Abstract
Human cardiac stem/progenitor cells and their potential for repair of heart injury are a current hot topic of research. CD117 has been used frequently as a marker for identification of stem/progenitor cells in the heart. However, cardiac mast cells, which are also CD117+, have not been excluded by credible means when selecting putative cardiac progenitors by using CD117 as a marker. We evaluated the relationship between CD117+ cells and mast cells in the left ventricle of human hearts (n=5 patients, ages 1 week–75 years) with the well-established mast cell markers tryptase, toluidine blue, and thionine. A large number (85–100%) of CD117+ cells in the human heart were specifically identified as mast cells. In addition, mast cells showed weak or moderate CD45 immunostaining signals. These results indicate that the majority of CD117+ cells in the heart are mast cells and that these cells are distinctly positive for CD45, although staining was weak or moderate. These results strongly suggest that the newly reported CD117+/CD45dim/moderate putative cardiac progenitor cells are mast cells. The significance of this observation in stem cell research of the heart is discussed. (J Histochem Cytochem 58:309–316, 2010)
Keywords: immunohistochemistry, CD117, CD45, progenitor cell, mast cell
Until recently, the perception was that the adult mammalian heart was an organ without regenerative capacity. However, in the past number of years, a series of reports of various putative cardiac progenitor cells which reside in the human heart or originate from outside the cardiovascular system have been reported (Martin-Puig et al. 2008; Reinecke et al. 2008).
Among the various markers for stemness used in the investigation of cardiac progenitor cells, CD117 has played a key role. For example, Beltrami et al. (2003) first reported a resident cardiac stem cell population that is positive for CD117 in the adult rat heart and described this cell as negative for blood lineage markers (Lin−), multipotent, and capable of giving rise to endothelial cells, smooth muscle cells, and functional cardiomyocytes. Since then, CD117 has been used frequently as a marker to isolate and identify these cells in the hearts of various species (Urbanek et al. 2003,2005; Dawn et al. 2005; Linke et al. 2005). It has been reported that such a CD117+ stem cell population could be identified and isolated from human heart (Bearzi et al. 2007).
On the other hand, the capability of CD117+ hematopoietic bone marrow cells to act as cardiac progenitors and transdifferentiate into cardiomyocytes has also been widely studied (Orlic et al. 2001; Kajstura et al. 2005; Rota et al. 2007; Scherschel et al. 2008). Although the cardiogenic potential of hematopoietic bone marrow cells is still in dispute, it has been asserted that CD117+ bone marrow cells engrafted within the host myocardium rapidly lose the hematopoietic CD45 phenotype and acquire a cardiomyocyte phenotype (Rota et al. 2007). A high proportion of these CD117+ cells isolated from normal and failing human hearts dimly or moderately coexpressed the pan leukocyte antigen (CD45), and this marker was also interpreted as reflecting cardiac progenitor cells' bone marrow origin (Kubo et al. 2008).
Mast cells arise from multipotent hematopoietic progenitors in the bone marrow (Kirshenbaum et al. 1991; Fodinger et al. 1994; Okayama and Kawakami 2006) and reside in connective tissues throughout the body, including the heart (Sperr et al. 1994; Bankl et al. 1995; Patella et al. 1998; Palladini et al. 2003; Shiota et al. 2003). Mast cells, including human cardiac mast cells, are CD117+ (Sperr et al. 1994), raising the possibility that they could potentially be mistaken for CD117+ cardiac progenitor cells. However, this potential source of confusion has rarely been specifically excluded by credible and specific mast cell markers when heart progenitor cells (both resident and transdifferentiated types) are experimentally isolated and/or identified using the criterion of CD117 positivity.
Based on the foregoing findings and studies, we hypothesized that regardless of age, a significant proportion of the CD117+ cells in the human heart are neither cardiac stem cells nor progenitor cells of bone marrow origin, and we raise the possibility that they are instead mast cells. In addition, the newly reported CD117+/CD45dim/moderate cardiac progenitors may be mast cells as well, and the CD45 positivity would not be qualified in distinguishing mast cells form Lin−/CD117+ cardiac stem cells with certainty as mast cells possess weak CD45 immunophenotype.
