Abstract
Markers of β-cell maturity would be useful in staging the differentiation of stem/progenitor cells to β-cells whether in vivo or in vitro. We previously identified markers for newly formed β-cells in regenerating rat pancreases after 90% partial pancreatectomy. To test the generality of these markers of newly formed β-cells, we examined their expression during the perinatal period, a time of recognized β-cell immaturity. We show by semiquantitative RT-PCR and immunostaining over the time course from embryonic day 18/20 to birth, 1 day, 2 days, 3 days, 7 days, and adult that MMP-2, CK-19, and SPD are truly markers of new and immature β-cells and that their expression transiently peaks in the perinatal period and is not entirely synchronous. The shared expression of these markers among fetal, newborn, and newly regenerated β-cells, but not adult, strongly supports their use as potential markers for new β-cells in the assessment of both the maturity of stem cell–derived insulin-producing cells and the presence of newly formed islets (neogenesis) in the adult pancreas. (J Histochem Cytochem 58:369–376, 2010)
Keywords: islet cells, β-cells, immunohistochemistry
Functional differences between neonatal and adult β-cells have been known for many years. Until 4 to 8 days after birth, islets lack the glucose-responsive insulin secretion characteristic of a functional β-cell (Bosco et al. 1995). Initially during development, insulin-expressing cells express the transcription factor MafB, which is restricted to adult glucagon-positive cells, and only later express MafA, the transcription factor found selectively in adult (mature) β-cells (Nishimura et al. 2006; Artner et al. 2007). Thus, there is probably a changing phenotype, from progenitor cell to immature β-cell, and finally, to mature adult β-cell. With the promise of generating new insulin-producing cells from stem cells of various types, we need a better understanding of the transition through the lifetime of β-cells. The hematopoietic and neurobiology fields have shown that having cell surface markers, even without known function, is useful for staging cells or sorting subpopulations (Uchida et al. 2000; Ye et al. 2005). Having markers that stage the process of differentiation from stem/progenitor cell to pancreatic β-cell would be useful in assessing the maturity of insulin-producing cells derived from stem/progenitor cells.
Recently, using gene expression profiling, we found identifiable differences in phenotype between young and mature β-cells, with the phenotype of new β-cells transitional between ductal cells and mature β-cells (Toschi et al. 2003). In that study, to identify candidate markers for newly formed β-cells, we used the powerful technique of laser capture microdissection (LCM) to excise the β-cell–enriched cores of both new and mature islets from the same pancreases of 90% partially pancreatectomized rats and compared them to the gene expression profiles on Affymetrix microarrays of β-cells excised from sham-operated rats. We used the 90% partially pancreatectomized rat because it is a well-established model in which regeneration occurs via both β-cell replication and differentiation of progenitors (Bonner-Weir et al. 1983,1993; Bonner-Weir 1997). By comparing the gene profiles of β-cell cores of newly regenerated and mature islets, we found 87 genes/expressed sequence tags (ESTs) that were more highly expressed in new islets than in mature islets. Of interest, over a third of the enhanced genes/ESTs in new islets were highly expressed in pancreatic ducts but not in adult islets. To assess their potential use as markers of the immature β-cell, eight genes were further studied by RT-PCR and by immunostaining. Of these, cytokeratin-19 (CK-19), matrix metalloproteinase-2 (MMP-2), frizzled-2, surfactant protein-D (SPD), and CD-24 were expressed at the protein level selectively in β-cells of new islets, with little to no expression in mature islets from the same pancreas or in pancreases of sham-operated adult rats. These differentially expressed genes thereby provide potential markers for determining β-cell immaturity.
To test the generality of these candidate markers of newly formed β-cells, we examined in the current study their expression during the perinatal period, a time of recognized β-cell immaturity. We show that MMP-2, CK-19, and SPD are truly markers of new and immature β-cells and that their expression is transient and not entirely synchronous. Furthermore, we found a unique pattern of expression for each marker in perinatal pancreases. These results support the potential value of these markers for assessing the maturity of insulin-producing cells and in identifying newly formed β-cells in the pancreas.
