequence for target gene silencing. For example, AAV serotypes 1 and 8 have much higher transduction efficiency in the CNS than rAAV2 (22, 48, 53). These serotypes may be combined with the self-complementary AAV (scAAV) technique to further enhance the neuronal transduction efficiency (22). For the eventual human application, further advantages may be gained by using nonhuman AAV serotypes or in vitro evolved AAV vectors, which can avoid pre-existing immunity against the human AAVs and have optimized neuronal affinity (52).
Thus, much work remains to bring the viral vector-delivered RNAi therapy for ALS to the clinic. While progress will be made in this front, an alternative strategy is to deliver RNAi therapy by directly infusing synthetic siRNA into the CNS. Several groups have tested this strategy using different neurodegenerative disease models, including models for Huntington disease, Parkinson disease, and ALS (15, 31, 54). These experiments have shown that the infused siRNA can knockdown the expression of the target disease genes such as mutant huntingtin, α-synuclein, and SOD1, and in some cases, display modest therapeutic efficacy. An important feature of these works is the use of chemically modified siRNA, which has enhanced stability and cell penetration properties. Compared with the delivery using viral vectors, direct siRNA infusion has the advantage of being able to control the dose of the siRNA, which enables better control of any possible adverse effects. Other advantages associated with the chemical modifications include application of special modifications that can enhance RNAi efficiency against specific disease targets, reduce the effects of the siRNA on nonspecific targets. and minimize the siRNA's immunogenic properties and interference with the endogenous miRNA pathways (56). The disadvantage of this strategy is that the patient has to carry the infusion device and the siRNA has to be administered continuously for life, adding to the cost and inconvenience.
In summary, data from our studies and from other labs have demonstrated in principle that RNAi can be delivered using viral vectors expressing shRNAs or chemically modified siRNAs to treat ALS and other neurodegenerative disorders. For ALS, nerve injection can be a highly effective way to deliver RNAi-expressing viral vectors into spinal cord motor neurons. Critical issues still to be addressed are further enhancing the delivery efficiency using improved viral vectors that have a low or no toxicity and the capability of sustaining target gene silencing for a long term. Alternatively, RNAi may be delivered using chemically modified siRNA. The critical issues in this approach are to further improve cellular uptake, to enhance RNAi efficacy, and to reduce off-target and toxic effects. With persistent efforts, clinical application of RNAi therapy is hopeful in the near future.
Acknowledgments
We thank Ms. Sili Zhou for genotyping and assessing end disease stage, Ms. Maria Scheel at Gene Transfer Vector Core at the University of Iowa for producing AAV2 vector, Dr. Beverly Davidson for advice, and members of Xu lab for discussion and suggestions. This work is supported by grants from the Robert Packard Center for ALS Research at Johns Hopkins, the ALS Association and NIH/NINDS (RO1NS048145 and R21NS053770) to ZX and MGC. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Abbreviations
AAV2, adeno-associated virus serotype 2; ALS, amyotrophic lateral sclerosis; DAB, diaminobenzidine; dsRNA, double-stranded RNA; HRP-CTB, horseradish peroxidase conjugated cholera toxin subunit B; LPS, lipopolysaccharide; miRNA, micro-RNA; pfu, plaque-forming unit; pre-miRNA, precursor miRNA; pri-miRNA, primary miRNA; Rad, recombinant adenovirus; RCA, replication-competent adenovirus; RISC, RNA-induced silencing complex; RLC, RISC-loading complex; RNAi, RNA interference; shRNA, short hairpin RNA; siRNA, small interfering RNA; SOD1, Cu, Zn superoxide dismutase; SOD1G93A, Cu, Zn superoxide dismutase with G to A mutation at the 93rd codon; TDP-43, TAR DNA binding protein 43 KD; VAPB, VAMP-associated protein B.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Alisky JM. van de Wetering CI. Davidson BL. Widespread dispersal of cholera toxin subunit b to brain and spinal cord neurons following systemic delivery. Exp Neurol. 2002;178:139–146. doi: 10.1006/exnr.2002.8031. [DOI] [PubMed] [Google Scholar]
