Abstract
Acrolein is a potent fixative that provides both excellent preservation of ultrastructural morphology and retention of antigenicity, thus it is frequently used for immunocytochemical detection of antigens at the electron microscopic level. However, acrolein is not commonly used for fluorescence microscopy because of concerns about possible autofluorescence and destruction of the luminosity of fluorescent dyes. Here we describe a simple protocol that allows fine visualization of two fluorescent markers in 40-μm sections from acrolein-perfused rat brain. Autofluorescence was removed by pretreatment with 1% sodium borohydride for 30 min, and subsequent incubation in a 50% ethanol solution containing 0.3% hydrogen peroxide enhanced fluorescence labeling. Thus, fluorescence labeling can be used for high-quality detection of markers in tissue perfused with acrolein. Furthermore, adjacent acrolein-fixed sections from a single experiment can be processed to produce high-quality results for electron microscopy or fluorescence labeling. (J Histochem Cytochem 58:359–368, 2010)
Keywords: sodium borohydride, endogenous peroxidase inactivation, autofluorescence, ethanol, enhanced penetration, hydrogen peroxide, aldehyde fixation
Acrolein (propenal or acrylic aldehyde) is a three-carbon α-β-unsaturated monoaldehyde that provides outstanding stabilization of proteins, similar to glutaraldehyde, but penetrates tissue more rapidly (Flitney 1966; Saito and Keino 1976), providing excellent morphological preservation of fine structure for electron microscopy (EM) studies (Sabatini et al. 1963). Although fixation of tissue blocks or cultured cells with acrolein can degrade enzymatic activity or antigenicity (Sabatini et al. 1963; van den Eijnden-van Raaij et al. 1988), acrolein has been frequently used for immunocytochemistry for EM-level studies (King et al. 1983; Pickel et al. 1986a,b,c; Sesack et al. 1998; Pinto et al. 2003; Alvira-Botero and Garzon 2006; Mengual et al. 2008; Pinto and Sesack 2008; Lessard et al. 2009) because degradation is not excessive when fixation times are short (Flitney 1966), such as during transcardial perfusion (King et al. 1983; Leranth and Pickel 1989). Furthermore, the rapid penetration and strong cross-linking abilities of acrolein quickly stabilize proteins, retaining even small peptides that can be successfully immunolabeled (King et al. 1983; Pickel et al. 1986c).
However, highly reactive aldehyde fixatives like glutaraldehyde or acrolein are not often used for fluorescence studies. While there are some reports of fluorescence labeling after glutaraldehyde fixation, to our knowledge, acrolein has been seldom used because of concerns about possible high-background autofluorescence levels, as described for glutaraldehyde (Cande et al. 1977; Weber et al. 1978; Clancy and Cauller 1998) and fluorophore degradation (Herzog and Kummel 2000). Nevertheless, we sought to extract connectional and neurochemical information from valuable tissue samples that had been labeled in vivo with an anterograde tracer and subsequently fixed with acrolein with the original intent of analysis with EM but where the number of labeled axons of interest were too sparse for EM detection.
Several treatments have been used to remove tissue fluorescence caused by other fixatives, mostly glutaraldehyde, but only partial reductions have been achieved (Weber et al. 1978; Beisker et al. 1987; Clancy and Cauller 1998; Haraguchi and Yokota 2002; Ngwenya et al. 2005). For example, a 30% reduction of background autofluorescence was reported for paraformaldehyde-fixed sections after immersion in sodium borohydride (NaBH4), an aldehydic reducing agent (Abdel-Akher et al. 1952), and somewhat lower in glutaraldehyde-fixed tissue (Clancy and Cauller 1998), whereas lower reductions have been achieved with treatments involving glycine, ammonium chloride, or Sudan black B (Ngwenya et al. 2005). In addition, in situ hybridization visualized with fluorescence labeling has been shown to benefit, in some cases, from incubation in an alcohol-containing quenching solution (Barroso-Chinea et al. 2007).
We report here that pretreatment with NaBH4 and subsequent incubation in 50% ethanol allows successful two-color fluorescence labeling of acrolein-fixed tissue and that the quality of detection is similar to that obtained for EM studies. Thus, fluorescence labeling can be used for high-quality detection of markers in tissue perfused with acrolein. Furthermore, fluorescence techniques and ultrastructural studies can be carried out with adjacent acrolein-fixed sections from the same tissue block.
Materials and Methods
Surgical Procedure and Perfusion of Animals
All experiments were conducted using adult male Wistar rats (250–300 g; Harlan, San Pietro al Natisone, Italy) in accordance with the national and European regulations for animal care (Spanish Royal Decree 223/1988 and European Council Directive 86/609/EEC) and were approved by the institutional animal care and use committee of the University of Navarra. Prior to surgery, rats were deeply anesthetized with a mixture of ketamine [(Imalgène 500) 75 mg/kg; Merial, Barcelona, Spain], xylacine [(Rompún 2%) 10 mg/kg; Bayer, Leverkusen, Germany), and atropine (0.05 mg/kg; B. Braun Medical, Barcelona, Spain) intraperitoneally and placed in a stereotaxic frame (model 1730; David Kopf Instruments, Tujunga, CA). A local anesthetic [(Xilonibsa) 2% lidocaine; Inibsa, Barcelona, Spain] was also administered in the external acoustic meati and scalp. After the craniotomy, 2% biotinylated dextran amine (BDA-10.000; Molecular Probes Europe, Leiden, The Netherlands) in phosphate buffer (PB), 0.01 M (pH 7.4), was iontophoretically administered in the ventral pallidum through glass micropipettes (inner tip diameter, 30–60 μm), using a 5-μA positive-pulsed direct current (7 sec on/7 sec off) over 10–20 min.
