Abstract
Axotrophin/MARCH-7 was first identified in mouse embryonic stem cells as a neural stem cell gene. Using the axotrophin/MARCH-7 null mouse, we discovered profound effects on T lymphocyte responses, including 8-fold hyperproliferation and 5-fold excess release of the stem cell cytokine leukemia inhibitory factor (LIF). Our further discovery that axotrophin/MARCH-7 is required for targeted degradation of the LIF receptor subunit gp190 implies a direct role in the regulation of LIF signaling. Bioinformatics studies revealed a highly conserved RING-CH domain in common with the MARCH family of E3-ubiquitin ligases, and accordingly, axotrophin was renamed “MARCH-7.” To probe protein expression of human axotrophin/MARCH-7, we prepared antibodies against different domains of the protein. Each antibody bound its specific target epitope with high affinity, and immunohistochemistry cross-validated target specificity. Forty-eight human tissue types were screened. Epithelial cells stained strongly, with trophoblasts having the greatest staining. In certain tissues, specific cell types were selectively positive, including neurons and neuronal progenitor cells in the hippocampus and cerebellum, endothelial sinusoids of the spleen, megakaryocytes in the bone marrow, crypt stem cells of the small intestine, and alveolar macrophages in the lung. Approximately 20% of central nervous system neuropils were positive. Notably, axotrophin/MARCH-7 has an expression profile that is distinct from that of other MARCH family members. This manuscript contains online supplemental material at http://www.jhc.org. Please visit this article online to view these materials. (J Histochem Cytochem 58:301–308, 2010)
Keywords: axotrophin/MARCH-7, E3-ligase, LIF, adult stem cells
Protein synthesis is critically balanced by protein degradation to maintain functional homeostasis within eukaryotic cells as they respond to and then recover from microenvironmental stimuli. Specificity in degradation involves the ubiquitin E3-ligases (Hershko and Ciechanover 1998) that recognize their target substrates and mediate transfer of ubiquitin from an E2-ubiquitin-conjugating enzyme, either by catalysis (involving the HECT family) or by providing a docking scaffold between the E2 and the substrate (RING family). The RING family is encoded by some 600 genes, where specific family members may function either to induce degradation of their protein target by the proteasome system or to modify the target, thereby modulating its function and/or cellular location (Deshaies and Joazeiro 2009). Despite their great biological importance, our understanding of the E3 ligases is in its infancy.
The membrane-associated RING-CH (MARCH) E3 ligases were first discovered as a family of viral transmembrane proteins that function to downregulate critical cell surface receptors, including major histocompatability class I molecules, providing a mechanism for immune evasion of poxviruses and γ-2 herpes viruses (Lehner et al. 2005). At least 11 cellular orthologs of the MARCH family are known (Bartee et al. 2004; Morokuma et al. 2007), one being encoded by a neuronal stem cell gene on chromosome 2 (murine and human) originally termed axotrophin and subsequently renamed MARCH-7. The functional importance of axotrophin/MARCH-7 in normal development initially appeared to be relatively mild since, in the axotrophin/MARCH-7 null mouse, the only defects detected were agenesis of the corpus callosum and early axonal degeneration of dorsal root ganglia (Metcalfe et al. 2005). Subsequently, a role for axotrophin/MARCH-7 in immunity was suspected when, in a full subtractive gene array experiment, it was one of only eight genes (8/36,000) specifically linked to immune tolerance (Metcalfe and Muthukumarana 2005). This led to the discovery that, when T-cell–mediated immunity is activated in the absence of axotrophin/MARCH-7, there is failure of feedback regulation of the T lymphocyte, with 8-fold hyperproliferation and 5-fold overproduction of T-cell–derived leukemia inhibitory factor (LIF). In marked contrast, axotrophin-null B lymphocytes appear normal (Metcalfe et al. 2005). Thus axotrophin/MARCH-7 proved to be a specific negative regulator of activated T lymphocytes.
