Abstract
Intrafusal fibers within muscle spindles retain features characteristic of immaturity, unlike the larger and more numerous extrafusal fibers constituting the bulk of skeletal muscle. Satellite cells (SCs), myogenic progenitors, are detected on the surfaces of both intrafusal and extrafusal fibers, but little is known of spindle SCs. We have recently demonstrated that, like their extrafusal counterparts, SCs in muscle spindles of posthatch chickens express paired box transcription factor 7 (Pax7) protein. During vertebrate embryogenesis, myogenic progenitors express both Pax7 and Pax3 proteins. In postnatal mice, Pax3 appears in rare SC subsets, whereas Pax7 is expressed by all SCs within extrafusal fibers. Here we test the hypothesis that Pax3 protein maintains localized expression within SCs of muscle spindles. Immunohistochemical techniques were used to identify SCs by their Pax7 expression within anterior latissimus dorsi muscle excised from posthatch chickens of various ages. A greater percentage of SCs express Pax3 within intrafusal than extrafusal fibers at each age, and the proportion of SCs expressing Pax3 declines with aging. This is the first study to localize Pax3 expression in posthatch avian muscle and within SCs of muscle spindles. We suggest that Pax3-positive SCs are involved in fiber maintenance. (J Histochem Cytochem 58:317–327, 2010)
Keywords: muscle spindles, intrafusal, extrafusal, Pax7, Pax3, satellite cells, ALD
Muscle spindles are minute mechanoreceptors embedded within skeletal muscles (Kokkorogiannis 2004; MacIntosh et al. 2006). They relay static and dynamic stretch information about each skeletal muscle to the central nervous system (Barker et al. 1974; MacIntosh et al. 2006). Muscle spindles contain from one to several intrafusal muscle fibers enclosed within a fusiform-shaped connective tissue capsule (De Anda and Rebollo 1967; Maier 1992). This capsule separates the spindle from the much larger extrafusal muscle fibers that make up the bulk of the muscle (Ovalle et al. 1999). Intrafusal fibers are believed to persist in a comparatively immature state. Unlike extrafusal fibers, intrafusal fibers maintain developmental myosin heavy chain isoform expression (slow developmental, embryonic, and/or neonatal) characteristic of early myotubes with the potential to become either intrafusal or extrafusal fibers (Walro and Kucera 1999; Liu et al. 2002). Myonuclei (MN) of intrafusal fibers maintain Myf5 gene activity, which is normally downregulated in the MN of mature extrafusal fibers (Zammit et al. 2004). In addition, while extrafusal fibers continue to show robust growth until maturity, intrafusal fiber growth is arrested shortly after birth, and fibers remain comparatively small (Kozeka and Ontell 1981).
Satellite cells (SCs) are myogenic stem cells located on the surface of skeletal muscle fibers, between plasmalemmae and basal laminae (Mauro 1961; Anderson 2006). SCs can be mobilized during muscle growth, maintenance, or repair to replicate and to contribute new MN (Charge and Rudnicki 2004; Collins et al. 2005; Zammit et al. 2006). During the early phases of embryogenesis, myogenic progenitors in most muscles express both paired box transcription factor 3 (Pax3) and Pax7 proteins, which are required for normal muscle development (Relaix et al. 2005; Buckingham and Relaix 2007; Galli et al. 2008; Sambasivan et al. 2009). In mature muscle, SCs are commonly labeled with an antibody against the Pax7 protein (Zammit et al 2006; Day et al. 2007), which is expressed by all quiescent, activated, and proliferating SCs but not by MN (Seale et al. 2000; Halevy et al. 2004; Shefer et al. 2006; Day et al. 2009). Pax3 protein expression, however, is repressed after embryonic day 13.5 in most mouse trunk and limb muscles (Horst et al. 2006). Pax3 is expressed only in a varying proportion of SCs within a minority of muscles, such as the diaphragm and gracilis, in postnatal mice (Montarras et al. 2005; Relaix et al. 2006; Day et al. 2007).
We have previously shown that intrafusal fibers maintain greater concentrations of SCs than their surrounding extrafusal fibers (Kirkpatrick et al. 2008). Almost all that is known about SCs has been derived from studies of extrafusal fibers. Little is known about the characteristics of muscle spindle SCs. This study elucidates the presence and frequency of Pax3 protein expression during posthatch muscle development. Based upon the rationale that intrafusal fibers are comparatively immature and that Pax3 is normally expressed by myoblasts in most developing muscle, the hypothesis tested is that Pax3 protein maintains localized expression within SCs of muscle spindles. The model studied is anterior latissimus dorsi (ALD) muscle of chicken, which we utilized previously to demonstrate relatively high numbers of SCs by their Pax7 expression within intrafusal fibers (Kirkpatrick et al. 2008). In the current study, immunohistochemical techniques were used to identify SCs by their Pax7 expression and label Pax3 protein within the nuclei of intrafusal and extrafusal fibers of ALD of several posthatch ages. Large numbers of SCs, and a few MN, within intrafusal fibers were found to express Pax3.
Materials and Methods
Experimental Model
White Leghorn chickens (Gallus gallus; Hy-Line W-36; Clark Hy-Line, Brandon, Canada) were hatched and raised under identical conditions at the Department of Animal and Poultry Science, University of Saskatchewan, Saskatoon, Canada. Birds were raised in a floor pen on a 23-hr-light:1-hr-dark cycle at an initial temperature of 35C. Temperature was decreased by 3.5C per week until a final temperature of 21C was reached when the birds were 5 weeks posthatch. Birds had free access to water and were fed ad libitum with commercial chick starter until 42 days posthatch, when they were then fed with a commercial chick grower feed (Federated Cooperatives Ltd.; Saskatoon, Canada). Following Canadian Council on Animal Care Guidelines and with approval of the University of Saskatchewan Committee on Animal Care and Supply, five birds were killed at 9, 30, 62, and 145 days posthatch. Birds of the oldest group were killed by inhalation of carbon dioxide and birds in other groups by cervical dislocation. This difference was due to the ready availability of carbon dioxide in the area of the facility where the birds where kept when they grew older.
The ALD muscle, more formally termed latissimus dorsi pars cranialis (Vanden Berge and Zweers 1993), is a superficial muscle on the dorsal aspect of the thorax (George and Berger 1966). It is a slow-tonic contracting muscle that originates on several cervical and thoracic vertebrae and inserts on the humerus (George and Berger 1966; Harvey and Marshall 1986). The ALD acts to fix the folded avian wing against the body (Raikow 1985; Meyers 1992).