Many of the markers found on human cardiac mast cells, including IgE receptor, CD117 (the receptor for stem cell factor), p24 antigen, Pgp-l homing receptor (CD44), and the ICAM-1 antigen (CD54) (Sperr et al. 1994) can be expressed by many other types of cells and are not cell type specific. On the other hand, toluidine blue (Sperr et al. 1994; Bankl et al. 1995; Noack et al. 2005; Frangogiannis and Entman 2006) and thionine histochemical stains (Cook 1961; Trotter et al. 1989; Victor et al. 2004) are commonly used for histochemical identification of mast cells in tissue sections and are recognized as having a high degree of specificity for this purpose. In addition, tryptase is a mast cell enzyme, and anti-tryptase antibody is highly specific for identification of mast cells by immunohistochemistry (Sperr et al. 1994; Bankl et al. 1995; Noack et al. 2005; Frangogiannis and Entman 2006). In this study, we therefore employed immunohistochemistry (CD117 and tryptase) and mast cell–specific histochemistry (toluidine blue and thionine stains) to investigate the relationship between CD117+ cells and mast cells in the human heart. To evaluate the CD45 immunophenotype of cardiac mast cells and shed further light on the CD117+/CD45dim/moderate cells which have been identified as cardiac progenitor cells, we employed immunohistochemistry (CD45 and tryptase) and evaluated the staining intensity of CD45 in cells which were also positive for tryptase.
Materials and Methods
Specimens
Five human hearts from patients of ages ranging from 7 days to 75 years were obtained from autopsies performed by the Department of Pathology, Beijing University Health Science Center. All of these hearts were normal based on preautopsy clinical diagnosis, gross anatomic finding, and histopathologic evaluation. Morphological features that disqualified specimens from entering into this study included severe atherosclerosis of the aorta and/or coronary arteries, coronary occlusion, myocardial infarction, metastatic neoplasia, and any evidence of diffuse myocardial injury. Brief clinical information of these cases is provided in Table 1.
Table 1.
Clinical information and immunohistochemical results with cell counts in human subjects
| % of positivity in CD117+ cells |
% of CD45 intensity of tryptase+ cells |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Case | Gender | Age | Cause of death | Tryptase | Toluidine blue | Thionine | Moderate | Weak | Negative |
| 5752 | Male | 7 days | ARDSb | 90 | 100 | 89.6 | 4 | 94.8 | 1.2 |
| 5855 | Male | 6 years | Unknown | 98.1 | 95.4 | 87.3 | 3.8 | 93.2 | 3 |
| 4911 | Female | 28 years | Amnionic fluid embolism | 100 | 97.8 | 97.1 | 6.6 | 91.2 | 2.2 |
| 5856 | Male | 45 years | Acute pancreatitis | 95.7 | 91.4 | 97.1 | 4.5 | 93 | 2.5 |
| 5804 | Female | 75 years | ARDS | 100 | 100 | 85 | 6.4 | 92.3 | 1.3 |
500–1000 CD117+ and 200–250 tryptase+ cells were counted in five sections of the left ventricle of each human heart (n=5 cases), and these sections were of similar sizes. No mast cells with strong CD45 signals were found. ARDS, acute respiratory distress syndrome.
The apex and anterior and lateral walls of the left ventricle were dissected and fixed in 10% formalin, followed by paraffin embedding. Consecutive tissue sections of 4-μm thickness were cut.
This investigation conforms to the principles outlined in the Declaration of Helsinki. The use of human specimens and all related ethical issues were reviewed and approved by the Research Administration Committee of Peking University.
Immunohistochemistry and Controls
Immunohistochemistry was performed according to the method of Ye et al. (2007). Briefly, paraffin-embedded tissue sections were deparaffinized and immersed in 3% hydrogen peroxide to eliminate endogenous peroxidase activity. Antigen retrieval was performed according to the instructions of the manufacturers by heating the tissue sections at 96C in 0.01 mol/liter citrate buffer (pH 6.0) or EDTA solution (pH 8.0) for 20 min and then cooling them to room temperature. Primary antibodies to CD117, tryptase, and CD45 were used. The sources, dilutions, and incubation times of each primary antibody are listed in Table 2. Following primary antibody incubation and rinsing, the sections were then incubated with a secondary antibody labeled with peroxidase or alkaline phosphatase at 37C for 20 min and colorized with the enzyme substrate 3-amino-9-ethylcarbazole (AEC) (Dako Cytomation; Glostrup, Denmark) for peroxidase or nitro blue tetrazolium/5-bromo-4-choloro-3-indolyl phosphate (Promega; Madison, WI) for alkaline phosphatase. Following every step, sections were rinsed with 0.01M PBS three times for 5 min each. Positive cells showed red staining (peroxidase) or purple-blue staining (alkaline phosphatase). Slides were lightly counterstained with hematoxylin.