After a survival period of 12–15 days, the animals were deeply anesthetized with 10% chloral hydrate (10 ml/kg, intraperitoneally) and perfused through the aortic arch with (1) 5–10 ml of heparin-saline, (2) 50 ml of 3.75% acrolein (catalog ref. 01680; Fluka, Steinheim, Germany) in 2% paraformaldehyde, and (3) 200 ml of 2% paraformaldehyde. The fixative solutions were prepared in 0.1 M PB (pH 7.4). Brains were removed from the cranium, blocked into 2–4-mm coronal slices of tissue and postfixed in a 2% paraformaldehyde solution for 30 min at 4C. Subsequently, brains were immersed in PB and sectioned rostrocaudally at a thickness of 40 μm on a vibratome (VT 1000S; Leica Microsystems, Nussloch, Germany). Sections were serially collected in cryoprotective solution (30% ethylene glycol, 30% glycerol in 0.05 M PB) and stored at −20C.
Sodium Borohydride Pretreatment
In a first set of experiments, we analyzed the effect of pretreatment with sodium borohydride (NaBH4) on acrolein-fixed tissue. Two serial brainstem sections from five animals that had undergone surgery and subsequent acrolein fixation were selected and rinsed thoroughly in PB at room temperature to remove all cryoprotectant solution. One series of sections was subsequently incubated in a freshly prepared 1% solution of NaBH4 in PB for 30 min under agitation at room temperature and thoroughly rinsed in PB to eliminate all bubbles, until the sections laid flat at the bottom of the crucible (Leranth and Pickel 1989; Mengual and Pickel 2002). The other series of sections were kept in PB under agitation at room temperature for the equivalent time of treatment and rinses. Finally, both sets of sections were mounted onto subbed slides, air-dried, dehydrated, and defatted in toluene (12 min), and coverslipped with mounting medium (DPX; VWR International Ltd., Poole, England).
All sections were examined with a confocal laser scanning microscope (LSM 510 META model; Zeiss, Göttingen, Germany) using a 40× oil immersion lens with differential interference contrast. Green Alexa 488 fluorescence (Molecular Probes) was excited using a 488-nm argon laser, and emitted fluorescence was detected with a 505–530-nm-band-pass filter. Red Alexa 568 was excited with a 543-nm helium/neon laser, and emission was detected with a 560-nm-band-pass filter. Two images were taken at random from each section; all images were captured with the same acquisition settings so that comparisons could be made between the two groups. The background intensity was measured both in the red and green channels with Zeiss LSM software and was calculated from three areas per section with the formula Σ (intensity × absolute frequency)/Σ absolute frequency. Mean values from treated sections were compared to those from non-treated sections, using the Wilcoxon non-parametric test for two dependent samples (SPSS software, version 15.0; Chicago, IL).
Incubation in a Quenching Solution and Two-color Fluorescence Labeling
After sections were pretreated with NaBH4, a series of sections were rinsed three times in PB and three times in PBS (0.1 M; pH 7.4) and incubated for 30 min at room temperature in a 50% ethanol solution containing 0.3% H2O2 (Prensa and Parent 2001). Treated and non-treated sections were subsequently rinsed three times in PBS and processed for two-color fluorescence labeling as follows. All incubations were carried out in the dark at room temperature and under agitation, unless otherwise specified. Sections were incubated in streptavidin-Alexa 488 (1:250 dilution; Molecular Probes) in PBS for 2 hr, followed by three washes in PBS and incubation for 40 min in a solution containing 4% normal rabbit serum, 0.05% Triton X-100, and 4% BSA. Subsequently, sections were incubated overnight at 4C in the same solution containing a goat polyclonal antibody against choline acetyltransferase [(ChAT] 1:150 dilution; Chemicon International Inc., Temecula, CA). After sections were washed three times in PBS, they were incubated for 2 hr in a solution containing 0.5% normal rabbit serum, 2% BSA, and Alexa Fluor 568 rabbit anti-goat IgG (1:200 dilution; Invitrogen). After another three washes in PBS, sections were mounted on gelatin-coated slides, air dried, dehydrated in toluene, and coverslipped. Slides were examined with a confocal microscope (LSM 510 META; Zeiss).
In a second series of experiments, the contribution of each component of the quenching solution to the fluorescent labeling was analyzed. Thus, four serial brainstem sections from the same five animals used previously were treated with NaBH4, and after three washes in PB followed by an additional three washes in PBS, the sections were sorted into four groups, each of which was incubated in one of the following solutions: (1) PBS only (negative control); (2) 50% ethanol; (3) 0.3% H2O2 in deionized water; and (4) 50% ethanol plus 0.3% H2O2. After incubation, all sections were again rinsed three times in PBS and then processed for two-color fluorescence labeling. Once sections were mounted as described above, slides were analyzed with confocal microscopy. One image of the cholinergic mesopontine tegumentum was captured per section, with the same acquisition settings in all cases. Since neurons displayed different degrees of intensity, three to four neurons that showed higher luminosity were selected; their intensities were calculated with the formula Σ (intensity × absolute frequency)/Σ absolute frequency, and the mean value for the image was obtained. Likewise, three to four neurons with a lighter labeling were randomly selected and the same calculations were made. Statistical comparisons of the mean values were carried out among the four different groups, using the Friedman non-parametric k-dependent samples test, followed by a Wilcoxon test with Bonferroni's correction (SPSS software, version 15.0).