- 2.Arai T. Hasegawa M. Akiyama H. Ikeda K. Nonaka T. Mori H. Mann D. Tsuchiya K. Yoshida M. Hashizume Y. Oda T. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 3.Azzouz M. Ralph GS. Storkebaum E. Walmsley LE. Mitrophanous KA. Kingsman SM. Carmeliet P. Mazarakis ND. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429:413–417. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- 4.Barcia C. Jimenez–Dalmaroni M. Kroeger KM. Puntel M. Rapaport AJ. Larocque D. King GD. Johnson SA. Liu C. Xiong W. Candolfi M. Mondkar S. Ng P. Palmer D. Castro MG. Lowenstein PR. One-year expression from high-capacity adenoviral vectors in the brains of animals with pre-existing anti-adenoviral immunity: Clinical implications. Mol Ther. 2007;15:2154–2163. doi: 10.1038/sj.mt.6300305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boillee S. Van de Velde C. Cleveland DW. ALS: A disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Boillee S. Yamanaka K. Lobsiger CS. Copeland NG. Jenkins NA. Kassiotis G. Kollias G. Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- 7.Borchelt DR. Guarnieri M. Wong PC. Lee MK. Slunt HS. Xu Z–S. Sisodia SS. Price DL. Cleveland DW. Superoxide dismutase 1 subunits with mutations linked to familial amyotrophic lateral sclerosis do not affect wild-type subunit function. J Biol Chem. 1995;270:3234–3238. doi: 10.1074/jbc.270.7.3234. [DOI] [PubMed] [Google Scholar]
- 8.Borchelt DR. Lee MK. Slunt HS. Guarnieri M. Xu ZS. Wong PC. Brown RHJ. Price DL. Sisodia SS. Cleveland DW. Superoxide dismutase 1 with mutations linked to familial amyotrophic lateral sclerosis possesses significant activity. Proc Natl Acad Sci USA. 1994;91:8292–8296. doi: 10.1073/pnas.91.17.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borchert GM. Lanier W. Davidson BL. RNA polymerase III transcribes human microRNAs. Nat Struct Mol Biol. 2006;13:1097–1101. doi: 10.1038/nsmb1167. [DOI] [PubMed] [Google Scholar]
- 10.Bruijn LI. Houseweart MK. Kato S. Anderson KL. Anderson SD. Ohama E. Reaume AG. Scott RW. Cleveland DW. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild type SOD1. Science. 1998;281:1851–4. doi: 10.1126/science.281.5384.1851. [DOI] [PubMed] [Google Scholar]
- 11.Caplen NJ. Parrish S. Imani F. Fire A. Morgan RA. Specific inhibition of gene expression by small double-stranded RNAs in invertebrate and vertebrate systems. Proc Natl Acad Sci USA. 2001;98:9742–9847. doi: 10.1073/pnas.171251798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dal Canto MC. Gurney ME. Neuropathological changes in two lines of mice carrying a transgene for mutant human Cu, Zn SOD, and in mice overexpressing wild type human SOD: A model of familial amyotrophic lateral sclerosis (FALS) Brain Res. 1995;676:25–40. doi: 10.1016/0006-8993(95)00063-v. [DOI] [PubMed] [Google Scholar]
- 13.Davidson BL. Boudreau RL. RNA interference: A tool for querying nervous system function and an emerging therapy. Neuron. 2007;53:781–788. doi: 10.1016/j.neuron.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 14.Deng H-X. Shi Y. Furukawa Y. Zhai H. Fu R. Liu E. Gorrie GH. Khan MS. Hung W-Y. Bigio EH. Lukas T. Dal Canto MC. O'Halloran TV. Siddique T. Conversion to the amyotrophic lateral sclerosis phenotype is associated with intermolecular linked insoluble aggregates of SOD1 in mitochondria. Proc Natl Acad Sci USA. 2006;103:7142–7147. doi: 10.1073/pnas.0602046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiFiglia M. Sena–Esteves M. Chase K. Sapp E. Pfister E. Sass M. Yoder J. Reeves P. Pandey RK. Rajeev KG. Manoharan M. Sah DWY. Zamore PD. Aronin N. Therapeutic silencing of mutant huntingtin with siRNA attenuates striatal and cortical neuropathology and behavioral deficits. Proc Natl Acad Sci USA. 2007;104:17204–17209. doi: 10.1073/pnas.0708285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding H. Schwarz DS. Keene A. Affar el B. Fenton L. Xia X. Shi Y. Zamore PD. Xu Z. Selective silencing by RNAi of a dominant allele that causes amyotrophic lateral sclerosis. Aging Cell. 2003;2:209–217. doi: 10.1046/j.1474-9728.2003.00054.x. [DOI] [PubMed] [Google Scholar]
