Abstract
Blood vessels respond to changes in mechanical load from circulating blood in the form of shear stress and mechanical strain as the result of heart propulsions by changes in intracellular signaling leading to changes in vascular tone, production of vasoactive molecules, and changes in vascular permeability, gene regulation, and vascular remodeling. In addition to hemodynamic forces, microvasculature in the lung is also exposed to stretch resulting from respiratory cycles during autonomous breathing or mechanical ventilation. Among various cell signaling pathways induced by mechanical forces and reported to date, a role of reactive oxygen species (ROS) produced by vascular cells receives increasing attention. ROS play an essential role in signal transduction and physiologic regulation of vascular function. However, in the settings of chronic hypertension, inflammation, or acute injury, ROS may trigger signaling events that further exacerbate smooth muscle hypercontractility and vascular remodeling associated with hypertension and endothelial barrier dysfunction associated with acute lung injury and pulmonary edema. These conditions are also characterized by altered patterns of mechanical stimulation experienced by vasculature. This review will discuss signaling pathways regulated by ROS and mechanical stretch in the pulmonary and systemic vasculature and will summarize functional interactions between cyclic stretch- and ROS-induced signaling in mechanochemical regulation of vascular structure and function. Antioxid. Redox Signal. 11, 1651–1667.
Introduction
Blood vessels are permanently exposed to hemodynamic forces in the form of shear stress and circumferential mechanical strain, which act on the vascular wall and play an important role in the regulation of vascular structure, myogenic tone, and functional responses to vasoactive agonists. Vascular cells can sense changes in mechanical forces and transduce the mechanical signal into a biological response (mechanotransduction). However, the mechanisms of mechanotransduction remain to be elucidated. Several mechanisms that have been proposed to be involved in mechano-sensation include stretch-activated ion channels, integrins, cytoskeletal meshwork (tensegrity model), and receptor tyrosine kinases (45, 64, 66, 73, 80, 120, 142). A role of signaling by reactive oxygen species (ROS), although less investigated, clearly attracts increasing attention (7, 123, 137, 167). Recent reports indicate that altered levels of shear stress or changes in cyclic stretch induce profound changes in ROS levels that may trigger vascular contraction in chronic hypertension (123, 152), induce vascular barrier dysfunction in pulmonary circulation associated with ventilator-induced lung injury (1, 34, 88), or stimulate vascular remodeling or angiogenesis via ROS-mediated transcriptional activation of growth factors, extracellular matrix proteins, matrix metalloproteinases, or bioactive peptides (60, 62, 103). Thus, stretch-induced regulation of ROS production and synergistic effects of mechanical and chemical stimuli on ROS generation under pathologic and physiologic conditions represent a highly promising area of research directed at the understanding of molecular mechanisms of vascular remodeling and barrier regulation. These studies may result in the development of novel therapeutic approaches in the treatment of pathologic conditions such as arterial and pulmonary hypertension, atherosclerosis, ventilator-induced lung injury, and adult respiratory distress syndrome (ARDS).
Biology of ROS
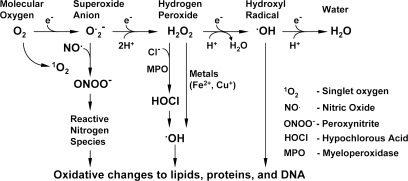
Cellular respiration in an oxygen-rich environment generates abundant derivatives of partially reduced oxygen, collectively termed reactive oxygen species (ROS). Produced by a gradual reduction of molecular oxygen, ROS include both unstable free radicals (chemical species having unpaired electrons in their outermost shell), such as the superoxide or the hydroxyl radical, and longer-lived nonfree radical oxidants, such as hydrogen peroxide, H2O2 (111), which are generally capable of oxidizing molecular targets. Since hydrogen peroxide does not possess an unpaired electron in its outer shell, it is more stable and less reactive with other tissue radicals (Fig. 1). Based on its chemical properties and mechanisms of production, H2O2 has been considered as a cellular second messenger capable of modulating both contractile and growth-promoting pathways (10). Also, H2O2 may act on neighboring cells in a paracrine fashion. As a host defense mechanism, ROS are produced by neutrophils upon their activation by bacterial material (oxidative burst), but they also may affect surrounding tissues and other cells. Thus, under basal conditions, ROS serve as an integral component of cellular signaling pathways; however, when these highly reactive metabolic products are in excess, they impose an oxidant stress on the cellular environment, leading to pathologic cell responses (111, 146). Increased production of ROS has been implicated in the pathogenesis of cardiovascular diseases such as atherosclerosis, restenosis, hypertension, diabetic vascular complications, and heart failure (29, 32, 152).
FIG. 1.
Metabolism of reactive oxygen species.
ROS Metabolism and Vascular Wall
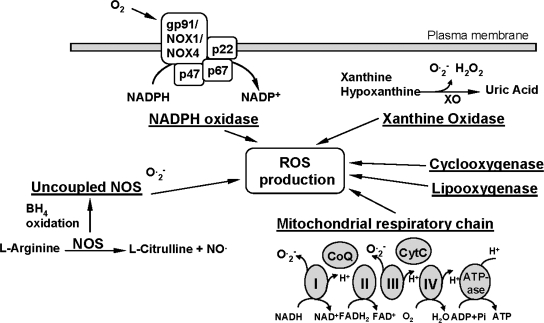
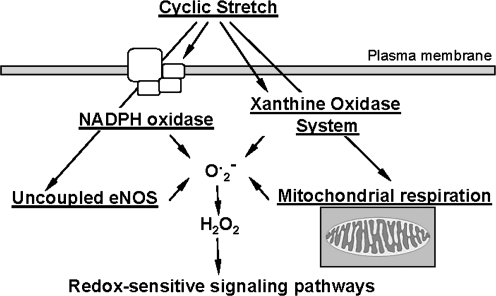
ROS are produced by all vascular cell types, including endothelial, smooth muscle, and adventitial cells, and can be generated from both metabolic and enzymatic sources (Fig. 2). These sources include mitochondrial respiration, xanthine oxidoreductase, cyclooxygenases and lipoxygenases, NADPH oxidases, and uncoupled nitric oxide synthases (32). The most relevant sources of ROS with respect to vascular disease and hypertension appear to be xanthine oxidoreductase, uncoupled endothelial NO synthase, and NADPH oxidase.
FIG. 2.
Metabolic and enzymatic sources of ROS. NOS, nitric oxide synthase; ROS, reactive oxygen species; XOR, xanthine oxidase.
NADPH oxidases
Many studies have shown that the major source of ROS in the vascular wall is nonphagocytic NADPH oxidase, which utilizes NADH/NADPH as the electron donor to reduce molecular oxygen and produce O2•−. Activation of this enzyme requires the assembly of both cytosolic (p47phox, p67phox, or homologues) and membrane bound (gp91phox/Nox1/Nox4 and p22phox) subunits to form a functional enzyme complex (47). In the vasculature the NADPH oxidase complex is at least partly pre-assembled, as a significant proportion of NADPH oxidase subunits are colocalized intracellularly in endothelial cells (61, 96). Various endogenous and external stimuli modulate the NADPH oxidase subunits expression and/or activity. Although endothelial and vascular oxidases appear to be constantly active, generating low levels of ROS, they are regulated by humoral factors as demonstrated for cytokines, growth factors, and vasoactive agents, as well as by physical factors including stretch, pulsatile strain, and shear stress (89). Interestingly, hydrogen peroxide and lipid peroxides can stimulate activity of the NADPH oxidases in vascular smooth muscle cells, leading to a feed-forward increase in ROS production in vascular wall. Expression of one or more of NADPH oxidase subunits is upregulated in HUVEC culture in response to angiotensin II, endothelin-1, oxidized low density lipoproteins, pulsatile shear stress, and phorbol esters (51, 102). They are conversely downregulated by treatment with statins, PPAR agonists, or estradiol (51, 138). Angiotensin II, TGFβ, TNFα, serum, PDGF, prostaglandin F2α, phorbol esters, and low density lipoproteins upregulated various NADPH oxidase subunits expression in the case of cultured arterial smooth muscle cells (89, 90).
Xanthine oxidase
Another source of vascular ROS is the xanthine oxidoreductase enzyme system. Xanthine oxidoreductase, also termed as xanthine oxidase (XOR) is an enzyme that catalyzes the last steps of purine metabolism: the transformation of hypoxanthine and xanthine to uric acid, with superoxide/H2O2 generated as by-products (77). There are two isoforms of XOR. The xanthine dehydrogenase activity present in vascular endothelium is readily converted into xanthine oxidase by processes including thiol oxidation and/or proteolysis. The ratio of xanthine oxidase to xanthine dehydrogenase in the cell is therefore critical to determine the amount of ROS produced by these enzymes (28, 58). Although both isoforms have ROS-generating potential, in vivo xanthine oxidase metabolizes hypoxanthine, xanthine, and NADH to form O2−· and H2O2 and appears to be an important source of ROS production in ischemia/reperfusion and hypercholesterolemia (59, 68). Analysis of xanthine oxidase in vascular wall showed xanthine oxidase immunoreactivity in human small vessel arterial endothelium, and xanthine oxidase mRNA was identified in cultured rat pulmonary arterial endothelial cells (52, 97). Moreover, measurable xanthine oxidase activity has been detected in various disease states in arteries or cultured endothelial cells. The addition of xanthine/xanthine oxidase or uric acid in cell culture modifies cell growth and proliferation (132). Thus, xanthine oxidase has the potential to be an important source of ROS production under certain pathophysiological conditions.
Nitric oxide synthase
Nitric oxide synthase (NOS), the enzyme responsible for NO generation, has three isoforms: NOS1 (the neuronal NOS), NOS2 (the inducible NOS, calcium-independent, transcriptionally regulated isoform found in macrophages), and NOS3 or eNOS (the endothelial NOS) (162, 173). Recently, all three isoforms have been identified in arteries and veins, as well as in endothelial cell culture. A reduction of NOS activity in the arterial endothelium-dependent vascular relaxation, defined as endothelial dysfunction, has been documented in atherosclerosis and hypertension (13, 30). In physiological conditions, NOS shuttles electrons from the reductase domain to the oxidase domain and catalyzes the transformation of L-arginine into L-citrulline and NO, using several cofactors. Under some conditions, NOS generates superoxide rather than NO (9, 162), a phenomenon that is known as NOS uncoupling, meaning that electrons flowing from the NOS reductase domain to the oxygenase domain are diverted to molecular oxygen rather than to L-arginine. (16). For endothelial NOS, this process can be triggered in vitro through the absence of the co-factors L-arginine and tetrahydrobiopterin (87, 162).
Mitochondrial respiratory chain
The mitochondrial respiratory chain is the main energy source for the cell. Situated in the inner mitochondrial membrane, it catalyzes electron transfer using more than 80 peptides organized in four complexes. The transfer of electrons, shuttled by coenzyme Q and cytochrome C, usually leads to the formation of ATP by the fifth complex. However, a certain amount (1–2% in vitro) of electrons leak generating superoxide (35, 55). The contribution of mitochondria to the production of ROS in vascular wall is less understood. Recent studies have implicated mitochondria as a source of ROS responsible for mediating flow-induced dilation in coronary arteries (101) and intercellular communication in vascular smooth muscle cells subjected to stretch (44). Endothelial cells lacking a functional electron transport chain lose the ability to increase oxidant signaling in response to cyclic stretch and fail to activate NFκB, yet they retain the ability to respond to other stimuli such as lipopolysaccharide (6). Mitochondria do not appear to contribute significantly to total vascular ROS production (121). This is most likely due to the relative metabolic inactivity of quiescent blood vessels. However, stretch-induced perturbation of mitochondria could then trigger release of ROS to the cytosol, thereby activating downstream effector molecules involved in the mechanotransduction signaling pathway (5, 6).