Table 2.
Antibodies for IHC
| Primary antibody | Supplied by | Dilution or concentration | Species, clones, and isotypes | Incubation, temperature, and period |
|---|---|---|---|---|
| CD117 | Dako Cytomation | Ready to use | Polyclonal rabbit anti-human | 4C, overnight |
| CD117 | Invitrogen; Carlsbad, CA | Ready to use | Monoclonal mouse anti-human, clone 2E4, IgG1 | 4C, overnight |
| Tryptase | Dako Cytomation | Ready to use | Monoclonal mouse anti-human, clone AA1, IgG1 | 4C, overnight |
| CD45 | Dako Cytomation | Ready to use | Monoclonal mouse anti-human, clone 2B11 + PD7/26, IgG1 | 4C, overnight |
| Insulin (negative control) | Invitrogen | Ready to use | Monoclonal mouse anti-human, clone Z006, IgG1 | 4C, overnight |
To show clear views of each of the markers coexpressed on individual cells, destaining–restaining was performed according to the technique of Deng et al. (2008) and Glass et al. (2009). Briefly, graded alcohols (100–75%) were used to dissolve the red AEC precipitate used for visualizing the first target antigen, and the antigen retrieval procedure was performed to denature any undetached primary antibody. After incubation with the second primary antibody, slides were processed as for routine immunohistochemistry (IHC). Positive and negative controls were treated as described previously (Deng et al. 2008; Glass et al. 2009).
For routine IHC technique, primary antibody was omitted or replaced by an irrelevant antibody (mouse anti-human insulin) of the isotype class (IgG1) of the primary antibody as a negative control. This served to exclude the possibility of nonspecific staining of mast cells particularly in view of the fact that mast cells generally express low levels of FcγRI (Okayama et al. 2000). Controls for destaining–restaining included omission of the first primary antibody or the second primary antibody and staining of sections with one antibody only to ascertain that the staining procedure did not interfere in any way with staining specificity or cell identification.
Histochemical Staining
Mast cells in the tissue samples were identified with toluidine blue staining using the technique of Bankl et al. (1995). Deparaffinized tissue sections were stained with 0.2% toluidine blue (0.2 g of toluidine blue dissolved in a mixture of 60 ml of 100% alcohol and 40 ml of ether) for 2 min, rinsed in water, dehydrated in acetone for 2 min, and cover-slipped for observation. Mast cells showed purple granules in the cytoplasm.
For thionine staining, deparaffinized sections were first immersed in 0.2% thionine solution (thionine dissolved in distilled water) for 10 min and then rinsed in water, dehydrated, and cover-slipped for observation. Mast cells showed purple granules in the cytoplasm.
CD117+ Cell Identification
All staining results were analyzed with a research-type microscope (Olympus BX51; Olympus Optical, Tokyo, Japan). To evaluate the proportion of CD117+ cells, which are mast cells in the human heart, tryptase immunostaining, toluidine blue staining, and thionine staining steps were carried out for mast cell identification of consecutive tissue sections adjacent to those immunostained with CD117 antibody. The positive cells in these serial sections were carefully compared. Alternatively, CD117 immunohistochemistry and tryptase immunohistochemistry or histochemical staining for mast cells was performed with the same tissue section using the destaining–restaining technique. Briefly, sections were first stained with tryptase for mast cells, and numerous photomicrographs were taken. The tryptase signal was then destained, and CD117 immunostaining was performed with these same tissue sections, followed by photomicrography of areas of interest. This procedure was also carried out in reverse order, first with CD117 immunohistochemistry, followed by photomicrography, then followed by tryptase immunostaining and histochemical staining and photomicrography of the destained slide. More than 50 fields were examined on each tissue section with a 20× objective lens. At least five sections from each heart were examined. In this manner, 500 to 1000 CD117+ cells were counted in each case. Data are expressed as a percentage of total CD117+ cells. Cell–cell identity in each pair of stains that was compared (both for destaining–restaining and serial sections) was established by microscopic evaluation of cell morphology and tissue structure.