Although axotrophin/MARCH-7 is now recognized as a regulator of neuronal stem cells and of T lymphocytes, little is known about its molecular biology, other than two recent reports showing that (1) it is required for degradation of the LIF receptor gp190 subunit (Gao et al. 2009) and that (2) it is tightly regulated by both auto-ubiquitinylation (degradation) and by deubiquitination (preservation) via the specific deubiquitination enzymes USP-7 and USP-9× (Nathan et al. 2008). The only functional association at the molecular level is with the E2, “E2-25K” (Flierman et al. 2006), also known as huntingtin-interacting protein, since it can ubiquitinate huntingtin, the gene product for Huntington's disease.
Given the potential importance of axotrophin/MARCH-7, we reasoned that the expression profile of the protein will inform on the functional significance of axotrophin/MARCH-7 in vivo, but, until now, lack of antibody has prevented expression mapping. Working with the Human Proteome Resource, Stockholm, where a human protein atlas is being generated to complement the human genome atlas (Berglund et al. 2008b; Hober and Uhlén 2008; Uhlen 2008), we raised rabbit-anti-human axotrophin/MARCH-7 antibody and screened 45 human tissue types for expression of the axotrophin/MARCH-7 protein. Certain tissue-specific patterning and cell-specific positivity were revealed, including those of neural progenitor cells, intestinal crypt stem cells, megakaryocytes, trophoblasts, some lymphocytes, and the splenic sinusoid.
Materials and Methods
Preparation of Anti-human Axotrophin/MARCH-7
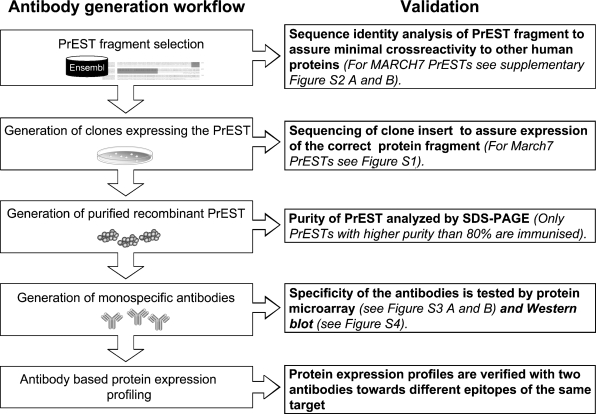
An overview of antibody generation and validation is shown in Figure 1. Rabbit antibody to human axotrophin/MARCH-7 was raised using antigenic fragments prepared as follows. First, as shown in Supplementary Figures S1 and S2, two protein epitope signature tags (PrEST) were selected based on information from ENSEMBL database, using a Prestige visualization tool (Berglund et al. 2008a). The selected fragments were amplified using total human RNA and then cloned into a vector for expression as recombinant proteins in Escherichia coli. These were used to immunize rabbits, and then monospecific antibodies were prepared from the collected antisera, using affinity purification in a two-step procedure as described by Nilsson et al. (2005). The specificity of the monospecific antibody was assessed by (1) staining of protein microarrays consisting of 384 spotted recombinant PrESTs (see Supplementary Figure S3) and by (2) Western blot analysis using tissue lysates (liver and tonsil), cell line extracts (RT-4 and U-251MG), and human plasma. Although only one of two selected anti-axotrophin antibodies gave a strong positive Western blot compared to its target, comparative studies permitted cross-validation of specificity and revealed that (1) both of the antibodies showed similar immunohistochemical staining profiles and that (2) both antibodies differed from the immunohistochemical staining profiles of other MARCH proteins, including MARCH-10.
Figure 1.
Flow chart of validation steps during antibody generation. More details are provided in Supplementary Figures S1–S3.
Immunohistochemistry
Human tissue microarrays were generated as described by Kampf (Kampf et al. 2004) and used first to validate the antibodies and thereafter to screen protein expression of axotrophin/MARCH-7. The staining protocol was optimized using 25 different human tissue types and 11 different cell lines, after which protein expression profiles were generated by staining of (1) 48 normal tissue types, (2) 47 different cell lines, and (3) 12 clinical cell samples. For initial screening, stained tissue microarrays were scanned using an automatic image acquisition system, and images were stored for subsequent annotation by certified pathologists. The full profile of immunohistochemistry for human axotrophin/MARCH-7 protein expression will be made publicly available via the Human Protein Atlas (www.proteinatlas.org), together with data regarding the corresponding antibody.