- 17.Elbashir SM. Harborth J. Lendeckel W. Yalcin A. Weber K. Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 18.Gil J. Esteban M. Induction of apoptosis by the dsRNA-dependent protein kinase (PKR): mechanism of action. Apoptosis. 2000;5:107–114. doi: 10.1023/a:1009664109241. [DOI] [PubMed] [Google Scholar]
- 19.Gurney ME. Pu H. Chiu AY. Dal Canto MC. Polchow CY. Alexander DD. Caliendo J. Hentati A. Kwon YW. Deng H-X. Chen W. Zhai P. Sufit RL. Siddique T. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 20.Harper SQ. Staber PD. He X. Eliason SL. Martins IH. Mao Q. Yang L. Kotin RM. Paulson HL. Davidson BL. RNA interference improves motor and neuropathological abnormalities in a Huntington's disease mouse model. Proc Natl Acad Sci USA. 2005;102:5820–5825. doi: 10.1073/pnas.0501507102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins CM. Jung C. Xu Z. ALS-associated mutant SOD1G93A causes mitochondrial vacuolation by expansion of the intermembrane space and by involvement of SOD1 aggregation and peroxisomes. BMC Neurosci. 2003;4:16. doi: 10.1186/1471-2202-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollis ER., 2nd Kadoya K. Hirsch M. Samulski RJ. Tuszynski MH. Efficient retrograde neuronal transduction utilizing self-complementary AAV1. Mol Ther. 2008;16:296–301. doi: 10.1038/sj.mt.6300367. [DOI] [PubMed] [Google Scholar]
- 23.Jaarsma D. Haasdijk ED. Grashorn JAC. Hawkins R. van Duijn W. Verspaget HW. London J. Holstege JC. Human Cu/Zn superoxide dismutase (SOD1) overexpression in mice causes mitochondrial vacuolization, axonal degeneration, and premature motoneuron death and accelerates motoneuron disease in mice expressing a familial amyotrophic lateral sclerosis mutant SOD1. Neurobiol Dis. 2000;7:623–643. doi: 10.1006/nbdi.2000.0299. [DOI] [PubMed] [Google Scholar]
- 24.Jonsson PA. Graffmo KS. Andersen PM. Brannstrom T. Lindberg M. Oliveberg M. Marklund SL. Disulphide-reduced superoxide dismutase-1 in CNS of transgenic amyotrophic lateral sclerosis models. Brain. 2006;129:451–464. doi: 10.1093/brain/awh704. [DOI] [PubMed] [Google Scholar]
- 25.Kabashi E. Valdmanis PN. Dion P. Spiegelman D. McConkey BJ. Velde CV. Bouchard J-P. Lacomblez L. Pochigaeva K. Salachas F. Pradat P-F. Camu W. Meininger V. Dupre N. Rouleau GA. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet. 2008;40:572–574. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- 26.Kaspar BK. Llado J. Sherkat N. Rothstein JD. Gage FH. Retrograde viral delivery of IGF-1 prolongs sSurvival in a mouse ALS model. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- 27.Kim VN. MicroRNA biogenesis: Coordinated cropping and dicing. Nat Rev Mol Cell Biol. 2005;6:376–385. doi: 10.1038/nrm1644. [DOI] [PubMed] [Google Scholar]
- 28.Kimmelman J. Recent developments in gene transfer: risk and ethics. BMJ. 2005;330:79–82. doi: 10.1136/bmj.330.7482.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King GD. Muhammad AKMG. Xiong W. Kroeger KM. Puntel M. Larocque D. Palmer D. Ng P. Lowenstein PR. Castro MG. High-capacity adenovirus vector-mediated anti-glioma gene therapy in the presence of systemic antiadenovirus immunity. J Virol. 2008;82:4680–4684. doi: 10.1128/JVI.00232-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong J. Xu Z. Peripheral axotomy slows motoneuron degeneration in a transgenic mouse line expressing mutant SOD1 G93A. J Comp Neurol. 1999;412:373–380. doi: 10.1002/(sici)1096-9861(19990920)412:2<373::aid-cne13>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 31.Lewis J. Melrose H. Bumcrot D. Hope A. Zehr C. Lincoln S. Braithwaite A. He Z. Ogholikhan S. Hinkle K. Kent C. Toudjarska I. Charisse K. Braich R. Pandey RK. Heckman M. Maraganore DM. Crook J. Farrer MJ. In vivo silencing of alpha-synuclein using naked siRNA. Mol Neurodegener. 2008;3:19. doi: 10.1186/1750-1326-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowenstein PR. Immunology of viral-vector-mediated gene transfer into the brain: An evolutionary and developmental perspective. Trends Immunol. 2002;23:23–30. doi: 10.1016/s1471-4906(01)02063-4. [DOI] [PubMed] [Google Scholar]