CD45 Staining and Intensity
To evaluate CD45 immunostaining signals of human cardiac mast cells, CD45 and tryptase IHC were performed with the same tissue section using the destaining–restaining technique as described previously. Microphotographs of CD45 staining intensity of cells positive and negative for tryptase were then compared. In this manner, mast cells and CD45+ non-mast cells were compared. We used five tissue sections from each case for this investigation, with 50 photomicrographs taken with a 40× objective lens and compared in each section. We classified the CD45 staining intensity into four degrees as strong, moderate, weak, and negative, and for each case, the CD45 staining intensity of 200 to 250 tryptase positive cells was evaluated.
Results
CD117+ Cells and Tryptase+ Cells in the Heart
CD117+ cells were dispersed in the matrix of all tissue sections in hearts from all age groups examined, and no significant differences were observed when polyclonal antibody or monoclonal antibody was used. CD117+ cells were slightly more prevalent in the apex (4.9 ± 1.3 cells/mm2) than in the anterior (4.2 ± 0.8cells/mm2) and the lateral (3.9 ± 0.6 cells/mm2) walls of the left ventricle. The shape of CD117+ cells varied from round, to oval, to spindled, and cell diameters ranged from 6 to 15 μm. Tryptase-positive, toluidine blue-positive, and thionine-positive cells were also identified in these sections.
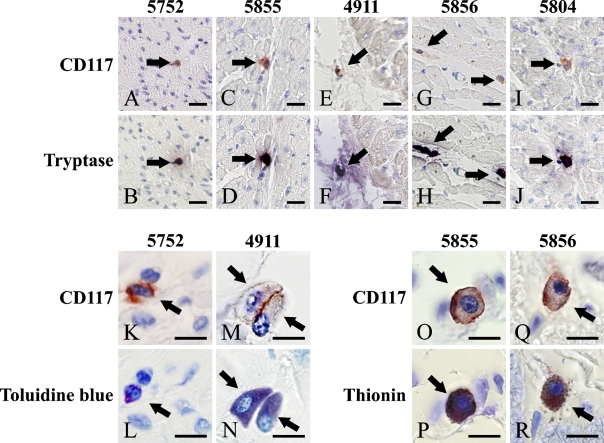
The four staining methods which were used to identify mast cells (CD117, tryptase, toluidine blue, and thionine) stained extensively overlapping populations of cells (Table 1). Figures 1A–1J show that the majority (90–100%) of these cells were both CD117+ (red membranous staining) and tryptase+ (purple-blue cytoplasmic staining). Most of the CD117+ cells (85–100%) showed purple granules in the cytoplasm with both toluidine blue staining and thionine staining, as shown in Table 1 and Figures 1K–1R.
Figure 1.
CD117+ cells in human hearts showing mast cell characteristics. (A,C,E,G,I) CD117+ cells (red, arrows) in cardiac sections of individuals of different ages; (B,D,F,H,J) mast cell–specific enzyme tryptase-positive cells (black purple, arrows). (A and B, C and D, E and F, G and H, I and J) The same fields from the same slide are shown. (K,M,O,Q) Higher power image of CD117+ cells (red, arrows). (L,N,P,R) Mast cell–specific histochemistry, toluidine blue staining and thionine staining, in which mast cells have purple cytoplasm (arrows). (K and L, M and N, O and P, Q and R) The same slide and the same field are shown. Bars: A–J = 20 μm; K–R = 10 μm.
It was noted that none of these three mast cell markers showed staining of 100% of CD 117+ cells in any cases (Table 1). Among mast cell–specific staining techniques in these five cases, a very small portion (0–15%) of the CD117+ cells were negative for tryptase with toluidine blue or thionine staining (Table 1).