ROS and Vascular Disease
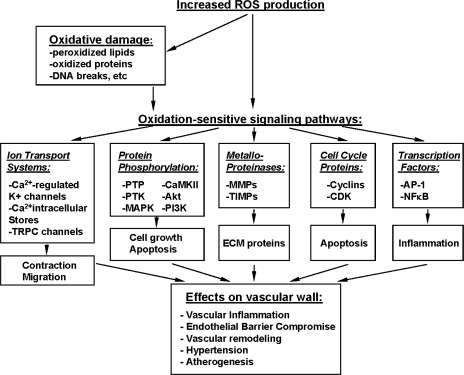
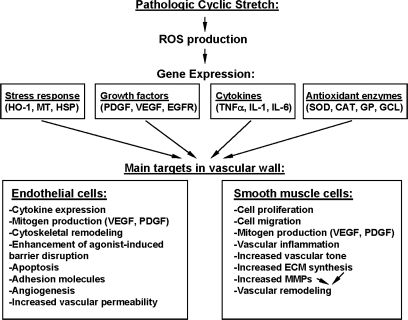
Although the sources described above are primarily responsible for ambient ROS production and basal cellular homeostatic function, when vascular disease states ensue, redox balance in the vessel wall is compromised because of increased ROS production by these sources. When this is coupled with decreased antioxidant defense, as may occur in the setting of an oxidative enzymopathy, the net result is an accumulation of superoxide anions in the vessel wall, where they are free to react and form a number of pathophysiologically relevant reactive species. These species include hydrogen and lipid peroxides, peroxynitrites and peroxynitrous acids, and hypochlorite and hypochlorous acid, which, in turn, may have deleterious consequences for vascular function. Therefore, the imbalance between prooxidant and antioxidant factors, defined as oxidative stress, can affect cellular homeostasis either through direct oxidative damage of basic cellular components (proteins, lipids, and nucleic acids) or through the activation of various redox-sensitive signaling pathways, leading to defective cellular function, aging, disease, or apoptosis (111, 118). ROS-mediated pathways leading to vascular remodeling, contractility or inflammation are summarized in Fig. 3. The presence of increased markers of oxidative stress (peroxidized lipids, oxidized proteins, increased GSSG, 8-oxoguanine, DNA breaks, etc.) has been identified in many pathophysiological situations (55). Oxidative stress can modulate vascular function through direct oxidative damage; endothelial dysfunction; decreased NO bioavailability; impaired contractility; platelet aggregation; and ROS-mediated inflammation, proliferation, and remodeling (31, 55, 107). ROS or oxidative stress involvement in cellular signaling include activation of major signaling pathways, such as MAPK, PI3K/Akt, NFκB, ERK, JNK, and p53. Recently it was shown that in vascular endothelium intracellular oxidant production mediated by NADPH oxidase is involved in TNFα-induced EC dysfunction via cadherin phosphorylation and JNK activation (115). However, the differences in the effects of oxidative stress on arterial and venous function remain to be elucidated.
FIG. 3.
Regulation of vascular structure and function by ROS.
ROS and hypertension
An increasing body of evidence supports the idea that ROS are involved in the pathogenesis of hypertension. Increased markers of oxidative stress are found in human hypertensive subjects, as well as in various animal models of hypertension (133, 141, 151). Increased NADPH oxidase and xanthine oxidase expression or activity has been observed in some experimental models of hypertension (33, 85). Treatment of these models with ROS scavengers (141), inhibitors of NADPH oxidase (18, 135), inhibitors of xanthine oxidase (110), SOD mimetics, BH4 (151) or targeted gene delivery of SOD (110), or NADPH oxidase inhibitors (50, 98) normalizes blood pressure or prevents the development of hypertension and in some cases improves vascular and renal function. Furthermore, genetic deficiency in ROS-generating enzymes protects some animals from experimental hypertension (86). In contrast, lack of antioxidant capacity causes increased hypertension in others (53, 150). The role of ROS in pathogenesis of hypertension includes both vasoconstriction and vascular hypertrophic effects. Contractile effects of ROS on vasculature are achieved via H2O2-induced influx of extracellular Ca2+ and release of intracellular Ca2+ pools, leading to activation of Ca2+-dependent myosin light chain kinase that triggers phosphorylation of myosin light chains and vascular smooth muscle contraction (22, 57, 126, 178). Rho-associated kinase (Rho-kinase) and stress-activated p38 MAP kinase is another group of redox-sensitive kinase activated by ROS, which also contribute to vasoconstriction by direct stimulation of MLC phosphorylation and by activating integrin-linked kinase and ZIP-kinase, which inactivate myosin light chain phosphatase and thus increase levels of phosphorylated MLC (54, 109, 163). Hypertrophic effects of ROS on the vasculature are associated with ROS-induced of c-Src, which in turn transactivates receptor tyrosine kinases EGF-R and PDGF-R. This process also involves activation of phosphatidyl inositol (PI)3-kinase (140). The resulting tyrosine phosphorylation leads to downstream activation of Rho kinase, MAP kinases (JNK, big MAPK or ERK5, and p38 MAP kinase) and transcription factors (2, 159, 177). ROS-induced activation of p38 MAP kinase stimulates the Akt/protein kinase B pathway, leading to cellular hypertrophy (60, 159). It is important to note that both endogenous ROS generated by NAD(P)H oxidase upon vascular smooth muscle cell stimulation by angiotensin-II and exogenously administered H2O2 does modulate ERK1/2 phosphorylation in vascular cells (27, 114, 117).
ROS and pulmonary vascular dysfunction
Adult respiratory distress syndrome (ARDS) or acute lung injury (ALI) is a common response of the lung to diverse clinical insults, including sepsis, pneumonia, trauma, aspiration, and ventilator-induced lung injury (VILI) (105). Acute lung injury (and ARDS) in its initial phases is characterized by an acute inflammatory response, with recruitment and activation of alveolar macrophages and neutrophils, which together with activated alveolar epithelial and vascular endothelial cells produce significant amounts of ROS, one of the key substances modulating the pulmonary vascular endothelial damage associated with ARDS (34, 160). Increased ROS levels further augment lung vascular leak by stimulating endothelial permeabiltiy (125, 158, 179) and exacerbate pulmonary edema and inflammation.
Increased ROS production has been also implicated in the hypoxia-induced pulmonary hypertension. Chronic hypoxia caused the generation of ROS, which is obliterated in NADPH oxidase (gp91phox) knockout mice. Furthermore, pathological changes associated with hypoxia-induced pulmonary arterial hypertension (mean right ventricular pressure, medial wall thickening of small pulmonary arteries, and right heart hypertrophy) were completely abolished in NADPH oxidase (gp91phox) knockout mice (99). Hypoxia potentiated vasoconstrictor responses of isolated intrapulmonary arteries to both 5-hydroxytryptamine (5-HT) and the thromboxane mimetic U-46619. Administration of CuZn superoxide dismutase to isolated intrapulmonary arteries significantly decreased hypoxia-induced ROS levels and reduced the hypoxia-enhanced vasoconstriction to 5-HT and U-46619 (99). Similarly, hypoxia-induced ROS production and vasoconstrictor activity of intrapulmonary arteries were markedly reduced in NADPH oxidase knockout mice. Development of pulmonary hypertension in utero is also associated with elevated production of the H2O2 and alterations in antioxidant capacity, which lead to decreased soluble guanylate cyclase expression, unpaired vasodilating response to NO, and increased vasoconstriction and vascular remodeling. Studies by Wedgwood et al. demonstrated that the addition of the ROS scavenger catalase to isolated pulmonary arteries normalized the vasodilator responses to exogenous NO (168).
Biology of Cyclic Stretch
Blood vessels are permanently exposed to mechanical stresses, and alterations in these forces are thought to be important in vascular remodeling in both physiological conditions, such as exercise training, and in pathological conditions, such as hypertension, atherosclerosis, and diabetes (4, 81, 93). The two main forces acting on the blood vessel wall are shear stress (generated by movement of blood through the vessel lumen) and stretch (determined by luminal pressure). Mechanical stretch experienced by vascular cells from systemic and pulmonary circulation is a superposition of pulsatile and tonic components. Tensile stress is imposed on vascular wall by hydrostatic pressure counteracted by tonic contraction of vascular smooth muscle cells and elastic components of extracellular matrix in the vessel wall. In addition, cyclic stretch (CS) is imposed by heart propulsions. Pulsatile distension of the arterial wall in systemic circulation normally does not exceed 10–12%, whereas various vasomotor reactions may change diameter of smaller caliber “resistance” arteries to 60% of initial diameter or more and last minutes or hours (82). Physiologic levels of cyclic stretch and intraluminal pressure are essential for the maintenance of vascular smooth muscle cell contractile phenotype (20), regulation of vascular tone, and expression of native constituents of vascular wall extracellular matrix (14). In contrast to pathologic CS, physiologic stretch also inhibits apoptosis in vascular endothelium and causes mitotic arrest of vascular smooth muscle cells (36, 69, 100). However, the role of CS in the endothelial function has not been yet characterized in detail. Our studies demonstrate magnitude-dependent effects of CS on pulmonary EC cytoskeletal remodeling and indices of EC permeability (23, 25). These data indicate an important role of cyclic stretch in regulation of mass transport across the vessel wall.
Cyclic stretch and vascular diseases
The increase in vascular wall stress associated with hypertension has been implicated in the pathogenesis of cardiovascular diseases. While physiological cyclic stretch causes cell cycle arrest in the vascular smooth muscle cells (69), chronically increased blood pressure and vascular transmural stress activates vascular cell proliferation, collagen and fibronectin synthesis which results in thickening of the vascular wall as a feature of hypertension-induced vascular remodeling (41, 71, 116). These changes can lead to increases of wall thickness and media-to-lumen ratio, and a decrease of luminal diameter. Such remodeling of the arteries would alter their compliance. The involvement of resistance vessels in these changes results in overall increased vascular resistance (83). Thus, given the role for ROS in regulation of redox-sensitive signaling leading to vascular smooth muscle hypertrophy and vascular remodeling described above, it is highly likely that stretch-mediated regulation of vascular tone and vascular remodeling may be controlled by stretch-induced ROS generation.
Cyclic stretch in pulmonary vasculature
In pulmonary circulation, pathological overdistension of the lung may induce inflammatory processes triggered by mechanical activation of macrophages, epithelial, and endothelial cells, which may cause alveolar and endothelial barrier dysfunction, vascular leak, and culminate in ventilator-induced lung injury (VILI) syndrome or pulmonary edema (49, 154). Experimental models of mechanical ventilation at high tidal volumes further confirmed activation of inflammatory events and increased vascular leak (112, 124). Direct measurements of interstitial/vascular distension in the mechanically ventilated lung are not currently available because of complexity of local distension patterns in the lung parenchyma further complicated by uneven regional lung distension observed during inflammation and lung injury. Morphometric estimations performed by Tschumperlin and Margulies (153) suggest 34–35% increases in the alveolar epithelial cell surface area if lung volume increases from 40% to 100% of total lung capacity. Similar distension is apparently experienced by terminal lung capillary endothelial cells which form tight contacts with alveolar epithelium. Studies of cellular mechanisms underlying ventilation-induced lung injury demonstrate several potential pathways leading to vascular dysfunction, including activation of interleukin-8 production (128, 165), leukocyte infiltration, or cell membrane disruption (147, 164). Established in vitro models mimicking effects of pathologic cyclic stretch and inflammatory agents on lung cells reproduced cell responses such as cytokine production and exacerbation of agonist-induced endothelial barrier dysfunction by high amplitude cyclic stretch observed in the injured lung (23, 49, 128, 165). These models are now intensively utilized in studies of pathophysiological mechanotransduction and gene expression, which will be described below.
Stretch-Activated Cellular Signaling
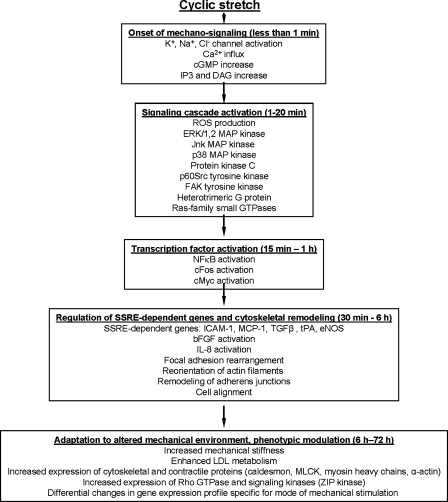
Cell membranes, cell attachment sites, and cytoskeletal network directly experience hemodynamic forces, and most likely serve as primary mechanosensors (45). Cells adhere to neighboring cells and to the extracellular matrix via transmembrane receptors of cadherin (cell-to-cell) and integrin (cell-to-substrate) families. In the cytoplasmic domain, these receptors are coupled to protein complexes that link receptors with cytoskeleton and also mediate mechanical signal transduction via activation of signaling molecules such as tyrosine (focal adhesion kinase, p60Src) kinases, serine (Erk-1,2, JNK, and p38 MAP kinases) protein kinases, inositol lipid kinases (phospholipase C), and some growth factor receptors (VEGF and PDGF receptors) (37, 41, 45, 134). Activation of mechanoreceptors triggers multiple signal cascades with ion channels (Na+ channel, K+ channel, chloride-selective channel) and heterotrimeric G proteins (Gαq) being activated within seconds of mechanical stimulation, and protein kinases (protein kinase C, MAP kinases, nonreceptor protein tyrosine kinases) activated within minutes of stimulation (reviewed in (45)). Protein kinase-mediated phosphorylation of specific cytoskeletal and cell contact proteins, other enzymes, and transcription factors induce cytoskeletal remodeling and stimulate gene expression in vascular cells. In vascular EC, cyclic stretch induced tyrosine dephosphorylation of occludin and serine/threonine phosphorylation of ZO-1 mediated by unidentified tyrosine kinases and PKCδ, respectively, and upregulated tight junction assembly associated with increased barrier integrity (43). Figure 4 summarizes major signal pathways and cellular responses induced by cyclic stretch.
FIG. 4.
Major signaling pathways and cellular responses induced by cyclic stretch.