- 33.Lowenstein PR. Mandel RJ. Xiong WD. Kroeger K. Castro MG. Immune responses to adenovirus and adeno-associated vectors used for gene therapy of brain diseases: the role of immunological synapses in understanding the cell biology of neuroimmune interactions. Curr Gene Ther. 2007;7:347–660. doi: 10.2174/156652307782151498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manabe Y. Nagano I. Gazi MS. Murakami T. Shiote M. Shoji M. Kitagawa H. Setoguchi Y. Abe K. Adenovirus-mediated gene transfer of glial cell line-derived neurotrophic factor prevents motor neuron loss of transgenic model mice for amyotrophic lateral sclerosis. Apoptosis. 2002;7:329–334. doi: 10.1023/a:1016123413038. [DOI] [PubMed] [Google Scholar]
- 35.Mello CC. Conte D. Revealing the world of RNA interference. Nature. 2004;431:338–342. doi: 10.1038/nature02872. [DOI] [PubMed] [Google Scholar]
- 36.Neumann M. Sampathu DM. Kwong LK. Truax AC. Micsenyi MC. Chou TT. Bruce J. Schuck T. Grossman M. Clark CM. McCluskey LF. Miller BL. Masliah E. Mackenzie IR. Feldman H. Feiden W. Kretzschmar HA. Trojanowski JQ. Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 37.Pasinelli P. Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci. 2006;7:710–723. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- 38.Ralph GS. Radcliffe PA. Day DM. Carthy JM. Leroux MA. Lee DCP. Wong L-F. Bilsland LG. Greensmith L. Kingsman SM. Mitrophanous KA. Mazarakis ND. Azzouz M. Silencing mutant SOD1 using RNAi protects against neurodegeneration and extends survival in an ALS model. Nat Med. 2005;11:429–433. doi: 10.1038/nm1205. [DOI] [PubMed] [Google Scholar]
- 39.Rana TM. Illuminating the silence: Understanding the structure and function of small RNAs. Nat Rev Mol Cell Biol. 2007;8:23–36. doi: 10.1038/nrm2085. [DOI] [PubMed] [Google Scholar]
- 40.Raoul C. Abbas–Terki T. Bensadoun J–C. Guillot S. Haase G. Szulc J. Henderson CE. Aebischer P. Lentiviral-mediated silencing of SOD1 through RNA interference retards disease onset and progression in a mouse model of ALS. Nat Med. 2005;11:423–428. doi: 10.1038/nm1207. [DOI] [PubMed] [Google Scholar]
- 41.Reaume AG. Elliott JL. Hoffman EK. Kowall NW. Ferrante RJ. Siwek DF. Wilcox HM. Flood DG. Beal MF. Brown RH., Jr. Scott RW. Snider WD. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat Genet. 1996;13:43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- 42.Segura MM. Alba R. Bosch A. Chillon M. Advances in helper-dependent adenoviral vector research. Curr Gene Ther. 2008;8:222–235. doi: 10.2174/156652308785160647. [DOI] [PubMed] [Google Scholar]
- 43.Semizarov D. Frost L. Sarthy A. Kroeger P. Halbert DN. Fesik SW. Specificity of short interfering RNA determined through gene expression signatures. Proc Natl Acad Sci USA. 2003;100:6347–6352. doi: 10.1073/pnas.1131959100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi Y. Mammalian RNAi for the masses. Trends Genet. 2003;19:9–12. doi: 10.1016/s0168-9525(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 45.Singer O. Marr RA. Rockenstein E. Crews L. Coufal NG. Gage FH. Verma IM. Masliah E. Targeting BACE1 with siRNAs ameliorates Alzheimer disease neuropathology in a transgenic model. Nat Neurosci. 2005;8:1343–1349. doi: 10.1038/nn1531. [DOI] [PubMed] [Google Scholar]
