Abstract
Responsible for the majority of excitatory activity in the central nervous system (CNS), glutamate interacts with a range of specific receptor and transporter systems to establish a functional synapse. Excessive stimulation of glutamate receptors causes excitotoxicity, a phenomenon implicated in both acute and chronic neurodegenerative diseases [e.g., ischemia, Huntington's disease, and amyotrophic lateral sclerosis (ALS)]. In physiology, excitotoxicity is prevented by rapid binding and clearance of synaptic released glutamate by high-affinity, Na+-dependent glutamate transporters and amplified by defects to the glutamate transporter and receptor systems. ALS pathogenetic mechanisms are not completely understood and characterized, but excitotoxicity has been regarded as one firm mechanism implicated in the disease because of data obtained from ALS patients and animal and cellular models as well as inferred by the documented efficacy of riluzole, a generic antiglutamatergic drug, has in patients. In this article, we critically review the several lines of evidence supporting a role for glutamate-mediated excitotoxicity in the death of motor neurons occurring in ALS, putting a particular emphasis on the impairment of the glutamate-transport system. Antioxid. Redox Signal. 11, 1587–1602.
Glutamate in the Central Nervous System
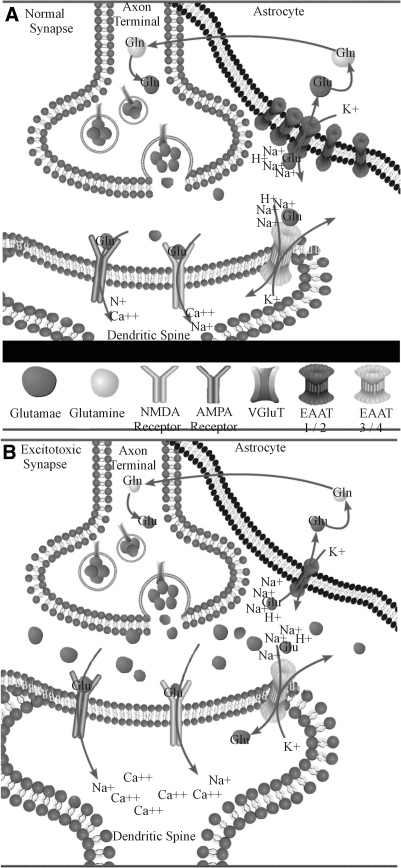
L-Glutamate is the predominant excitatory neurotransmitter in the central nervous system (CNS). A nonessential amino acid, glutamate is continuously converted to α-ketoglutarate through deamination by glutamate dehydrogenase or by transamination by one of the transaminases and metabolized through the tricarboxylic acid cycle to succinate, fumarate, and malate, successively. Glutamate is also the product of the deamination of glutamine by phosphate-activated glutaminase, a mitochondrial and possibly neuron-specific enzyme (80). Synaptically released glutamate activates a family of ligand-gated ion channels (ionotropic receptors) and G protein–coupled receptors (metabotropic receptors), and its action is terminated by specific reuptake systems located mainly in astrocytes surrounding the synapse. In astrocytes, glutamate is then converted into glutamine, which does not have neurotransmitter properties and can be released and made available for neurons to convert it back to glutamate through a glutamine-reuptake system. Glutamate is then packed by vesicular glutamate transporters in synaptic vesicles, ready to be released again (35, 129) (Fig. 1).
FIG. 1.
(A) In a normally functioning synapse, glutamate released from the presynaptic terminals activates the NMDA and AMPA receptors, resulting in an influx of Na and Ca ions into the postsynaptic element, depolarization of the neuron, and ultimately, an action potential. The neurotransmitter action is then terminated by glutamate transporters located in the nearby astroglia cells, as well as in the postsynaptic elements. (B) Excitotoxicity can be induced by an elevation of synaptic glutamate concentration. This can be caused by an increased released of glutamate and/or an impaired glutamate uptake. The excessive stimulation of the glutamate receptors that results from this increased synaptic glutamate gives rise to an increased intracellular concentration of Ca ions, resulting in neuronal death. Neuronal cell loss resulting from this process can cause a further increase in extracellular glutamate and amplifies the excitotoxic damage.
Three major classes of metabotropic and three of ionotropic receptors for glutamate are known (Table 1). Both receptor families localize to different structures of the excitatory synapse, including the presynaptic terminal and the postsynaptic element, and astrocytes that envelop the synapse (75, 100). The ionotropic receptor complexes are classified according to their responsiveness and affinity to exogenous agonists; N-methyl-d-aspartic acid (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and kainate (KA). Classically, a demarcation existed between the Ca2+-permeable NMDA receptors and the Ca2+-impermeable AMPA and KA receptors. However, AMPA receptors missing the GluR2 subunit have been shown to be Ca2+ permeable (70, 152). GluR2-deficient AMPA receptors are expressed in the motor neurons and are implicated in excitotoxic degeneration (34, 73, 145).
Table 1.
Classification of Glutamate Receptors
| Receptor class | Subunits | Permeability/second messenger system | Antagonist | Special properties |
|---|---|---|---|---|
| NMDA (ionotropic) | N1 | Na+ | APV | Mg2+ block |
| N2a | Ca2+ | Gly coactivator | ||
| N2b | ||||
| N2c | ||||
| N2d | ||||
| N3a | ||||
| N3b | ||||
| AMPA (ionotropic) | GluR1GluR2 | Na+(Ca2+)* | CNQX | Q/R editing in GluR subunit |
| GluR3 | ||||
| GluR4 | ||||
| Kainate (ionotropic) | GluR5 | Na+ | CNQX | |
| GluR6 | ||||
| GluR7 | ||||
| KA1 | ||||
| KA2 | ||||
| Class I (metabotropic) | mGluR1, | IP3, Ca2+ | LY393675, | |
| mGluR5 | MPEP | |||
| Class II (metabotropic) | mGluR2, | cAMP | LY341495 | |
| mGluR3 | ||||
| Class III (metabotropic) | mGluR4, | cAMP | CPPG | |
| mGluR6, | ||||
| mGluR7, | ||||
| mGluR8 |
GluR2 dependent.
Both ionotropic and metabotropic receptors comprises three functional defined classes made up of several individual subunits, each encoded by a different gene. Abbreviations: AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid; APV, (2R)-amino-5-5phosphonovaleric acid; CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione.
The levels of glutamate in the mammalian CNS are very high compared with the levels of all other neurotransmitters, ranging between 5 and 10 mmol/kg of tissue (22). Excitotoxicity is caused by the excessive and dysregulated activation of glutamate receptors. Prolonged exposure of these receptors to high or persistently increased concentrations of glutamate can lead the cell expressing these receptors to death (27). In physiologic conditions, extracellular levels of glutamate are maintained at submicromolar concentrations, more likely in the nanomolar concentration range (64), which is too low to cause activation of the high-affinity glutamate receptors. During synaptic release events, glutamate concentration can increase up to the millimolar range (32). Excitotoxicity is propagated primarily through the Ca2+-permeable receptors. Influx of Ca2+ is buffered by the endoplasmic reticulum (ER) and the mitochondria, but in the presence of excess Ca2+ influx, these systems can be overwhelmed. Ca2+ overload or perturbations of intracellular Ca2+ compartmentalization can activate or enhance mechanisms leading to cell death. An imbalance between Ca2+ influx and efflux from cells is the initial signal leading to Ca2+ overload and death of neurons (Fig. 1). In addition, alterations in intracellular Ca2+ storage can integrate with death signals that do not initially require Ca2+, to promote processing of cellular components and death by apoptosis or necrosis. Finally, Ca2+ can directly activate catabolic enzymes such as proteases, phospholipases, and nucleases that directly cause cell demise and tissue damage. When the mitochondrial buffering system fails, the cell becomes highly vulnerable to mitochondria-mediated apoptosis, reactive oxygen species (ROS), production and electron-chain dysfunction (30, 43).
Glutamate is cleared from the intersynaptic milieu by specialized transporters for a normal, nonpathogenic functioning of the synapses (69). Unlike acetylcholine at the neuromuscular junction, which is enzymatically degraded, no evidence exists for the presence of extracellular synaptic enzymes that can inactivate glutamate (69). Clearance of glutamate is accomplished by a family of glutamate-transporter proteins. Five high-affinity, Na+-dependent glutamate transporters have been identified and termed EAAT1–5, also known in rodents as GLAST, GLT-1, EAAC1, and rodent EAAT4–5 (Table 2). These transporters share ∼50–60% amino acid sequence similarity and have varying cellular and anatomic distributions (6, 18, 72). EAAT3–5 are expressed by neurons throughout the brain. Notably, EAAT4 and EAAT5 are specifically located in Purkinje cells in the cerebellum and the retina, respectively. EAAT1 and EAAT2 are located mainly on astrocytes, although they also are expressed by other glia cells like oligodendroglia and macrophages, with EAAT1 primarily expressed in the cerebellum, and EAAT2 widespread throughout the CNS (36). Splice variants of EAAT2 have also been cloned, but their abundance is relatively low, and their specific purpose still unclear (25, 26). The EAATs have structural differences and varying affinity for glutamate and sensitivities to glutamate-receptor agonists, which create physiological differences in activity. The KA-receptor agonist kainic acid, and its dehydrogenated form, dihydrokainic acid (DHK), specifically block, with high affinity, EAAT2, which highlights a significant difference between the classes of transporters (6). Crystallographic studies recently shed light on the architecture of glutamate transporters. Yernool and colleagues (156) crystallized a glutamate transporter (Glt-ph) from the obligate anerobe, Pyrococcus horikoshii, which shares ∼40% homology with the eukaryotic glutamate transporter EAAT2. The protomer structure contains eight transmembrane regions, which are predominantly α-helical (regions 4 and 7 are segmental α-helices), a large extracellular loop connecting transmembrane region 3 and 4, and two hairpin regions. These data, combined with biochemical evidence, also predict that the functional glutamate transporter has a homotrimeric quaternary structure, which is conserved in bacterial and human transporters (53, 156).
Table 2.
Classification and distribution of glutamate transporters in the nervous system
| Human gene name | Rodent gene name | Cellular expression | Anatomical distribution |
|---|---|---|---|
| EAAT1 | GLAST | Astrocytes | Cerebellum |
| EAAT2 | GLT1 | Astrocytes | Widespread throughout CNS |
| EAAT3 | EAAC1 | Neurons | Widespread throughout CNS |
| EAAT4 | Rodent | Neurons | Purkinje cells of the cerebellum |
| EAAT4 | |||
| EAAT5 | Rodent | Neurons | Retina |
| EAAT5 |
EAAT, excitatory amino acid transporter; GLT, glutamate transporter; GLAST, glutamate-aspartate transporter.
Glutamate transporters account for the bulk transport of glutamate across the plasma membrane of cells and act quickly to buffer synaptically released glutamate (140). To accomplish these tasks, glia cells express transporters in abundance, whereas neurons express fewer transporters, although they appear to be precisely located near or at the synapses. Several lines of evidence suggest that neuronal transporters work by controlling activation of metabotropic glutamate receptors at the postsynapse (17) and by limiting glutamate spillover between adjacent synapses (38) whereas their glial counterparts function as the main glutamate sinks for all released glutamate. The glial glutamate transporter EAAT2 is very abundant in the brain, representing up to 1% of total brain proteins (35), and it is therefore thought to be primarily responsible for the removal of glutamate from the synapse (113). A clear understanding of the contribution of EAAT2 to total glutamate transport in the CNS came from studies performed in synaptosomes prepared from EAAT2-knockout mice. Tanaka and colleagues (130) found that glutamate uptake in cortical crude synaptosomes of EAAT2(−/−) mice was reduced to 5.8% of that measured in synaptosomes from wild-type mice, indicating that EAAT2 is responsible for the greatest proportion of glutamate transport in the CNS. Phenotypically, EAAT2-knockout mice are hyperexcitable and die prematurely (50.0% survival after 6 weeks) with occurrence of spontaneous epileptic seizures and behavioral patterns similar to those of N-methyl-d-aspartate (NMDA)–induced seizures, underscoring the role for EAAT2 in maintaining functional excitatory neurotransmission. By using long-term antisense oligonucleotide administration, in vitro and in vivo, Rothstein and colleagues (115) demonstrated that loss of the glial glutamate transporters EAAT1 or EAAT2 produced elevated extracellular glutamate levels, neurodegeneration characterized by excitotoxicity, and a progressive paralysis in rats. These studies suggest that glial glutamate transporters could provide the majority of functional glutamate transport and are essential for maintaining low extracellular glutamate and for preventing chronic glutamate neurotoxicity.