Tissue Preparation and Sectioning
The ALD was excised bilaterally from each 9-, 30-, and 62-day bird and from the left side of each 145-day bird. Muscles were weighed, coated with Tissue-Tek optimal cutting temperature compound (Sakura Finetek; Torrance, CA), and immediately frozen in isopentane cooled by liquid nitrogen (Sewry and Dubowitz 2001). Muscle samples of ∼0.5–1.0 cm × 2–4 cm × thickness of muscle were removed such that the long axis of each sample was parallel to the direction of the muscle fibers. Right and left ALD muscles of the 9-, 30-, and 62-day birds were grouped together for analyses, and the left ALD muscle of each 145-day bird was analyzed. Samples were stored at −80C. Serial cross-sections were cut at 12-μm thickness at −20C by using a cryostat. Three serial sections were placed on each ProbeOn Plus-charged microscope slide (Fisher Scientific; Nepean, Canada), and sequential slides were numbered and stored at −20C.
Immunohistochemistry
Immunohistochemical techniques were used to analyze cryosections from various locations along the length of each muscle. This method ensured that a sufficient number of spindles would be located and reduced the likelihood of sampling the same intrafusal fiber more than once. Several consecutive slides were labeled at each location along the muscle, permitting comparison and tracking of intrafusal fibers from one section to the next. In analyzing extrafusal fibers, only one location along the muscle was evaluated so that fibers would not be sampled more than once along their lengths. The extrafusal fibers sampled were juxtaposed to one another within fascicles, and anything that could have been considered an extracapsular portion of an intrafusal fiber (Maier 1992) was avoided.
Primary antibodies used were anti-myosin, anti-laminin, anti-Pax7, and anti-Pax3. Anti-myosin (NA4; a gift from Dr. E. Bandman, University of California, Davis, CA, now available at Developmental Studies Hybridoma Bank/DSHB, Iowa City, IA), a mouse monoclonal antibody developed against chicken myosin was used to detect all myosins of avian skeletal muscle fibers (Moore et al. 1992). Anti-laminin (L9393; Sigma Chemical, St. Louis, MO), a rabbit polyclonal raised against the glycoprotein laminin of mice, was used to label basal laminae of skeletal muscle fibers (Vater et al. 1994). Anti-Pax7 (DSHB), an IgG1 isotype mouse monoclonal antibody developed against the chicken Pax7 protein, was used to identify SCs (Kawakami et al. 1997). Anti-Pax3 (DSHB), an IgG2a isotype mouse monoclonal antibody raised against the quail Pax3 protein, was used to detect the Pax3 protein within nuclei (Venters et al. 2004). According to information supplied by the vendors, the specificity of each primary antibody used included the proper chicken antigen.
The blocking solution consisted of 1% BSA and 5 mM EDTA in PBS [0.02 M sodium phosphate buffer, 0.15 M sodium chloride (pH 7.2)] and was applied to each slide for 15–20 min at room temperature. The blocking agent was then drained from each slide, and a primary antibody cocktail was applied overnight at 4C. The primary antibody cocktail consisted of either (a) 1:400 anti-laminin and 1:5000 anti-myosin diluted in blocking solution or (b) 1:400 anti-laminin, 1:30 anti-Pax7, and 1:30 anti-Pax3 diluted in blocking solution. Primary antibody cocktails A and B were alternated with each serial slide. The slides were transferred to Coplin jars and rinsed three times for 5 minutes each with PBS.
The secondary antibody cocktail added to those slides receiving the first primary cocktail (a) consisted of Alexa Fluor 546 goat anti-rabbit IgG (A11010; Invitrogen, Carlsbad, CA), which labels the rabbit anti-laminin red, and Alexa Fluor 488 goat anti-mouse IgG (A11001; Invitrogen), which labels the mouse anti-myosin green. The secondary antibody cocktail applied to slides receiving the other primary cocktail (b) included Alexa Fluor 350 goat anti-rabbit IgG (A21068; Invitrogen), which labels the rabbit anti-laminin blue, Alexa Fluor 568 goat anti-mouse IgG1 (A21124; Invitrogen), which labels the mouse anti-Pax7 red, and Alexa Fluor 488 goat anti-mouse IgG2a (A21131; Invitrogen), which labels the mouse anti-Pax3 green. Secondary antibodies in each cocktail were diluted 1:200 in PBS. The appropriate secondary antibody cocktail was applied to each serial slide at room temperature for 30 min in the dark. Secondary antibody cocktails were drained, and slides were rinsed three times for 5 minutes each with PBS. Hoechst 33258 (bisbenzimide; Sigma Chemical) was applied to each slide for five minutes at 1:1,500,000 in PBS to label DNA within nuclei blue under epifluorescence. The blue of the bisbenzimide was a lighter hue than the blue of the goat anti-rabbit IgG-labeled anti-laminin, so the structures were easily distinguishable. Slides were drained and rinsed twice for 5 minutes each with PBS. Finally, 4% formaldehyde in PBS was applied to each slide for 3 minutes, followed by draining and rinsing twice for 5 minutes each with PBS. Slides were mounted in Geltol (Thermo Scientific; Pittsburgh, PA) or Aqua-Mount (Lerner Laboratories; Pittsburgh, PA), and stored at 4C in the dark. Appropriate controls, including primary antibodies without secondary antibodies and vice versa, were included in each experiment.
Imaging and Analysis
Images of results were obtained using a Zeiss Axioskop 20 microscope (Carl Zeiss; Oberkochen, Germany) equipped for epifluorescence, attached to a Sony Cyber-Shot DSC-V3 digital still camera (Sony; Tokyo, Japan). Muscle spindles were located and photographed from sections of serial slides such that the total number of intrafusal fibers analyzed for each muscle ranged from 30 to 85 fibers. Epifluorescent images (red, green, and/or blue), each viewed through a different wavelength filter, were taken from each muscle spindle on adjacent serial slides. Images were transferred to an iMac computer (Apple Computers; Cupertino, CA) and layered using Adobe Photoshop (Adobe Systems; San Jose, CA) such that final images consisted of (a) myosin in green and basal laminae in red and (b) basal laminae in deep blue, all nuclei in light blue, SCs (Pax7) in red, and Pax3 in green.