CD45 Staining of Tryptase+ Cells
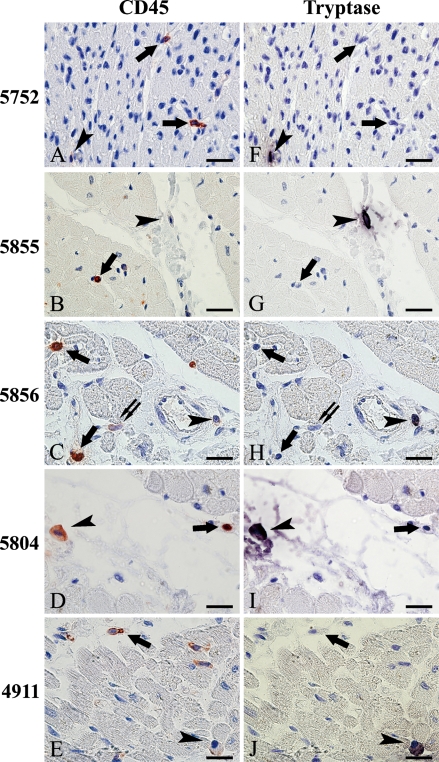
CD45+ cells were also found dispersed in cardiac tissue sections in all cases. Figures 2A–2E show CD45+ immunostaining signals visualized with AEC that vary from weak to strong. Moreover, a comparison of CD45 staining (Figures 2A–2E) with tryptase staining (Figures 2F–2J) shows that most (97–98.8%) of the tryptase+ cells (mast cells) also contained a positive CD45 signal. When cells were positive for both CD45 and tryptase, the CD45 immunoreactivity was generally (91.2 –94.8%) weak but distinct (Table 1; Figure 2).
Figure 2.
Tryptase-positive cells in human hearts stain weakly for CD45. (A–E) CD45+ cells in cardiac sections of individuals of different ages, with differing staining intensity (red, arrows, arrowheads, and double arrows). (F–J) Mast cell–specific tryptase-positive cells (black purple, arrowheads) and cells negative for tryptase but positive for CD45 (arrows and double arrows). (A and F, B and G, C and H, D and I, E and J) The same fields of one section are shown. Cells positive for tryptase in panels B, D, F, H, and J (black purple, arrowheads) show weaker CD45 staining intensity than those in panels A–D, and E (red, arrowheads) than most of the other CD45+ cells (red, arrows). Most of the tryptase-positive cells show weak staining (91.2–94.8%; red, arrowheads in A, C, and E). Occasionally, CD45 showed moderate staining (3.8–6.6%; red, arrowheads in D) or staining which was almost negative (1.2–3%; red, arrowheads in B). There were no strongly CD45 staining tryptase-positive cells. Cells indicated by arrows in panels A–E are strongly CD45+ but tryptase negative. However, the presence of weak or moderate CD45 staining did not guarantee tryptase positivity (C, double arrows). Bar = 20 μm.
All negative and positive controls gave appropriate results. No positive signal was noticed as mouse anti-human insulin was used as an irrelevant primary antibody for negative control.
Discussion
Our study has raised significant questions about the identity of isolated CD117+ cells from the human heart, which have heretofore been regarded as progenitor cells (Kubo et al. 2008) and the criterion used in excluding mast cells from the CD117+ stem cells (Bearzi et al. 2007) .
Results from this study demonstrate clearly that in human hearts of various ages, the majority (85–100%) of CD117+ cells showed distinct characteristics of mast cells including positive toluidine blue and thionine histochemical staining and tryptase immunoreactivity. In addition, cardiac mast cells identified by positive tryptase IHC characteristically showed weakly stained CD45 (CD45weak) IHC reactivity in our study.
In this study, the destaining–restaining method was employed to show coexpression of markers on individual cells on paraffin-embedded tissue slides (CD117 and tryptase or CD45 and tryptase). This method was chosen instead of using serial sections as cells of small size, like mast cells, would likely be lost or potentially mismatched in the 4-μm thickness between adjacent sections. Meanwhile, compared with double staining, the destaining–restaining method offered better visualization of each of the antigen staining results. The negative controls also ruled out the possibility of nonspecific staining of mast cells.
Kubo et al. (2008) reported that a CD117+/CD45+ phenotype from both normal and failing human hearts represented approximately 87% of the CD117+ cell population overall. Moreover, these putative CD45+ progenitors displayed a CD45dim/moderate phenotype, which was interpreted as reflecting the bone marrow origin of these cells. This interpretation seems reasonable in view of the time-dependent decrease in CD45 positivity in transdifferentiating bone marrow cells migrating to become cardiac progenitors observed in experimental murine myocardial infarction (Mouquet et al. 2005; Rota et al. 2007). However, based on our observation that a large proportion of mast cells were present among the CD117+ cardiac cells and characteristically showed CD45 weak positivity, it is likely that CD117+/CD45weak cardiac mast cells have been mistakenly identified as progenitor cells of bone marrow origin.