Changes in expression and activity of glutamate transporters have been reported in many neurodegenerative diseases such as Huntington's disease, Parkinson's disease, Alzheimer's disease, and amyotrophic lateral sclerosis (ALS), but also in astrogliomas, epilepsy, and in more-acute neuropathologic events like stroke and ischemia (90). In ALS, the role of the glutamate transporter EAAT2 has been investigated more thoroughly. In chronic neurodegenerative diseases, however, it is not clear whether these dysfunctions in the glutamate-transport system contribute to the pathogenesis or whether they are more a secondary event consequential to primary pathologic insults. This is not to say that, in the latter case, glutamate-transporter dysfunction does not play a role in the overall pathologic manifestation of the diseases. However, the temporal correlation between glutamate-transporter dysfunction and pathology is a question that must be addressed more thoroughly, as it may have important therapeutic and mechanistic implications.
Amyotrophic lateral sclerosis and glutamate
ALS is a fatal paralytic disorder characterized by selective death of motor neurons. Approximately 10% of ALS cases are inherited (FALS), and 90% are sporadic (SALS). About 25% of the FALS cases are caused by missense mutations in the ubiquitously expressed enzyme Cu2+/Zn2+ superoxide dismutase (SOD1). The symptoms and pathology of SOD1-FALS closely resemble the rest of ALS cases, raising considerable enthusiasm for the transgenic animal models expressing human SOD1 mutations (mutSOD1), in the hope that these models could provide insights into the pathogenic mechanisms of both FALS and SALS. MutSOD1-mediated toxicity results from the impairment of multiple cellular functions (12, 104). Ubiquitous expression of high levels of mutSOD1 causes progressive motor neuron disease in transgenic mice (i.e., SOD1-G93A, G37R, and G85R) and rats (H46R and G93A) that recapitulates most of the clinical features of human ALS (20, 60, 68, 98, 154). Although the cause of paralysis in ALS is the death of motor neurons, the cell autonomy of the pathogenesis has been questioned by studies in which selective excision of mutSOD1 from microglia and astrocytes emphasized the role of these cells as key contributors in ALS pathogenesis (13, 31, 155). Several factors originating from different cell types were also investigated as potential toxic molecules that could mediate motor-neuron death (12, 104). Among these, a role for the dysregulation of glutamate homeostasis in ALS-mediated neurodegeneration has been established, based on the following evidence:
Motor neurons showed a marked vulnerability to glutamate excitotoxicity (112, 144–147). In vitro experiments showed that motor neurons in spinal cord organotypic cultures are particularly vulnerable to increased glutamate levels or to AMPA-receptor–mediated excitotoxicity (116, 119). Similarly, induction of motor-neuron death was also achieved by activating Ca2+-permeable AMPA receptors both in vitro, in a co-culture system consisting of motor neurons seeded on an astrocytic monolayer (145), and in vivo by delivery in the mouse spinal cord of selective agonists. (66, 71, 99, 136).
Increased plasma levels of glutamate (1, 107), decreased glutamate uptake, decreased expression levels of the glial glutamate-transporter EAAT2 (47, 117), and altered glutamine synthetase (14) have been documented in ALS patients.
Cerebrospinal fluid (CSF) collected from ALS patients, but not from healthy controls, was shown to cause excitotoxicity in neuronal cultures, which is blocked by glutamate-receptor antagonists. This implies that the levels of glutamate released in the extracellular milieu are higher in patients with ALS (29, 62, 124).
The only effective treatment available today for ALS is the antiglutamatergic drug riluzole, which is routinely prescribed for ALS patients. Riluzole regulates glutamate release, postsynaptic receptor activation, and inhibits voltage-sensitive channels (2, 10). Riluzole also was found to increase significantly glutamate uptake in a dose-dependent manner in the mouse CNS, facilitating the buffering of excessive extracellular glutamate and suggesting that the neuroprotective action of riluzole might be partly mediated by its activating effect on glutamate uptake (51). Treatment with riluzole decreased the plasma levels of excitatory amino acids during late stages of ALS in patients (101), although these data should not be considered conclusive (2). Riluzole remains the only FDA-approved drug for ALS, based on the 3-month improvement in survival observed in two large clinical trials (10, 82, 83).
A role for astrocytes and impairment of the astroglial glutamate-transporter EAAT2 in ALS
Astrocytes intimately interact with neurons to provide trophic support and actively participate in neuronal excitability by controlling the extracellular levels of ions and neurotransmitters (149). Astrocytes also exert potent trophic influences on motor neurons through a variety of proteins and molecules. In response to injury, astrocytes and microglia display characteristic phenotypic changes characterized as astrocytosis or gliosis and respond to pathologic stress by proliferating and adopting a reactive phenotype, which is characterized morphologically by hypertrophic nuclei and cell bodies, and elaboration of distinctly long and thick processes with increased content of glial fibrillary acidic protein (GFAP). In addition, reactive astrocytes express a wide variety of markers, such as cytoskeleton proteins, cell-surface and matrix molecules, proteases, protease inhibitors, several growth factors, and cytokines (111). By secreting diffusible factors, damaged neurons or activated astrocytes interact in a complex manner with immune cells and microglia. Activated microglia, in turn, secrete proinflammatory peptides, nitric oxide (NO), and excitotoxins that further induce astrocytosis or aggravate neuronal damage, therefore perpetuating and amplifying a local pathogenic process (56). Recent evidence indicates the existence of mechanisms by which activated astrocytes may contribute either to the death of neurons or to their survival in response to damage (7, 106, 132). Understanding these processes and the interaction between neurons and glia may help to explain the induction and the propagation of motor-neuron loss in ALS.
Astroglia dysfunction in ALS occurs through different synergistic mechanisms
Cytokine production by astrocytes
Much of the research on the pathology of neurodegenerative diseases has been focused on neuroinflammatory mechanisms. In ALS, neuroinflammation involves the entire motor system (63). Important functional interactions have been described between IL-1β expression by glial cells and the occurrence of excitotoxic mechanisms and neuronal death in diverse forms of neurodegeneration, which could be relevant in ALS pathophysiology. Interestingly, cytokine signaling can induce iNOS, COX-2, and NMDA-receptor phosphorylation, with different consequences in glial and neuronal cells. Activation of iNOS in astrocytes by IL-1β potentiates NMDA-mediated neurotoxicity in mixed cortical cultures (65).
Production of nitric oxide (NO°) and peroxynitrite (ONOO−)
Free radical damage is a characteristic of ALS tissues (46). Several reports have shown that reactive astrocytes in culture may contribute to free-radicals formation and neuronal death. In particular, induction of iNOS by lipopolysaccharide (LPS) or cytokines seems to be required for astrocytes to promote neuronal death (126). Barbeito and colleagues (24, 105) reported that production of NO° by reactive astrocytes is required for the induction of motor-neuron apoptosis in a co-culture model. Apoptotic motor neurons were immunoreactive for nitrotyrosine, suggesting a role for ONOO−. NO° itself cannot nitrate tyrosine, which implies that it was transformed into peroxynitrite by reaction with superoxide. Nitrotyrosine staining has been reported in cultured motor neurons undergoing apoptosis (44, 45), in spinal cord of mutSOD1 mice, and in sporadic and familial cases of ALS (46).
Production of apoptotic factors
Cytokines and trophic factors produced by reactive astrocytes such as FasL, TNF-α, and NGF, are capable of activating death receptors expressed in the diseased CNS. Receptor-mediated apoptosis could play a role in motor neuron loss in ALS without the direct involvement of the immune system. These factors show a dual function, promoting cell survival or death, depending on gene expression and activation state of the target cell (i.e., motor neurons) (8). Another potential apoptotic candidate released by astrocytes is NGF. Clearly, NGF is critical for the differentiation and survival of specific neuronal populations during development and for neural plasticity in the mature CNS (121). Whereas NGF can signal through activation of the high-affinity TrkA receptor, it also can activate the nonselective neurotrophin receptor p75NTR, a member of the tumor necrosis factor–receptor superfamily. Motor neurons are generally unresponsive to NGF because they lack the specific TrkA receptor. Signaling through p75NTR, in the absence of the corresponding Trk receptor, has been shown to promote apoptosis in specific neuronal types during normal CNS development (49) and is probably used to eliminate damaged neurons and oligodendrocytes in the mature CNS. Motor neurons express p75NTR during the embryonic period of naturally occurring cell death when more than half of motor neurons die, but its expression gradually ends after birth. Although p75NTR is not present in mature motor neurons, the receptor can be re-expressed after nerve injury (110). Moreover, p75NTR is found in motor neurons of ALS patients (88), suggesting that re-expression of the receptor might modulate the death of neurons in damaged areas. Astrogliosis is associated with increased expression and release of several growth factors and cytokines, including NGF (42). Little is known about the expression of NGF in ALS, although increased NGF levels were reported in muscle of ALS patients (127). Thus, it is conceivable that NGF signaling between astrocytes and p75NTR-expressing motor neurons may contribute to the induction of neuronal apoptosis in ALS.
Downregulation and impairment of the glutamate transporter EAAT2
The downregulation of EAAT2 expression and activity levels in ALS suggests a connection between this disease and synaptic glutamate homeostasis. Expression of EAAT2 is dramatically decreased in postmortem spinal cord specimens of ALS patients, particularly in the ventral horn, where motor neurons are found (86). The first demonstration of an impaired glutamate-transport system was obtained by direct measurements of 3H-l-glutamate uptake in synaptosomes prepared from different CNS areas of sporadic ALS patients. The patients displayed a marked decrease in the maximal transport velocity (Vmax) for glutamate in synaptosomes prepared from spinal cord (−59%), motor cortex (−70%), and somatosensory cortex (−39%), but not in synaptosomes prepared from regions not affected by the disease, such as visual cortex, striatum, or hippocampus, or when compared with the corresponding regions in unaffected individuals or other neurodegenerative disease patients (117). The decrease of glutamate uptake (47, 117) has been linked specifically to a decrease in the levels of EAAT2 expression (19, 120). Although clear alterations in EAAT2 levels are found in patients with ALS, it is not likely that these reductions could have a genetic cause. With single-strand conformation polymorphism analysis of genomic DNA, Aoki and colleagues (137) identified one novel mutation in the EAAT2 gene in a single sporadic ALS patient and two novel mutations in two affected familial non-SOD1 ALS siblings. In the sporadic ALS patient, the mutation substitutes serine for an asparagine and removes one N-linked glycosylation site in the EAAT2 protein, affecting the normal function of the transporter. In the two affected individuals in the ALS family, a mutation in the 5' end of intron 7 and a silent G → A transition at codon 234 in exon 5 was also reported (4). However, no suggestion has been made that this polymorphism is widely represented among the ALS population or can cause the disease.
Abnormal variants of EAAT2 mRNA resulting from incorrect splicing were found in the affected CNS areas of ALS patients (86). These intron-retention and exon-skipping mRNA species encoded truncated EAAT2 fragments thought to have dominant-negative effects on the expression and activity of EAAT2 and claimed to be the cause of EAAT2 downregulation found in ALS patients. However, subsequent studies have contradicted these findings and showed that abnormal EAAT2 transcripts were also found in areas of the CNS unaffected by ALS, and in normal subjects, thus questioning the proposed link between intron-retention and exon-skipping EAAT2 mRNA variants as a cause for EAAT2 loss in ALS pathogenesis (48, 67, 95, 97).
