Abstract
Can dendrites grow in mature cortex? Here we address this question using chronic in vivo imaging to follow pyramidal neurons before and after cortical deletion of the Pten tumor suppressor gene in mature mice. We find that Pten/mTOR signaling uniquely regulates growth of layer 2/3 apical dendrites; no effects of gene deletion were observed on basal dendrites of these pyramidal neurons or along layer 5 apical dendrites.
Cortical dendrites are dynamic integrators of synaptic input to the neuronal soma. Their integrative properties are critically affected by the branching pattern of the dendritic tree1. In mice, the final form of the dendritic tree is laid down in the first two weeks of postnatal development during a period of maximum afferent innervation and synapse formation2, 3. Thereafter, large-scale dendritic structure is remarkably stable 4–6.
The PI-3 kinase/Pten signaling pathway regulates dendritic hypertrophy in developing cortex7, 8, but its action in mature cortex is unclear. To directly examine the potential, extent, dynamics, and molecular mechanisms of dendritic growth in the mature cortex in vivo we generated mice with a conditional cortical deletion of the Pten tumor suppressor gene (αCamKII-Cre+/−; Ptenloxp/loxp; Thy1-GFP). Experiments were approved by the University of California Los Angeles Office for Protection of Research Subjects and the Chancellor’s Animal Research Committee. Gene deletion significantly increases around the 8th postnatal week in these mice (Fig. S1).
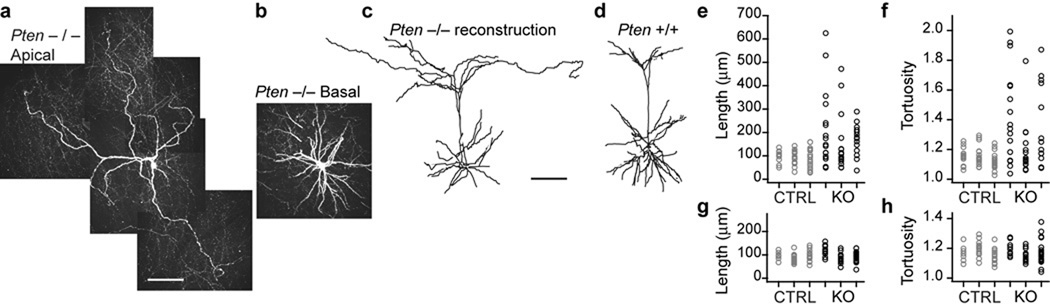
We imaged pyramidal neurons in cortical layer 2/3 in their entirety in whole mount brains of 3.5–4 month old mice and reconstructed in three dimensions (Fig. 1). The apical dendrites of neurons in Pten−/− mice were much longer (Fig. 1a,c,e) and more tortuous (Fig. 1a,f) than those in controls. Total apical arbor length was on average 1.6mm longer in Pten−/− mice than in controls, representing an approximately 80% expansion of the apical dendritic tree relative to normal (Total arbor lengths: Pten−/− 3.1mm, 3.2mm, 4.6mm; WT 1.5mm, 2.1mm, 2.4mm). Notably, basal dendritic length (Fig. 1g) and tortuosity (Fig. 1h) from these same neurons were not measurably affected by Pten deletion. Nor did we find any significant difference in spine density between the two groups (density: 7.3±0.8 spines per 10µm in controls vs. 7.5±0.9 spines per 10µm in Pten−/−; P=0.29. n=1798 spines in control and 1989 in Pten−/− mice). All imaged neurons in Pten−/− mice showed signs of robust apical dendritic growth.
Figure 1. Compartment-specific dendritic growth.
(a) Photomontage of maximum intensity projections of the apical dendritic tree of a layer 2/3 pyramidal neuron imaged in a 4 month old Pten−/− mouse. (b) Basal dendrites of the same neuron in (a). (c) Coronal reconstruction of the full dendritic tree of the cell in (a) and (b). (d) Similar view of a layer 2/3 pyramidal neuron from a control mouse. Scale bars are 100µm. (e,g) Lengths and (f,h) tortuosities of the terminal apical and basal dendrites, respectively, from 3 neurons in control (CTRL) and Pten−/− (KO) mice.
To examine the kinetics of apical dendritic growth we repeatedly imaged these dendrites in vivo. Imaging began at 7 or 8 weeks of age when cortical Cre expression was rapidly increasing in Cre+/− mice (Fig. S1b). In agreement with previous reports 6, the apical dendrites of layer 2/3 neurons in control mice (n=59 dendrites from 4 neurons in 3 mice) were quite stable when imaged over a 1 month interval (Fig. 2a,c). Although small elongations and retractions could be observed these amounted to a net loss of only 1–2% of apical dendritic length.