Materials and Methods
Animals
Pancreases between embryonic day 18 (E18) up to 7 days after birth were excised from Sprague-Dawley rats (Taconic Farms; Germantown, NY), in which the time of gestation is ∼22–23 days, with islet formation occurring between E17–E19. Adult pancreases from Sprague-Dawley rats older than 2 months were used as negative controls. Timed-pregnant Sprague-Dawley rats were anesthetized, the uterus removed to chilled (4C) PBS, and the mother euthanized. Each fetus was decapitated, and then the pancreas was excised. All procedures were approved by the Joslin Institutional Animal Care and Use Committee. For neonatal rats, birth was considered time 0, and the pups were euthanized by decapitation at less than 12 hr (birth), 1 day, 2 days, 3 days, and 7 days. Excised pancreases were placed in 15 ml chilled Minimal Essential Medium Eagle (MEM) (Mediatech Cellgro; Manassas, VA) without serum for islet isolation or for immunostaining, in tissue cassettes, fixed by immersion in 4% paraformaldehyde for 2 hr at room temperature then overnight at 4C, and stored in PBS until embedded in paraffin.
Islet Isolation
Neonatal/fetal islets were isolated by a modification of the Hellerstrom technique (Hellerstrom and Swenne 1991). Pancreases from a litter (10 animals) were minced to obtain 1–2-mm pieces. Fifteen ml Liberase RI (12.5 mg/50 ml, Roche Applied Sciences; Indianapolis, IN) was added, and the tissue was digested for 20 min in a water bath at 37C, with vigorous shaking for 10 sec every 5 min. Cold MEM (25 ml) with 10% newborn calf serum was added to stop the digestion. Tissue was centrifuged, washed three times with MEM with serum, and then filtered through a 500-μm mesh. Islet fractions were collected at the interface of a gradient separation using 15 ml Histopaque 1077 (Sigma-Aldrich; St. Louis, MO) and 10 ml MEM without serum after centrifugation at 2500 rpm for 20 min. Islets from adult male Sprague-Dawley rats (older than 2 months) were isolated by Liberase digestion, as described by Montana et al. (1994). All islets were double hand-picked under a dissecting microscope, washed three times in fresh MEM with serum, then in calcium-magnesium–free PBS three times, and stored in 1 ml Trizol (Invitrogen; Carlsbad, CA) at −80C until extraction according to the manufacturer's instructions. Adult common pancreatic ducts, the portions of the common bile ducts that pass through the pancreas, were isolated manually by a modification of adult islet isolation (Sharma et al. 1999).
RNA Extraction, cDNA Synthesis, and PCR
Total RNA from islets from individual adult rats or from a litter of animals (up to 10 animals) for fetal and neonatal rats was extracted by Trizol and converted into cDNA with SuperScript Choice cDNA. It was necessary to pool fetal or neonatal islets from one litter to have enough RNA for analysis. Reverse transcription and semi-quantitative PCR were performed as previously described (Jonas et al. 1999). PCR conditions were set so that the number of cycles used was in the exponential phase of amplification and that each PCR product increased linearly. All primers (see Table 1) were designed with Eugene version 2.2 software (Daniben Systems; Cincinnati, OH) designed specifically for amplified RNA to confirm the array data. Each gene of interest was amplified in parallel with either ribosomal S25 or α-tubulin as internal control; we had verified that these housekeeping genes did not change over this time period. PCR was performed on RT-negative samples to exclude genomic DNA contamination for each cDNA preparation. PCR products were separated on a 6% polyacrylamide gel in Tris borate EDTA buffer. The gel was dried under vacuum at 80C for 1 hr and exposed overnight to a PhosphoImager screen (Molecular Dynamics; Sunnyvale, CA). Band intensity was quantified with ImageQuant software (Molecular Dynamics). Each marker was normalized to the internal control gene run in parallel. The expression levels of adult and neonatal islets were compared using four or five samples each.
Table 1.