Scoring of Axotrophin/MARCH-7 Protein Expression
For detailed assessment, the full library of 48 human tissue images stained for axotrophin/MARCH-7 (antibody HPA014275) were downloaded from the Human Protein Atlas (collaborator version). The images were reviewed in random order, independently, by two experienced histopathologists. Each image was assessed with regard to the types of cells positively stained, the number of cells positively stained, the intensity of staining, and any specific subcellular staining. The resultant analyses were compared and discussed and agreement reached by using an appropriate score for each of the parameters for each image. Scoring of the protein expression consisted of an estimation of the percentage of cells that were positive (0–100%) and the intensity of the positivity (from + to +++++). The agreed scores are presented in Table 1.
Table 1 .
Expression of axotrophin/MARCH-7 in human tissues
| Tissue | Cell or tissue type | % of positive cells | Intensity |
|---|---|---|---|
| Adrenal | Medulla | 100 | ++++ |
| Appendix | Glandular epithelium | 100 | ++++ |
| Lymphocytes | 50 | ++ | |
| Bone marrow | Megakaryocyte | 100 | ++++ |
| Other | 40 | ++++ | |
| Breast | Ductal epithelial | 100 | +++++ |
| Bronchus | Glandular epithelium | 100 | +++++ |
| Plasma cells | 100 | ++++ | |
| Cerebellum | Neurons | 100 | ++++ |
| Glia | Negative | Negative | |
| Cervix | Squamous epithelium | 100 | + |
| Colon | Glandular epithelium | 100 | +++++ |
| Plasma cells | 100 | ++++ | |
| Duodenum | Glandular epithelium | 100 | +++++ |
| Plasma cells | 70 | ++ | |
| Epididymis | Ciliated glandular epithelium | 100 | +++ |
| Esophagus | Squamous epithelium | 100 | +++++ |
| Fallopian tube | Ciliated glandular epithelium | 100 | +++++ |
| Gall bladder | Glandular epithelium | 100 | ++++ |
| Heart muscle | Myocytes | 100 | ++++ |
| Hippocampus | Neurons | 100 | +++++ |
| Glia | Negative | Negative | |
| Neuropil | 20 | + | |
| Kidney | Tubular epithelium | 100 | ++++ |
| Glomeruli | Negative | Negative | |
| Lateral ventricle | Neuropil | 20 | ++ |
| Glia | Negative | Negative | |
| Lung | Pneumocytes | Negative | Negative |
| Alveolar macrophages | 20 | + | |
| Lymph node | Lymphocytes (some T-cell patches) | 20 | +++ |
| B cell areas | Negative | Negative | |
| Nasopharynx | Glandular epithelium | 100 | ++++ |
| Oral mucosa | Squamous epithelium | 20 | + |
| Ovary | Endothelium | 50 | +++ |
| Scattered stromal cells | 30 | ++ | |
| Pancreas | Acinar | 100 | +++++ |
| Islets | 100 | +++ | |
| Prostate | Glandular epithelium | 100 | ++++ |
| Rectum | Glandular epithelium | 100 | ++++ |
| Salivary gland | Acinar and ductal epithelium | 100 | ++++ |
| Seminal vesicle | Ductal epithelium | 100 | ++++ |
| Skeletal muscle | Myocytes | 100 | ++++ |
| Skin | Keratinocytes | 50 | ++ |
| Spleen | Endothelial sinusoids | 100 | ++++ |
| Lymphocytes | 5 | ++ | |
| Lymphocytes | 90 | Negative | |
| Small intestine | Glandular epithelium | 100 | +++++ |