These images were used to count the number of SCs and MN within each muscle fiber cross-sectional profile and to calculate the proportion of SCs and MN expressing Pax3. Each fiber boundary was demarcated by its basal lamina. SCs were identified as light-blue nuclei expressing red Pax7 (Pax7 positive). Pax3-expressing SCs were observed as light-blue nuclei coexpressing red Pax7 and green Pax3 (Pax3 positive). Pax3-expressing MN were recognized as light-blue nuclei expressing only green Pax3. Using the features of Adobe Photoshop, each colored layer could be either augmented or diminished in intensity to assist with on-screen interpretation and counting. All intrafusal fibers examined and 200 adjacent extrafusal fibers from each muscle studied were analyzed in this way. The ellipse minor axis of these same fibers was measured using Scion Image software version 1.63 (developed by the US National Institutes of Health). The ellipse minor axis of Scion Image is identical to a lesser fiber diameter (Rosser et al. 2000), which is defined as the maximum aspect across the lesser aspect of a fiber. Lesser fiber diameter is routinely used to overcome distortion that may result if a muscle fiber is cut obliquely rather than transversely (Dubowitz and Sewry 2007).
The average numbers of SC nuclei (SCN) and MN per fiber were used to calculate the frequency of SCs in both intrafusal and extrafusal fibers of each muscle studied, using the formula SCN frequency = [no. of SCN/(no. of SCN + no. of MN)] × 100% (Schmalbruch and Hellhammer 1977; Hikida et al. 1998; Allouh et al. 2008). The proportion of SCs or MN containing Pax3 in both intrafusal and extrafusal fibers of each muscle studied was calculated using the formulae Pax3-positive SCN frequency = (no. of Pax3 positive SCN/no. of all SCN) × 100% or Pax3-positive MN frequency = (no. of Pax3-positive MN/no. of all MN) × 100%. The number of SCs assessed in intrafusal fibers of 9-, 30-, 62-, and 145-day birds ranged, respectively, from 14 to 23, 21 to 29, 11 to 20, and 19 to 26 fibers. In extrafusal fibers, the number of SCs studied from 9-, 30-, 62-, and 145-day birds ranged, respectively, from 37 to 54, 50 to 77, 85 to 180, and 76 to 111 fibers.
Statistics
ALD muscles from five animals at 9-, 30-, 62-, and 145-days posthatch were studied. Data are presented as means and standard errors. The muscle weight, ellipse minor axis, SC frequency, percentage of Pax3-positive SCs, and percentage of Pax3-positive MN were analyzed at each age. An arcsine transformation was applied to percentage data prior to statistical analysis (Zar 1999; van Emden 2008). Homogeneity of variance was tested via Levene's test, and group means were subsequently evaluated using a one-way ANOVA test at 5% level of significance (p≤0.05). Bonferroni's pairwise comparison between group means was used for post hoc analysis when variances were equal and Tamhane's pairwise comparison when variances were unequal. Statistical tests were performed using SPSS version 16.0 software for Windows (SPSS; Chicago, IL).
Results
Identifying Structures
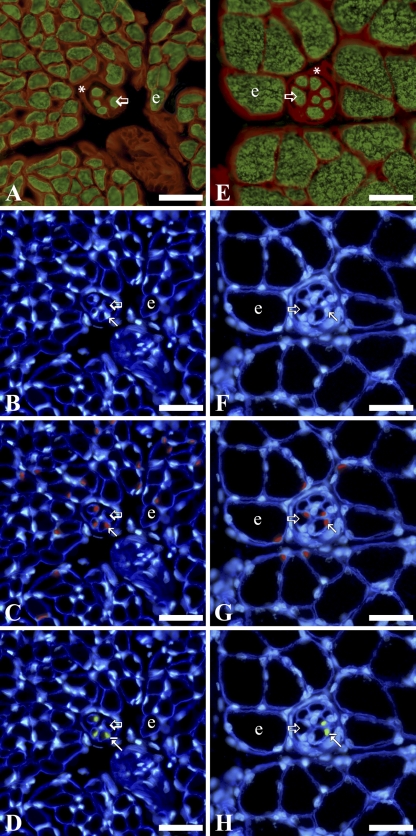
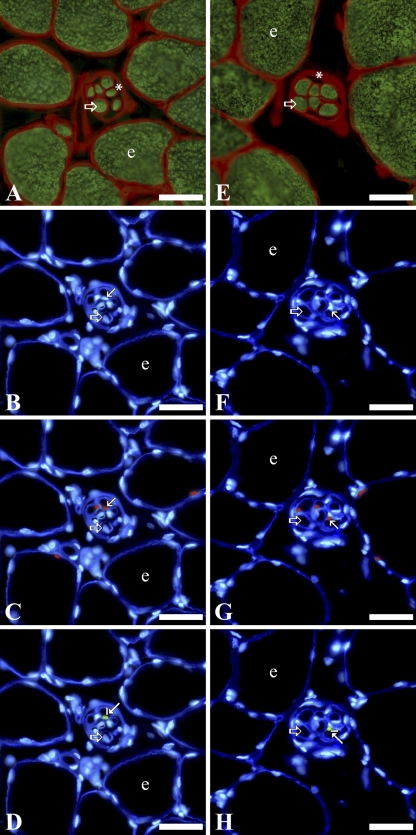
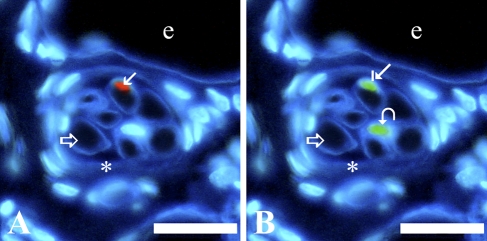
Laminin is a major component of the basement membrane in a variety of cell types, including endothelium, nerve, and muscle cells (Tzu and Marinkovich 2008). Muscle fibers were located based on their myosin heavy chain content and laminin-rich basement membrane (Figures 1A and 1E and 2A and 2E). To distinguish intrafusal from extrafusal fibers, the thickened, laminin-rich connective tissue capsule of muscle spindles was identified surrounding intrafusal fibers (Figures 1 and 2). SCs in both intrafusal and extrafusal fibers were identified by their Pax7 content and standard position beneath basal laminae, whereas MN, also located beneath basal laminae, were recognized by their lack of Pax7 expression (Figures 1C and 1G and 2C and 2G). Pax3 protein was localized primarily in a subpopulation of SC nuclei within intrafusal fibers (Figures 1D and 1H and 2D and 2H). Pax3 was expressed less frequently in SC nuclei of extrafusal fibers and rarely within MN of intrafusal or extrafusal fibers (Figure 3). Nuclei located outside basal laminae of muscle fibers did not express Pax3.
Figure 1.