Although the majority of putative mechanosensors and mechanotransduction pathways are stimulated by both shear stress and stretch, the nature and magnitude of mechanical forces may differentially affect certain signaling systems. Interestingly, cyclic but not steady mechanical strain activates vascular FAK, and Src and integrins are involved in steady pressure-induced FAK activation in the vessels (92). Furthermore, laminar shear stress (5–20 dynes/cm2) and low magnitude cyclic stretch (5% elongation) selectively activates small GTPase Rac that results in peripheral translocation of actin polymerization proteins and enhancement of cortical actin cytoskeleton (21, 169), whereas high magnitude cyclic stretch (18% elongation) stimulates small GTPase Rho without affecting Rac and potentiates stress fiber formation and barrier dysfunction induced by edemagenic agonists (23, 25, 129, 176). Excessive stretch also activated NFκB pathway in rat and in cell models of VILI via PI3K-dependent manner (95, 155) and exacerbated sepsis-induced NFkB activation in the isolated lung cells (95). Remarkably, selective inhibition of VILI-induced NFκB signaling is possible without inhibiting the NF-kappaB signaling activated by endotoxin. (155). Most protein kinases and ion channels involved in mechanochemical signaling exhibit amplitude-dependent activation which may explain the above described amplitude-dependent cell responses and alternatively regulated gene expression described below.
Cyclic Stretch-Regulated Gene Expression
Studies on the effects of mechanical stretch on vascular cells indicate that mechanical stretch has significant effects on the expression of genes related to vascular remodeling and cell functions such as cell proliferation, apoptosis, migration, and control of cell phenotype. Phenotypic responses of vascular cells exposed to cyclic stretch in vitro include increased expression of contractile and cytoskeletal proteins (myosin light chain kinase, smooth muscle myosin heavy chains, desmin, h-caldesmon) (24, 143, 144), and increased expression of thrombin receptor PAR1 (113) in vascular smooth muscle cells. A number of bioactive proteins regulated by cyclic stretch have been also identified in endothelial cells and macrophages and include IL-8, TGFβ, VEGF, and monocyte chemotactic protein-1 (128, 172, 181). Analysis of vascular gene expression regulated by mechanical strain reveals differential responses to physiological and pathophysiological (increased) levels of mechanical strain. For example, release of FGF-2, a growth factor involved in cellular reparation after injury, is induced in vascular smooth muscle cells stretched at 14% and 33% elongation, but not at 5% elongation (39). Significant increase in IL-8 production is observed in endothelial cells exposed to cyclic stretch at 15% elongation, whereas stretch at 6% elongation did not affect IL-8 levels. In pulmonary circulation, pathological overdistension of the lung may induce inflammatory processes triggered mechanical activation of macrophages, epithelial and endothelial cells, which may cause alveolar and endothelial barrier dysfunction, vascular leak, and culminate in pulmonary edema. Recently, microarray DNA technologies have been applied to assess time- and amplitude-dependence of cyclic stretch effects on the gene expression profile in human pulmonary endothelial cells (23). The results showed (19) that cyclic stretch at physiologically relevant and pathological amplitudes (5% and 18% elongation, respectively) induced distinct expression patterns of genes involved in signal transduction, cytoskeletal remodeling, cell adhesion, inflammatory responses, and regulation of endothelial barrier function (Table 1).
Table 1.
Selected Genes Differentially Regulated by Low (5%) and High (18%) Amplitude Cyclic Stretch
| Fold change at 5% | Fold change at 18% | |
|---|---|---|
| Signal transduction | ||
| Inducible T-cell kinase | — | 17.1 |
| Nuclear receptor subfamily 1, group D, member 2 | — | 3.2 |
| Angiopoietin 2 | 1.8 | 2.1 |
| HMG-CoA-synthase | NC | 4.0 |
| Proteinase-activated receptor 2 | 1.4 | 2.1 |
| Proteinase-activated receptor 1 | NC | −1.2 |
| Rho B GTPase | 1.87 | 2.1 |
| Rho C GTPase | NC | 1.4 |
| Cell adhesion | ||
| Gap junction protein, alpha 5 | — | 2.8 |
| CD54 | NC | 2.0 |
| Beta 3 integrin | 1.5 | 2.1 |
| Beta-catenin | NC | 2.1 |
| cadherin-13 | 8.5 | 1.7 |
| Cell–cell signaling | ||
| Placental growth factor, VEGF-related | 1.8 | 2.0 |
| Ephrin A1 | 1.2 | 2.1 |
| Ephrin B2 | 1.7 | 2.0 |
| Cytoskeleton | ||
| Smooth muscle myosin heavy chain | NC | 1.4 |
| Filamin | NC | 1.7 |
| Inflammation/remodeling | ||
| Human cyclooxygenase-2 | NC | 3.0 |
| TGF-beta superfamily protein | 1.6 | 2.3 |
| Proteinase-activated receptor 2 | 1.4 | 2.1 |
| ZIP-kinase | 1.3 | 1.7 |
Expression profiling was performed using Affymetrix GeneChip® system. Samples obtained from cells exposed to 5% or 18% cyclic stretch (48 h) were hybridized to the Affymetrix HGU95Av2 Array (∼12,000 full-length genes). Affymetrix Microarray Suite software was used to determine relative gene expression. GeneSpring and MAPPFinder software (48) were used for microarray data analysis. Results q1 represent fold increase in cDNA signal in cyclic stretch-preconditioned cells over static control. (NC, no change; — signal absent). (Adapted from ref. 19).
Recent studies indicate involvement of ROS signaling in cyclic stretch-induced gene regulation. For example, Ali et al. reported that cyclic stretch-induced increases in NFκB activation and VCAM-1 mRNA expression during strain were prevented by antioxidants (6). This study demonstrated that mitochondria may function as mechanotransducers in endothelium by increasing ROS signaling that is required for strain-induced increase in VCAM-1 expression via NFκB. Grote et al. showed that in cell culture model of arterial hypertension cyclic stretch induced mRNA MM-2 (matrix metalloproteinase) expression in a NADPH/ROS-dependent manner (62). Studies by Mata-Greenwood et al. (103) show that cyclic stretch upregulates VEGF expression via the TGFβ1-dependent activation of NADPH oxidase and increased generation of ROS.
Cyclic Stretch and Control of ROS Production
Indeed, vascular stretch is capable of stimulating release of ROS (174, 175) and activating redox-sensitive signaling pathways (67, 167). In injured vessels, vascular stretch affects both the endothelium and vascular smooth muscle. Increased production of ROS in response to cyclic mechanical stretch has been described in endothelial cells (6, 104, 170, 171), vascular smooth muscle cells (62, 74, 113), fibroblasts (8), and cardiac myocytes (4). Superoxide appears to be the initial species generated in these cell types. Several mechanisms have all been implicated as potential sources for increased superoxide production in response to mechanical stress (Fig. 5), including the NADPH oxidase system (46, 62, 104, 106), mitochondrial production (5, 6, 72), and the xanthine oxidase system (1, 106). ROS production by NO synthase has been implicated in pathogenesis of pulmonary hypertension (26). However, whether these potential sources are activated directly or indirectly by mechanical stress is unclear. Little data are available demonstrating increased ROS production by stretched alveolar or airway epithelial cells (76, 157), and the sources of increased ROS production and species generated in pulmonary epithelial and vascular cells exposed to mechanical stretch remain to be determined. However, increased ROS production in response to elevated stretch may contribute to the onset of VILI (65, 76, 167).
FIG. 5.
Mechanisms of stretch-induced ROS production.
ROS in Stretch-Induced Vascular Remodeling
Increased pressure in the vascular system is associated with cyclic or sustained stretch of vascular endothelial and smooth muscle cells. Stretch-induced ROS production was detected in vascular endothelial, smooth muscle cells, and fibroblasts (5, 8, 103). Sustained stretch of systemic vascular cells or vessels perfused and pressurized ex vivo has been attributed to arterial hypertension in vivo (14, 94), whereas high magnitude cyclic stretch of pulmonary EC relates to lung capillary strain associated with mechanical ventilation at high tidal volumes (23, 25). The vascular production of ROS is increased by chronic hypertension (86, 123, 145, 151, 156). In many cases of hypertension, either circulating or local levels of angiotensin II are increased, and this hormone can directly activate NADPH oxidase in both endothelial and vascular smooth muscle cells (131). In turn, mechanical stretch also activates NADPH oxidase (87, 103), leading to ROS production and oxidation of the NO synthase co-factor tetrahydrobiopterin (87). In the absence of tetrahydrobiopterin, eNOS becomes uncoupled such that superoxide rather than NO is formed. These events form a vicious circle of vascular contraction associated with arterial hypertension. Passive stretch of endothelium-denuded coronary vascular smooth muscle strips induces their contraction via the activation of NADPH oxidase and ERK1/2 (117). High intraluminal pressures also cause superoxide production via activation of NADPH oxidase in intact isolated vessels, an effect that is independent of the local renin–angiotensin system (156). Activation of mechanically sensitive redox signaling pathways may thus contribute to some of the maladaptive responses to altered hemodynamics in hypertension.
Stretch-induced increase in ROS production by vascular cells has been implicated in activation of NFκB, activation of matrix metalloproteases (MMPs), MAP kinase activation, angiogenesis, LDL oxidation, and altered vasomotion. Using an organ model of isolated mouse arteries, it was shown that high-pressure-induced activation of NFκB pathway is critically dependent on NADPH-mediated increased ROS production (94, 127). In cardiac myocytes, high-amplitude cyclic strain induced increase in ROS, associated with activation of JNK and Erk1/2 and induction of apoptotic phenotype (38). In vascular smooth muscle cells, cyclic stretch induced ROS production by rapid activation of NADPH oxidase, leading to activation of MAPK signaling including p38, JNK, and Erk1/2, and inhibition of NADPH or p38 prevented strain-induced cell alignment (38). These studies delineated the role of ROS-sensitive p38 activation in vascular remodeling by CS. Similar results were obtained using an organ culture model of rabbit aorta. It was shown that pulsatile stretch induces Erk1/2 activation via ROS production (91). Other studies suggested additional mechanisms and demonstrated that in cell culture model of arterial hypertension CS induced ROS production via NADPH oxidase and led to vascular remodeling via matrix metalloproteinase activation (62). In pulmonary arterial SM cells, CS contributed to vascular remodeling via increase in VEGF expression, which was mediated by CS-induced activation of NADPH oxidase and elevation of ROS production (103). Therefore, increased oxidative stress in response to stretch contributes to activation of pro-inflammatory transcription factors, activation of growth-promoting MAP kinases, upregulation of pro-fibrogenic mediators and altered vascular tone, important processes contributing to the vascular phenotype associated with hypertension (Fig. 6).
FIG. 6.
Role of ROS in cyclic stretch-induced vascular remodeling and endothelial activation.
Cyclic Stretch and ROS-Dependent Endothelial Activation
Mechanical ventilation with oxygen-enriched gas mixtures is a strategy widely employed to improve arterial oxygenation in patients with acute hypoxemic respiratory failure. However, combination of excessive ventilation and hyperoxia can damage normal lung tissue and initiate or exacerbate lung injury. Ventilation at high tidal volumes combined with hyperoxia significantly increased edema formation and neutrophil migration into the lungs (130), indicating critical changes in pulmonary vascular permeability.
In endothelium, mechanical stretch has been shown to increase ROS production leading to the upregulation of cell adhesion molecules and chemokines (40, 171). Several mechanisms of ROS production in EC have been described. Cyclic stretch stimulated ROS production via increased expression of ROS-generating enzymes: NADPH oxidase and NO synthase-3 (eNOS) (11, 12, 70). Kuebler et al. reported that circumferential stretch activates NO production in pulmonary EC via activation of PI3K, Akt, and eNOS-dependent signaling cascade (84). Using pulmonary endothelial cells exposed to high magnitude cyclic stretch in vitro and animal models of ventilation at high tidal volumes, we found that endothelial xanthine oxireductase (XOR) is also upregulated by cyclic stretch, and stretch-induced stimulation of XOR enzymatic activity was dependent on p38 and ERK1/2 MAP kinase activation. We suggested that these mechanisms also may contribute to the development of VILI (1).
Ali et al. proposed that endothelial cells detect cyclic strain by transmitting externally applied force via the cytoskeleton to the mitochondria, stimulating an increase in the release of ROS signals to the cytosol (6). Moreover, it was demonstrated that increase in oxidant signaling leads to the activation of NFκB, which then triggers subsequent gene expression. It was concluded that mitochondria function as mechanotransducers in endothelium by increasing ROS signaling, which is required for strain-induced increase in VCAM-1 expression via NFκB (6). Further studies by this group reported that mitochondrial oxidants generated in response to endothelial strain trigger FAK phosphorylation through a signaling pathway that involves PKC (5). These results suggest an interesting possibility for mitochondria being functional mechanotransducers in endothelial cells, regulating pulmonary vascular barrier function via a ROS-mediated effect on redox-sensitive signaling protein kinases. Taken together, these studies suggest an important role of ROS signaling in mechanochemical regulation of endothelial cell remodeling and pulmonary vascular permeability.