Similar to sporadic ALS patients, mouse models of ALS also show a clear and consistent reduction in glutamate-transport activity and EAAT2 protein levels. In mutant SOD1 mice, several studies have shown decreased EAAT2 protein and downregulation of glutamate-transport activity in affected CNS areas (9, 15, 20, 23, 41, 151, 153, 155). Similar results are found in the SOD1-G93A and H46R transgenic rats (41, 68) (see also Fig. 2). One exception is a study from Heiman-Patterson and colleagues (37), in which the authors showed that EAAT2 levels in sensorimotor cortex, brainstem, and cervical and lumbar spinal cord of G93A mice did not differ significantly from controls, either at presymptomatic, early at onset, or at the end stage. Although puzzling, this latter study is interesting because these authors found retarded gel mobility of EAAT2 in the brainstem, cortex, and spinal cord of SOD1-G93A mice compared with controls. EAAT1 and EAAT3 were unchanged in both amount and mobility. The changes in EAAT2 mobility and distribution indicate that this transporter could be posttranslationally altered in mice with the SOD1 mutation. Evidence in the literature thus far has shown no decrease of EAAT2 mRNA levels in the spinal cords of transgenic mice, even at stages in which EAAT2 protein could be lost (9). In addition, no quantitative change in mRNA for EAAT1, EAAT2, or EAAT3 was found in the motor cortex of ALS patients, including patients with a large loss of EAAT2 protein (95% decrease compared with control) and decreased tissue glutamate transport (73% decrease compared with control), suggesting that the dramatic abnormalities in EAAT2 expression levels may be due to translational or posttranslational processes. In support of posttranslational–mediated impairment and loss of EAAT2, several lines of evidence exist. EAAT2 is a selective molecular target for some of the pathologic mechanisms occurring in ALS. Oxidative or nitrosative stressors produce rapid inactivation of the transporter activity (109, 139). When cultured astrocytes expressing endogenous levels of EAAT2, MDCK cells transiently expressing EAAT2, or Xenopus oocytes expressing EAAT2 are transfected with ALS-causing SOD1 mutations, a marked reduction in transporter activity and protein levels is seen (133, 138, 148). Analysis of chimeric transporters indicates that the EAAT2 cytosolic C-terminus domain could drive the specific degradation and removal of the transporter from the plasma membrane (148). The mechanisms of EAAT2 downregulation in vivo in ALS are, however, not completely understood.
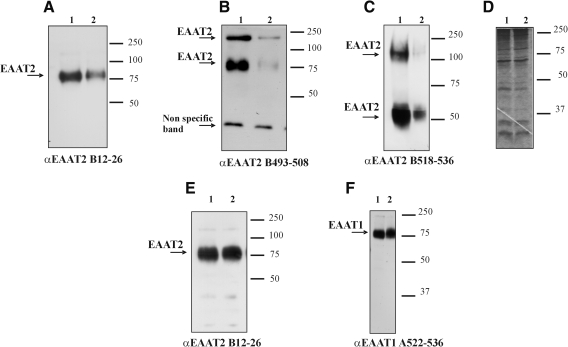
FIG. 2.
Expression levels of EAAT2 are decreased in spinal cord homogenates prepared from SOD1-H46R rat model of ALS. Representative results from three different experiments showing Western blot analysis of spinal cord homogenates (A–D) and hippocampus (E, F) of SOD1-H46R rats at presymptomatic stage (lane 1) and disease end-stage (lane 2). Spinal cords and hippocampi were collected and immediately homogenized on ice (glass-Teflon homogenizer; 1,000 rpm) in 30 volumes of extraction buffer containing SDS (1%), 150 mM NaCl, 10 mM NaPi (pH 7.4), and Complete protease inhibitor mix with EDTA (Roche). “Crude” extracts were incubated for 10 min at room temperature, briefly sonicated, centrifuged (1,000 g, 4 min) to remove unsolubilized material, and immediately analyzed or stored at −80°C. The homogenates prepared with this protocol were termed SDS extracts (15). The rat model of ALS (SOD1-H46R line 4) was generated by Nagai and colleagues, and the phenotype was described in (98). Spinal cord and hippocampus homogenates were collected at 120 days of age for the presymptomatic stage and 160 days of age for the end stage. Blots were probed with affinity-purified polyclonal antibodies against peptides of the glutamate transporter EAAT1–2, referred to by capital letters A (EAAT1) and B (EAAT2), followed by numbers indicating the corresponding peptide of the rat transporter sequence. For this study, we used A522–541 (0.2 μg/ml), B12–26 (0.2 μg/ml), B493–508 (0.1 μg/ml), and B518–536 (0.2 μg/ml). (D) Coomassie Blue staining to show equal total proteins content in lanes 1–2 of the spinal cord homogenates.
Several studies in the mouse model of ALS showed that changes in the EAAT2 expression levels and glutamate-uptake activity are found only in the ventral horn of the affected spinal cord at a late stage of disease (9, 23, 41). An interpretation of these studies points to a noncausal role for EAAT2 impairment in ALS, because if downregulation of EAAT2 is seen only at a late stage of the disease, that is, after motor neurons have been for the most part lost, then these changes may be more a consequence of the motor-neuron loss and not a key to their demise. An additional clue supporting this interpretation may be offered by the evidence that the expression levels of EAAT2 are downregulated in purified astrocytes in primary culture where the influences of neurons are removed, suggesting that expression of EAAT2 is critically dependent on the presence of neurons or at least a soluble factor released from the neurons (122, 128).
Nevertheless, not all CNS diseases associated with neuronal death display loss of EAAT2. For example, spinal muscular atrophy, another motor-neuron disease characterized by motor-neuron degeneration, has no associated loss of EAAT2 (5, 125). If EAAT2 downregulation is a consequence of motor-neuron loss rather then the cause, then the claim that motor neurons could succumb to excitotoxic damage caused by decreased glutamate-transport activity in ALS also should be revisited. Indeed, few reports in the literature do not support glutamate-transport deficits as a cause of neuronal death in vivo (33, 66, 93). In a recent study, Tovar-y-Romo and colleagues (135) attempted to verify whether blockade of glutamate transporters could result in hyperexcitation and loss of neurons exposed to the accumulation of extracellular glutamate in the SOD1-G93A mouse model of ALS (135). The expectations were that, in the disease, mouse motor neurons and, in general, neuronal cells would be more susceptible to excitotoxicity because of the associated oxidative environment caused by the presence of disease-causing mutant SOD1 proteins (11). Infusion by reverse microdialysis of l-trans-2,4-pyrrolidin-dicarboxylic acid (PDC) (25 mM for 1 h), a transportable nonselective inhibitor of glutamate transporters, directly into the hippocampus or motor cortex of SOD1-G93A mice and SOD1–wild-type control mice caused a consistent (about sixfold) increase in extracellular glutamate. In both mouse models and despite the marked increase in extracellular glutamate, histologic examinations showed that no overt neuronal loss occurred in the hippocampus and motor cortex of either SOD1–wild-type or, even more surprisingly, SOD1-G93A ALS mice treated with PDC. These results are quite in contrast with the hypothesis of a causal role for glutamate-transporter impairment in ALS, because neither increased neuronal susceptibility to excitotoxicity in the diseased SOD1-G93A mice nor a correlation between elevation in extracellular glutamate mediated by glutamate-transport blockade and neuronal death in vivo was found. However, some considerations should be pointed out regarding this study: (a) the analysis of the neuronal loss was only limited to 24 h after infusion of PDC; (b) although no increased neuronal loss was detected, the study does not indicate whether the neurons exposed to high concentrations of glutamate were indeed starting to become dysfunctional and still progress to dead motor neurons if observed over a longer temporal scale; and (c) extracellular GABA levels were not reported. An increase in GABA release, which could have been induced concurrent with the PDC-mediated increase in extracellular glutamate, would considerably dampen the excitation of neurons and effectively protect them from the excitotoxic insult produced by the blockade of glutamate transporters (28, 77, 158).
Other reports in the literature, however, showed that the loss of the glutamate-transporter EAAT2 in the mouse and rat models of ALS occurs also at presymptomatic and early symptomatic stages of the disease when no overt loss of motor neurons has occurred, suggesting perhaps that additional mechanisms could be responsible for the selective loss of EAAT2, independent of neuronal inputs (54, 68, 114). In the SOD1-G93A rat model of ALS, focal loss of the EAAT2 glutamate transporter in the ventral horn of the spinal cord coincides with gliosis, but appears before motor neuron/axon degeneration. At end-stage disease, gliosis increases, and EAAT2 loss in the ventral horn exceeded 90%, suggesting a role for this transporter in the events leading to cell death in ALS (68). In further support of this, direct manipulation of EAAT2 expression levels has effects on both cellular and animal models of ALS. Overexpression of EAAT2 was shown to be protective in vitro (92) and to slow disease progression in vivo (58). However, it does not prevent disease onset or death. In one study, transgenic SOD1-G93A mice, which expressed twice the normal levels of the EAAT2 glutamate transporter and had twice the normal glutamate-uptake capacity in the spinal cord, had better-preserved motor function and delayed death of spinal motor neurons, but not delayed onset of ALS symptoms, suggesting that EAAT2 overexpression could indeed afford some protection and that loss of EAAT2 may contribute to, but does not cause, motor-neuron degeneration in ALS (58). In this study, Guo and colleagues (50) coupled the expression of the EAAT2 transgene to a ∼2-Kb fragment of the promoter of the astrocyte-specific GFAP gene, a rather weak promoter that becomes active at or around disease onset (50), and thus the EAAT2 expression was progressively increased only when the disease was beginning to manifest (102). This may explain the partial protection offered by elevating EAAT2 levels. In another study, Pardo and colleagues (103) took the opposite approach and investigated whether a further reduction in EAAT2 expression levels could accelerate motor-neuron degeneration. They crossed the SOD1-G93A mouse line with a mouse heterozygous for EAAT2 (EAAT2(+/−)). SOD1-G93A::EAAT2(+/−) bigenic mice exhibited a significant reduction in transporter protein and increased rate of motor decline accompanied by earlier motor-neuron loss and reduction in survival, again underscoring a role for EAAT2 loss in ALS (103). More recently, an elegant study from Maragakis's group (85) demonstrated that a significant level of motor-neuron protection could also be achieved by transplanting glia-restricted precursor cells (GRPs) in the spinal cord of the SOD1-G93A rat, an interesting application that can have therapeutic implications. What makes this study relevant to excitotoxicity is the evidence that neuroprotection and therapeutic efficacy of GRP cells could have been mediated in part by the astrocytic glutamate-transporter EAAT2 expressed in these cells.
Compounds such as the β-lactam antibiotic ceftriaxone and GPI-1046, an immunophilin ligand, were discovered to increase the levels of EAAT2 in astrocytes in culture, in spinal cord organotypic cultures, and in vivo (52, 118). Ceftriaxone increases EAAT2 transcription in astrocytes through the nuclear factor-κB (NF-κB) signaling pathway by promoting nuclear translocation of p65 and activation of NF-κB (84). The mechanism of action of GPI-1046 is still not clear and must be investigated more in detail, although the initial indication suggests that immunophilins are involved in this upregulation (52). Both ceftriaxone and GPI-1046 have been reported to prolong survival and protect motor neurons in mutant SOD1 transgenic mice. Again, this may suggest that EAAT2 dysfunction contributes to disease progression and that these drugs were neuroprotective because of their EAAT2-enhancing effect, although no proof has been provided that this mechanism was indeed directly responsible for the neuroprotection. Despite affording some degree of neuroprotection, the overall effect on the disease phenotype of these drugs is quite unsatisfactory, casting doubts on the relevance of the glutamate transporter–mediated excitotoxic pathway in the degeneration of motor neurons in ALS. In this respect, one consideration should be made in light of a recent study in which we reported that nor-dihydroguaiaretic acid (NDGA), an antiinflammatory compound that is also a potent glutamate-uptake enhancer both in vitro and in vivo (16), failed to increase glutamate uptake in the ALS mice because of poor CNS bioavailability, likely caused by a disease-driven increased expression of the multidrug-efflux transporter, P-glycoprotein (16). P-glycoproteins, along with other drug-efflux transporters, work by expelling from the cells toxins and xenobiotics, including many potential therapeutic compounds, limiting their effectiveness (87). It is therefore possible that poor bioavailability of ceftriaxone and GPI-1046 was responsible for their modest therapeutic efficacy. The specific mechanisms by which the expression and function of multidrug transporters are regulated in neurologic disorders like ALS should also be investigated in detail and, if necessary, should reconsider many clinical trials that have been attempted in the mutant SOD1 animal model in which the increased expression of multidrug transporters could have compromised a positive outcome.
One theory to explain the depressed levels of EAAT2 expression and activity during the progression of ALS is that aberrantly formed splice variants of the EAAT2 transcript may prevent proper EAAT2 expression (57). Originally, these aberrant transcripts were selectively found in ALS patients. However, subsequent studies found these variants also in normal controls (95) and in white matter far from the areas of depressed EAAT2 expression (89). Another possibility is that, although EAAT2 could be properly translated and expressed, it loses function in ALS. This hypothesis is supported by the evidence that a lower Vmax for EAAT2 is measured in mutant SOD1 animal models (41). In addition, reactive oxygen species (ROS) formed in motor neurons after glutamate-receptor activation seem to be able to diffuse out of the motor neurons and induce oxidation and disruption of EAAT2-mediated glutamate uptake in neighboring astrocytes (108, 109).
Correspondingly, in a transgenic mouse model of ALS, protein oxidation was increased in regions immediately surrounding motor neurons. These results provide a mechanism that can account for the focal loss of glial glutamate transport seen in the disease (68) and lend support for a feed-forward model involving reciprocal interactions between motor neurons and glia, which may prove useful in understanding ALS pathogenesis (109).