| Crypt basal cells | ++++ | ||
| Smooth muscle | Myocytes | Negative | Negative |
| Vascular endothelium | Endothelial | 30 | ++ |
| Stromal spindle cells | Negative | Negative | |
| Fat | Adipocytes | Negative | Negative |
| Peripheral nerve | Nerve | Negative | Negative |
| Lymphocytes | Negative | Negative | |
| Plasma cells | 5 | +++++ | |
| Stomach | Glandular epithelium | 100 | +++++ |
| Testis (from 26-year-old) | Sertoli cells | Negative | Negative |
| Early germ cells | 20 | ++ | |
| Maturing sperm cells | Negative | Negative | |
| Testis (from 26-year-old) | Spermatozoa | Negative | Negative |
| Leydig cells | 100 | ++++ | |
| Testis (from 46-year-old) | Sertoli cells | 100 | ++ |
| Germ cells | 100 | ++ | |
| Leydig cells | 100 | ++++ | |
| Thyroid gland | Follicular epithelium | 100 | ++++ |
| Trophoblast | Epithelium | 100 | ++++ |
| Trophoblast | 100 | +++++ | |
| Urinary bladder | Transitional epithelium | 100 | ++++ |
| Vagina | Squamous epithelium | Negative | Negative |
| Vulva | Epidermis | Negative | Negative |
Results
Table 1 shows that, in general, most normal tissues showed moderate cytoplasmic positivity for the axotrophin/MARCH-7 protein, with glandular epithelial cells showing high intensity. In particular, the trophoblast showed marked positivity as did ciliated respiratory epithelial cells of the nasopharynx and ciliated glandular epithelium of the fallopian tube. The majority of stromal tissue showed little if any expression.
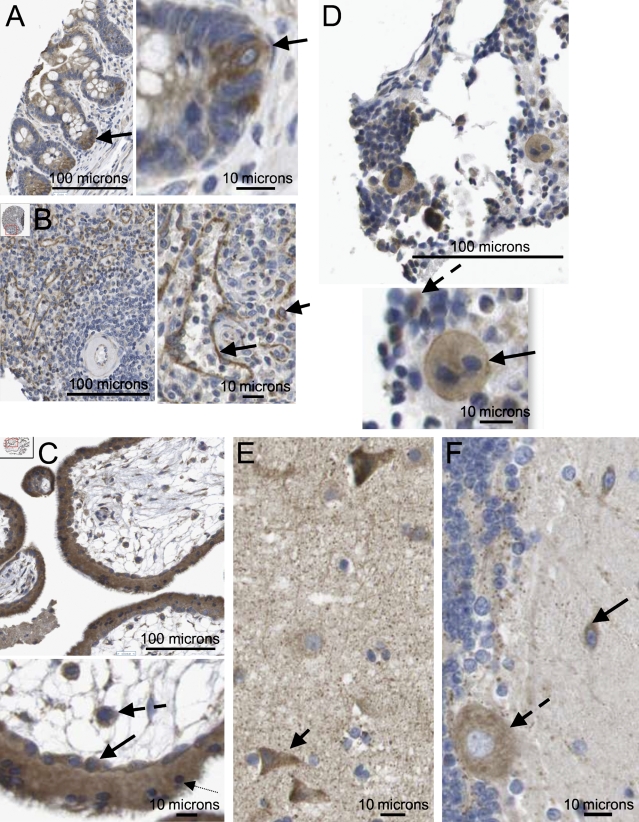
Certain tissues revealed cell-specific patterns of expression that may be linked to specific activities. In the small intestine, the cytoplasm of specific crypt cells of the villus endothelium was notably positive, while adjacent cells showed relatively weak expression (Figure 2A). In the spleen, the splenic sinusoid structure was clearly delineated by strong cytoplasmic expression of axotrophin/MARCH-7 (Figure 2B), while the surrounding lymphoid tissue was mostly negative. Within the trophoblast, both the syncitiotrophoblast and the cytotrophoblast strongly expressed axotrophin, as did the cells within the vascular mesoderm of the villus (Figure 2C). In bone marrow, specific functional cell types were positive for axotrophin/MARCH-7, notably megakaryocytes and certain mononuclear cells (Figure 2D). Highly specific staining was also found in central nervous system (CNS), with neurons being positive in contrast to negative glia (Figures 2E and 2F), while in the hippocampus, neuronal precursor cells were also stained. In contrast to the CNS, peripheral nerve tissue did not express axotrophin/MARCH-7. Notably, some 20% of neuropil structures appeared to be positive; here, the reported functional association with the huntingtin-interacting E2-25K protein is relevant (Flierman et al. 2006). In the lymph node and tonsil, most lymphocytic cells were also negative, but there were some larger cells with light cytoplasmic staining (Supplementary Figure S4).
