Abstract
Ro 23-9424 is a novel ester-linked codrug of fleroxacin (Ro 23-6240; AM-833) and the cefotaxime metabolite desacetylcefotaxime. Its potency was determined against over 1,000 organisms and found to be intermediate between those of the two components. More than 99% of members of the family Enterobacteriaceae were inhibited by greater than or equal micrograms of Ro 23-9424 per ml; its MIC for 50% of strains tested ranged from greater than or equal to 0.06 to 1 micrograms/ml. Staphylococci, streptococci, Branhamella catarrhalis, Corynebacterium jeikeium, Bacillus spp., Haemophilus influenzae, Listeria monocytogenes, and the pathogenic Neisseria spp., including oxacillin-resistant Staphylococcus aureus, beta-lactamase-producing strains, and penicillin-resistant pneumococci, were also inhibited by Ro 23-9424. Pseudomonas aeruginosa, Enterococcus spp., and Bacteroides fragilis group isolates were more refractory to Ro 23-9424 (the MIC for 90% of strains tested was less than or equal to 32 micrograms/ml). Overall, Ro 23-9424 inhibited 97% of the aerobic strains, compared with 90% for ceftazidime and 92% for cefoperazone. Ro 23-9424 was bactericidal, was relatively stable to inoculum effects on MICs at 10(7) CFU/ml, and was determined to be highly active against organisms resistant to fluoroquinolones or ceftazidime. Preliminary quality control guidelines were determined, and a 30-micrograms disk concentration appears to be the most usable form.
Full text
PDF
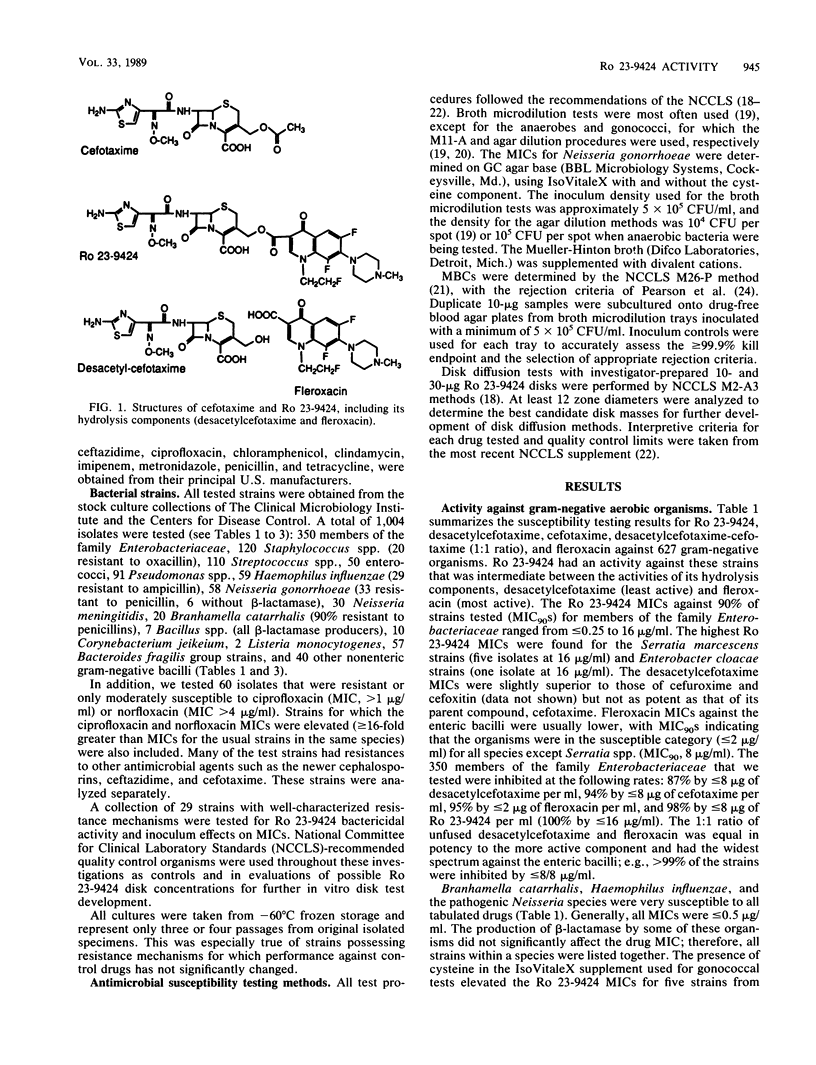
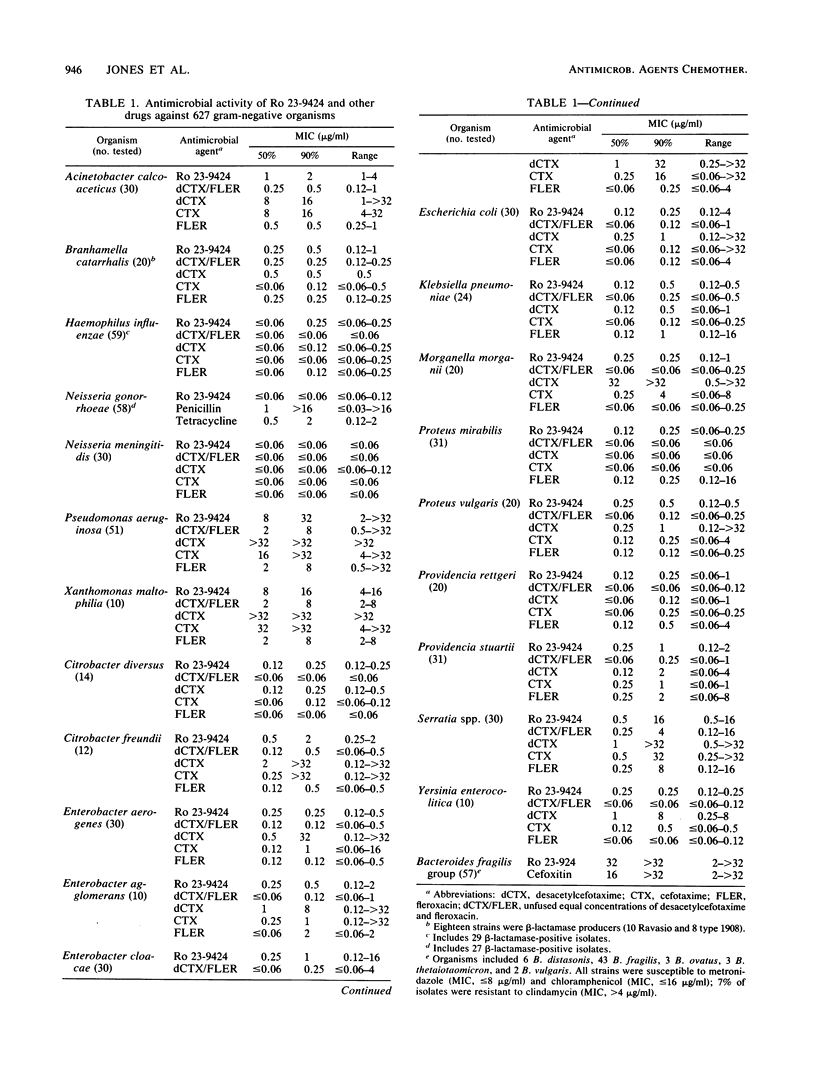
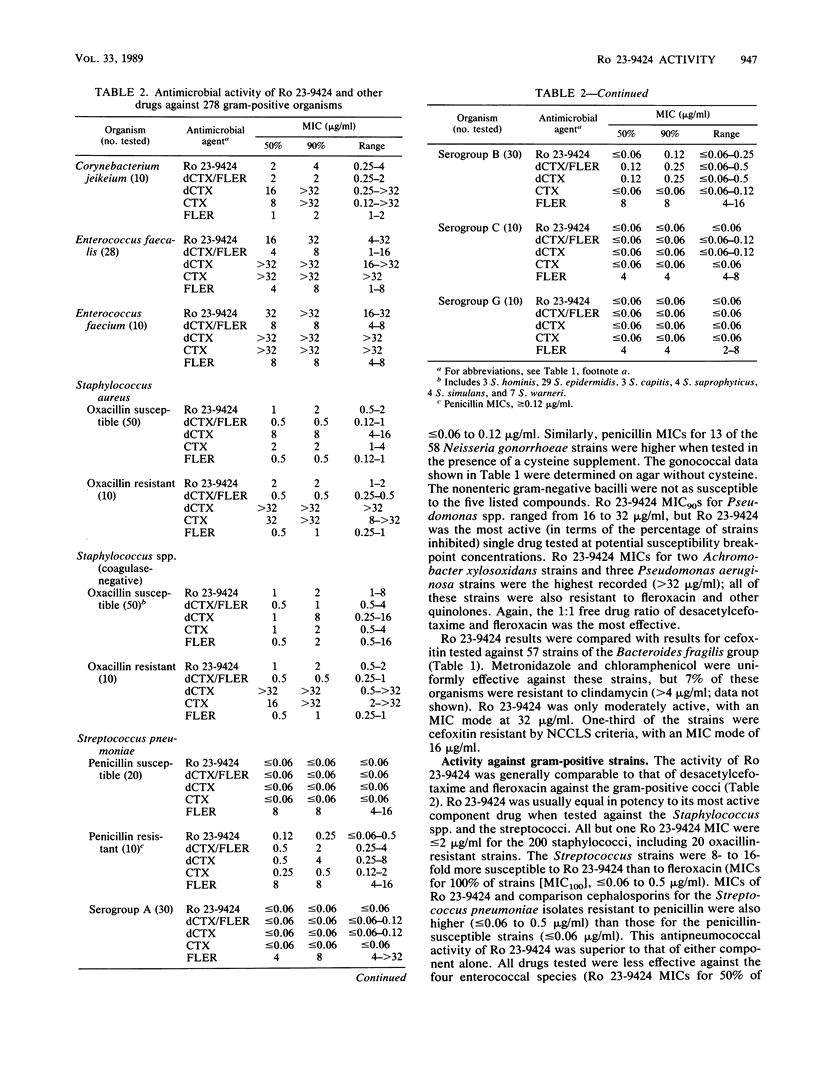



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball A. P., Fox C., Ghosh D. Sultamicillin (CP-49, 952): evaluation of two dosage schedules in urinary infection. J Antimicrob Chemother. 1984 Oct;14(4):395–401. doi: 10.1093/jac/14.4.395. [DOI] [PubMed] [Google Scholar]