Primers and conditions used for semiquantitative PCR
| Gene | Size product | 5′ Oligonucleotide (forward primer) | 3′ Oligonucleotide (reverse primer) | Annealing temperature | Cycles |
|---|---|---|---|---|---|
| SPD | 118 bp | AGCAAAGCTGCTTTCCTGAG | CTCCATTGTTGTTGGGCTC | 58C | 32 |
| MMP-2 | 166 bp | GTTGCCTTTGCTGTTG | TGGAGGAAGTGACTGGAGTTG | 57C | 32 |
| Cytokeratin-19 | 150 bp | TCCACACTACGCAGATCCAG | ATCTCTGCCACAGTGCCTTC | 57C | 32 |
| CD-24 | 168 bp | TCACACACTGATGCTTGCC | ATTCGGGTATGAGGCAAGG | 58C | 32 |
| Frizzled-2 | 125 bp | AGGAGGTGGACTGACTGATG | AACACACTGCCGTGAATCC | 57C | 32 |
| α-Tubulina | 451 bp | CTCGCATCCACTTCCCTC | ATGCCCTCACCCACGTAC | 57C | 32 |
| S-25 | 190 bp | CGGGACAAGC TGAACAATC | TGTGCTTTGA AACCAGCTTG | 58C | 32 |
As previously reported by Jonas et al. (1999).
Immunohistochemistry
Paraffin sections (5-μm) from the time course (E18 and E20, birth, 1 day, 2 days, 7 days, and adult) were immunostained for each candidate marker and then for insulin to identify the β-cells. More specifically, after antigen retrieval consisting of microwaving in 0.1 M citric acid buffer for 15 min (three separate 5-min pulses) and then cooling for 30 min, sections were blocked for nonspecific staining with 1.5% donkey serum for 20 min at room temperature and for endogenous biotin with a biotin blocking kit (Vector Laboratories; Burlingame, CA). The primary antibodies used were: CK-19 (rck108 mouse anti-human, 1:100; DAKO, Carpinteria, CA) using 5-min treatment with 0.1% trypsin as antigen retrieval; SPD (sc13980 rabbit anti-human-120, 1:50; Santa Cruz Biotechnology, Santa Cruz, CA), MMP-2, (ab7032 mouse anti-human,1:100; Abcam, Cambridge, MA), and frizzled-2 (af1307 goat anti-mouse 1:100; R and D Systems, Minneapolis, MN). After overnight incubation with primary antibodies at 4C, sections were incubated with the secondary antibody, donkey biotinylated anti-mouse or anti-rabbit IgG (1:400; Jackson Immunochemicals, West Grove, PA) and then with streptavidin-conjugated Alexafluor green (1:400; Invitrogen). Sections were then double stained for insulin by incubating overnight at 4C with guinea pig anti-bovine insulin (1:200; Linco Research, St. Charles, MO), and then Texas Red–conjugated Affinipure donkey anti–guinea pig IgG (1:400; Jackson Immunochemicals). Slides were mounted with DABCO-glycerol anti-fade medium. Non-immune serum was used as negative controls.
For each marker, sections were stained and photographed in parallel with identical settings for the complete time course of E18, E20, birth, 1 day, 2 days, 7 days, and adult. Thus, any differences seen among the images reflect the level of protein expression. Images were taken on a Zeiss 410 LSM in confocal mode with fluorescent filters for Alexafluor and Texas Red. Adobe Photoshop was used to make final figures with all manipulations done in parallel. Multiple sections from multiple animals at each time point were examined for each marker.