Immunohistochemical labeling of serial cross-sections of anterior latissimus dorsi muscle: 9 days (A–D), and 30 days (E–H) posthatch. In each series, the first image (A or E) is from a serial section adjacent to the cross-section from which the subsequent three images (B–D or F–H) are taken. In the first image (A or E), laminin is red and myosin green. In subsequent images, laminin is deep blue and nuclei are light blue (B–D or F–H), Pax7 is red (C or G), and Pax3 is green (D or H). Several intrafusal fibers clustered within a spindle capsule are near the center of each image, and are smaller in diameter than the larger extrafusal fibers that are always situated outside of the capsule. Open/large arrows indicate intrafusal fibers; small arrows show satellite cells; small arrows with line show a satellite cell expressing Pax3; asterisk, capsule of muscle spindle; e, extrafusal fiber. The same representative intrafusal fibers, extrafusal fibers, and satellite cell nuclei are labeled in each image throughout each series (A–D, E–H). Bar = 30 μm.
Figure 2.
Immunohistochemical labeling of serial cross-sections of anterior latissimus dorsi muscle: 62 days (A–D), and 145 days (E–H) posthatch. In each series, the first image (A or E) is from a serial section adjacent to the cross-section from which the subsequent three images (B–D or F–H) are taken. In the first image (A or E), laminin is red, and myosin is green. In subsequent images, laminin is deep blue, and nuclei are light blue (B–D or F–H), Pax7 is red (C or G), and Pax3 is green (D or H). Labeling scheme is the same as that used in Figure 1. Bar = 30 μm.
Figure 3.
Pax7-positive/Pax3-positive and Pax7-positive/Pax3-negative cells detected by immunohistochemical labeling of serial cross-sections of anterior latissimus dorsi muscle from a 62-day posthatch chicken. (A) Laminin is deep blue, nuclei are light blue, and Pax7-labeled nuclei are red. (B) Laminin is deep blue, nuclei are light blue, and Pax3-labeled nuclei are green. Curved arrow indicates a myonucleus expressing Pax3. The rest of the labeling scheme is the same as that used in Figure 1. Bar = 20 μm.
Anterior Latissimus Dorsi Muscle Weight
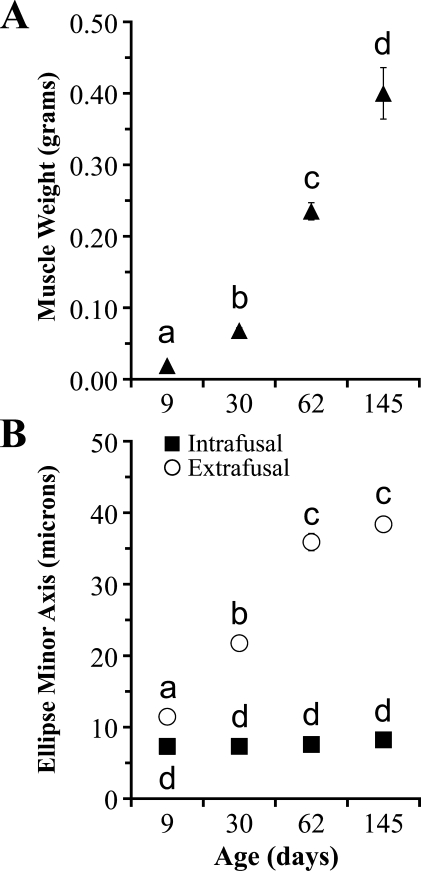
Wet weight of ALD muscle increased over 20-fold from 9 to 145 days posthatch (Figure 4A). Mean ALD weight was 0.019 ± 0.001 g (range, 0.008–0.030 g) at 9 days, 0.068 ± 0.004 g (range, 0.057–0.082 g) at 30 days, 0.235 ± 0.012 g (range, 0.192–0.268 g) at 62 days, and 0.400 ± 0.036 g (range, 0.352–0.544 g) at 145 days. Muscle weight was significantly different among all ages: 9 vs 30 days, p<0.001; 9 vs 62 days, p<0.001; 9 vs 145 days, p=0.003; 30 vs 62 days, p<0.001; 30 vs 145 days, p=0.005; and 62 vs 145 days, p=0.05.
Figure 4.
Anterior latissimus dorsi muscle weight and fiber size data. Muscle weight (A) increases with age. Ellipse minor axis (B) of extrafusal fibers is greater than that of intrafusal fibers. Ellipse minor axis of extrafusal fibers increases with age but remains constant in intrafusal fibers. Within each graph (A or B), symbols with the same lowercase letter are not significantly different (p>0.05) from one another. Symbols with different lowercase letters are significantly different (p≤0.05) from one another. Each value is expressed as mean ± SE (n=5). Error bars that are not visible are smaller than the symbol used to denote each mean.
Ellipse Minor Axis
Mean ellipse minor axis of extrafusal fibers increased more than 3-fold from 9 to 145 days posthatch; at 9 days, 11.46 ± 0.27 μm (range, 10.65–12.08 μm); at 30 days, 21.75 ± 0.30 μm (range, 20.62–22.29 μm); at 62 days, 35.89 ± 1.20 μm (range, 33.95–40.53 μm); and at 145 days, 38.38 ± 0.39 μm (range, 37.62–39.68 μm) (Figure 4B). Extrafusal fiber ellipse minor axis values were significantly different among all ages, except at 62 and 145 days posthatch (p=0.960); 9 vs 30 days, p<0.001; 9 vs 62 days, p<0.001; 9 vs 145 days, p<0.001; 30 vs 62 days, p=0.005; and 30 vs 145 days, p<0.001. Conversely, ellipse minor axis values of intrafusal fibers did not change with age (Figure 4B): 9 vs 30 days, p=1.000; 9 vs 62 days, p=1.000; 9 vs 145 days, p=0.800; 30 vs 62 days, p=1.000; 30 vs 145 days, p=0.238; and 62 vs 145 days, p=0.933. The overall mean intrafusal fiber ellipse minor axis for all ages was 7.64 ± 0.28 μm (range, minimum 6.50 to maximum 8.83 μm).