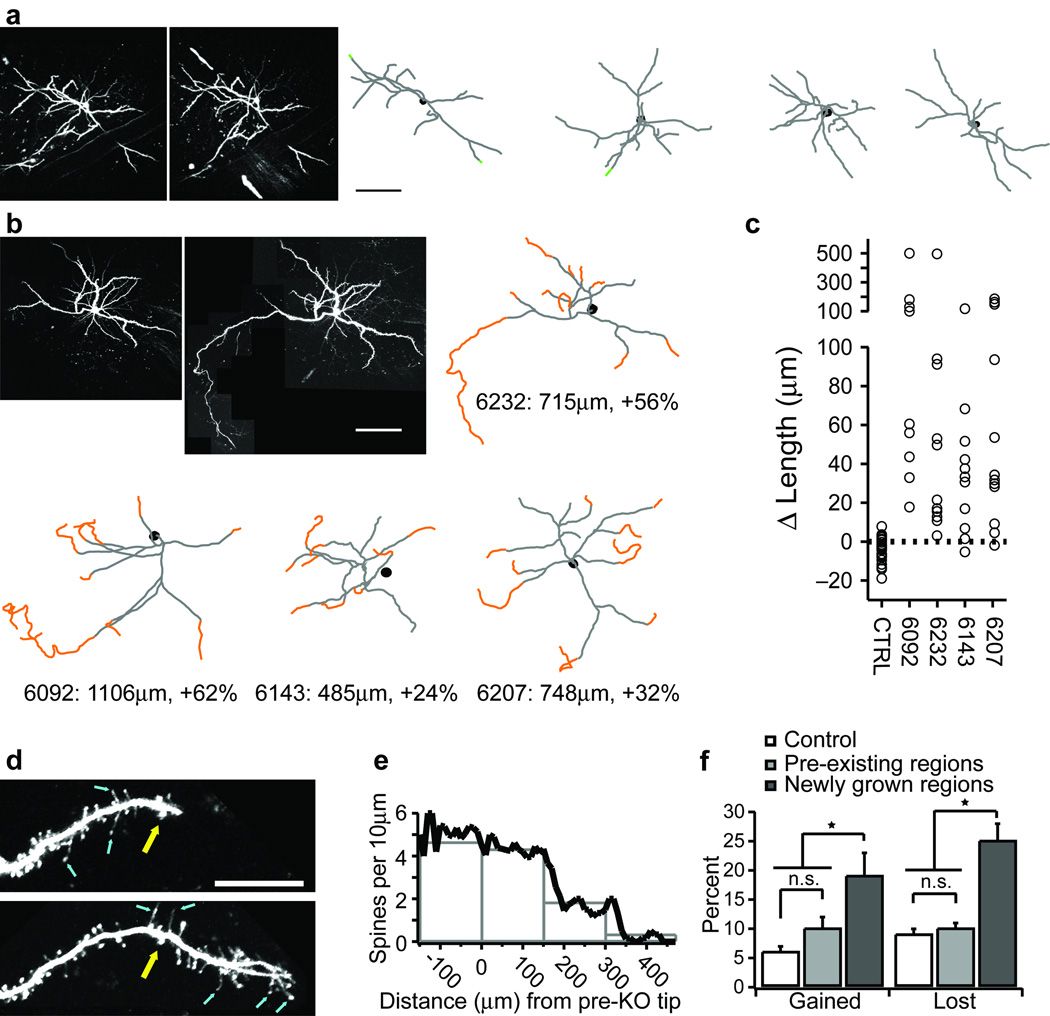
Figure 2. Dynamics of apical dendritic growth in vivo.
Low magnification views of the same layer 2/3 (L2/3) apical dendrites imaged in vivo at PW8 and PW12 in control (a) and Pten−/− (b) mice. Reconstructions of these cells and 3 additional control and Pten−/− cells are shown. Dendritic growths over this period are shown in orange, retractions are shown in green. (c) Total growth and fractional change in the apical tree over a one-month interval. Controls are pooled. Each neuron in (b) is plotted separately. Each circle represents one dendrite (control: n=59 dendrites; Pten−/−: n=43 dendrites). (d) High magnification time-series of a growing L2/3 apical dendrite following Pten deletion. Top image acquired at PW9, bottom at PW10. Yellow arrow identifies a fiducial spine. Blue arrows identify filopodia. (e) Spine density as a function of distance grown. (f) Fractional spine gain and loss over a 2-week interval from control, pre-existing, and newly grown dendrites. * significance at P<0.01. Error bars are s.d. Scale bars: a&b, 100µm; d, 20µm.