Modulation of Stretch-Induced ROS Production and Vascular Functions
Like other tissue, blood vessels experience a complex pattern of mechanical and chemical stimulations in physiological and pathological conditions. Lung vascular permeability to water and proteins is also controlled by vascular endothelial growth factor (VEGF). VEGF overexpression in the lungs or injection of purified VEGF increases endothelial permeability in vivo (79, 136). However, the role of VEGF in lung pathology is controversial. VEGF is primarily produced by type II alveolar epithelial cells and is a survival factor for the lung microvascular endothelial cells (166). In healthy human subjects, VEGF is highly compartmentalized to the lung with alveolar VEGF protein levels 500 times higher than in plasma (78). However, under conditions of stress or injury such as in ALI or VILI, because of anatomic proximity between alveolar epithelial and microvascular endothelial cells, VEGF may literally spill onto pulmonary EC, increasing permeability and leading to interstitial and pulmonary edema (78, 108). High tidal volume ventilation and cyclic stretch of vascular endothelial and smooth muscle cells in vitro also stimulates VEGF and VEGF receptor expression (63, 103, 180). It is proposed, but not tested experimentally, that mechanical forces associated with mechanical ventilation may possess synergistic effects on the VEGF-induced ROS production. Furthermore, the crosstalk between physiologically and pathologically relevant amplitudes of cyclic stretch and VEGF effects on pulmonary vascular permeability is not yet clear.
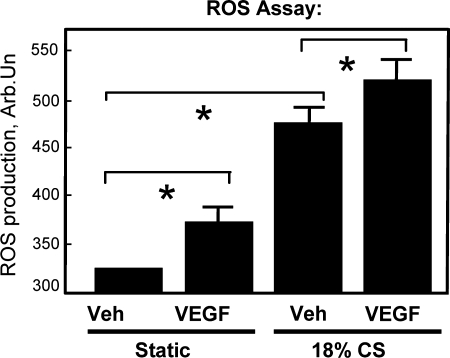
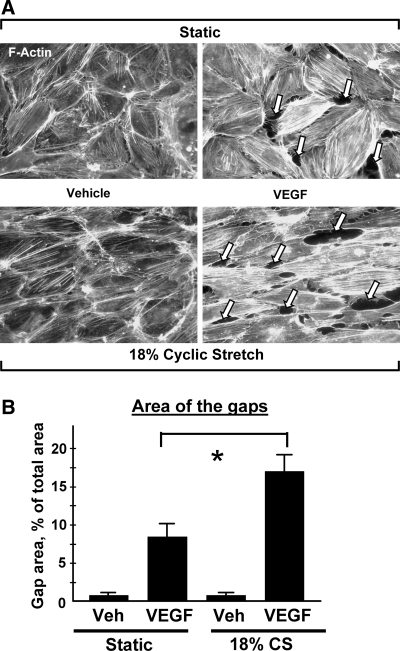
VEGF induces activation of small GTPase RhoA and its recruitment to the cell membrane in pulmonary EC (161). RhoA activation is necessary for the VEGF-induced reorganization of the F-actin cytoskeleton, EC migration (161), and increased permeability (148). VEGF may also increase vascular permeability via PI3K/Akt-dependent induction via stimulation of eNOS activity and NO production. Other reports suggest a role for Src (119), Erk-1,2 and p38 MAP kinases (15, 75), as well as in PI3K/Akt-dependent induction of eNOS activity (119, 149) in increased vascular permeability by VEGF. Cyclic stretch stimulation of pulmonary arterial smooth muscle cells contributed to vascular remodeling via increase in VEGF expression that was mediated by stretch-induced activation of NADPH oxidase and elevation of ROS production (103). Our results show that similar to smooth muscle cells, cyclic stretch stimulation of pulmonary endothelial cells promoted VEGF-induced EC barrier dysfunction in part via synergistic effects on ROS production (Figs. 7 and 8). Inhibition of ROS production by N-acetyl cysteine significantly decreased endothelial cytoskeletal remodeling and barrier dysfunction induced by VEGF and pathologic amplitudes of cyclic stretch (Fig. 9). These functional interactions between VEGF- and cyclic stretch-mediated pathways and delineation of the role of ROS production in endothelial signaling, cytoskeletal regulation, and increased pulmonary vascular permeability are in the focus of our current studies.
FIG. 7.
Synergistic effect of high magnitude cyclic stretch and VEGF on ROS production by pulmonary endothelial cells. Static controls or cells preconditioned at 18% CS were exposed to vehicle or VEGF (200 ng/ml, 15 min), and ROS production was measured using EC preincubation with fluorescent ROS sensor DCFDA followed by fluorimetric analysis. Shown are mean ± SD of three independent experiments, *p < 0.05.
FIG. 8.
Role of ROS in pulmonary endothelial barrier disruption induced by high magnitude cyclic stretch and VEGF. EC were left static or exposed to cyclic stretch at 18% linear elongation for 2 h, followed by stimulation with VEGF (200 ng/ml, 15 min). (A) Immunoflourescence staining of F-actin was performed using Texas Red conjugated phalloidin. Arrows indicate cyclic stretch- and VEGF-induced paracellular gap formation. (B) Quantitative image analysis of gap formation induced by 18% CS and VEGF. Shown are mean ± SD of four independent experiments, *p < 0.05.
FIG. 9.
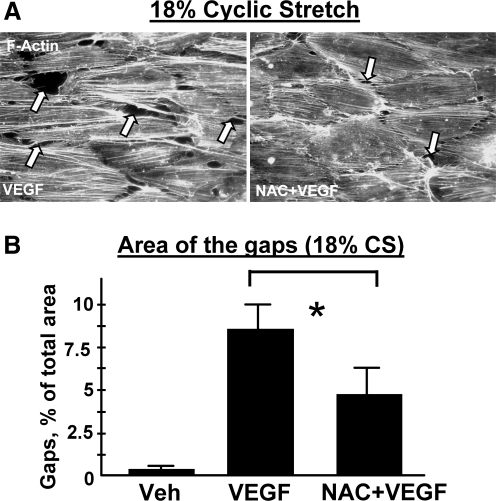
Attenuation of pulmonary endothelial barrier disruption induced by high magnitude cyclic stretch and VEGF by ROS scavenger N-acetyl cysteine. EC exposed to 18% CS were pretreated with NAC (1 mM, 30 min) prior to VEGF stimulation (200 ng/ml, 15 min). (A) Immunoflourescence staining of F-actin was performed using Texas Red conjugated phalloidin. Arrows indicate cyclic stretch- and VEGF-induced paracellular gap formation. (B) Quantitative analysis of gap formation induced by 18% CS and VEGF in untreated and NAC-pretreated cells. Shown are mean ± SD of three independent experiments, *p < 0.05.
This review summarized mechanisms of ROS production induced by cyclic stretch or agonists (angiotensin-II). However, in physiologic milieu, signaling by chemical and mechanical stimuli is highly interconnected. Thus, interactions between cyclic stretch and agonist stimulation may represent a fundamental mechanism of mechanochemical control of vascular remodeling and barrier function. For example, our previous studies have shown synergistic effect of high magnitude cyclic stretch on thrombin-induced pulmonary endothelial barrier dysfunction, which resulted from increased actomyosin contraction mediated by the Rho pathway (23, 25). In contrast, agonist-induced EC barrier dysfunction was attenuated in EC exposed to cyclic stretch at physiologic magnitudes (5% CS) (25). Because thrombin-mediated signal transduction also involves ROS-dependent mechanism (17), potentiation of thrombin-induced endothelial permeability by high magnitude cyclic stretch may share a common mechanism with stretch-VEGF signaling described above.
The other part of stretch-induced modulation of ROS signaling is feedback regulation of antioxidant systems. Some vascular genes encoding antioxidant enzymes appear to be upregulated by exercise training. For example, exercise training leading to increased shear and pressure imposed on the vessel walls increased the expression of potentially atheroprotective vascular proteins such as eNOS, extracellular superoxide dismutase (ecSOD, SOD3), and Co/Zn-SOD (SOD1) (56, 139). These enzymes convert superoxide to the less active ROS compound, hydrogen peroxide. In turn, potentially pro-oxidant and atherogenic vascular proteins such as subunits of endothelial and vascular smooth muscle NADPH oxidase and angiotensin receptor type I were downregulated by exercise training (3, 139). The mechanisms by which cyclic stretch regulates the antioxidant enzyme expression remain poorly understood. Our recent studies revealed involvement of the transcription factor Nrf2 in this process (122). The transcription factor Nrf2, via the antioxidant response element (ARE), alleviates pulmonary toxicant- and oxidant-induced oxidative stress by upregulating the expression of several antioxidant enzymes (42). Cyclic stretch exposure stimulated ARE-driven transcriptional responses and subsequent expression of antioxidant enzymes such as glutathione peroxidase 2, glutamate-cysteine ligase, heme oxygenase −1, and glutamate cysteine ligase in stretched pulmonary EC (122). We further demonstrated that cyclic stretch transactivates epithelial growth factor receptor (EGFR), and the PI3K-Akt pathway acts as the downstream effector of EGFR and regulates CS-induced ARE activation in an oxidative stress-dependent manner. These novel findings suggest that EGFR-activated signaling and actin remodeling act in concert to regulate the stretch-induced Nrf2-ARE transcriptional response and subsequent AOE expression. It is important to note that NADPH oxidase inhibitor, which inhibits the generation of ROS, N-acetyl cysteine and other flavoproteins that regulate ROS production blocked CS-induced EGFR phosphorylation, suggesting that ROS-mediated signaling regulates EGFR activation in response to cyclic stretch. These findings again reflect tight relations between cyclic stretch and ROS in mechanochemical regulation of vascular function.
Conclusions
Shear stress and tensile forces are now well-recognized factors that regulate endothelial signaling, cytoskeletal remodeling, gene expression, and physiological responses. The rapidly growing body of evidence indicates that endothelial cells discriminate between steady and cyclic, low and high amplitude mechanical strain. Moreover, the pattern of mechanical stimulation determines whether endothelial cells will develop pro- or anti-inflammatory cell responses and also may differentially regulate endothelial barrier regulation and vascular remodeling. Experimental and analytical tools are being developed to assess the stress distribution throughout cell structures that might be involved in mechanotransduction. Studies by several groups suggest that in acute settings agonist-induced ROS production may be further enhanced by cyclic stretch. These synergistic effects may exacerbate pathologic reactions in the vasculature, for example, vascular leak during mechanical lung ventilation with ongoing oxidative stress caused by neutrophil activation or inflammatory cytokine production. Another potential situation is the pulmonary hypertension with elevated angiotensin II levels, where increased luminal pressure may further potentiate ROS production and vascular remodeling triggered by angiotensin II. Redox balance in the pulmonary circulation is even more delicate in clinical settings of mechanical ventilation with oxygen-rich gas formulations. Recent studies suggest direct involvement of ROS in stretch-induced secretion of angiogenic factors and vascular remodeling illustrated by a loop where cyclic stretch stimulates NADPH-dependent ROS production essential for increased VEGF synthesis. In turn, VEGF itself triggers ROS signaling and remodeling in vascular cells. Finally, synergistic effects of VEGF and high magnitude stretch may further promote endothelial dysfunction and vascular remodeling associated with hemodynamic perturbations and acute vascular injury conditions. However, stretch- and agonist-induced oxidative stress in the vasculature appears to be counterbalanced by upregulation of antioxidant enzymes via negative feedback signaling loops, and involvement of Nrf2 in stretch-induced antioxidant enzyme transcriptional regulation appears to be a plausible mechanism. A challenging task of future studies will be to address a role of specific patterns of mechanical forces experienced by vasculature in physiological and pathological conditions (acute injury, inflammation, hypertension, ventilator-induced lung injury), delineate synergistic mechanisms of mechanical and chemical stimulation in the redox regulation of vascular function, and will identify key cellular targets for drug design and gene therapy.
Abbreviations
ARDS, adult respiratory distress syndrome; bFGF, fibroblast growth factor-b; CaMKII, Ca2+/calmodulin-dependent protein kinase; CS, cyclic stretch; CDK, cyclin dependent kinase; CAT, catalase; EC, endothelial cells; ECM, extracellular matrix; EGFR, epithelial growth factor receptor; eNOS, endothelial NO synthase; ERK1/2, extracellular signal regulated kinase 1/2; FAK, focal adhesion kinase; GCL, glutamate cysteine ligase; GP, glutathione peroxidase; HO-1, heme oxygenase-1; HSP, heat shock proteins; ICAM-1, intracellular adhesion molecule-1; IL-8, interleukin-8; Jnk, Jun N-terminal kinase; LDL, low density lipoproteins; LPS, bacterial wall lipopolysacharide; MAPK, mitogen activated protein kinases; MCP-1, monocyte chemoattractant protein-1; MLC, regulatory myosin light chains; MLCK, myosin light chain kinase; MMP, matrix metalloproteinases; NFκB, nuclear factor-kappaB; NOS, nitric oxide synthase; Nrf2, nuclear factor E2-related factor; PDGF, platelet derived growth factor; PI3-kinase, 1-phosphatidylinositol 3-kinase; PKC, protein kinase C; PTK, protein tyrosine kinase; PTP, protein tyrosine phosphatase; ROS, reactive oxygen species; SOD, superoxide dismutase; SSRE, shear stress response element; TGFβ, transforming growth factor β; TIMP, tissue inhibitor of matrix metalloproteinases; TNFα, tissue necrosis factor α; tPA, tissue plasminogen activator; VEGF, vascular endothelial growth factor; VILI, ventilator induced lung injury; XOR, xanthine oxidoreductase.