Figure 2.
Axotrophin/MARCH-7 protein expression in selected tissue types. (A) Small intestine showing strong positive, precise, and consistent cytoplasmic staining of crypt basal progenitor cells (arrow); nucleus is negative. Adjacent cells higher up the crypt show relatively weak cytoplasmic staining. (B) In the spleen, the sinusoidal endothelium shows strong positive, precise, and consistent staining of the cytoplasm (longer arrow). In the splenic pulp, most lymphocytes are negative, apart from a small number of cells with specific cytoplasmic staining (shorter arrow). Small arteriole is negative. (C) First trimester placenta; here, there is strong, precise, and consistent positive staining of both the cytotrophoblast (solid arrow) and the syncitiotrophoblast (dotted arrow); a few cells also show nuclear positivity. Cells within the vascular mesoderm of the villus are also strongly positive (dashed arrow). (D) In bone marrow, megakaryocytes are present and show clear moderate granular expression of axotrophin/MARCH-7, which is increasing at the plasma membrane (solid arrow). Some 40% of small mononuclear cells also show cytoplasmic positivity (dashed arrow). (E) In the CNS, the cerebral cortex neurons show a range of moderate to strong cytoplasmic positivity (arrow). A fine network of lightly stained neuropils is evident. The glia is negative. (F) CNS cerebellum showing moderate cytoplasmic positivity in neurons (solid arrow) and Purkinje cells (dashed arrow). The glia is negative.
Since the MARCH family member closest to axotrophin/MARCH-7 is MARCH-10, we compared their expression profiles in kidney, testis, lung, and pancreas. Supplementary Figure S5 shows details of axotrophin/MARCH-7 staining in these tissues where alveolar macrophages are specifically stained in the lung, while the pancreatic tissue is positive, with islet cells being relatively weakly stained compared with the surrounding exocrine tissue. Supplementary Figure S6 shows a comparison of axotrophin/MARCH-7 and MARCH-10 and reveals clear differences in expression profiles, as described in the legend. Similarly, axotrophin/MARCH-7 differed from MARCH-1, MARCH-2, and MARCH-4 expression (data not shown). Supplementary Figure S7 compares the same tissues (kidney, testis, lung, and pancreas) for the presence of LIF protein. Here, strong positivity of alveolar macrophages occurs for both axotrophin/MARCH-7 and LIF. In the pancreas there was marked expression of LIF in the islet cells, especially at the plasma membrane, and in ductal cells of the exocrine tissue, while the exocrine tissue itself was only faintly positive: this is an inverse profile to that observed for axotrophin/MARCH-7.
Given the intense cytoplasmic expression of axotrophin/MARCH-7 in syncitiotrophoblast and cytotrophoblast cells, we compared expression of the LIF receptor (LIF-R) gp190, where a similar pattern was found (Supplementary Figure S8B). In contrast, MARCH-10 showed a distinct pattern with strongly positive nuclei (Supplementary Figure S8C). Since axotrophin is linked to cell cycle regulation in T lymphocytes (Metcalfe et al. 2005), we also looked for expression of the cyclin B2 cell cycle protein in the trophoblast. Here, expression was highly specific to a few cytotrophoblasts, indicating cell cycle-specific expression in contrast to the constitutive expression of axotrophin/MARCH-7, LIF-R, and MARCH-10.
Discussion
This is the first report of axotrophin/MARCH-7 protein expression in a full profile of human tissues; it is also the first report of expression in any mammalian species. Our findings suggest specific candidates for functional involvement of axotrophin/MARCH-7 and, in particular, stem cells and precursor cells of the brain and small intestinal crypts. Here, we speculate that axotrophin/MARCH-7's E3 ligase activity linked to degradation of the LIF-R may play a regulatory role to prevent excessive LIF signaling. In addition to adult stem cells, the finding of significant axotrophin/MARCH-7 transcript levels in three different hESC cell lines (de Sousa and Pells, unpublished data) demonstrates that this putative regulatory role may extend to control of LIF-R in embryonic stem cells.