- Baltzer B., Binderup E., von Daehne W., Godtfredsen W. O., Hansen K., Nielsen B., Sørensen H., Vangedal S. Mutual pro-drugs of beta-lactam antibiotics and beta-lactamase inhibitors. J Antibiot (Tokyo) 1980 Oct;33(10):1183–1192. doi: 10.7164/antibiotics.33.1183. [DOI] [PubMed] [Google Scholar]
- Boisvert W., Cheung K. S., Lerner S. A., Johnston M. Mechanisms of action of chloroalanyl antibacterial peptides. Identification of the intracellular enzymes inactivated on treatment of Escherichia coli JSR-O with the dipeptide beta Cl-LAla-beta Cl-LAla. J Biol Chem. 1986 Jun 15;261(17):7871–7878. [PubMed] [Google Scholar]
- Chin N. X., Brittain D. C., Neu H. C. In vitro activity of Ro 23-6240, a new fluorinated 4-quinolone. Antimicrob Agents Chemother. 1986 Apr;29(4):675–680. doi: 10.1128/aac.29.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin N. X., Neu H. C. Cefotaxime and desacetylcefotaxime: an example of advantageous antimicrobial metabolism. Diagn Microbiol Infect Dis. 1984 Jun;2(3 Suppl):21S–31S. [PubMed] [Google Scholar]
- Greenwood D., O'Grady F. Dual-action cephalosporin utilizing a novel therapeutic principle. Antimicrob Agents Chemother. 1976 Aug;10(2):249–252. doi: 10.1128/aac.10.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding S. M., Williams P. E., Ayrton J. Pharmacology of Cefuroxime as the 1-acetoxyethyl ester in volunteers. Antimicrob Agents Chemother. 1984 Jan;25(1):78–82. doi: 10.1128/aac.25.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley S., Wise R. A three-way crossover study to compare the pharmacokinetics and acceptability of sultamicillin at two dose levels with that of ampicillin. J Antimicrob Chemother. 1982 Jul;10(1):49–55. doi: 10.1093/jac/10.1.49. [DOI] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Hosaka M., Oomori Y., Niwata Y., Suzue S., Irikura T. In vitro and in vivo antibacterial activity of AM-833, a new quinolone derivative. Antimicrob Agents Chemother. 