Acknowledgments
The author thanks Dr. Anna Birukova for invaluable comments in preparing the manuscript and for superior assistance in figure preparation. This study was supported by National Institutes of Health NHLBI grants HL075349 and HL076259.
References
- 1.Abdulnour RE. Peng X. Finigan JH. Han EJ. Hasan EJ. Birukov KG. Reddy SP. Watkins JE., 3rd Kayyali US. Garcia JG. Tuder RM. Hassoun PM. Mechanical stress activates xanthine oxidoreductase through MAP kinase-dependent pathways. Am J Physiol Lung Cell Mol Physiol. 2006;291:L345–353. doi: 10.1152/ajplung.00453.2005. [DOI] [PubMed] [Google Scholar]
- 2.Abe J. Kusuhara M. Ulevitch RJ. Berk BC. Lee JD. Big mitogen-activated protein kinase 1 (BMK1) is a redox-sensitive kinase. J Biol Chem. 1996;271:16586–16590. doi: 10.1074/jbc.271.28.16586. [DOI] [PubMed] [Google Scholar]
- 3.Adams V. Linke A. Krankel N. Erbs S. Gielen S. Mobius-Winkler S. Gummert JF. Mohr FW. Schuler G. Hambrecht R. Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation. 2005;111:555–562. doi: 10.1161/01.CIR.0000154560.88933.7E. [DOI] [PubMed] [Google Scholar]
- 4.Aikawa R. Nagai T. Tanaka M. Zou Y. Ishihara T. Takano H. Hasegawa H. Akazawa H. Mizukami M. Nagai R. Komuro I. Reactive oxygen species in mechanical stress-induced cardiac hypertrophy. Biochem Biophys Res Commun. 2001;289:901–907. doi: 10.1006/bbrc.2001.6068. [DOI] [PubMed] [Google Scholar]
- 5.Ali MH. Mungai PT. Schumacker PT. Stretch-induced phosphorylation of focal adhesion kinase in endothelial cells: Role of mitochondrial oxidants. Am J Physiol Lung Cell Mol Physiol. 2006;291:L38–45. doi: 10.1152/ajplung.00287.2004. [DOI] [PubMed] [Google Scholar]
- 6.Ali MH. Pearlstein DP. Mathieu CE. Schumacker PT. Mitochondrial requirement for endothelial responses to cyclic strain: Implications for mechanotransduction. Am J Physiol Lung Cell Mol Physiol. 2004;287:L486–496. doi: 10.1152/ajplung.00389.2003. [DOI] [PubMed] [Google Scholar]
- 7.Ali MH. Schumacker PT. Endothelial responses to mechanical stress: Where is the mechanosensor? Crit Care Med. 2002;30:S198–206. doi: 10.1097/00003246-200205001-00005. [DOI] [PubMed] [Google Scholar]
- 8.Amma H. Naruse K. Ishiguro N. Sokabe M. Involvement of reactive oxygen species in cyclic stretch-induced NF-kappaB activation in human fibroblast cells. Br J Pharmacol. 2005;145:364–373. doi: 10.1038/sj.bjp.0706182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrew PJ. Mayer B. Enzymatic function of nitric oxide synthases. Cardiovasc Res. 1999;43:521–531. doi: 10.1016/s0008-6363(99)00115-7. [DOI] [PubMed] [Google Scholar]
- 10.Ardanaz N. Pagano PJ. Hydrogen peroxide as a paracrine vascular mediator: regulation and signaling leading to dysfunction. Exp Biol Med (Maywood) 2006;231:237–251. doi: 10.1177/153537020623100302. [DOI] [PubMed] [Google Scholar]
- 11.Awolesi MA. Sessa WC. Sumpio BE. Cyclic strain upregulates nitric oxide synthase in cultured bovine aortic endothelial cells. J Clin Invest. 1995;96:1449–1454. doi: 10.1172/JCI118181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babior BM. The NADPH oxidase of endothelial cells. IUBMB Life. 2000;50:267–269. doi: 10.1080/713803730. [DOI] [PubMed] [Google Scholar]
- 13.Bachetti T. Comini L. Curello S. Bastianon D. Palmieri M. Bresciani G. Callea F. Ferrari R. Co-expression and modulation of neuronal and endothelial nitric oxide synthase in human endothelial cells. J Mol Cell Cardiol. 2004;37:939–945. doi: 10.1016/j.yjmcc.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Bardy N. Merval R. Benessiano J. Samuel JL. Tedgui A. Pressure and angiotensin II synergistically induce aortic fibronectin expression in organ culture model of rabbit aorta. Evidence for a pressure-induced tissue renin-angiotensin system. Circ Res. 1996;79:70–78. doi: 10.1161/01.res.79.1.70. [DOI] [PubMed] [Google Scholar]
- 15.Becker PM. Verin AD. Booth MA. Liu F. Birukova A. Garcia JG. Differential regulation of diverse physiological responses to VEGF in pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1500–1511. doi: 10.1152/ajplung.2001.281.6.L1500. [DOI] [PubMed] [Google Scholar]
- 16.Beckman JS. Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am J Physiol. 1996;271:C1424–1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 17.BelAiba RS. Djordjevic T. Petry A. Diemer K. Bonello S. Banfi B. Hess J. Pogrebniak A. Bickel C. Gorlach A. NOX5 variants are functionally active in endothelial cells. Free Radic Biol Med. 2007;42:446–459. doi: 10.1016/j.freeradbiomed.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 18.Beswick RA. Dorrance AM. Leite R. Webb RC. NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension. 2001;38:1107–1111. doi: 10.1161/hy1101.093423. [DOI] [PubMed] [Google Scholar]
- 19.Birukov KG. Regulation of vascular endothelial cell signal transduction and phenotype by mechanical factors. In: Shepro D, editor. Microvascular Research: Biology and Pathology. San Diego, CA: Academic Press; 2005. pp. 209–215. Editor-in-Chief. [Google Scholar]
- 20.Birukov KG. Bardy N. Lehoux S. Merval R. Shirinsky VP. Tedgui A. Intraluminal pressure is essential for the maintenance of smooth muscle caldesmon and filamin content in aortic organ culture. Arterioscler Thromb Vasc Biol. 1998;18:922–927. doi: 10.1161/01.atv.18.6.922. [DOI] [PubMed] [Google Scholar]
- 21.Birukov KG. Birukova AA. Dudek SM. Verin AD. Crow MT. Zhan X. DePaola N. Garcia JG. Shear stress-mediated cytoskeletal remodeling and cortactin translocation in pulmonary endothelial cells. Am J Respir Cell Mol Biol. 2002;26:453–464. doi: 10.1165/ajrcmb.26.4.4725. [DOI] [PubMed] [Google Scholar]
- 22.Birukov KG. Csortos C. Marzilli L. Dudek S. Ma SF. Bresnick AR. Verin AD. Cotter RJ. Garcia JG. Differential regulation of alternatively spliced endothelial cell myosin light chain kinase isoforms by p60(Src) J Biol Chem. 2001;276:8567–8573. doi: 10.1074/jbc.M005270200. [DOI] [PubMed] [Google Scholar]
- 23.Birukov KG. Jacobson JR. Flores AA. Ye SQ. Birukova AA. Verin AD. Garcia JG. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol Lung Cell Mol Physiol. 2003;285:L785–797. doi: 10.1152/ajplung.00336.2002. [DOI] [PubMed] [Google Scholar]
- 24.Birukov KG. Shirinsky VP. Stepanova OV. Tkachuk VA. Hahn AW. Resink TJ. Smirnov VN. Stretch affects phenotype and proliferation of vascular smooth muscle cells. Mol Cell Biochem. 1995;144:131–139. doi: 10.1007/BF00944392. [DOI] [PubMed] [Google Scholar]
- 25.Birukova AA. Chatchavalvanich S. Rios A. Kawkitinarong K. Garcia JG. Birukov KG. Differential regulation of pulmonary endothelial monolayer integrity by varying degrees of cyclic stretch. Am J Pathol. 2006;168:1749–1761. doi: 10.2353/ajpath.2006.050431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Black SM. Fineman JR. Oxidative and nitrosative stress in pediatric pulmonary hypertension: Roles of endothelin-1 and nitric oxide. Vascul Pharmacol. 2006;45:308–316. doi: 10.1016/j.vph.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Blanc A. Pandey NR. Srivastava AK. Distinct roles of Ca2+, calmodulin, and protein kinase C in H2O2-induced activation of ERK1/2, p38 MAPK, and protein kinase B signaling in vascular smooth muscle cells. Antioxid Redox Signal. 2004;6:353–366. doi: 10.1089/152308604322899422. [DOI] [PubMed] [Google Scholar]
- 28.Borges F. Fernandes E. Roleira F. Progress towards the discovery of xanthine oxidase inhibitors. Curr Med Chem. 2002;9:195–217. doi: 10.2174/0929867023371229. [DOI] [PubMed] [Google Scholar]
- 29.Brandes RP. Kreuzer J. Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc Res. 2005;65:16–27. doi: 10.1016/j.cardiores.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Buchwalow IB. Podzuweit T. Bocker W. Samoilova VE. Thomas S. Wellner M. Baba HA. Robenek H. Schnekenburger J. Lerch MM. Vascular smooth muscle and nitric oxide synthase. FASEBJ. 2002;16:500–508. doi: 10.1096/fj.01-0842com. [DOI] [PubMed] [Google Scholar]
- 31.Cai H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc Res. 2005;68:26–36. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 32.Cai H. Griendling KK. Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci. 2003;24:471–478. doi: 10.1016/S0165-6147(03)00233-5. [DOI] [PubMed] [Google Scholar]
- 33.Callera GE. Tostes RC. Yogi A. Montezano AC. Touyz RM. Endothelin-1-induced oxidative stress in DOCA-salt hypertension involves NADPH-oxidase-independent mechanisms. Clin Sci (Lond) 2006;110:243–253. doi: 10.1042/CS20050307. [DOI] [PubMed] [Google Scholar]
- 34.Chabot F. Mitchell JA. Gutteridge JM. Evans TW. Reactive oxygen species in acute lung injury. Eur Respir J. 1998;11:745–757. [PubMed] [Google Scholar]
- 35.Chance B. Sies H. Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 36.Chapman GB. Durante W. Hellums JD. Schafer AI. Physiological cyclic stretch causes cell cycle arrest in cultured vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2000;278:H748–754. doi: 10.1152/ajpheart.2000.278.3.H748. [DOI] [PubMed] [Google Scholar]
- 37.Chen KD. Li YS. Kim M. Li S. Yuan S. Chien S. Shyy JY. Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins, and Shc. J Biol Chem. 1999;274:18393–18400. doi: 10.1074/jbc.274.26.18393. [DOI] [PubMed] [Google Scholar]
- 38.Chen Q. Li W. Quan Z. Sumpio BE. Modulation of vascular smooth muscle cell alignment by cyclic strain is dependent on reactive oxygen species and P38 mitogen-activated protein kinase. J Vasc Surg. 2003;37:660–668. doi: 10.1067/mva.2003.95. [DOI] [PubMed] [Google Scholar]
- 39.Cheng GC. Briggs WH. Gerson DS. Libby P. Grodzinsky AJ. Gray ML. Lee RT. Mechanical strain tightly controls fibroblast growth factor-2 release from cultured human vascular smooth muscle cells. Circ Res. 1997;80:28–36. doi: 10.1161/01.res.80.1.28. [DOI] [PubMed] [Google Scholar]
- 40.Cheng JJ. Wung BS. Chao YJ. Wang DL. Cyclic strain-induced reactive oxygen species involved in ICAM-1 gene induction in endothelial cells. Hypertension. 1998;31:125–130. doi: 10.1161/01.hyp.31.1.125. [DOI] [PubMed] [Google Scholar]
- 41.Chien S. Li S. Shyy YJ. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension. 1998;31:162–169. doi: 10.1161/01.hyp.31.1.162. [DOI] [PubMed] [Google Scholar]
- 42.Cho HY. Reddy SP. Kleeberger SR. Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal. 2006;8:76–87. doi: 10.1089/ars.2006.8.76. [DOI] [PubMed] [Google Scholar]
- 43.Collins NT. Cummins PM. Colgan OC. Ferguson G. Birney YA. Murphy RP. Meade G. Cahill PA. Cyclic strain-mediated regulation of vascular endothelial occludin and ZO-1: Influence on intercellular tight junction assembly and function. Arterioscler Thromb Vasc Biol. 2006;26:62–68. doi: 10.1161/01.ATV.0000194097.92824.b3. [DOI] [PubMed] [Google Scholar]