Mast cells have been reported to show increases of nearly 4-fold in hearts failing with ischemic cardiomyopathy (18.4 ± 1.5 cells/mm2) and idiopathic dilated cardiomyopathy (18.4 ± 1.6 cells/mm2) compared with normal controls (5.3 ± 0.7 cells/mm2) (Patella et al. 1998), and this coincides with almost the same extent of increase as that reported for cells identified as cardiac progenitors (Kubo et al. 2008). Thus, not only is the authenticity of the CD117+/CD45dim/moderate cells of bone marrow origin identified as a cardiac progenitor open to question but also the assumption that cardiac progenitor cell increase in hearts failing with ischemic cardiomyopathy and idiopathic dilated cardiomyopathy based mainly on observations of the putative cardiac progenitor cell CD117+/CD45dim/moderate phenotype may be erroneous.
Although the interpretation of Kubo et al. (2008) regarding the identity of CD45+/CD117+ cells in the human heart differs from ours (mast cell vs progenitor cell), both of these results are in contrast with the report by Bearzi et al. (2007), in which hematopoietic markers (including CD45) were not detected in CD117+ cells isolated from human heart. However, it is certain that the heart contains a CD117+/CD45+ cardiac mast cell population. Therefore, as the CD45 mast cell positivity is generally weak, mast cells may be interpreted as CD45− if the threshold for positivity was set too high. It is therefore questionable how many isolated CD117+ cells were authentic Lin−/CD117+cardiac stem cell in the study by Bearzi et al. (2007).
Several studies of CD117+ stem cells in the human heart (Urbanek et al. 2003,2005; Bearzi et al. 2007) exclude their hematopoietic nature by demonstrating a CD45− immunophenotype. However, the CD45 immunophenotype is not a credible criterion for exclusion of mast cells, as in our study mast cells were only weakly positive or even negative for CD45 immunostaining. Methods including tryptase immunostaining or toluidine blue and thionine staining must be employed to distinguish CD117+ mast cells from other CD117+cells.
Pouly et al. (2008) reported the presence of CD117+ cells in human hearts that showed characteristic mast cell staining. However, their findings differed from ours in that all of the CD117+ cells found in biopsy samples of the endomyocardium and the right appendage of the hearts, they reported coexpression of CD117 and tryptase and that all of these cardiac CD117+ cells were thus interpreted as mast cells. In our study, a small subpopulation (0–15%) of cells positive for CD117 and negative for tryptase or toluidine blue or thionine staining was also observed, and the possibility that these cells represent stem or progenitor cells cannot be excluded. This speculation is supported by another study in which a population of CD117+ cells was found to be self-renewing, clonogenic, and multipotent (Bearzi et al. 2007), further suggesting the existence of true progenitor cells in the human heart.
Cardiac progenitor cells have a putative capacity for differentiation into cardiomyocytes and serve in rebuilding tissues to restore cardiac function lost due to cardiovascular disease. On the other hand, the majority of available data show mast cells may play a detrimental role in myocardial remodeling following myocardial injury (Janicki et al. 2006; Balakumar et al. 2008). Cardiac mast cells have been reported to increase in failing human hearts (Patella et al. 1998; Batlle et al. 2006) and animal models of myocardial infarction (Engels et al. 1995). Both human failing hearts and animal myocardial infarction models have been used as models in cardiac progenitor research. This makes the distinction of CD117+ cardiac mast cells from true CD117+ cardiac progenitors extremely important, from both a clinical and a research perspective.
In conclusion, our results clearly demonstrate that most CD117+ cells in the human heart are mast cells but not cardiac stem cells or progenitors. As most cardiac mast cells have a weakly positive CD45 phenotype, the CD117+/CD45weak phenotype could not be regarded as the identity of cardiac stem cells or progenitors with bone marrow origin. CD45 positivity is not a good criterion for discriminating mast cells from Lin−/CD117+ cardiac stem cells either. In addition, our results also provide evidence for the possibility that a subpopulation of CD117+ cardiac cells may be authentic stem/progenitor cells. It is evident that a standardized set of criteria is still needed for identification of authentic cardiac stem and/or progenitor cells. Such criteria must be able to exclude mast cells, particularly when CD117 and/or CD45 are used as markers for isolation and identification of a cardiac progenitor population.