Satellite Cell Frequency
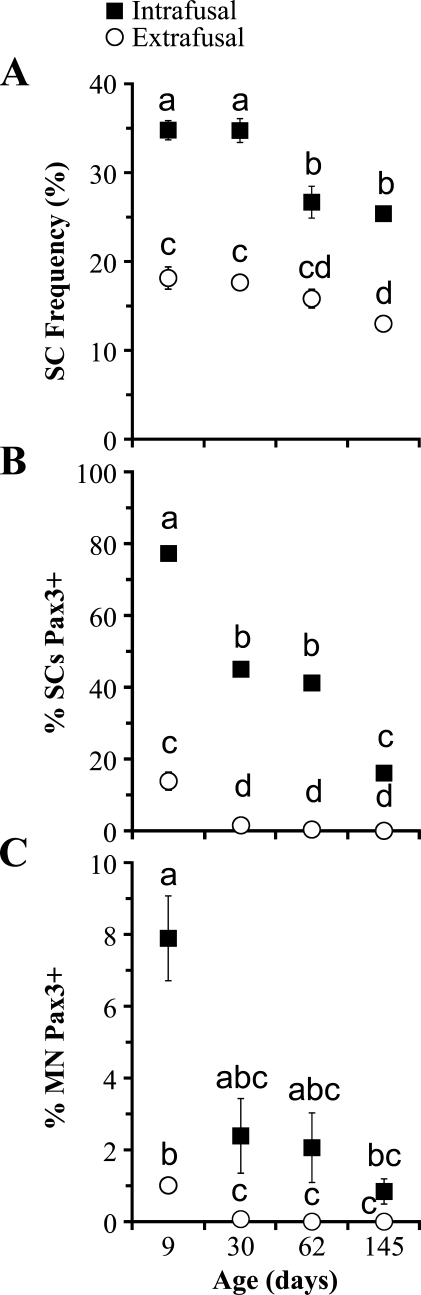
Mean frequency of SCs (Figure 5A) was significantly (p≤0.001) higher in intrafusal than extrafusal fibers at each of the four ages studied. Mean SC frequency for all ages was 30.40 ± 2.32% (range, 22.64–38.46%) in intrafusal fibers and 16.15 ± 1.26% (range, 11.89–20.68%) in extrafusal fibers. Overall, SC frequency decreased within both intrafusal and extrafusal fibers with age. Within intrafusal fibers, SC frequency decreased significantly from 30 to 62 days posthatch (p≤0.001). SC frequency in intrafusal fibers stayed constant from 9 to 30 days (p=1.000) and from 62 to 145 days (p=1.000) posthatch. Within extrafusal fibers, SC frequency decreased significantly by 145 days posthatch: 9 vs 30 days, p=1.000; 9 vs 62 days, p=1.000; 9 vs 145 days, p=0.017; 30 vs 62 days, p=1.000; 30 vs 145 days, p=0.040; and 62 vs 145 days, p=1.000.
Figure 5.
Anterior latissimus dorsi muscle intrafusal and extrafusal fiber nuclei data. Satellite cell (SC) frequency (A) is greater in intrafusal than extrafusal fibers at each age studied and, with aging, decreases in both intrafusal and extrafusal fibers. The percentage of SCs expressing Pax3 (B) is also higher in intrafusal than extrafusal fibers at each age studied and also decreases with age. The percentage of myonuclei (MN) expressing Pax3 (C) is initially higher in intrafusal than extrafusal fibers but decreases with age to be statistically the same in intrafusal and extrafusal fibers. Within each graph (A–C), symbols with the same lowercase letter are not significantly different (p>0.05) from one another. Symbols with different lowercase letters are significantly different (p≤0.05) from one another. Symbols with more than one lower case letter are not significantly different from other symbols that bear one or more of the same letters. Each value is expressed as mean ± SE (n=5). Error bars that are not visible are smaller than the symbol used to denote each mean.
Satellite Cells Expressing Pax3
Mean percentage of SCs expressing Pax3 (percent Pax3-positive SCs) (Figure 5B) was significantly (p≤0.009) higher in intrafusal than extrafusal fibers at each age studied and at all ages except for comparisons of 9-day extrafusal and 145-day intrafusal fibers (p=1.000). Within intrafusal fibers, the mean percent Pax3-positive SCs was highest at 9 days (range, 73.91–82.35%; mean, 77.26 ± 1.49%), decreasing to 38.10–50.00% (average, 45.00 ± 2.00%) at 30 days, 36.36–45.00% (mean, 41.18 ± 1.38%) at 62 days, and 12.00–19.05% (average, 16.08 ± 1.23%) at 145 days posthatch. Within intrafusal fibers, the mean percent Pax3-positive SCs was significantly (p<0.001) different among all ages, with the exception of 30 and 62 days posthatch (p=0.992). Within extrafusal fibers, the mean percent Pax3-positive SCs at 9 days was 13.85 ± 2.53% (range, 9.26–21.62%). However, the mean percent Pax3-positive SCs declined to less than 2% by 30 days and became zero by 145 days posthatch. The mean percent Pax3-positive SCs within extrafusal fibers at 9 days posthatch was significantly (p≤0.013) different from that in extrafusal fibers at each of the other ages studied but did not change from 30 days onward: 30 vs 62 days, p=0.919; 30 vs 145 days, p=0.400; and 62 vs 145 days, p=0.996.
Myonuclei Expressing Pax3
The mean percentage of MN expressing Pax3-positive MN (percent Pax3-positive MN) (Figure 5C) was highest in the 9-day intrafusal fibers, at 7.89 ± 1.18% (range, 5.71–12.00%), which was significantly (p=0.008) different from that of the 9-day extrafusal fibers, at 1.01 ± 0.19% (range, 0.45–1.60%). After 9 days, the mean intrafusal fiber percent Pax3-positive MN declined to 2.39 ± 1.04% (range, 0.00–4.88%) at 30 days, 2.06 ± 0.97% (range, 0.00–4.55%) at 62 days, and 0.84 ± 0.35% (range, 0.00–1.52%) at 145 days posthatch. In extrafusal fibers, the mean percent Pax3-positive MN decreased to 0.07 ± 0.07% (range, 0.00–0.33%) at 30 days and became zero for all five animals at both 62 and 145 days posthatch. No significant difference in the mean percent Pax3-positive MN was found between intrafusal and extrafusal fibers at 30 (p=0.939), 62 (p=0.904), or 145 (p=0.872) days posthatch. Within intrafusal fibers, except between 9 and 145 days (p=0.015), there was no significant differences in mean percent Pax3-positive MN among the various ages studied: 9 vs 30 days, p=0.539; 9 vs 62 days, p=0.392; 30 vs 62 days, p=1.000; 30 vs 145 days, p=1.000; and 62 vs 145 days, p=1.000. Within extrafusal fibers, the mean percent Pax3-positive MN at 9 days was significantly different from that of 30 (p=0.013), 62 (p=0.016), and 145 (p=0.016) days posthatch. However, in extrafusal fibers, the mean percent Pax3-positive MN was not significantly different (p=1.000) among the 30-, 62-, and 145-day posthatch birds.