- 44.Cowan DB. Jones M. Garcia LM. Noria S. del Nido PJ. McGowan FX., Jr Hypoxia and stretch regulate intercellular communication in vascular smooth muscle cells through reactive oxygen species formation. Arterioscler Thromb Vasc Biol. 2003;23:1754–1760. doi: 10.1161/01.ATV.0000093546.10162.B2. [DOI] [PubMed] [Google Scholar]
- 45.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Keulenaer GW. Chappell DC. Ishizaka N. Nerem RM. Alexander RW. Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: Role of a superoxide-producing NADH oxidase. Circ Res. 1998;82:1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- 47.Decoursey TE. Ligeti E. Regulation and termination of NADPH oxidase activity. Cell Mol Life Sci. 2005;62:2173–2193. doi: 10.1007/s00018-005-5177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doniger SW. Salomonis N. Dahlquist KD. Vranizan K. Lawlor SC. Conklin BR. MAPPFinder: using Gene Ontology and GenMAPP to create a global gene-expression profile from microarray data. Genome Biol. 2003;4:R7. doi: 10.1186/gb-2003-4-1-r7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dos Santos CC. Slutsky AS. Invited review: mechanisms of ventilator-induced lung injury: a perspective. J Appl Physiol. 2000;89:1645–1655. doi: 10.1152/jappl.2000.89.4.1645. [DOI] [PubMed] [Google Scholar]
- 50.Dourron HM. Jacobson GM. Park JL. Liu J. Reddy DJ. Scheel ML. Pagano PJ. Perivascular gene transfer of NADPH oxidase inhibitor suppresses angioplasty-induced neointimal proliferation of rat carotid artery. Am J Physiol Heart Circ Physiol. 2005;288:H946–953. doi: 10.1152/ajpheart.00413.2004. [DOI] [PubMed] [Google Scholar]
- 51.Duerrschmidt N. Wippich N. Goettsch W. Broemme HJ. Morawietz H. Endothelin-1 induces NAD(P)H oxidase in human endothelial cells. Biochem Biophys Res Commun. 2000;269:713–717. doi: 10.1006/bbrc.2000.2354. [DOI] [PubMed] [Google Scholar]
- 52.Dupont GP. Huecksteadt TP. Marshall BC. Ryan US. Michael JR. Hoidal JR. Regulation of xanthine dehydrogenase and xanthine oxidase activity and gene expression in cultured rat pulmonary endothelial cells. J Clin Invest. 1992;89:197–202. doi: 10.1172/JCI115563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Faraci FM. Didion SP. Vascular protection: Superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol. 2004;24:1367–1373. doi: 10.1161/01.ATV.0000133604.20182.cf. [DOI] [PubMed] [Google Scholar]
- 54.Feng J. Ito M. Kureishi Y. Ichikawa K. Amano M. Isaka N. Okawa K. Iwamatsu A. Kaibuchi K. Hartshorne DJ. Nakano T. Rho-associated kinase of chicken gizzard smooth muscle. J Biol Chem. 1999;274:3744–3752. doi: 10.1074/jbc.274.6.3744. [DOI] [PubMed] [Google Scholar]
- 55.Finkel T. Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 56.Fukai T. Siegfried MR. Ushio–Fukai M. Cheng Y. Kojda G. Harrison DG. Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J Clin Invest. 2000;105:1631–1639. doi: 10.1172/JCI9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garcia JG. Lazar V. Gilbert–McClain LI. Gallagher PJ. Verin AD. Myosin light chain kinase in endothelium: Molecular cloning and regulation. Am J Respir Cell Mol Biol. 1997;16:489–494. doi: 10.1165/ajrcmb.16.5.9160829. [DOI] [PubMed] [Google Scholar]
- 58.Granger DN. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. Am J Physiol. 1988;255:H1269–1275. doi: 10.1152/ajpheart.1988.255.6.H1269. [DOI] [PubMed] [Google Scholar]
- 59.Granger DN. Ischemia-reperfusion: Mechanisms of microvascular dysfunction and the influence of risk factors for cardiovascular disease. Microcirculation. 1999;6:167–178. [PubMed] [Google Scholar]
- 60.Griendling KK. Sorescu D. Lassegue B. Ushio–Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol. 2000;20:2175–2183. doi: 10.1161/01.atv.20.10.2175. [DOI] [PubMed] [Google Scholar]
- 61.Griendling KK. Sorescu D. Ushio-Fukai M. NAD(P)H oxidase: Role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 62.Grote K. Flach I. Luchtefeld M. Akin E. Holland SM. Drexler H. Schieffer B. Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase-2 (MMP-2) via NAD(P)H oxidase-derived reactive oxygen species. Circ Res. 2003;92:e80–86. doi: 10.1161/01.RES.0000077044.60138.7C. [DOI] [PubMed] [Google Scholar]
- 63.Gurkan OU. O'Donnell C. Brower R. Ruckdeschel E. Becker PM. Differential effects of mechanical ventilatory strategy on lung injury and systemic organ inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2003;285:L710–718. doi: 10.1152/ajplung.00044.2003. [DOI] [PubMed] [Google Scholar]
- 64.Haga JH. Li YS. Chien S. Molecular basis of the effects of mechanical stretch on vascular smooth muscle cells. J Biomech. 2007;40:947–960. doi: 10.1016/j.jbiomech.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 65.Hammerschmidt S. Schiller J. Kuhn H. Meybaum M. Gessner C. Sandvoss T. Arnold K. Wirtz H. Influence of tidal volume on pulmonary NO release, tissue lipid peroxidation and surfactant phospholipids. Biochim Biophys Acta. 2003;1639:17–26. doi: 10.1016/s0925-4439(03)00126-1. [DOI] [PubMed] [Google Scholar]
- 66.Han B. Lodyga M. Liu M. Ventilator-induced lung injury: role of protein–protein interaction in mechanosensation. Proc Am Thorac Soc. 2005;2:181–187. doi: 10.1513/pats.200501-008AC. [DOI] [PubMed] [Google Scholar]
- 67.Harrison DG. Widder J. Grumbach I. Chen W. Weber M. Searles C. Endothelial mechanotransduction, nitric oxide and vascular inflammation. J Intern Med. 2006;259:351–363. doi: 10.1111/j.1365-2796.2006.01621.x. [DOI] [PubMed] [Google Scholar]
- 68.Harrison R. Physiological roles of xanthine oxidoreductase. Drug Metab Rev. 2004;36:363–375. doi: 10.1081/dmr-120037569. [DOI] [PubMed] [Google Scholar]
- 69.Hipper A. Isenberg G. Cyclic mechanical strain decreases the DNA synthesis of vascular smooth muscle cells. Pflugers Arch. 2000;440:19–27. doi: 10.1007/s004240000246. [DOI] [PubMed] [Google Scholar]
- 70.Hishikawa K. Luscher TF. Pulsatile stretch stimulates superoxide production in human aortic endothelial cells. Circulation. 1997;96:3610–3616. doi: 10.1161/01.cir.96.10.3610. [DOI] [PubMed] [Google Scholar]
- 71.Hu Y. Bock G. Wick G. Xu Q. Activation of PDGF receptor alpha in vascular smooth muscle cells by mechanical stress. FASEB J. 1998;12:1135–1142. doi: 10.1096/fasebj.12.12.1135. [DOI] [PubMed] [Google Scholar]
- 72.Ichimura H. Parthasarathi K. Quadri S. Issekutz AC. Bhattacharya J. Mechano-oxidative coupling by mitochondria induces proinflammatory responses in lung venular capillaries. J Clin Invest. 2003;111:691–699. doi: 10.1172/JCI17271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ingber DE. Tensegrity: The architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- 74.Inoue N. Kawashima S. Hirata KI. Rikitake Y. Takeshita S. Yamochi W. Akita H. Yokoyama M. Stretch force on vascular smooth muscle cells enhances oxidation of LDL via superoxide production. Am J Physiol. 1998;274:H1928–1932. doi: 10.1152/ajpheart.1998.274.6.H1928. [DOI] [PubMed] [Google Scholar]
- 75.Issbrucker K. Marti HH. Hippenstiel S. Springmann G. Voswinckel R. Gaumann A. Breier G. Drexler HC. Suttorp N. Clauss M. p38 MAP kinase–a molecular switch between VEGF-induced angiogenesis and vascular hyperpermeability. FASEB J. 2003;17:262–264. doi: 10.1096/fj.02-0329fje. [DOI] [PubMed] [Google Scholar]
- 76.Jafari B. Ouyang B. Li LF. Hales CA. Quinn DA. Intracellular glutathione in stretch-induced cytokine release from alveolar type-2 like cells. Respirology. 2004;9:43–53. doi: 10.1111/j.1440-1843.2003.00527.x. [DOI] [PubMed] [Google Scholar]
- 77.Jarasch ED. Grund C. Bruder G. Heid HW. Keenan TW. Franke WW. Localization of xanthine oxidase in mammary-gland epithelium and capillary endothelium. Cell. 1981;25:67–82. doi: 10.1016/0092-8674(81)90232-4. [DOI] [PubMed] [Google Scholar]
- 78.Kaner RJ. Crystal RG. Compartmentalization of vascular endothelial growth factor to the epithelial surface of the human lung. Mol Med. 2001;7:240–246. [PMC free article] [PubMed] [Google Scholar]
- 79.Kaner RJ. Ladetto JV. Singh R. Fukuda N. Matthay MA. Crystal RG. Lung overexpression of the vascular endothelial growth factor gene induces pulmonary edema. Am J Respir Cell Mol Biol. 2000;22:657–664. doi: 10.1165/ajrcmb.22.6.3779. [DOI] [PubMed] [Google Scholar]
- 80.Katsumi A. Orr AW. Tzima E. Schwartz MA. Integrins in mechanotransduction. J Biol Chem. 2004;279:12001–12004. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- 81.Kojda G. Hambrecht R. Molecular mechanisms of vascular adaptations to exercise. Physical activity as an effective antioxidant therapy? Cardiovasc Res. 2005;67:187–197. doi: 10.1016/j.cardiores.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 82.Koller A. Signaling pathways of mechanotransduction in arteriolar endothelium and smooth muscle cells in hypertension. Microcirculation. 2002;9:277–294. doi: 10.1038/sj.mn.7800142. [DOI] [PubMed] [Google Scholar]
- 83.Korner PI. Angus JA. Bobik A. Jennings GL. Amplifier function of resistance vessels and the left ventricle in hypertension. J Hypertens Suppl. 1991;9:S31–40. doi: 10.1097/00004872-199112002-00005. discussion S40–31. [DOI] [PubMed] [Google Scholar]
- 84.Kuebler WM. Uhlig U. Goldmann T. Schael G. Kerem A. Exner K. Martin C. Vollmer E. Uhlig S. Stretch activates nitric oxide production in pulmonary vascular endothelial cells in situ. Am J Respir Crit Care Med. 2003;168:1391–1398. doi: 10.1164/rccm.200304-562OC. [DOI] [PubMed] [Google Scholar]
- 85.Laakso JT. Teravainen TL. Martelin E. Vaskonen T. Lapatto R. Renal xanthine oxidoreductase activity during development of hypertension in spontaneously hypertensive rats. J Hypertens. 2004;22:1333–1340. doi: 10.1097/01.hjh.0000125441.28861.9f. [DOI] [PubMed] [Google Scholar]
- 86.Landmesser U. Cai H. Dikalov S. McCann L. Hwang J. Jo H. Holland SM. Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40:511–515. doi: 10.1161/01.hyp.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Landmesser U. Dikalov S. Price SR. McCann L. Fukai T. Holland SM. Mitch WE. Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lang JD. McArdle PJ. O'Reilly PJ. Matalon S. Oxidant-antioxidant balance in acute lung injury. Chest. 2002;122:314S–320S. doi: 10.1378/chest.122.6_suppl.314s. [DOI] [PubMed] [Google Scholar]
- 89.Lassegue B. Clempus RE. Vascular NAD(P)H oxidases: Specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R277–297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 90.Lassegue B. Sorescu D. Szocs K. Yin Q. Akers M. Zhang Y. Grant SL. Lambeth JD. Griendling KK. Novel gp91(phox) homologues in vascular smooth muscle cells: Nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 91.Lehoux S. Esposito B. Merval R. Loufrani L. Tedgui A. Pulsatile stretch-induced extracellular signal-regulated kinase 1/2 activation in organ culture of rabbit aorta involves reactive oxygen species. Arterioscler Thromb Vasc Biol. 2000;20:2366–2372. doi: 10.1161/01.atv.20.11.2366. [DOI] [PubMed] [Google Scholar]