In Pten−/− mice, the apical dendrites of pyramidal neurons imaged at 7 or 8 weeks of age were not measurably different from those in age-matched controls (Terminal branch point lengths: 91±46µm in controls, 104±52µm in Pten−/−; P=0.239; Tortuosity: 1.15±0.11 in controls, 1.19±0.14 in Pten−/−; P=0.20). Nor was there any significant difference in spine density between the two groups (control: 5.7±0.7 spines per 10µm; Pten−/−: 5.5±1.4 spines per 10µm; P=0.66). Over the next month these apical dendrites grew hundreds of microns, expanding the apical arborizations by 24% to 62% per imaged neuron (Fig. 2b,c). Again, this apical dendritic growth occurred in every layer 2/3 pyramidal neuron that we were able to locate in Pten−/− mice. This growth always occurred from the tips of existing dendrites and was characterized by the presence of filopodia-like protrusions at the growing tips (Fig. 2d). Spine density over the first 150µm of any newly grown dendrite was not significantly different from that along the original dendritic regions of these same neurons or in controls. However, spine density was significantly reduced along more distal dendritic regions (Fig. 2e). This new spine growth resulted in the addition of between 220 and 404 spines per imaged neuron. These newly formed spines were extremely labile; spine formation and elimination along newly grown dendrites was approximately two-fold greater than along pre-existing dendritic regions or on dendrites in control mice (Fig. 2f). This rate of growth and elimination is similar to that seen during early postnatal development when cortical circuits are refined by activity and experience 9.
Dendritic regions with spine densities less than 2 spines per 10µm ultimately retracted. This retraction was often associated with the presence of large swellings near the dendritic tips that were similar in appearance to “retraction bulbs” that have been described at the tips of retracting motor axons (10; Fig. S2). Thus, it appears that synaptic innervation, or at least the presence of spines is required for the maintenance of newly grown dendrites in mature cortex, much as in developing cortex 2.
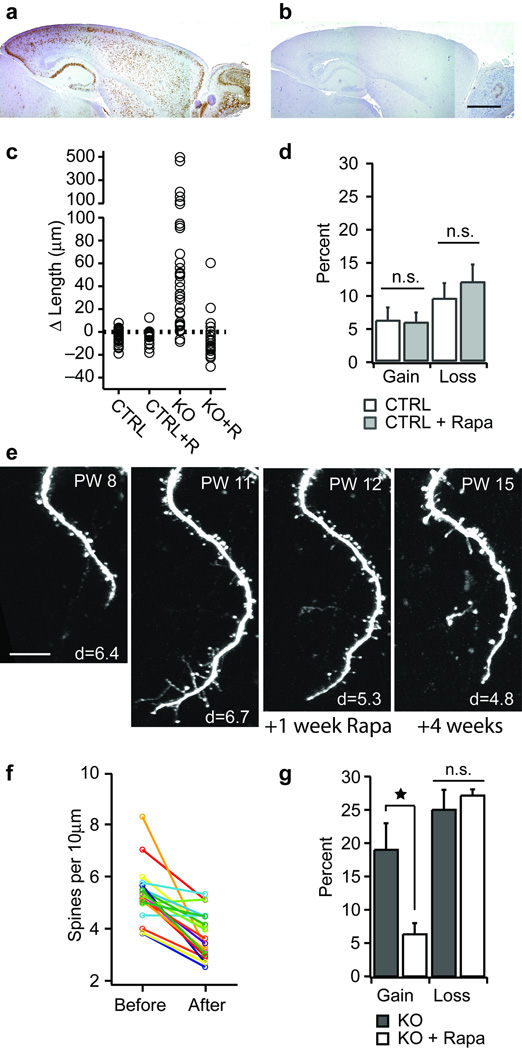
Pten deficiency leads to increased activity of the mTOR kinase and a concomitant increase in the activity of S6 kinase (Fig. S1c), resulting in increased protein translation and cell growth 11. Daily intraperitoneal injections of rapamycin antagonized cortical mTOR signaling in vivo (Fig. 3a,b) and completely prevented the robust dendritic growth normally seen following Pten deletion (Fig. 3c). In control mice rapamycin had no effect on dendrite length (Fig. 3c) or spine kinetics (Fig. 3d), indicating that the effects of rapamycin are specific to growing dendrites.
Figure 3. Effect of rapamycin.
(a) pS6 staining in a sagittal section from a PW14 Pten−/− mouse. (b) pS6 staining in a PW14 Pten−/− mouse given rapamycin for 2 weeks. (c) Dendritic length changes observed over a 4 week interval in control (CTRL; n=4 cells, 59 dendrites), control given rapamycin (CTRL+R; n=3 cells, 23 dendrites), Pten−/− (KO, n=4 cells, 43 dendrites), and Pten−/− given rapamycin (KO+R; n=3 cells, 26 dendrites). (d) Spine gain and loss in control (CTRL, n=362 spines) mice and mice given daily rapamycin (CTRL + Rapa, n=291 spines). (e) Time series showing the growth of a dendrite following Pten deletion and subsequent treatment with rapamycin. Rapamycin treatment began immediately after the image was acquired at PW11. Note the retraction between PW11 and PW12. Age at imaging is stamped in the upper right corner of each panel. Spine density per 10µm for each image is stamped in the lower right corner. (f) Spine density measured along the same dendrites repeatedly imaged in vivo over a 2-week period of rapamycin treatment. Each circle is a separate dendrite. (g) Fractional gain and loss of spines along L2/3 apical dendrites imaged over a 2-week period in Pten−/− mice (KO; n=407 spines), and Pten−/− mice given daily rapamycin (KO + Rapa; n=536 spines). Error bars are s.d. * denotes significance at P<0.01. Scale bars: a&b, 1mm; e, 20µm.