Loss of EAAT2 activity could also be mediated by a direct or indirect effect of mutant SOD1 proteins on the transporter. We have demonstrated that mutant SOD1 proteins linked to familial ALS inactivate the function of EAAT2 (138). Exposure of Xenopus oocyte cells expressing SOD1-A4V, I113T, or SOD1 wild-type as control and the glutamate-transporter EAAT2 to the biologic oxidant hydrogen peroxide led to a rapid and consistent inhibition of the transporter activity when either one of the two mutant SOD1s, but not wild-type proteins, were expressed with EAAT2. The molecular determinant(s) of the EAAT2 inhibition resided in the cytoplasmic C-terminal domain of the transporter. The inhibition was blocked and even partially reversed by antioxidants such as Mn(III)TBAP, a manganese porphyrin with SOD1-mimetic free radical–scavenging properties, suggesting oxidation of critical residues within the C-terminal domain of EAAT2 as a possible mechanism of inactivation. The precise site(s) of oxidation is not yet defined, but it seems unlikely that a single amino acid residue could be responsible for the loss of activity. However, oxidation of the transporter mediated by mutant SOD1 proteins did not alter the transport properties of EAAT2, such as the affinity for glutamate and transport coupling coefficient, suggesting that oxidized transport molecules are either nonfunctional or form more-rigid structures that impair the overall transport dynamics.
Interestingly, a specific disulfide reducing agent like dithiothreitol (DTT) was found effective in halting the inhibition of EAAT2 but ineffective in reversing it, ruling out a major role for disulfide bridge formation among cysteine residues as the culprit mechanism for the inhibition. Among possible targets of oxidation are aromatic rings of tyrosine, histidine, and tryptophan residues, which are possibly vulnerable to dimerization. More-extensive oxidation may also result in modification of the thiol groups within the EAAT2 C-terminus, leading to the formation of sulfenic, sulfininc, and sulfonic acids, which are not reducible by DTT. Also important is the selectivity of the inhibitory action of mutant SOD1 proteins toward EAAT2. The neuronal glutamate-transporter EAAT3 does not display the same sensitivity to oxidant stressors and mutant SOD1 proteins. Swapping the cytosolic C-terminus domain of EAAT2 with the same domain of EAAT3 generated a chimeric EAAT2 transporter that was insensitive to the same inhibitory paradigm.
Searching for the molecular determinant(s) of EAAT2 sensitivity, we recently discovered that caspase-3 can cleave EAAT2 at a unique site located in the cytosolic C-terminus of the transporter, inactivating the transporter activity, a finding that could link excitotoxicity and activation of caspase-3 in astrocytes as converging mechanisms in the pathogenesis of ALS. Interestingly, mutant SOD1 protein–mediated inhibition of EAAT2 is also largely, although not completely (≥60%), blocked by a specific inhibitor of caspase-3 and partially prevented by disruption of the unique caspase-3 consensus site in the cytosolic C-terminal domain on EAAT2 by site-directed mutagenesis (15), suggesting that biologic oxidants like H2O2 in the presence of FALS-linked mutSOD1 proteins could lead to activation of caspase-3, which in turn cleaves within the C-terminus of EAAT2, inactivating the transporter.
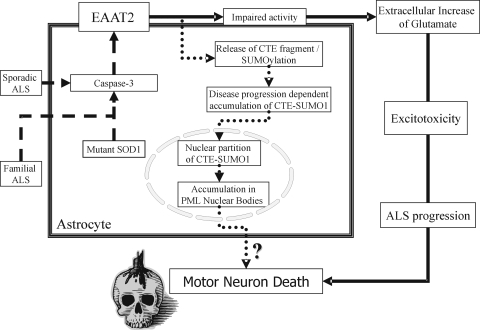
What is emerging from these studies is a critical role for the C-terminal domain of EAAT2 in regulating the transporter activity. Whether oxidation and caspase-3 cleavage occur concurrently or whether to be cleaved by caspase-3, the C-terminus domain would have to be first oxidized, remains to be elucidated. In addition, whether these processes are responsible, partially or totally, for the loss of EAAT2 in ALS also remains to be established. In a further follow-up to these studies, we demonstrated that a proteolytic fragment of EAAT2 derived from caspase-3 cleavage of the EAAT2 cytoplasmic C-terminus is SUMO1 conjugated (termed CTE-SUMO1), progressively accumulates in the spinal cord of SOD1-G93A mice, starting as early as the presymptomatic stage, and this accumulation is disease specific, as it does not occur, for instance, in the mouse model for Huntington disease (54). Moreover, we found that CTE-SUMO1 accumulates in the nucleus of astrocytes, where it interacts with promyelocytic leukemia protein (PML) in nuclear structures called PML-nuclear bodies (PML-NBs) (54). Several lines of evidence indicate that SUMOylation of polypeptides and proteins can affect protein stability, protein–protein interactions, subcellular relocalization, and transcriptional regulation (61), and that PML-NBs interact with chromatin to regulate gene expression and DNA repair and transcription (55). This leads to the speculation that CTE-SUMO1 may be involved in the dysregulation of nuclear processes in astrocytes. Because astrocytes and neurons interact in physiology, accumulation of CTE-SUMO1 in astrocytes may disturb or alter this relation and ultimately lead to neuronal damage and degeneration (Fig. 3).
FIG. 3.
Posttranslational processing of the glutamate transporter EAAT2 in ALS. Activated caspase-3, in mutant SOD1-expressing astrocytes, cleaves the glutamate transporter EAAT2 at a unique site in the cytoplasmic C-terminal domain. Activation of caspase-3 could be mediated by the toxic gain-of-function of mutant SOD1 in familial ALS, or it might occur through some other still-unidentified pathway in sporadic ALS or the non-SOD1 component of familial ALS (dashed-line pathway). The cleavage inactivates the transporter, leading to decreased clearance of synaptically released glutamate and persistent activation of glutamate receptors (excitotoxicity), which could contribute to the death of motor neurons in ALS (solid-line pathway). This proteolytic cleavage also releases a C-terminal fragment of EAAT2 (CTE), which we found to be posttranslationally modified by SUMO1 (CTE-SUMO1). SUMOylation of the CTE can occur before or after the fragment has been released from the full-length transporter. Once released, CTE-SUMO1 accumulates in the nucleus of the astrocytes, where it associates with promyelocytic leukemia protein (PMLs) in subnuclear structures called PML-nuclear bodies (dotted-line pathway). Within these nuclear structures, CTE-SUMO1 may disrupt their normal functions, altering the genotypic profiles of the astrocytes, indirectly contributing to motor-neuron death or dysfunction. Both solid- and dotted-line pathways may together participate in the death processes of motor neurons in ALS.
Glutamate Receptor–Mediated Excitotoxicity in ALS
A chronic build-up of synaptic glutamate can cause excitotoxicity. It has been shown that excitotoxic disorders are capable of causing a dying-back neuronal phenotype similar to that seen in ALS. Evidence of the excitotoxic aspect of ALS is supported by the fact that the CSF of patients is excitotoxic to cultured motor neurons compared with that from normal controls (3, 124). In vitro, it was shown that motor neurons are selectively vulnerable to slow excitotoxic death (94). Motor neurons die from an influx of Ca2+ and mitochondria-mediated apoptosis. The large uncontrolled influx of Ca2+ causes stress to the Ca2+-buffering systems in the cell, in particular the mitochondria. When the mitochondria are overly stressed by cytosolic Ca2+ levels, an apoptotic cascade can be activated concomitant with increased ROS production. The stressed mitochondria are not capable of maintaining proper electron activity and ROS escape into the cytoplasm (43). Activation of glutamate (AMPA) receptors by delivery in the mouse spinal cord of selective agonists induced motor-neuron death. In vivo, intrathecal injection, osmotic minipump infusion, or microdialysis perfusion of AMPA in the spinal cord of adult rats also produced vast motor-neuron death associated with severe motor dysfunctions (71, 99, 136). Similarly, AMPA-mediated motor-neuron death can be achieved in vivo in rat by long-term blockade of glutamate transporters by Threo-β-hydroxyaspartate (THA) (66).
The Q/R editing site of the AMPA-receptor complex subunit GluR2 is a major aspect of the AMPA-receptor ability to block massive Ca2+ influx (123). The GluR2 subunit is subject to posttranscriptional RNA editing, which results, under normal circumstances, in a change of the glutamine residue (Q) in the Q/R site of the AMPA-receptor subunit GluR2 to arginine (R), a substitution that renders the AMPA-receptor complex Ca2+ impermeable when activated (39, 96). However, if this editing is prevented, the resultant AMPA receptor is permeable to Ca2+. The importance of edited GluR2 in neuronal survival is indicated by the phenotype of transgenic mice in which the RNA editing at the Q/R site is reduced. This generates a lethal phenotype characterized by seizures and acute neurodegeneration (21). GluR2-editing defects also have implications in ALS, because a significant reduction in RNA editing of GluR2 at the Q/R site occurred specifically in motor neurons of five patients with sporadic ALS (74, 81).
In support of a crucial role for GluR2 in controlling Ca2+ influx through the AMPA-receptor complex, the evidence indicates that the overexpression of a GluR2-deficient Ca2+-permeable AMPA receptor in mice leads to a late-onset motor-neuron degenerative disorder, which is exacerbated when coexpressed with the mutant SOD1 transgene (78, 79). To assess the role of Ca2+-permeable AMPA receptors on the evolution of ALS pathology in vivo, Yin and colleagues (157) examined the effects of prolonged intrathecal infusion of the AMPA channel blocker, 1-naphthyl acetylspermine (NAS), in SOD1-G93A transgenic rat. In wild-type animals, immunoreactivity for EAAT2 was particularly strong around ventral horn motor neurons. However, a marked loss of ventral-horn EAAT2 was observed, along with substantial motor-neuron damage, before onset of symptoms (90–100 days) in the SOD1-G93A rats. Compared with sham-treated SOD1-G93A animals, 30-day NAS infusions (starting at approximately 70 days of age) markedly diminished the loss of both motor neurons and of astrocytic EAAT2 labeling (157). Interestingly, a recent study showed that, under normal conditions, astrocytes regulate the expression and subunit composition of the AMPA receptors in motor neurons. However, under disease conditions, this regulatory action is decreased, and the level of GluR2-deficient AMPA receptors increases (141).
Although most attention has been focused on the AMPA receptor, involvement of other glutamatergic receptors in ALS has been noted. The glutamate receptor mGluR5 is implicated in morphologic disarrangement restricted to astrocytes directly surrounding spinal motor neurons in the mutant SOD1 mouse model of ALS (114). This degenerative process of the astrocytes manifests early at onset and becomes significant concomitant with the loss of motor neurons and the appearance of clinical symptoms. Blocking this receptor in vivo slows astrocytic degeneration, delays onset of ALS, and slightly extends survival in SOD1-G93A transgenic mice. The group 1 metabotropic receptors have also been implicated in the CSF-mediated toxicity in cultured motor neurons. Treatment with the specific group 1 mGluR antagonist 1-aminoindan-1,5-dicarboxylic acid selectively protects motor neurons from cell death when treated with the CSF of ALS patients (3).
Concluding Remarks
During the course of ALS, problems in both glutamate-receptor and glutamate-transporter systems culminate in an excitotoxic disorder, which could adversely affect the motor neurons. Both genetic and pharmacologic lines of evidence suggest that the glutamatergic system is crucial to the normal functioning of the synapses in the spinal cord, and its dysregulation could play a role in the disease of the motor system (Tables 3 and 4). Over the last 15-year period, many pathogenic mechanisms have been proposed to take part in ALS pathogenesis, and excitotoxicity is only one among them (104). This multitude of factors and mechanisms, however, indicates that not everything in ALS pathogenesis can be related to excitotoxicity, but what is emerging is that at least some of these mechanisms are interconnected. For example, several lines of evidence indicate that excessive stimulation of glutamate receptors, perhaps due to impairment to the glutamate-transport system and, in particular, of the astroglial transporter EAAT2, could lead to Ca2+ overload in mitochondria, resulting in overproduction of ROS and oxidative stress–mediated motor-neuron damage. In addition, motor neurons could become more sensitive to glutamate-mediated excitotoxicity in the presence of mutant SOD1 in mitochondria (76).
Table 3.