- 92.Lehoux S. Esposito B. Merval R. Tedgui A. Differential regulation of vascular focal adhesion kinase by steady stretch and pulsatility. Circulation. 2005;111:643–649. doi: 10.1161/01.CIR.0000154548.16191.2F. [DOI] [PubMed] [Google Scholar]
- 93.Lehoux S. Tedgui A. Signal transduction of mechanical stresses in the vascular wall. Hypertension. 1998;32:338–345. doi: 10.1161/01.hyp.32.2.338. [DOI] [PubMed] [Google Scholar]
- 94.Lemarie CA. Tharaux PL. Esposito B. Tedgui A. Lehoux S. Transforming growth factor-alpha mediates nuclear factor kappaB activation in strained arteries. Circ Res. 2006;99:434–441. doi: 10.1161/01.RES.0000237388.89261.47. [DOI] [PubMed] [Google Scholar]
- 95.Levine GK. Deutschman CS. Helfaer MA. Margulies SS. Sepsis-induced lung injury in rats increases alveolar epithelial vulnerability to stretch. Crit Care Med. 2006;34:1746–1751. doi: 10.1097/01.CCM.0000218813.77367.E2. [DOI] [PubMed] [Google Scholar]
- 96.Li JM. Shah AM. Intracellular localization and preassembly of the NADPH oxidase complex in cultured endothelial cells. J Biol Chem. 2002;277:19952–19960. doi: 10.1074/jbc.M110073200. [DOI] [PubMed] [Google Scholar]
- 97.Linder N. Rapola J. Raivio KO. Cellular expression of xanthine oxidoreductase protein in normal human tissues. Lab Invest. 1999;79:967–974. [PubMed] [Google Scholar]
- 98.Liu J. Ormsby A. Oja–Tebbe N. Pagano PJ. Gene transfer of NAD(P)H oxidase inhibitor to the vascular adventitia attenuates medial smooth muscle hypertrophy. Circ Res. 2004;95:587–594. doi: 10.1161/01.RES.0000142317.88591.e6. [DOI] [PubMed] [Google Scholar]
- 99.Liu JQ. Zelko IN. Erbynn EM. Sham JS. Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox) Am J Physiol Lung Cell Mol Physiol. 2006;290:L2–10. doi: 10.1152/ajplung.00135.2005. [DOI] [PubMed] [Google Scholar]
- 100.Liu XM. Ensenat D. Wang H. Schafer AI. Durante W. Physiologic cyclic stretch inhibits apoptosis in vascular endothelium. FEBS Lett. 2003;541:52–56. doi: 10.1016/s0014-5793(03)00285-0. [DOI] [PubMed] [Google Scholar]
- 101.Liu Y. Zhao H. Li H. Kalyanaraman B. Nicolosi AC. Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res. 2003;93:573–580. doi: 10.1161/01.RES.0000091261.19387.AE. [DOI] [PubMed] [Google Scholar]
- 102.Madamanchi NR. Vendrov A. Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 103.Mata–Greenwood E. Grobe A. Kumar S. Noskina Y. Black SM. Cyclic stretch increases VEGF expression in pulmonary arterial smooth muscle cells via TGF-beta1 and reactive oxygen species: A requirement for NAD(P)H oxidase. Am J Physiol Lung Cell Mol Physiol. 2005;289:L288–289. doi: 10.1152/ajplung.00417.2004. [DOI] [PubMed] [Google Scholar]
- 104.Matsushita H. Lee Kh K. Tsao PS. Cyclic strain induces reactive oxygen species production via an endothelial NAD(P)H oxidase. J Cell Biochem. 2001;81:99–106. doi: 10.1002/jcb.1094. [DOI] [PubMed] [Google Scholar]
- 105.Matthay MA. Zimmerman GA. Esmon C. Bhattacharya J. Coller B. Doerschuk CM. Floros J. Gimbrone MA., Jr. Hoffman E. Hubmayr RD. Leppert M. Matalon S. Munford R. Parsons P. Slutsky AS. Tracey KJ. Ward P. Gail DB. Harabin AL. Future research directions in acute lung injury: Summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med. 2003;167:1027–1035. doi: 10.1164/rccm.200208-966WS. [DOI] [PubMed] [Google Scholar]
- 106.McNally JS. Davis ME. Giddens DP. Saha A. Hwang J. Dikalov S. Jo H. Harrison DG. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol. 2003;285:H2290–2297. doi: 10.1152/ajpheart.00515.2003. [DOI] [PubMed] [Google Scholar]
- 107.Mueller CF. Laude K. McNally JS. Harrison DG. ATVB in focus: Redox mechanisms in blood vessels. Arterioscler Thromb Vasc Biol. 2005;25:274–278. doi: 10.1161/01.ATV.0000149143.04821.eb. [DOI] [PubMed] [Google Scholar]
- 108.Mura M. dos Santos CC. Stewart D. Liu M. Vascular endothelial growth factor and related molecules in acute lung injury. J Appl Physiol. 2004;97:1605–1617. doi: 10.1152/japplphysiol.00202.2004. [DOI] [PubMed] [Google Scholar]
- 109.Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol. 2006;68:345–374. doi: 10.1146/annurev.physiol.68.040504.094707. [DOI] [PubMed] [Google Scholar]
- 110.Nakazono K. Watanabe N. Matsuno K. Sasaki J. Sato T. Inoue M. Does superoxide underlie the pathogenesis of hypertension? Proc Natl Acad Sci USA. 1991;88:10045–10048. doi: 10.1073/pnas.88.22.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Napoli C. de Nigris F. Palinski W. Multiple role of reactive oxygen species in the arterial wall. J Cell Biochem. 2001;82:674–682. doi: 10.1002/jcb.1198. [DOI] [PubMed] [Google Scholar]
- 112.Narimanbekov IO. Rozycki HJ. Effect of IL-1 blockade on inflammatory manifestations of acute ventilator-induced lung injury in a rabbit model. Exp Lung Res. 1995;21:239–254. doi: 10.3109/01902149509068830. [DOI] [PubMed] [Google Scholar]
- 113.Nguyen KT. Frye SR. Eskin SG. Patterson C. Runge MS. McIntire LV. Cyclic strain increases protease-activated receptor-1 expression in vascular smooth muscle cells. Hypertension. 2001;38:1038–1043. doi: 10.1161/hy1101.092840. [DOI] [PubMed] [Google Scholar]
- 114.Niwa K. Inanami O. Ohta T. Ito S. Karino T. Kuwabara M. p38 MAPK and Ca2+ contribute to hydrogen peroxide-induced increase of permeability in vascular endothelial cells but ERK does not. Free Radic Res. 2001;35:519–527. doi: 10.1080/10715760100301531. [DOI] [PubMed] [Google Scholar]
- 115.Nwariaku FE. Liu Z. Zhu X. Nahari D. Ingle C. Wu RF. Gu Y. Sarosi G. Terada LS. NADPH oxidase mediates vascular endothelial cadherin phosphorylation and endothelial dysfunction. Blood. 2004;104:3214–3220. doi: 10.1182/blood-2004-05-1868. [DOI] [PubMed] [Google Scholar]
- 116.O'Callaghan CJ. Williams B. Mechanical strain-induced extracellular matrix production by human vascular smooth muscle cells: Role of TGF-beta(1) Hypertension. 2000;36:319–324. doi: 10.1161/01.hyp.36.3.319. [DOI] [PubMed] [Google Scholar]
- 117.Oeckler RA. Kaminski PM. Wolin MS. Stretch enhances contraction of bovine coronary arteries via an NAD(P)H oxidase-mediated activation of the extracellular signal-regulated kinase mitogen-activated protein kinase cascade. Circ Res. 2003;92:23–31. doi: 10.1161/01.res.0000051860.84509.ce. [DOI] [PubMed] [Google Scholar]
- 118.Ogita H. Liao J. Endothelial function and oxidative stress. Endothelium. 2004;11:123–132. doi: 10.1080/10623320490482664. [DOI] [PubMed] [Google Scholar]
- 119.Olsson AK. Dimberg A. Kreuger J. Claesson–Welsh L. VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 120.Orr AW. Helmke BP. Blackman BR. Schwartz MA. Mechanisms of mechanotransduction. Dev Cell. 2006;10:11–20. doi: 10.1016/j.devcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 121.Pagano PJ. Ito Y. Tornheim K. Gallop PM. Tauber AI. Cohen RA. An NADPH oxidase superoxide-generating system in the rabbit aorta. Am J Physiol. 1995;268:H2274–2280. doi: 10.1152/ajpheart.1995.268.6.H2274. [DOI] [PubMed] [Google Scholar]
- 122.Papaiahgari S. Yerrapureddy A. Hassoun PM. Garcia JG. Birukov KG. Reddy SP. EGFR-activated signaling and actin remodeling regulate cyclic stretch-induced NRF2-ARE activation. Am J Respir Cell Mol Biol. 2007;36:304–312. doi: 10.1165/rcmb.2006-0131OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Paravicini TM. Touyz RM. Redox signaling in hypertension. Cardiovasc Res. 2006;71:247–258. doi: 10.1016/j.cardiores.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 124.Parker JC. Inhibitors of myosin light chain kinase and phosphodiesterase reduce ventilator-induced lung injury. J Appl Physiol. 2000;89:2241–2248. doi: 10.1152/jappl.2000.89.6.2241. [DOI] [PubMed] [Google Scholar]
- 125.Pearse DB. Shimoda LA. Verin AD. Bogatcheva N. Moon C. Ronnett GV. Welsh LE. Becker PM. Effect of cGMP on lung microvascular endothelial barrier dysfunction following hydrogen peroxide. Endothelium. 2003;10:309–317. doi: 10.1080/10623320390272307. [DOI] [PubMed] [Google Scholar]
- 126.Pfitzer G. Invited review: Regulation of myosin phosphorylation in smooth muscle. J Appl Physiol. 2001;91:497–503. doi: 10.1152/jappl.2001.91.1.497. [DOI] [PubMed] [Google Scholar]
- 127.Pimentel DR. Amin JK. Xiao L. Miller T. Viereck J. Oliver–Krasinski J. Baliga R. Wang J. Siwik DA. Singh K. Pagano P. Colucci WS. Sawyer DB. Reactive oxygen species mediate amplitude-dependent hypertrophic and apoptotic responses to mechanical stretch in cardiac myocytes. Circ Res. 2001;89:453–460. doi: 10.1161/hh1701.096615. [DOI] [PubMed] [Google Scholar]
- 128.Pugin J. Dunn I. Jolliet P. Tassaux D. Magnenat JL. Nicod LP. Chevrolet JC. Activation of human macrophages by mechanical ventilation in vitro. Am J Physiol. 1998;275:L1040–1050. doi: 10.1152/ajplung.1998.275.6.L1040. [DOI] [PubMed] [Google Scholar]
- 129.Putnam AJ. Cunningham JJ. Pillemer BB. Mooney DJ. External mechanical strain regulates membrane targeting of Rho GTPases by controlling the state of microtubule polymerization. Am J Physiol Cell Physiol. 2002;284:C627–639. doi: 10.1152/ajpcell.00137.2002. [DOI] [PubMed] [Google Scholar]
- 130.Quinn DA. Moufarrej RK. Volokhov A. Hales CA. Interactions of lung stretch, hyperoxia, and MIP-2 production in ventilator-induced lung injury. J Appl Physiol. 2002;93:517–525. doi: 10.1152/japplphysiol.00570.2001. [DOI] [PubMed] [Google Scholar]
- 131.Rajagopalan S. Kurz S. Munzel T. Tarpey M. Freeman BA. Griendling KK. Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rao GN. Corson MA. Berk BC. Uric acid stimulates vascular smooth muscle cell proliferation by increasing platelet-derived growth factor A-chain expression. J Biol Chem. 1991;266:8604–8608. [PubMed] [Google Scholar]
- 133.Redon J. Oliva MR. Tormos C. Giner V. Chaves J. Iradi A. Saez GT. Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension. 2003;41:1096–1101. doi: 10.1161/01.HYP.0000068370.21009.38. [DOI] [PubMed] [Google Scholar]
- 134.Resnick N. Yahav H. Shay–Salit A. Shushy M. Schubert S. Zilberman LC. Wofovitz E. Fluid shear stress and the vascular endothelium: For better and for worse. Prog Biophys Mol Biol. 2003;81:177–199. doi: 10.1016/s0079-6107(02)00052-4. [DOI] [PubMed] [Google Scholar]
- 135.Rey FE. Cifuentes ME. Kiarash A. Quinn MT. Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(-) and systolic blood pressure in mice. Circ Res. 2001;89:408–414. doi: 10.1161/hh1701.096037. [DOI] [PubMed] [Google Scholar]
- 136.Roberts WG. Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995;108:2369–2379. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- 137.Rojas A. Figueroa H. Re L. Morales MA. Oxidative stress at the vascular wall. Mechanistic and pharmacological aspects. Arch Med Res. 2006;37:436–448. doi: 10.1016/j.arcmed.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 138.Rueckschloss U. Galle J. Holtz J. Zerkowski HR. Morawietz H. Induction of NAD(P)H oxidase by oxidized low-density lipoprotein in human endothelial cells: antioxidative potential of hydroxymethylglutaryl coenzyme A reductase inhibitor therapy. Circulation. 2001;104:1767–1772. doi: 10.1161/hc4001.097056. [DOI] [PubMed] [Google Scholar]