The neuropil intensity was also measured, both in the red and the green channels. Three to four areas devoid of neurons, consisting of only fiber-containing neuropil, were chosen and delineated in the same images previously acquired from the mesopontine tegmentum, and the intensities were obtained with the same software and formula as described above. Mean values from each section were then calculated, and statistical comparisons were carried out among the four different groups using the Friedman test and followed by the Wilcoxon test with Bonferroni's correction (SPSS software, version 15.0).
The possible effect of the components of the quenching solution after NaBH4 treatment was also tested with unlabeled tissue. Thus, four serial brainstem sections from the same five rats were selected, treated with NaBH4 and rinsed, sorted into four groups, and incubated for 30 min at room temperature in one of the four solutions mentioned above. After incubation, all sections were rinsed three times in PBS and mounted as described above. Slides were analyzed using a regular epifluorescence microscope (Eclipse E800M model; Nikon, Tokyo, Japan).
Results
To determine whether double-labeling fluorescence techniques could be successfully performed on acrolein-fixed tissue, we first tested the effect of pretreatment with NaBH4 on both unlabeled and fluorescence-labeled sections. In subsequent experiments, the effect of a quenching solution was analyzed after two-color fluorescence labeling. A summary of the different trials and their results is shown in Table 1.
Table 1.
Summary of treatments tested on acrolein-fixed tissue and results
| Treatment 1 | Treatment 2 | Fluorescence labeling | Result |
|---|---|---|---|
| NaBH4 | — | No | NaBH4 eliminates tissue autofluorescence |
| PBS | — | No | |
| NaBH4 | EtOH 50% + 0.3% H2O2 | Intense | Ethanol + H2O2 increases fluorescent detection |
| PBS | Poor | ||
| NaBH4 | PBS | Poor | Ethanol is the component that accounts for the enhanced detection |
| EtOH 50% | Intense | ||
| EtOH 50% + 0.3% H2O2 | Intense | ||
| H2O + 0.3% H2O2 | Poor |
Effect of Pretreatment With NaBH4 on Tissue Fluorescence After Acrolein Fixation
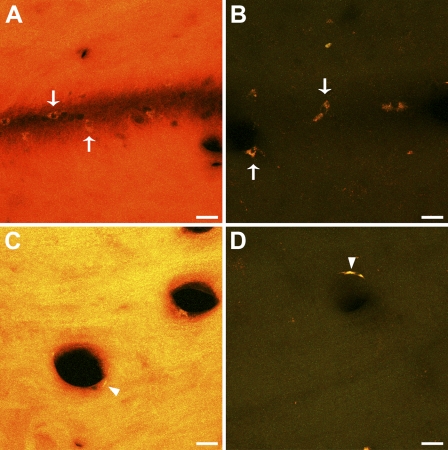
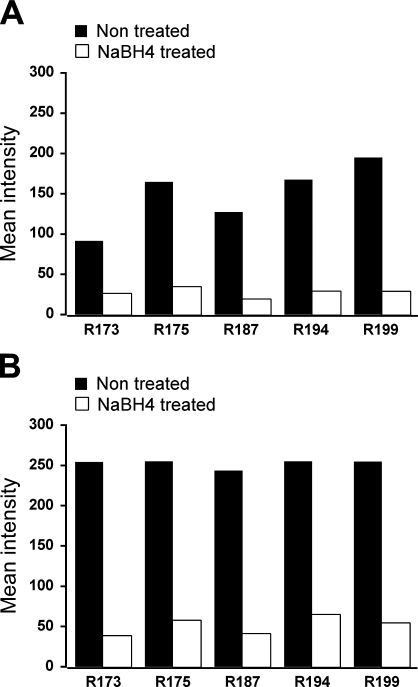
Qualitative observation of acrolein-fixed sections with a fluorescence microscope revealed a strong tissue fluorescence, consistent with previous reports showing autofluorescence produced by other strong fixatives like glutaraldehyde (Figures 1A and 1C). In contrast NaBH4-treated sections displayed little or no autofluorescence (Figures 1B and 1D). In a quantitative analysis, the mean values of fluorescence intensity in NaBH4-treated sections were, in all cases, much lower than those in non-treated sections, both in the green and the red channels (Figures 2A and 2B). The statistical comparison between the two groups showed that fluorescence in non-treated sections was significantly higher than in the treated ones (Wilcoxon test, p=0.043 for both channels). These results show that pretreatment with NaBH4 efficiently reduces tissue fluorescence due to acrolein fixation. However, sporadic cells exhibiting strong autofluorescence were occasionally observed in close relationship to blood vessels (Figures 1A–1D, arrows and arrowheads).
Figure 1.
Effect of sodium borohydride pretreatment on unlabeled acrolein-fixed sections. Representative confocal microscopy stacks from pairs of adjacent sections from different animals, obtained with the same scanning settings, are shown. (A,C) show strong tissue fluorescence in non-treated sections. (B,D) NaBH4-treated sections display no tissue fluorescence. Note that sporadic autofluorescent blood cells adhered to the blood vessel wall are still observed after the treatment (A,B, arrows). Putative endothelial cells also displayed autofluorescence before and after incubation in NaBH4 (C,D, arrowheads). Bar = 20 μm.