Results
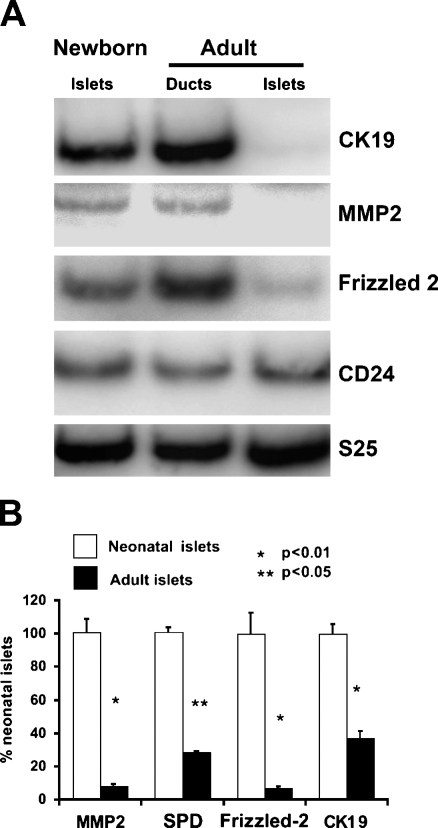
We have previously reported substantial regeneration in the adult rat pancreas after 90% partial pancreatectomy (Px) due to enhanced replication of the preexisting exocrine and endocrine cells, as well as differentiation of progenitors to form new lobes. Using this model, we found differentially expressed genes that are potential cell markers for determining β-cell immaturity. To validate these as true markers of newly formed β-cells, we examined the level of expression of these markers in neonatal rat islets in comparison to adult islets and adult ducts. By semi-quantitative RT-PCR, CK-19, MMP-2, and frizzled-2 were differentially expressed in neonatal islets compared with adult islets (Figure 1A). These markers of new β-cells were also strongly expressed in adult ducts. In contrast, CD-24 was not differentially expressed (Figure 1A) and so was not studied further. Quantification of these differential expressions was performed on five independent samples from neonatal islets (each a pool of 2 litters or ∼20 day-1 pancreases) and from adult islets (each a pool from two pancreases at age 2 months), as shown in Figure 1B. The ribosomal protein S25 was used as the control because we had found that it did not change between adult and neonatal islets.
Figure 1.
Differential expression of marker mRNAs in neonatal (1-day-old) islets, adult ducts, and adult islets by RT-PCR. (A) In this representative gel, CK-19 and MMP-2 are differentially expressed in newborn islets compared with adult islets; they are also expressed in adult pancreatic ducts. (B) Gene expression of markers is higher in 1-day-old neonatal islets (gray) than in adult islets (black) normalized to the internal control ribosomal S25. n=4–5 samples for each time point. Error bars represent standard error of the mean.
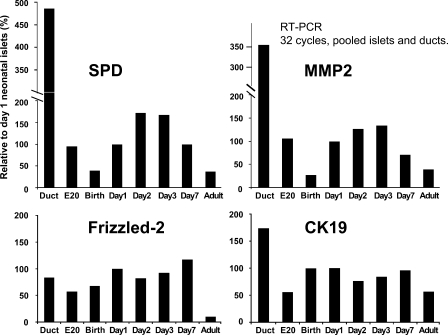
Neonatal islets have been reported to be immature at birth and to develop glucose-induced insulin secretion only after day 7, so we examined the expression of the potential markers from E20 to day 7 in two additional sets of pooled islets and compared their expression to that of adult islets and pancreatic ducts (Figure 2). Each marker had its own pattern of expression. Expression of both SPD and MMP-2 increased by day 1, peaked by day 3, and waned by day 7. CK-19 had almost equal expression across the perinatal period. Frizzled-2 had little change throughout the examined neonatal period but was greatly reduced in adult islets. Interestingly, the mRNA of all these markers was highly expressed in adult ducts.
Figure 2.
Each candidate marker had a distinct pattern of gene expression, as seen in this representative graph from one of two complete time courses of samples from islets pooled from multiple pups. α-Tubulin was used as internal control for normalization. RT-PCR, 32 cycles each. All expression values were normalized to the housekeeping gene, α-tubulin, and then shown relative to day 1 expression level.