Some of the therapeutic trials on transgenic SOD1-G93A mice with compounds involved in glutamate-mediated excitotoxicity
| Agent (mechanism of action with respect to glutamate homeostasis) | Dose | Route | Start of therapy (days) | Onset change (%) | Survival change | Reference |
|---|---|---|---|---|---|---|
| Carboxyfullerene (block excitotoxicity mediated NMDA and AMPA receptors) | 15 mg/kg/day | i.p. | 73 | Increase | Increase | 40 |
| Ceftriaxone (increase expression levels and activity of EAAT2) | 200 mg/kg/day | i.p. | 42 | Increase | +11% | 118 |
| Gabapentin (decrease of glutamate release) | 3% diet | diet | 50 | − | +5% | 59 |
| GPI-1046 (increase expression levels and activity of EAAT2) | 50 mg/kg twice a day | p.o. | 150 - SOD1-G93A low expressors | − | +12% | 52 |
| Memantine (noncompetitive NMDA antagonist) | 10 mg/kg twice a day | s.c. | 70 | − | +7% | 150 |
| NBQX (AMPA receptor antagonist) | 8 mg/kg 5 times a week | i.p. | 70 | Increase | +10 | 143 |
| Riluzole (decrease of glutamate release, increase of glutamate uptake) | 50 mg/kg/day | p.o. | 50 | − | +10% | 59 |
| Topiramate (decrease of glutamate release, block of AMPA receptors) | 50 mg/kg | p.o | 30 | − | − | 91 |
| NDGA (increase of glutamate transport) | 1 mg/day | s.c. | 90 | − | − | 16 |
| MPEP (block of mGluR5) | 30 mg/kg/day | i.p. | 40 | Increase | Increase | 114 |
| ZK 187638 (noncompetitive antagonist of AMPA receptor) | 140 mg/kg once in 2 days | p.o. | 77 | Increase | Increase | 134 |
i.p., intraperitoneal; p.o., per osmosis; s.c., subcutaneous; NBQX, 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzoquinoxaline-2,3-dione; MPEP, 2-methyl-6-(phenylethylnyl)pyridine; NDGA, nordihydroguaiaretic acid.
Table 4.
Modifier genes of transgenic mutant SOD1 mice involved in Excitotoxicity
| Model | Change in onset | Change in survival | Reference |
|---|---|---|---|
| EAAT2(GFAP) × SOD1-G93A | +19% | − | 58* |
| EAAT2(+/−) × SOD1-G93A | − | −4% | 103* |
| GluR2(−/−) × SOD1-G93A | −15% | −15% | 142* |
| GluR2(ChAT) × SOD1-G93A | +20% | +14% | 131* |
| GluR2-R607N × SOD1-G93A | − | −7% | 78* |
The promoter element used to drive expression of the transgene.
Drugs targeted to increase EAAT2 activity, the glutamate-transport system in general, or to block the AMPA receptors have been shown to prevent excitotoxicity in several models and could be potential treatments for disorders like ALS (Table 3). However, considering the poor therapeutic efficacy of these compounds in vivo, it is not clear whether targeting the excitotoxic pathways in ALS could result in a therapy for patients. In recent years, important advances have been made in understanding basic molecular mechanisms governing the expression and activity of glutamate transporters, their translational and posttranslational processing, and their involvement in regulating and shaping the excitatory neurotransmission. Considerable advances have been also made in the field of glutamate receptors with the design of more-specific inhibitors that can affect the different subclasses and subtypes of receptors. These achievements are expected to facilitate further studies on the role of individual transporter and receptor subtypes and to develop new strategies for the treatment of ALS and other diseases associated with malfunctioning of glutamate transporters and dysregulation of glutamatergic neurotransmission.
Acknowledgments
This work was supported by grants from NIH (NS044993) and Muscular Dystrophy Association. The Weinberg Unit for ALS research at Thomas Jefferson University is supported by the Farber Family Foundation.
Abbreviations
ALS, Amyotrophic lateral sclerosis; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid; CTE, C-terminus of EAAT2; EAAT, excitatory amino acid transporter; ONOO−, peroxynitrite; SOD1, superoxide dismutase 1; SUMO, small ubiquitin modifier; ROS, reactive oxygen species.
References
- 1.Andreadou E. Kapaki E. Kokotis P. Paraskevas GP. Katsaros N. Libitaki G. Petropoulou O. Zis V. Sfagos C. Vassilopoulos D. Plasma glutamate and glycine levels in patients with amyotrophic lateral sclerosis. In Vivo. 2008;22:137–141. [PubMed] [Google Scholar]
- 2.Andreadou E. Kapaki E. Kokotis P. Paraskevas GP. Katsaros N. Libitaki G. Zis V. Sfagos C. Vassilopoulos D. Plasma glutamate and glycine levels in patients with amyotrophic lateral sclerosis: the effect of riluzole treatment. Clin Neurol Neurosurg. 2008;110:222–226. doi: 10.1016/j.clineuro.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Anneser JM. Chahli C. Borasio GD. Protective effect of metabotropic glutamate receptor inhibition on amyotrophic lateral sclerosis-cerebrospinal fluid toxicity in vitro. Neuroscience. 2006;141:1879–1886. doi: 10.1016/j.neuroscience.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 4.Aoki M. Lin CL. Rothstein JD. Geller BA. Hosler BA. Munsat TL. Horvitz HR. Brown RH., Jr Mutations in the glutamate transporter EAAT2 gene do not cause abnormal EAAT2 transcripts in amyotrophic lateral sclerosis. Ann Neurol. 1998;43:645–653. doi: 10.1002/ana.410430514. [DOI] [PubMed] [Google Scholar]
- 5.Araki S. Hayashi M. Tamagawa K. Saito M. Kato S. Komori T. Sakakihara Y. Mizutani T. Oda M. Neuropathological analysis in spinal muscular atrophy type II. Acta Neuropathol (Berl) 2003;106:441–448. doi: 10.1007/s00401-003-0743-9. [DOI] [PubMed] [Google Scholar]
- 6.Arriza JL. Fairman WA. Wadiche JI. Murdoch GH. Kavanaugh MP. Amara SG. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci. 1994;14:5559–5569. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbeito LH. Pehar M. Cassina P. Vargas MR. Peluffo H. Viera L. Estevez AG. Beckman JS. A role for astrocytes in motor neuron loss in amyotrophic lateral sclerosis. Brain Res Brain Res Rev. 2004;47:263–274. doi: 10.1016/j.brainresrev.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Becher B. D'Souza SD. Troutt AB. Antel JP. Fas expression on human fetal astrocytes without susceptibility to fas-mediated cytotoxicity. Neuroscience. 1998;84:627–634. doi: 10.1016/s0306-4522(97)00455-7. [DOI] [PubMed] [Google Scholar]
- 9.Bendotti C. Tortarolo M. Suchak SK. Calvaresi N. Carvelli L. Bastone A. Rizzi M. Rattray M. Mennini T. Transgenic SOD1 G93A mice develop reduced GLT-1 in spinal cord without alterations in cerebrospinal fluid glutamate levels. J Neurochem. 2001;79:737–746. doi: 10.1046/j.1471-4159.2001.00572.x. [DOI] [PubMed] [Google Scholar]
- 10.Bensimon G. Lacomblez L. Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis: ALS/Riluzole Study Group [see comments] N Engl J Med. 1994;330:585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- 11.Boillee S. Cleveland DW. Revisiting oxidative damage in ALS: microglia, Nox, and mutant SOD1. J Clin Invest. 2008;118:474–478. doi: 10.1172/JCI34613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boillee S. Vande Velde C. Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Boillee S. Yamanaka K. Lobsiger CS. Copeland NG. Jenkins NA. Kassiotis G. Kollias G. Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- 14.Bos IW. Hoogland G. Meine Jansen CF. Willigen G. Spierenburg HA. van den Berg LH. de Graan PN. Increased glutamine synthetase but normal EAAT2 expression in platelets of ALS patients. Neurochem Int. 2006;48:306–311. doi: 10.1016/j.neuint.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Boston-Howes W. Gibb SL. Williams EO. Pasinelli P. Brown RH Jr. Trotti D. Caspase-3 cleaves and inactivates the glutamate transporter EAAT2. J Biol Chem. 2006;281:14076–14084. doi: 10.1074/jbc.M600653200. [DOI] [PubMed] [Google Scholar]
- 16.Boston-Howes W. Williams EO. Bogush A. Scolere M. Pasinelli P. Trotti D. Nor-dihydroguaiaretic acid increases glutamate uptake in vitro and in vivo: therapeutic implications for amyotrophic lateral sclerosis. Exp Neurol. 2008;213:229. doi: 10.1016/j.expneurol.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brasnjo G. Otis TS. Neuronal glutamate transporters control activation of postsynaptic metabotropic glutamate receptors and influence cerebellar long-term depression. Neuron. 2001;31:607–616. doi: 10.1016/s0896-6273(01)00377-4. [DOI] [PubMed] [Google Scholar]
- 18.Bridges RJ. Esslinger CS. The excitatory amino acid transporters: pharmacological insights on substrate and inhibitor specificity of the EAAT subtypes. Pharmacol Ther. 2005;107:271–285. doi: 10.1016/j.pharmthera.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Bristol LA. Rothstein JD. Glutamate transporter gene expression in amyotrophic lateral sclerosis motor cortex. Ann Neurol. 1996;39:676–679. doi: 10.1002/ana.410390519. [DOI] [PubMed] [Google Scholar]
- 20.Bruijn LI. Becher MW. Lee MK. Anderson KL. Jenkins NA. Copeland NG. Sisodia SS. Rothstein JD. Borchelt DR. Price DL. Cleveland DW. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- 21.Brusa R. Zimmermann F. Koh DS. Feldmeyer D. Gass P. Seeburg PH. Sprengel R. Early-onset epilepsy and postnatal lethality associated with an editing-deficient GluR-B allele in mice. Science. 1995;270:1677–1680. doi: 10.1126/science.270.5242.1677. [DOI] [PubMed] [Google Scholar]
- 22.Butcher SP. Hamberger A. In vivo studies on the extracellular, and veratrine-releasable, pools of endogenous amino acids in the rat striatum: effects of corticostriatal deafferentation and kainic acid lesion. J Neurochem. 1987;48:713–721. doi: 10.1111/j.1471-4159.1987.tb05575.x. [DOI] [PubMed] [Google Scholar]
- 23.Canton T. Pratt J. Stutzmann JM. Imperato A. Boireau A. Glutamate uptake is decreased tardively in the spinal cord of FALS mice. Neuroreport. 1998;9:775–778. doi: 10.1097/00001756-199803300-00001. [DOI] [PubMed] [Google Scholar]
- 24.Cassina P. Peluffo H. Pehar M. Martinez-Palma L. Ressia A. Beckman JS. Estevez AG. Barbeito L. Peroxynitrite triggers a phenotypic transformation in spinal cord astrocytes that induces motor neuron apoptosis. J Neurosci Res. 2002;67:21–29. doi: 10.1002/jnr.10107. [DOI] [PubMed] [Google Scholar]
- 25.Chen W. Aoki C. Mahadomrongkul V. Gruber CE. Wang GJ. Blitzblau R. Irwin N. Rosenberg PA. Expression of a variant form of the glutamate transporter GLT1 in neuronal cultures and in neurons and astrocytes in the rat brain. J Neurosci. 2002;22:2142–2152. doi: 10.1523/JNEUROSCI.22-06-02142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W. Mahadomrongkul V. Berger UV. Bassan M. DeSilva T. Tanaka K. Irwin N. Aoki C. Rosenberg PA. The glutamate transporter GLT1a Is expressed in excitatory axon terminals of mature hippocampal neurons. J Neurosci. 2004;24:1136–1148. doi: 10.1523/JNEUROSCI.1586-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi DW. Glutamate receptors and the induction of excitotoxic neuronal death. Prog Brain Res. 1994;100:47–51. doi: 10.1016/s0079-6123(08)60767-0. [DOI] [PubMed] [Google Scholar]
- 28.Matthews CC. Zielke HR. Fishman PS. Remington MP. Bowen TG. Glutamate decarboxylase protects neurons against excitotoxic injury. J Neurosci Res. 2007;85:855–859. doi: 10.1002/jnr.21187. [DOI] [PubMed] [Google Scholar]