Figure 2.
Graphs show mean intensity values in NaBH4-treated (gray) vs non-treated (black) unlabeled sections from five animals. Mean intensity values ranged from 0 (black) to 255 (maximum intensity). (A) Values obtained in the green channel. (B) Values obtained in the red channel. R number = rat subject number.
Effect of Incubation in an H2O2 Solution on Fluorescence Labeling
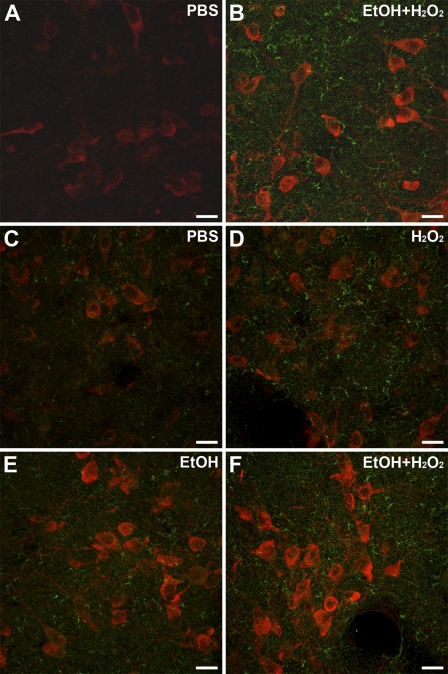
Two-color fluorescence labeling was then carried out with another set of sections previously treated with NaBH4. ChAT-labeled neurons were visible in the red channel and BDA-containing fibers were observed in the green channel. Since immunofluorescence detection of in situ hybridization is enhanced by prior incubation in a quenching solution (Barroso-Chinea et al. 2007), we determined whether the two-color fluorescence labeling would benefit from incubation in a 50% ethanol solution plus H2O2. Indeed, qualitative observations showed that incubation in the ethanol/H2O2 solution enhanced the fluorescence detection of labeled neurons and fibers (Figures 3A and 3B). In further experiments designed to quantitatively determine the improved detection, and also whether a single component of the incubation solution was causing it, the intensity of both neurons and neuropil was estimated, analyzing intensely labeled and lightly labeled neurons separately. Comparisons of mean values among groups showed statistically significant differences within both populations (Friedman test, p=0.014 and p=0.021, intensely labeled and lightly labeled neurons, respectively). Comparisons between groups revealed no differences, either between PBS and H2O2 in water or between ethanol only and ethanol plus H2O2 (Wilcoxon test, p=0.345, and p=0.138, intensely labeled neurons, respectively; p=0.50, p=0.56, lightly labeled neurons, respectively), suggesting that H2O2 is not the cause of the enhanced detection of neurons. In contrast, a trend toward a statistically significant difference was found between PBS and ethanol plus H2O2 and also between ethanol plus H2O2 and H2O2 in water, in both intensely labeled and lightly labeled neurons (Wilcoxon test, p=0.043, and p=0.043, intensely labeled neurons; p=0.08, and p=0.043, lightly labeled neurons, respectively), indicating that the presence of ethanol is likely to account for the enhanced neuronal labeling.
Figure 3.
Effects of incubation in ethanol plus H2O2, ethanol only, or H2O2 only, on two-color fluorescence labeling. (A–F) Merged images from confocal microscopy of double-labeled sections from the lateral dorsal tegmental nucleus showing cholinergic neurons in red and BDA-labeled fibers in green. All images were obtained with the same acquisition settings. (A,B) sections from rat R175 without (A) and with (B) incubation in a 50% ethanol plus 0.3% H2O2, prior to the two-color fluorescence labeling. (C–F) Four serial sections from rat R194 showing the effect of different incubation solutions: (C) PBS; (D) water plus 0.3% H2O2; (E) 50% ethanol only; (F) 50% ethanol 0.3% plus H2O2. BDA, biotinylated dextran amine. Bar = 20 μm.
In relation to the neuropil, mean values were also obtained in the green and the red channels. Comparisons of mean values showed no differences among the four groups in the red channel (Friedman test, p=0.266), although a statistically significant difference was observed in the green channel (Friedman test, p=0.006), consistent with the qualitative observation of enhanced detection of green BDA-labeled fibers. Comparisons between groups showed no differences, either between PBS and H2O2 in water or between ethanol only and ethanol plus H2O2, as previously observed for labeled neurons. In contrast, a trend toward a statistically significant difference was found again in PBS vs ethanol plus H2O2 and between ethanol plus H2O2 and H2O2 in water (Wilcoxon test, p=0.043 and p=0.043, respectively). Thus, ethanol seems to be contributing significantly to the enhanced fiber labeling in the green channel.