Discussion
This study is the first to localize Pax3 expression within SCs of muscle spindles and the first to demonstrate Pax3 expression in posthatch avian muscle. It is generally held that Pax3 expression is downregulated before birth (Kassar-Duchossoy et al. 2005; Horst et al. 2006). Thus, most previous investigations of Pax3 expression in muscle have been largely restricted to embryos (Keller et al. 2004; Kassar-Duchossoy et al. 2005; Relaix et al. 2005; Horst et al. 2006; Otto et al. 2006; Galli et al. 2008). Only subsets of SCs have been found to maintain Pax3 expression within extrafusal fibers of a minority of postnatal murine muscles (Montarras et al. 2005; Relaix et al. 2006). Northern blotting analysis has been applied to show Pax3 in homogenates of adult guinea pig diaphragm (Jin et al. 2007). Immunocytochemical techniques have been used to identify abundant Pax3-expressing SCs within the diaphragm and gracilis of 3-week postnatal mice (Relaix et al. 2006). The proportion of SCs expressing Pax3 in the diaphragm of these mice was reported to be 85%. Percentages of Pax3-expressing SCs in other muscles were not quantified but were said to be lower than that of the diaphragm. SCs in most ventral trunk muscles and about half of the forelimb muscles studied expressed Pax3, while Pax3 expression was scarce in hindlimb muscles. Reasons for the differential expression of Pax3 between muscles of mice are unclear, as it was concluded that Pax3 expression was not related to fiber type, innervation, or embryological origin (Relaix et al. 2006).
A greater percentage of Pax3-expressing SCs are associated with intrafusal fibers of muscle spindles than with the surrounding extrafusal fibers, regardless of posthatch age studied and despite a decline in the percentage of Pax3 expressing SCs with aging. The reason for greater Pax3 in muscle spindles may be concomitant with other factors indicative of muscle spindle immaturity. Developmental myosin heavy chains (Liu et al. 2002), Myf5 expression (Zammit et al. 2004), greater SC frequency (Kirkpatrick et al. 2008), and small diameters (Kozeka and Ontell 1981) are all indicators of immaturity in muscle spindles. The adaptive advantage in intrafusal fibers maintaining a comparatively immature state is unknown. Muscle spindles are constantly monitoring length changes in skeletal muscle and intrafusal fibers frequently adjusting their own lengths (Dorward 1970; Vallbo 1974; Maier 1992). This may subject intrafusal fibers to a greater degree of wear and damage than experienced by the large majority of extrafusal fibers. Maintaining numerous factors associated with early muscle development, such as Pax3, may aid in intrafusal fiber repair and preservation. During maturation, Pax3-expressing SCs decline in number, just as overall SC frequency declines. The decrease of SC numbers with maturation has been previously shown in intrafusal and extrafusal fibers (Kirkpatrick et al. 2008) and has been related to a weakened capacity for growth, regeneration, and repair (Shefer et al. 2006; Brack and Rando 2007).
Wet ALD muscle weight was shown to increase during the developmental period studied. This posthatch growth occurs through increases in diameter and/or length in preexisting muscle fibers (hypertrophy), rather than an increase in the number of muscle fibers (hyperplasia) (Gollnick et al. 1981,1983; Antonio and Gonyea 1993). This hypertrophy is restricted to extrafusal fibers whose size increases with age (Ono et al. 1993; Tamaki and Uchiyama 1995), as intrafusal fibers reach their maximum size and number shortly after birth (Kozeka and Ontell 1981). The results of our study concur, as throughout posthatch development extrafusal fiber diameter increases dramatically, while intrafusal fiber size remains consistently small.
This study also shows Pax3-expressing MN at a greater frequency in intrafusal fibers than in the surrounding mature extrafusal fibers. Pax3-positive MN have been identified previously in mature mouse diaphragm (Relaix et al. 2006). The role of these nuclei in mature muscle remains to be established. As Pax3 is known to function upstream of MyoD (Tajbakhsh et al. 1997; Relaix et al. 2006; Collins et al. 2009), a key protein in the differentiation of SCs into MN (Zammit et al. 2006), Pax3-positive/Pax7-negative nuclei may be in transition from SCs to MN. Pax3 is also known to regulate c-Met (Epstein et al. 1996; Keller et al. 2004), a receptor critical in migration of myogenic progenitors (Birchmeier and Brohmann 2000). Perhaps MN express Pax3 as part of their migration from SCs on the surface of muscle fibers to nuclei within fibers. Labeling sections with Pax3, Pax7, and dystrophin could help to somewhat resolve the nature of the Pax3-positive/Pax7-negative nuclei observed in our study. Dystrophin, a structural protein located on the cytoplasmic face of the plasmalemma, is superficial to myonuclei but deep to SCs (Rosser et al. 2002). Labeling for dystrophin could determine whether Pax3-positive/Pax7-negative nuclei are in the normal SC and/or myonuclear compartments of the fibers.
Pax3 expression in the SCs of postnatal or posthatch muscles may be more than simply a remnant of early development. Skeletal muscles show decreased numbers of SCs with maturation (Shefer et al. 2006; Allouh et al. 2008, Kirkpatrick et al. 2008). Earlier studies have also shown that SCs from aged muscle demonstrate decreased activity and reduced proliferative potential in response to normal activating stimuli (Schultz and Lipton 1982; Gopinath and Rando 2008). SC numbers certainly decreased in our current study, and the disproportionate decrease of the number of Pax3-positive SCs during maturation could be correlated with a decrease in SC activity. The disproportionate decrease is more noticeable in intrafusal than extrafusal fibers, as at all ages studied intrafusal fibers have not only more SCs but also significantly higher proportions of Pax3-positive SCs. These higher numbers are presumably due to the greater need for repair and maintenance of intrafusal fibers, which would be sustained through the activation, proliferation, and differentiation of SCs. If Pax3 did not perform some function in maturing muscle and were merely a developmental remnant, the number of Pax3-positive and Pax3-negative SCs would decline at comparable rates in maturing muscle spindles. If, however, Pax3 expression is correlated with SC activation and proliferation in muscle spindles, one might expect to see a greater decline in the proportion of Pax3-positive SCs as spindles age. Based upon our observations, it seems reasonable to postulate that Pax3 may play a role in the activation and proliferation of SCs within the spindles of maturing muscle.
Acknowledgments
A Discovery Grant awarded to B.W.C.R. from the Natural Sciences and Engineering Research Council (NSERC) of Canada provided funds for this study. Additional funds to B.W.C.R. were provided by the College of Medicine, University of Saskatchewan. Two undergraduate student research awards and an Alexander Graham Bell Canada graduate scholarship from NSERC funded L.J.K. Z.Y.R is supported by the National Institute on Aging (AG013798, AG021566, AG035377) and the Muscular Dystrophy Association (MDA 135908) and had previous support from the USDA Cooperative State Research, Education and Extension Service (NRI, 2003-35206-12843).
Dr. Everett Bandman at the Department of Food Sciences and Technology, University of California, provided the anti-myosin antibody. Antibodies against Pax7 and Pax3 developed by, respectively, A. Kawakami and C.P. Ordahl, were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the Department of Biological Sciences, University of Iowa, Iowa City, IA.