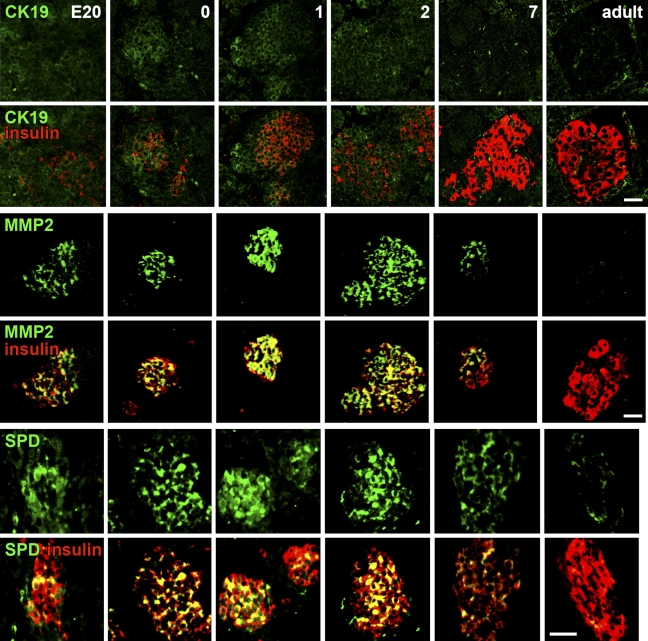
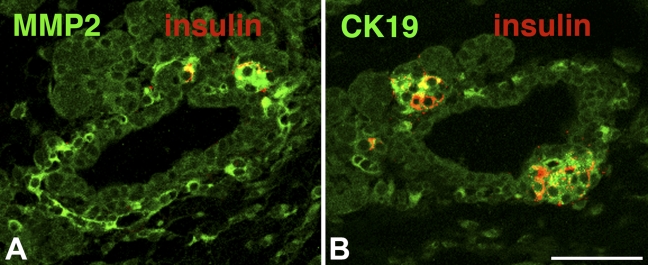
Because there can be discrepancies between mRNA and protein expression, we next examined the protein level of these potential markers during the time course from E20 to adult using immunohistochemistry (Figure 3). We found specific patterns of protein expression for each marker. Around birth, CK-19 is initially limited to expression in islets and some duct cells. CK-19 expression then peaks and encompasses more of the area around the islet at day 1. By day 2, CK-19 expression expands throughout the pancreas. At day 7, the pattern is similar in expression to the adult pancreas, where it is mainly expressed in the pancreatic ducts, with slight expression in acinar cells, and essentially is not present in the islet.
Figure 3.
Time course (E20 to adult) of the protein expression of the markers by immunostaining. For each marker (green) and insulin (red), immunostaining and confocal microscopy were done in parallel at the same settings for all samples, so the relative intensity reflects the protein expression. Colocalization of the marker and insulin is seen as yellow. For each marker, the top panel of images is that of the marker alone and the bottom panel has the merged image of both channels. Bar = 50 μm.
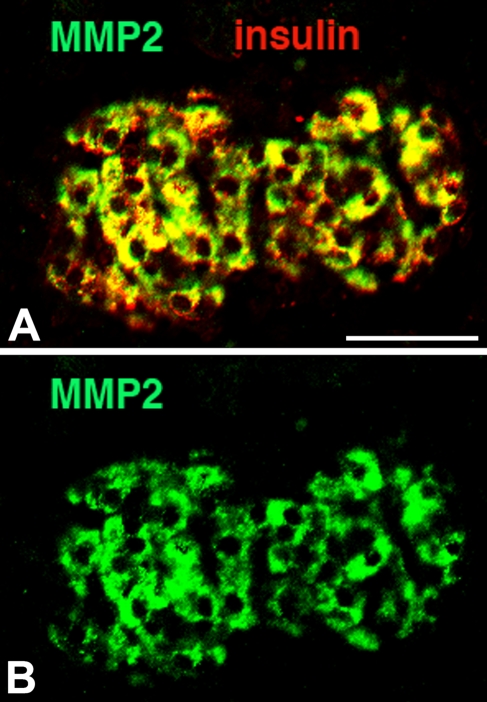
MMP-2 expression in islets appears in the embryonic stage by E20 and is greatest at birth; within the islet, its expression overlaps with that of insulin (Figures 3 and 4). After 48 hours, MMP-2 expression starts to decrease and disappears by day 7; it is absent in adult islets. We also examined the expression of CK-19 and MMP-2 more closely around birth (Figure 5). At birth, islets budding from ducts coexpress both CK-19 and insulin, but cells one or two cells away from the lumen express only CK-19. Intriguingly, MMP-2 expression is similarly found in those cells one to two cells away from the lumen, perhaps in delaminating endocrine progenitors.