- 46.Southgate T. Kroeger KM. Liu C. Lowenstein PR. Castro MG. Gene transfer into neural cells in vitro using adenoviral vectors. Curr Protoc Neurosci. 2008;Chapter 4(Unit 4):23. doi: 10.1002/0471142301.ns0423s45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sreedharan J. Blair IP. Tripathi VB. Hu X. Vance C. Rogelj B. Ackerley S. Durnall JC. Williams KL. Buratti E. Baralle F. de Belleroche J. Mitchell JD. Leigh PN. Al-Chalabi A. Miller CC. Nicholson G. Shaw CE. TDP-43 Mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Storek B. Harder NM. Banck MS. Wang C. McCarty DM. Janssen WG. Morrison JH. Walsh CE. Beutler AS. Intrathecal long-term gene expression by self-complementary adeno-associated virus type 1 suitable for chronic pain studies in rats. Mol Pain. 2006;2:4. doi: 10.1186/1744-8069-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas CE. Schiedner G. Kochanek S. Castro MG. Lowenstein PR. Preexisting antiadenoviral immunity is not a barrier to efficient and stable transduction of the brain, mediated by novel high-capacity adenovirus vectors. Hum Gen Ther. 2001;12:839–846. doi: 10.1089/104303401750148829. [DOI] [PubMed] [Google Scholar]
- 50.Tomari Y. Zamore PD. Perspective: Machines for RNAi. Genes Dev. 2005;19:517–529. doi: 10.1101/gad.1284105. [DOI] [PubMed] [Google Scholar]
- 51.Urabe M. Ding C. Kotin RM. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum Gen Ther. 2002;13:1935–1943. doi: 10.1089/10430340260355347. [DOI] [PubMed] [Google Scholar]
- 52.Vandenberghe LH. Wilson JM. Gao G. Tailoring the AAV vector capsid for gene therapy. Gene Ther. 2009;16:311–319. doi: 10.1038/gt.2008.170. [DOI] [PubMed] [Google Scholar]
- 53.Wang C. Wang CM. Clark KR. Sferra TJ. Recombinant AAV serotype 1 transduction efficiency and tropism in the murine brain. Gene Ther. 2003;10:1528–1534. doi: 10.1038/sj.gt.3302011. [DOI] [PubMed] [Google Scholar]
- 54.Wang H. Ghosh A. Baigude H. Yang C–S. Qiu L. Xia X. Zhou H. Rana TM. Xu Z. Therapeutic gene silencing delivered by a chemically modified small interfering RNA against mutant SOD1 slows amyotrophic lateral sclerosis progression. J Biol Chem. 2008;283:15845–15852. doi: 10.1074/jbc.M800834200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J. Xu G. Gonzales V. Coonfield M. Fromholt D. Copeland NG. Jenkins NA. Borchelt DR. Fibrillar inclusions and motor neuron degeneration in transgenic mice expressing superoxide dismutase 1 with a disrupted copper-binding site. Neurobiol Dis. 2002;10:128–138. doi: 10.1006/nbdi.2002.0498. [DOI] [PubMed] [Google Scholar]
- 56.Watts JK. Deleavey GF. Damha MJ. Chemically modified siRNA: Tools and applications. Drug Discov Today. 2008;13:842–855. doi: 10.1016/j.drudis.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 57.Wong PC. Pardo CA. Borchelt DR. Lee MK. Copeland NG. Jenkins NA. Sisodia SS. Cleveland DW. Price DL. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- 58.Xia H. Mao Q. Eliason SL. Harper SQ. Martins IH. Orr HT. Paulson HL. Yang L. Kotin RM. Davidson BL. RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat Med. 2004;10:816–820. doi: 10.1038/nm1076. [DOI] [PubMed] [Google Scholar]
- 59.Xia X. Zhou H. Huang Y. Xu Z. Allele-specific RNAi selectively silences mutant SOD1 and achieves significant therapeutic benefit in vivo. Neurobiol Dis. 2006;23:578–586. doi: 10.1016/j.nbd.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 60.Xia XG. Zhou H. Zhou S. Yu Y. Wu R. Xu Z. An RNAi strategy for treatment of amyotrophic lateral sclerosis caused by mutant Cu,Zn superoxide dismutase. J Neurochem. 2005;92:362–367. doi: 10.1111/j.1471-4159.2004.02860.x. [DOI] [PubMed] [Google Scholar]
- 61.Xiao X. Li J. Samulski RJ. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiong W. Goverdhana S. Sciascia SA. Candolfi M. Zirger JM. Barcia C. Curtin JF. King GD. Jaita G. Liu C. Kroeger K. Agadjanian H. Medina–Kauwe L. Palmer D. Ng P. Lowenstein PR. Castro MG. Regulatable gutless adenovirus vectors sustain inducible transgene expression in the brain in the presence of an immune response against adenoviruses. J Virol. 2006;80:27–37. doi: 10.1128/JVI.80.1.27-37.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou H. Xia XG. Xu Z. An RNA polymerase II construct synthesizes short-hairpin RNA with a quantitative indicator and mediates highly efficient RNAi. Nucl Acids Res. 2005;33:e62. doi: 10.1093/nar/gni061. [DOI] [PMC free article] [PubMed] [Google Scholar]