In addition to stem and precursor cells, we found that certain differentiated cells were positive for axotrophin/MARCH-7. LIF is a pleiotropic cytokine that activates both the STAT-3 and the mitogen-activated protein-kinase signaling pathways to induce various effects in a cell-specific manner. Cell type specificity is possible because, although LIF may activate STAT-3-mediated gene transcription (Heinrich et al. 2003), the LIF response genes available to STAT-3 are cell type specific. Cellular differentiation down a given pathway that is associated with chromatin remodeling and epigenetic profiling will establish a signature of STAT-3-responsive genes specific for the cell in question, qualifying its response to LIF. Thus, while axotrophin/MARCH-7 activity will qualify the strength of the LIF signal, the differences in chromatin structure will qualify the effect of the LIF signal.
We had initially expected axotrophin to be linked to lymphoid tissue, given its profound regulatory role in T lymphocytes and in tolerogenic LIF signaling (Metcalfe 2005; Muthukumarana et al. 2006,2007) and the fact that LIF is required for maintenance of hematopoietic stem cells (Escary et al. 1993). However, high lymphoid tissue expression was not found. For active regulatory-tolerant T (Treg) cells, controlled expression of the LIF-R will permit controlled responses to LIF where tolerance is operating, including in self-tolerance: axotrophin/MARCH-7 may play a role in this control mechanism (Gao et al. 2009). The observed small percentage of positive lymphocytes may reflect LIF activity in Treg cells, known to have a relative abundance of 5% to 10% (Wan and Flavell 2005). Here, the hierarchical role of axotrophin/MARCH-7 relative to that of other E3 ligases involved in immune regulation (Lin and Mak 2007) needs to be investigated.
Of the other systems studied, there were several notable features. In bone marrow, LIF is a growth factor for maturation of megakaryocytic progenitor cells, and these express cell surface LIF-R (Metcalf et al. 1991), a feature that may be linked to the coexpression of axotrophin/MARCH-7 seen in bone marrow megakaryocytes. In the CNS, the staining of some neuropils may indicate functional activity linked to huntingtin-interacting protein, and we plan to probe brain from Huntington's disease patients to look for correlates with disease. In trophoblasts, LIF activity is required for implantation of trophoblastic tissue (Cullinan et al. 1996; Fouladi-Nashta et al. 2005) and is also associated with immune tolerance at the fetomaternal interface (Piccinni et al. 2001); here, the high level of axotrophin/MARCH-7 expression may reflect the need to regulate LIF signaling and prevent abnormal autocrine-positive feedback of LIF activity that may lead to pathogenic changes within the placenta.
The expression of high levels of cytoplasmic axotrophin/MARCH-7 in the splenic sinusoids was of interest, since here too, a regulatory role effect upon LIF signaling may occur. De novo formation of blood vessels, vasculogenesis, is integral to embryogenesis and occurs by differentiation of angioblasts, and there is evidence for cooperation between basic fibroblast growth factor (bFGF) and LIF in the vasculogenic process (Gendron et al. 2006). LIF may be associated with endothelial pathogenic processes, and abnormally high levels of LIF can be detected in patients with giant-cell arteritis (Lecron et al. 1993), a degenerative disease of the blood vessel wall, supporting the notion that LIF signaling needs to be tightly regulated within the healthy vessel. One observation that would suggest a role for axotrophin in such regulation is the observed vascular leak in pulmonary vessels of the aged axotrophin null mouse (Metcalfe, unpublished data), raising the possibility that a lack of axotrophin and associated high LIF signaling activity within the pulmonary vessel may be causative.