Figure 4.
Expression of MMP-2 (green) in neonatal islets is seen in insulin-positive (red) β-cells (overlap is seen as yellow) (A). In the pancreas at birth, there is almost complete colocalization of insulin and MMP-2 expression. B shows only MMP2 expression (green). Bar = 50 μm.
Figure 5.
MMP-2 (green) (A) and CK-19 (green) (B) are localized in cells one to two cells away from the lumen of the main ducts and in insulin-positive cells (red) budding from these ducts at day 1. Coexpression is seen as yellow. Bar = 50 μm.
SPD pattern of expression is similar to that of MMP-2. At E20, SPD protein is expressed in ductal cells and β-cells. Among the three candidate markers, SPD has the most intense staining at E20. At 1 and 2 days, SPD is expressed in almost every β-cell, but by day 7, decreases, and is hardly detectable in the adult.
By immunochemistry, frizzled-2 colocalizes uniformly with insulin, with no differential expression at any age (data not shown). However, this antibody had not been verified by Western blot for specificity.
Discussion
With the generation of functional β-cells from stem/progenitor cells, there is a need to be able to assess the stage of differentiation toward mature, functional β-cells. Previous work by our lab using the 90% Px model identified markers of newly regenerated β-cells (Toschi et al. 2003). In the current study, the generality of several of these markers has been confirmed by showing their transient expression in β-cells for the time course E20 to day 7 in the perinatal period. We found that CK-19, MMP-2, and SPD mRNA and proteins are transiently expressed in β-cells of neonatal islets but not of adult islets. Each marker has its own unique pattern of expression such that a combination of such markers may help identify β-cells at more-precise differentiation stages.
Our time course was chosen to encompass two very important perinatal periods of growth: the time of islet morphogenesis, which occurs around E17–E19 in the rodent (Miralles et al. 1998), and the first week of life, when neogenesis occurs (Kaung 1994). Functionally, fetal and neonatal islets are different from adult islets, in that they are unresponsive to glucose (Bliss and Sharp 1992), but they gradually acquire glucose-induced insulin secretion, the hallmark of functional, mature β-cells. We propose that there are phenotypic differences that can be identified with cell markers.
We confirmed that these putative markers are highly expressed in the pancreatic ducts. Although we cannot be sure that their mRNA expression in the neonatal islets is not due to contamination of ductal tissue, we do not believe this to be the case, for the following reasons: (1) our microarray data from LCM of the β-cell cores identified these markers initially; (2) the islets were hand-picked twice to ensure purity; (3) the amount of RNA per cell in ducts was considerably less than in islets, so the amount of duct necessary to skew the PCR data would be far larger than what could have been included as contamination; and (4) the immunostaining supports the expression of these proteins within the islets.
Our proposed markers, CK-19, MMP-2, and SPD, have previously been shown to have roles in pancreatic development, but their functions remain unclear. CK-19, an intermediate filament specific for epithelial cells, has been proposed to characterize cells that are in a flexible state of differentiation (Stasiak et al. 1989). It is expressed in the basal layer of epidermis, in which the progenitor cells are found, whereas the differentiated cells lack CK-19 expression. Weak CK-19 immunoreactivity has been observed in human islets until the end of gestation, suggesting that β-cells arise from duct-like cells (Bouwens et al. 1997). Additionally, Bouwens et al. (1994) reported CK-19 expressed in a peripheral mantle zone continuous with the ductal epithelium surrounding newborn rat islets and suggested that these cells were the islet progenitors. Like Bouwens et al. (1994), we found CK-19 coexpression with insulin, particularly in budding islets at birth and in cells one or two cell distances away from the ductal epithelium. Thus, our findings support the idea that β-cells arise from ductal epithelial cells. However, shortly after birth, the expression of CK-19 broadens to the expanding acinar tissue.