Having shown that rapamycin prevents dendritic growth; we next investigated how rapamycin affects growing dendrites. To examine this we performed an in vivo wash-in experiment. Dendrites were first imaged weekly for 4 weeks (PW8–11). At the period of most rapid growth (PW11 in this mouse) we began rapamycin treatment. Imaging continued weekly for 4 additional weeks (PW 12–15). One week after the onset of treatment all dendritic growth had stopped (7 of 7 imaged dendrites), and all filopodial protrusions were gone (Fig. 3e; compare 2nd and 3rd panels). Rapamycin also reduced spine density on the newly grown dendrites (Fig. 3f) by inhibiting spine gain (Fig. 3g). Prolonging rapamycin treatment did not reverse dendritic growth significantly (cf. last panel Fig. 3e). Mild growth resumed when rapamycin was withdrawn.
Notably, the apical dendrites of layer 5 pyramidal neurons in Pten−/− mice displayed no measurable differences in dendritic growth, spine density, or spine kinetics relative to controls. Laser capture rtPCR and immunostaining confirmed the absence of Pten RNA and protein in layer 5 pyramidal neurons in Pten−/− mice. Rapamycin had no measurable effect on these neurons in control mice or in Pten−/− mice. These data (Fig. S3), suggest that Pten/mTOR signaling does not regulate dendritic growth of layer 5 pyramidal neurons at this age.
We draw three major conclusions from this study. First, dendrites in mature cortex retain the capacity for large-scale growth. Second, growing dendrites appear to behave much like dendrites in younger cortex. Third, the regulation of this growth by the Pten/mTOR signaling pathway is restricted to layer 2/3 apical dendrites in mature cortex. Lastly, our results suggest that growth-promoting pathways are constitutively active in adult cortical neurons; the growth we observed was induced not by activating a dormant growth pathway, but by removing a barrier to an active pathway.
Supplementary Material
Acknowledgements
We thank EM Callaway and K Bochmann for providing the ZnG reporter mice. This work was supported by the US National Eye Institute (EY016052), The Esther and Joseph Klingenstein Foundation, and by the US National Institute for Mental Health (MH068172).
Footnotes
Author Contributions:
DKC, MG, and JTT designed the experiments. DKC and JTT performed all imaging experiments, analyzed the data, and wrote the manuscript. MP performed the pS6 immunostaining. MM and STC performed the laser capture qrtPCR. MP, and XL provided the mice.
REFERENCES
- 1.Hausser M, Spruston N, Stuart GJ. Diversity and dynamics of dendritic signaling. Science. 2000;290:739–744. doi: 10.1126/science.290.5492.739. [DOI] [PubMed] [Google Scholar]
- 2.Wong RO, Ghosh A. Activity-dependent regulation of dendritic growth and patterning. Nat Rev Neurosci. 2002;3:803–812. doi: 10.1038/nrn941. [DOI] [PubMed] [Google Scholar]
- 3.Cline HT. Dendritic arbor development and synaptogenesis. Curr Opin Neurobiol. 2001;11:118–126. doi: 10.1016/s0959-4388(00)00182-3. [DOI] [PubMed] [Google Scholar]
- 4.Trachtenberg JT, et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- 5.Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441:979–983. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- 6.Lee WC, et al. Dynamic remodeling of dendritic arbors in GABAergic interneurons of adult visual cortex. PLoS Biol. 2006;4:e29. doi: 10.1371/journal.pbio.0040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraser MM, Bayazitov IT, Zakharenko SS, Baker SJ. Phosphatase and tensin homolog, deleted on chromsome 10 deficiency in brain causes defects in synaptic structure, transmission and plasticity, and myelination abnormalities. Neuroscience. 2008;151:476–488. doi: 10.1016/j.neuroscience.2007.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon CH, et al. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holtmaat A, et al. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45:279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Bishop DL, Misgeld T, Walsh MK, Gan WB, Lichtman JW. Axon branch removal at developing synapses by axosome shedding. Neuron. 2004;44:651–661. doi: 10.1016/j.neuron.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Guertin DA, Sabatini DM. An expanding role for mTOR in cancer. Trends Mol Med. 2005;11:353–361. doi: 10.1016/j.molmed.2005.06.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.