- 29.Cid C. Alvarez-Cermeno JC. Regidor I. Salinas M. Alcazar A. Low concentrations of glutamate induce apoptosis in cultured neurons: implications for amyotrophic lateral sclerosis. J Neurol Sci. 2003;206:91–95. doi: 10.1016/s0022-510x(02)00339-8. [DOI] [PubMed] [Google Scholar]
- 30.Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 31.Clement AM. Nguyen MD. Roberts EA. Garcia ML. Boillee S. Rule M. McMahon AP. Doucette W. Siwek D. Ferrante RJ. Brown RH Jr. Julien JP. Goldstein LS. Cleveland DW. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 2003;302:113–117. doi: 10.1126/science.1086071. [DOI] [PubMed] [Google Scholar]
- 32.Clements JD. Lester RA. Tong G. Jahr CE. Westbrook GL. The time course of glutamate in the synaptic cleft. Science. 1992;258:1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- 33.Corona JC. Tapia R. AMPA receptor activation, but not the accumulation of endogenous extracellular glutamate, induces paralysis and motor neuron death in rat spinal cord in vivo. J Neurochem. 2004;89:988–997. doi: 10.1111/j.1471-4159.2004.02383.x. [DOI] [PubMed] [Google Scholar]
- 34.Corona JC. Tapia R. Ca2+-permeable AMPA receptors and intracellular Ca2+ determine motoneuron vulnerability in rat spinal cord in vivo. Neuropharmacology. 2007;52:1219–1228. doi: 10.1016/j.neuropharm.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 36.Danbolt NC. Lehre KP. Dehnes Y. Chaudhry FA. Levy LM. Localization of transporters using transporter-specific antibodies. Methods Enzymol. 1998;296:388–407. doi: 10.1016/s0076-6879(98)96028-1. [DOI] [PubMed] [Google Scholar]
- 37.Deitch JS. Alexander GM. Del Valle L. Heiman-Patterson TD. GLT-1 glutamate transporter levels are unchanged in mice expressing G93A human mutant SOD1. J Neurol Sci. 2002;193:117–126. doi: 10.1016/s0022-510x(01)00656-6. [DOI] [PubMed] [Google Scholar]
- 38.Diamond JS. Neuronal glutamate transporters limit activation of NMDA receptors by neurotransmitter spillover on CA1 pyramidal cells. J Neurosci. 2001;21:8328–8338. doi: 10.1523/JNEUROSCI.21-21-08328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dingledine R. Borges K. Bowie D. Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 40.Dugan LL. Turetsky DM. Du C. Lobner D. Wheeler M. Almli CR. Shen CK. Luh TY. Choi DW. Lin TS. Carboxyfullerenes as neuroprotective agents. Proc Natl Acad Sci U S A. 1997;94:9434–9439. doi: 10.1073/pnas.94.17.9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dunlop J. Beal McIlvain H. She Y. Howland DS. Impaired spinal cord glutamate transport capacity and reduced sensitivity to riluzole in a transgenic superoxide dismutase mutant rat model of amyotrophic lateral sclerosis. J Neurosci. 2003;23:1688–1696. doi: 10.1523/JNEUROSCI.23-05-01688.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eddleston M. de la Torre JC. Oldstone MB. Loskutoff DJ. Edgington TS. Mackman N. Astrocytes are the primary source of tissue factor in the murine central nervous system: a role for astrocytes in cerebral hemostasis. J Clin Invest. 1993;92:349–358. doi: 10.1172/JCI116573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Emerit J. Edeas M. Bricaire F. Neurodegenerative diseases and oxidative stress. Biomed Pharmacother. 2004;58:39–46. doi: 10.1016/j.biopha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Estevez AG. Crow JP. Sampson JB. Reiter C. Zhuang Y. Richardson GJ. Tarpey MM. Barbeito L. Beckman JS. Induction of nitric oxide-dependent apoptosis in motor neurons by zinc-deficient superoxide dismutase. Science. 1999;286:2498–2500. doi: 10.1126/science.286.5449.2498. [DOI] [PubMed] [Google Scholar]
- 45.Estevez AG. Spear N. Manuel SM. Radi R. Henderson CE. Barbeito L. Beckman JS. Nitric oxide and superoxide contribute to motor neuron apoptosis induced by trophic factor deprivation. J Neurosci. 1998;18:923–931. doi: 10.1523/JNEUROSCI.18-03-00923.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferrante RJ. Browne SE. Shinobu LA. Bowling AC. Baik MJ. MacGarey U. Kowall NW. Brown RH. Beal MF. Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. J Neurochem. 1997;69:2064–2074. doi: 10.1046/j.1471-4159.1997.69052064.x. [DOI] [PubMed] [Google Scholar]
- 47.Ferrarese C. Sala G. Riva R. Begni B. Zoia C. Tremolizzo L. Galimberti G. Millul A. Bastone A. Mennini T. Balzarini C. Frattola L. Beghi E. Decreased platelet glutamate uptake in patients with amyotrophic lateral sclerosis. Neurology. 2001;56:270–272. doi: 10.1212/wnl.56.2.270. [DOI] [PubMed] [Google Scholar]
- 48.Flowers JM. Powell JF. Leigh PN. Andersen P. Shaw CE. Intron 7 retention and exon 9 skipping EAAT2 mRNA variants are not associated with amyotrophic lateral sclerosis. Ann Neurol. 2001;49:643–649. [PubMed] [Google Scholar]
- 49.Frade JM. Rodriguez-Tebar A. Barde YA. Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature. 1996;383:166–168. doi: 10.1038/383166a0. [DOI] [PubMed] [Google Scholar]
- 50.Fujita H. Sato K. Wen TC. Peng Y. Sakanaka M. Differential expressions of glycine transporter 1 and three glutamate transporter mRNA in the hippocampus of gerbils with transient forebrain ischemia. J Cereb Blood Flow Metab. 1999;19:604–615. doi: 10.1097/00004647-199906000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Fumagalli E. Funicello M. Rauen T. Gobbi M. Mennini T. Riluzole enhances the activity of glutamate transporters GLAST, GLT1 and EAAC1. Eur J Pharmacol. 2008;578:171. doi: 10.1016/j.ejphar.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 52.Ganel R. Ho T. Maragakis NJ. Jackson M. Steiner JP. Rothstein JD. Selective up-regulation of the glial Na + −dependent glutamate transporter GLT1 by a neuroimmunophilin ligand results in neuroprotection. Neurobiol Dis. 2006;21:556. doi: 10.1016/j.nbd.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 53.Gendreau S. Voswinkel S. Torres-Salazar D. Lang N. Heidtmann H. Detro-Dassen S. Schmalzing G. Hidalgo P. Fahlke C. A trimeric quaternary structure Is conserved in bacterial and human glutamate transporters. J Biol Chem. 2004;279:39505–39512. doi: 10.1074/jbc.M408038200. [DOI] [PubMed] [Google Scholar]
- 54.Gibb SL. Boston-Howes W. Lavina SZ. Gustincich S. Brown RH Jr. Pasinelli P. Trotti D. A caspase-3 cleaved fragment of the glial glutamate transporter EAAT2 is sumoylated and targeted to promyelocytic leukemia nuclear bodies in mutant SOD1 linked ALS. J Biol Chem. 2007;282:32480–32490. doi: 10.1074/jbc.M704314200. [DOI] [PubMed] [Google Scholar]
- 55.Gill G. SUMO and ubiquitin in the nucleus: different functions, similar mechanisms? Genes Dev. 2004;18:2046–2059. doi: 10.1101/gad.1214604. [DOI] [PubMed] [Google Scholar]
- 56.Giulian D. Baker TJ. Peptides released by ameboid microglia regulate astroglial proliferation. J Cell Biol. 1985;101:2411–2415. doi: 10.1083/jcb.101.6.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo H. Lai L. Butchbach ME. Lin CL. Human glioma cells and undifferentiated primary astrocytes that express aberrant EAAT2 mRNA inhibit normal EAAT2 protein expression and prevent cell death. Mol Cell Neurosci. 2002;21:546–560. doi: 10.1006/mcne.2002.1198. [DOI] [PubMed] [Google Scholar]
- 58.Guo H. Lai L. Butchbach ME. Stockinger MP. Shan X. Bishop GA. Lin CL. Increased expression of the glial glutamate transporter EAAT2 modulates excitotoxicity and delays the onset but not the outcome of ALS in mice. Hum Mol Genet. 2003;12:2519–2532. doi: 10.1093/hmg/ddg267. [DOI] [PubMed] [Google Scholar]
- 59.Gurney ME. Cutting FB. Zhai P. Doble A. Taylor CP. Andrus PK. Hall ED. Benefit of vitamin E, riluzole, and gabapentin in a transgenic model of familial amyotrophic lateral sclerosis. Ann Neurol. 1996;39:147–157. doi: 10.1002/ana.410390203. [DOI] [PubMed] [Google Scholar]
- 60.Gurney ME. Pu H. Chiu AY. Dal Canto MC. Polchow CY. Alexander DD. Caliendo J. Hentati A. Kwon YW. Deng H-X. Chen W. Zhai P. Sufit RL. Siddique T. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation [see comments] [published erratum appears in Science 269(5221):149] Science. 1994;1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 61.Hay RT. SUMO: a history of modification. Mol Cell. 2005;18:1–12. doi: 10.1016/j.molcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 62.Heath PR. Shaw PJ. Update on the glutamatergic neurotransmitter system and the role of excitotoxicity in amyotrophic lateral sclerosis. Muscle Nerve. 2002;26:438–458. doi: 10.1002/mus.10186. [DOI] [PubMed] [Google Scholar]
- 63.Hensley K. Mhatre M. Mou S. Pye QN. Stewart C. West M. Williamson KS. On the relation of oxidative stress to neuroinflammation: lessons learned from the G93A-SOD1 mouse model of amyotrophic lateral sclerosis. Antioxid Redox Signal. 2006;8:2075–2087. doi: 10.1089/ars.2006.8.2075. [DOI] [PubMed] [Google Scholar]
- 64.Herman MA. Jahr CE. Extracellular glutamate concentration in hippocampal slice. J Neurosci. 2007;27:9736–9741. doi: 10.1523/JNEUROSCI.3009-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hewett SJ. Csernansky CA. Choi DW. Selective potentiation of NMDA-induced neuronal injury following induction of astrocytic iNOS. Neuron. 1994;13:487–494. doi: 10.1016/0896-6273(94)90362-x. [DOI] [PubMed] [Google Scholar]
- 66.Hirata A. Nakamura R. Kwak S. Nagata N. Kamakura K. AMPA receptor-mediated slow neuronal death in the rat spinal cord induced by long-term blockade of glutamate transporters with THA. Brain Res. 1997;771:37. doi: 10.1016/s0006-8993(97)00709-9. [DOI] [PubMed] [Google Scholar]
- 67.Honig LS. Chambliss DD. Bigio EH. Carroll SL. Elliott JL. Glutamate transporter EAAT2 splice variants occur not only in ALS, but also in AD and controls. Neurology. 2000;55:1082–1088. doi: 10.1212/wnl.55.8.1082. [DOI] [PubMed] [Google Scholar]
- 68.Howland DS. Liu J. She Y. Goad B. Maragakis NJ. Kim B. Erickson J. Kulik J. DeVito L. Psaltis G. DeGennaro LJ. Cleveland DW. Rothstein JD. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS) Proc Natl Acad Sci U S A. 2002;29:29. doi: 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang YH. Bergles DE. Glutamate transporters bring competition to the synapse. Curr Opin Neurobiol. 2004;14:346–352. doi: 10.1016/j.conb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 70.Isaac JT. Ashby M. McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 71.Juan Carlos Corona RT. AMPA receptor activation, but not the accumulation of endogenous extracellular glutamate, induces paralysis and motor neuron death in rat spinal cord in vivo. J Neurochem. 2004;89:988–997. doi: 10.1111/j.1471-4159.2004.02383.x. [DOI] [PubMed] [Google Scholar]
- 72.Kanai Y. Hediger MA. The glutamate/neutral amino acid transporter family SLC1: molecular, physiological and pharmacological aspects. Pflugers Arch. 2004;447:469–479. doi: 10.1007/s00424-003-1146-4. [DOI] [PubMed] [Google Scholar]