MMP-2 (gelatinase A), a member of a family of zinc-requiring neutral endopeptidases that cleaves most extracellular matrix proteins (Pirila et al. 2003), has its activity highest at E17 to E19, when islet morphogenesis occurs (Kaung 1994). Although we found MMP-2 expression at the E18–E20 stage, its expression was highest at birth. MMP-2 has been shown to have a role in islet morphogenesis: in explant cultures, chemical MMP antagonists resulted in aberrant islet formation (Miralles et al. 1998). Furthermore, targeted deletion of the epidermal growth factor in mice resulted in decreased MMP-2, impaired epithelial branching, reduced β-cell proliferation, and defective islet morphogenesis (Miettinen et al. 2000). Our MMP-2 staining pattern suggests a role of remodeling the extracellular matrix, allowing delamination of the newly forming islet cells.
In contrast, SPD has not previously been shown in neonatal endocrine pancreas. SPD, a member of the C-type lectin family, is no longer considered lung-specific, having been found in several tissues, including fetal and adult human exocrine pancreas (Stahlman et al. 2002). SPD was expressed early in development, increased its expression, and colocalized with insulin between birth and 2 days. By day 7, SPD expression had disappeared. Thus, the transient expression of SPD may be useful as a candidate marker for identifying newly formed β-cells.
In conclusion, using gene expression and immunohistochemistry, we found phenotypic differences among late-embryonic, neonatal, and adult β-cells in a series of markers with transient expression patterns in the perinatal period. The expression pattern of each marker was unique. Their strongest expression occurs closest to birth, with little protein expression in adult islets. There should be a continuum of β-cell phenotypes from islet progenitor cells that perhaps goes through a ductal phase to an immature β-cell and finally to an adult β-cell. The overlapping expression of marker genes in both neonatal β-cells and ductal cells concurs with our lineage-tracing data (Inada et al. 2008) and supports the concept that ducts are an in vivo source of pancreatic progenitors after birth. Using just one of our candidate markers probably could not identify newly formed β-cells, but using combinations of them may do so. There are probably additional cell surface markers to identify this unique population of β-cells, and further investigation of such markers will be important.
Acknowledgments
The authors thank Dr. Gordon Weir for helpful discussions.
This article is a JHC article of the month. This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication with the exception of the JHC articles of the month which are immediately released for public access.
This research was supported by National Institutes of Health Grants DK-44523 and DK-66056 (to SBW), DK-61251, β-cell Biology Consortium (to DS), and the cores of the Joslin Diabetes Endocrine Research Center (DK-36836), by the Juvenile Diabetes Research Foundation (JDRF 1-2004-120 to SBW and 1-2000-389 to AS), and an important group of private donors. T.A. was recipient of an Endocrine Fellow Foundation award and a Lawson-Wilkins Fellowship.