This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication.
References
- Allouh MZ, Yablonka-Reuveni Z, Rosser BWC (2008) Pax7 reveals a greater frequency and concentration of satellite cells at the ends of growing skeletal muscle fibers. J Histochem Cytochem 56:77–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JE (2006) The satellite cell as a companion in skeletal muscle plasticity: currency, conveyance, clue, connector and colander. J Exp Biol 209:2276–2292 [DOI] [PubMed] [Google Scholar]
- Antonio J, Gonyea WJ (1993) Skeletal muscle fiber hyperplasia. Med Sci Sports Exerc 25:1333–1345 [PubMed] [Google Scholar]
- Barker D, Hunt CC, McIntyre AK (1974) Muscle receptors. In Autrum H, Jung R, Loewenstein WR, MacKay DM, Teuber HL, eds. Handbook of Sensory Physiology, vol. 3, no. 2. Berlin, Springer-Verlag, 1–310
- Birchmeier C, Brohmann H (2000) Genes that control the development of migrating muscle precursor cells. Curr Opin Cell Biol 12:725–730 [DOI] [PubMed] [Google Scholar]
- Brack AS, Rando TA (2007) Intrinsic changes and extrinsic influences of myogenic stem cell function during aging. Stem Cell Rev 3:226–237 [DOI] [PubMed] [Google Scholar]
- Buckingham M, Relaix F (2007) The role of Pax genes in the development of tissues and organs: Pax3 and Pax7 regulate muscle progenitor cell functions. Annu Rev Cell Dev Biol 23:645–673 [DOI] [PubMed] [Google Scholar]
- Charge SBP, Rudnicki MA (2004) Cellular and molecular regulation of muscle regeneration. Physiol Rev 84:209–238 [DOI] [PubMed] [Google Scholar]
- Collins CA, Gnocchi VF, White RB, Boldrin L, Perez-Ruiz A, Relaix F, Morgan JE, et al. (2009) Integrated functions of Pax3 and Pax7 in the regulation of proliferation, cell size and myogenic differentiation. PLoS One 4:e4475. Published online February 16, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE (2005) Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 122:289–301 [DOI] [PubMed] [Google Scholar]
- Day K, Paterson B, Yablonka-Reuveni Z (2009) A distinct profile of myogenic regulatory factor detection within Pax7(+) cells at S Phase supports a unique role of Myf5 during posthatch chicken myogenesis. Dev Dyn 238:1001–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day K, Shefer G, Richardson JB, Enikolopov G, Yablonka-Reuveni Z (2007) Nestin-GFP reporter expression defines the quiescent state of skeletal muscle satellite cells. Dev Biol 304:246–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Anda G, Rebollo MA (1967) The neuromuscular spindles in the adult chicken. I. Morphology. Acta Anat (Basel) 67:437–451 [DOI] [PubMed] [Google Scholar]
- Dorward PK (1970) Response characteristics of muscle afferents in the domestic duck. J Physiol 211:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubowitz V, Sewry CA (2007) Muscle Biopsy: A Practical Approach. 3rd ed. Philadelphia, Elsevier, 83–84
- Epstein JA, Shapiro DN, Cheng J, Lam PYP, Maas RL (1996) Pax3 modulates expression of the c-Met receptor during limb muscle development. Proc Natl Acad Sci USA 93:4213–4218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli LM, Knight SR, Barnes TL, Doak AK, Kadzik RS, Burrus LW (2008) Identification and characterization of subpopulations of Pax3 and Pax7 expressing cells in developing chick somites and limb buds. Dev Dyn 237:1862–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George JC, Berger AJ (1966) Avian Myology. New York, Academic Press
- Gollnick PD, Parsons D, Riedy M, Moore RL (1983) Fiber number and size in overloaded chicken anterior latissimus dorsi muscle. J Appl Physiol 54:1292–1297 [DOI] [PubMed] [Google Scholar]
- Gollnick PD, Timson BF, Moore RL, Riedy M (1981) Muscular enlargement and number of fibers in skeletal muscles of rats. J Appl Physiol 50:936–943 [DOI] [PubMed] [Google Scholar]
- Gopinath SD, Rando TA (2008) Stem cell review series: aging of the skeletal muscle stem cell niche. Aging Cell 7:590–598 [DOI] [PubMed] [Google Scholar]
- Halevy O, Piestun Y, Allouh MZ, Rosser BWC, Rinkevich Y, Reshef R, Rozenboim I, et al. (2004) Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Dev Dyn 231:489–502 [DOI] [PubMed] [Google Scholar]
- Harvey AL, Marshall IG (1986) Muscle. In Sturkie PD, ed. Avian Physiology. 4th ed. New York, Springer-Verlag, 74–86
- Hikida RS, Walsh S, Barylski N, Campos G, Hagerman FC, Staron RS (1998) Is hypertrophy limited in elderly muscle fibers? A comparison of elderly and young strength-trained men. BAM 8:419–427 [Google Scholar]
- Horst D, Ustanina S, Sergi C, Mikuz G, Juergens H, Braun T, Vorobyov E (2006) Comparative expression analysis of Pax3 and Pax7 during mouse myogenesis. Int J Dev Biol 50:47–54 [DOI] [PubMed] [Google Scholar]
- Jin Z, Mannstrom P, Jarlebark L, Ulfendahl M (2007) Malformation of stria vascularis in the developing inner ear of the German waltzing guinea pig. Cell Tissue Res 328:257–270 [DOI] [PubMed] [Google Scholar]
- Kassar-Duchossoy L, Giacone E, Gayraud-Morel B, Jory A, Gomes D, Tajbakhsh S (2005) Pax3/Pax7 mark a novel population of primitive myogenic cells during development. Genes Dev 19:1426–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami A, Kimura-Kawakami M, Nomura T, Fujisawa H (1997) Distributions of PAX6 and PAX7 suggest their involvement in both early and late phases of chick brain development. Mech Dev 66:119–130 [DOI] [PubMed] [Google Scholar]
- Keller C, Hansen MS, Coffin CM, Capecchi MR (2004) Pax3:Fkhr interferes with embryonic Pax3 and Pax7 function: implications for alveolar rhabdomyosarcoma cell of origin. Genes Dev 18:2608–2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick LJ, Allouh MZ, Nightingale CN, Devon HG, Yablonka-Reuveni Z, Rosser BWC (2008) Pax7 shows higher satellite cell frequencies and concentrations within intrafusal fibers of muscle spindles. J Histochem Cytochem 56:831–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkorogiannis T (2004) Somatic and intramuscular distribution of muscle spindles and their relation to muscular angiotypes. J Theor Biol 229:263–280 [DOI] [PubMed] [Google Scholar]