Study Limitations
We did not seek further to identify the small number of cells positive for CD117 which did not costain for mast cell markers, as these cells could be identified only by isolation and functional analysis, and we had no fresh heart tissue biopsy material available for that study. In addition, it should be noted that only left ventricles of the human hearts were sampled for analysis in this study (right ventricles, atria, and septa were not sampled), although the observations would most likely represent the whole heart. However, the data must be interpreted with caution when investigating mast cells and stem cells of the entire heart.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (code 30370745 to JG) and the 111 Project (grant B07001), and the general support of the LiFu Educational Foundation is acknowledged.
We are grateful to Ruishu Deng, Yong Guo, Qi Cao, and Yan Tang for helpful discussions and technical assistance.
This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication.
References
- Balakumar P, Singh AP, Ganti SS, Krishan P, Ramasamy S, Singh M (2008) Resident cardiac mast cells: are they the major culprit in the pathogenesis of cardiac hypertrophy? Basic Clin Pharmacol Toxicol 102:5–9 [DOI] [PubMed] [Google Scholar]
- Bankl HC, Radaszkiewicz T, Klappacher GW, Glogar D, Sperr WR, Grossschmidt K, Bankl H, et al. (1995) Increase and redistribution of cardiac mast cells in auricular thrombosis. Possible role of kit ligand. Circulation 91:275–283 [DOI] [PubMed] [Google Scholar]
- Batlle M, Roig E, Perez-Villa F, Lario S, Cejudo-Martin P, Garcia-Pras E, Ortiz J, et al. (2006) Increased expression of the renin-angiotensin system and mast cell density but not of angiotensin-converting enzyme II in late stages of human heart failure. J Heart Lung Transplant 25:1117–1125 [DOI] [PubMed] [Google Scholar]
- Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, et al. (2007) Human cardiac stem cells. Proc Natl Acad Sci USA 104:14068–14073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, et al. (2003) Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114:763–776 [DOI] [PubMed] [Google Scholar]
- Cook HC (1961) A modified thionin technique for mast cells in tissue sections. J Med Lab Technol 18:188–189 [PubMed] [Google Scholar]
- Dawn B, Stein AB, Urbanek K, Rota M, Whang B, Rastaldo R, Torella D, et al. (2005) Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci USA 102:3766–3771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng R, Lu M, Korteweg C, Gao Z, McNutt MA, Ye J, Zhang T, et al. (2008) Distinctly different expression of cytokines and chemokines in the lungs of two H5N1 avian influenza patients. J Pathol 216:328–336 [DOI] [PubMed] [Google Scholar]
- Engels W, Reiters PH, Daemen MJ, Smits JF, van der Vusse GJ (1995) Transmural changes in mast cell density in rat heart after infarct induction in vivo. J Pathol 177:423–429 [DOI] [PubMed] [Google Scholar]
- Fodinger M, Fritsch G, Winkler K, Emminger W, Mitterbauer G, Gadner H, Valent P, et al. (1994) Origin of human mast cells: development from transplanted hematopoietic stem cells after allogeneic bone marrow transplantation. Blood 84:2954–2959 [PubMed] [Google Scholar]
- Frangogiannis NG, Entman ML (2006) Identification of mast cells in the cellular response to myocardial infarction. Methods Mol Biol 315:91–101 [DOI] [PubMed] [Google Scholar]
- Glass G, Papin JA, Mandell JW (2009) SIMPLE: a sequential immunoperoxidase labeling and erasing method. J Histochem Cytochem 57:899–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicki JS, Brower GL, Gardner JD, Forman MF, Stewart JA Jr, Murray DB, Chancey AL (2006) Cardiac mast cell regulation of matrix metalloproteinase-related ventricular remodeling in chronic pressure or volume overload. Cardiovasc Res 69:657–665 [DOI] [PubMed] [Google Scholar]
- Kajstura J, Rota M, Whang B, Cascapera S, Hosoda T, Bearzi C, Nurzynska D, et al. (2005) Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion. Circ Res 96:127–137 [DOI] [PubMed] [Google Scholar]
- Kirshenbaum AS, Kessler SW, Goff JP, Metcalfe DD (1991) Demonstration of the origin of human mast cells from CD34+ bone marrow progenitor cells. J Immunol 146:1410–1415 [PubMed] [Google Scholar]