Effect of the H2O2 Solution on Unlabeled Tissue
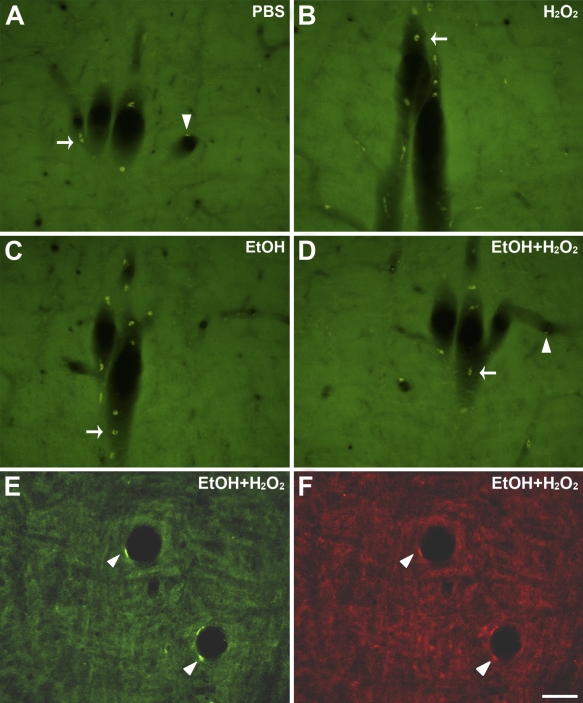
As noted above, sporadic autofluorescent cells continued to be present after NaBH4 treatment, which were in sharp contrast to the dark background. Some cells resembled blood cells and appeared mainly along longitudinally sectioned blood vessels, adhered to remnants of blood vessel wall (Figure 1B, arrows). Other cells had an elongated cell body that lined the blood vessel lumen, suggesting an endothelial cell phenotype (Figure 1D, arrowhead). Subsequent incubation of NaBH4-treated sections in the quenching solution or each of its components showed no effect of either ethanol or H2O2 or both on removal of cell autofluorescence (Figure 4). However, blood vessels were scarce within individual sections and only occasionally fell within the area of interest. Moreover, the morphology and location of the autofluorescent cells were obviously distinguishable from the labeled neurons and fibers and did not interfere with them.
Figure 4.
Effect of incubation in ethanol plus H2O2, ethanol only, or H2O2 only, on autofluorescent cells in NaBH4-treated acrolein-fixed sections. (A–D) Serial sections showing the same longitudinally sectioned blood vessel, incubated in different solutions: (A) PBS; (B) water plus 0.3% H2O2; (C) 50% ethanol only; (D) 50% ethanol 0.3% plus H2O2. Blood cells were visible in the four different conditions but were generally associated with remnants of blood vessel wall (A–D, arrows). Autofluorescent endothelial cells were also observed in the four conditions but were scarcer than blood cells (e.g., arrowheads in A and D). (E,F) Images of a single section incubated in ethanol plus H2O2, obtained in the green (E) or red (F) channel, showing a putative endothelial autofluorescent cell (arrowheads) that displays a stronger luminosity in the green channel. Bar = 50 μm.
Discussion
In the present study we report a simple procedure that allows fine visualization of two fluorescent markers in acrolein-fixed brain tissue. Antigenicity was preserved despite the potency of the fixative; the background fluorescence produced by the fixative was virtually eliminated; and the fluorescent signal was enhanced, providing clear detection of thin fibers and neurons.
Preservation of Antigenicity After Acrolein Fixation
To our knowledge, few studies have used acrolein as a fixative for fluorescence labeling. One of the few studies reported that fixation of cell cultures with 0.1% acrolein for 1 h at room temperature eliminated antigenicity (van den Eijnden-van Raaij et al. 1988). However, much faster acrolein fixation achieved by perfusion preserves antigenicity in brain tissue subsequently analyzed by EM (Leranth and Pickel 1989; Mengual and Pickel 2002). We now report a procedure for obtaining excellent fluorescence labeling with anti-ChAT antibodies after a similarly fast perfusion with acrolein, which differs from the procedure described in a previous report (Leranth and Pickel 1989) for unknown reasons.
Effect of Sodium Borohydride Pretreatment on Acrolein vs Glutaraldehyde Autofluorescence
Aldehyde fixatives typically give rise to tissue fluorescence (Cande et al. 1977; Weber et al. 1978; Clancy and Cauller 1998), which seems to be due to the formation of conjugated Schiff bases between aldehydes and amino groups of proteins yielding fluorescent compounds (Nascimento and Cilento 1989; Tuma et al. 1991; García Fraile and Teso Vilar 1992; Hoffmann et al. 1993). The use of the aldehyde-reducing agent NaBH4 in aqueous solution (Abdel-Akher et al. 1952; Schachner et al. 1977) has provided modest reductions in tissue fluorescence produced by glutaraldehyde (Weber et al. 1978; Beisker et al. 1987; Hirsch and Helke 1988; Clancy and Cauller 1998; Haraguchi and Yokota 2002; Ngwenya et al. 2005). In addition, conveniently, NaBH4 has also been used frequently during sample processing for EM studies after acrolein fixation (Leranth and Pickel 1989; Mengual and Pickel 2002) but has not been previously tested alone as a single treatment for reducing tissue autofluorescence. In the present study, we used the same protocol (Mengual and Pickel 2002,2004) and found a dramatic reduction in the autofluorescence induced by acrolein fixation, which enabled fine detection of both cholinergic neurons and BDA-containing fibers. The autofluorescence reduction did not require treatment with sodium metaperiodate prior to NaBH4 treatment, as reported for the single study carried out with brain tissue after acrolein fixation (Hirsch and Helke 1988).