- 139.Rush JW. Turk JR. Laughlin MH. Exercise training regulates SOD-1 and oxidative stress in porcine aortic endothelium. Am J Physiol Heart Circ Physiol. 2003;284:H1378–1387. doi: 10.1152/ajpheart.00190.2002. [DOI] [PubMed] [Google Scholar]
- 140.Seshiah PN. Weber DS. Rocic P. Valppu L. Taniyama Y. Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: Upstream mediators. Circ Res. 2002;91:406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 141.Shokoji T. Nishiyama A. Fujisawa Y. Hitomi H. Kiyomoto H. Takahashi N. Kimura S. Kohno M. Abe Y. Renal sympathetic nerve responses to tempol in spontaneously hypertensive rats. Hypertension. 2003;41:266–273. doi: 10.1161/01.hyp.0000049621.85474.cf. [DOI] [PubMed] [Google Scholar]
- 142.Shyy JY. Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res. 2002;91:769–775. doi: 10.1161/01.res.0000038487.19924.18. [DOI] [PubMed] [Google Scholar]
- 143.Smith PG. Moreno R. Ikebe M. Strain increases airway smooth muscle contractile and cytoskeletal proteins in vitro. Am J Physiol. 1997;272:L20–27. doi: 10.1152/ajplung.1997.272.1.L20. [DOI] [PubMed] [Google Scholar]
- 144.Smith PG. Roy C. Fisher S. Huang QQ. Brozovich F. Selected contribution: mechanical strain increases force production and calcium sensitivity in cultured airway smooth muscle cells. J Appl Physiol. 2000;89:2092–2098. doi: 10.1152/jappl.2000.89.5.2092. [DOI] [PubMed] [Google Scholar]
- 145.Somers MJ. Mavromatis K. Galis ZS. Harrison DG. Vascular superoxide production and vasomotor function in hypertension induced by deoxycorticosterone acetate-salt. Circulation. 2000;101:1722–1728. doi: 10.1161/01.cir.101.14.1722. [DOI] [PubMed] [Google Scholar]
- 146.Stocker R. Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 147.Stroetz RW. Vlahakis NE. Walters BJ. Schroeder MA. Hubmayr RD. Validation of a new live cell strain system: characterization of plasma membrane stress failure. J Appl Physiol. 2001;90:2361–2370. doi: 10.1152/jappl.2001.90.6.2361. [DOI] [PubMed] [Google Scholar]
- 148.Sun H. Breslin JW. Zhu J. Yuan SY. Wu MH. Rho and ROCK signaling in VEGF-induced microvascular endothelial hyperpermeability. Microcirculation. 2006;13:237–247. doi: 10.1080/10739680600556944. [DOI] [PubMed] [Google Scholar]
- 149.Takahashi H. Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond) 2005;109:227–241. doi: 10.1042/CS20040370. [DOI] [PubMed] [Google Scholar]
- 150.Tanito M. Nakamura H. Kwon YW. Teratani A. Masutani H. Shioji K. Kishimoto C. Ohira A. Horie R. Yodoi J. Enhanced oxidative stress and impaired thioredoxin expression in spontaneously hypertensive rats. Antioxid Redox Signal. 2004;6:89–97. doi: 10.1089/152308604771978381. [DOI] [PubMed] [Google Scholar]
- 151.Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: What is the clinical significance? Hypertension. 2004;44:248–252. doi: 10.1161/01.HYP.0000138070.47616.9d. [DOI] [PubMed] [Google Scholar]
- 152.Touyz RM. Schiffrin EL. Reactive oxygen species in vascular biology: Implications in hypertension. Histochem Cell Biol. 2004;122:339–352. doi: 10.1007/s00418-004-0696-7. [DOI] [PubMed] [Google Scholar]
- 153.Tschumperlin DJ. Margulies SS. Alveolar epithelial surface area-volume relationship in isolated rat lungs. J Appl Physiol. 1999;86:2026–2033. doi: 10.1152/jappl.1999.86.6.2026. [DOI] [PubMed] [Google Scholar]
- 154.Uhlig S. Ventilation-induced lung injury and mechanotransduction: sStretching it too far? Am J Physiol Lung Cell Mol Physiol. 2002;282:L892–896. doi: 10.1152/ajplung.00124.2001. [DOI] [PubMed] [Google Scholar]
- 155.Uhlig U. Fehrenbach H. Lachmann RA. Goldmann T. Lachmann B. Vollmer E. Uhlig S. Phosphoinositide 3-OH kinase inhibition prevents ventilation-induced lung cell activation. Am J Respir Crit Care Med. 2004;169:201–208. doi: 10.1164/rccm.200303-343OC. [DOI] [PubMed] [Google Scholar]
- 156.Ungvari Z. Csiszar A. Huang A. Kaminski PM. Wolin MS. Koller A. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation. 2003;108:1253–1258. doi: 10.1161/01.CIR.0000079165.84309.4D. [DOI] [PubMed] [Google Scholar]
- 157.Upadhyay D. Correa–Meyer E. Sznajder JI. Kamp DW. FGF-10 prevents mechanical stretch-induced alveolar epithelial cell DNA damage via MAPK activation. Am J Physiol Lung Cell Mol Physiol. 2003;284:L350–359. doi: 10.1152/ajplung.00161.2002. [DOI] [PubMed] [Google Scholar]
- 158.Usatyuk PV. Vepa S. Watkins T. He D. Parinandi NL. Natarajan V. Redox regulation of reactive oxygen species-induced p38 MAP kinase activation and barrier dysfunction in lung microvascular endothelial cells. Antioxid Redox Signal. 2003;5:723–730. doi: 10.1089/152308603770380025. [DOI] [PubMed] [Google Scholar]
- 159.Ushio–Fukai M. Alexander RW. Akers M. Griendling KK. p38 Mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II. Role in vascular smooth muscle cell hypertrophy. J Biol Chem. 1998;273:15022–15029. doi: 10.1074/jbc.273.24.15022. [DOI] [PubMed] [Google Scholar]
- 160.van der Vliet A. Cross CE. Oxidants, nitrosants, and the lung. Am J Med. 2000;109:398–421. doi: 10.1016/s0002-9343(00)00479-4. [DOI] [PubMed] [Google Scholar]
- 161.van Nieuw Amerongen GP. Koolwijk P. Versteilen A. van Hinsbergh VW. Involvement of RhoA/Rho kinase signaling in VEGF-induced endothelial cell migration and angiogenesis in vitro. Arterioscler Thromb Vasc Biol. 2003;23:211–217. doi: 10.1161/01.atv.0000054198.68894.88. [DOI] [PubMed] [Google Scholar]
- 162.Vasquez–Vivar J. Kalyanaraman B. Martasek P. Hogg N. Masters BS. Karoui H. Tordo P. Pritchard KA., Jr Superoxide generation by endothelial nitric oxide synthase: The influence of cofactors. Proc Natl Acad Sci USA. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Velasco G. Armstrong C. Morrice N. Frame S. Cohen P. Phosphorylation of the regulatory subunit of smooth muscle protein phosphatase 1M at Thr850 induces its dissociation from myosin. FEBS Lett. 2002;527:101–104. doi: 10.1016/s0014-5793(02)03175-7. [DOI] [PubMed] [Google Scholar]
- 164.Vlahakis NE. Hubmayr RD. Invited review: Plasma membrane stress failure in alveolar epithelial cells. J Appl Physiol. 2000;89:2490–2496; discussion 2497. doi: 10.1152/jappl.2000.89.6.2490. [DOI] [PubMed] [Google Scholar]
- 165.Vlahakis NE. Schroeder MA. Limper AH. Hubmayr RD. Stretch induces cytokine release by alveolar epithelial cells in vitro. Am J Physiol. 1999;277:L167–173. doi: 10.1152/ajplung.1999.277.1.L167. [DOI] [PubMed] [Google Scholar]
- 166.Voelkel NF. Vandivier RW. Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol. 2006;290:L209–221. doi: 10.1152/ajplung.00185.2005. [DOI] [PubMed] [Google Scholar]
- 167.Waters CM. Reactive oxygen species in mechanotransduction. Am J Physiol Lung Cell Mol Physiol. 2004;287:L484–485. doi: 10.1152/ajplung.00161.2004. [DOI] [PubMed] [Google Scholar]
- 168.Wedgwood S. Steinhorn RH. Bunderson M. Wilham J. Lakshminrusimha S. Brennan LA. Black SM. Increased hydrogen peroxide downregulates soluble guanylate cyclase in the lungs of lambs with persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol. 2005;289:L660–666. doi: 10.1152/ajplung.00369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wojciak–Stothard B. Ridley AJ. Shear stress-induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3-kinases. J Cell Biol. 2003;161:429–439. doi: 10.1083/jcb.200210135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wung BS. Cheng JJ. Chao YJ. Hsieh HJ. Wang DL. Modulation of Ras/Raf/extracellular signal-regulated kinase pathway by reactive oxygen species is involved in cyclic strain-induced early growth response-1 gene expression in endothelial cells. Circ Res. 1999;84:804–812. doi: 10.1161/01.res.84.7.804. [DOI] [PubMed] [Google Scholar]
- 171.Wung BS. Cheng JJ. Hsieh HJ. Shyy YJ. Wang DL. Cyclic strain-induced monocyte chemotactic protein-1 gene expression in endothelial cells involves reactive oxygen species activation of activator protein 1. Circ Res. 1997;81:1–7. doi: 10.1161/01.res.81.1.1. [DOI] [PubMed] [Google Scholar]
- 172.Wung BS. Cheng JJ. Shyue SK. Wang DL. NO modulates monocyte chemotactic protein-1 expression in endothelial cells under cyclic strain. Arterioscler Thromb Vasc Biol. 2001;21:1941–1947. doi: 10.1161/hq1201.099428. [DOI] [PubMed] [Google Scholar]
- 173.Xia Y. Tsai AL. Berka V. Zweier JL. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem. 1998;273:25804–25808. doi: 10.1074/jbc.273.40.25804. [DOI] [PubMed] [Google Scholar]
- 174.Yamamoto N. Fukuda K. Matsushita T. Matsukawa M. Hara F. Hamanishi C. Cyclic tensile stretch stimulates the release of reactive oxygen species from osteoblast-like cells. Calcif Tissue Int. 2005;76:433–438. doi: 10.1007/s00223-004-1188-4. [DOI] [PubMed] [Google Scholar]
- 175.Yamazaki K. Fukuda K. Matsukawa M. Hara F. Matsushita T. Yamamoto N. Yoshida K. Munakata H. Hamanishi C. Cyclic tensile stretch loaded on bovine chondrocytes causes depolymerization of hyaluronan: Involvement of reactive oxygen species. Arthritis Rheum. 2003;48:3151–3158. doi: 10.1002/art.11305. [DOI] [PubMed] [Google Scholar]
- 176.Yano Y. Saito Y. Narumiya S. Sumpio BE. Involvement of rho p21 in cyclic strain-induced tyrosine phosphorylation of focal adhesion kinase (pp125FAK), morphological changes and migration of endothelial cells. Biochem Biophys Res Commun. 1996;224:508–515. doi: 10.1006/bbrc.1996.1057. [DOI] [PubMed] [Google Scholar]
- 177.Yoshizumi M. Abe J. Haendeler J. Huang Q. Berk BC. Src and Cas mediate JNK activation but not ERK1/2 and p38 kinases by reactive oxygen species. J Biol Chem. 2000;275:11706–11712. doi: 10.1074/jbc.275.16.11706. [DOI] [PubMed] [Google Scholar]
- 178.Yuan SY. Protein kinase signaling in the modulation of microvascular permeability. Vascul Pharmacol. 2002;39:213–223. doi: 10.1016/s1537-1891(03)00010-7. [DOI] [PubMed] [Google Scholar]
- 179.Zhao Y. Davis HW. Hydrogen peroxide-induced cytoskeletal rearrangement in cultured pulmonary endothelial cells. J Cell Physiol. 1998;174:370–379. doi: 10.1002/(SICI)1097-4652(199803)174:3<370::AID-JCP11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 180.Zheng W. Christensen LP. Tomanek RJ. Stretch induces upregulation of key tyrosine kinase receptors in microvascular endothelial cells. Am J Physiol Heart Circ Physiol. 2004;287:H2739–2745. doi: 10.1152/ajpheart.00410.2004. [DOI] [PubMed] [Google Scholar]
- 181.Zheng W. Seftor EA. Meininger CJ. Hendrix MJ. Tomanek RJ. Mechanisms of coronary angiogenesis in response to stretch: role of VEGF and TGF-beta. Am J Physiol Heart Circ Physiol. 2001;280:H909–917. doi: 10.1152/ajpheart.2001.280.2.H909. [DOI] [PubMed] [Google Scholar]