- 73.Kawahara Y. Kwak S. Sun H. Ito K. Hashida H. Aizawa H. Jeong SY. Kanazawa I. Human spinal motoneurons express low relative abundance of GluR2 mRNA: an implication for excitotoxicity in ALS. J Neurochem. 2003;85:680–689. doi: 10.1046/j.1471-4159.2003.01703.x. [DOI] [PubMed] [Google Scholar]
- 74.Kawahara Y. Sun H. Ito K. Hideyama T. Aoki M. Sobue G. Tsuji S. Kwak S. Underediting of GluR2 mRNA, a neuronal death inducing molecular change in sporadic ALS, does not occur in motor neurons in ALS1 or SBMA. Neurosci Res. 2006;54:11–14. doi: 10.1016/j.neures.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 75.Kim CH. Lee J. Lee JY. Roche KW. Metabotropic glutamate receptors: phosphorylation and receptor signaling. J Neurosci Res. 2008;86:1–10. doi: 10.1002/jnr.21437. [DOI] [PubMed] [Google Scholar]
- 76.Kong J. Xu Z. Massive mitochondrial degeneration in motor neurons triggers the onset of amyotrophic lateral sclerosis in mice expressing a mutant SOD1. J Neurosci. 1998;18:3241–3250. doi: 10.1523/JNEUROSCI.18-09-03241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kristensen BW. Noraberg J. Zimmer J. The GABAA receptor agonist THIP is neuroprotective in organotypic hippocampal slice cultures. Brain Res. 2003;973:303. doi: 10.1016/s0006-8993(03)02550-2. [DOI] [PubMed] [Google Scholar]
- 78.Kuner R. Groom AJ. Bresink I. Kornau HC. Stefovska V. Muller G. Hartmann B. Tschauner K. Waibel S. Ludolph AC. Ikonomidou C. Seeburg PH. Turski L. Late-onset motoneuron disease caused by a functionally modified AMPA receptor subunit. Proc Natl Acad Sci U S A. 2005;102:5826–5831. doi: 10.1073/pnas.0501316102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuner R. Groom AJ. Muller G. Kornau HC. Stefovska V. Bresink I. Hartmann B. Tschauner K. Waibel S. Ludolph AC. Ikonomidou C. Seeburg PH. Turski L. Mechanisms of disease: motoneuron disease aggravated by transgenic expression of a functionally modified AMPA receptor subunit. Ann N Y Acad Sci. 2005;1053:269–286. doi: 10.1196/annals.1344.024. [DOI] [PubMed] [Google Scholar]
- 80.Kvamme E. Torgner IA. Roberg B. Evidence indicating that pig renal phosphate-activated glutaminase has a functionally predominant external localization in the inner mitochondrial membrane. J Biol Chem. 1991;266:13185–13192. [PubMed] [Google Scholar]
- 81.Kwak S. Kawahara Y. Deficient RNA editing of GluR2 and neuronal death in amyotrophic lateral sclerosis. J Mol Med. 2005;83:110–120. doi: 10.1007/s00109-004-0599-z. [DOI] [PubMed] [Google Scholar]
- 82.Lacomblez L. Bensimon G. Leigh PN. Guillet P. Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis: Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996;347:1425–1431. doi: 10.1016/s0140-6736(96)91680-3. [DOI] [PubMed] [Google Scholar]
- 83.Lacomblez L. Bensimon G. Leigh PN. Guillet P. Powe L. Durrleman S. Delumeau JC. Meininger V. A confirmatory dose-ranging study of riluzole in ALS: ALS/Riluzole Study Group II. Neurology. 1996;47:S242–S250. doi: 10.1212/wnl.47.6_suppl_4.242s. [DOI] [PubMed] [Google Scholar]
- 84.Lee SG. Su ZZ. Emdad L. Gupta P. Sarkar D. Borjabad A. Volsky DJ. Fisher PB. Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes. J Biol Chem. 2008;283:13116–13123. doi: 10.1074/jbc.M707697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lepore AC. Rauck B. Dejea C. Pardo AC. Rao MS. Rothstein JD. Maragakis NJ. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat Neurosci. 2008;11:1294–1301. doi: 10.1038/nn.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lin C-LG. Bristol LA. Jin L. Dykes-Hoberg M. Crawford T. Clawson L. Rothstein JD. Aberrant RNA processing in a neurodegenerative disease: the cause for absent EAAT2, a glutamate transporter, in amyotrophic lateral sclerosis. Neuron. 1998;20:589–602. doi: 10.1016/s0896-6273(00)80997-6. [DOI] [PubMed] [Google Scholar]
- 87.Loscher W. Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci. 2005;6:591–602. doi: 10.1038/nrn1728. [DOI] [PubMed] [Google Scholar]
- 88.Lowry KS. Murray SS. McLean CA. Talman P. Mathers S. Lopes EC. Cheema SS. A potential role for the p75 low-affinity neurotrophin receptor in spinal motor neuron degeneration in murine and human amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2001;2:127–134. doi: 10.1080/146608201753275463. [DOI] [PubMed] [Google Scholar]
- 89.Macnab LT. Pow DV. Expression of the exon 9-skipping form of EAAT2 in astrocytes of rats. Neuroscience. 2007;150:705–711. doi: 10.1016/j.neuroscience.2007.09.049. [DOI] [PubMed] [Google Scholar]
- 90.Maragakis NJ. Dykes-Hoberg M. Rothstein JD. Altered expression of the glutamate transporter EAAT2b in neurological disease. Ann Neurol. 2004;55:469–477. doi: 10.1002/ana.20003. [DOI] [PubMed] [Google Scholar]
- 91.Maragakis NJ. Jackson M. Ganel R. Rothstein JD. Topiramate protects against motor neuron degeneration in organotypic spinal cord cultures but not in G93A SOD1 transgenic mice. Neurosci Lett. 2003;338:107–110. doi: 10.1016/s0304-3940(02)01386-1. [DOI] [PubMed] [Google Scholar]
- 92.Maragakis NJ. Rao MS. Llado J. Wong V. Xue H. Pardo A. Herring J. Kerr D. Coccia C. Rothstein JD. Glial restricted precursors protect against chronic glutamate neurotoxicity of motor neurons in vitro. Glia. 2005;50:145–159. doi: 10.1002/glia.20161. [DOI] [PubMed] [Google Scholar]
- 93.Massieu L. Morales-Villagran A. Tapia R. Accumulation of extracellular glutamate by inhibition of its uptake is not sufficient for inducing neuronal damage: an in vivo microdialysis study. J Neurochem. 1995;64:2262–2272. doi: 10.1046/j.1471-4159.1995.64052262.x. [DOI] [PubMed] [Google Scholar]
- 94.Matyja E. Taraszewska A. Naganska E. Rafalowska J. Gebarowska J. Astroglial alterations in amyotrophic lateral sclerosis (ALS) model of slow glutamate excitotoxicity in vitro. Folia Neuropathol. 2006;44:183–190. [PubMed] [Google Scholar]
- 95.Meyer T. Fromm A. Munch C. Schwalenstocker B. Fray AE. Ince PG. Stamm S. Gron G. Ludolph AC. Shaw PJ. The RNA of the glutamate transporter EAAT2 is variably spliced in amyotrophic lateral sclerosis and normal individuals. J Neurol Sci. 1999;170:45–50. doi: 10.1016/s0022-510x(99)00196-3. [DOI] [PubMed] [Google Scholar]
- 96.Myers SJ. Dingledine R. Borges K. Genetic regulation of glutamate receptor ion channels. Annu Rev Pharmacol Toxicol. 1999;39:221–241. doi: 10.1146/annurev.pharmtox.39.1.221. [DOI] [PubMed] [Google Scholar]
- 97.Nagai M. Abe K. Okamoto K. Itoyama Y. Identification of alternative splicing forms of GLT-1 mRNA in the spinal cord of amyotrophic lateral sclerosis patients. Neurosci Lett. 1998;244:165–168. doi: 10.1016/s0304-3940(98)00158-x. [DOI] [PubMed] [Google Scholar]
- 98.Nagai M. Aoki M. Miyoshi I. Kato M. Pasinelli P. Kasai N. Brown RH Jr. Itoyama Y. Rats expressing human cytosolic copper-zinc superoxide dismutase transgenes with amyotrophic lateral sclerosis: associated mutations develop motor neuron disease. J Neurosci. 2001;21:9246–9254. doi: 10.1523/JNEUROSCI.21-23-09246.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nakamura R. Kamakura K. Kwak S. Late-onset selective neuronal damage in the rat spinal cord induced by continuous intrathecal administration of AMPA. Brain Res. 1994;654:279. doi: 10.1016/0006-8993(94)90490-1. [DOI] [PubMed] [Google Scholar]
- 100.Newpher TM. Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–497. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Niebroj-Dobosz I. Janik P. Kwiecinski H. Effect of riluzole on serum amino acids in patients with amyotrophic lateral sclerosis. Acta Neurol Scand. 2002;106:39–43. doi: 10.1034/j.1600-0404.2002.00206.x. [DOI] [PubMed] [Google Scholar]
- 102.Noriyuki S. Transgenic mouse model for familial amyotrophic lateral sclerosis with superoxide dismutase-1 mutation. Neuropathology. 2001;21:82–92. doi: 10.1046/j.1440-1789.2001.00361.x. [DOI] [PubMed] [Google Scholar]
- 103.Pardo AC. Wong V. Benson LM. Dykes M. Tanaka K. Rothstein JD. Maragakis NJ. Loss of the astrocyte glutamate transporter GLT1 modifies disease in SOD1 (G93A) mice. Exp Neurol. 2006;201:120–130. doi: 10.1016/j.expneurol.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 104.Pasinelli P. Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci. 2006;7:710–723. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- 105.Pehar M. Martinez-Palma L. Peluffo H. Kamaid A. Cassina P. Barbeito L. Peroxynitrite-induced cytotoxicity in cultured astrocytes is associated with morphological changes and increased nitrotyrosine immunoreactivity. Neurotox Res. 2002;4:87–93. doi: 10.1080/10298420290015818. [DOI] [PubMed] [Google Scholar]
- 106.Pehar M. Vargas MR. Cassina P. Barbeito AG. Beckman JS. Barbeito L. Complexity of astrocyte-motor neuron interactions in amyotrophic lateral sclerosis. Neurodegener Dis. 2005;2:139–146. doi: 10.1159/000089619. [DOI] [PubMed] [Google Scholar]
- 107.Plaitakis A. Constantakakis E. Altered metabolism of excitatory amino acids, N-acetyl-aspartate and N-acetyl-aspartyl-glutamate in amyotrophic lateral sclerosis. Brain Res Bull. 1993;30:381–386. doi: 10.1016/0361-9230(93)90269-h. [DOI] [PubMed] [Google Scholar]
- 108.Rao SD. Weiss JH. Excitotoxic and oxidative cross-talk between motor neurons and glia in ALS pathogenesis. Trends Neurosci. 2004;27:17–23. doi: 10.1016/j.tins.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 109.Rao SD. Yin HZ. Weiss JH. Disruption of glial glutamate transport by reactive oxygen species produced in motor neurons. J Neurosci. 2003;23:2627–2633. doi: 10.1523/JNEUROSCI.23-07-02627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rende M. Giambanco I. Buratta M. Tonali P. Axotomy induces a different modulation of both low-affinity nerve growth factor receptor and choline acetyltransferase between adult rat spinal and brainstem motoneurons. J Comp Neurol. 1995;363:249–263. doi: 10.1002/cne.903630207. [DOI] [PubMed] [Google Scholar]
- 111.Ridet JL. Malhotra SK. Privat A. Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- 112.Robberecht W. Van Den Bosch L. Vleminckx V. Amyotrophic lateral sclerosis: pathogenesis. Acta Neurol Belg. 2000;100:181–187. [PubMed] [Google Scholar]
- 113.Robinson MB. The family of sodium-dependent glutamate transporters: a focus on the GLT-1/EAAT2 subtype. Neurochem Int. 1998;33:479–491. doi: 10.1016/s0197-0186(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 114.Rossi D. Brambilla L. Valori CF. Roncoroni C. Crugnola A. Yokota T. Bredesen DE. Volterra A. Focal degeneration of astrocytes in amyotrophic lateral sclerosis. Cell Death Differ. 2008;15:1691–1700. doi: 10.1038/cdd.2008.99. [DOI] [PubMed] [Google Scholar]
- 115.Rothstein JD. Dykes-Hoberg M. Pardo CA. Bristol LA. Jin L. Kuncl RW. Kanai Y. Hediger MA. Wang Y. Schielke JP. Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 116.Rothstein JD. Jin L. Dykes-Hoberg M. Kuncl RW. Chronic inhibition of glutamate uptake produces a model of slow neurotoxicity. Proc Natl Acad Sci U S A. 1993;90:6591–6595. doi: 10.1073/pnas.90.14.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rothstein JD. Martin LJ. Kuncl RW. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis [see comments] N Engl J Med. 1992;326:1464–1468. doi: 10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- 118.Rothstein JD. Patel S. Regan MR. Haenggeli C. Huang YH. Bergles DE. Jin L. Dykes Hoberg M. Vidensky S. Chung DS. Toan SV. Bruijn LI. Su ZZ. Gupta P. Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 119.Saroff D. Delfs J. Kuznetsov D. Geula C. Selective vulnerability of spinal cord motor neurons to non-NMDA toxicity. Neuroreport. 2000;11:1117–1121. doi: 10.1097/00001756-200004070-00041. [DOI] [PubMed] [Google Scholar]