References
- Artner I, Blanchi B, Raum JC, Guo M, Kaneko T, Cordes S, Sieweke M, et al. (2007) MafB is required for islet beta cell maturation. Proc Natl Acad Sci USA 104:3853–3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss CR, Sharp GW (1992) Glucose-induced insulin release in islets of young rats: time-dependent potentiation and effects of 2-bromostearate. Am J Physiol 263:E890–896 [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S (1997) Partial pancreatectomy as a model of regeneration. In Sarvetnick N, ed. Pancreatic Growth and Regeneration. Basel, Switzerland, Karger Landes Systems, 138–153
- Bonner-Weir S, Baxter L, Schuppin GT, Smith FE (1993) A second pathway for regeneration of adult exocrine and endocrine pancreases: a possible recapitulation of embryonic development. Diabetes 42:1715–1720 [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S, Trent DF, Weir GC (1983) Partial pancreatectomy in the rat and subsequent defect in glucose-induced insulin release. J Clin Invest 71:1544–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco D, Meda P, Thorens B (1995) Heterogeneous secretion of individual β cells in response to D-glucose and to nonglucidic nutrient secretagogues. Am J Physiol 268:C611–618 [DOI] [PubMed] [Google Scholar]
- Bouwens L, Lu WG, De Krijger R (1997) Proliferation and differentiation in the human fetal endocrine pancreas. Diabetologia 40:398–404 [DOI] [PubMed] [Google Scholar]
- Bouwens L, Wang RN, De Blay E, Pipeleers DG, Kloppel G (1994) Cytokeratins as markers of ductal cell differentiation and islet neogenesis in the neonatal rat pancreas. Diabetes 43:1279–1283 [DOI] [PubMed] [Google Scholar]
- Hellerstrom C, Swenne I (1991) Functional maturation and proliferation of fetal pancreatic beta-cells. Diabetes 40(suppl. 2):89–93 [DOI] [PubMed] [Google Scholar]
- Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, Morita R, Sharma A, et al. (2008) Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci USA 105:19915–19919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas JC, Sharma A, Hasenkamp W, Ilkova H, Patane G, Laybutt R, Bonner-Weir S, et al. (1999) Chronic hyperglycemia triggers loss of pancreatic beta cell differentiation in an animal model of diabetes. J Biol Chem 274:14112–14121 [DOI] [PubMed] [Google Scholar]
- Kaung HL (1994) Growth dynamics of pancreatic islet cell populations during fetal and neonatal development of the rat. Dev Dyn 200:163–175 [DOI] [PubMed] [Google Scholar]
- Miettinen P, Huotari M, Koivisto T, Ustivnov J, Pagli J, Rasilainen S, Lehtonen E, et al. (2000) Impaired migration and delayed differentiation of pancreatic islet cells in mice lacking EGF-receptors. Development 127:2617–2627 [DOI] [PubMed] [Google Scholar]
- Miralles F, Battelino T, Czernichow P, Shcarfmann R (1998) TGF-beta plays a key role in morphogenesis of the pancreatic islets of Langerhans by controlling the activity of the matrix metalloproteinase MMP-2. J Cell Biol 143:827–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montana E, Bonner-Weir S, Weir GC (1994) Transplanted beta-cell replication and mass increase after 95% pancreatectomy. J Clin Invest 93:1577–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura W, Kondo T, Salameh T, El Khattabi I, Dodge R, Bonner-Weir S, Sharma A (2006) A switch from MafB to MafA expression accompanies differentiation to pancreatic beta-cells. Dev Biol 293:526–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirila E, Ramamurthy NS, Sorsa T, Salo T, Hietanen J, Maisi P (2003) Gelatinase A (MMP-2), collagenase-2 (MMP-8), and laminin-5 gamma2-chain expression in murine inflammatory bowel disease (ulcerative colitis). Dig Dis Sci 48:93–98 [DOI] [PubMed] [Google Scholar]
- Sharma A, Zangen D, Reitz P, Taneja M, Lissauer M, Miller C, Weir G, et al. (1999) The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes 48:507–513 [DOI] [PubMed] [Google Scholar]
- Stahlman MT, Gray ME, Hull WM, Whitsett JA (2002) Immunolocalization of surfactant protein-D (SP-D) in human fetal, newborn, and adult tissues. J Histochem Cytochem 50:651–660 [DOI] [PubMed] [Google Scholar]
- Stasiak PC, Purkis PE, Leigh IM, Lane EB (1989) Keratin 19: predicted amino acid sequence and broad tissue distribution suggest it evolved from keratinocyte keratins. J Invest Dermatol 92:707–716 [DOI] [PubMed] [Google Scholar]
- Toschi E, Gaudet J, Sharma A, Weir G, Sgroi D, Bonner-Weir S (2003) Delineation of phenotypic differentiation between new and old beta cells. Diabetes 52(suppl.):A363 [Google Scholar]
- Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, et al. (2000) Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA 97:14720–14725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZJ, Kluger Y, Lian Z, Weissman SM (2005) Two types of precursor cells in a multipotential hematopoietic cell line. Proc Natl Acad Sci USA 102:18461–18466 [DOI] [PMC free article] [PubMed] [Google Scholar]