- Kozeka K, Ontell M (1981) The 3-dimensional cytoachitecture of developing murine muscle spindles. Dev Biol 87:133–147 [DOI] [PubMed] [Google Scholar]
- Liu JX, Eriksson PO, Thornell LE, Pedrosa-Domellof F (2002) Myosin heavy chain composition of muscle spindles in human biceps brachii. J Histochem Cytochem 50:171–183 [DOI] [PubMed] [Google Scholar]
- MacIntosh BR, Gardier PF, McComas AJ (2006) Skeletal Muscle: Form and Function. 2nd ed. Windsor, Human Kinetics
- Maier A (1992) The avian muscle spindle. Anat Embryol (Berl) 186:1–25 [DOI] [PubMed] [Google Scholar]
- Mauro A (1961) Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9:493–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers RA (1992) The morphological basis of folded-wing posture in the American kestrel (Falco sparverius). Anat Rec 232:493–494 [DOI] [PubMed] [Google Scholar]
- Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, et al. (2005) Direct isolation of satellite cells for skeletal muscle regeneration. Science 309:2064–2067 [DOI] [PubMed] [Google Scholar]
- Moore LA, Arrizubieta MJ, Tidyman WE, Herman LA, Bandman E (1992) Analysis of the chicken fast myosin heavy chain family. Localization of isoform-specific antibody epitopes and regions of divergence. J Mol Biol 225:1143–1151 [DOI] [PubMed] [Google Scholar]
- Ono Y, Iwamoto H, Takahara H (1993) The relationship between muscle growth and the growth of different fiber types in the chicken. Poult Sci 72:568–576 [DOI] [PubMed] [Google Scholar]
- Otto A, Schmidt C, Patel K (2006) Pax3 and Pax7 expression and regulation in the avian embryo. Anat Embryol (Berl) 211:293–310 [DOI] [PubMed] [Google Scholar]
- Ovalle WK, Dow PR, Nahirney PC (1999) Structure, distribution and innervation of muscle spindles in avian fast and slow skeletal muscle. J Anat 194:381–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raikow RJ (1985) Locomotor systems. In King AS, McLelland J, eds. Form and Functions of Birds, vol. 3. New York, Academic Press, 57–146
- Relaix F, Montarras D, Zaffran S, Gayraud-Morel B, Rocancourt D, Tajbakhish S, Mansouri A, et al. (2006) Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol 172:91–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Rocancourt D, Mansouri A, Buckingham M (2005) A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature 435:948–953 [DOI] [PubMed] [Google Scholar]
- Rosser BWC, Dean MS, Bandman E (2002) Myonuclear domain size varies along the lengths of maturing skeletal muscle fibers. Int J Dev Biol 46:747–754 [PubMed] [Google Scholar]
- Rosser BWC, Farrar CM, Crellin NK, Andersen LB, Bandman E (2000) Repression of myosin isoforms in developing and denervated skeletal muscle fibers originates near motor endplates. Dev Dyn 217:50–61 [DOI] [PubMed] [Google Scholar]
- Sambasivan R, Gayraud-Morel B, Dumas G, Cimper C, Paisant S, Kelly R, Tajbakhsh S (2009) Distinct regulatory cascades govern extraocular and pharyngeal arch muscle progenitor cell fates. Dev Cell 16:810–821 [DOI] [PubMed] [Google Scholar]
- Schmalbruch H, Hellhammer U (1977) The number of nuclei in adult rat muscles with special reference to satellite cells. Anat Rec 189:169–176 [DOI] [PubMed] [Google Scholar]
- Schultz E, Lipton BH (1982) Skeletal muscle satellite cells: changes in proliferation potential as a function of age. Mech Ageing Dev 20:377–383 [DOI] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA (2000) Pax7 is required for the specification of myogenic satellite cells. Cell 102:777–786 [DOI] [PubMed] [Google Scholar]
- Sewry CA, Dubowitz V (2001) Histochemistry and immunocytochemistry of muscle in health and disease. In Karpati G, Hilton-Jones D, Griggs RC, eds. Disorders of Voluntary Muscle. 7th ed. Cambridge, Cambridge University Press, 251–282
- Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z (2006) Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol 294:50–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajbakhsh S, Rocancourt D, Cossu G, Buckingham M (1997) Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell 89:127–138 [DOI] [PubMed] [Google Scholar]
- Tamaki T, Uchiyama S (1995) Absolute and relative growth of rat skeletal muscle. Physiol Behav 57:913–919 [DOI] [PubMed] [Google Scholar]
- Tzu J, Marinkovich MP (2008) Bridging structure with function: structural, regulatory, and developmental role of laminins. Int J Biochem Cell Biol 40:199–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo AB (1974) Afferent discharge from human muscle spindles in non-contracting muscles: steady state impulse frequency as a function of joint angle. Acta Physiol Scand 90:303–318 [DOI] [PubMed] [Google Scholar]
- Vanden Berge JC, Zweers GA (1993) Myologia. In Baumel JJ, King AS, Breazille JE, Evan HE, Vanden Berge JC, eds. Handbook of Avian Anatomy: nomina anatomica avium. Cambridge, Nuttall Ornithological Club, 189–247
- van Emden HF (2008) Statistics for Terrified Biologists. Malden, Blackwell Publishing, 40
- Vater R, Cullen MJ, Harris JB (1994) The expression of vimentin in satellite cells of regenerating skeletal muscle in vivo. Histochem J 26:916–928 [PubMed] [Google Scholar]
- Venters SJ, Argent RE, Deegan FM, Perez-Baron G, Wong TS, Tidyman WE, Denetclaw WF, et al. (2004) Precocious terminal differentiation of pre-migratory limb muscle precursor cells requires positive-signalling. Dev Dyn 229:591–599 [DOI] [PubMed] [Google Scholar]
- Walro JM, Kucera J (1999) Why adult mammalian intrafusal and extrafusal fibers contain different myosin heavy-chain isoforms. Trends Neurosci 22:180–184 [DOI] [PubMed] [Google Scholar]
- Zammit PS, Carvajal JJ, Golding JP, Morgan JE, Summerbell D, Zolnerciks J, Partridge TA, et al. (2004) Myf5 expression in satellite cells and spindles in adult muscle is controlled by separate genetic elements. Dev Biol 273:454–465 [DOI] [PubMed] [Google Scholar]
- Zammit PS, Partridge TA, Yablonka-Reuveni Z (2006) The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem 54:1177–1191 [DOI] [PubMed] [Google Scholar]
- Zar JH (1999) Biostatistical Analysis. 4th ed. Upper Saddle River, Prentice-Hall, 278–280