- 120.Sasaki S. Komori T. Iwata M. Excitatory amino acid transporter 1 and 2 immunoreactivity in the spinal cord in amyotrophic lateral sclerosis [In Process Citation] Acta Neuropathol (Berl) 2000;100:138–144. doi: 10.1007/s004019900159. [DOI] [PubMed] [Google Scholar]
- 121.Schinder AF. Poo M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000;23:639–645. doi: 10.1016/s0166-2236(00)01672-6. [DOI] [PubMed] [Google Scholar]
- 122.Schlag BD. Vondrasek JR. Munir M. Kalandadze A. Zelenaia OA. Rothstein JD. Robinson MB. Regulation of the glial Na + −dependent glutamate transporters by cyclic AMP analogs and neurons. Mol Pharmacol. 1998;53:355–369. doi: 10.1124/mol.53.3.355. [DOI] [PubMed] [Google Scholar]
- 123.Seeburg PH. Hartner J. Regulation of ion channel/neurotransmitter receptor function by RNA editing. Curr Opin Neurobiol. 2003;13:279–283. doi: 10.1016/s0959-4388(03)00062-x. [DOI] [PubMed] [Google Scholar]
- 124.Sen I. Nalini A. Joshi NB. Joshi PG. Cerebrospinal fluid from amyotrophic lateral sclerosis patients preferentially elevates intracellular calcium and toxicity in motor neurons via AMPA/kainate receptor. J Neurol Sci. 2005;235:45–54. doi: 10.1016/j.jns.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 125.Shaw PJ. Chinnery RM. Ince PG. [3H]D-aspartate binding sites in the normal human spinal cord and changes in motor neuron disease: a quantitative autoradiographic study. Brain Res. 1994;655:195–201. doi: 10.1016/0006-8993(94)91614-4. [DOI] [PubMed] [Google Scholar]
- 126.Skaper SD. Facci L. Leon A. Inflammatory mediator stimulation of astrocytes and meningeal fibroblasts induces neuronal degeneration via the nitridergic pathway. J Neurochem. 1995;64:266–276. doi: 10.1046/j.1471-4159.1995.64010266.x. [DOI] [PubMed] [Google Scholar]
- 127.Stuerenburg HJ. Kunze K. Tissue nerve growth factor concentrations in neuromuscular diseases. Eur J Neurol. 1998;5:487–490. doi: 10.1046/j.1468-1331.1998.550487.x. [DOI] [PubMed] [Google Scholar]
- 128.Swanson RA. Liu J. Miller JW. Rothstein JD. Farrell KA. Stein B. Longuemare MC. Neuronal regulation of glutamate transporter subtype expression in astrocytes. J Neurosci. 1997;17:932–940. doi: 10.1523/JNEUROSCI.17-03-00932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Takamori S. VGLUTs: `Exciting' times for glutamatergic research? Neurosci Res. 2006;55:343. doi: 10.1016/j.neures.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 130.Tanaka K. Watase K. Manabe T. Yamada K. Watanabe M. Takahashi K. Iwama H. Nishikawa T. Ichihara N. Kikuchi T. Okuyama S. Kawashima N. Hori S. Takimoto M. Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- 131.Tateno M. Sadakata H. Tanaka M. Itohara S. Shin RM. Miura M. Masuda M. Aosaki T. Urushitani M. Misawa H. Takahashi R. Calcium-permeable AMPA receptors promote misfolding of mutant SOD1 protein and development of amyotrophic lateral sclerosis in a transgenic mouse model. Hum Mol Genet. 2004;13:2183–2196. doi: 10.1093/hmg/ddh246. [DOI] [PubMed] [Google Scholar]
- 132.Taylor AR. Gifondorwa DJ. Newbern JM. Robinson MB. Strupe JL. Prevette D. Oppenheim RW. Milligan CE. Astrocyte and muscle-derived secreted factors differentially regulate motoneuron survival. J Neurosci. 2007;27:634–644. doi: 10.1523/JNEUROSCI.4947-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tortarolo M. Crossthwaite AJ. Conforti L. Spencer JP. Williams RJ. Bendotti C. Rattray M. Expression of SOD1 G93A or wild-type SOD1 in primary cultures of astrocytes down-regulates the glutamate transporter GLT-1: lack of involvement of oxidative stress. J Neurochem. 2004;88:481–493. doi: 10.1046/j.1471-4159.2003.02208.x. [DOI] [PubMed] [Google Scholar]
- 134.Tortarolo M. Grignaschi G. Calvaresi N. Zennaro E. Spaltro G. Colovic M. Fracasso C. Guiso G. Elger B. Schneider H. Seilheimer B. Caccia S. Bendotti C. Glutamate AMPA receptors change in motor neurons of SOD1G93A transgenic mice and their inhibition by a noncompetitive antagonist ameliorates the progression of amytrophic lateral sclerosis-like disease. J Neurosci Res. 2006;83:134–146. doi: 10.1002/jnr.20715. [DOI] [PubMed] [Google Scholar]
- 135.Tovar-y-Romo LB. Tapia R. Cerebral neurons of transgenic ALS mice are vulnerable to glutamate release stimulation but not to increased extracellular glutamate due to transport blockade. Exp Neurol. 2006;199:281–290. doi: 10.1016/j.expneurol.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 136.Tovar YRLB. Zepeda A. Tapia R. Vascular endothelial growth factor prevents paralysis and motoneuron death in a rat model of excitotoxic spinal cord neurodegeneration. J Neuropathol Exp Neurol. 2007;66:913–922. doi: 10.1097/nen.0b013e3181567c16. [DOI] [PubMed] [Google Scholar]
- 137.Trotti D. Aoki M. Pasinelli P. Berger UV. Danbolt NC. Brown RH Jr. Hediger MA. Amyotrophic lateral sclerosis-linked glutamate transporter mutant has impaired glutamate clearance capacity. J Biol Chem. 2000;276:576–582. doi: 10.1074/jbc.M003779200. [DOI] [PubMed] [Google Scholar]
- 138.Trotti D. Rolfs A. Danbolt NC. Brown RH Jr. Hediger MA. SOD1 mutants linked to amyotrophic lateral sclerosis selectively inactivate a glial glutamate transporter [published erratum appears in Nat Neurosci Sep 2: 848, 1992] Nat Neurosci. 1999;2:427–433. doi: 10.1038/8091. [DOI] [PubMed] [Google Scholar]
- 139.Trotti D. Rossi D. Gjesdal O. Levy LM. Racagni G. Danbolt NC. Volterra A. Peroxynitrite inhibits glutamate transporter subtypes. J Biol Chem. 1996;271:5976–5979. doi: 10.1074/jbc.271.11.5976. [DOI] [PubMed] [Google Scholar]
- 140.Tzingounis AV. Wadiche JI. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci. 2007;8:935–947. doi: 10.1038/nrn2274. [DOI] [PubMed] [Google Scholar]
- 141.Van Damme P. Bogaert E. Dewil M. Hersmus N. Kiraly D. Scheveneels W. Bockx I. Braeken D. Verpoorten N. Verhoeven K. Timmerman V. Herijgers P. Callewaert G. Carmeliet P. Van Den Bosch L. Robberecht W. Astrocytes regulate GluR2 expression in motor neurons and their vulnerability to excitotoxicity. Proc Natl Acad Sci U S A. 2007;104:14825–14830. doi: 10.1073/pnas.0705046104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Van Damme P. Braeken D. Callewaert G. Robberecht W. Van Den Bosch L. GluR2 deficiency accelerates motor neuron degeneration in a mouse model of amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 2005;64:605–612. doi: 10.1097/01.jnen.0000171647.09589.07. [DOI] [PubMed] [Google Scholar]
- 143.Van Damme P. Leyssen M. Callewaert G. Robberecht W. Van Den Bosch L. The AMPA receptor antagonist NBQX prolongs survival in a transgenic mouse model of amyotrophic lateral sclerosis. Neurosci Lett. 2003;343:81–84. doi: 10.1016/s0304-3940(03)00314-8. [DOI] [PubMed] [Google Scholar]
- 144.Van Den Bosch L. Robberecht W. Different receptors mediate motor neuron death induced by short and long exposures to excitotoxicity. Brain Res Bull. 2000;53:383–388. doi: 10.1016/s0361-9230(00)00371-3. [DOI] [PubMed] [Google Scholar]
- 145.Van Den Bosch L. Vandenberghe W. Klaassen H. Van Houtte E. Robberecht W. Ca(2+)-permeable AMPA receptors and selective vulnerability of motor neurons. J Neurol Sci. 2000;180:29–34. doi: 10.1016/s0022-510x(00)00414-7. [DOI] [PubMed] [Google Scholar]
- 146.Vandenberghe W. Ihle EC. Patneau DK. Robberecht W. Brorson JR. AMPA receptor current density, not desensitization, predicts selective motoneuron vulnerability. J Neurosci. 2000;20:7158–7166. doi: 10.1523/JNEUROSCI.20-19-07158.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vandenberghe W. Robberecht W. Brorson JR. AMPA receptor calcium permeability, GluR2 expression, and selective motoneuron vulnerability. J Neurosci. 2000;20:123–132. doi: 10.1523/JNEUROSCI.20-01-00123.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vanoni C. Massari S. Losa M. Carrega P. Perego C. Conforti L. Pietrini G. Increased internalisation and degradation of GLT-1 glial glutamate transporter in a cell model for familial amyotrophic lateral sclerosis (ALS) J Cell Sci. 2004;117:5417–5426. doi: 10.1242/jcs.01411. [DOI] [PubMed] [Google Scholar]
- 149.Volterra A. Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- 150.Wang R. Zhang D. Memantine prolongs survival in an amyotrophic lateral sclerosis mouse model. Eur J Neurosci. 2005;22:2376–2380. doi: 10.1111/j.1460-9568.2005.04431.x. [DOI] [PubMed] [Google Scholar]
- 151.Warita H. Manabe Y. Murakami T. Shiote M. Shiro Y. Hayashi T. Nagano I. Shoji M. Abe K. Tardive decrease of astrocytic glutamate transporter protein in transgenic mice with ALS-linked mutant SOD1. Neurol Res. 2002;24:577–581. doi: 10.1179/016164102101200384. [DOI] [PubMed] [Google Scholar]
- 152.Williams TL. Day NC. Ince PG. Kamboj RK. Shaw PJ. Calcium-permeable alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors: a molecular determinant of selective vulnerability in amyotrophic lateral sclerosis. Ann Neurol. 1997;42:200–207. doi: 10.1002/ana.410420211. [DOI] [PubMed] [Google Scholar]
- 153.Wilson JM. Khabazian I. Pow DV. Craig UK. Shaw CA. Decrease in glial glutamate transporter variants and excitatory amino acid receptor down-regulation in a murine model of ALS-PDC. Neuromol Med. 2003;3:105–118. doi: 10.1385/NMM:3:2:105. [DOI] [PubMed] [Google Scholar]
- 154.Wong PC. Pardo CA. Borchelt DR. Lee MK. Copeland NG. Jenkins NA. Sisodia SS. Cleveland DW. Price DL. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- 155.Yamanaka K. Chun SJ. Boillee S. Fujimori-Tonou N. Yamashita H. Gutmann DH. Takahashi R. Misawa H. Cleveland DW. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11:251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yernool D. Boudker O. Jin Y. Gouaux E. Structure of a glutamate transporter homologue from Pyrococcus horikoshii. Nature. 2004;431:811–818. doi: 10.1038/nature03018. [DOI] [PubMed] [Google Scholar]
- 157.Yin HZ. Tang DT. Weiss JH. Intrathecal infusion of a Ca(2+)-permeable AMPA channel blocker slows loss of both motor neurons and of the astrocyte glutamate transporter, GLT-1 in a mutant SOD1 rat model of ALS. Exp Neurol. 2007;207:177–185. doi: 10.1016/j.expneurol.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang F. Li C. Wang R. Han D. Zhang QG. Zhou C. Yu HM. Zhang GY. Activation of GABA receptors attenuates neuronal apoptosis through inhibiting the tyrosine phosphorylation of NR2A by Src after cerebral ischemia and reperfusion. Neuroscience. 2007;150:938. doi: 10.1016/j.neuroscience.2007.09.070. [DOI] [PubMed] [Google Scholar]