1986 Jun;29(6):1059–1066. doi: 10.1128/aac.29.6.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L., Thornsberry C. Antimicrobial activity of desacetylcefotaxime alone and in combination with cefotaxime: evidence of synergy. Rev Infect Dis. 1982 Sep-Oct;4 (Suppl):S366–S373. doi: 10.1093/clinids/4.supplement_2.s366. [DOI] [PubMed] [Google Scholar]
- Jones R. N., Thornsberry C. Cefotaxime: a review of in vitro antimicrobial properties and spectrum of activity. Rev Infect Dis. 1982 Sep-Oct;4 (Suppl):S300–S315. doi: 10.1093/clinids/4.supplement_2.s300. [DOI] [PubMed] [Google Scholar]
- Mobashery S., Johnston M. Inactivation of alanine racemase by beta-chloro-L-alanine released enzymatically from amino acid and peptide C10-esters of deacetylcephalothin. Biochemistry. 1987 Sep 8;26(18):5878–5884. doi: 10.1021/bi00392a045. [DOI] [PubMed] [Google Scholar]
- Mobashery S., Johnston M. Reactions of Escherichia coli TEM beta-lactamase with cephalothin and with C10-dipeptidyl cephalosporin esters. J Biol Chem. 1986 Jun 15;261(17):7879–7887. [PubMed] [Google Scholar]
- O'Callaghan C. H., Sykes R. B., Staniforth S. E. A new cephalosporin with a dual mode of action. Antimicrob Agents Chemother. 1976 Aug;10(2):245–248. doi: 10.1128/aac.10.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. D., Steigbigel R. T., Davis H. T., Chapman S. W. Method of reliable determination of minimal lethal antibiotic concentrations. Antimicrob Agents Chemother. 1980 Nov;18(5):699–708. doi: 10.1128/aac.18.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrinner E., Limbert M., Seeger K., Seibert G., Novick W. J., Jr The in vitro antimicrobial activity of desacetylcefotaxime compared to other related beta-lactams. Diagn Microbiol Infect Dis. 1984 Jun;2(3 Suppl):13S–20S. [PubMed] [Google Scholar]
- Wise R. After pro-drug -- mutual pro-drugs. J Antimicrob Chemother. 1981 May;7(5):503–504. doi: 10.1093/jac/7.5.503. [DOI] [PubMed] [Google Scholar]