Autofluorescence is removed by NaBH4 most likely by reduction of Schiff bases formed after reaction of aldehydes with an amino group, which then become non-fluorescent (Beppu et al. 1987); the alternate possibility that NaBH4 instead reduces alkenes is considered unlikely (Morrison and Boyd 1976). However, why NaBH4 is much better at eliminating autofluorescence in acrolein-fixed than in glutaraldehyde-fixed tissue is not known. One possibility is the 2× faster tissue penetration speed of acrolein (Saito and Keino 1976). The longer time needed by glutaraldehyde to stabilize proteins may promote, or be caused by, formation of additional bonds with its free aldehyde group, creating steric impediments for NaBH4 penetration; this would require longer exposure time to the reducing agent, thereby altering the compromise between reduction of autofluorescence and quality of the tissue (Clancy and Cauller 1998; Ngwenya et al. 2005). In addition, since we do not know the amount of reducible Schiff base formed from glutaraldehyde in the tissue, the amount of NaBH4 used could be stoichiometrically insufficient to reduce all susceptible bonds, which would be more abundant in the case of glutaraldehyde (including imine and carbonyl groups). On the other hand, differences in concentrations and volumes of fixative cannot be ruled out. In any case, our observation that acrolein autofluorescence is totally abolished with incubation in 1% NaBH4 without altering the quality of the tissue, whereas glutaraldehyde autofluorescence is not, even with a longer incubation (Clancy and Cauller 1998; Ngwenya et al. 2005), suggests that acrolein may be a more convenient fixative than glutaraldehyde for fluorescence labeling techniques.
Enhancement of the Fluorescence Signal After Incubation in Ethanol and H2O2
We additionally found that sections treated with ethanol prior to fluorescence labeling displayed an enhanced signal in comparison to control sections, in both neuronal bodies and thin fibers. This is consistent with many reports that ethanol and methanol enhance tissue penetration of immunoreagents (Llewellyn-Smith and Minson 1992; Deere et al. 1998; Krutzik and Nolan 2003; Yokouchi et al. 2003; Pearson et al. 2007). Other treatments have also been used to facilitate penetration of immunoglobulins into the tissue, such as preincubation with dimethylsulfoxide (King et al. 1983). The results, however, are not comparable, since only cell bodies, and not fibers, were labeled in the single report that used dimethylsulfoxide on acrolein-fixed tissue (Hirsch and Helke 1988).
Lack of Effect of Ethanol or H2O2 on Artifactual Autofluorescent Cells
Scattered autofluorescent cells could occasionally be observed in close relation with blood vessels in non-treated and in NaBH4-treated sections. This is consistent with a previous study reporting resilient autofluorescence in blood cells, as well as in pial cells, after NaBH4 treatment in glutaraldehyde-fixed tissue (Clancy and Cauller 1998). The present study adds putative endothelial cells to the list of cell types displaying autofluorescence that was not removed with NaBH4 treatment. Moreover, neither ethanol nor H2O2 had any effect on this artifactual fluorescence. Blood cells display autofluorescence that increases after aldehyde fixation (Hoffman and Hansen 1981; Nascimento and Cilento 1989; Stewart and Stewart 2001; Stewart et al. 2007), and endothelial cells are known to contain lipofuscin (Lamar et al. 1980; Monma et al. 1988), a molecule that has a characteristic fluorescence with broad excitation and emission spectra (Mochizuki et al. 1995; Marmorstein et al. 2002). Lipofuscin pigment appears to be the fluorescent end product of a free radical-induced cross-linking of proteins with oxidized lipids (Kikugawa and Beppu 1987). Brain lipofuscin is not eliminated by incubation with either H2O2 or NaBH4 but only by treatment with CuSO4 (Schnell et al. 1999). In our sample sections, endothelial fluorescence was detected in both the green and the red ranges, although less intensely in the last range, consistent with the emission spectrum of lipofuscin.
In summary, we have developed a simple protocol that allows simultaneous detection of two markers by means of two-color fluorescence labeling in acrolein-fixed brain tissue. A pretreatment with 1% NaBH4 for 30 min eliminates the background autofluorescence otherwise produced by acrolein, whereas subsequent incubation in 50% ethanol for 30 min greatly enhances the detection of specific fluorescence labeling. This new protocol enables high-quality detection of markers on acrolein-fixed tissue by using fluorescence labeling, hence, broadening the technical approaches that can be used with acrolein-fixed tissue, which to date has been used almost exclusively for EM studies. Furthermore, the two approaches can be performed in parallel, with different, including adjacent, tissue sections from the same experiment.
Acknowledgments
This work was supported by the Spanish Ministry of Education and Science (MEC, BFU2004-06825), Gobierno de Navarra 2004, and the Unión Temporal de Empresas project CIMA.
The authors thank Pedro Barroso-Guinea for helpful technical comments, Carmen Sanmartín for advice on organic chemistry, and Isabel Pérez-Otaño and John F. Wesseling for critical reading of the manuscript.
This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication.