Overall, by mapping expression of axotrophin in normal human tissues for the first time, we provide a baseline for normal expression from which to assess pathological conditions for potential involvement of axotrophin/MARCH-7 activity. In addition, we reveal a putative link with the regulation of LIF activity in several tissue-specific contexts, including stem cells and precursor cells of the gut and brain; the site of embryo implantation that is also immune privileged; glandular epithelial tissues; and specific cells of blood lineage including megakaryocytes and a small proportion of lymphocytes that may make up the Treg population. These links may be reflected in those diseases where reparative adult stem cell activity is compromised, including neurodegenerative diseases, while the link to huntingtin-interacting protein indicates a further potential role in the CNS, distinct from that involving adult stem cells.
Acknowledgments
We thank Dr. Mike Taussig of the Babraham Institute, Cambridge, for early input into this work; Prof. Twink Allen, Paul Mellon Laboratory, Newmarket, for advice on the trophoblast's structural elements; Dr. Toby Gibson, The European Molecular Biology Laboratory Heidelberg, for constructive advice; and Dr. Willem Ouwehand, Department of Haematology, University of Cambridge, for advice on the bone marrow image. S.M.M. holds the University of Cambridge Grimshaw Parkinson Lectureship.
This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication.
References
- Bartee E, Mansouri M, Hovey Nerenberg BT, Gouveia K, Fruh K (2004) Downregulation of major histocompatibility complex class I by human ubiquitin ligases related to viral immune evasion proteins. J Virol 78:1109–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund L, Björling E, Jonasson K, Rockberg J, Fagerberg L, Al-Khalili Szigyarto C, Sivertsson A, et al. (2008a) A whole-genome bioinformatics approach to selection of antigens for systematic antibody generation. Proteomics 8:2832–2839 [DOI] [PubMed] [Google Scholar]
- Berglund L, Björling E, Oksvold P, Fagerberg L, Asplund A, Szigyarto CA, Persson A, et al. (2008b) A gene-centric human protein atlas for expression profiles based on antibodies. Mol Cell Proteomics 10:2019–2027 [DOI] [PubMed] [Google Scholar]
- Cullinan EB, Abbondanzo SJ, Anderson PS, Pollard JW, Lessey BA, Stewart CL (1996) Leukemia inhibitory factor (LIF) and LIF receptor expression in human endometrium suggests a potential autocrine/paracrine function in regulating embryo implantation. Proc Natl Acad Sci USA 93:3115–3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies RJ, Joazeiro CA (2009) RING domain E3 ubiquitin ligases. Annu Rev Biochem 78:399–434 [DOI] [PubMed] [Google Scholar]
- Escary JL, Perreau J, Dumenil D, Ezine S, Brulet P (1993) Leukaemia inhibitory factor is necessary for maintenance of haematopoietic stem cells and thymocyte stimulation. Nature 363:361–364 [DOI] [PubMed] [Google Scholar]
- Flierman D, Coleman CS, Pickart CM, Rapoport TA, Chau V (2006) E2–25K mediates US11-triggered retro-translocation of MHC class I heavy chains in a permeabilized cell system. Proc Natl Acad Sci USA 103:11589–11594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouladi-Nashta AA, Jones CPJ, Nijjar N, Mohamet L, Smith A, Chambers I, Kimber SJ (2005) Characterization of the uterine phenotype during the peri-implantation period for LIF-null, MF1 strain mice. Dev Biol 281:1–2 [DOI] [PubMed] [Google Scholar]
- Gao W, Thompson HL, Zhou Q, Putheti P, Fahmy TM, Strom TB, Metcalfe SM (2009) Treg versus Th17 lymphocyte lineages are cross-regulated by LIF versus IL-6. Cell Cycle 8:1444–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron RL, Tsai FY, Paradis H, Arceci RJ (2006) Induction of embryonic vasculogenesis by bFGF and LIF in vitro and in vivo. Dev Biol 177:332–346 [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F (2003) Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 374:1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A (1998) The ubiquitin system. Annu Rev Biochem 67:425–479 [DOI] [PubMed] [Google Scholar]