- Kubo H, Jaleel N, Kumarapeli A, Berretta RM, Bratinov G, Shan X, Wang H, et al. (2008) Increased cardiac myocyte progenitors in failing human hearts. Circulation 118:649–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke A, Muller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, et al. (2005) Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci USA 102:8966–8971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Puig S, Wang Z, Chien KR (2008) Lives of a heart cell: tracing the origins of cardiac progenitors. Cell Stem Cell 2:320–331 [DOI] [PubMed] [Google Scholar]
- Mouquet F, Pfister O, Jain M, Oikonomopoulos A, Ngoy S, Summer R, Fine A, et al. (2005) Restoration of cardiac progenitor cells after myocardial infarction by self-proliferation and selective homing of bone marrow-derived stem cells. Circ Res 97:1090–1092 [DOI] [PubMed] [Google Scholar]
- Noack F, Kruger S, Thorns C, Finas D, Stocker W, Diedrich K, Horny HP (2005) Application of novel tissue microarrays to investigate expression of tryptase, chymase and KIT protein in placental mast cells. Arch Gynecol Obstet 272:223–228 [DOI] [PubMed] [Google Scholar]
- Okayama Y, Kawakami T (2006) Development, migration, and survival of mast cells. Immunol Res 34:97–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama Y, Kirshenbaum AS, Metcalfe DD (2000) Expression of a functional high-affinity IgG receptor, Fc gamma RI, on human mast cells: up-regulation by IFN-gamma. J Immunol 164:4332–4339 [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, et al. (2001) Bone marrow cells regenerate infarcted myocardium. Nature 410:701–705 [DOI] [PubMed] [Google Scholar]
- Palladini G, Tozzi R, Perlini S (2003) Cardiac mast cells in the transition to heart failure: innocent bystanders or key actors? J Hypertens 21:1823–1825 [DOI] [PubMed] [Google Scholar]
- Patella V, Marino I, Arbustini E, Lamparter-Schummert B, Verga L, Adt M, Marone G (1998) Stem cell factor in mast cells and increased mast cell density in idiopathic and ischemic cardiomyopathy. Circulation 97:971–978 [DOI] [PubMed] [Google Scholar]
- Pouly J, Bruneval P, Mandet C, Proksch S, Peyrard S, Amrein C, Bousseaux V, et al. (2008) Cardiac stem cells in the real world. J Thorac Cardiovasc Surg 135:673–678 [DOI] [PubMed] [Google Scholar]
- Reinecke H, Minami E, Zhu WZ, Laflamme MA (2008) Cardiogenic differentiation and transdifferentiation of progenitor cells. Circ Res 103:1058–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rota M, Kajstura J, Hosoda T, Bearzi C, Vitale S, Esposito G, Iaffaldano G, et al. (2007) Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci USA 104:17783–17788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherschel JA, Soonpaa MH, Srour EF, Field LJ, Rubart M (2008) Adult bone marrow-derived cells do not acquire functional attributes of cardiomyocytes when transplanted into peri-infarct myocardium. Mol Ther 16:1129–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota N, Rysa J, Kovanen PT, Ruskoaho H, Kokkonen JO, Lindstedt KA (2003) A role for cardiac mast cells in the pathogenesis of hypertensive heart disease. J Hypertens 21:1935–1944 [DOI] [PubMed] [Google Scholar]
- Sperr WR, Bankl HC, Mundigler G, Klappacher G, Grossschmidt K, Agis H, Simon P, et al. (1994) The human cardiac mast cell: localization, isolation, phenotype, and functional characterization. Blood 84:3876–3884 [PubMed] [Google Scholar]
- Trotter CM, Carney AS, Wilson JA (1989) Mast cell distribution and morphology in human nasal turbinates following decalcification. Rhinology 27:81–89 [PubMed] [Google Scholar]
- Urbanek K, Quaini F, Tasca G, Torella D, Castaldo C, Nadal-Ginard B, Leri A, et al. (2003) Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy. Proc Natl Acad Sci USA 100:10440–10445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanek K, Torella D, Sheikh F, De Angelis A, Nurzynska D, Silvestri F, Beltrami CA, et al. (2005) Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci USA 102:8692–8697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor R, Ravindranath R, Padmalatha K, Venkatraman BV, Thomas IM (2004) A modified thionin acridine-orange stain for mast cells. Indian J Pathol Microbiol 47:168–169 [PubMed] [Google Scholar]
- Ye J, Zhang B, Xu J, Chang Q, McNutt MA, Korteweg C, Gong E, et al. (2007) Molecular pathology in the lungs of severe acute respiratory syndrome patients. Am J Pathol 170:538–545 [DOI] [PMC free article] [PubMed] [Google Scholar]