References
- Abdel-Akher M, Hamilton J, Montgomery R, Smith F (1952) A new procedure for the determination of the fine structure of polysaccharides. J Am Chem Soc 74:4970–4971 [Google Scholar]
- Alvira-Botero MX, Garzon M (2006) Cellular and subcellular distributions of delta opioid receptor activation sites in the ventral oral pontine tegumentum of the cat. Brain Res 1123:101–111 [DOI] [PubMed] [Google Scholar]
- Barroso-Chinea P, Aymerich MS, Castle MM, Perez-Manso M, Tunon T, Erro E, Lanciego JL (2007) Detection of two different mRNAs in a single section by dual in situ hybridization: a comparison between colorimetric and fluorescent detection. J Neurosci Methods 162:119–128 [DOI] [PubMed] [Google Scholar]
- Beisker W, Dolbeare F, Gray JW (1987) An improved immunocytochemical procedure for high-sensitivity detection of incorporated bromodeoxyuridine. Cytometry 8:235–239 [DOI] [PubMed] [Google Scholar]
- Beppu M, Murakami K, Kikugawa K (1987) Detection of oxidized lipid-modified erythrocyte membrane proteins by radiolabeling with tritiated borohydride. Biochim Biophys Acta 897:169–179 [DOI] [PubMed] [Google Scholar]
- Cande WZ, Lazarides E, McIntosh JR (1977) A comparison of the distribution of actin and tubulin in the mammalian mitotic spindle as seen by indirect immunofluorescence. J Cell Biol 72:552–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Cauller LJ (1998) Reduction of background autofluorescence in brain sections following immersion in sodium borohydride. J Neurosci Methods 83:97–102 [DOI] [PubMed] [Google Scholar]
- Deere D, Vesey G, Milner M, Williams K, Ashbolt N, Veal D (1998) Rapid method for fluorescent in situ ribosomal RNA labelling of Cryptosporidium parvum. J Appl Microbiol 85:807–818 [DOI] [PubMed] [Google Scholar]
- Flitney FW (1966) The time course of the fixation of albumin by formaldehyde, glutaraldehyde, acrolein and other higher aldehydes. J R Micros Soc 85:353 [Google Scholar]
- García Fraile A, Teso Vilar E (1992) Análisis Orgánico. Madrid, UNED
- Haraguchi CM, Yokota S (2002) Immunofluorescence technique for 100-nm-thick semithin sections of Epon-embedded tissues. Histochem Cell Biol 117:81–85 [DOI] [PubMed] [Google Scholar]
- Herzog J, Kummel H (2000) Fixation of transsynaptically transported WGA-HRP and fluorescent dyes used in combination. J Neurosci Methods 101:149–156 [DOI] [PubMed] [Google Scholar]
- Hirsch MD, Helke CJ (1988) Bulbospinal thyrotropin-releasing hormone projections to the intermediolateral cell column: a double fluorescence immunohistochemical-retrograde tracing study in the rat. Neuroscience 25:625–637 [DOI] [PubMed] [Google Scholar]
- Hoffman RA, Hansen WP (1981) Immunofluorescent analysis of blood cells by flow cytometry. Int J Immunopharmacol 3:249–254 [DOI] [PubMed] [Google Scholar]
- Hoffmann T, Meyer RJ, Sorrell MF, Tuma DJ (1993) Reaction of acetaldehyde with proteins: formation of stable fluorescent adducts. Alcohol Clin Exp Res 17:69–74 [DOI] [PubMed] [Google Scholar]
- Kikugawa K, Beppu M (1987) Involvement of lipid oxidation products in the formation of fluorescent and cross-linked proteins. Chem Phys Lipids 44:277–296 [DOI] [PubMed] [Google Scholar]
- King JC, Lechan RM, Kugel G, Anthony EL (1983) Acrolein: a fixative for immunocytochemical localization of peptides in the central nervous system. J Histochem Cytochem 31:62–68 [DOI] [PubMed] [Google Scholar]
- Krutzik PO, Nolan GP (2003) Intracellular phospho-protein staining techniques for flow cytometry: monitoring single cell signaling events. Cytometry A 55:61–70 [DOI] [PubMed] [Google Scholar]
- Lamar CH, McKinley GA, Hinsman EJ (1980) The fine structure of lipofuscin in the mouse hippocampus. Anat Anz 147:215–219 [PubMed] [Google Scholar]
- Leranth C, Pickel V (1989) Electron microscopic pre-embedding double immunohistochemical methods. In Heimer L, Zaborszky L, eds. Neuroanatomical Tract Tracing Methods 2. Recent Progress. New York, Plenum, 129–172
- Lessard A, Savard M, Gobeil F Jr, Pierce JP, Pickel VM (2009) The neurokinin-3 (NK3) and the neurokinin-1 (NK1) receptors are differentially targeted to mesocortical and mesolimbic projection neurons and to neuronal nuclei in the rat ventral tegumental area. Synapse 63:484–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Minson JB (1992) Complete penetration of antibodies into vibratome sections after glutaraldehyde fixation and ethanol treatment: light and electron microscopy for neuropeptides. J Histochem Cytochem 40:1741–1749 [DOI] [PubMed] [Google Scholar]
- Marmorstein AD, Marmorstein LY, Sakaguchi H, Hollyfield JG (2002) Spectral profiling of autofluorescence associated with lipofuscin, Bruch's membrane, and sub-RPE deposits in normal and AMD eyes. Invest Ophthalmol Vis Sci 43:2435–2441 [PubMed] [Google Scholar]
- Mengual E, Chan J, Lane D, San Luciano Palenzuela M, Hara Y, Lessard A, Pickel VM (2008) Neurokinin-1 receptors in cholinergic neurons of the rat ventral pallidum have a predominantly dendritic distribution that is affected by apomorphine when combined with startle-evoking auditory stimulation. Neuroscience 151:711–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengual E, Pickel VM (2002) Ultrastructural immunocytochemical localization of the dopamine D2 receptor and tyrosine hydroxylase in the rat ventral pallidum. Synapse 43:151–162 [DOI] [PubMed] [Google Scholar]