- Hober S, Uhlén M (2008) Human protein atlas and the use of microarray technologies. Curr Opin Biotechnol 19:30–35 [DOI] [PubMed] [Google Scholar]
- Kampf C, Andersson AC, Wester K, Björling E, Uhlen M, Ponten F (2004) Antibody-based tissue profiling as a tool for clinical proteomics. Clin Proteomics 1:285–289 [Google Scholar]
- Lecron JC, Roblot P, Chevalier S, Morel F, Alderman E, Gombert J, Gascan H (1993) High circulating leukaemia inhibitory factor (LIF) in patients with giant cell arteritis: Independent regulation of LIF and IL-6 under corticosteroid therapy. Clin Exp Immunol 92:22–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner PJ, Hoer S, Dodd R, Duncan LM (2005) Downregulation of cell surface receptors by the K3 family of viral and cellular ubiquitin E3 ligases. Immunol Rev 207:112–125 [DOI] [PubMed] [Google Scholar]
- Lin AE, Mak TW (2007) The role of E3 ligases in autoimmunity and the regulation of autoreactive T cells. Curr Opin Immunol 19:665–673 [DOI] [PubMed] [Google Scholar]
- Metcalf D, Hilton D, Nicola NA (1991) Leukaemia inhibitory factor can potentiate murine megakaryocyte production in vitro. Blood 15:2150–2153 [PubMed] [Google Scholar]
- Metcalfe SM (2005) Axotrophin and leukaemia inhibitory factor (LIF) in transplantation tolerance. Philos Trans R Soc Lond B Biol Sci 360:1687–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe SM, Muthukumarana DSPA (2005) Transplantation tolerance: gene expression profiles comparing allotolerance vs. allorejection. Int Immunopharmacol 5:33–39 [DOI] [PubMed] [Google Scholar]
- Metcalfe SM, Muthukumarana PA, Thompson HL, Haendel MA, Lyons GE (2005) Leukaemia inhibitory factor (LIF) is functionally linked to axotrophin and both LIF and axotrophin are linked to regulatory immune tolerance. FEBS Lett 579:609–614 [DOI] [PubMed] [Google Scholar]
- Morokuma Y, Nakamura N, Kato A, Notoya M, Yamamoto Y, Sakai Y, Fukuda H, et al. (2007) MARCH-XI, a novel trans-membrane ubiquitin ligase implicated in ubiquitin-dependent protein sorting in developing spermatids. J Biol Chem 282:24806–24815 [DOI] [PubMed] [Google Scholar]
- Muthukumarana P, Chae WJ, Maher S, Rosengard BR, Bothwell AL, Metcalfe SM (2007) Regulatory transplantation tolerance and “stemness”: evidence that Foxp3 may play a regulatory role in SOCS-3 gene transcription. Transplantation 84(suppl 1):S6–11 [DOI] [PubMed] [Google Scholar]
- Muthukumarana PA, Lyons GE, Miura Y, Thomson LH, Watson T, Green CJ, Shurey S, et al. (2006) Evidence for functional inter-relationships between FOXP3, leukaemia inhibitory factor, and axotrophin/MARCH-7 in transplantation tolerance. Int Immunopharmacol 6:1993–2001 [DOI] [PubMed] [Google Scholar]
- Nathan JA, Sengupta S, Wood S, Admon A, Markson G, Sanderson C, Lehner PL (2008) The Ubiquitin E3 ligase MARCH7 is differentially regulated by the deubiquitylating enzymes USP7 and USP9X. Traffic 9:1130–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson P, Paavilainen L, Larsson K, Odling J, Sundberg M, Andersson AC, Kampf C, et al. (2005) Towards a human proteome atlas: high-throughput generation of mono-specific antibodies for tissue profiling. Proteomics 5:4327–4337 [DOI] [PubMed] [Google Scholar]
- Piccinni M-P, Scaletti C, Vultaggio A, Maggi E, Romagnani S (2001) Defective production of LIF, M-CSF and Th2-type cytokines by T cells at fetomaternal interface is associated with pregnancy loss. J Reprod Immunol 52:35–43 [DOI] [PubMed] [Google Scholar]
- Uhlen M (2008) A new era for proteomics research? Genome Biol 9:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan YY, Flavell RA (2005) Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci USA 102:5126–5131 [DOI] [PMC free article] [PubMed] [Google Scholar]