- Mengual E, Pickel VM (2004) Regional and subcellular compartmentation of the dopamine transporter and tyrosine hydroxylase in the rat ventral pallidum. J Comp Neurol 468:395–409 [DOI] [PubMed] [Google Scholar]
- Mochizuki Y, Park MK, Mori T, Kawashima S (1995) The difference in autofluorescence features of lipofuscin between brain and adrenal. Zoolog Sci 12:283–288 [DOI] [PubMed] [Google Scholar]
- Monma N, Satodate R, Suzuki H, Ujiie T (1988) Ceroid-lipofuscinosis. Report of two autopsy cases. Acta Pathol Jpn 38:1191–1203 [DOI] [PubMed] [Google Scholar]
- Morrison R, Boyd R (1976) Química Orgánica. Boston, Fondo Educativo Interamericano
- Nascimento AL, Cilento G (1989) Schiff base formation with amino acids enhances light emission and damage induced in neutrophils by phenylacetaldehyde. Biochim Biophys Acta 991:50–55 [DOI] [PubMed] [Google Scholar]
- Ngwenya LB, Peters A, Rosene DL (2005) Light and electron microscopic immunohistochemical detection of bromodeoxyuridine-labeled cells in the brain: different fixation and processing protocols. J Histochem Cytochem 53:821–832 [DOI] [PubMed] [Google Scholar]
- Pearson RJ, Gatti PJ, Sahibzada N, Massari VJ, Gillis RA (2007) Ultrastructural evidence for selective noradrenergic innervation of CNS vagal projections to the fundus of the rat. Auton Neurosci 136:31–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Ganten D (1986a) Dual peroxidase and colloidal gold-labeling study of angiotensin converting enzyme and angiotensin-like immunoreactivity in the rat subfornical organ. J Neurosci 6:2457–2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Milner TA (1986b) Autoradiographic detection of [125I]-secondary antiserum: a sensitive light and electron microscopic labeling method compatible with peroxidase immunocytochemistry for dual localization of neuronal antigens. J Histochem Cytochem 34:707–718 [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Park DH, Joh TH, Milner TA (1986c) Ultrastructural localization of phenylethanolamine N-methyltransferase in sensory and motor nuclei of the vagus nerve. J Neurosci Res 15:439–455 [DOI] [PubMed] [Google Scholar]
- Pinto A, Jankowski M, Sesack SR (2003) Projections from the paraventricular nucleus of the thalamus to the rat prefrontal cortex and nucleus accumbens shell: ultrastructural characteristics and spatial relationships with dopamine afferents. J Comp Neurol 459:142–155 [DOI] [PubMed] [Google Scholar]
- Pinto A, Sesack SR (2008) Ultrastructural analysis of prefrontal cortical inputs to the rat amygdala: spatial relationships to presumed dopamine axons and D1 and D2 receptors. Brain Struct Funct 213:159–175 [DOI] [PubMed] [Google Scholar]
- Prensa L, Parent A (2001) The nigrostriatal pathway in the rat: a single-axon study of the relationship between dorsal and ventral tier nigral neurons and the striosome/matrix striatal compartments. J Neurosci 21:7247–7260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini DD, Bensch K, Barrnett RJ (1963) Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol 17:19–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Keino H (1976) Acrolein as a fixative for enzyme cytochemistry. J Histochem Cytochem 24:1258–1269 [DOI] [PubMed] [Google Scholar]
- Schachner M, Hedley-Whyte ET, Hsu DW, Schoonmaker G, Bignami A (1977) Ultrastructural localization of glial fibrillary acidic protein in mouse cerebellum by immunoperoxidase labeling. J Cell Biol 75:67–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell SA, Staines WA, Wessendorf MW (1999) Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue. J Histochem Cytochem 47:719–730 [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI (1998) Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci 18:2697–2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CC, Stewart SJ (2001) Cell preparation for the identification of leukocytes. Methods Cell Biol 63:217–251 [DOI] [PubMed] [Google Scholar]
- Stewart JC, Villasmil ML, Frampton MW (2007) Changes in fluorescence intensity of selected leukocyte surface markers following fixation. Cytometry A 71:379–385 [DOI] [PubMed] [Google Scholar]
- Tuma DJ, Hoffman T, Sorrell MF (1991) The chemistry of acetaldehyde-protein adducts. Alcohol Alcohol Suppl 1:271–276 [PubMed] [Google Scholar]
- van den Eijnden-van Raaij AJ, van Maurik P, Boonstra J, van Zoelen EJ, de Laat SW (1988) Ultrastructural localization of platelet-derived growth factor and related factors in normal and transformed cells. Exp Cell Res 178:479–492 [DOI] [PubMed] [Google Scholar]
- Weber K, Rathke PC, Osborn M (1978) Cytoplasmic microtubular images in glutaraldehyde-fixed tissue culture cells by electron microscopy and by immunofluorescence microscopy. Proc Natl Acad Sci USA 75:1820–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokouchi H, Takeyama H, Miyashita H, Maruyama T, Matsunaga T (2003) In situ identification of symbiotic dinoflagellates, the genus Symbiodinium with fluorescence-labeled rRNA-targeted oligonucleotide probes. J Microbiol Methods 53:327–334 [DOI] [PubMed] [Google Scholar]